Abstract
This article describes why the safety and efficacy assessment of non-nutrient bioactives for reducing chronic disease risk is so complicated, especially for dietary supplements and traditional medicines. Scientists, regulators, and the public have different and sometimes opposing perspectives about bioactives. Drug, food, and traditional medicine models used for bioactive safety assessment are based on different assumptions and use different processes. Efficacy assessment is seldom based on clinical trials of boactives’ effects in reducing chronic disease risk. It usually consists of application of quality assurance measures and evaluation of label claims and commercial speech about ingredients or products to ensure conformity to regulations. Harmonization of safety and efficacy assessment on a global basis is difficult because of differences within and between regulatory systems. The recommendations provided may open the way for bioactives to play a larger health role in the future, fill gaps in data needed for crafting authoritative dietary guidance on intakes, and speed harmonization of global standards.
Keywords: Bioactives, Nutraceuticals, Functional ingredients, Safety, Effectiveness, Dietary Recommendations, Regulation, Policy
1. Introduction
The foods we eat contain thousands of bioactive chemicals. Historically, scientists focused first on characterizing the few dozen nutrients that were essential to human life. They studied nutrient effects and developed intake recommendations. Non-nutrient bioactives were disregarded as “dark matter” and largely ignored, except for those that possessed special properties affecting food safety, stability, taste, or appearance. Times have changed. Now the beneficial health effects of many non-nutrient bioactives are receiving attention. Some scientists declare that at last knowledge about the safety and efficacy of these non-nutrient bioactives has “come of age” and that “bioactive”, “functional” and “nutraceutical” products or diets rich in them will bring major future advances in preventing chronic degenerative disease. But have they really come of age? Others are not so sure, pointing to their variable quality, unvetted safety and dubious claims that are made about many products containing them. The problem today is to examine past progress, gaps, and how best to move forward in the face of such controversy.
This article examines why definitions and regulations for assessing safety and efficacy of non-nutrient bioactive ingredients in foods and dietary supplements are so difficult to handle within regulatory frameworks and harmonize globally. It describes the different scientific, cultural, and philosophical assumptions that contribute to the continuing controversy about bioactives’ safety, efficacy, and the advisability of providing authoritative dietary guidance about them, particularly in the United States (US). Current processes and their limitations are presented. The article concludes with recommendations for dealing with remaining challenges.
2. What are bioactives?
2.1. Historical context.
As the chemistry of living things developed in the late 19th and early 20th centuries, organic chemists categorized the chemicals necessary for the life of the plant as primary metabolites. The others, first characterized as secondary metabolites of uncertain utility, were later divided further into compounds that affected human beings either as drugs or poisons, and remaining compounds that were viewed as having no known human health effects (Bourgaud et al 2001). When epidemiological studies found that some of those seemingly inert, innocuous secondary metabolites were associated with positive long term health outcomes in epidemiological studies, the current concept of bioactives was born.
2.2. Why is it important to assess the safety and efficacy of bioactives?
As humans co-evolved with plants over the millennia they developed the ability to eat all sorts of xenobiotics that they were constantly exposed to in their diets without being poisoned. Over the course of evolution, some plants reproduced more efficiently and survived better than others because they developed specific bioactives such as the polyphenols that deflected potential herbivores or pathogens. Polyphenols have mostly structural functions in plants as complex polyphenolic glycosides, but they also serve as redox agents to protect chloroplasts from being damaged by ultraviolet radiation, as toxins that deflect potential herbivores or pathogens, and probably have other as yet unknown activities. Polyphenols certainly predate humans and most other members of the animal kingdom. For humans, the survival benefit over time of access to nutrients from plants has far outweighed the risks of being exposed to compounds like the polyphenols that are basically plant defense chemicals. It is possible that humans co-evolved with these phytochemicals to the point that some of these bioactives might reduce chronic disease risk and promote human health. However, such claims must be examined in the context of what is known about human biology (Li et al., 2022 and Cardoso et al. 2022). For all of human history except the past century, most human beings died of the infectious diseases of childhood, and not from chronic degenerative diseases such as coronary artery disease and cancers. Thus, it is highly unlikely that man co-evolved with these bioactives in a way that was always beneficial in preventing chronic disease. Surely it is possible that some bioactives have preventive benefits, and indeed it has been demonstrated in some cases (Sesso et al 2022). However, the pathways and targets are likely to be much more complex than is currently recognized, underscoring the importance of safety and efficacy assessment.
2.3. Perspectives about bioactives vary by scientific discipline
All ingested molecules influence living organisms to some extent, and all are thus “bioactive”, but such a broad definition is meaningless operationally. Working definitions are narrower, although they vary from one scientific discipline to another.
2.3.1. Biochemical
Bioactives are structurally complex, highly heterogeneous, diverse, nonnutritive organic mixtures of compounds that are frequently derived from plants. Their chemical structures are often difficult to determine because they often contain very large polymers and stereochemical elements that affect their activity. Some biochemists use the term bioactive to refer only refer to compounds that exist in nature or in crude extracts, while others include highly processed and synthetic organic compounds. Lists of bioactives usually include classes, subclasses and individual phytochemicals (e.g., polyphenols, flavonoids, phytosterols, saponins, phytates, caffeine, glucosinolates, isothiocyanates, and others) as well as some zoochemicals (e.g., choline, Coenzyme Q, and others).
2.3.2. Nutritional
Nutrition scientists distinguish between nutrient and non-nutrient bioactives on the basis of their essentiality for life and other characteristics described in Table 1. The U.S. Office of Dietary Supplements (ODS), National Institutes of Health (NIH) has tentatively defined the non-nutrient bioactives as ingredients in foods or dietary supplements other than those needed to meet basic human nutritional needs (i.e., nutrients) which are responsible for changes in health status (U.S. Department of Health and Human Services, 2004). The term bioactives will be used to refer to these non-nutrient bioactives in the remainder of this article.
Table 1.
Differences between nutrient and non-nutrient bioactives
| Characteristic | Nutrients | Non-Nutrients |
|---|---|---|
| Chemical structures known | Yes | For some, not all |
| Xenobiotics | Yes | Yes |
| Multiple functions, pathways and targets known | Yes | Incomplete |
| Essential for human life | Yes | No but may have health effects |
| Functions and metabolism known | Yes | Incompletely described |
| Safety assessment models available and agreed upon internationally | Yes | No |
| Adequate intakes known | Yes | No |
| Excessive intakes known | Yes | No |
| Efficacy assessment models available and agreed upon internationally | Yes | No |
| Intakes reducing chronic disease risk known | For some (dietary fiber, sodium, potassium) | Possibly for a few |
| Authoritative guidance on intakes is available in US | Yes | Not in US, but for selected bioactives in Korea and China (Lupton et al 2014) |
| Statutory definitions exist | Yes | No |
2.3.3. Natural products chemistry
Natural product chemists and pharmacognosists focus broadly on the thousands of bioactives in natural products that have human health effects, rather than solely on a few dozen of paramount interest to human nutritionists. They regard both nutrient and non-nutrient bioactives as xenobiotics, since both are foreign to the human body, not synthesized by it, and involve multiple pathways, functions, targets, bioactivities, and side effects with health implications.
2.4. Popular and regulatory definitions of bioactives differ as well
Table 2 summarizes some of the many terms in common use referring to bioactive ingredients or products rich in them. These include functional foods and ingredients, nutraceuticals, traditional complementary medicines, and herbals. Conventional descriptions of bioactives in common use imply that use of the ingredients or products containing them will impart a beneficial health effect while safety and adverse effects are often unmentioned.
Table 2.
Terms commonly used to describe ingredients and products containing non-nutrient bioactives.
| Term | Definition and examples |
|---|---|
| Functional ingredient | Ingredients with positive effects or functions beyond those of nutrients alone. |
| Functional food | A food claimed to have an additional beneficial function by adding new ingredients or more of existing ingredients that have this function. The term also applies to any modified food or food ingredient that may provide a health benefit beyond that of the traditional nutrients it contains, including foods with traits purposely bred into existing edible plants. |
| Nutraceutical | A substance, food or part of a food and produces a health benefit (Das et al., 2012). |
| Traditional or herbal medicine (Chinese, Kampo, Ayurvedic and herbal medicines. Still others are called herbal medicines, plants, and plant extracts) | Products involving the knowledge, skills and practices based on the theories, beliefs, and experiences indigenous to different cultures, used in the maintenance of health and in the prevention, diagnosis, improvement, or treatment of physical and mental illness. (World Health Organization 2019) |
2.5. Regulatory definitions are lacking
The term “bioactive” is not officially defined by any authoritative scientific body. Statutory and regulatory definitions of bioactives or terms for products containing them are not provided in U.S. law, nor in the laws of many other countries. The regulations for dealing with bioactives differ by intended use and vary greatly from country to country (Shao, 2017). Figure 1 shows the different regulatory categories into which they are placed and that determine how they are assessed.
Figure 1.
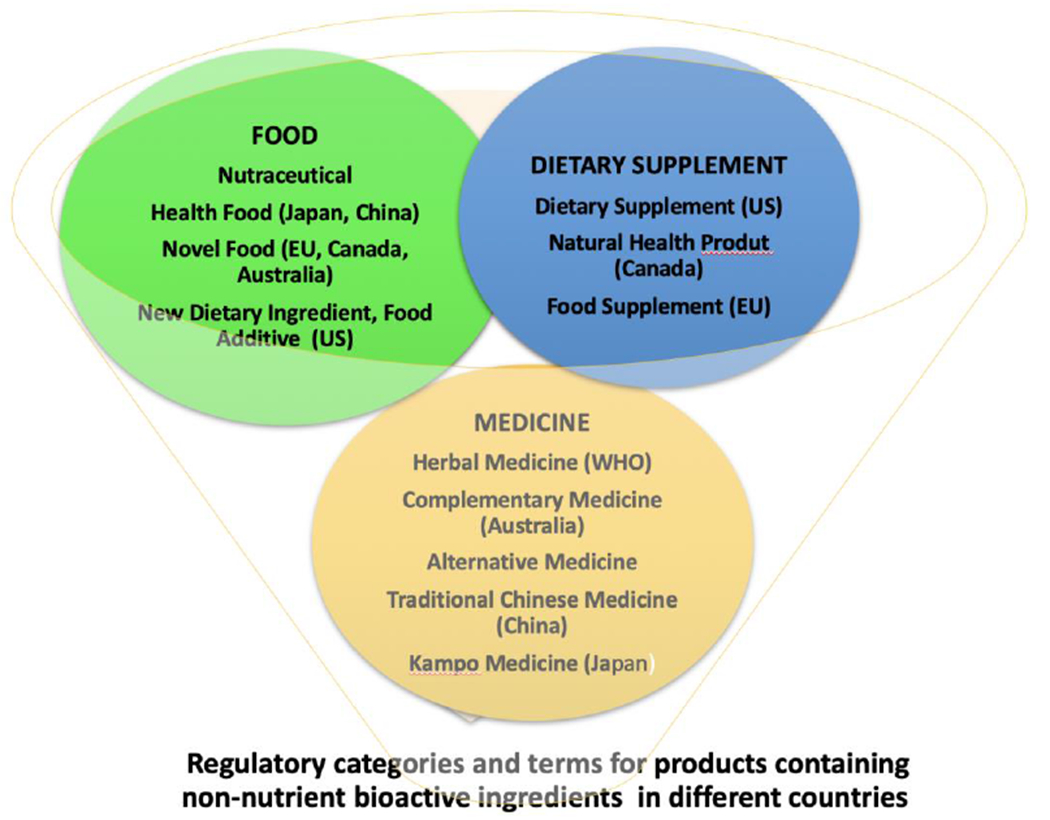
Different Regulatory Categories into which Bioactives are Placed in various countries
2.6. Implications
Safety and efficacy assessment are hindered by the diverse definitions of bioactives, their lack of chemical characterization, the diversity of bioactive ingredients and products, and different regulatory standards. The same products are categorized differently by regulations in various countries, sometimes as foods, sometimes as medicines, and in others as free-standing traditional medicines, each triggering different safety assessment procedures. Confusion is rampant, hampering global harmonization and regulation. For example, Ginkgo biloba is regulated as an herbal medicine in the European Union (EU), as a traditional medicine in China and Thailand, as a health food in Japan, and as a dietary supplement in the United States (Jiratchariyakul and Mahady, 2013).
3. How do underlying scientific, societal, and cultural assumptions affect safety assessment of bioactives?
Safety and efficacy are scientific concepts but they are not amenable solely to scientific resolution because they involve value laden societal, cultural, and legal views.
3.1. What underlying assumptions affect safety assessment?
Countries define what safety is and who decides how safe is “safe enough” based on societal and cultural views as well as science. Their responses influence laws, regulations and safety assessment processes that are adopted. Table 3 shows that starting assumptions vary both on the likely harmfulness of bioactive and how best to handle the risks they may pose. Assumptions that absolute safety can be achieved by precaution applies to all regulatory categories and may lead to processes that virtually prohibit use of some bioactives. Views that the risks bioactives pose can be attenuated to achieve a low probability of harm lead to risk- assessment and management strategies. Views that bioactives are inherently safe owing to a long history of use lead to processes stressing traditional methods of preparation to ensure safety. International consensus on the appropriate processes is difficult to achieve because these underlying views about safety vary so much.
Table 3.
Assumptions About Bioactives and Achieving Safety
| Regulatory Category | |||||
|---|---|---|---|---|---|
| All categories | Drug | Food | Dietary Supplement | Traditional and Complementary Medicine | |
| Assumption |
Highly unsafe |
Unsafe until proven safe |
Safe until proven unsafe |
||
| Goal | Precaution : Apply the precautionary principle to achieve absolute safety, and prohibit until proven safe | Risk Assessment : Assure safety by achieving a low probability of harm |
Safe History of Use: Assume that established tradition and long history of safe use ensures safety |
||
|
Assumption |
Bioactives must demonstrate no appreciable risk of harm Avoid uncertain hazards by invoking the precautionary principle when hazards and outcomes are uncertain Permit only new bioactives in foods or supplements only that are highly unlikely to result in significant harm (Stewart, 2002). Do not offset risks with benefits Use the best available technology to minimize risk of harm. Include a margin of safety below the predicted or observed no adverse effect level (NOEL) in regulations. Do not use lack of scientific evidence as an excuse for inaction when damage is predicted to be serious and irreversible |
Bioactives possibly pose health risks and therefore risk assessment is necessary to estimate the magnitude and probability of harm by the hazard. Risk management must then demonstrate that potential risks can be avoided or if not eliminated, so greatly attenuated that harm is extremely unlikely |
Food contains thousands of bioactives and is inherently safe. Only new ingredients need to be assessed | Includes different traditional ethnobotanical natural products used in some countries to treat various diseases and conditions (some recognized in Western allopathic medical classifications of diseases, others not | |
3.2. Possible premature application of the precautionary principle
The precautionary principle was first invoked by the European Union (EU) in dealing with threats of prion disease (bovine spongiform encephalopathy or “mad cow” disease) and more recently to prevent the use of bioengineered ingredients (genetically modified organisms or GMOs) in foods owing to their potential threats to biodiversity. In applying the principle, a good estimate of the risk (safety) is needed to avoid premature application and prohibition of a bioactive solely on speculative evidence. The goal of minimizing risk is difficult to apply in practice because the precautionary principle poorly defines the plausible uncertainties and risks that are threatening enough to trigger it. The strictest interpretation of the precautionary principle treats hazards as if they were risks and gives excessive weight to potential negative impacts and may dampen development of new ingredients (Hoetkamp, 2006).
4. Safety Assessment Processes for Bioactives
Regulatory agencies rightly give priority to safety concerns about bioactives. Bioactives’ effects are much more complicated physiologically than was originally thought, and their safety cannot automatically be assumed, as translational efforts over the last 50 years have shown (Box 1).
Box 1. A Half Century of Efforts to Translate Intakes of Non-Nutrient Bioactives into Decreased Chronic Disease Risk in Humans.
By the mid 20th century, antioxidant, and other functions of many non-nutrient bioactives were described in vitro, and cytochrome 450 enzymes in the liver were found to deactivate reactive molecules. Epidemiological studies later reported protective effects of plant-based diets containing them on chronic degenerative diseases
Hoping that bioactives might reverse oxidative or other types of human tissue damage and protect the body from xenobiotics with adverse effects, scientists fed experimental animals single bioactives thought to be the “magic bullets” responsible for beneficial effects using reductionist approaches based on drug discovery techniques with some promising results. However, difficulties arose in translating these findings to humans. The early randomized clinical trials of relatively short duration frequently failed to support effects reported in longer cohort studies (Weaver, 2014). Larger clinical trials were also often null and occasionally produced adverse effects, as in the Carotene and Retinol Efficacy Trial (CARET) study (Omenn et al., 1996). Also, many environmental factors other than diet such as compounds produced by sunlight, oxygen, cigarette smoke, and anaerobic exercise damaged human tissues. While liver enzymes deactivated some harmful xenobiotics, they activated some otherwise inert compounds, making them potentially dangerous. For example, work in the 1990s showed that polyphenols hydroxylated in one position up-regulated the activating enzymes, while others down-regulated those same enzymes (Zhai et al 1998, Canivenc-Lavier et al 1996). The chemical means of damaging bodily structures clearly went far beyond oxidation or any other single process. morbidity and mortality associated with use of ephedra products in the early 2000s that led to its ban provided further evidence that bioactives could not be automatically assumed to be safe and effective became evident with the morbidity and mortality associated with ephedra product use in the early 2000s that eventually led to its ban in the US (Palamar, 2011).
4.1. Processes
Bioactive safety assessment processes vary by regulatory category of intended use, and each category has its own special requirements. Table 4 describes the objectives rationale, and safety assessment processes for intended uses in drugs, foods, dietary supplements, and traditional medicines. The compound itself, the form in which the bioactive occurs, its’ chemical characteristics, and whether it occurs singly or in mixtures are all important determinants of the appropriate methods to employ.
Table 4.
Safety Assessment Processes for Bioactives
| Regulatory Category | ||||
|---|---|---|---|---|
| Model | Risk Assessment | History of Use | ||
| Drugs | Foods | Dietary Supplements | Traditional, Complementary and Herbal Medicines | |
|
Objective |
Use risk assessment processes for bioactive ingredients that are also used for drugs. Bioactive is unsafe until proven safe | Enforce good manufacturing practices to make sure the food is not adulterated. In the United States, a food is deemed adulterated (unsafe) if it bears any poisonous or deleterious substances that may render it injurious to health, or if the substance is an added ingredient even if the amount does not render it injurious to health. | Ensure that the dietary supplement is not adulterated. In the US, dietary supplements are considered adulterated (unsafe) if they present a significant or unreasonable risk of illness or injury under conditions of use suggested on labels or as specified in the U.S. Code of Federal regulations. The focus is on new dietary ingredients not in use prior to passage of DSHEA. | Ensure product integrity and the safety of traditional medical products containing bioactives by ascertaining that they have been produced with fidelity to classical techniques. (Liang et al., 2021). |
| Processes | Identify the bioactive or actives, including those that have been altered or optimized to enhance efficacy. The characteristics, activity and safety of the ingredient are validated and assessed. Next, preclinical testing determines the safety and efficacy of the ingredient/s in cells and experimental animals, including toxicological and pharmacologic studies on animals to elucidate the pharmacokinetics and pharmacodynamics. In the US and some other countries, before clinical studies begin, an investigational new drug submission (IND) must be approved if endpoints involving disease prevention, treatment, mitigation, or cure, and/or if a vulnerable population is being studied (e.g., children, pregnant women, and the elderly and frail). Other nutrition studies of bioactives do not require an IND, however. Human clinical studies commence in phases. In phase 0, micro doses are provided to assess toxicity. In phase 1 full doses are given to further identify possible toxicities and optimal doses. In phase 2, full doses are used in a small group to identify the compound’s benefits and side effects, then in phase 3 in a larger group, and in phase 4 for still longer. In phase 5, the ingredient is given over an even still longer time with continued surveillance to further monitor benefits and side effects. |
New food ingredients: In the US, Food and Drug Administration (FDA) premarket approval is required for new food ingredients. They must be assessed for safety before they are used, and major adverse events must be reported after marketing. In the US, bioactive ingredients in food use prior to 1955 were “grandfathered” as being generally recognized as safe (GRAS) by regulatory authorities. They are permitted in foods based on their prior history of safe use and do not require additional safety assessment. Isolated phytochemicals that are added back to conventional foods must either be GRAS or approved food additives. GRAS ingredients must be used only under conditions that are specified in the GRAS notifications, and in the minimum amount to achieve the desired technical effect. Mandatory listing of ingredients is required before food products enter the market. Databases of product contents are publicly available. In the US, manufacturers are a required to follow good manufacturing practices (GMPs) to ensure product safety. If requested, they must provide evidence that the product is safe. FDA has the power to inspect/audit productions facilities to ensure that good manufacturing practices (GMP) are being followed. Safety enforcement includes a variety of measures up to criminal charges |
Mandatory preapproval of dietary ingredients is not required except for new ingredients not present in dietary supplements at the time of the Dietary Supplement and Health Education Act (DSHEA) of 1994’s passage Product listing prior to marketing is not required, as it is for foods. Publicly available databases of dietary supplement products are now available (Dwyer et al 2018). FDA has inspection/audit/and enforcement power but first it must provide evidence of harm caused by a product or ingredient Note: Many bioactives such as quercetin do not meet standards for foods and thus are not permitted in foods, although they are allowed in dietary supplements Their safety cannot be automatically assumed. |
No standardized system exists globally for assessing the safety of these products. However, simplified assessment pathways leading to registration are similar in many Asian countries (Liang et al., 2021). Generally, less safety testing is required for traditional products and all countries place heavy weight on the long history of use, quality control that involves faithful adherence to traditional production and processing practices to ensure quality, product integrity, and safety. More testing is required for the nontraditional products. Both China and Korea have issued quantitative recommendations for selected bioactives although they rarely include data from animal toxicity or human clinical trials. (Lupton et al 2014) Methods for assessing adverse events occurring with traditional remedies already on sale in some European countries such as Italy include significant event audits (SEAs) and failure modes and effects analysis (FEMAs) (Rossi et al., 2017). that involves both risk assessment and risk management |
4.2. Limitations
The limitations of these processes are several, and include the following:
4.2.1. Bioactives differ from drugs, complicating application of the risk assessment model based on drug safety testing.
The bioactives’ inherent variability and degree of purity vary depending on their structure, stability, processing, and difficulty of synthesis. Preparations often consist of multiple compounds that may have effects as mixtures rather than as the single, highly purified compounds present in modern drugs. In many natural products, the identity of the actual bioactive is often unknown and the entire plant or extract is assumed to be the “bioactive.” In other instances, the active compound(s) is based on a mechanistic hypothesis that may or may not be backed up by in-vitro data (e.g., antioxidants) or only on guesswork. Other shortcomings of the drug assessment model are that some safety risks of bioactives are difficult to ascertain (e.g., end-organ damage and reproductive toxicology, or long-term harm, such as cancer). Benefits may also be elusive (e.g., lifetime reduction of risk or cancer or heart disease), and risk-benefit tradeoffs ultimately represent value judgements.
4.2.2. A history of use is an unreliable indicator of safety, particularly for traditional medicines and dietary supplements
Some traditional medicinal preparations contain only complex “classical” mixtures of natural products, while others include variations introduced by later processes and purified synthetic compounds (Shipkowski et al, 2018). Table 5 shows that the diverse types of products marketed as traditional medicines are placed in many different regulatory categories and that it further varies by country. Adverse health effects may arise with both traditional medicines and dietary supplements owing to different conditions of use, including uncontrolled self-medication, use at incorrect dosages or times, and use with certain drugs or foods that may generate adverse interactions. For example, Hypericum perforatum L. (St. John’s wort) reduces the blood levels of cyclosporine, cyclosporine, digitalis, theophylline, anti-retroviral medications, and oral anticoagulants. (Rossi et al., 2017).
Table 5.
Regulatory Category Placement of Different Types of Traditional, Complementary and Herbal Medicines For Selected Countries
| Regulatory Category | ||||
|---|---|---|---|---|
| Product | Drug | Special Pharmaceutical with various exemptions | Food/Health Food | Dietary Supplement |
| “Classical “or Traditional Medicine Preparations. (Ayurveda, Kampo, traditional Chinese and others) | United States: (if disease claims are made about them) |
China uses a tiered system of requirements for different categories of traditional and other medicines. “Classical Chinese Medicine Formulations” are exempt from producing evidence on mechanisms of action or from clinical trials for efficacy but must include formula composition, manufacturing details, dosage form, administration route compatible with classical use, dosage, as well required labeling statements on medication safety and restrictions for some users. Predefined lists of classic formulas from prescriptions in ancient medical textbooks are used as the basis for quality setting (Liang et al., 2021). Other tiers or categories of traditional medicines include innovative Chinese medicines, modified new Chinese medicines, and generic Chinese medicines, each with its own set of efficacy assessment criteria, some of which require additional stability, quality and safety tests. Japan: Classifies “ethical” or “prescribed” Kampo medicine products in this category. They are crude drugs, available by prescription from a physician and reimbursed by Japanese health insurance. They were “grandfathered” in 1986 and approved based solely on their chemistry and quality control with no required preclinical or clinical studies. |
Japan (some 200 or mor other Kampo products that are not reimbursable) | United States (if no disease claims are made |
| “Newer” traditional preparations (containing highly purified compounds and extracts of natural products and/or synthetic compounds) |
United States: Human studies and clinical trials are required to demonstrate safety and efficacy. If phases 3 to 5 of assessments for safety are successful a new drug application NDI is issued by FDA. After review and approval of data from the clinical trials, it can be marketed European Union: Products making medicinal claims about treating major health conditions are regulated under the pharmacological regulations of medicinal uses of products originating from plants. They must be registered as conventional medicines and are required to have a marketing authorization demonstrating quality, safety and efficacy. (Knöss and Chinoau, 2012). Japan: Since 1986 new products wishing to claim Kampo prescription status are required to evaluate their preparations as drugs, with clinical trials to demonstrate safety, but no additional products have been approved. Some other Asian countries also require data that benefits clearly outweigh accompanying risks and adverse effects (Thakkar et al., 2020). |
|||
| Herbal medicines (containing only herbal ingredients0 |
European Union: Products must meet regulatory standards for efficacy of the Committee on Herbal Medicinal Products of the European Medicines Agency (EMA) (Knöss and Chinoau, 2012). Japan: 200 over-the-counter Kampo products that are not reimbursed by health insurance are exempt from preclinical and clinical safety data (Maegawa et al., 2014). |
|||
4.2.3. Dietary supplements differ from foods, complicating the application of a food paradigm to them
The unique characteristics that pose potential safety concerns and challenges for bioctives in dietary supplements are described in Table 6. Although they are regulated more like foods than drugs in some countries, including the US, the bioactives in dietary supplements often behave differently than they do in foods. The father of toxicology, Paracelsus, said the poison depends on the dose. In foods bioactives are usually present in low concentrations and it is unlikely that hazardous amounts of bioactive constituents will be consumed. The food’s energy providing ingredients, water, and other ingredients promote satiety, and consumption of several pounds of food in a single serving would be required to yield a dangerous amount of the bioactive. In contrast, ingredients in dietary supplements are present in more highly isolated, purified, and concentrated forms which are easily ingested in very large quantities, often in a single bolus dose and in high amounts. The combinations of ingredients, matrices, physical forms of products containing the bioactive, dosage form performance (i.e., whether and how a supplement dissolves, disintegrates, and goes into solution) and conditions of use are also different in dietary supplements and affect. ingredient bioavailability and bioaccessability. The combinations of ingredients also influence other medications taken at the same time, altering amounts of the bioactive reaching target sites of action. They may be present owing to the composition of the preparations themselves or differences in conditions of use, especially when the products are ingested chronically may both pose risks. Some types of dietary supplements such as condition specific performance enhancers, body building and weight loss products are particularly liable to violations in good manufacturing practice, (GMP) adulteration, and spiking with active drugs. (Crawford et al., 2022). The lack of clinical trials to assess safety of dietary supplements prior to marketing them makes it difficult to anticipate the presence and causes of adverse effects. Staffing limitations limit the number of GMP regulatory inspections that can be done. For example, in the US, the Food and Drug Administration’s Office of Dietary Supplement Programs currently employs a few dozen personnel to review an estimated 80,000 products produced by 2,000 firms. Safety problems are further compounded because in the US FDA must provide evidence that a bioactive is unsafe before it can institute enforcement measures for dietary supplements.
Table 6.
Differences between Dietary Supplements and Foods
| Characteristics | Dietary Supplements | Foods |
|---|---|---|
| Concentration of bioactives | Higher and in more highly isolated, purified, and concentrated forms | Low |
| Presence of other ingredients | Varies, generally fewer | Energy providing ingredients, water, and other ingredients dilute bioactive and may promote satiety, |
| Ease of ingesting a large quantity of bioactive | Easily ingested in very large quantities, often in a single bolus dose in high amounts | Unlikely that hazardous amounts of bioactive will be consumed in a single serving |
| Dosage form performance | Matrices (tablets, capsules, soft gels and liquids) and ingredients differ in dissolution and disintegration | Matrices vary |
| Conditions of use | Bolus doses and on empty stomach common, sometimes with food | Usually with other foods or beverages |
| Regulation | Less highly regulated than foods | Highly regulated |
4.2.4. Traditional medicines differ from conventional drugs and it is difficult to ensure their safety and product integrity
Traditional medicines have many characteristics that differ from drugs and therefore safety assessment processes for drugs may need to be modified when applied to them. The bioactives responsible for the beneficial effects of traditional ethnobotanical medicines are often unknown. The sources of the natural products are difficult to access, supplies are unreliable, and product composition varies by season and environmental factors, especially if the bioactives are secondary metabolites in plants. In some preparations, the extent to which the bioactives have been isolated from their natural sources, and the bioactivity of these isolates is uncertain. Modern, rather than the “traditional” botanical preparation and processing techniques are often used today and so product quality or potency may be different than classical preparations (Funk and Schneider, 2021). Also, the use of traditional medicines today differs from classical indications. Traditional medicines are not being used over a short time for classical ailments but for longer periods of time to treat chronic degenerative diseases such as coronary artery disease that were rare in antiquity, increasing the possibility of delayed adverse events and cumulative organ damage. Some but not all countries have adopted regulations to ensure product integrity and safety, especially for the “newer” traditional medicine formulations to deal with concerns about quality and possible adverse effects.
4.2.6. One country’s traditional medicine is often regulated as a drug or dietary supplement elsewhere
For example, in the United States, if a treatment or cure claim is made about a traditional Chinese medicine, FDA treats it as a drug. If no treatment claim is made, products used as traditional and herbal medicines are regulated as dietary supplements.
4.2.6. Internationally harmonized safety assessment standards for dietary supplements and traditional medicines are difficult to achieve.
In different countries, the same product may be classified in many different regulatory categories. Resolution of these international differences is complicated because not only the regulatory categories themselves but the assessment processes required within each category vary from one country to another and are enshrined in laws and regulations. Discrepancies from country to country for traditional and complementary medicine safety assessment processes are described in Table 5, and they are provided elsewhere for dietary supplements (Dwyer Coates and Smith 2018). A few other examples will suffice. Herbal preparations are regulated as a prescription only medicines, traditional herbal medicines, herbal medicinal products, food supplements, dietary supplements and homeopathic or anthroposophical medical products in different countries (Bilia et al., 2021). Even within the EU itself, despite having a common legislative framework, member states often regulate the same products in different categories. Efforts to harmonize regulations for popular botanicals such as St. John’s wort, valerian, ginkgo, ginseng, and green tea failed because different countries positioned the botanicals’ differently in their regulatory frameworks and local laws.
In Europe, botanicals were initially defined as “other substances” by the European Food Safety Agency (EFSA) in 2001 and subsequently examined more fully to determine which ingredients were toxic (and should be prohibited), and which should be regulated as medicines. However, each country has different laws and regulations on what constitutes toxic or medicine-like characteristics, and despite several EU harmonization efforts, botanicals remain unharmonized today (Vettorazzi et al., 2020). In the US, those same botanicals (including herbs, spices, algae, fungi, and lichens) are regulated as foods and dietary supplements, and not as medicines as long as they do not make disease claims. If they do make such claims they are regulated as drugs. The profusion of different regulations for safety assessment makes it difficult for regulators to deal with dangerous or irrational product formulations that are either unsafe themselves or that jeopardize the metabolism of drugs or other compounds. It also complicates the process of locating and disciplining noncompliant producers across national borders.
5. Efficacy
5.1. Background.
Legislators and regulators everywhere and want bioactives to be efficacious and to pose minimal risk. But they disagree on how efficacy should be defined, whether and how it should be assessed, and who should decide. Also, it has been difficult to implement appropriate models for translating in vitro findings on bioactives into clinical trials that show reduced chronic disease risk in humans. Initially too little attention may have been paid to the fact bioactives’ health effects were much smaller than the large, easily observed effects of a few compounds purified from natural products that are now used in drugs like atropine, digoxin, and insulin. Humans consume bioactives in low doses, chronically, with cumulative effects that are likely to be small, and in mixtures, with the activity of one compound often dependent on the presence of others. Clinical trials using well characterized mixtures of bioactives may sometimes have failed to demonstrate efficacy not because they lacked a true effect, but because they recruited insufficient numbers of participants, administered the intervention over short time frames, and lacked definitive endpoints. For reducing risks of most chronic diseases, years-long interventions with tens of thousands of subjects are required to have sufficient power to detect such small effects. Many such clinical trials have now been carried out on bioactives in dietary supplements and foods, including the Age Related Eye Disease Study (AREDS), Vitamin D and Omega Three Trial (VITAL), Women’s Health Initiative (WHI), Selenium Vitamin E and Cancer Trial (SELECT), and Cocoa Supplement and Multivitamin Outcomes Study (COSMOS) (Chew, 2014; Chew, 2021; Leboff et al., 2018; Prentice, 2021; Klein et al., 2011; and Sesso et al., 2022). However, the work is difficult, expensive, and often, but not always, results are discouraging. Nevertheless, clinical trials of some bioactives are proceeding, some with positive results. For example, using a well characterized cocoa extract standardized for cocoa flavanols the recently completed COSMOS clinical trial showed beneficial effects on cardiovascular disease outcomes (Sesso et al., 2022).
5.2. Evidence of Efficacy
The efficacy standards and processes vary markedly for bioactives in different regulatory categories. ( e.g., drugs, traditional medicines, foods, and dietary supplements). They currently rely chiefly on ensuring product integrity through enforcement of good manufacturing practices (GMP) to ensure quality coupled with regulation of claims made about bioactives on labels or in commercial speech. In contrast, definitive evidence from clinical trials showing chronic disease risk reduction is rarely required for foods, dietary supplements and traditional medicines, while it is for drugs before they can be sold. Regulatory agencies lack the clear statutory authority to regulate the other categories that they do for drugs.
5.1. Drugs
5.1.1. Process.
Once risk assessment has been successfully completed, risk management is employed to find ways to reduce and manage the potential risks before the bioactive is marketed (see Table 3). In the US, the manufacturer must submit evidence that the new ingredient is safe in the manner it will be used, and FDA must grant approval before it is marketed.
5.1.2. Limitations
The process for approval is costly and time consuming, and few incentives exist for manufacturers to seek it.
5.2. Traditional Medicines
5.2.1. Process
Traditional medicines sold today include “classical” traditional medicines with both herbal and non-herbal ingredients, herbal medicines containing only herbal ingredients and newer preparations containing both extracts and highly purified active compounds that resemble drugs. There is no single process for evaluating their efficacy varies greatly from country to country. The “classical” traditional and herbal medicines are often treated by regulators in their countries of origin as a special category of pharmaceuticals for which mechanisms of action and supportive data from clinical trials are not required. (Liang et al., 2017).
5.2.2. Limitation: Absence of clinical trials makes it difficult to evaluate effects of traditional medicines and dietary supplements on chronic disease risk
Since clinical trials are not required for traditional medicines, if beneficial effects are found they may be from the bioactive preparation itself, concomitant interventions in lifestyle that are prescribed along with their use, placebo effects or all of these (Funk and Schneider, 2021).
5.3. Foods
5.3.1. Process
Foods are regarded as efficacious by definition because they have nutritional effects and therefore the efficacy of most bioactives in foods is usually based on quality assessment. However, claims about special benefits of bioactives in foods beyond those of nutritional value alone trigger requirements for evidence to back them up. The statements that are allowed in labeling and marketing as well as quality and product integrity standards vary from country to country. For example, in the United States the types of evidence required vary depending on the intended use of the bioactive ingredient (e.g., whether it is classified as a drug, food, or dietary supplement), and the claims being made about it. Permissible statements about bioactives on labels include content, dietary guidance statements, and health claims of various sorts. Table 7 presents allowable claims for bioactives in foods and dietary supplements in the US. Note that structure-function claims allowed on dietary supplements are not evaluated by FDA, although all such claims must be truthful and not misleading. Table 8 provides examples of some unqualified and qualified health claims for bioactives in the US. In the EU there are three types of health related claims. “Functional health claims’(Article 13 claims) that relate to growth, development, bodily, psychological, and behavioral functions, slimming or weight control treat botanicals and botanicals differently. “Risk reduction” claims on reducing a risk factor in the development of a disease (Article 14(1)(a) claims) are like the United States’ structure-function claims, (e.g., Plant stand esters have been shown to reduce blood cholesterol. Blood cholesterol is a risk factor in the development of coronary heart disease). The third category of EU health claims refers specifically to child growth and development (Article 14(1)(b) claims). Manufacturers are allowed to promote nutritional and/or scientifically proven health benefits of their food products after the European Food Safety Agency (EFSA) positively reviews the scientific evidence for them (Vettorazzi et al., 2020).
Table 7.
Types of claims allowed about the Efficacy of Bioactives in Foods and Dietary Supplements in the USA
| Allowed In: | Comments | ||
|---|---|---|---|
| Food | Dietary Supplements | ||
| Claim and Description | |||
|
Nutrient Content Claims |
√ | √ | The content of a bioactive in can be provided but standardized terms and units are required for nutrients but no other bioactives |
|
Dietary Guidance Statements (based on consensus recommendations from reports such as the Dietary Guidelines for Americans to suggest that ingredient helps maintain a nutritious dietary pattern) |
√ | . | FDA premarket review not required Not encouraged on supplements because authoritative sources recommend that consumers meet their nutrient needs from whole food sources |
|
Authorized Health Claims (sometimes referred to as unqualified health claims, are based on “significant scientific agreement” (SSA or unqualified health claims). Approved by FDA for use based on strong scientific consensus on the evidence. |
√ | √ | Authorized health claims communicate more specific information on the relationship between a food substance such as a bioactive ingredient and reduced risk of a disease or condition |
|
Qualified Health Claims Describe the role of a bioactive in maintaining normal healthy bodily structures or functions Refer specifically to how an ingredient impacts a disease or condition |
√ | √ | Used when evidence is less than that needed to support the SSA standard. Supported by some scientific evidence, but the quality and amount of science are less definitive, FDA conditionally permits their use only after premarket review and when specific wording is applied. |
|
Structure Function Claims In the United States, when the botanicals label states how they might help a bodily function, they are regulated as dietary supplements, but if they claim to treat a disease or illness, they are regulated as drugs (Dwyer et al., 2021 and Dwyer et al., 2018). |
√ (only for claims based on nutrients | √ | Must be truthful, not misleading, and include a disclaimer that the claim has not been evaluated by FDA (Burdock Group, 2021. |
Key: √= yes
Table 8.
Authorized (SSA) and Qualified Health Claims about Bioactives in the United States
| Type of Claim | Condition | Health Claim |
|---|---|---|
| Authorized (Significant Scientific Agreement) Health Claims | ||
| Cancer | • fiber containing grain products, fruits and vegetables and decreased risk of some cancers • Fruits and Vegetables and Cancer • Dietary Lipids (Fat) and Cancer |
|
| Cardiovascular Disease | • fruits, vegetables, and grains containing fiber, particularly soluble fiber, and decreased risk of coronary heart disease. • soluble fiber from certain foods and risk of coronary heart disease (barley, Oatrim, psyllium husk, soluble fibers, and beta glucans from whole oats) • stanols and sterols and reduced risk of coronary heart disease. • FDA published a notification in 2017 that it intended to remove the authorized SSA health claim for soy protein and coronary heart disease but it has not yet been formalized • Dietary Saturated Fat and Cholesterol and Risk of Coronary Heart Disease |
|
| Dental caries | • dietary noncariogenic carbohydrate sweeteners and dental caries (including D tagatose and sugar alcohols). | |
| Hypertension | • sodium and hypertension | |
| Neural tube defects | • folic acid and neural tube defects | |
| Osteoporosis | • Calcium, Vitamin D, and Osteoporosis | |
| Qualified Health Claims | ||
| Atopic dermatitis | • 100% Whey-Protein Partially Hydrolyzed Infant Formula and Reduced Risk of Atopic Dermatitis | |
| Cancers | • Green Tea and Risk of Breast Cancer • Prostate Cancer Health Claim • Selenium and a Reduced Risk of Site-specific Cancers • Tomatoes and Prostate, Ovarian, Gastric, and Pancreatic Cancers • Tomatoes and Prostate Cancer • Calcium and Colon/Rectal Cancer and Calcium and Colon/Rectal Polyps • Selenium and Certain Cancers • Selenium and Certain Cancers • Antioxidant Vitamins and Risk of Certain Cancers |
|
| Cardiovascular disease | • Oleic Acid for the Oleic Acid from Edible Oils and Coronary Heart Disease Claim • Folic Acid, Vitamin B6, and Vitamin B12 and Vascular Disease • Health Claim Relating B Vitamins and Vascular Disease • Nuts and Coronary Heart Disease • Macadamia Nuts and Reduced Risk of Coronary Heart Disease • Walnuts and Coronary Heart Disease • Nuts and Coronary Heart Disease • Omega-3 Fatty AcidsOmega-3 Fatty Acids and Reduced Risk of Coronary Heart Disease • Omega-3 Fatty Acids and Reduced Risk of Coronary Heart Disease • Soybean Oil and Reduced Risk of Coronary Heart Disease • Corn Oil and Corn Oil-Containing Products and a Reduced Risk of Heart Disease • Unsaturated Fatty Acids from Canola Oil and Reduced Risk of Coronary Heart Disease • Monounsaturated Fatty Acids from Olive Oil and Coronary Heart Disease |
|
| Cognitive function | • Phosphatidylserine and Cognitive Function and Dementia Qualified Health Claim | |
| Diabetes | • High-Amylose Maize Starch and Reduced Risk Type 2 Diabetes Mellitus • Psyllium Husk and a Reduced Risk of Type 2 Diabetes) • Whole Grains and a Reduced Risk of Diabetes Mellitus Type 2 • Chromium Picolinate and a Reduced Risk of Insulin Resistance, Type 2 Diabetes |
|
| Hypertension | • Eicosapentaenoic Acid and Docosahexaenoic Acid and Reduction of Blood Pressure in the General Population • Calcium and Hypertension, Pregnancy-Induced Hypertension, and Preeclampsia |
|
| Peanut Allergy | • Ground Peanuts and Reduced Risk of Developing Peanut Allergy | |
| Urinary Tract Infection | • Cranberry Juice Beverages and Cranberry Dietary Supplements and Reduced Risk of Recurrent Urinary Tract Infection in Healthy Women |
5.3.2. Limitations: Rigorous standards for the efficacy of bioactives on chronic disease risk reduction are lacking, especially for dietary supplements and traditional medicines
In Western countries today, demonstrations of efficacy of bioactives in chronic disease risk reduction with more rigorous standards of causal inference are increasingly being required. Many studies of poor quality are insufficient to support efficacy claims. For example, in the US, FDA now requires greater detail on how studies were conducted, and more credence is placed on demonstrations of efficacy in randomized double blind clinical trials than on observational studies within the totality of evidence. Also, currently accepted and validated surrogate endpoints must be cited in research intended to support health claims (Liska and Olsen, 2018). Since there are still very few well conducted randomized clinical trials available on bioactive ingredients and products, it is understandable that regulatory authorities have granted few petitions.
5.4. Dietary supplements
Countries differ on what products are regulated as dietary supplements. For example, In the EU the dietary (food) supplement category is restricted largely to nutrients, and herbals are regulated either as drugs or traditional medicines, depending on their constituents. In the United States, nutrients, herbals, and many other bioactives are regulated as dietary supplements, as long as they do not make disease claims
5.4.1. Process
In the US, efficacy assessment consists of assuring the quality of bioactive containing dietary supplement products and regulating claims about them, as is done for foods. Use in dietary supplements does not require efficacy assessment unless they contain “new” dietary ingredients that were introduced after passage of the Dietary Supplement and Health Education Act (DSHEA) of 1994. To ensure quality, FDA provides guidance on current GMPs for preparation, packaging, labeling, storage practices, and documentation of ingredients in dietary supplements that meet specifications for purity, composition, and strength. (Bailey, 2018). FDA-authorized health claims, qualified health claims, structure-function claims, and nutrient content claims are all permitted on dietary supplements, along with many other marketing statements (Hua et al., 2021). If dietary supplements are marketed with ‘disease’ claims (e.g., ‘this supplement shrinks tumors’) they are regulated as drugs.
5. 4.2. Limitations
5. 4.2.1. Product integrity is vital for assessing efficacy, but it is often problematic, particularly in multi-ingredient products
Efficacy assessment of multi-ingredient products such as dietary supplements and traditional medicines is impossible when product integrity is lacking. This lack has plagued many clinical trials containing mixtures of natural products such as turmeric, cranberry, green tea, and spices. (Panel on Dietetic Products, Nutrition, and Allergies, 2009 and Raz et al., 2006). For example, botanical preparations were poorly characterized in studies testing turmeric, an anti-inflammatory used in Ayurvedic medicine to lessen inflammation for its effects in obesity related conditions and musculoskeletal diseases, making it difficult to evaluate the results (Raz et al., 2006). Also, products were poorly characterized in some clinical trials of cranberry preparations and dietary supplements. Thus, it was unclear if the null results were due to lack of product integrity or to a true lack of effect (Panel on Dietetic Products, Nutrition, and Allergies, 2009 and Maddan et al., 2021). Later, a qualified health claim for cranberries was obtained after more rigorous clinical trials with well characterized products (see Table 8).
5. 4.2. Efficacy assessment of bioactives in dietary supplements using validated endpoints or surrogates is infrequent
Clinical trials demonstrating an impact of a bioactive on reducing validated outcomes or surrogates of chronic disease risk are rarely done for bioactives in dietary supplements. The law does not require it and incentives are lacking for manufacturers to invest the time and money required to conduct such efficacy assessments when patent protection for an ingredient or product unlikely and marketing advantages are unclear. Health claims are made instead based on less definitive changes such as a change in an intermediary biomarker of metabolism. However, a few bioactives sold as dietary supplements have now been assessed using the drug standard
6. Authoritative statements on recommended intakes of bioactives in the US
Scientists and regulators in the US have struggled for many years about the level of evidence required and the wisdom of making authoritative recommendations to consumers on safe and potentially beneficial intakes of bioactives. Today, regulatory processes involving the efficacy of bioactives’ impacts on chronic disease risk depend on what regulatory authorities deem to be permissible in labeling and commercial speech about bioactives, and that in turn is based on what authoritative statements that are available. Some countries in Asia have made such recommendations for selected bioactives and there is considerable interest in developing such recommendations elsewhere, but the devil is in the details about acceptable standards for safety and efficacy (Lupton et al 2014) The timeline of events and statements about bioactives made by authoritative US bodies and others in recent years provided below traces thinking on the subject and where recommendations stand today
6.1. Background
The Recommended Dietary Allowances (RDA) for nutrients issued by the Food and Nutrition Board (FNB) of the National Academy of Sciences(NAS) provided authoritative guidance on “safe and adequate levels of intake” of some non-nutrient bioactives from the 1970s to 1980s (National Research Council 1989a). Growing recognition of the contributions of nutrient and non-nutrient bioactives in reducing chronic disease risk and their importance in healthful dietary patterns was also apparent in Diet and Health, another consensus report of the National Academies of (National Research Council, 1989b). It concluded that increased chronic disease risk was likely for alcohol but not significant for coffee, tea, or some other non-nutrient bioactives including food additives, environmental and naturally occurring contaminants. At the same time scientists recognized that while many bioactives appeared to have beneficial physiological effects, the lack of data about bioactives made it impossible to estimate exposures and their links to health and disease (Kris-Etherton et al., 2002). Examples included caffeine on attention, fiber on laxation, flavan-3-ols on blood pressure and others such as lycopene, resveratrol, lignans, tannins, and indoles. They called for more research to identify foods and dietary components that altered the risk of chronic diseases and elucidation of their mechanisms of action and development of measures of nutritional status or outcomes might be more appropriate for evaluating their effects than the classical functional measures used with nutrients.
Until the early 1990s the bioactives permitted in dietary supplements were relatively few. The number of bioactive ingredients allowed in supplements then greatly expanded with enactment of the Dietary Supplement Health and Education Act (DSHEA) of 1994 that created a whole new regulatory category of “dietary ingredients” (DIs) that could be included in dietary supplements. Some DIs were nutrients (such as, vitamins, minerals, and amino acids). Most were non-nutrient bioactives (e.g., herbs or other botanicals, a ‘dietary substance for use by man to supplement the diet by increasing total dietary intake, or a concentrate, metabolite, constituent, or extract’). Although DSHEA allowed non-nutrient DIs in dietary supplements and permitted claims of “nutritional support”‘(e.g., structure-function claims) about them, listing of amounts were not required on dietary supplement labels.
In the 1990’s, a few years after the passage of DSHEA, the designation of safe and adequate intakes of bioactives was omitted in the new NAS Dietary Reference Intakes (DRI) model adopted for nutrients (Institute of Medicine 1998). The DRI Committee originally intended to address safe and adequate ranges of intakes of non-nutrient bioactive ingredients in foods and evidence about reduction of chronic disease risk. However, in the end it did not do so because it quickly recognized that the relationships between nutrient intakes and chronic disease risk were much more challenging to model than the relationships between nutrient adequacy to prevent deficiency disease and toxicity. The DRI model used the term “adequate intake” (AI) differently. It now referred to the recommended average daily intake level of nutrients based on observed or experimentally determined approximations or estimates of nutrient intake by a group(s) of apparently healthy people assumed to be adequate when an RDA could not be determined. The new DRI model did not easily accommodate the likely effects of non-nutrient bioactives. It assumed that, like nutrients, bioactives had a relatively large effect and worked quickly, which might not be so for the smaller and slower effects of some non-nutrient bioactives. It also focused on functional indices of nutrient adequacy and toxicity and devoted little attention to biomarkers of chronic disease risk reduction or biomarkers of health. (Weaver, 2014).
In the early 2000s an ad hoc federal working group of representatives from all the relevant federal agencies was convened and led by the National Institutes of Health’s Office of Disease Prevention and Health Promotion. It defined bioactive components and explored next steps but failed to reach consensus on what to do next (U.S. DHHS, 2004). Conferees were uncertain about appropriate processes and standards for evaluating the safety and the efficacy of many DIs that were now permitted in dietary supplements and were reluctant to make recommendations until such standards were in place.
A private sector effort later produced one possible framework for developing a DRI like process for evaluating the safety and efficacy of bioactives. It included Joanne Lupton PhD, to whose memory this issue of the journal is devoted (Lupton et al., 2014). The criteria developed to evaluate a bioactive included an accepted definition, a reliable analytic method, a food database with known amounts of the bioactive, cohort studies, clinical trials on metabolic processes, clinical trials for dose-response and efficacy, safety data, systematic reviews and/or meta-analyses supporting the findings, and a plausible biological rationale. Another private sector group evaluated lutein using these criteria, found that it met them, and urged that recommendations be issued to encourage intakes of lutein-containing foods and raise public awareness about its potential health benefits (Ranard et al., 2017).
The next definitive step forward was development of authoritative recommendations on guiding principles for developing recommendations on decreasing chronic disease risk laid out in an FNB consensus report (National Academies of Sciences, Engineering, and Medicine, 2017). It described the principles involved and high quality evidence needed for making authoritative recommendations on decreasing chronic disease risk. The report established a new set of Dietary Reference Intakes (DRI) values, called ‘nutrients and other food substances’ (NOFS). The purpose of NOFS values was to provide estimated amounts of these compounds for reducing risk of specific chronic diseases with a full vetting of each bioactive by qualified experts, and to estimate the mounts associated with health benefits. The criteria and standards for demonstrating associations with chronic disease risk reduction recommended for the NOFS were identical for nutrients and non-nutrients (National Academies of Sciences, Engineering, and Medicine, 2017). Current US regulations for making health claims on bioactives in dietary supplements do not require bioactives to document such associations using the specified principles and criteria before making qualified health claims or structure-function claims for bioactives. However, the report suggests a likely future regulatory direction for bioactives. Building on the Food and Nutrition Board’s (FNB) work, another framework was proposed in 2021 that included a process for developing recommendations for intakes of bioactives. It was proposed by an ad hoc committee of the International Life Sciences Institute of North America (ILSI NA), that later continued its work under the aegis of the Institute for Advancement of Food and Nutrition Science (IAFNS). This more specific framework focused on developing recommendations for intakes of bioactive dietary substances in foods that were consistent with the FNB 2017 report (Yates et al., 2021). It emphasized four key decision-making steps in developing consumer guidance for intakes of bioactives in foods (Figure 2). These were: characterization of the bioactive; determination of the amounts in specific food sources, and quantification of intakes; evaluation of safety; quantification of the causal relationship between the specific bioactive and accepted markers of health or normal function using systematic evidence reviews and finally translation of the evidence into a quantified bioactive intake statement (Yates et al., 2021). One major omission in the 2021 report was that the framework did not address the unique challenges involved with the safety and efficacy of bioactives in dietary supplements and traditional medicines that are discussed elsewhere in this article
Figure 2.

Framework for Developing Recommended Intakes of Bioactive Dietary Substances in Foods (courtesy of IFANS).
6.2. Current status
At present, only a few bioactive ingredients in foods have approved (SSA “unqualified”) health claims. There are nearly two dozen qualified health claims subject to FDA’s enforcement discretion and dozens and dozens of structure-function claims on dietary supplements and foods.
7. What is needed for bioactives to demonstrate that they can play a larger role in chronic disease risk reduction?
If bioactives are to realize their potential for improving health and preventing chronic disease, action is needed on several fronts. Table 9 presents recommendations for improving the safety and efficacy assessment and dietary guidance about them. Key points are highlighted below.
Table 9.
Recommendations for the Path Forward for Safety and Efficacy Assessment of Bioactives
| Goal | Objective | Comments |
|---|---|---|
|
Improve Quality and Product Integrity |
Accelerate botanical and chemical characterization of single ingredients and mixtures (Mudge et al. 2016). | Develop robust methods of analysis and reference materials especially for bioactives in proprietary blends, dietary supplements and traditional medicines. Characterized and standardized extracts of cocoa flavanols and robust methods of analysis were used in the recently completed COSMOS clinical trial (Bussy et al., 2022). |
| Adopt standardized nomenclature for ingredients | Use a common terminology with standard names and definitions, expressed in the same units, especially for bioactives in natural products such as botanicals and medicinal plants (Frank et al., 2020). (National Academies of Science, Engineering, and Medicine, 2018). | |
| Develop databases of ingredients and products | Use databases for bioactives with possibly beneficial health effects to improve exposure estimates (Betz et al., 2018). | |
|
Ensure Safety Assessment |
Elucidate mechanisms of action and pathways responsible for bioactives’ health effects (Ribeiro et al., 2019) | |
| Document pharmacokinetics, pharmacodynamics, and dose-response profiles of bioactives over a broad range of intakes for ingredients occurring alone and in combinations ( Cazarin and Bicas 2022 ) | Associations between intakes and health effects may not be linear, as the U-shaped relationship between selenium intakes and type 2 diabetes mellitus exhibited(Cardoso et al., 2022). | |
| Encourage industry self-regulation | Examples include product integrity standards, third party certification and certificates of analysis required by some trade associations and internet marketers before products can use their platforms. | |
| Register ingredient lists of products with regulatory authorities | This is done for foods, but not yet for traditional medicines or dietary supplements | |
| Review amounts of bioactives originally approved compared to those used in herbal medicines and dietary supplements | Doses, matrices, and conditions of use of some bioactives differ from approved uses and may have adverse health effects. | |
| Enforce safety standards more vigorously at national and international levels | Methods include enhanced enforcement capacity, seizure of violative products and assets of offending companies, legislation stipulating premarket approval of products. Outside actors such as lawsuits against offenders by plaintiff’s lawyers might also enforce safety standards. | |
| Activate global frameworks for safety surveillance especially of bioactive ingredients and products marketed in many countries spanning different regulatory systems | Draw upon existing collaborations with the Food and Agricultural Organization, the World Health Organization, Codex Alimentarius and other groups that already exist between countries for foods (Thakkar et al.2020) | |
| Enhance surveillance of dietary supplements and traditional preparations marketed globally over the internet | Prioritize safety effort for global online sales of “nutraceuticals” and dietary supplements such as performance and weight-control drugs often spiked with active pharmaceuticals and otherwise adulterated(Low et al., 2017). | |
| Achieve consensus on safety assessment processes for bioactives in natural products | Modify existing processes or develop new ones to overcome their limitations(Wallace et al., 2015). | |
| Resolve discrepancies between countries in safety standards and placement in regulatory categories for traditional/herbal medicines, and dietary supplements | Simplify regulation and ease compliance of multinational marketers with local regulations | |
| Increase international collaboration in safety a surveillance to detect adulteration and fraud ( Low et al 2017 ) | Highest priority is ingredients of safety concern in dietary supplements and traditional /herbal/medicines and botanicals | |
| Develop a tiered global system prioritizing “problem” bioactives likely to pose greatest safety risk and disseminate wisely | The top tier is ingredients and products most likely to pose greatest risk, such as pyrrolizidine alkaloids (Colegate et al., 2018). Wider dissemination of bioactives in dietary supplements and traditional medicines with safety concerns beyond the international regulatory community would be helpful, as is done with EFSA’s botanical compendium (Low et al., 2017). | |
| Work toward eventual adoption of global safety assessment processes and standards | Scientific and regulatory approval of food safety assessment standards, testing, inspection, data collection, monitoring and global harmonization activities are all essential | |
| Improve Efficacy Assessment |
Build in regulatory incentives to promote high quality clinical trials Prioritize promising “best bet” bioactive ingredients and preparations with strong ethnobotanical and preclinical evidence for assessment (Funk and Schneider, 2021). |
These may be widely used traditional botanical preparations with strong ethnobotanical and preclinical evidence of efficacy |
| Apply more rigorous standards in clinical trials of bioactives |
Improve designs in studies of bioactives Develop supportive preclinical study data, use of appropriate animal models, dosing levels and routes of administration and predetermined endpoints to translate evidence from animal experiments appropriately for planning human clinical trials of bioactives in foods, dietary supplements and traditional medicines (Floyd et al., 2022 and Sorkin et al., 2020) (Floyd et al., 2022). Use biomarkers of exposure to the bioactive both before and after the intervention Adopt validated biomarkers of chronic disease outcome for assessing nutrient and non-nutrient compounds for decreasing chronic disease risk Use the same validated surrogate biomarkers of chronic disease prevention for both nutrient and non-nutrient bioactives (Institute of Medicine, 2010 and National Academies of Science, Engineering, and Medicine, 2017). Use validated qualified surrogate biomarkers of chronic disease risk Surrogate markers of chronic disease risk allow smaller clinical studies over a shorter time.(Sesso et al., 2022). They must be analytically validated, on a causal pathway in the pathogenesis of the disease and be significantly associated with the disease in the target population in a defined context of use. They must consistently change in response to the intervention with a change health outcome and explain a substantial proportion of the disease response to the intervention (Institute of Medicine, 2010 and National Academies of Science, Engineering, and Medicine, 2017). Develop validated intermediary surrogate biomarkers of decreased chronic disease risk Intermediary surrogate biomarkers must reflect critical intermediate steps in the bioactive’s metabolic activity, correlate with decreased disease risks, and measure all adverse effects associated with the bioactive. |
|
| Improve communications about bioactive safety and efficacy to consumers | ||
| Work toward developing guidance on bioactive intakes | Amass more information about the safety and efficacy of common bioactives to make it possible to frame evidence-based recommendations for their consumption in foods and dietary supplements (Weaver, 2014). |
7.1. Enhanced safety assessment
This era of increasingly interconnected and global supply chains requires product integrity, quality and safety assessment standards coupled with inspection, testing, data collection, monitoring surveillance and enforcement systems at national and international levels.
7.2. Evidence of efficacy in reducing chronic disease risk based on more rigorous clinical trials and studies
The efficacy of bioactives showing potential for improving health and decreasing chronic disease risk must be better assessed and documented (Sorkin et al., 2020; Sorkin et al., 2016; Ankliam et al., 2021). After all, claims about decreasing chronic disease risk imply effects appear to be drug like, at least under US law.
7.3. Eventual development of authoritative guidance on bioactive intakes
Recommendations are available for a process to assess the safety and efficacy of bioactive ingredients to develop dietary recommendations on intakes of bioactives in foods (Yates et al 2021) (Weaver, 2014). However, intakes in dietary supplements and traditional medicines must still be addressed before authoritative guidance on intakes of specific bioactives for decreasing chronic disease risk can be crafted. In the meantime, much remains to be done by many actors to better convey information about bioactives to consumers. Scientists involved in bioactive research can help by eschewing overblown statements about bioactives that are then broadcast further over social media platforms (Antoakis, 2017). Health professionals can share evidence-based information about bioactives with the public (Rogge et al., 2022). Mass media can better vet and consumers can become more sophisticated in their evaluation of assertions about bioactives (Rowe and Alexander, 2022).
8. Conclusions
At present, only a few bioactives have “come of age” and conclusively demonstrated both their safety and efficacy. More bioactives must “grow up” if they are to play a larger role in health promotion and chronic disease prevention (Gundrum et al., 2022). More rigorous safety assessment of bioactive ingredients and mixtures is needed in dietary supplements and traditional medicines. It will be necessary to demonstrate that bioactives truly reduce chronic disease risk human clinical trials, especially for those in dietary supplements and traditional medicines using validated surrogate endpoints. Global harmonization of safety and efficacy assessment continues to be difficult to achieve but it is vital in this interconnected world, particularly for dietary supplements and traditional medicines. The recommendations provided for resolving the remaining challenges bioactives face may help them fill a larger future role in health promotion and chronic disease risk reduction.
The glad tidings that emerge from this review is that existing problems are fixable. Foods and diets are very much more complicated than a single compound or group of bioactives. They consist of complex mixtures of different bioactives, nutrients and many other compounds. Good health probably depends on consuming complex and varied mixtures of bioactives in foods every day. In addition to protective effects that some bioactives may provide, the mixtures help to tamp down the side effects of other natural products with negative effects that may be in the mix.
Acknowledgements
The author acknowledges with many thanks the helpful comments on drafts of this paper provided by Joseph M Betz PhD Acting Director, Office of Dietary Supplements, National Institutes of Health. As always, his perspectives greatly enriched the paper. The author also thanks Yolanda L. Jones, NIH Library, for her very helpful editing assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests: Dr. Dwyer is Senior Nutrition Scientist (contractor) at the Office of Dietary Supplements, National Institutes of Health, and Senior Scientist, Senior Scientist at the Jean Mayer USDA Human Nutrition Research Center and a Professor at the School of Medicine, Tufts University. She is editor-in-chief of the journal Nutrition Today. She is a member of the Scientific Advisory Board of the McCormick Science Institute, the Mushroom Council, and Bay State Milling Inc, and the *STAR Singapore Institute of Science and Technology is a nonpaid advisor to a committee of the Institute of Food and Nutrition Science (IFANS) and chair of the governance committee of the International Life Sciences(ILSI) US-Canada Research Program. She holds stock in several food and drug companies and was a one-time consultant to Nestle Inc., in 2020, on an issue unrelated to the content of this article and holds no grants or contracts.
References
- Anklam E, Bahl MI, Ball R, et al. 2021. Emerging technologies and their impact on regulatory science. Exp Biol Med (Maywood). Nov 16:15353702211052280. doi: 10.1177/15353702211052280. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonakis J 2017. On doing better science: from thrill of discovery to policy implications. Leadership Quarterly 28 (1):5–21. https://serval.unil.ch/resource/serval:BIB_8EF01CB8)A64.P001/REF.pdf doi: 10.1016/j.leaqua.2017.01.006 [DOI] [Google Scholar]
- Bailey RL.2018. Current regulatory guidelines and resources to support research of dietary supplements in the United States. Crit Rev Food Sci Nutr. 2020;60(2):298–309. doi: 10.1080/10408398.2018.1524364. Epub 2018 Nov 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz JM, Rimmer CA, Saldanha LG, Phillips MM, Andrews KW, Wise SA, Wood LJ, Kuszak AJ, Gusev PA and Pehrsson PR, 2018. Challenges in developing analytically validated laboratory-derived dietary supplement databases. The Journal of Nutrition, 148(suppl_2), pp.1406S–1412S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilia AR, Costa MDC. 2021. Medicinal plants and their preparations in the European market: Why has the harmonization failed? The cases of St. John’s wort, valerian, ginkgo, ginseng, and green tea. Phytomedicine. Jan;81:153421. doi: 10.1016/j.phymed.2020.153421. Epub 2020 Nov 24. [DOI] [PubMed] [Google Scholar]
- Bourgaud F, Gravot A, Milesi S, Gontier E 2001. Production of plant secondary metabolites: a historical perspective Plant Science 161: 839–851 [Google Scholar]
- Burdock Group 2021. Dietary supplements vs. functional foods/ safety and .labeling April 15, 2021 access at https://www.burdockgroup.com/dietary-supplements-vs-functional-foods-safety-and-labeling
- Canivenc-Lavier M-C, Bentejac M, Miller ML, Leclerc J, Siess MH, Latruffe N, Suschetet M (1996) Differential effects of oonhydroxylated flavonoids as induscer of cytochrome P450 1A and 2B isozymes in rat liver Toxicol Appl Pharmaol 136: 348–353 [DOI] [PubMed] [Google Scholar]
- Cazarin CBB, Bicas JL, Pastore GM, Marostica MR, 2022. Chapter 1 - Introduction, in Editor(s): Cazarin CBB, Bicas JL Pastore GL, Marostica MR Jr, Bioactive Food Components Activity in Mechanistic Approach, Academic Press, Pages 1–3, [Google Scholar]
- Cardoso BR, Cominetti C, Seale LA.2022. Editorial: Selenium, Human Health, and Chronic disease Frontiers in Nutrition 8 2022 URL=https://www.frontiersin.org/article/10.3389/fnut.2021.827759 doi: 10.3389/fnut.2021.827759 ISSN=2296-861X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew EY, Clemons TE, Agrón E, Sperduto RD, Sangiovanni JP, Davis MD, Ferris FL 2014. ; Age-Related Eye Disease Study Research Group. Ten-year follow-up of age-related macular degeneration in the age-related eye disease study: AREDS report no. 36. JAMA Ophthalmol. Mar;132(3):272–7.doi: 10.1001/jamaophthalmol.2013.6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew EY, Clemons TE, Keenan TDL, Agron E, Malley CE, Domalpally A 2021The Results of the 10 Year Follow-on Study of the Age-Related Eye Disease Study 2 (AREDS2). Invest. Ophthalmol. Vis. Sci 62(8):1215. [Google Scholar]
- Choiniere CJ, Verrill L 2009. Experimental Study of Qualified Health Claims: Consumer Inferences about Monounsaturated Fatty Acids from Olive Oil, EPA and DHA Omega-3 Fatty Acids, and Green Tea OMB No. 0910-0952 Office of Regulations, Policy and Social Science Center for Food Safety and Applied Nutrition Food and Drug Administration; November 9, 2009 [Google Scholar]
- Colegate SM, Upton R, Gardner DR, Panter KE and Betz JM, 2018. Potentially toxic pyrrolizidine alkaloids in Eupatorium perfoliatum and three related species. Implications for herbal use as boneset. Phytochemical Analysis, 29(6), pp.613–626. [DOI] [PubMed] [Google Scholar]
- Crawford C, Boyd C, Brown L, Costello R, Cordell J, Frushour K, Junker C, Khan I, Jared Ross, Deuster PA 2021. Prioritized research recommendations and potential solutions: addressing gaps surrounding dietary supplement ingredients for boosting brain health and optimizing cognitive performance, Nutrition Research 96 9–19 2 ISSN 02715317, 10.1016/j.nutres.2021.10.001. (https://www.sciencedirect.com/science/article/pii/S0271531721000592 [DOI] [PubMed] [Google Scholar]
- Crawford D, Walter AR, Arula B, Lindsey A, Hunter AM, Khan I, Duester PA 2022. Relative safety, and quality of various dietary supplement products US service members ask about Clinical Toxicology doi: 10.1080/15563650.2022.2036751 [DOI] [PubMed] [Google Scholar]
- Das L, Bhaumik E, Raychaudhuri U, Chakraborty R. Role of nutraceuticals in human health 2012. J Food Sci Technol. 49(2):173–83. doi: 10.1007/s13197-011-0269-4. Epub 2011 Feb 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo, Sorkin BC, Lucarini M, Gusev PA, Kuszak AJ, Crawford C, Boyd C, Deuster PA, Saldanha LG, Gurley BJ, Pehrsson PR, Harnly JM, Turrini A, Andrews KW, Lindsey AT, Heinrich M, Dwyer JT.2022. Analytical Challenges and Metrological Approaches to Ensuring Dietary Supplement Quality: International Perspectives Frontiers in Pharmacology 12 2022 :3956 https://www.frontiersin.org/article/10.3389/fphar.2021.714434 doi: 10.3389/fphar.2021.714434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JT, Bailen RA, Saldanha LG, Gahche JJ, Costello RB, Betz JM, Davis CD, Bailey RL, Potischman N, Ershow AG, Sorkin BC, Kuszak AJ, Rios-Avila L, Chang F, Goshorn J, Andrews KW, Pehrsson PR, Gusev PA, Harnly JM, Hardy CJ, Emenaker NJ, Herrick KA 2018. The Dietary Supplement Label Database: Recent developments and applications, The Journal of Nutrition, 148, (Suppl_2),: 1428S–1435S, 10.1093/in/nxv082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer J, Saldanha L, Bailen R, Durazzo A, Le Donne C, Piccinelli R, Andrews K, Pehrsson P, Gusev P, Calvillo A, Connor E, Goshorn J, Sette S, Lucarini M, D’Addezio L, Camilli E, Marleta L, Turrini 2021. A Commentary: An impossible dream? Integrating dietary supplement label databases: needs, challenges, next steps J. Food Comp Analysis 102;102882 [Google Scholar]
- Dwyer JT, Coates PM, Smith MJ 2018. Dietary Supplements: regulatory challenges and research resources Nutrients 10(1) 41 10.3390/nu100010041 Published online 2018 Jan 4, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Administration (2016). Analysis of EFSA methodological needs for evidence use in scientific assessments European Food Safety Authority 14 September 2016PUBLISHED: 31 October 2016 www.efsa.europa.eu/publications Supporting publication2016:EN-109 www.efsa.europa.eu/publicationsEFSA Supporting publication2016:EN-1092
- Floyd ZE, Ribnicky DM, Raskin I, Hsia DS, Rood JC, Gurley B 2022. Designing a Clinical Study with Dietary Supplements: It’s all in the details Frontiers in Nutrition 8 https://www.frontiersin.org/article/10.3389/fnut.2021.779486 doi: 10.3389/fnut.2021.779486 ISSN=2296-861X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank J, Fukagawa NK, Bilia AR, Johnson EJ, Kwon jO, Prakash V, Miyazawa T, Clifford MN, Kay CD, Crazier A, Erdman JQ, Shao A, and Williamson G 2020. Terms and nomenclature used for plant derived components in nutrition and related research effort toward harmonization Nutrition Reviews 78 (6) 451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk JL, Schneider C 2021. Perspective on Improving the Relevance, Rigor, and Reproducibility of Botanical Clinical Trials: Lessons Learned From Turmeric Trials. Front Nutr. 3;8:782912. doi: 10.3389/fnut.2021.782912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundmann O, Kumar P, Rogge M 2022. On behalf of the ACCP Public Policy Committee Regulation of dietary supplements and nutraceutical products in the United States: An argument for greater oversight and uniform standards Journal of Clinical Pharmacology 62(1) 14–16 2021 doi: 10.1002/jcph.1982 [DOI] [PubMed] [Google Scholar]
- Hoetkamp JC 2006. The precautionary principle: a critique in the context of the EU Food Supplements Directive Env. Liability 2;43–50 10.1016/S0002-9343(01)00995-0. [DOI] [Google Scholar]
- Institute of Medicine 2010. Evaluation of Biomarkers and Surrogate Endpoints in Chronic Disease Washington DC: National Academies Press; https://www.nap.edu/catalog/12869/evaluation-of-biomarkers-and-surrogate-endpoints-in-chronic-disease. Accessed 9 April 2022 [PubMed] [Google Scholar]
- Institute of Medicine 1998 Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington (DC): National Academies Press (US); 1998.: https://www.ncbi.nlm.nih.gov/books/NBK114332/ [PubMed] [Google Scholar]
- Jiratchariyakul W, Mahady GB 2013. Overview of botanical status in EU, USA, and Thailand”, Evidence-Based Complementary and Alternative Medicine, 10.1155/2013/480128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein EA, Thompson IM, Tangen CM, et al. 2011. Vitamin E and the Risk of Prostate Cancer: Results of The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 306(14) 1549–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knöss W, Chinou I. 2012. Regulation of medicinal plants for public health--European community monographs on herbal substances. Planta Med. 2012 Aug;78(12):1311–6. doi: 10.1055/S-0031-1298578. Epub 2012 May 22. [DOI] [PubMed] [Google Scholar]
- Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, Griel AE, Etherton TD 2002,Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer, American Journal of Medicine, 113 (9), 71–88,ISSN 0002-9343, [DOI] [PubMed] [Google Scholar]
- LeBoff MS, Murata EM, Cook NR, Cawthon P, Chou SH, Kotler G, Bubes V, Buring JE, Manson JE, 2020. VITamin D and OmegA-3 TriaL (VITAL): Effects of Vitamin D Supplements on Risk of Falls in the US Population, The Journal of Clinical Endocrinology & Metabolism 105: (9)2929–2938, 10.1210/clinem/dgaa311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang W, Laden F, Curhan GC, Rimm EB, Guo X, Hart JE, Wu S 2022, Dietary nitrate intake and vegetable consumption, ambient particulate matter, and risk of hypertension in the Nurses’ Health study, Environment International, 161 10.1016/j.envint.2022.107100 107100, ISSN 0160-4120, 10.1016/j.envint.2022.107100. [DOI] [PubMed] [Google Scholar]
- Liang Z, Hu H, Li J, Yao D, Wang Y, Oi Lam Ung V 2021. Advancing the regulation of traditional and complementary medicine products: A comparison of five regulatory systems on traditional medicines with a long history of use Evidence-Based Complementary and Alternative Medicine 10.1155/2021/5833945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liska DA, Olsen M 2018. Evaluating science for health claims: An evolving landscape Posted 23 July 2018 | RAPS.org
- Low TY, Wong KO, Yap ALL, De Haan LHJ, Rietjens IMCM. 2017. The regulatory framework across international jurisdictions for risks associated with consumption of botanical food supplements. Compr Rev Food Sci Food Saf. 16(5):821–834. doi: 10.1111/1541-4337.12289. Epub 2017 Aug 10. [DOI] [PubMed] [Google Scholar]
- Lupton JR, Atkinson SA, Chang N, Fraga CG, Levy J, Messina M, Richardson DP, van Ommen B, Yang Y, Griffiths JC, Hathcock J. 2014. Exploring the benefits and challenges of establishing a DRI-like process for bioactives. Eur J Nutr 53 Suppl 1 (Suppl 1):1–9. doi: 10.1007/s00394-014-0666-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden E, McLachlan C, Oketch-Rabah H Calderón AI 2021. Safety of cranberry: evaluation of evidence of kidney stone formation and botanical drug-interactions Planta Med 87: 803–817. DOI 10.1055/a-1497-6241 ISSN 0032-094(https://www.sciencedirect.com/science/article/pii/S0002934301009950) [DOI] [PubMed] [Google Scholar]
- Mudge EM, Betz JM, Brown PN 2016, The importance of method selection in determining product integrity for nutrition research, Advances in Nutrition, 7:(2)390–398, 10.3945/an.115.010611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering and Medicine 2017. Guiding Principles for Developing Dietary Reference Intakes Based on Chronic Disease Washington DC: National Academies Press; doi: https://doi.org/10.17226124828 [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. 2018. Global Harmonization of Methodological Approaches to Nutrient Intake Recommendations: Proceedings of a Workshop—in Brief. Washington, DC: The National Academies Press. 10.17226/24989. [DOI] [Google Scholar]
- National Research Council (US) Subcommittee on the Tenth Edition of the Recommended Dietary Allowances. 1989. Recommended Dietary Allowances: 10th Edition. Washington (DC): National Academies Press (US). [PubMed] [Google Scholar]
- Hooker NH, Ratapol T 2008. Dissecting qualified health claims: Evidence from experimental studies, Critical Reviews in Food Science and Nutrition, 48:2, 160–176, DOI: 10.1080/10408390601177704 [DOI] [PubMed] [Google Scholar]
- Maegawa H, Nakamura T, Saito K. 2014. Regulation of traditional herbal medicinal products in Japan. J Ethnopharmacol. 158 Pt B:511–5. doi: 10.1016/j.jep.2014.07.012. Epub 2014 Jul 17. [DOI] [PubMed] [Google Scholar]
- National Research Council 1989. Committee on Diet and Health. Diet and Health: Implications for Reducing Chronic Disease Risk. Washington (DC): National Academies Press (US) [PubMed] [Google Scholar]
- Oketch-Rabah HA, Roe AL, Rider CV, Bonkovsky HL, Giancaspro GI, Navarro V, Paine MF, Betz JM, Marles RJ, Casper S and Gurley B, 2020. United States Pharmacopeia (USP) comprehensive review of the hepatotoxicity of green tea extracts. Toxicology Reports, 7, pp.386–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oketch-Rabah HA, Madden EF, Roe AL and Betz JM, 2021. United States Pharmacopeia (USP) safety review of gamma-aminobutyric acid (GABA). Nutrients, 13(8), p.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, Glass A, Keogh JP, Meyskens FL Jr, Valanis B, Williams JH Jr, Barnhart S, Cherniack MG, Brodkin CA, Hammar S 1996. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 88 (21):1550–9. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- Palamar J 2011. How ephedrine escaped regulation in the United States: a historical review of misuse and associated policy Health Policy 99:1–9 [DOI] [PubMed] [Google Scholar]
- Prentice RL, Floward BV, Van Horn L, Neuhouser ML, Anderson GL, Tinker LF, Lampe JW, Raftery D, Pettinger M, Aragaki AK, Thomson CA, Mossavar-Rahmani Y, Stefanick ML, Cauley JA, Rossouw JE, Manson JE, Chlebowski RT 2021. Nutritional epidemiology and the Women’s Health Initiative: a review. Am J Clin Nutr. May 8;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz R, Chazan B, Dan M 2004, Cranberry juice and urinary tract infection, Clinical Infectious Diseases, 38 (10)1413–1419, 10.1086/386328 [DOI] [PubMed] [Google Scholar]
- Ranard KM, Jeon S, Mohn ES, Griffiths JC, Johnson EJ, Erdman JW 2017. Dietary guidance for lutein: consideration for intake recommendations is scientifically supported Dur J Nutrition 56 (Suppl 3):537–542 10.1007/s00394-017-1580-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro PVM, Andrade PA, Hermsdorff HHM, Dos Santos CA, Cotta RMM, Estanislau JASG, Campos AAO, Rosa COB 2019. Dietary non-nutrients in the prevention of non-communicable diseases: Potentially related mechanisms. Nutrition. 2019 Oct;66:22–28 doi: 10.1016/j.nut.2019.03.016. Epub 2019 Apr 25. [DOI] [PubMed] [Google Scholar]
- Rogge M, Kumar P, Grundmann O, On behalf of the ACCP Public Policy Committee 2022. Front-line health care professionals lack critical knowledge in dietary supplement and nutraceutical products: A call to action for comprehensive educational opportunities Journal of Clinical Pharmacology 62(1) 17–19 [DOI] [PubMed] [Google Scholar]
- Rowe S, Alexander N 2022. Fighting nutrition and health misinformation: enlisting the public’s help Nutrition Today. Nutrition Today 57(1) 34–37 [Google Scholar]
- Rowe S, and Alexander N 2017. On Post truth, fake news, and trust Nutrition Today 52(4): 170–182 [Google Scholar]
- Panel on Dietetic Products, Nutrition and Allergies 2009. Scientific Opinion of the Panel on Dietetic Products, Nutrition and Allergies on a request from Ocean Spray International Services Limited (UK), related to the scientific substantiation of a health claim on Ocean Spray Cranberry Products® and reduced risk of urinary tract infection in women by inhibiting the adhesion of certain bacteria in the urinary tract. EFSA Journal 943, 1–16 [Google Scholar]
- Shao A 2017. Chapter 15 – Global market entry regulations for nutraceuticals, functional foods, dietary/food/health supplements. In: Bagchi D, Nair S editors. Developing new functional food and nutraceutical products. Amsterdam: Academic Press. p 279–90. [Google Scholar]
- Sharp KJ, Vitagliano JA, Weitzman ER, Fitzgerald S, Dahlberg SE, Austin SB. 2021. Peer-to-peer social media communication about dietary supplements used for weight loss and sports performance among military personnel: pilot content analysis of 11 years of posts on Reddit. JMIR Form Res. ;5(10):e28957. doi: 10.2196/28957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipkowski KA, Betz JM, Birnbaum LS, Bucher JR, Coates PM, Hopp DC, MacKay D, Oketch-Rabah H, Walker NJ, Welch C, Rider CV 2018. Naturally complex: Perspectives and challenges associated with botanical dietary supplement safety assessment. Food Chem Toxicol. 2018 Aug;118:963–971. doi: 10.1016/j.fct.2018.04.007. Epub 2018 Apr 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi EG, Bellandi T, Picchi M, et al. 2017. Patient safety in complementary medicine through the application of clinical risk management in the public health system. Medicines (Basel). 2017;4(4):93. Published 2017. Dec 16. doi: 10.3390/medicines4040093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesso HD, Manson JE, Aragaki AK, Rist PM, Johnson LG, Friedenberg G, Copeland T, Clar A, Mora S, Moorthy MV, Sarkissian A, Carrick WR, Anderson GL, COSMOS Research Group (2022) Effect of cocoa flavanol supplementation for the prevention of cardiovascular disease events: the COcoa Supplement and Multivitamin Outcomes Study (COSMOS) randomized clinical trial, The American Journal of Clinical Nutrition, 115 (6): 1490–1500. 10.1093/ajcn/nqac055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin BC, Kuszak AJ, Hoffman FA, Jafari M, Bushman FD, Casper SJ, Ferruzzi MG, Hopp DC, MacMillan JB, Meltzer DO, Pritchett Corning K, Quinney SK, Rehermann B, Setchell KR, Sipes NS, Stephens JM, Taylor DL, Tiria H, Walters MA, Xi D, Zappala G Pauli GF, Bloss B, Fukagawa NK, Barrett B, Brow PN, Chilton FH, Coffey CS, Kiely M, Lakens D, Pahor M, Paul J 2020. Improving natural product research translation: From source to clinical trial FASEB J doi 10.1096/fj.201902143R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin BC, Kuszak AJ, Williamson JS, Hopp DC, and Betz JM 2016. The challenge of reproducibility and accuracy in nutrition research: resources and pitfalls Adv Nutr 7:383–389 Doi: 1010.1096/fj.201902143R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RB (2002). “Environmental Regulatory Decision Making Under Uncertainty”. Research in Law and Economics. 20: 76. 2002 [Google Scholar]
- Thakkar S, Anklam E, Xu A, Ulberth F, Li J, Li B, Hugas M, Sarma N, Crerar S, Swift S, Hakamatsuka T, Curtui V, Yan W, Geng X, Slikker W, Tong W, 2020. Regulatory landscape of dietary supplements and herbal medicines from a global perspective, Regulatory Toxicology and Pharmacology, 114, 104647,ISSN 0273-2300, 10.1016/j.yrtph.2020.104647. (https://www.sciencedirect.com/science/article/pii/S0273230020300738) [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services (USDHHS) 2004. Solicitation of written comments on a proposed definition of “bioactive food components” Federal Register 69 No 170 Sept 16, pp 55821–55822 [Google Scholar]
- Vettorazzi A, López de Cerain A, Sanz-Serrano J, Gil AG, Azqueta A.2020. European regulatory framework and safety assessment of food-related bioactive compounds. Nutrients; 12(3):613. 10.3390/nu12030613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace TC, Blumberg JB, Johnson EJ, Shao A. 2015. Dietary bioactives: establishing a scientific framework for recommended intakes. Adv Nutr.;6(1):1–4. doi: 10.3945/an.114.007294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CM 2014. Bioactive foods and ingredients for health Adv. Nutr 5: 306S–311S, [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization 2019. WHO Global Report on Traditional and Complementary Medicine 2019 World Health Organization; Geneva: 226 pp. [Google Scholar]
- Yates AA, Dwyer JT, Erdman JW, King JC, Lyle BJ, Schneeman BO, Weaver CM 2021. serving as an ad hoc working group on a framework for developing recommended intakes for dietary bioactives 2021 perspective: framework for developing recommended intakes of bioactive dietary substances Adv Nutr 2021; 12:1087–1099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai S, Dai R, Fridsent PK, Vestal RE (1998) Comparative inhibition of human cytochromes P450 1A1 and 1A2 by flavanoids Drug Metabolism and Disposiiton 26 (10) 989–992 [PubMed] [Google Scholar]


