Abstract
The acute coronavirus disease-2019 (COVID-19) pandemic has had a significant impact on the incidence and prevalence of acute kidney injury and chronic kidney disease globally and in low-income settings. Chronic kidney disease increases the risk of developing COVID-19 and COVID-19 causes acute kidney injury directly or indirectly and is associated with high mortality in severe cases. Outcomes of COVID-19–associated kidney disease were not equitable globally owing to a lack of health infrastructure, challenges in diagnostic testing, and management of COVID-19 in low-income settings. COVID-19 also significantly impacted kidney transplant rates and mortality among kidney transplant recipients. Vaccine availability and uptake remains a significant challenge in low- and lower-middle–income countries compared with high-income countries. In this review, we explore the inequities in low- and lower-middle–income countries and highlight the progress made in the prevention, diagnosis, and management of patients with COVID-19 and kidney disease. We recommend further studies into the challenges, lessons learned, and progress made in the diagnosis, management, and treatment of patients with COVID-19–related kidney diseases and suggest ways to improve the care and management of patients with COVID-19 and kidney disease.
Keywords: COVID-19, chronic kidney disease, acute kidney injury, low-income setting, vaccine equity
Graphical abstract
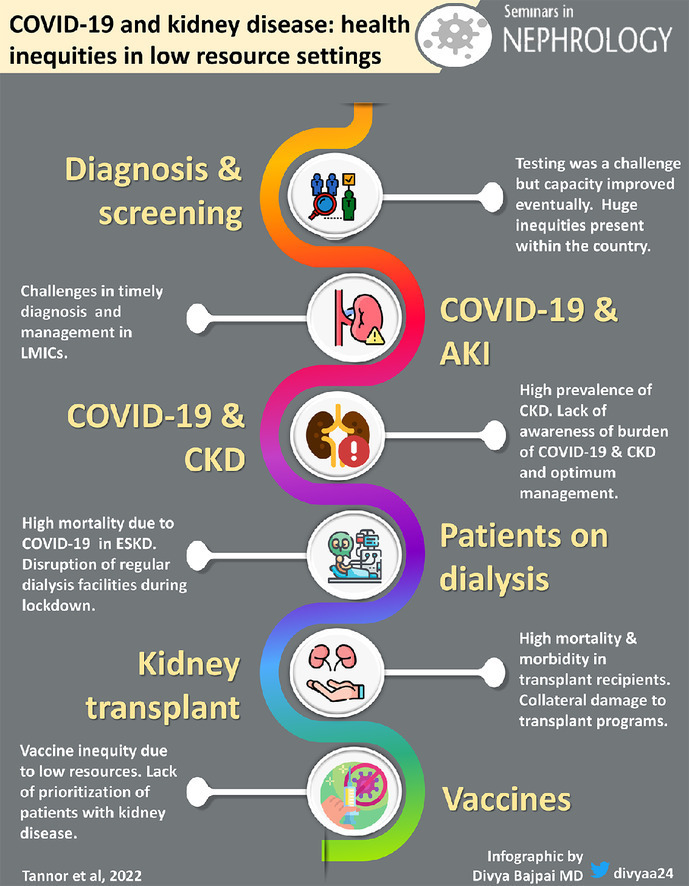
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causing coronavirus disease-2019 (COVID-19) has had a significant impact on the burden and management of patients with kidney diseases globally and in low- and lower-middle–income countries (LLMICs).1, 2, 3, 4, 5 COVID-19 increases the risk of proteinuria, hematuria, tubulopathies, acute kidney injury (AKI), and chronic kidney disease (CKD)5, 6, 7 AKI and CKD is also associated with severe COVID-19 disease.2 , 4 , 8 When COVID-19 infection is complicated by AKI, or in patients with underlying CKD or kidney failure, is associated with increased mortality, especially in low-income settings.3 , 9, 10, 11, 12 The care and management of patients on kidney replacement therapy (KRT) also was affected significantly by the COVID-19 pandemic, leading to missing dialysis sessions. Transplant programs were suspended, with uncertainties in immunosuppression dosing among some kidney transplant recipients (KTR), which was associated with increased morbidity and mortality.13, 14, 15, 16, 17
The diagnosis and management of patients with COVID-19–associated kidney disease has been greatly affected as a result of the COVID-19 pandemic.18 , 19 Inequitable distribution of resources needed for the screening, diagnosis, and optimal management of COVID-19–associated kidney disease has been highlighted, coupled with the looming vaccine inequity in LLMICs, although high vaccine rollout was the most effective remedy for the prevention of COVID-19 transmission and the decrease in COVID-19 severity.20, 21, 22
In this review, we describe the impact of COVID-19 on kidney disease and the progress made in the care and management of patients with COVID-19–associated kidney disease and provide suggestions to improve the diagnosis and management of COVID-19–associated kidney disease in LLMICs.
COVID-19 AND KIDNEY DISEASE
The effect of COVID-19 on the kidneys ranges from asymptomatic proteinuria, hematuria, AKI, and progression to CKD and kidney failure.1, 2, 3, 4, 5 Kidney failure in patients with underlying CKD and severe cases of AKI in patients with severe COVID-19 may require KRT.9 SARS-CoV-2 can have a direct or indirect effect on the kidneys. Directly, it can cause AKI by cytokine storm, angiotensin 2 pathway activation, dysregulation of the complement system, hypercoagulability, microangiopathy, and collapsing glomerulopathy.5, 6, 7 Indirectly, COVID-19 increases the risk of AKI through hemodynamic instability, hypoxemia, sepsis, and exposure to nephrotoxins.5 COVID-19 may lead to CKD as a consequence of clinical or subclinical AKI, or as a result of residual inflammation associated with SARS-CoV-2 infection.5
The pooled incidence of AKI was reported in a meta-analysis to be 10% (95% CI, 7.0%-12.0%),23 with a pooled prevalence of 17% (range, 0.5%-80.5%) among hospitalized patients with COVID-19.24 The wide variations result from varying AKI definitions, differences in gender, race, carrier status of APOL-1 risk alleles, comorbidities, severity of COVID-19, and changes in the virulence of SARS-CoV-2 with different mutations over time.25
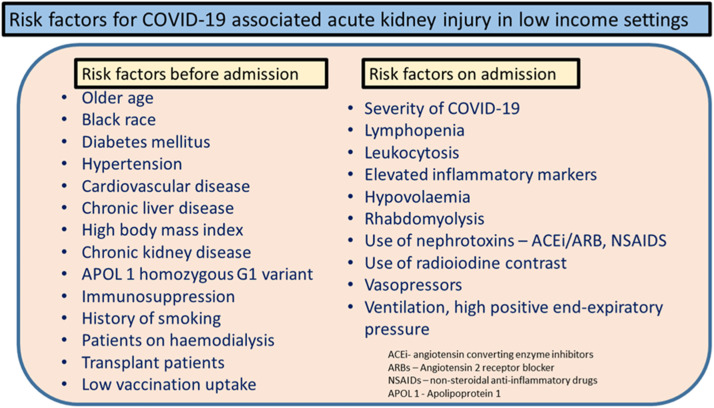
The consensus report of the 25th Acute Disease Quality Initiative summarized all of these risk factors for COVID-19 into three broad categories: demographic risk factors, AKI risk factors before admission, and AKI risk factors during hospitalization.5 We have expanded the list of risk factors to include those relevant to low-income settings (Fig. 1 ).1 , 3 , 12 , 26 Some medications, such as vancomycin, aminoglycosides, and angiotensin-converting enzyme inhibitor, also are associated with AKI.27 , 28 COVID-associated AKI is associated with increased mortality, especially in severe cases.12 , 29
Figure 1.
Risk factors for acute kidney injury in patients with coronavirus disease-2019 (COVID-19) in low-income settings.
Proteinuria occurs in 24% to 84% of patients with COVID-19 in the presence or absence of AKI, and it is a predictor of mortality with or without AKI and/or microscopic hematuria.30 Proteinuria also may suggest subclinical kidney pathology or underlying comorbidities such as diabetes mellitus and/or hypertension.31 The first documented COVID-19–related glomerular diseases were case reports describing collapsing glomerulopathy6 , 32 , 33 found mostly in Black patients who were carriers with APOL1 risk alleles presenting with nephrotic range proteinuria and AKI.25 , 32 Minimal change glomerular injury, anti–glomerular basement membrane glomerulonephritis, and membranous glomerulonephritis all have been described in patients with COVID-19 disease. Subnephrotic proteinuria is mainly of tubular origin, predominantly resulting from 1-α microglobulin.34 , 35 Proximal tubulopathy with acute Fanconi syndrome also has been described and is associated with severe disease. Tubulopathy has been shown to precede the onset of AKI and disappeared with kidney recovery.36, 37, 38
INEQUITY AND PROGRESS IN THE SCREENING AND DIAGNOSIS OF COVID-19
The diagnostic polymerase chain reaction test is preferred to antigen testing for diagnosing SARS-CoV-2 infection,39 and intensified polymerase chain reaction testing decreased the rate of transmission during the first wave.40 The diagnosis and management of kidney disease at the onset of the COVID-19 pandemic was very challenging, especially in LLMICs, given the inability to perform rapid polymerase chain reaction testing as a result of the lack of resourced laboratories and technical staff.41 The International Society of Nephrology and the Dialysis Outcome and Practice Pattern (ISN-DOPPS) survey highlighted gross disparities in the testing for COVID-19 during the pandemic.19 Diagnostic polymerase chain reaction testing was reportedly unavailable or of limited availability in low-income countries (LICs) (72%) and lower-middle–income countries (LMICs) (68%) compared with 20% in high-income countries at the peak of the pandemic.41 Rates decreased during the period of the ISN-DOPPS survey in November 2020 to March 2021 from 68% to 21% in LMICs, but only marginally from 72% to 62% in LICs compared with the peak of the pandemic in March 2022. The turnaround time for receiving diagnostic test results also was delayed.42 Same-day results were received in 60% in high-income countries compared with 13% to 21% in LLMICs according to respondents, whereby those infected had a delayed diagnosis and a possibility of impacting higher transmission rates. The low testing rates supported the hypothesis that rates of COVID-19 and mortality in Africa were low owing to undertesting and not from a low infection rate.43, 44, 45
During the peak of the pandemic, a mandatory negative test was required before discharge of patients from hospital, this criterion later was revised by the World Health Organization (WHO) on May 27, 2020, in which patients were considered to have recovered after 2 weeks without symptoms. This new criterion was useful in LLMICs because of challenges with frequent testing,46 as the previous criteria for discharge from isolation was clinical recovery and two negative reverse-transcriptase polymerase chain reaction results on sequential results taken at least 24 hours apart.39 These changes were recommended by the WHO based on limited laboratory supplies, equipment, and personnel in low-income settings.
Testing capacity and rapid results of diagnostic tests improved through the pandemic as tests were made readily available for the diagnosis of COVID-19 by some governments. In the Philippines and Kenya, the cost for testing was between $11 and $55 for diagnostic testing, however, in India, Ghana, and Zambia, it was free in public institutions. Even within a country, there was wide variation of costs, leading to even more misinformation. Within the same country prices ranged from $0 to $91 in Kenya, $0 to $99 in Zambia, $0 to $104 in the Philippines, and $0 to $14 in India, depending on whether the facility is public or private. Stigmatization and government- and/or employer-enforced isolation after a positive test often discouraged people from availing themselves for testing even when the test was readily available.44
Rates of COVID-19 in Africa, for example, were favorable in terms of infection and mortality rates compared with the rest of the world. At the peak of the pandemic in August 2020, although Africa comprises 17.2% of the world's population, it accounted for only 5% of COVID-19 cases and 3% of COVID-19–related mortalities. Seeding effect, low testing capacity, low population density, a more youthful population, exposure to previous infections, and environmental conditions were postulated as reasons for this phenomenon.43 LLMICs, however, have unique challenges owing to poverty and low infrastructure, leading to poor outcomes in severe cases.11 , 47 The gross inequities in the screening and diagnosis of COVID-19 in kidney failure patients on hemodialysis during the COVID-19 pandemic, however, were associated with higher increased mortality.19
COVID-19 AND AKI IN LOW-INCOME SETTINGS
Generally, LLMICs face challenges in the diagnosis of AKI as a result of a small nephrology workforce and an absence of quality laboratory infrastructure and quality histopathology even during nonpandemic times.48 The pandemic widened the gap in care in LLMICs. Furthermore, the lack of adequate specialized medical/laboratory personnel, significant knowledge gaps among primary health care providers, and suboptimal diagnostic capacity limits the detection and optimization in management of AKI.49
AKI occurred in 5% to 15% of COVID-19 patients and it is associated with increased mortality of up to 90%, especially in patients admitted into intensive care units (ICUs) in China during the onset of the pandemic.50 A retrospective study in New York, however, showed that 87.2% of most patients with AKI attained full recovery of kidney function in the ICU.51 A recent study in South Africa reported that AKI occurred in 33.9% of patients admitted with COVID-19 and 24.3% were admitted to the ICU.12 The risk of AKI during the pandemic was associated with the severity of COVID-19, high comorbidity burden, underlying CKD, decreased vaccination rate, high-risk carriers of APOL1, and decreased access to specialized care.52
Prerenal and acute tubular necrosis were the most common causes of AKI indirectly, but collapsing glomerulopathy, also termed COVID-19–associated nephropathy, was suggested to be caused directly by SARS-CoV-2.6 , 53 There also have been reports of pauci-immune crescentic necrotizing glomerulonephritis as a result of COVID-19 vaccination.54 Such diagnosis requires adequate nephropathology, which may be lacking in most low-income settings. Where histopathology is available, most countries in LLMICs use light microscopy without immunofluorescence and electron microscopy.55
A recent introduction of the Extended Kidney Disease Improving Global Outcome AKI criteria was adopted by the ISN AKI 0by25 studies for COVID-19. The new definition includes the decrease of serum creatinine of 26.5 μmol/L in 48 hours or a decrease of 1.5 times from baseline in 7 days, which identified twice as many cases of AKI compared with the traditional criteria (31.7% versus 16.8%).56 This classification has significant logistic challenges if implemented in LLMICs because it requires frequent measurement of serum creatinine levels even in stable cases, which is prohibitive owing to cost.
Optimal management of AKI in the light of COVID-19 is impossible without an adequate nephrology workforce. The nephrologists per million population in high-income countries is 28.5 compared with 2.4 per million population in LMICs and 0.31 per million population in LICs. This is a direct result of the dearth of training programs to train both physicians and nurses. Africa has 9 of the 10 countries with the lowest nephrology workforce globally.57, 58, 59 There also are challenges in access to KRT in low-income settings in Africa, leading to increased mortality.60 There is inequity in the distribution of hemodialysis services in most countries in low-income settings, even within the same country.61 Patients pay out of pocket for hemodialysis sessions in some countries and mortality increases in the absence of KRT in some low-income settings.
During the COVID-19 pandemic, patients with underlying CKD or AKI with COVID-19 were prevented from accessing ICU care when needed owing to a perceived poor prognosis during triaging.18 , 19 This resulted in increased mortality from treatable causes of AKI in low-income settings.19 Although the vaccination rollout decreased the incidence and prevalence of COVID-19 and COVID-19–associated AKI worldwide, there has not been any marked documented improvement in access to KRT because only 7% of those on KRT live in LLMICs.62
Some suggested solutions to improve AKI diagnosis include engaging local and regional stakeholders in health care financing, developing educational programs and guidelines, training health care workers, providing adequate health care resources, linking with regional health care projects, and improving research opportunities in low-income settings.63
COVID-19 AND CKD IN LOW-INCOME SETTINGS
CKD increases the risk of AKI and AKI can lead to CKD and progression to kidney failure in both hospitalized and nonhospitalized patients with COVID-19.64 , 65 COVID-19 infection could be due to the presence of an undiagnosed CKD prior to hospitalization.66 There is a high global prevalence of CKD of 11%,67 which is even higher in low-income continents such as Africa, with a prevalence of 13.9% to 15.8%.68, 69, 70
COVID-19 survivors also may develop long-term kidney issues.65 A Chinese study reported a 35% reduction in estimated glomerular filtration rate 6 months after COVID-19 hospitalization.66 Even without documented evidence of AKI on admission, 13% of patients showed a reduction in estimated glomerular filtration rate during follow-up evaluation.71 Although many patients have normalization of their creatinine level after AKI, the kidneys may not recover completely and increase the risk of CKD over time.64 Progression of kidney disease in COVID-19 likely is multifactorial and could be driven by continuous inflammation, intrinsic tubular lesions, or improper repair.65 In most patients with CKD in LICs, progression to kidney failure requiring dialysis increases mortality as a result of the absence of KRT and increased cost associated with poor outcomes.61 , 72 The risk factors associated with a worse outcome with CKD include increasing age, diabetes mellitus, hypertension, the unavailability of an adequate nephrology workforce, and the absence of adequate diagnostic tools.8
AWARENESS OF CKD IN LOW-INCOME SETTINGS
There has not been much progress made in increasing awareness for the prevention and management of risk factors for COVID-19–associated kidney disease in low-income settings. Moreover, the incidence and prevalence of the noncommunicable diseases such as diabetes and hypertension that can lead to kidney disease also are increasing, and account for 80% of global deaths in LLMICs.73 Governments must invest more in health to decrease the burden of noncommunicable diseases and conditions that impact kidney health. Public health interventions such as education, a healthy dietary lifestyle, and exercise should be part of a national agenda to prevent CKD. There has been poor governmental support in this regard with few sustainable programs to decrease the burden of kidney disease. Such public health interventions have been championed by individuals and some nongovernmental organizations such as the ISN's World Kidney Day activities.74, 75, 76 Such initiatives should be encouraged to decrease the kidney disease burden with or without COVID-19. The reduction of the burden of kidney disease in patients with COVID-19 is based on the decrease in the transmission of SARS-CoV-2. This calls for optimization of vaccinations around the globe, with early detection and appropriate management of cases to prevent AKI and CKD.77
INEQUITY IN KIDNEY FAILURE PATIENTS ON CHRONIC HEMODIALYSIS WITH COVID-19 IN LOW-INCOME SETTINGS
Patients on chronic dialysis are at increased risk of COVID-19 because of their decreased immune status, frequent hospital visits for hemodialysis sessions, and difficulty in maintaining social distancing during hemodialysis sessions, especially in constrained spaces among dialysis units from low-income settings.9 , 17 , 78 , 79 The incidence of SARS-CoV-2 infection range from 1% to 19.9% in dialysis populations and had significant mortality of 10% to 41% during the peak of the pandemic.17 , 24 , 80, 81, 82, 83, 84 In the peak of the pandemic, as a result of lockdown measures, it was reported that patients in LICs missed their dialysis sessions more frequently than those in high-income countries. According to the ISN-DOPPS survey, hemodialysis sessions reportedly were missed in 66% to 67% in LLMICs compared with 20% to 33% in upper-middle–income countries and high-income countries.19 Transportation to and from hemodialysis units reportedly affected more patients in LLMICs. However, this improved with easing of lockdown restrictions anecdotally, but published evidence is lacking.
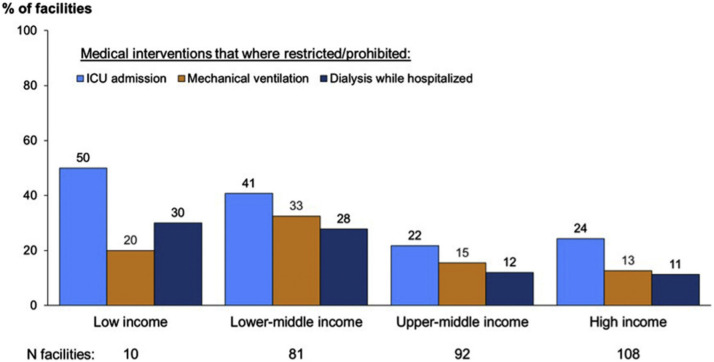
There were also major supply chain disruptions of hemodialysis consumables in LICs during the pandemic. Intensive care admission, mechanical ventilation, and dialysis during hospitalization were more restricted or prohibited in patients with kidney failure on chronic dialysis in low-income settings compared with high-income settings during the peak of the pandemic (Fig. 2 ).19
Figure 2.
Medical interventions such as intensive care unit (ICU) admission, mechanical ventilation, and dialysis while hospitalized became more restricted or prohibited for chronic dialysis patients admitted to the hospital with coronavirus disease-2019 (COVID-19), per World Bank income category.19
Patients with kidney failure were at increased risk of infection as a result of frequent center visits for hemodialysis sessions. They also are particularly at increased risk because of the decrease in immunity as a result of kidney failure. Close proximity of patients during transportation as well as long durations in close proximity during hemodialysis sessions further increased their risk.79
Guidelines were instituted by many societies85, 86, 87, 88 to help decrease the spread of infections in hemodialysis units but this could not be strictly adhered to in most low-income settings owing to poor testing and an unavailability of resources for transmission prevention. Some dialysis centers reportedly were turning away dyspneic patients for fear of COVID-19 transmission to other patients because testing was not readily available, but this improved when testing was made more readily available. The vaccination rollout in patients with kidney failure on dialysis also was filled with challenges regarding uptake because of various myths about the vaccines, but vaccine hesitancy has improved with time based on better education.41 , 89
Health care personnel preferred under poor conditions and were exposed to infections as a result of the absence of the required personal protective equipment. Diagnostic polymerase chain reaction and rapid antigen testing were not readily available for asymptomatic and symptomatic testing of staff in most low-income settings when required.19 , 90 Staff in hemodialysis units in low-income settings were tested infrequently when asymptomatic: 11% to 14% were tested compared with 28% in high-income countries,19 which lead to stress, psychological problems, and stigmatization among health care workers.91 , 92
COVID-19 AND KIDNEY TRANSPLANTATION
The rapid evolution of the COVID-19 pandemic had a two-fold effect on KTRs and programs. On one hand, KTRs are more vulnerable to severe COVID-19–related morbidity and mortality,93, 94, 95 there is a decrease in transplant capacity and volume due to the need for more equitable distribution of scarce resources.96 , 97 The pandemic exposed the pre-existing inequities and deficiencies in health care worldwide,98 but countries with fewer resources suffered to a greater extent.19
CLINICAL OUTCOMES OF COVID-19 IN KTRs
The presence of medical comorbidities (eg, hypertension, diabetes, cardiovascular disease) in KTRs makes it difficult to assess the attributable impact of transplantation on morbidity and mortality. The mortality resulting from COVID-19 remains high in KTRs, even after adjustment for comorbidities.93, 94, 95 , 99 In a systematic review of 74 studies (between March 2020 and January 2021) including 5,559 KTRs with COVID-19, the mortality rate was 23% (95% CI, 21%-27%), which remained high after adjustment for age, sex, and comorbidities.100 However, when comparing 2,307 KTRs with 231,047 nontransplant controls who were propensity-matched for age and comorbidities in a multicenter study, there was no difference in the mortality rate, although transplant recipients had higher rates of hospitalization and AKI.100
The effect of immunosuppression on clinical outcomes of COVID-19 remains obscure. The harmful impact of COVID-19 is a result of the complex interplay of direct viral injury and the host's immune response, with evidence that dysregulated and intense immune responses cause severe disease.101 Because immunosuppressive drugs modulate host responses, the intensity or type of immunosuppression potentially can affect the severity of COVID-19. The adjustment of immunosuppression in KTRs with COVID-19 is complex and needs to be tailored according to disease activity and risk of graft rejection. Lymphopenia, which is a risk factor for severe COVID-19, can be potentiated by antimetabolites, which are recommended to be discontinued. Calcineurin inhibitors usually are continued because they inhibit interleukin-6 and interleukin-1, which is instrumental in causing a dysregulated immune response leading to severe COVID-19. Specific drugs such as mammalian target of rapamycin inhibitors have some biological activity against SARS-CoV-2, but this needs further evaluation.102 Other strategies for the management of COVID-19 remain similar to the general population. Remdesivir is reported to be safe in KTRs.103 , 104
Compared with nontransplant populations, KTRs are at a higher risk of COVID-19–associated AKI (50% higher risk; 95% CI, 44%-56% in a systematic review of 74 studies involving 5,559 KTRs) even after adjustment for comorbidities.100 This can adversely affect long-term graft outcomes. In a multicenter prospective study from India evaluating KTRs with COVID-19–associated AKI, complete graft recovery at 3 months was seen in 40.5% of patients, with high rates of proteinuria (47%) and graft failure (14.3%).105 This underscores kidney involvement in long COVID-19 syndromes and the need for long-term close monitoring in this high-risk population.
EVIDENCE ON CLINICAL OUTCOMES FROM LOW-RESOURCE SETTINGS
Evidence on the clinical outcomes of patients with COVID-19 with kidney disease in low-resource settings is scarce. In a systematic review of 74 studies published from March 2020 to January 2021, 86% of studies were from North America and Europe, while 14% were from Asia-Pacific and none were from Latin America and Africa.100 They reported lower mortality rates in the United States (estimated proportion of deaths, 18%; 95% CI, 14%-23%) than in Asia-Pacific, but this difference was not statistically significant.100 Initial reports from India documented higher mortality rates (27%-30%) in KTRs compared with the general population, with 100% mortality in ventilated patients.15 , 106 A multicenter cohort of 250 KTRs with COVID-19 from India reported 10% mortality, which is lower than that reported in high-income countries.107 A large cohort from South East Asia of 259 KTRs showed a change in the clinical spectrum of COVID-19 over subsequent pandemic waves.108 Improvement in clinical outcomes after the first wave of the pandemic can be attributable to advances in health care infrastructure and the success of vaccination programs; for example, India had more than 1 billion individuals vaccinated by May 15, 2022. In a head-to-head comparison by a propensity score–matched cohort from India there was no difference in mortality among solid-organ transplant recipients with COVID-19 compared with nontransplant patients.109
In a narrative review evaluating 6 studies from Latin America, mortality from COVID-19 in KTRs ranged from 14.3% to 35.4%; and from 25.5% to 40.9% in KTRs hospitalized with COVID-19.110 Similarly, a report from South Africa documented 20% mortality in KTRs.111
Another malady that struck LLMICs (especially in South East Asia) was COVID-19–associated mucormycosis.112 In the largest multicenter cohort of COVID-19–associated mucormycosis in KTRs (61 KTRs from 18 centers), the incidence of COVID-19–associated mucormycosis was 4.4% and the mortality rate was 26.2%.113 The emergence of such opportunistic infections can further strain the already overwhelmed health care resources. Thus, there is a need for a high level of preparedness for timely diagnosis and management of such emerging infections during the pandemic.
DEVELOPMENT OF MANAGEMENT GUIDELINES FROM LMICs
The COVID-19 pandemic was marked by the exponential growth of misinformation, alarming misbeliefs against emerging therapies, and dilemmas in decision making that hindered health care delivery. In India, the National Organ and Tissue Transplant Organization, the apex body governing transplantation, published guidelines on transplant-specific issues on COVID-19 early in the pandemic.114 The Indian Society of Organ Transplantation and Latin America formulated local guidelines on transplantation in donors and recipients recovering from COVID-19.115 , 116
COLLATERAL DAMAGE TO TRANSPLANT PROGRAM DEVELOPMENT
According to the Global Observatory on Organ Donation and Transplantation, all regions of the world suffered a decrease in transplant volume.117 However, there was a stark difference in the speed with which the high-income countries could rebuild their transplant volume and maintain their total number of transplants per year, unlike LLMICs.97 In Latin America in 2020, for instance, there was a 32% to 64% reduction in the number of transplants, which was significantly higher than the global average of 16% in high-income countries.97 The worst hit was the deceased donor program, which is only in its nascent stages in the developing world.118
A multicenter study from India reported a dramatic impact on transplant programs in general, however, the centers in the public sector were more affected compared with private institutions.119 Most of these centers were converted into dedicated COVID-19 centers, which was the need of the hour. Thus, regulatory policies, health care capacity, state of SARS-CoV-2 transmission, and public awareness governed this collateral damage. The long-term sequelae of delaying transplantation still is unclear. Limited evidence has suggested that the risk of hospitalization and mortality associated with COVID-19 is higher in patients on the waitlist compared with KTRs.14 Studies from India have documented favorable outcomes and stressed the importance of continuing elective transplant procedures amid the pandemic if health care facilities are not overwhelmed.120 , 121 Furthermore, a study from India reported a serendipitous silver lining of reduced infections in liver transplants performed amid the pandemic as a result of health policy changes to control SARS-CoV-2 spread.122 This is especially vital for transplant programs from LLMICs, where infections are the primary cause of death with a functioning graft. The pandemic taught the need for concerted efforts at a global level to facilitate improved pandemic preparedness so that transplant programs can be sustainable even in pandemics.
COVID-19 VACCINE EQUITY
Vaccination against SARS-CoV-2 remains the cornerstone of preventing severe COVID-19 infection and its complications among individuals with kidney disease despite the decreased antibody responses observed among these patients compared with the general population.20 , 123 To maximize the benefits of COVID-19 vaccines globally, they should be produced in adequate amounts, made affordable, distributed equitably so that they are available where needed, and deployed effectively at individual, country, and regional levels. This is challenging, especially in the LLMICs of Asia, Africa, and Latin America.
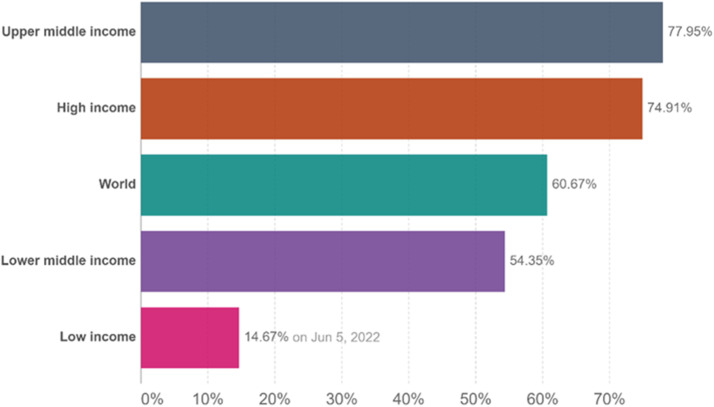
Low-income settings were disadvantaged significantly in the vaccine rollout.124 In some countries such as the United Arab Emirates where 99% of the citizens have received one dose of the vaccine as of May 11, 2022, LMICs such as Ghana and Nigeria have 30.5% and 13%, respectively.125 Although high-income countries and upper-middle–income countries have 74.9% to 77.9% of their population fully vaccinated, LLMICs have 14.7% to 54.4% of their population fully vaccinated as of June 4, 2022 (Fig. 3 ).126 There was a modest improvement in vaccine rollout but this is far from optimal. It was suggested that vaccine equity is necessary to win the war on COVID-19 and patients on chronic dialysis should be prioritized.79 LLMICs do not have the same ability to purchase vaccines, do not have the technology and capacity to produce their own vaccines, and lack the infrastructure to deploy these vaccines efficiently. This has led to large disparities in COVID-19 vaccination, especially among patients with kidney disease, between high- and low-income countries throughout the world.
Figure 3.
The number of people fully vaccinated per World Bank income status.127 Abbreviation: COVID-19, coronavirus disease-2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The COVID Vaccine Global Access (COVAX) facility was created by the WHO in April 2021 to facilitate the access of COVID-19 vaccines to both high- and low-income countries equally.127 The key elements of this program are that vaccination should proceed in stages, and priority should be given to high-risk individuals before proceeding to vaccinate others in the general population. All participating countries initially would receive a stock of vaccines sufficient to vaccinate 20% of the population. COVAX was helpful in initiating the COVID vaccination programs, especially in some LLMICs, by being able to procure and provide vaccines at affordable prices. However, the amount of vaccines provided through COVAX was inadequate to meet the demand, resulting in most of the high-income countries leaving the program and purchasing vaccines directly from the manufacturers. This has resulted in vaccines developed in the United States and Europe becoming less available for the LLMICs in Asia, Africa, and Latin America. Furthermore, the global inequity of vaccine availability is compounded by certain high-income countries starting to administer booster doses of the COVID vaccine despite the majority of the population in LICs not yet having received a single dose.126
The presence of an infrastructure enabling efficient distribution and administration of the vaccines is a key requirement for the success of the COVID vaccination program. Most of the LLMICs do not have formal vaccination programs, lack data and records of individuals in need of vaccination, and do not have storage and distribution facilities to maintain the ultra-cold chains required by some of the messenger RNA vaccines.128 , 129
Vaccine hesistance in LMICs
Vaccine hesitancy has added to the challenge of the COVID-19 vaccine deployment. Although vaccine hesitancy is prevalent in both high- and low-income countries alike, certain factors such as mistrust in the allopathic medicines, suspicion of individuals from LICs being used as guinea pigs, and low literacy rates may influence vaccine hesitancy more in LLMICs. The resulting inefficient deployment of vaccines has led to vaccine waste (eg, South Sudan and Malawi) or vaccine redeployment to avoid vaccine waste (eg, the Democratic Republic of Congo to Ghana and Madagascar) in some of these countries.89
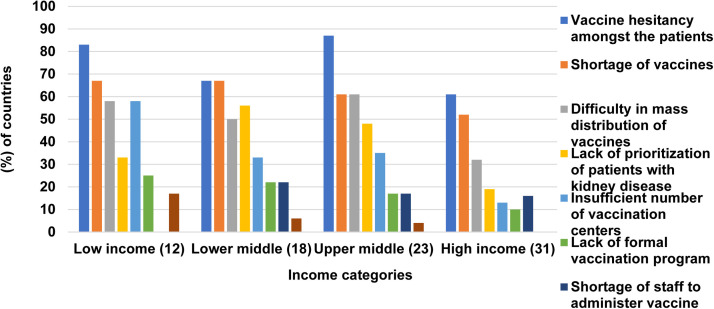
A survey was conducted recently by ISN and DOPPS to ascertain the availability, access, and prioritization of COVID-19 vaccines globally.77 This survey showed that at least one COVID vaccine was available in almost all of the countries surveyed. Patients with stage 4/5 CKD, dialysis, and KTR were identified as a priority group for COVID-19 vaccination and were vaccinated during the first two phases of the vaccination in 51%, 71%, and 62% of the respondent countries. By August 2021, more than half of the countries surveyed had a vaccination rate of more than 50% for people with stage 4/5 CKD, dialysis, and kidney transplants. However, there were significant variations according to the region and World Bank income categories. For example, all respondents from Western Europe reported that more than 50% of all kidney transplant recipients were vaccinated, whereas in Africa and South East Asia, only a minority of respondents reported a more than 50% vaccination rate among KTRs. The most common barriers to vaccination of kidney patients reported by respondents were vaccine hesitancy (74%), vaccine shortages (61%), and vaccine mass distribution challenges (48%) (Fig. 4 ).
Figure 4.
Challenges encountered in coronavirus disease-2019 (COVID-19) vaccines to patients with kidney disease according to income categories. The percentage frequencies are based on responses by countries to: “How frequently are the following major challenges encountered in delivering COVID-19 vaccines to patients with kidney disease in your country?” If the response to the question on a particular challenge was either always, often, or sometimes, it was considered a major challenge. The total number of countries per income group is indicated in parentheses.
More equitable distribution of COVID-19 vaccines is the key to control the pandemic. According to a report by the United Nations as of September 2021, slightly more than 3% of people in LICs were vaccinated with at least one dose, compared with 60.18% in high-income countries.130 By March 2022, of the more than 10 billion COVID-19 vaccines given out worldwide, only 1% had been administered in high-income countries, highlighting the widening global inequality of COVID-19 vaccination.
We need to do more work to overcome these increasing inequalities in vaccination. Apart from strengthening the existing frameworks, such as COVAX, the high-income countries need to share their knowledge regarding manufacturing COVID-19 vaccines with LICs, to set up production facilities in these countries.
One such initiative is the COVID-19 Technology Access Pool, launched by the WHO in May 2020.131 It provides a single platform for the developers of COVID-19 vaccines, tests, devices, and medicines to share their data, knowledge, and technologies with manufacturers. Policy makers should engage with communities to improve public confidence and trust by combating the misinformation surrounding COVID-19 vaccination. Organizations such as the United Nations Children's Fund, which already is involved with well-conducted immunization programs in most of the LICs, could support the development of large-scale COVID-19 vaccination programs in these countries.
CONCLUSIONS
Inequities in kidney care preceded the pandemic in LLMICs, however, the COVID-19 pandemic widened the gap even further and showed a clear need for a global approach to care. Although LLMICs are improving in the diagnosis and management of COVID-19, barriers still remain. Efforts should be placed on improving the capacity of LLMICs to increase kidney care in general and vaccine equity to prevent transmission and decease the burden of COVID-19 infection.
Advocacy to improve the care and management of kidney disease in LLMICs is needed for COVID-19 and beyond. The sequel of COVID-19 is still not clear and could lead to an increased prevalence of CKD. Further studies to assess the progress made to date in low-income settings also will improve the care and management of COVID-19–associated kidney disease. Preparedness for future pandemics also is critical given the mortality and morbidity burden among patients with CKD.
Footnotes
Financial disclosure and conflict of interest statements: none.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.semnephrol.2023.151318.
Appendix. APPENDIX A. SUPPLEMENTARY MATERIAL
REFERENCES
- 1.Alessandri F, Pistolesi V, Manganelli C, et al. Acute kidney injury and COVID-19: a picture from an intensive care unit. Blood Purif. 2021;50:767–771. doi: 10.1159/000513153. [DOI] [PubMed] [Google Scholar]
- 2.Carlson N, Nelveg-Kristensen KE, Freese Ballegaard E, et al. Increased vulnerability to COVID-19 in chronic kidney disease. J Intern Med. 2021;290(1):166–178. doi: 10.1111/joim.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–836. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henry BM, Lippi G. Chronic kidney disease is associated with severe coronavirus disease 2019 (COVID-19) infection. Int Urol Nephrol. 2020;52(6):1193–1194. doi: 10.1007/s11255-020-02451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nadim MK, Forni LG, Mehta RL, et al. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat Rev Nephrol. 2020;16(12):747–764. doi: 10.1038/s41581-020-00356-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Velez JCQ, Caza T, Larsen CP. COVAN is the new HIVAN: the re-emergence of collapsing glomerulopathy with COVID-19. Nat Rev Nephrol. 2020;16(10):565–567. doi: 10.1038/s41581-020-0332-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peleg Y, Kudose S, D'Agati V, et al. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int. 2020;5(6):940–945. doi: 10.1016/j.ekir.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Marco L, Puchades MJ, Romero-Parra M, et al. Coronavirus disease 2019 in chronic kidney disease. Clin Kidney J. 2020;13(3):297–306. doi: 10.1093/ckj/sfaa104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adapa S, Aeddula NR, Konala VM, et al. COVID-19 and renal failure: challenges in the delivery of renal replacement therapy. J Clin Med Res. 2020;12(5):276. doi: 10.14740/jocmr4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumenthal D, Fowler EJ, Abrams M, Collins SR. Covid-19—implications for the health care system. N Engl J Med. 2020;383(15):1483–1488. doi: 10.1056/NEJMsb2021088. [DOI] [PubMed] [Google Scholar]
- 11.Bong C-L, Brasher C, Chikumba E, McDougall R, Mellin-Olsen J, Enright A. The COVID-19 pandemic: effects on low-and middle-income countries. Anesth Analg. 2020;131(1):86–92. doi: 10.1213/ANE.0000000000004846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diana NE, Kalla IS, Wearne N, et al. Acute kidney injury during the COVID-19 pandemic–experience from two tertiary centres in South Africa. Wits J Clin Med. 2020;2(3):189–198. [Google Scholar]
- 13.Achilonu OJ, Fabian J, Musenge E. Modeling long-term graft survival with time-varying covariate effects: an application to a single kidney transplant centre in Johannesburg, South Africa. Front Public Health. 2019;7:201. doi: 10.3389/fpubh.2019.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Craig-Schapiro R, Salinas T, Lubetzky M, et al. COVID-19 outcomes in patients waitlisted for kidney transplantation and kidney transplant recipients. Am J Transplant. 2021;21(4):1576–1585. doi: 10.1111/ajt.16351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godara S, Saraf KK, Sadasukhi T, et al. COVID-19 infection in kidney transplant recipients: a single centre study from Northern India. Indian J Nephrol. 2021;31(6):531–535. doi: 10.4103/ijn.IJN_571_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hadi YB, Naqvi SF, Kupec JT, Sofka S, Sarwari A. Outcomes of COVID-19 in solid organ transplant recipients: a propensity-matched analysis of a large research network. Transplantation. 2021;105(6):1365–1371. doi: 10.1097/TP.0000000000003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilbrands LB, Duivenvoorden R, Vart P, et al. COVID-19-related mortality in kidney transplant and dialysis patients: results of the ERACODA collaboration. Nephrol Dial Transplant. 2020;35(11):1973–1983. doi: 10.1093/ndt/gfaa261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aylward R, Bieber B, Guedes M, et al. The global impact of the COVID-19 pandemic on in-center hemodialysis services: an ISN-dialysis outcomes practice patterns study survey. Kidney Int Rep. 2021;7(3):397–409. doi: 10.1016/j.ekir.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tannor E, Bieber B, Shah D, et al. COVID-19 pandemic highlights global inequities in chronic hemodialysis care: a DOPPS/ISN survey. J Am Soc Nephrol. 2021;32:87–88. [Google Scholar]
- 20.Hou Y-C, Lu K-C, Kuo K-L. The efficacy of COVID-19 vaccines in chronic kidney disease and kidney transplantation patients: a narrative review. Vaccines. 2021;9(8):885. doi: 10.3390/vaccines9080885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Asundi A, O'Leary C, Bhadelia N. Global COVID-19 vaccine inequity: the scope, the impact, and the challenges. Cell Host Microbe. 2021;29(7):1036–1039. doi: 10.1016/j.chom.2021.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi EM. COVID-19 vaccines for low-and middle-income countries. Trans R Soc Trop Med Hyg. 2021;115(5):447–456. doi: 10.1093/trstmh/trab045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z, Tang Y, Huang Q, et al. Systematic review and subgroup analysis of the incidence of acute kidney injury (AKI) in patients with COVID-19. BMC Nephrol. 2021;22(1):1–10. doi: 10.1186/s12882-021-02244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Valeri AM, Robbins-Juarez SY, Stevens JS, et al. Presentation and outcomes of patients with ESKD and COVID-19. J Am Soc Nephrol. 2020;31(7):1409–1415. doi: 10.1681/ASN.2020040470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hung AM, Shah SC, Bick AG, et al. APOL1 risk variants, acute kidney injury, and death in participants with African ancestry hospitalized with COVID-19 from the Million Veteran Program. JAMA Intern Med. 2022;182(4):386–395. doi: 10.1001/jamainternmed.2021.8538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meena P, Bhargava V, Rana DS, Bhalla AK, Gupta A. COVID-19 and the kidney: a matter of concern. Curr Med Res Pract. 2020;10(4):165–168. doi: 10.1016/j.cmrp.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pannu N, Nadim MK. An overview of drug-induced acute kidney injury. Crit Care Med. 2008;36(4):S216–S223. doi: 10.1097/CCM.0b013e318168e375. [DOI] [PubMed] [Google Scholar]
- 28.See YP, Young BE, Ang LW, et al. Risk factors for development of acute kidney injury in COVID-19 patients: a retrospective observational cohort study. Nephron. 2021;145(3):256–264. doi: 10.1159/000514064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kolhe NV, Fluck RJ, Selby NM, Taal MW. Acute kidney injury associated with COVID-19: a retrospective cohort study. PLoS Med. 2020;17(10) doi: 10.1371/journal.pmed.1003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mohamed MM, Velez JCQ. Proteinuria in COVID-19. Clin Kidney J. 2021;14(suppl 1):i40–i47. doi: 10.1093/ckj/sfab036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kissling S, Rotman S, Gerber C, et al. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. 2020;98(1):228–231. doi: 10.1016/j.kint.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meijers B, Hilbrands LB. The clinical characteristics of coronavirus-associated nephropathy. Nephrol Dial Transplant. 2020;35(8):1279–1281. doi: 10.1093/ndt/gfaa197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kudose S, Batal I, Santoriello D, Xu K, Barasch J, Peleg Y, et al. Kidney biopsy findings in patients with COVID-19. J Am Soc Nephrol. 2020;31(9):1959–1968. doi: 10.1681/ASN.2020060802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huart J, Bouquegneau A, Lutteri L, et al. Proteinuria in COVID-19: prevalence, characterization and prognostic role. J Nephrol. 2021;34(2):355–364. doi: 10.1007/s40620-020-00931-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Werion A, Belkhir L, Perrot M, et al. SARS-CoV-2 causes a specific dysfunction of the kidney proximal tubule. Kidney Int. 2020;98(5):1296–1307. doi: 10.1016/j.kint.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morelle J, Wérion A, Belkhir L, Chen Z, Jadoul M, Devuyst O. L'infection à SARS-CoV-2 cause une dysfonction tubulaire proximale du rein. Louvain Med. 2020;139:496. [Google Scholar]
- 38.Kormann R, Jacquot A, Alla A, et al. Coronavirus disease 2019: acute Fanconi syndrome precedes acute kidney injury. Clin Kidney J. 2020;13(3):362–370. doi: 10.1093/ckj/sfaa109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization, Laboratory testing of human suspected cases of novel coronavirus (nCoV) infection Interim guidance, 2019. Accessed May 8, 2022. https://apps.who.int/iris/bitstream/handle/10665/330374/WHO-2019-nCoV-laboratory-2020.1-eng.pdf
- 40.Rannan-Eliya RP, Wijemunige N, Gunawardana J, et al. Increased intensity of PCR testing reduced COVID-19 transmission within countries during the first pandemic wave: study examines increased intensity of reverse transcription–polymerase chain reaction (PCR) testing and its impact on COVID-19 transmission. Health Aff (Millwood) 2021;40(1):70–81. doi: 10.1377/hlthaff.2020.01409. [DOI] [PubMed] [Google Scholar]
- 41.Tannor EK. Challenges in kidney care in a lower middle income country during the COVID-19 pandemic–the Ghanaian perspective. Kidney Int Rep. 2021;6:2014–2016. doi: 10.1016/j.ekir.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Torres I, Sippy R, Sacoto F. Assessing critical gaps in COVID-19 testing capacity: the case of delayed results in Ecuador. BMC Public Health. 2021;21(1):1–8. doi: 10.1186/s12889-021-10715-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bamgboye EL, Omiye JA, Afolaranmi OJ, et al. COVID-19 pandemic: is Africa different? J Natl Med Assoc. 2020;113(3):324–335. doi: 10.1016/j.jnma.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.A Connor, N Hariharan, S Carson, KC Sanders, KB Vosburg and O Sabot, Access to COVID-19 testing in low- and middle-income countries is still critical to achieving health equity, 2021. Accessed May 2, 2022. https://www.healthaffairs.org/do/10.1377/forefront.20211026.483412
- 45.Lone SA, Ahmad A. COVID-19 pandemic–an African perspective. Emerg Microbes Infect. 2020;9(1):1300–1308. doi: 10.1080/22221751.2020.1775132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Organization Criteria for releasing COVID-19 patients from isolation. 17th June 2020. Scientific brief. Accessed May 12, 2022. https://www.who.int/publications/i/item/criteria-for-releasing-covid-19-patients-from-isolation.
- 47.Hopman J, Allegranzi B, Mehtar S. Managing COVID-19 in low-and middle-income countries. JAMA. 2020;323(16):1549–1550. doi: 10.1001/jama.2020.4169. [DOI] [PubMed] [Google Scholar]
- 48.Cerdá J, Mohan S, Garcia-Garcia G, et al. Acute kidney injury recognition in low-and middle-income countries. Kidney Int Rep. 2017;2(4):530–543. doi: 10.1016/j.ekir.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Igiraneza G, Dusabejambo V, Finklestein FO, Rastegar A. Challenges in the recognition and management of acute kidney injury by hospitals in resource-limited settings. Kidney Int Rep. 2020;5(7):991–999. doi: 10.1016/j.ekir.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naicker S, Yang C-W, Hwang S-J, Liu B-C, Chen J-H, Jha V. The novel coronavirus 2019 epidemic and kidneys. Kidney Int. 2020;97(5):824–828. doi: 10.1016/j.kint.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hittesdorf E, Panzer O, Wang D, et al. Mortality and renal outcomes of patients with severe COVID-19 treated in a provisional intensive care unit. J Crit Care. 2021;62:172–175. doi: 10.1016/j.jcrc.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Long JD, Strohbehn I, Sawtell R, Bhattacharyya R, Sise ME. COVID-19 survival and its impact on chronic kidney disease. Transl Res. 2021;241:70–82. doi: 10.1016/j.trsl.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nlandu YM, Makulo J-RR, Pakasa NM, et al. First case of COVID-19-associated collapsing glomerulopathy in sub-Saharan Africa. Case Rep Nephrol. 2020;2020 doi: 10.1155/2020/8820713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shakoor MT, Birkenbach MP, Lynch M. ANCA-associated vasculitis following Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis. 2021;78(4):611–613. doi: 10.1053/j.ajkd.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bijol V, Farag YM, Harris DC, et al. Renal pathology practice globally: identifying needs and meeting the challenge. Kidney Int. 2019;96(2):258–261. doi: 10.1016/j.kint.2018.10.040. [DOI] [PubMed] [Google Scholar]
- 56.Wainstein M, MacDonald S, Fryer D, et al. Use of an extended KDIGO definition to diagnose acute kidney injury in patients with COVID-19: a multinational study using the ISARIC–WHO clinical characterisation protocol. PLoS Med. 2022;19(4) doi: 10.1371/journal.pmed.1003969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wearne N, Okpechi IG, Swanepoel CR. Nephrology in South Africa: not yet ubuntu. Kidney Dis. 2019;5(3):189–196. doi: 10.1159/000497324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Osman MA, Alrukhaimi M, Ashuntantang GE, et al. Global nephrology workforce: gaps and opportunities toward a sustainable kidney care system. Kidney Int Suppl. 2018;8(2):52–63. doi: 10.1016/j.kisu.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharif MU, Elsayed ME, Stack AG. The global nephrology workforce: emerging threats and potential solutions! Clin Kidney J. 2016;9(1):11–22. doi: 10.1093/ckj/sfv111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Naicker S. End-stage renal disease in sub-Saharan Africa. Ethn Dis. 2009;19(suppl 1):S1-13-5. [PubMed] [Google Scholar]
- 61.Tannor E, Awuku Y, Boima V, Antwi S. The geographical distribution of dialysis services in Ghana. Ren Replace Ther. 2018;4(1):3. [Google Scholar]
- 62.Harris DC, Davies SJ, Finkelstein FO, et al. Increasing access to integrated ESKD care as part of universal health coverage. Kidney Int. 2019;95(4):S1–S33. doi: 10.1016/j.kint.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 63.Lunyera J, Kilonzo K, Lewington A, Yeates K, Finkelstein FO. Acute kidney injury in low-resource settings: barriers to diagnosis, awareness, and treatment and strategies to overcome these barriers. Am J Kidney Dis. 2016;67(6):834–840. doi: 10.1053/j.ajkd.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 64.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371(1):58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bowe B, Xie Y, Xu E, Al-Aly Z. Kidney outcomes in long COVID. J Am Soc Nephrol. 2021;32(11):2851–2862. doi: 10.1681/ASN.2021060734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cheng Y, Zhang N, Luo R, et al. Risk factors and outcomes of acute kidney injury in critically ill patients with coronavirus disease 2019. Kidney Dis. 2021;7(2):111–119. doi: 10.1159/000512270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41(1):1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 68.Stanifer JW, Jing B, Tolan S, et al. The epidemiology of chronic kidney disease in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(3):e174–e181. doi: 10.1016/S2214-109X(14)70002-6. [DOI] [PubMed] [Google Scholar]
- 69.Stanifer JW, Muiru A, Jafar TH, Patel UD. Chronic kidney disease in low-and middle-income countries. Nephrol Dial Transplant. 2016;31(6):868–874. doi: 10.1093/ndt/gfv466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaze AD, Ilori T, Jaar BG, Echouffo-Tcheugui JB. Burden of chronic kidney disease on the African continent: a systematic review and meta-analysis. BMC Nephrol. 2018;19(1):125. doi: 10.1186/s12882-018-0930-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ashuntantang G, Osafo C, Olowu WA, et al. Outcomes in adults and children with end-stage kidney disease requiring dialysis in sub-Saharan Africa: a systematic review. Lancet Glob Health. 2017;5(4):e408–e417. doi: 10.1016/S2214-109X(17)30057-8. [DOI] [PubMed] [Google Scholar]
- 73.Alwan A, MacLean DR. A review of non-communicable disease in low-and middle-income countries. Int Health. 2009;1(1):3–9. doi: 10.1016/j.inhe.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 74.Tannor EK, Calice-Silva V. Kidney health for all-efforts in low-income settings to enhance community engagement, kidney health awareness and screening. Kidney Int Rep. 2022;7:359–362. doi: 10.1016/j.ekir.2021.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kumela Goro K, Desalegn Wolide A, Kerga Dibaba F, et al. Patient awareness, prevalence, and risk factors of chronic kidney disease among diabetes mellitus and hypertensive patients at Jimma University Medical Center, Ethiopia. BioMed Res Int. 2019;2019 doi: 10.1155/2019/2383508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oluyombo R, Ayodele O, Akinwusi P, et al. Awareness, knowledge and perception of chronic kidney disease in a rural community of South‑West Nigeria. Niger J Clin Pract. 2016;19(2):161–169. doi: 10.4103/1119-3077.175960. [DOI] [PubMed] [Google Scholar]
- 77.Wijewickrama E, Bajpai D, Hafidz M, et al. Availability and prioritization of covid-19 vaccines among patients with chronic kidney disease and kidney transplant-a global survey by the international society of nephrology. Kidney Int Rep. 2022;7(2):S426. [Google Scholar]
- 78.Weinhandl ED, Wetmore JB, Peng Y, Liu J, Gilbertson DT, Johansen KL. Initial effects of COVID-19 on patients with ESKD. J Am Soc Nephrol. 2021;32(6):1444–1453. doi: 10.1681/ASN.2021010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Combe C, Kirsch AH, Alfano G, et al. At least 156 reasons to prioritize COVID-19 vaccination in patients receiving in-centre haemodialysis. Nephrol Dial Transplant. 2021;36(4):571–574. doi: 10.1093/ndt/gfab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jager KJ, Kramer A, Chesnaye NC, et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98(6):1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scarpioni R, Manini A, Valsania T, et al. Covid-19 and its impact on nephropathic patients: the experience at Ospedale “Guglielmo da Saliceto” in Piacenza. G Ital Nefrol. 2020;37(2):1–5. [PubMed] [Google Scholar]
- 82.Alberici F, Delbarba E, Manenti C, et al. A report from the Brescia Renal COVID Task Force on the clinical characteristics and short-term outcome of hemodialysis patients with SARS-CoV-2 infection. Kidney Int. 2020;98(1):20–26. doi: 10.1016/j.kint.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Meester J, De Bacquer D, Naesens M, Meijers B. Couttenye MM, De Vriese AS. Incidence, characteristics, and outcome of COVID-19 in adults on kidney replacement therapy: a regionwide registry study. J Am Soc Nephrol. 2021;32(2):385–396. doi: 10.1681/ASN.2020060875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Robinson BM, Guedes M, Alghonaim M, et al. Worldwide early impact of COVID-19 on dialysis patients and staff and lessons learned: a DOPPS roundtable discussion. Kidney Med. 2021;3(4):619–634. doi: 10.1016/j.xkme.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Basile C, Combe C, Pizzarelli F, et al. Recommendations for the prevention, mitigation and containment of the emerging SARS-CoV-2 (COVID-19) pandemic in haemodialysis centres. Nephrol Dial Transplant. 2020;35(5):737–741. doi: 10.1093/ndt/gfaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Centre for Disease Control and Prevention . 2020. Accessed April 30, 2022.. Interim additional guidance for infection prevention and control for patients with suspected or confirmed COVID-19 in nursing homes.https://www.cdc.gov/coronavirus/2019-ncov/hcp/long-term-care.html [Google Scholar]
- 87.Elsayed HM, Wadee S, Zaki MS, et al. Guidelines for the prevention, detection and management of the renal complications of COVID-19 in Africa. Afr J Nephrol. 2020;23(1):109–126. [Google Scholar]
- 88.National Institute of Health and Care Excellence . 2021. Accessed June 3, 2021.. COVID-19 rapid guideline: managing COVID-19.https://www.nice.org.uk/guidance/ng191 [PubMed] [Google Scholar]
- 89.Mwai P. Covid-19 vaccines: why some African states can’t use their vaccines, 8th June 2021, BBC News, 8. Accessed July 8, 2021. https://www.bbc.com/news/56940657
- 90.Scohy A, Anantharajah A, Bodéus M, Kabamba-Mukadi B, Verroken A, Rodriguez-Villalobos H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bagcchi S. Stigma during the COVID-19 pandemic. Lancet Infect Dis. 2020;20(7):782. doi: 10.1016/S1473-3099(20)30498-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Uvais NA, Aziz F, Hafeeq B. COVID-19-related stigma and perceived stress among dialysis staff. J Nephrol. 2020;33:1121–1122. doi: 10.1007/s40620-020-00833-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Caillard S, Anglicheau D, Matignon M, Durrbach A, Greze C, Frimat L. On behalf of the French SOT COVID registry. An initial report from the French SOT COVID registry suggests high mortality due to Covid−19 in recipients of kidney transplants. Kidney Int. 2020;98(6):1549–1558. doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pereira M, Mohan S, Cohen D. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fernández-Ruiz M, Andrés A, Loinaz C, et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant. 2020;20(7):1849–1858. doi: 10.1111/ajt.15929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Emanuel JE, Persad G. Upshur R, et al. Fair Allocation Of Scarce Medical Resources In The Time Of Covid-19. N Engl Med J. 2020;382(21):2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 97.Aubert O, Yoo D, Zielinski D, et al. COVID-19 pandemic and worldwide organ transplantation: a population-based study. Lancet Public Health. 2021;6(10):e709–e719. doi: 10.1016/S2468-2667(21)00200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Paremoer L, Nandi S, Serag H, Baum F. Covid-19 pandemic and the social determinants of health. BMJ. 2021;372:n129. doi: 10.1136/bmj.n129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Roberts MB, Izzy S, Tahir Z, Al Jarrah A, Fishman JA, El Khoury J. COVID-19 in solid organ transplant recipients: dynamics of disease progression and inflammatory markers in ICU and non-ICU admitted patients. Transplant Infect Dis. 2020;22(5):e13407. doi: 10.1111/tid.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sargazi M, Shenkin A, Roberts NB. Aluminium-induced injury to kidney proximal tubular cells: effects on markers of oxidative damage. J Trace Elem Med Biol. 2006;19(4):267–273. doi: 10.1016/j.jtemb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 101.Chen G, Wu D, Guo W, et al. Clinical and immunologic features in severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Conti P, Ronconi G, Caraffa A, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34(2):327–331. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 103.Elec F, Magnusson J, Elec A, et al. COVID-19 and kidney transplantation: the impact of remdesivir on renal function and outcome-a retrospective cohort study. Int J Infect Dis. 2022;118:247–253. doi: 10.1016/j.ijid.2022.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thakare S, Gandhi C, Modi T, et al. Safety of remdesivir in patients with acute kidney injury or CKD. Kidney Int Rep. 2021;6(1):206–210. doi: 10.1016/j.ekir.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Divua B, Satarupa D, Sreyashi B, et al. Recovery of kidney function after AKI because of COVID-19 in kidney transplant recipients. Transpl Int. 2021;34(6):1074–1082. doi: 10.1111/tri.13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jasuja S, Sagar G, Bahl A, Verma S. COVID-19 infection clinical profile, management, outcome, and antibody response in kidney transplant recipients: a single centre experience. Int J Nephrol. 2021;2021 doi: 10.1155/2021/3129411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kute VB, Bhalla AK, Guleria S, et al. Clinical profile and outcome of COVID-19 in 250 kidney transplant recipients: a multicenter cohort study from India. Transplantation. 2021;105(4):851–860. doi: 10.1097/TP.0000000000003593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kute VB, Meshram HS, Navadiya VV, et al. Consequences of the first and second COVID-19 wave on kidney transplant recipients at a large Indian transplant centre. Nephrology. 2022;27(2):195–207. doi: 10.1111/nep.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kute VB, Meshram HS, Chauhan S, et al. A propensity-matched analysis of COVID-19 in kidney transplant recipients compared with non-kidney transplant patients: a single-center report from India. Exp Clin Transplant. 2021;19(12):1263–1270. doi: 10.6002/ect.2021.0438. [DOI] [PubMed] [Google Scholar]
- 110.Cristelli MP, Viana LA, Tedesco-Silva H, Medina-Pestana J. COVID-19 among kidney transplant recipients: a look into Latin America. Transplantation. 2022;106(3):e185. doi: 10.1097/TP.0000000000004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jones ES, Davidson BJ, Barday Z, et al. COVID-19 and the kidney: a South African state healthcare experience. Clin Nephrol. 2021;95(4):171. doi: 10.5414/CN110390. [DOI] [PubMed] [Google Scholar]
- 112.Muthu V, Agarwal R, Patel A, et al. Definition, diagnosis, and management of COVID-19-associated pulmonary mucormycosis: Delphi consensus statement from the Fungal Infection Study Forum and Academy of Pulmonary Sciences, India. Lancet Infect Dis. 2022;22(9):e240–e253. doi: 10.1016/S1473-3099(22)00124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Meshram HS, Kute VB, Yadav DK, et al. Impact of COVID-19-associated Mucormycosis in kidney transplant recipients: a multicenter cohort study. Transplant Direct. 2022;8(1):e1255. doi: 10.1097/TXD.0000000000001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kute V, Guleria S, Prakash J, et al. NOTTO transplant specific guidelines with reference to COVID-19. Indian J Nephrol. 2020;30(4):215. doi: 10.4103/ijn.IJN_299_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kute VB, Guleria S, Bhalla AK, et al. ISOT consensus statement for the kidney transplant recipient and living donor with a previous diagnosis of COVID-19. Indian J Transplant. 2021;15(2):131. doi: 10.4103/ijn.ijn_120_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Millán DAC, Fajardo-Cediel W, Tobar-Roa V, García-Perdomo HA, Autrán-Gómez AM. Strategies to mitigate the impact of COVID 19 pandemic on organ donation and kidney transplantation in Latin America. Curr Urol Rep. 2021;22(12):1–7. doi: 10.1007/s11934-021-01076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kute VB, Meshram HS, Mahillo B, Dominguez-Gil B. Transplantation in India and China during the COVID-19 pandemic. Lancet Public Health. 2022;7(1):e12. doi: 10.1016/S2468-2667(21)00280-2. [DOI] [PubMed] [Google Scholar]
- 118.Kute VB, Tullius SG, Rane H, Chauhan S, Mishra V, Meshram HS. Global impact of the COVID-19 pandemic on solid organ transplant. Transplant Proc. 2022;54(6):1412–1416. doi: 10.1016/j.transproceed.2022.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Meshram HS, Kute VB, Swarnalatha G, et al. Effect of coronavirus disease 2019 on transplantation and nephrology in India: a nationwide report from India. Transplant Proc. 2022;54(6):1429–1433. doi: 10.1016/j.transproceed.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Soin AS, Choudhary NS, Yadav SK, et al. Restructuring living-donor liver transplantation at a high-volume center during the COVID-19 pandemic. J Clin Exp Hepatol. 2021;11(4):418–423. doi: 10.1016/j.jceh.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Singhal A, Sahota GS, Srivastava P, et al. Living donor liver transplantation during the COVID-19 pandemic: “elective” but “necessary”. Transplantation. 2020;104(12):e351–e353. doi: 10.1097/TP.0000000000003449. [DOI] [PubMed] [Google Scholar]
- 122.Menon J, Hakeem AR, Rammohan A, et al. Living donor liver transplantation during the COVID-19 pandemic: a serendipitous silver lining! Transplantation. 2021;105(2):e20–e21. doi: 10.1097/TP.0000000000003574. [DOI] [PubMed] [Google Scholar]
- 123.Glenn DA, Hegde A, Kotzen E, et al. Systematic review of safety and efficacy of COVID-19 vaccines in patients with kidney disease. Kidney Int Rep. 2021;6(5):1407–1410. doi: 10.1016/j.ekir.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Akinbodewa AA, Adejumo OA. Awareness and practice of vaccination of chronic hemodialysis patients by specialist nephrology practitioners in Nigeria: a cross-sectional survey. J Epidemiol Glob Health. 2019;9(3):204–209. doi: 10.2991/jegh.k.190518.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Connor A, Hariharan N, Carson S, et al. Access to COVID-19 testing in low- and middle-income countries is still critical to achieving health equity, 2021, Health Affairs Blog, Accessed May 2, 2022.https://www.healthaffairs.org/do/10.1377/forefront.20211026.483412
- 126.Mathieu E, Ritchie H, Rodés-Guirao L, et al. Coronavirus (COVID-19) vaccinations 2022. Accessed May 19, 2022. https://ourworldindata.org/covid-vaccinations
- 127.Emanuel EJ, Luna F, Schaefer GO, Tan K-C, Wolff J. Enhancing the WHO's proposed framework for distributing COVID-19 vaccines among countries. Am J Public Health. 2021;111(3):371–373. doi: 10.2105/AJPH.2020.306098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Peacocke EF, Heupink LF, Frønsdal K, Dahl EH, Chola L. Global access to COVID-19 vaccines: a scoping review of factors that may influence equitable access for low and middle-income countries. BMJ Open. 2021;11(9) doi: 10.1136/bmjopen-2021-049505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wouters OJ, Shadlen KC, Salcher-Konrad M, et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021;397:1023–1034. doi: 10.1016/S0140-6736(21)00306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yarlagadda H, Patel MA, Gupta V, et al. COVID-19 vaccine challenges in developing and developed countries. Cureus. 2022;14(4):e23951. doi: 10.7759/cureus.23951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tisi A. Patent rights or patient rights? An assessment of intellectual property and right to health within the Covid-19 pandemic. University of Southern Denmark/Danish Institute for Human Rights Dissertation, 2021. Accessed May 19, 2022. http://hdl.handle.net/20.500.11825/2417
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.