Abstract
A man in his 70s visited our department for dyspnea with pulmonary infiltrate that was unresolved by antibiotics. He had been taking Sansoninto for five years and doubled its dose a month ago. After discontinuing Sansoninto without any additional medications, his symptoms gradually disappeared, and pulmonary infiltration improved. Drug lymphocyte stimulation tests showed a positive result for Sansoninto. We diagnosed this patient with Sansoninto-induced lung injury. Sansoninto is a combination drug that consists of sansonin, bukuryo, senkyo, chimo, and kanzo. This paper reports the first case of Sansoninto-induced lung injury and discusses the mechanism considering its components.
Keywords: drug-induced pneumonitis, Sansoninto, Chinese herb, kampo
Introduction
Any drug can cause lung injury, including pneumonitis, through direct cytotoxicity or an immune-mediated mechanism (1,2). The diagnosis of drug-induced pneumonitis is often challenging. However, it is comprehensively considered based on the history of recent medication, radiological features, biomarkers, drug lymphocyte stimulation test (DLST), clinical course after discontinuing suspected drug, and a re-exposure test if required (1).
Japanese herbal medicine, a traditional medicine with roots in ancient Chinese medicine, is prescribed or available over the counter for purposes widely ranging from self-care to treatment of chronic diseases (3). Herbal medicines may unexceptionally induce pneumonitis, such as Sho-saiko-to (minor bupleurum decoction) and Sairei-to (minor bupleurum decoction plus poria powder with five herbs). Among the components, Saiko (bupleuri radix) and Ougon (skullcap) are suspected as the crude agents associated with lung injury (4).
Sansoninto is a Japanese herbal medicine prescribed for patients experiencing physical and mental fatigue and insomnia. This drug is composed of sansonin (jujube seeds), bukuryou (Poria sclerotium), senkyuu (Cnidium rhizome), chimo (Anemarrhena rhizome), and kanzo (Glycyrrhiza), but not Saiko and Ougon.
We herein report the first case of a patient with Sansoninto-induced lung injury and discuss the mechanism considering its components.
Case Report
A man in his 70s visited our hospital because of dyspnea without a fever and cough, lasting for about 1 week. Chest X-ray showed bilateral pulmonary infiltration, and blood tests revealed a normal leukocyte count (7,110 cells/μL), slightly elevated serum C-reactive protein (CRP) levels (0.39 mg/dL) and no remarkable change as usual in brain natriuretic peptide (220 pg/mL). A polymerase chain reaction (PCR) test for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was negative. Clarithromycin was administered for five days, but his symptom and pulmonary infiltration did not improve. Consequently, he visited the Department of Respiratory Medicine two weeks after dyspnea started.
This patient was an ex-smoker (Brinkman index, 400) and had been exposed to neither dust nor pets, including birds. A cardiac pacemaker had been implanted for sick sinus syndrome 15 years earlier, and no underlying pulmonary diseases had been diagnosed. He had been taking Sansoninto (7.5 g/day) for general fatigue for 5 years and doubled its dose (15 g/day) in hope of further effects on the symptom under his own judgment 1 month ago, which he had continued until his visit to our department.
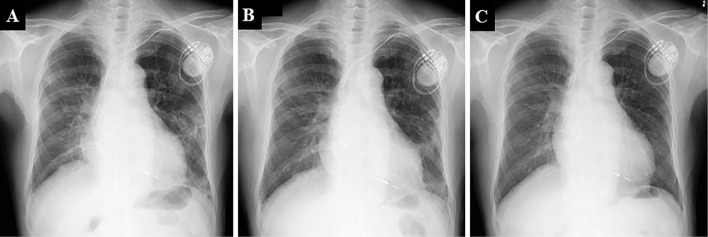
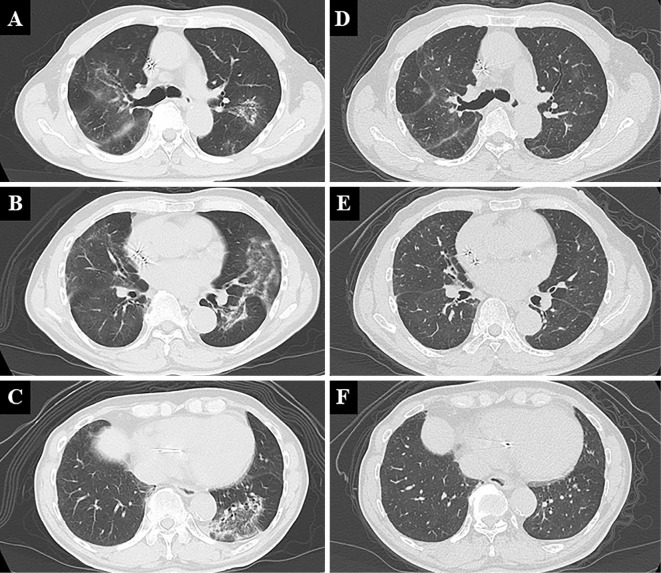
A physical examination revealed a body temperature of 36.0°C, an oxygen saturation (SpO2) of 95% without supplemental oxygenation, blood pressure of 140/90 mmHg, and heart rate of 83 beats/min. Fine crackles were heard bilaterally on chest auscultation. Laboratory tests revealed a normal leukocyte count (7,050 cells/μL), slightly elevated serum CRP levels (0.48 mg/dL) and lactate dehydrogenase (233 IU/L), and significantly elevated KL-6 (692 U/mL). Test results for antinuclear antibodies and other autoantibodies in the serum to screen for collagen vascular diseases were all negative. A sample of the patient's sputum was not available because of a lack of sputum symptoms. Chest X-ray demonstrated bilateral diffuse ground-glass attenuation, which were almost the same findings as seen on an image obtained one week earlier (Fig. 1A), and high-resolution computed tomography (HRCT) demonstrated non-segmental ground-glass attenuation with reversed halo signs in both lungs. However, neither honeycomb lesions nor emphysematous changes were observed (Fig. 2A-C). A pulmonary function test revealed a decreased percent volume capacity (59.8%).
Figure 1.
Chest X-ray features at the first visit to our department (A) and 1 month (B) and 6 months (C) after Sansoninto was withdrawn.
Figure 2.
Chest computed tomography features at the first visit to our department (A-C) and 6 months (D-F) after Sansoninto was withdrawn.
As he had been taking double doses of Sansoninto for a month and non-segmental ground-glass attenuation was found on HRCT, we suspected Sansoninto-induced lung injury. A bronchoscopic examination was considered for a further evaluation of the lung involvement, but it was foregone because of the coronavirus disease 2019 (COVID-19) pandemic. Since his oxygenation status was good, his treatment included only discontinuation of Sansoninto. Consequently, his symptoms and infiltration on chest X-ray gradually improved (Fig. 1B, C), and the KL-6 level declined to a normal value (499 U/mL and 291 U/mL at 2 and 4 months, respectively, after the withdrawal of Sansoninto). The non-segmental ground-glass attenuation on HRCT has nearly disappeared (Fig. 2D-F), and the percent volume capacity measured in pulmonary function test has also improved. A DLST showed a positive result for Sansoninto.
Discussion
To our knowledge, we presented the first case of Sansoninto-induced lung injury. First, whether or not the diagnosis is clinically correct needs to be discussed. Given the lack of clear diagnostic criteria for drug-induced pneumonia, the diagnosis was made based on the medication history, biomarkers, radiological features, and clinical course.
The patient started Sansoninto five years ago and had been taking doubled doses for the past month. A study reported that approximately 90% of cases of herbal medicine-induced pneumonitis occur within 3 months after drug administration (4). The interval from the initial drug administration to disease development appears long in this case. However, the duration (one month) since he had started taking a double dose is consistent with this report. Pulmonary infiltration unresolved by antibiotics, a normal or slightly elevated leukocyte count and CRP level, an elevated KL-6 level, and a decreased percent volume capacity on a pulmonary function test all support the diagnosis (5). HRCT showed non-segmental ground-glass attenuation, which indicates viral pneumonia or immune-mediated pneumonitis (6). As the patient was treated in the midst of the COVID-19 pandemic, we excluded SARS-CoV-2 infection by PCR first; a simultaneous bronchoscopic evaluation was not performed due to reluctance, as it was a high-risk procedure with the potential for transmission. The clinical course, whereby the symptoms and pulmonary infiltration apparently improved after the discontinuation of Sansoninto alone, also corresponded to drug-induced pneumonitis. DLST showed a positive result for Sansoninto, but the sensitivity and specificity for the diagnosis remain unknown. Based on these findings, the patient was diagnosed with Sansoninto-induced pneumonitis.
Generally, a dose-dependent reaction indicates direct cytotoxicity rather than an immune-mediated mechanism. Indeed, drug overdose is known to induce lung injury (7), and some drugs, including leflunomide and amiodarone, induce dose-dependent direct lung toxicity (1,8,9). Considering that Sansoninto overdose is suspected to accelerate pneumonitis, ground-glass attenuation on HRCT may reflect cytotoxic injury. However, HRCT showed an organizing pneumonia pattern, suggesting an immune-mediated mechanism. KL-6 is a known marker of interstitial pneumonitis or lung injury and unlikely to be increased in organizing pneumonia (5). However, KL-6 levels were slightly elevated in the current case. The fact that organizing pneumonia pattern with an increased KL-6 level may indicate lung injury through both immune-mediated and cytotoxic mechanisms, despite a lack of pathological evidence. Studies have investigated HRCT patterns and their prognostic value in drug-induced lung injury, and diffuse alveolar damage and nonspecific interstitial patterns have been reported as poor predictive features (9,10). By contrast, cases with nonextensive ground-glass attenuation are likely to fully recover (11). Indeed, the ground-glass attenuation disappeared in the present patient as the KL-6 level normalized.
Sansoninto does not include representative potential herbs, such as Saiko and Ougon. Nevertheless, as the first case, our patient was strongly suspected of having Sansoninto-induced lung injury. Notably, no cases of crude drug-induced lung injury have been reported even for the included components (i.e. sansonin, bukuryo, senkyu, chimo, and kanzo). Although some cases of lung injury induced by herbal medicines including these crude drugs, rather than sansonin, have been reported (12,13), which components are the causative ones remains uncertain. Physicians need to be aware that any drugs, even unwarranted herbal medicine, may cause lung injury, and appropriate doses of such drugs should be recommended.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank Dr. Eiichiro Anan (Sakai Hospital, Oita, Japan) for his advice.
References
- 1. Matsuno O. Drug-induced interstitial lung disease: mechanisms and best diagnostic approaches. Respir Res 13: 39, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Skeoch S, Weatherley N, Swift AJ, et al. Drug-induced interstitial lung disease: a systematic review. J Clin Med 7: 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Motoo Y, Yukawa K, Arai I, Hisamura K, Tsutani K. Use of complementary and alternative medicine in Japan: a cross-sectional internet survey using the Japanese version of the International Complementary and Alternative Medicine Questionnaire. JMA J 2: 35-46, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Enomoto Y, Nakamura Y, Enomoto N, Fujisawa T, Inui N, Suda T. Japanese herbal medicine-induced pneumonitis: a review of 73 patients. Respir Investig 55: 138-144, 2017. [DOI] [PubMed] [Google Scholar]
- 5. Ohnishi H, Yokoyama A, Yasuhara Y, et al. Circulating KL-6 levels in patients with drug induced pneumonitis. Thorax 58: 872-875, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Delli Pizzi A, Chiarelli AM, Chiacchiaretta P, et al. Radiomics-based machine learning differentiates “ground-glass” opacities due to COVID-19 from acute non-COVID-19 lung disease. Sci Rep 11: 17237, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kobayashi J, Kitamura S. KL-6: a serum marker for interstitial pneumonia. Chest 108: 311-315, 1995. [DOI] [PubMed] [Google Scholar]
- 8. Lee JS, Lee EY, Ha YJ, Kang EH, Lee YJ, Song YW. Serum KL-6 levels reflect the severity of interstitial lung disease associated with connective tissue disease. Arthritis Res Ther 21: 58, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mankikian J, Favelle O, Guillon A, et al. Initial characteristics and outcome of hospitalized patients with amiodarone pulmonary toxicity. Respir Med 108: 638-646, 2014. [DOI] [PubMed] [Google Scholar]
- 10. Cleverley JR, Screaton NJ, Hiorns MP, Flint JD, Müller NL. Drug-induced lung disease: high-resolution CT and histological findings. Clin Radiol 57: 292-299, 2002. [DOI] [PubMed] [Google Scholar]
- 11. Tomii K, Kato T, Takahashi M, et al. Pemetrexed-related interstitial lung disease reported from post marketing surveillance (malignant pleural mesothelioma/non-small cell lung cancer). Jpn J Clin Oncol 47: 350-356, 2017. [DOI] [PubMed] [Google Scholar]
- 12. Fujita T, Nagakawa H, Izawa T, et al. [Case of shakuyakukanzoto-induced CD4 dominant pneumonitis diagnosed on day eight of the challenge test]. Nihon Kokyuki Gakkai Zasshi (J Jpn Respir Soc) 46: 717-721, 2008. [PubMed] [Google Scholar]
- 13. Nakamura K. Keisibukuryo-gan-induced interstitial pneumonitis. Kampo no Rinsho (J Kampo Med) 46: 1842-1843, 1999. [Google Scholar]