Abstract
Objective
In patients with coronavirus disease 2019 (COVID-19), understanding the timeline of oxygen demand and severe respiratory failure, such as intensive care unit (ICU) admission, may clarify the therapeutic window when home-care treatment is possible and help determine the timing of treatment in hospitalized patients to improve the respiratory status. We examined the timeline of respiratory status in hospitalized patients with moderate-to-severe COVID-19 in terms of oxygen demand and ICU admission.
Methods
We retrospectively assessed all patients with COVID-19 who were admitted to our hospital between February 2020 and February 2021 and required supplemental oxygen. This study included 66 patients who were transferred to the ICU (ICU patients) and 144 patients who were not transferred to the ICU (non-ICU patients).
Results
In the total cohort, the median duration from symptom onset to the need for supplemental oxygen was 8 [interquartile range (IQR) 6-10] days. This duration was significantly shorter in ICU patients than in non-ICU patients [8 (IQR 6-9) vs. 9 (IQR 6-10) days, p=0.02]. The median duration from symptom onset to ICU admission was 9 (IQR 8-11) days in severely ill patients. The median duration from the initiation of supplemental oxygen to ICU admission was 1.0 (IQR 1-2.75) days. Only 2 of 66 patients (3.0%) were admitted to the ICU six days or later after the initiation of supplemental oxygen.
Conclusion
Physicians should carefully monitor each patient's condition after eight days from symptom onset. New therapies and their early administration are needed to reduce the frequency of respiratory failure in COVID-19 patients.
Keywords: COVID-19, oxygen, timeline, SARS-CoV-2, organizing pneumonia, corticosteroid
Introduction
Patients with coronavirus disease 2019 (COVID-19) often require supplemental oxygen during their illness. Understanding the timeline of oxygen demand and severe respiratory failure, such as intensive care unit (ICU) admission, may clarify the therapeutic window when home-care treatment is possible and help determine the timing of treatment in hospitalized patients to improve their respiratory status.
In a series of studies on hospitalized patients with COVID-19, acute respiratory distress syndrome developed a median of eight to nine days after symptom onset (1,2). However, the details concerning the timeline of oxygen demand in patients with COVID-19 pneumonia remain unclear. We examined the timeline of the respiratory status in hospitalized patients with moderate-to-severe COVID-19 in terms of their oxygen demand and ICU admission.
Materials and Methods
This single-center retrospective study was performed at Kanagawa Cardiovascular and Respiratory Center in Yokohama, Japan. We assessed 475 consecutive patients with COVID-19 admitted to our hospital between February 2020 and February 2021. Only patients who required supplemental oxygen during hospitalization were enrolled in this study. Clinical and laboratory data, treatment, and outcomes were retrospectively collected from patients' medical records.
We defined the day of the symptom onset (Day 0) as when the patient became aware of symptoms, such as a fever, cough, malaise, or headache, obtained through a detailed interview. Supplemental oxygen was administered when the oxygen saturation was ≤90% at rest. ICU admission was defined as oxygen saturation ≤90% at rest despite an oxygen flow of ≥6 L/min through a face mask or if a high-flow nasal cannula or mechanical ventilation (fraction of inspiratory oxygen ≥50%) was required to correct the hypoxemia. Patients with reduced oxygenation intake due to complications, such as acute pulmonary thromboembolism, were admitted to the ICU.
The study protocol was approved by the Institutional Review Board of Kanagawa Cardiovascular Respiratory Center (approval number KCRC-21-0008, approval date July 5, 2021). In addition, the ethics committee waived the requirement for informed consent due to the study's retrospective nature.
Categorical data are presented as numbers (percentages) and were compared using the Fisher's exact test. Continuous data are presented as medians [interquartile ranges (IQR)] and were compared using the Mann-Whitney U test. Statistical significance was set at p<0.05. To investigate the risk factors for ICU admission, univariate logistic regression analyses adjusted for age and sex were applied. Subsequently, variables with a p<0.05 in the univariate analysis were included in the multivariate analysis. All statistical analyses were performed using the SPSS software program, version 24 (IBM, Armonk, USA).
Results
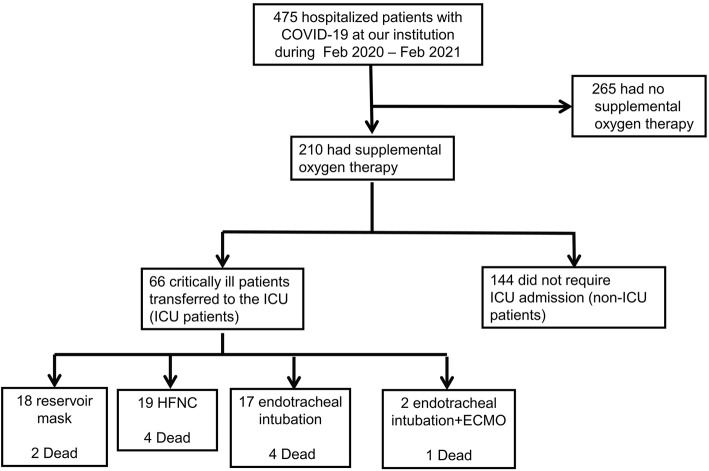
A total of 210 hospitalized COVID-19 patients who required supplemental oxygen were enrolled in this study (Fig. 1). Of these, 66 were transferred to the ICU, as they required a high-flow nasal cannula or mechanical ventilation to correct hypoxemia (ICU patients). The remaining 144 patients did not require ICU admission (non-ICU patients). In the ICU, 18 patients were given a reservoir mask, 19 a high-flow nasal cannula, 27 endotracheal intubation, and 2 endotracheal intubation and extracorporeal membrane oxygenation. Eleven patients died in these groups. One patient had acute pulmonary thromboembolism.
Figure 1.
Flow chart. ICU: intensive care unit, HFNC: high-flow nasal cannula, ECMO: extracorporeal membrane oxygenation
Table 1 shows the baseline features of the patients with COVID-19 requiring supplemental oxygen during hospitalization. The ICU patients included 51 men and 15 women (median age: 72.5 years old), while the non-ICU patients included 94 men and 50 women (median age: 69 years old). The median duration from symptom onset to admission was significantly shorter in ICU patients than in non-ICU patients [6 (IQR 3-8) vs. 7 (IQR 4-9) days, p=0.02]. In addition, ICU patients were significantly more likely to have interstitial lung disease than non-ICU patients [7 (4.9%) vs. 10 (15.2%), p=0.03], and the baseline platelet count was significantly lower in ICU patients than in non-ICU patients (p=0.02).
Table 1.
Baseline Features of Patients with COVID-19 Requiring Supplemental Oxygen during Hospitalization.
| Parameters | Total (n=210) | Non-ICU (n=144) | ICU (n=66) | p value |
|---|---|---|---|---|
| Age, years | 70 (60-78) | 69 (59-78) | 72.5 (62-78) | 0.35 |
| Men, n | 15 (69.0) | 94 (65.3) | 51 (77.3) | 0.11 |
| Body mass index, kg/m 2 | 25.4 (22.2-27.8) | 24.8 (22.0-28.0) | 25.6 (23.0-26.8) | 0.97 |
| Smoking, pack-year | 8 (0-40) | 3 (0-34) | 15 (0-41) | 0.09 |
| Days from symptom onset to admission, days | 6 (4-8) | 7 (4-9) | 6 (3-8) | 0.02 |
| Comorbidities | ||||
| Hypertension, n | 112 (53.3) | 79 (54.9) | 33 (50.0) | 0.55 |
| Diabetes, n | 66 (31.4) | 43 (7.1) | 23 (34.8) | 0.52 |
| Cardiovascular disease, n | 45 (21.4) | 33 (22.9) | 12 (18.2) | 0.48 |
| Cerebrovascular disease, n | 24 (11.4) | 14 (9.7) | 10 (15.2) | 0.25 |
| Chronic kidney disease, n | 8 (3.8) | 4 (2.8) | 4 (6.1) | 0.26 |
| Chronic obstructive pulmonary disease, n | 43 (20.0) | 25 (17.4) | 18 (27.3) | 0.14 |
| Interstitial lung disease, n | 17 (8.1) | 7 (4.9) | 10 (15.2) | 0.03 |
| Malignancy, n | 7 (3.3) | 4 (2.8) | 3 (4.5) | 0.68 |
| Laboratory data | ||||
| White blood cells, ×109/L | 5.9 (4.5-7.3) | 5.9 (4.5-7.4) | 5.8 (4.4-7.0) | 0.79 |
| Lymphocytes, ×109/L | 1.0 (0.7-1.2) | 1.0 (0.7-1.2) | 0.9 (0.7-1.2) | 0.51 |
| Platelets, ×109/L | 185 (155-224) | 187 (161-231) | 176 (138-209) | 0.02 |
| Aspartate aminotransferase, IU/L | 43 (29-57) | 42 (30-56) | 44.5 (28.0-62.5) | 0.31 |
| Alanine aminotransferase, IU/L | 31 (20-48) | 31 (19-48) | 29 (20.0-49.3) | 0.84 |
| Lactate dehydrogenase, IU/L | 302 (227-389) | 295 (226-379) | 310 (242-451) | 0.17 |
| Creatine, mg/dL | 0.88 (0.71-1.04) | 0.87 (0.71-1.05) | 0.88 (0.72-1.01) | 0.97 |
| C-reactive protein, mg/dL | 6.4 (3.3-11.8) | 6.3 (3.3-11.3) | 7.5 (2.7-13.5) | 0.35 |
| D-dimer, μg/mL | 1.2 (0.9-1.7) | 1.2 (0.9-1.7) | 1.2 (0.8-1.7) | 0.80 |
| KL-6, U/mL | 316 (232-496) | 315 (229-488) | 318 (246-602) | 0.52 |
| Ferritin, ng/mL | 606 (336-1,131) | 590 (289-1,204) | 650 (463-1,097) | 0.38 |
Categorical data are presented as numbers (percentages), and continuous data as medians (interquartile range). COVID-19: coronavirus disease 2019, ICU: intensive care unit, KL-6: Krebs von den Lungen 6
Combination therapy with corticosteroids, antiviral agents, and tocilizumab was used more frequently in ICU patients than in non-ICU patients (p<0.01) (Table 2). Tocilizumab was administered early in the course of the disease to prevent severe illness. The median duration from symptom onset to the initiation of corticosteroid administration was significantly shorter in ICU patients than in non-ICU patients [6 (IQR 4-8) vs. 7 (IQR 5-9) days, p<0.01]. There were no significant differences in oxygenation (oxygen saturation to a fraction of inspiratory oxygen ratio) at the initiation of corticosteroid administration, the number of patients who received pulse corticosteroids, or the type of corticosteroids in the two groups.
Table 2.
Treatment of Patients with COVID-19 Requiring Supplemental Oxygen during Hospitalization.
| Parameters | Total | Non-ICU | ICU | p value |
|---|---|---|---|---|
| (n=210) | (n=144) | ICU (n=66) | ||
| Regimen | ||||
| Corticosteroid* only, n | 8 (3.8) | 8 (5.6) | 0 (0) | 0.06 |
| Antiviral agents† only, n | 8 (3.8) | 6 (4.2) | 2 (3.0) | 1.00 |
| Corticosteroid*/antiviral agents†, n | 113 (53.8) | 85 (59.0) | 28 (42.4) | 0.03 |
| Corticosteroid*/antiviral agents†/tocilizumab, n | 45 (21.4) | 18 (12.5) | 27 (40.9) | <0.01 |
| Corticosteroid‡/antiviral agents§/baricitinib, n | 31 (14.8) | 24 (16.7) | 7 (10.6) | 0.30 |
| Corticosteroid therapy | ||||
| Days from symptom onset to the initiation of corticosteroid administration, days | 7 (5-9) | 7 (5-9) | 6 (4-8) | <0.01 |
| SpO2 to FiO2 ratio at the initiation of corticosteroid administration | 395.8 | 395.8 | 404.2 | 0.84 |
| (336.8-447.6) | (339.3-447.6) | (337.1-447.6) | ||
| Dose of corticosteroids (equivalent to methylprednisolone), mg | 80 (68-500) | 80 (76-500) | 80 (62-500) | 0.95 |
| Dose of methylprednisolone <80 mg, n | 42 (20.0) | 27 (18.8) | 15 (22.7) | 0.58 |
| 80 mg ≤ dose of methylprednisolone <250 mg, n | 65 (31.0) | 45 (31.3) | 20 (30.3) | 1.00 |
| 250 mg ≤ dose of methylprednisolone, n | 92 (43.8) | 63 (43.8) | 29 (43.9) | 1.00 |
| Type of corticosteroids | ||||
| Dexamethasone, n | 32 (15.2) | 20 (13.9) | 12 (18.2) | 0.42 |
| Prednisolone, n | 9 (4.3) | 7 (4.9) | 2 (3.0) | 0.72 |
| Methylprednisolone, n | 158 (75.2) | 108 (75.0) | 50 (75.8) | 1.00 |
| Outcome | ||||
| Dead, n | 11 (5.2) | 0 (0) | 11(16.7) | <0.01 |
Categorical data are presented as numbers (percentages), and continuous data as medians (interquartile range). COVID-19: coronavirus disease 2019, ICU: intensive care unit, SpO2: oxygen saturation, FiO2: a fraction of inspiratory oxygen. *Dexamethasone or prednisolone or methylprednisolone, †Favipiravir or remdesivir, ‡Dexamethasone or methylprednisolone, §Remdesivir
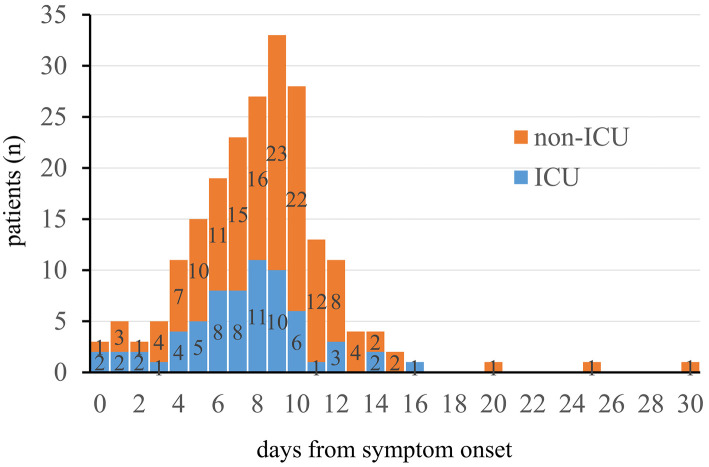
The median duration from symptom onset to the initiation of supplemental oxygen was significantly shorter in ICU patients than in non-ICU patients [8 (IQR 6-9) vs. 9 (IQR 6-10) days, p=0.02] (Fig. 2). Among the 210 patients, 38 (18.1%) initiated oxygen administration on day 11 or later after symptom onset. As shown in Fig. 2, the patient who initiated supplemental oxygen 25 days after symptom onset had chronic heart failure. Bilateral pleural effusion developed due to the exacerbation of chronic heart failure by COVID-19. The patient's conditions subsequently recovered with corticosteroid and diuretic therapies. The patient who initiated supplemental oxygen on day 30 from symptom onset had chest computed tomography findings showing dense subpleural consolidation in the bilateral upper and middle lobes. These findings were suggestive of post-COVID-19 organizing pneumonia. The contraction of dense subpleural consolidation reduced the lung volume, and oxygen therapy was initiated. The patient recovered with pulse methylprednisolone therapy.
Figure 2.
Duration from symptom onset to oxygen administration in patients with COVID-19 who required supplemental oxygen. The orange bar represents the number of non-ICU patients, and the blue bar represents the number of ICU patients. The day of symptom onset is defined as Day 0.
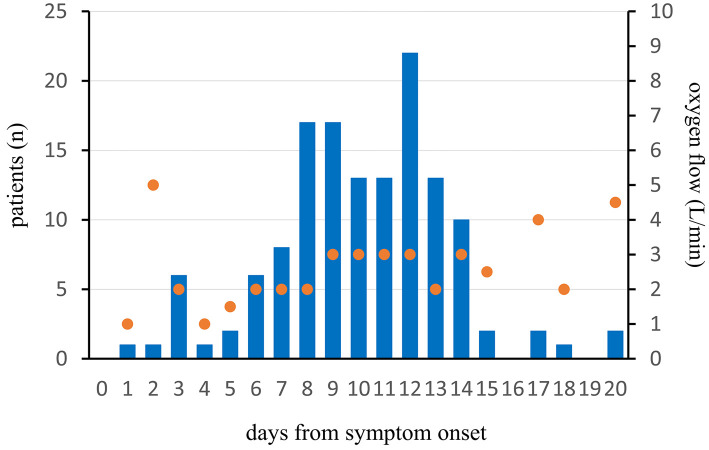
The mean duration from symptom onset to maximum supplemental oxygen administration in 144 non-ICU patients was 10 (IQR 8-12) days, and the peak oxygen flow was 3.0 L/min at that time (Fig. 3).
Figure 3.
Duration from symptom onset to the maximum supplemental oxygen and the mean of peak oxygen flow in 144 non-ICU patients. The blue bar represents the number of non-ICU patients, and the orange dot represents the mean of peak oxygen flow at that time. The day of symptom onset is defined as Day 0.
In the univariate logistic regression analysis, comorbidity with interstitial lung disease, lactate dehydrogenase, and combination therapy with corticosteroid, antiviral agents, and tocilizumab were identified as positive risk factors for ICU admission (Table 3). In contrast, the days from symptom onset to admission, the days from symptom onset to the initiation of corticosteroid administration, and platelet count were significant negative risk factors. In the multivariate logistic regression analysis, lactate dehydrogenase and combination therapy with corticosteroid, antiviral agents, and tocilizumab were significantly positively associated with ICU admission. Conversely, the days from symptom onset to the initiation of corticosteroid administration was a negative risk factor (p=0.03).
Table 3.
Univariate and Multivariate Logistic Regression Analyses of ICU Admission Adjusted for Age and Sex.
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Odds ratio | p value | Odds ratio | p value | ||
| (95% CI) | (95% CI) | ||||
| Body mass index | 1.06 (0.98-1.15) | 0.17 | |||
| Days from symptom onset to admission* | 0.90 (0.83-0.99) | 0.03 | |||
| Diabetes | 1.20 (0.64-2.25) | 0.57 | |||
| Cardiovascular disease | 0.73 (0.33-1.57) | 0.42 | |||
| Chronic kidney disease | 2.13 (0.50-8.98) | 0.31 | |||
| Chronic obstructive pulmonary disease | 1.46 (0.70-3.01) | 0.31 | |||
| Interstitial lung disease | 3.15 (1.12-8.86) | 0.03 | 2.76 (0.78-9.81) | 0.12 | |
| White blood cells | 1.000 (0.999-1.001) | 0.58 | |||
| Lymphocytes | 1.000 (0.999-1.001) | 0.99 | |||
| Platelets | 0.95 (0.91-0.99) | 0.04 | 0.99 (0.93-1.04) | 0.64 | |
| Lactate dehydrogenase | 1.003 (1.000-1.005) | 0.04 | 1.004 (1.001-1.007) | 0.01 | |
| Creatine | 0.88 (0.33-2.33) | 0.80 | |||
| C-reactive protein | 1.03 (0.99-1.08) | 0.20 | |||
| Corticosteroid/antiviral agents | 0.57 (0.31-1.03) | 0.06 | |||
| Corticosteroid/antiviral agents/tocilizumab | 4.12 (2.06-8.25) | <0.01 | 3.58 (1.69-7.56) | <0.01 | |
| Corticosteroid/antiviral agents/baricitinib | 0.67 (0.27-1.67) | 0.39 | |||
| Days from symptom onset to the initiation of corticosteroid administration | 0.85 (0.76-0.95) | <0.01 | 0.86 (0.75-0.99) | 0.03 | |
| SpO2 to FiO2 ratio at the initiation of corticosteroid administration | 0.998 (0.994-1.002) | 0.26 | |||
| Dose of methylprednisolone<80 mg | 1.18 (0.57-2.43) | 0.65 | |||
| 80 mg ≤ dose of methylprednisolone<250 mg | 1.08 (0.56-2.06) | 0.83 | |||
| 250 mg ≤ dose of methylprednisolone | 0.98 (0.54-1.77) | 0.94 | |||
| Dexamethasone | 1.28 (0.58-2.83) | 0.55 | |||
| Prednisolone | 0.56 (0.11-2.82) | 0.48 | |||
| Methylprednisolone | 1.15 (0.58-2.29) | 0.70 | |||
CI: confidence interval, ICU: intensive care unit, SpO2: oxygen saturation, FiO2: a fraction of inspiratory oxygen
*“Days from symptom onset to admission” was not included in the multivariate analysis due to co-linearity with “days from symptom onset to the initiation of corticosteroid administration” (r=0.860, p<0.01).
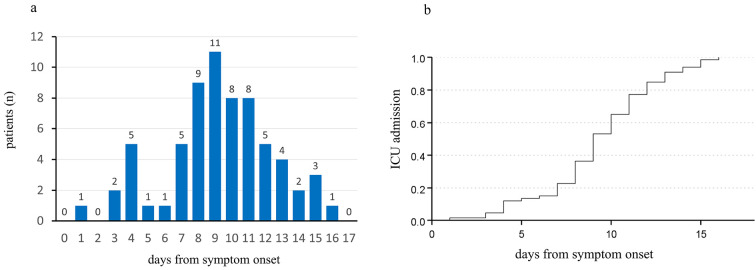
The median duration from symptom onset to ICU admission was 9 (IQR 8-11) days in severely ill patients (Fig. 4a, b). None of the patients were transferred to the ICU 17 days or later after symptom onset. As shown in Fig. 4a, the patient who was admitted to the ICU on day 1 from symptom onset had lung volume loss due to interstitial pneumonia and left upper lobectomy. The patient had been on home oxygen therapy at 3 L/min at rest through the nasal cannula before admission.
Figure 4.
a: Duration from symptom onset to ICU transfer in severely ill patients. The blue bar represents the number of ICU patients. The day of symptom onset is defined as Day 0. b: The median duration from symptom onset to ICU admission using the Kaplan-Meier method. The median duration from symptom onset to ICU admission was 9 (confidence interval 8.2-9.8) days.
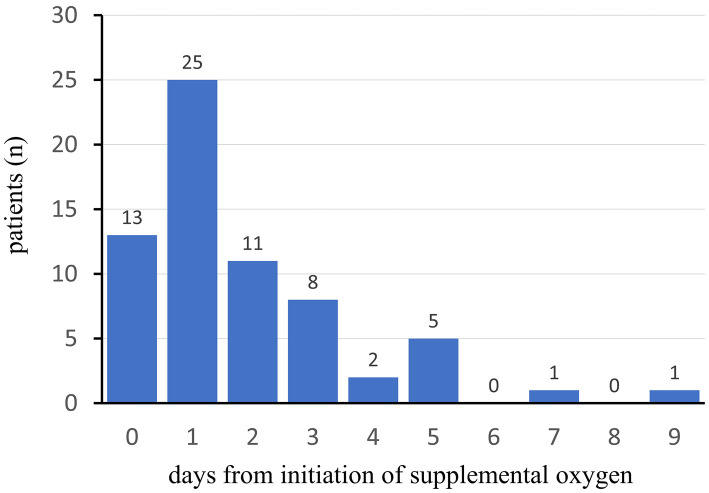
The median duration from the initiation of supplemental oxygen to ICU admission was 1.0 (IQR 1-2.75) days (Fig. 5). Only 2 of 66 patients (3.0%) were admitted to the ICU six days or later after the initiation of supplemental oxygen.
Figure 5.
The number of days from the initiation of supplemental oxygen to ICU admission in patients with severe COVID-19. The blue bar represents the number of ICU patients. The day of initiation of supplemental oxygen is defined as Day 0.
Discussion
In this retrospective study, we investigated the timeline of oxygen demand in hospitalized patients with moderate-to-severe COVID-19. Our results suggest two important clinical points. First, although the median duration from symptom onset to the initiation of supplemental oxygen was eight days, 18.1% of the patients in this study started the administration of supplemental oxygen 11 days after symptom onset. Therefore, home-care patients with COVID-19 should monitor their oxygen saturation for 11 days or longer from symptom onset. Second, most patients were admitted to the ICU nine days after symptom onset, suggesting that treatment for COVID-19 pneumonia should be intensified within nine days from symptom onset to prevent ICU admission.
Supplemental oxygen therapy was typically initiated nine days after symptom onset and peaked on day 10 in non-ICU patients. Our findings are similar to those of a previous study that demonstrated that the median duration from symptom onset to dyspnea was eight days (1). Among the 210 patients, 38 (18.1%) initiated supplemental oxygen from symptom onset on day 11 or later, possibly due to post-COVID-19 organizing pneumonia (3-5). Post-COVID-19 organizing pneumonia is a reparative response of the lung tissue to injury by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. The histopathologic lesion characteristic is an excessive proliferation of granulation tissue within small airways and alveolar ducts, along with inflammation in surrounding alveoli. COVID-19 pneumonia evolves rapidly from focal unilateral to diffuse bilateral ground-glass opacities that progress to consolidation within one to three weeks after symptom onset, peaking at around two weeks (6). The consolidation indicates the appearance of interstitial changes, suggesting post-COVID-19 organizing pneumonia. The reported computed tomography findings of COVID-19 suggested that post-COVID-19 organizing pneumonia may be occurring frequently (7,8).
Most patients in the present study were admitted to the ICU nine days after symptom onset, which is consistent with previous findings wherein the median duration from symptom onset to ICU admission was 9.5-10 days (9-11). The time from symptom onset can guide physicians in determining whether or not COVID-19 pneumonia treatment should be intensified. In other words, if the patient's condition deteriorates within nine days of symptom onset, the patient should be treated intensively to prevent ICU admission.
Given the high frequency of post-COVID-19 organizing pneumonia, corticosteroids are likely to be the primary drugs used to treat COVID-19 pneumonia (12). There remain few studies concerning the optimal dose and type of corticosteroids for treating COVID-19 pneumonia (13-16). Although corticosteroid therapy was administered to pre-ICU patients a median of six days from symptom onset at an oxygen saturation-to-fraction of inspiratory oxygen ratio of 404.2 (equivalent to an oxygen saturation of 85% while the patient was breathing ambient air at rest), it did not prevent ICU admission in this study. A meta-analysis reported that, compared to non-pulse dose steroids, pulse dose steroids are associated with a similar mortality rate and need for endotracheal intubation (17). Recently, a randomized control trial reported that no significant differences between pulse methylprednisolone and placebo arms were observed for admission to the ICU with orotracheal intubation or death (18). Our univariate analysis showed that a dose of methylprednisolone ≥250 mg was not significantly associated with preventing ICU admission. Pulse methylprednisolone therapy may therefore not be effective in patients with COVID-19 pneumonia who require supplemental oxygen to prevent ICU admission.
This study was unable to prove the efficacy of combination triple therapy with corticosteroids, antiviral agents, and tocilizumab for COVID-19 pneumonia because of the small number of patients who received the treatment. Combination triple therapy was shown to be a risk factor for ICU admission. However, this appears to be a spurious association, as the more severely ill patients received combination triple therapy of corticosteroid, antiviral agents, and tocilizumab. A recent report showed that combination therapy with baricitinib, remdesivir, and dexamethasone was effective in treating severe COVID-19 (19). Additional data on case series with combination triple therapy are needed to confirm the efficacy of this treatment in patients with COVID-19 pneumonia.
In this study, the “days from symptom onset to the initiation of corticosteroid administration” parameter was significantly negatively associated with ICU admission in the multivariate analysis. These results suggest that patients with a long duration from symptom onset to the administration of corticosteroids may have had less aggressive COVID-19 pneumonia than others and been less likely to be admitted to the ICU.
Several limitations associated with the present study warrant mention. First, this was a retrospective study with a small number of patients. Second, the various therapeutic agents administered at our hospital, such as corticosteroids and antiviral agents, may have influenced the natural course of COVID-19. Third, neutralizing antibodies for the treatment of COVID-19 were unavailable during the study period. Fourth, the SARS-CoV-2 in this study was presumed to be the Wuhan strain, SARS-CoV-2 variants B.1.1., B.1.1.284, and B.1.1.214, which were prevalent before the alpha variants. SARS-CoV-2 variants, such as the Delta or Omicron variants, are now prevalent globally. Thus, future studies will be needed to determine the timeline of oxygen demand for SARS-CoV-2 infection for these new variants. Finally, all patients were unvaccinated against COVID-19 at the time of the study. Given that vaccine effectiveness against severe, critical, or fatal diseases due to SARS-CoV-2 infection is very high (20), the duration from symptom onset to the need for supplemental oxygen may be longer for vaccinated patients with COVID-19 than for unvaccinated individuals.
In conclusion, the median duration from symptom onset to the initiation of supplemental oxygen was eight days, and that until ICU admission was nine days. Since some patients initiated supplemental oxygen 11 days after symptom onset, physicians should carefully monitor each patient's condition 8-11 days from symptom onset. New therapies and their early administration are needed to reduce the frequency of respiratory failure in COVID-19 patients.
The authors state that they have no Conflict of Interest (COI).
Acknowledgment
We would like to thank all of the staff at the Kanagawa Cardiovascular and Respiratory Center who supported us.
References
- 1. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497-506, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323: 1061-1069, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Myall KJ, Mukherjee B, Castanheira AM, et al. Persistent post-COVID-19 interstitial lung disease. An observational study of corticosteroid treatment. Ann Am Thorac Soc 18: 799-806, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bieksiene K, Zaveckiene J, Malakauskas K, Vaguliene N, Zemaitis M, Miliauskas S. Post COVID-19 organizing pneumonia: the right time to interfere. Medicina 57: 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chong WH, Saha BK, Chopra A. Does COVID-19 pneumonia signify secondary organizing pneumonia?: a narrative review comparing the similarities between these two distinct entities. Heart Lung 50: 667-674, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 20: 425-434, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fu F, Lou J, Xi D, et al. Chest computed tomography findings of coronavirus disease 2019 (COVID-19) pneumonia. Eur Radiol 30: 5489-5498, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vadász I, Husain-Syed F, Dorfmüller P, et al. Severe organising pneumonia following COVID-19. Thorax 76: 201-204, 2021. [DOI] [PubMed] [Google Scholar]
- 9. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 8: 475-481, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang F, Qu M, Zhou X, et al. The timeline and risk factors of clinical progression of COVID-19 in Shenzhen, China. J Transl Med 18: 270, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zayet S, Gendrin V, Klopfenstein T. Natural history of COVID-19: back to basics. New Microbes New Infect 38: 100815, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 384: 693-704, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ranjbar K, Moghadami M, Mirahmadizadeh A, et al. Methylprednisolone or dexamethasone, which one is superior corticosteroid in the treatment of hospitalized COVID-19 patients: a triple-blinded randomized controlled trial. BMC Infect Dis 21: 337, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pinzón MA, Ortiz S, Holguín H, et al. Dexamethasone vs methylprednisolone high dose for Covid-19 pneumonia. PLoS One 16: e0252057, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. López Zúñiga M, Moreno-Moral A, Ocaña-Granados A, et al. High-dose corticosteroid pulse therapy increases the survival rate in COVID-19 patients at risk of hyper-inflammatory response. PLoS One 16: e0243964, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jeronimo CMP, Farias MEL, Val FFA, et al. ; the Metcovid Team. Methylprednisolone as adjunctive therapy for patients hospitalized with coronavirus disease 2019 (COVID-19; Metcovid): a randomized, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis 72: e373-e381, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khokher W, Beran A, Iftikhar S, et al. Pulse versus nonpulse steroid regimens in patients with coronavirus disease 2019: a systematic review and meta-analysis. J Med Virol 94: 4125-4137, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salvarani C, Massari M, Costantini M, et al. Intravenous methylprednisolone pulses in hospitalised patients with severe COVID-19 pneumonia, A double-blind, randomised, placebo-controlled trial. Eur Respir J. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Izumo T, Kuse N, Awano N, et al. Clinical impact of combination therapy with baricitinib, remdesivir, and dexamethasone in patients with severe COVID-19. Respir Investig 59: 799-803, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 Covid-19 vaccine against the B.1.1.7 and B.1.351 variants. N Engl J Med 385: 187-189, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]