Abstract
Muramic acid serves as a marker for the presence of bacterial cell wall debris in mammalian tissues. There have been a number of controversial and sometimes conflicting results on assessing the levels of muramic acid in health and disease. The present report is the first to use the state-of-the art technique, gas chromatography-tandem mass spectrometry, to identify and quantify the levels of muramic acid in tissues. Muramic acid was not found in normal rat brain or spleen. However, when tissues were spiked with muramic acid, it was readily identified. The detection limit was <1 ng of muramic acid/100 mg (wet weight) of tissue. The levels of muramic acid reported in diseased human spleen and spleen of arthritic rats, previously injected with bacterial cell walls, were 100- to 1,000-fold higher. In the present study, muramic acid was also readily detected in the cerebrospinal fluid of patients with pneumococcal meningitis (6.8 to 3,900 ng of muramic acid/ml of cerebrospinal fluid). In summary, there can be an enormous difference in the levels of muramic acid found in different mammalian tissues and body fluids in health and disease. This report could have great impact in future studies assessing the role of bacterial cell wall remnants in the pathogenesis of certain human inflammatory diseases.
Humans are constantly exposed to microorganisms present in the environment. Physical barriers (e.g., epithelium of the skin and gastrointestinal tract) separate the internal milieu from the hostile external environment. In spite of host defenses, bacteria are capable of translocating across gut epithelium and can be cultured from organs of the reticuloendothelial system (RES) (5). Alternatively, bacteria may be digested in the gastrointestinal tract, and released cell wall products may pass into the circulation (3, 11, 22). Muramic acid is a constituent of the peptidoglycan (PG) backbones of gram-positive and gram-negative bacteria. This amino sugar (3-O-lactyl glucosamine) is not synthesized by mammalian enzyme systems and should therefore serve as a marker to indicate the presence of both viable bacteria and their nonviable cell wall remnants in tissues and body fluids (13, 20). In systemic infections, these remnants play a direct role in the disease process by activating cytokines and promoting acute inflammation (10). In chronic infections, bacterial products processed by the immune system may accumulate in organs of the reticuloendothelium.
The ability of bacterial debris to cause disease in animal models has been clearly demonstrated. The systemic injection of high-molecular-weight (MW) PG-polysaccharide complexes, cell wall constituents isolated from a variety of gram-positive bacterial species, results in chronic inflammation of the joint (9, 13, 20, 29, 40–42, 44). High-MW PG-polysaccharide complexes persist, causing chronic inflammation (12). In contrast, small subunits of PG are rapidly eliminated in vivo (1, 15, 35, 45) and cause acute but not chronic inflammation (8, 24, 27, 46). In either situation, it is extremely difficult to detect the inflammatory agent using standard microbiological techniques. The fate of degraded bacterial remnants has not been clearly elucidated.
Following administration, large quantities of muramic acid, as a component of bacterial debris, localize in the RES (5,000 ng of muramic acid/100 mg in spleen). This debris is slowly degraded over time (13, 20, 42). Much smaller quantities are found in peripheral tissues (<100 ng of muramic acid/100 mg in joints) (13, 20, 42). In these animal studies, muramic acid was not detected in normal tissues. Persistence of bacterial debris occurs despite partial degradation by mammalian enzymes, including lysozyme (19) and muramyl-l-alanine amidase (28). Solubilized cell wall fragments have also been shown to be excreted in the urine (45).
Certain forms of reactive arthritis in humans may result from a perpetuating immune response in the joint that is often associated with extra-articular bacterial infection. This occurs as nonpurulent joint inflammation developing after infection elsewhere in the body in which viable microbes can not be cultured from the joint (11, 22). For example, one complication of gram-negative bacillary dysentery can be a reactive arthritis (4, 6, 21, 22, 33). Detection of muramic acid in synovial fluids from patients with septic arthritis demonstrates the utility of nontraditional techniques for evaluation of the presence of bacteria in human tissue (7, 16, 30). In reactive arthritis, muramic acid was found in 2 of 14 synovial fluid specimens; however, in the vast majority the levels were so low that detection proved elusive (30). Muramic acid has not been found in noninfected control synovial fluids (7, 16, 30).
A noninflammatory physiological effect of muramyl peptides has been speculated to be the induction of slow-wave sleep after intestinal uptake of degraded bacterial flora. In early studies, a “sleep-promoting factor” (factor S) was extracted from the brains of sleep-deprived animals. Intraventricular injection of factor S into rats induced depressed locomotor activity and slow-wave sleep (34). Factor S was later purified from over 3,000 liters of human urine collected in large containers placed in lavatories for extended periods. The purified substance was reported to contain amino acids and muramic acid resembling bacterial PG (26). The authors suggested that this glycopeptide may be a natural sleep-promoting substance, synthesized in mammals and excreted in the urine. However, they admitted that it is possible that the substance isolated from urine was a result of bacterial contamination introduced during collection. As an extrapolation of these studies, it was reported that muramic acid is present at trace levels (<3 ng of muramic acid/100 mg) in the brains of normal rats (39). It should be noted that the levels of muramic acid reported to be present in normal mammalian tissues (including brain) are at least 1,000-fold lower than those in certain tissues from rats injected with cell wall material in models of chronic inflammation (13, 39).
There is a real need to categorically prove whether bacterial remnants are present and, if so, at what levels, not only during the infectious process but also in normal tissues and body fluids. Reports of muramic acid in mammalian tissues of different species cannot be reconciled, probably because of limitations of the methodologies employed. Studies have primarily used either gas chromatography-mass spectrometry (GC-MS) or liquid chromatography (both thin-layer chromatography and high-performance liquid chromatography) with fluorescence detection. GC-MS is highly selective in ignoring interfering substances common in complex biological matrices. Fluorescence-based trace detection is a highly sensitive technique; unfortunately, numerous contaminating compounds that coelute are commonly observed. In this study, we used an improved GC-tandem MS (GC-MS/MS) technique that dramatically improves the specificity and sensitivity of trace detection. The purpose of this study was to definitively assess the presence of muramic acid in normal rat brain, to detect its presence in the cerebrospinal fluid (CSF) in human infections, and to determine if muramic acid accumulates to detectable levels in normal rat spleens.
MATERIALS AND METHODS
Animal and human samples.
Female Sprauge-Dawley rats weighing ≈150 to 200 g were sacrificed, and the spleen and brain were removed and weighed. Approximately 10 ml of sterile H2O was added to each organ for homogenization. Homogenized organs were lyophilized and stored at −70°C until analysis. Human CSF was collected by lumbar puncture from pediatric patients during the period 1984 to 1987. Six children had otitis media with Streptococcus pneumoniae isolated from middle ear fluid but had negative cultures from blood and CSF. Six children had pneumococcal bacteremia with negative CSF cultures. Eight children had pneumococcal meningitis confirmed by culture. Samples were stored frozen at −70°C until analyzed for muramic acid levels. CSF samples from three patients with pseudo tumor cerebrei were pooled and supplied by K. V. Chalam, Department of Ophthalmology, University of South Carolina School of Medicine. This condition results in overproduction of otherwise normal CSF which is culture negative.
Sample preparation for GC-MS/MS.
The alditol acetate derivatization procedure for muramic acid has been described elsewhere (14, 18). Work from this laboratory has demonstrated the ubiquitous presence of muramic acid in surface and airborne dust (17, 25). Scrupulous attention was essential to eliminate environmental contamination of the samples. Glassware used in the procedure was first cleaned, soaked in 1 N HCl overnight, and baked at 246°C for a minimum of 24 h. Rigorous measures were employed to avoid cross contamination between samples. For example, during nitrogen evaporation used in various parts of the analytical procedure, sample droplets can be aerosolized, passing from one sample to the next. The evaporator was modified with plastic barriers placed between each sample. All samples were analyzed in duplicate. Samples were first hydrolyzed to release muramic acid from PG by treatment with 1 ml of 2 N sulfuric acid for 20 mg of lyophilized tissue (approximately 100 mg [wet weight]) or with 0.4 ml of 4 N sulfuric acid for 0.4 ml of CSF for 3 h at 100°C. 13C-labeled muramic acid was prepared in advance by hydrolyzing 40 mg of 13C-labeled cyanobacteria (Isotec, Miamisburg, Ohio) as described above. The bacteria were 0.4% muramic acid on a dry weight basis. Thirty-four nanograms of 13C-labeled muramic acid was added to each sample as an internal standard. Additionally, 50 μg of glucose was added to each sample as a carrier. External standards consisted of a known amount of muramic acid and a constant amount (34 ng) of 13C-labeled muramic acid. Blanks consisted of water spiked with 13C-labeled muramic acid. Following hydrolysis, samples were neutralized by mixing with 2 ml of N,N-dioctylmethylamine (Fluka, Buchs, Switzerland) in chloroform (50:50 vol/vol) and centrifuged. The aqueous phase was removed and passed through C18 octyldecyl silane columns (J&W Scientific, Folsom, Calif.) and reduced with 5 mg of sodium borohydride. To remove generated borate, methanol-acetic acid (200:1, vol/vol) was added continuously while evaporating under nitrogen. The samples were dried under vacuum. The alditols were acetylated at 100°C overnight. Acetic anhydride was decomposed with 0.75 ml of H2O. One milliliter of chloroform was added, and after mixing, the aqueous phase was discarded. A 0.8-ml volume of ammonium hydroxide in H2O (80:20, vol/vol) was added, the mixture was passed through a Chem Elut column (Varian, Walnut Creek, Calif.), and the organic phase was collected. The samples were evaporated to dryness and reconstituted in 10 μl of chloroform prior to analysis.
Instrumentation.
Samples were analyzed in the quantitation mode on a VG Quattro 1 triple-quadrupole tandem mass spectrometer (Micromass, Boston, Mass.) coupled to a Fisons 8000 GC equipped with an automated sample injector (A200s) and a nonpolar, DB-5ms fused silica capillary column (J&W Scientific). Quantitation was based upon the peak area ratio of the muramic acid to the internal standard (13C-labeled muramic acid) in the sample compared with the peak area ratio in the external standard mixture (containing a known amount of muramic acid and 13C-labeled muramic acid). Electron impact ionization was performed, followed by collision-induced disassociation of precursor ions with a mass of 403 for muramic acid and a mass of 412 for 13C-labeled muramic acid. Muramic acid and 13C-labeled muramic acid were detected using the mass transitions 403→198 and 412→205, respectively for quantitation.
In the identification mode, samples were analyzed on a GCQ ion trap tandem mass spectrometer (Finnigan, Atlanta, Ga.), also equipped with an A200s autosampler and a DB-5ms capillary column. Electron impact ionization was performed, followed by collision-induced disassociation of the dominant precursor ion (m/z 403) to obtain unique product ion spectra (fingerprints) for muramic acid.
RESULTS
GC-MS/MS employs a GC separation coupled with the exquisite selectively of MS/MS. In MS (monitoring/quantitation mode), background peaks are screened out. MS/MS (monitoring/quantitation mode) screens out background a second time, thus dramatically lowering the detection limit. Alternatively, in identification mode (MS/MS), a chemical fingerprint of the compound of interest allows definitive identification. This study combines the high-resolution separating power of capillary GC with the unequaled selectivity of MS/MS, lowering the detection limit for muramic acid to levels previously unattainable. In addition, extreme measures were taken to minimize contamination of samples with muramic acid, which is ubiquitous in the environment (17, 25).
Interpretation of GC-MS/MS data as applied to analysis of mammalian tissues and body fluids.
Natural [12C]muramic acid is first released from PG polymers by hydrolysis. Conversion of 12C muramic acid to a volatile derivative, muramicitol lactam pentaacetate (MW, 445), is essential for GC-MS/MS analysis.
(i) GC-MS/MS monitoring/quantitation mode.
MS/MS consists of two stages. In the first stage, the molecule is isolated essentially intact with an MW of 403 due to the loss of a ketene (loss of 42). Coeluting molecules of different MW are essentially eliminated; i.e., only molecules with an MW of 403 are permitted to pass into the next stage. In the second stage, molecules with an MW of 403 are broken into characteristic fragments, including one that contains the original lactam with an MW of 198 (17). Thus, in the second stage, only molecules with an MW of 198 are detected. The second time, background molecules of different MW that coelute are essentially eliminated.
The use of a stable isotope-labeled (13C-analog) of muramic acid in each sample verifies that there is no false-negative result. This ensures that muramic acid is not lost during the complex sample preparation or hidden within the background in the instrumental analysis. Although [12C]muramic acid and [13C]muramic acid have the same retention time on GC analysis, they can be discriminated in the tandem mass spectrometer. GC-MS/MS analysis of [13C]muramic acid is identical to that of natural [12C]muramic acid. However, the MWs in the first and second stages are correspondingly higher, i.e., 412 and 205, respectively. Thus, in the tandem mass spectrometer, it is possible to monitor two separate windows simultaneously, one for [13C]muramic acid (top window) and one for natural muramic acid (bottom window). Accurate quantitation is readily accomplished by comparing the ratio of the areas of [13C]muramic acid versus [12C]muramic acid. The present study is one of the first uses of 13C-labeled muramic acid in the analysis of tissues and body fluids for muramic acid. It has recently been used in the analysis of muramic acid in the urine of patients with a culture-confirmed urinary tract infection (2).
(ii) GC-MS/MS identification mode.
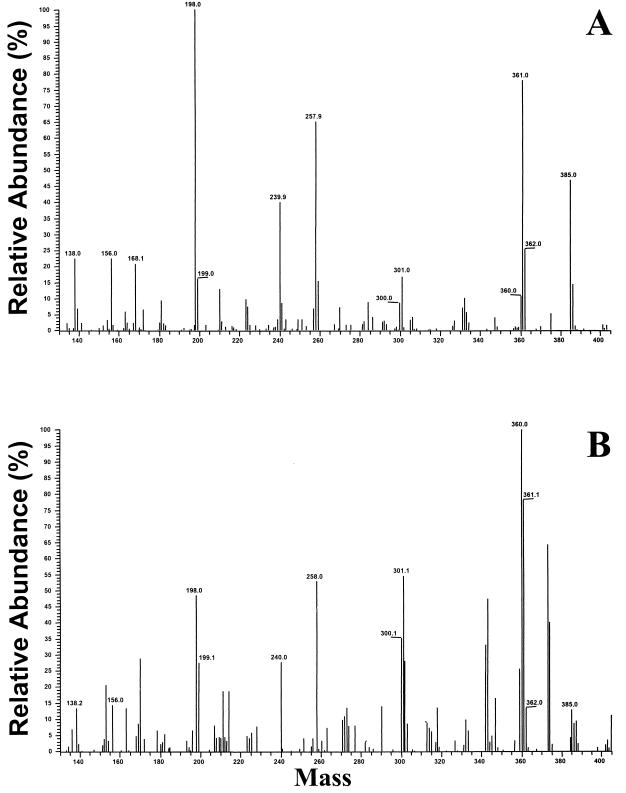
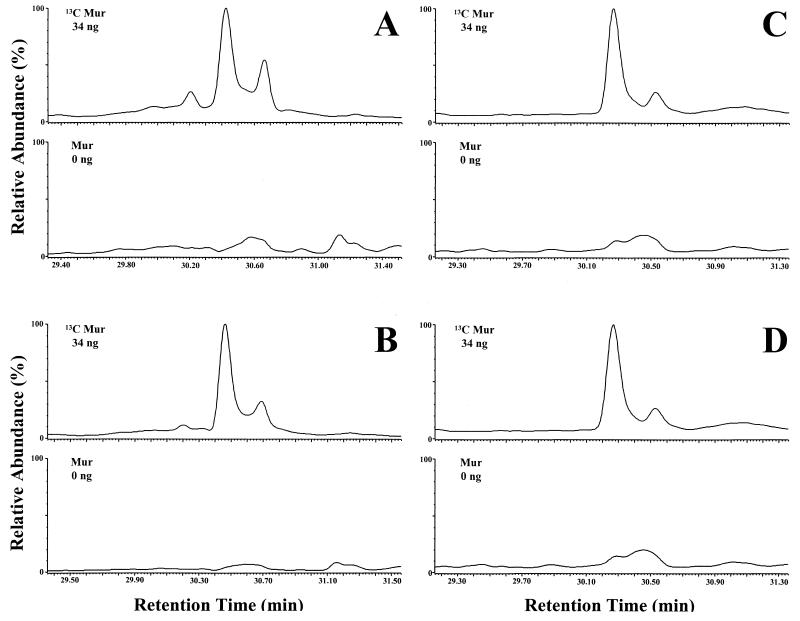
A chemical fingerprint is generated consisting of scission products, in the MS/MS instrument, characteristic of the compound of interest. In Fig. 1, the fingerprints of natural muramic acid (Fig. 1A) and natural muramic acid added to a brain homogenate (4 ng of muramic acid/100 mg of tissue) (Fig. 1B) are compared. This represents a categorical identification of muramic acid present in the spiked brain homogenate. It is noted that the same major fragments occur in both fingerprints. The parent molecule has an MW of 403. In each case the same major fragments are observed, with MWs of 361, 301, 258, 240, 198, 156, and 138: the 361-MW fragment results from the loss of a ketene (loss of 42), and the 301-MW fragment results from a subsequent loss of acetic acid (loss of 60). Breakage between C-4 and C-5 (loss of 145) would generate the 258-MW fragment, and further loss of acetic acid (loss of 60) would generate the 198-MW fragment. Loss of ketene or acetic acid from the 198-MW fragment results in the 156- and 138-MW fragments, respectively (17). There was insufficient muramic acid in samples spiked with 1 or 2 ng of muramic acid to obtain a confirmatory fingerprint.
FIG. 1.
GC-MS/MS analysis (identification mode) of authentic muramic acid (A) and muramic acid spiked in normal rat brain (4 ng/100 mg) (B). Note that the two fingerprints contain the same major masses.
Analysis of muramic acid in rat brain.
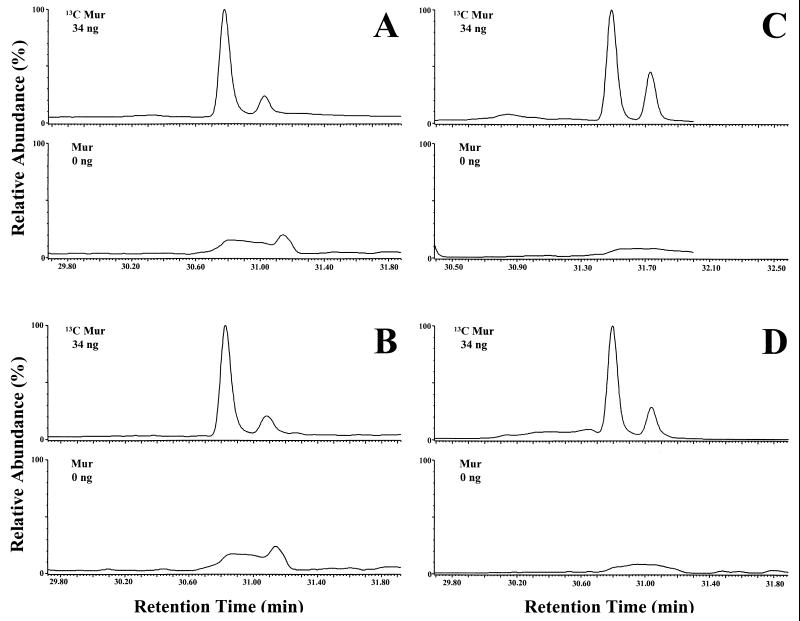
Brains from seven rats were analyzed in duplicate. Muramic acid was not detected in normal rat brain. In every case 34 ng of [13C]muramic acid, added to the samples as an internal standard, was readily detected in 100 mg (wet weight) of rat brain. Typical results for four different rat brains are depicted in Fig. 2. The top panel, for each, shows the presence of 13C-labeled muramic acid. Two peaks, characteristic of the alditol acetate of muramic acid, are observed. The major peak has been identified as muramicitol lactam pentaacetate (13). The minor peak has not been identified but is a by-product of the derivatization procedure. The bottom panels illustrate the absence of natural [12C]muramic acid in brain. Peaks in the two separate windows are normalized relative to the highest peak in either window. Since there is no clearly defined peak at the retention time for muramic acid present in the 12C window, only the baseline is displayed. Muramic acid was also not detected in identification mode in normal (unspiked) brain. In summary, using GC-MS/MS in both monitoring and identification modes, muramic acid was not detected in healthy rat brain.
FIG. 2.
GC-MS/MS analysis (monitoring/quantitation mode) of normal rat brain. Representative chromatograms of four normal rat brains are shown with 34 ng of 13C-labeled muramic acid (Mur) in the upper windows. The lower windows show the absence of normal [12C]muramic acid, since there is no peak at the retention time of muramic acid.
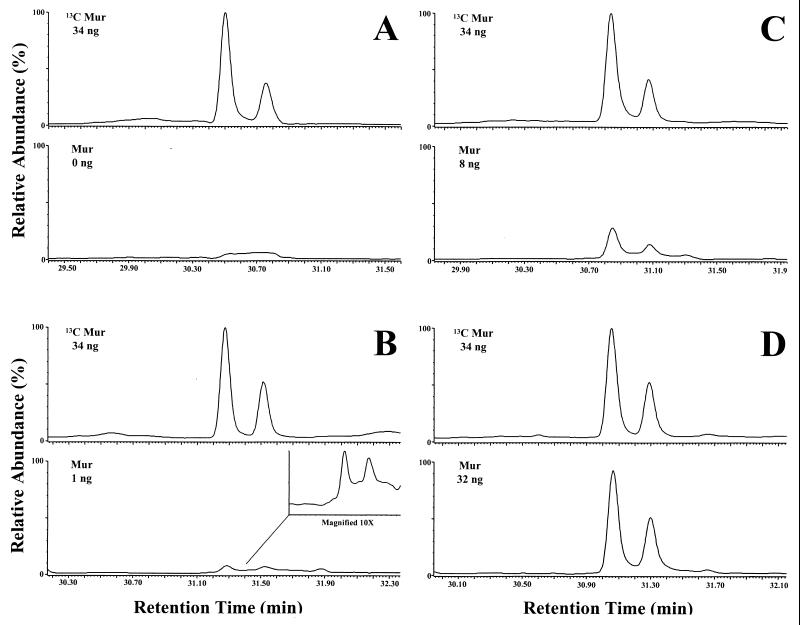
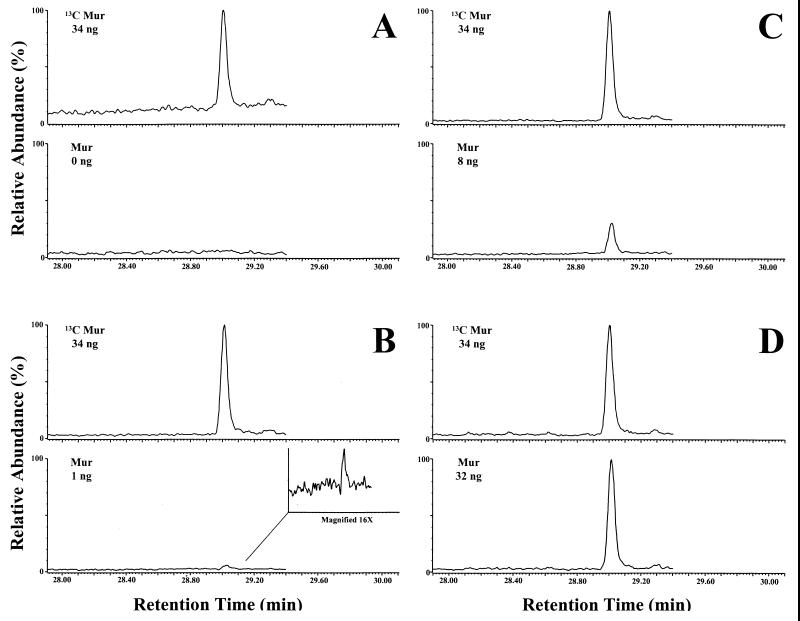
To determine the limit of detection, various amounts of muramic acid (0, 1, 2, 4, 8, 16, and 32 ng, each sample in duplicate) were added to a constant amount of normal rat brain (100 mg [wet weight]). In Fig. 3, again the top panel for each of the four pairs represents 34 ng of [13C]muramic acid. In Fig. 3A, the [12C]muramic acid window (bottom) again shows no discernible peak. Even 1 ng of natural muramic acid added to 100 mg of rat brain tissue clearly exhibits a peak at the retention time of the [13C]muramic acid internal standard. At the 8-ng level, the major and minor peaks are more pronounced. At the 32-ng level, both peaks are clearly observed, background noise is minimal, and the two windows are nearly identical. Thus, the present detection limit is in the range of 1 ng of muramic acid in 100 mg of brain. In a previous qualitative study, others observed that normal rat brain contained <3 ng of muramic acid/100 mg (wet weight) (39). The present quantitative procedure was linear from 0 to 32 ng of muramic acid/100 mg (wet wt) of brain tissue (r2 = 0.9992; y intercept = 0.0199).
FIG. 3.
GC-MS/MS analysis (monitoring/quantitation mode) of normal rat brain (100 mg [wet weight]) unspiked (A) and spiked with muramic acid (Mur) at 1 ng (B), 8 ng (C), and 32 ng (D). In each case, the upper chromatogram depicts the internal standard, i.e., 13C-labeled muramic acid (34 ng), and the lower chromatogram depicts normal [12C]muramic acid. Peaks at the retention time for muramic acid were not observed in the unspiked tissue (even with magnification).
Analysis of muramic acid in human CSF.
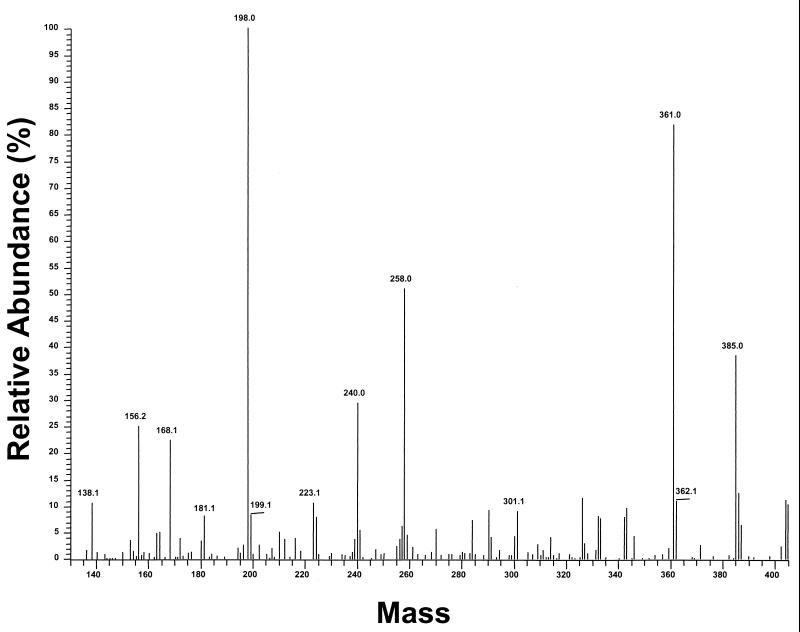
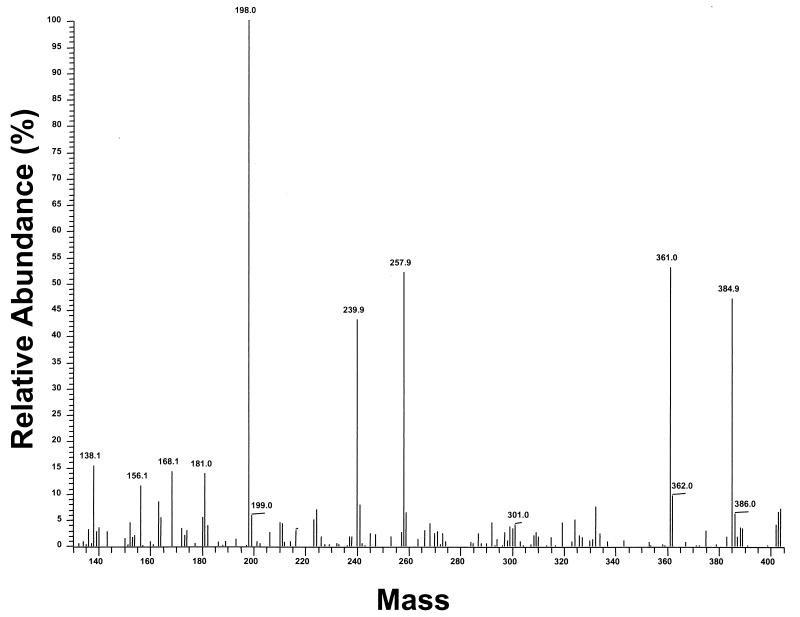
No muramic acid was detected in the CSF samples from any of the patients with otitis media or bacteremia, who did not have meningitis (Table 1). Muramic acid was detected in CSF from all but one of the children with meningitis. The levels of muramic acid in patients with meningitis ranged from 6.8 to 3,890 ng/ml (Table 2). In one sample muramic acid was present below the detection limit. Figure 4 shows a muramic acid fingerprint of CSF from a patient with pneumococcal meningitis (21.5 ng of muramic acid/ml of CSF). Note that the same major fragments are present as for the muramic acid standard (Fig. 1A). This represents the categorical identification of muramic acid in infected human CSF.
TABLE 1.
Detection of muramic acid in CSF of humans with culture-proven pneumococcal infections
| Diagnosis | No. of samples | No. in which muramic acid was detected |
|---|---|---|
| Otitis media (middle ear culture positive, blood and CSF cultures negative) | 6 | 0 |
| Bacteremia (blood culture positive but CSF culture negative) | 6 | 0 |
| Meningitis (CSF culture positive) | 8 | 7 |
TABLE 2.
Concentrations of muramic acid in CSF samples from patients with meningitis
| Patient | Concn of muramic acid (ng/ml) |
|---|---|
| A | NDa |
| B | 6.8 |
| C | 21.5 |
| D | 34.4 |
| E | 54.4 |
| F | 79.3 |
| G | 3,346.8 |
| H | 3,889.9 |
ND, not detected.
FIG. 4.
GC-MS/MS analysis (identification mode), showing the fingerprint of muramic acid present in human CSF from a patient with pneumococcal meningitis (21.5 ng/ml). Note that the fingerprint is essentially identical to that of standard muramic acid (Fig. 1A).
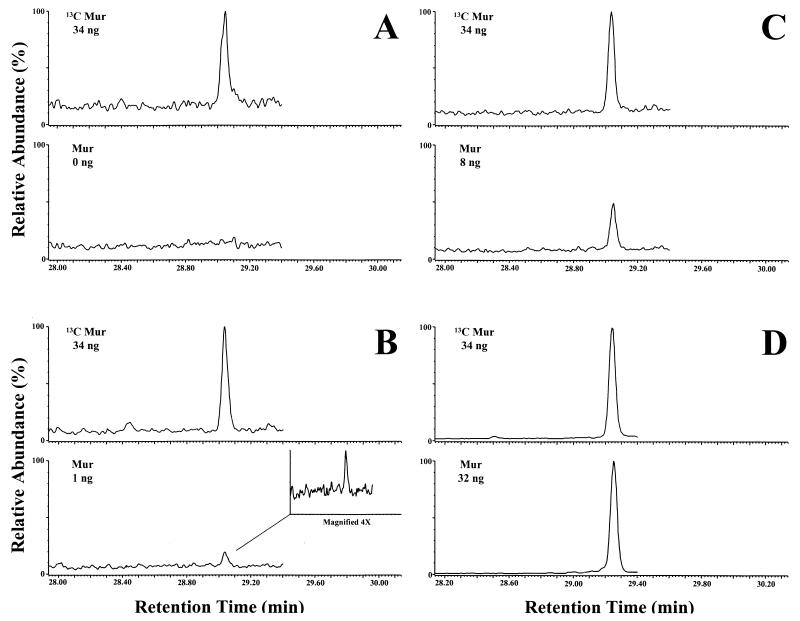
To determine the limit of detection in CSF, spiking experiments were also performed. A pool of culture-negative CSF from patients with pseudo tumor cerebrei was studied. Muramic acid was added in amounts of 0, 1, 2, 4, 8, 16, and 32 ng to 0.4 ml of CSF (each level in duplicate). Results were similar to those in the brain spiking experiment, although the minor muramic acid peak did not appear in any of the chromatograms of the internal standard or natural muramic acid windows. No identifiable peak is evident in the unspiked CSF sample (Fig. 5A). In contrast, at the 1-ng level the major peak is discernible (Fig. 5B). The 8-ng level (Fig. 5C) and the 32-ng level (Fig. 5D) show a clear peak with minimal background. Thus, the present detection limit is in the range of 2.5 ng of muramic acid/ml CSF. The procedure was linear from 0 to 32 ng of muramic acid/0.4 ml of CSF (r2 = 0.9990; y intercept = −0.001).
FIG. 5.
GC-MS/MS analysis (monitoring/quantitation mode) of CSF (0.4 ml) unspiked (A) and spiked with muramic acid (Mur) at 1 ng (B), 8 ng (C), and 32 ng (D). In each case, the upper chromatogram depicts the internal standard, i.e., 13C-labeled muramic acid (34 ng), and the lower chromatogram depicts normal [12C]muramic acid. Peaks at the retention time for muramic acid were not observed in the unspiked tissue (even with magnification).
Analysis of muramic acid in rat spleen.
To see if muramic acid might accumulate to detectable levels in normal rat reticuloendothelium, spleens from nine rats were analyzed by GC-MS/MS in the monitoring mode. Muramic acid was also not detected in normal rat spleen. Typical results for four different rat spleens are depicted in Fig. 6. In no case was muramic acid categorically detected. Samples showed no discernible peak (Fig. 6). It proved to be impossible to obtain a fingerprint confirming the presence of muramic acid in any of these samples. Thus, we can state that muramic acid is not detectable in normal rat spleen at the present analytical limits.
FIG. 6.
GC-MS/MS analysis (monitoring/quantitation mode) of normal rat spleen. Representative chromatograms of four normal rat spleens are shown with 34 ng of 13C-labeled muramic acid (Mur) in the upper windows. The lower windows show no discernible peak at the retention time of normal [12C]muramic acid.
Rat spleen was also spiked with muramic acid and analyzed by GC-MS/MS. Zero, 1, 2, 4, 8, 16, or 32 ng of muramic acid was added to 100 mg (wet weight) of spleen (each level in duplicate) and analyzed in the monitoring mode. The presence of 1 ng of muramic acid (Fig. 7B) can readily be distinguished from the unspiked sample (Fig. 7A). At the 8-ng level (Fig. 7C), a clear, discernible peak is observed. At 32 ng, the peak is well defined and has about the same relative area under the peak as for the [13C]muramic acid. The method proved to be linear over the range of 0 to 32 ng (r2 = 0.9773; y intercept = 0.0731). Furthermore, fingerprints of samples spiked with 8 ng of muramic acid or more categorically confirmed the presence of muramic acid (Fig. 8). It is noteworthy that previous studies have reported the levels of muramic acid in pathologic human spleen to be between 400 and 600 ng/100 mg (wet weight) of tissue (23, 38). The levels of muramic acid in diseased human spleen are thus over 100-fold higher than the current detection limit (1 ng/100 mg) for normal rat spleen. In the human studies, normal spleen was not analyzed; thus, normal rat spleen serves as a useful basis for comparison.
FIG. 7.
GC-MS/MS analysis (monitoring/quantitation mode) of normal rat spleen (100 mg [wet weight]) unspiked (A) and spiked with muramic acid (Mur) at 1 ng (B), 8 ng (C), and 32 ng (D). In each case, the upper chromatogram depicts the internal standard, i.e., 13C-labeled muramic acid (34 ng), and the lower chromatogram depicts normal [12C]muramic acid. Peaks at the retention time for muramic acid were not observed in the unspiked tissue (even with magnification).
FIG. 8.
GC-MS/MS analysis (identification mode), showing the fingerprint of 8 ng of muramic acid spiked in 100 mg (wet weight) of normal rat spleen. Note that the fingerprint is essentially identical to that of standard muramic acid (Fig. 1A).
DISCUSSION
The level of muramic acid present (even in grossly infected tissues and body fluids) can be so low that assay is extremely difficult. Detecting muramic acid in normal mammalian tissues (if indeed it is present) is an even more demanding task. This has resulted in some of the information available being confusing and sometimes contradictory. The primary purpose of these experiments was to investigate whether muramic acid is indeed present in normal rat tissues. Rat brain was analyzed to verify earlier reports claiming that muramic acid is present in this tissue (39). Human CSF specimens from patients with bacterial meningitis were studied to provide a comparison of the levels of muramic acid found in a documented infection. Finally, spleen was analyzed since it is a major organ of the RES and serves as a depot for bacterial debris in animal models of polyarthritis (13, 20). Muramic acid has also been reported in pathologic human spleen (23, 38). Whether muramic acid it is present in normal rat or human spleen remains to be determined.
In the present study, muramic acid was not found in normal rat brain using GC-MS/MS in the monitoring mode. Furthermore, it proved impossible to obtain a product ion spectrum (fingerprint) indicative of the presence of muramic acid in any of the normal rat brains analyzed. However, muramic acid was readily detected in spiked rat brain (1 ng of muramic acid added to 100 mg [wet weight] of tissue). The current detection limit in rat brain is therefore <1 ng/100 mg of spleen tissue. In the identification mode, the addition of 4 ng of muramic acid to 100 mg of brain tissue was readily detected by the characteristic fingerprint of muramic acid at the appropriate retention time. Thus, it can be stated that muramic acid is not detectable in normal rat brain at the present analytical limits.
The levels previously reported for normal rat brain (<3 ng/100 mg of tissue) (39) are within the limit of detection of the current GC-MS/MS method with the selectivity required to rule out a false-positive result. In the GC-MS/MS method, for a compound to be categorically identified as muramic acid, not only must it have the same retention time by GC as a 13C-labeled, chemically identical internal standard, but during MS/MS it must have the same MW in the first stage of the analysis and identical characteristic fragments in the second stage of the MS/MS analysis. In trace analysis of muramic acid in complex biological matrices, contaminating compounds are commonplace, masking detection and causing false positives. Therefore, observing a chromatographic peak at the correct retention time (using a nonselective detector) does not constitute definitive identification. One may merely be detecting a coeluting contaminant. In the previous study, an attempt was made to isolate muramic acid from normal brain using thin-layer chromatography. The fluorescamine derivative of muramic acid was identified by periodate oxidation (indicating the presence of a sugar or other diol) and alkaline release of lactic acid (which is characteristic but not specific for muramic acid). It is entirely possible that a substance or mixture of substances other than muramic acid was detected (39).
In our work, muramic acid was detected in the CSF of patients with pneumococcal meningitis but not in the CSF of those with otitis media, or even in those with bacteremia, who would be expected to have high levels of circulating cell wall remnants containing muramic acid. The levels of muramic acid found in the CSF of patients with meningitis (6.8 to 3,890 ng/ml) in the present study are consistent with the results from our earlier study of muramic acid levels in septic arthritis (16) and urinary tract infections (2). These are the only studies employing GC-MS/MS for the detection of muramic acid, or indeed any other bacterial constituent, in a human body fluid. In the earlier study, GC-MS/MS was used for “absolute” identification at trace levels of muramic acid in septic human synovial fluids. Fingerprints of muramic acid peaks (≈30 ng/ml) in infected human body fluids were identical to those of pure muramic acid. Muramic acid was positively identified in synovial fluids during infection (primarily with staphylococci) and was eliminated over time during antibiotic therapy, but it was absent from aseptic fluids. The present study confirms these earlier findings in that the levels of muramic acid present during an infection were similar in infected CSF samples but were absent in culture-negative CSF samples.
In addition to PG, pneumococcal cell envelopes contain lipoteichoic acid and teichoic acid (LTA-TA). The combined LTA-TA level in CSF from patients with pneumococcal meningitis has been determined using enzyme immunoassay (37). There was a clear correlation between the LTA-TA levels and severity of disease and outcome among 30 patients with pneumococcal meningitis. The levels of LTA-TA were of about the same order of magnitude (median, 285 ng/ml; range, 4.8 to 26,694 ng/ml) as values for muramic acid in the present study. Both LTA-TA and PG have been shown to strongly induce cytokine production in human monocytes (32, 36). Measurement of muramic acid may be able to provide information on the magnitude of cell wall material present in disease states (approximately 1 ng/106 gram-positive cocci). This is in line with the threshold concentration of cell wall material (PG plus LTA-TA, about 10 ng/ml or equivalent to about 105 bacterial cells) required to incite inflammation in vitro and in animal models of meningitis (36, 43).
In the present study, muramic acid was also not detected in normal rat spleen in the GC-MS/MS quantitation mode. However, muramic acid was readily detected in rat spleen spiked with 1 ng/100 mg of spleen tissue. In the identification mode, the addition of 8 ng of muramic acid to 100 mg of spleen tissue was readily detected by the characteristic fingerprint of muramic acid at the appropriate retention time. Thus, it can be stated that muramic acid is not detectable in normal rat spleen at current detection limits.
It is reasonable to assume that bacteria and bacterial debris that are processed by the host immune system eventually find their way into tissues of the RES. Muramic acid has been tentatively identified in the RES, using a nonselective methodology, in the livers of normal rats (>3 ng of muramic acid/100 mg) (39). Another study using a higher-resolution chromatographic separation was used for analysis of human spleens from five patients with gastric adenocarcinoma and one with splenic rupture (23, 38). Muramic acid was analyzed as a dansyl derivative, which detects amino sugars, amino acids, and other compounds containing amino groups. The peak isolated at the retention time for muramic acid on rechromatography contained numerous components (23). However, in a later study, using a more rigorous isolation procedure, a single peak at the retention time for muramic acid was identified in spleens from three patients with gastric carcinoma and four with splenomegaly due to hematologic disease (38). The levels of muramic acid reported (400 to 600 ng of muramic acid/100 mg of spleen) are more than 100-fold higher than those reported by Sen and Karnovsky (39) for normal rat tissues. However, the levels of muramic acid in human spleen are close to the levels seen in the spleens and livers of rats previously injected with cell walls. One study noted that the level in spleen 6 days after injection was 5,000 ng of muramic acid/100 mg (13). In a second study of levels of muramic acid in liver, it was noted that over a 63-day period the total amount declined 5.6-fold (approximately 1,000 ng/100 mg of tissue) (20). Taking the two studies together suggests that the levels found in the RESs of polyarthritic rats are similar to the levels reported to be present in pathologic human spleen.
In gastric carcinoma, modulation of the epithelium might cause an influx of bacteria or bacterial debris from the gastrointestinal flora. Splenomegaly might result from accumulation of bacteria or bacterial remnants from translocation across the gut epithelium (31). The levels of muramic acid found in the spleens of these patients are near those found in the spleens of rats experimentally injected with bacterial cell walls. Unfortunately, normal human tissues (e.g., from patients with trauma) were not included in these studies as negative controls. It is intriguing as to whether the muramic acid detected in human spleen was derived from the pathologic condition, rather than being naturally present in normal human spleen.
In conclusion, at this time we are unable to provide evidence for the presence of muramic acid in normal rat tissues. The current detection limits are approximately 1 ng of muramic acid/100 mg of tissue. However, muramic acid is readily detected in documented human infection, including pneumococcal meningitis. Muramic acid has also been reported by others to be present in diseased, although culture negative, human spleen. The levels are similar to those found in spleens of arthritic rats. Future work is needed to assess the significance of changes in the levels of muramic acid that occur in health and disease. In some instances, inflammatory bacterial cell wall debris may be directly involved in human pathology.
ACKNOWLEDGMENT
Michael P. Kozar was supported by a fellowship from the U.S. Army.
REFERENCES
- 1.Ambler L, Hudson A M. Pharmacokinetics and metabolism of muramyl dipeptide and nor-muramyl dipeptide [3H-labelled] in the mouse. Int J Immunopharmacol. 1984;6:133–139. doi: 10.1016/0192-0561(84)90008-0. [DOI] [PubMed] [Google Scholar]
- 2.Bal K, Larsson L. New and simple procedure for the determination of muramic acid in chemically complex environments by gas chromatography-ion trap tandem mass spectrometry. J Chromatogr B. 2000;738:57–65. doi: 10.1016/s0378-4347(99)00494-6. [DOI] [PubMed] [Google Scholar]
- 3.Bennett J C. The infectious etiology of rheumatoid arthritis. New considerations. Arthr Rheum. 1978;21:531–538. doi: 10.1002/art.1780210507. [DOI] [PubMed] [Google Scholar]
- 4.Berden J H M, Muytjens H L, Van de Putte L. Reactive arthritis associated with Campylobacter jejuni enteritis. Br Med J. 1979;2:380–381. doi: 10.1136/bmj.1.6160.380-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berg R D, Garlington A W. Translocation of certain indigenous bacteria from the gastrointestinal tract to the mesenteric lymph nodes and other organs in a gnotobiotic mouse model. Infect Immun. 1979;23:403–411. doi: 10.1128/iai.23.2.403-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calin A, Fries J F. An experimental epidemic of Reiter's syndrome revisited: follow-up evidence on genetic and environmental factors. Ann Intern Med. 1976;84:564–566. doi: 10.7326/0003-4819-84-5-564. [DOI] [PubMed] [Google Scholar]
- 7.Christensson B, Gilbart J, Fox A, Morgan S L. Mass spectrometric quantitation of muramic acid, a bacterial cell wall component, in septic synovial fluids. Arthr Rheum. 1989;32:1268–1272. doi: 10.1002/anr.1780321012. [DOI] [PubMed] [Google Scholar]
- 8.Cookson B T, Cho H-L, Herwaldt L, Goldman W E. Biological activities and chemical composition of purified tracheal cytoxin of Bordetella pertussis. Infect Immun. 1989;57:2223–2229. doi: 10.1128/iai.57.7.2223-2229.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cromartie W J, Craddock J G, Schwab J H, Anderle S K, Yang C-H. Arthritis in rats after systemic injection of streptococcal cells or cell walls. J Exp Med. 1977;146:1585–1602. doi: 10.1084/jem.146.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cundell D, Masure H R, Tuomanen E I. The molecular basis of pneumococcal infection: a hypothesis. Clin Infect Dis. 1995;21(Suppl. 3):S204–S212. doi: 10.1093/clind/21.supplement_3.s204. [DOI] [PubMed] [Google Scholar]
- 11.Fox A. Role of bacterial debris in inflammatory diseases of the joint and eye. Acta Pathol Microbiol Immunol Scand. 1990;98:957–968. doi: 10.1111/j.1699-0463.1990.tb05021.x. [DOI] [PubMed] [Google Scholar]
- 12.Fox A, Brown R R, Anderle S K, Chetty C, Cromartie W J, Gooder H, Schwab J H. Arthropathic properties related to the molecular weight of peptidoglycan-polysaccharide polymers of streptococcal cell walls. Infect Immun. 1982;35:1003–1010. doi: 10.1128/iai.35.3.1003-1010.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fox A, Schwab J H, Cochran T. Muramic acid detection in mammalian tissues by gas-liquid chromatography-mass spectrometry. Infect Immun. 1980;29:526–531. doi: 10.1128/iai.29.2.526-531.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox A, Black G. Identification and detection of carbohydrate markers for bacteria: derivatization and gas chromatography-mass spectrometry. In: Fenselau C, editor. Mass spectrometry for the characterization of microorganisms. Washington, D.C.: American Chemical Society; 1994. pp. 107–131. [Google Scholar]
- 15.Fox A, Fox K. Rapid elimination of a synthetic adjuvant peptide from the circulation after systemic administration and absence of detectable natural muramyl peptides in normal serum at current analytical limits. Infect Immun. 1991;59:1202–1205. doi: 10.1128/iai.59.3.1202-1205.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox A, Fox K, Christensson B, Harrelson D, Krahmer M. Absolute identification of muramic acid, at trace levels, in human septic synovial fluids in vivo and absence in aseptic fluids. Infect Immun. 1996;64:3911–3915. doi: 10.1128/iai.64.9.3911-3915.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox A, Krahmer M, Harrelson D. Monitoring muramic acid in air (after alditol acetate derivatization) using a gas chromatograph-ion trap tandem mass spectrometer. J Microbiol Methods. 1996;27:129–138. [Google Scholar]
- 18.Fox A, Morgan S L, Gilbart J. Preparation of alditol acetates and their analysis by gas chromatography and mass spectrometry. In: Biermann C J, McGinnis G, editors. Analysis of carbohydrates by GLC and MS. Boca Raton, Fla: CRC Press; 1988. pp. 87–117. [Google Scholar]
- 19.Gallis H A, Miller S E, Wheat R W. Degradation of 14C-labeled streptococcal cell walls by egg white lysozyme and lysosomal enzymes. Infect Immun. 1976;13:1459–1466. doi: 10.1128/iai.13.5.1459-1466.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbart J, Fox A. Elimination of group A streptococcal cell walls from mammalian tissues. Infect Immun. 1987;55:1526–1528. doi: 10.1128/iai.55.6.1526-1528.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Granfors K, Jalkanen S, Lindberg A A, Maki-Ikola O, Von Essen R, Lahesmaa-Rantala R, Isomaki, Saario R, Arnold W J, Toivanen A. Salmonella lipopolysaccharide in synovial cells from patients with reactive arthritis. Lancet. 1990;335:685–688. doi: 10.1016/0140-6736(90)90804-e. [DOI] [PubMed] [Google Scholar]
- 22.Granfors K, Jalkanen S, Von Essen R, Lahesmaa-Rantala R, Isomaki O, Pekkola-Heino K, Merilahti-Palo R, Saario R, Isomaki H, Toivanen A. Yersinia antigens in synovial-fluid cells from patients with reactive arthritis. N Engl J Med. 1989;320:216–221. doi: 10.1056/NEJM198901263200404. [DOI] [PubMed] [Google Scholar]
- 23.Hoijer M A, Melief M J, van Helden-Meeuwsen C G, Eulderink F, Hazenberg M P. Detection of muramic acid in a carbohydrate fraction of human spleen. Infect Immun. 1995;63:1652–1657. doi: 10.1128/iai.63.5.1652-1657.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johannsen L, Rosenthal R S, Martin S A, Cady A B, Obal F, Jr, Guinand M, Krueger J M. Somnogenic activity of O-acetylated and dimeric muramyl peptides. Infect Immun. 1989;57:2726–2732. doi: 10.1128/iai.57.9.2726-2732.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krahmer M, Fox K, Fox A, Saraf A, Larsson L. Total and viable airborne bacterial load in two different agricultural environments using gas chromatography-tandem mass spectrometry and culture: a prototype study. Am Ind Hyg Assoc J. 1998;59:524–531. doi: 10.1080/15428119891010695. [DOI] [PubMed] [Google Scholar]
- 26.Krueger J M, Pappenheimer J R, Karnovsky M L. The composition of sleep-promoting factor isolated from human urine. J Biol Chem. 1982;257:1664–1669. [PubMed] [Google Scholar]
- 27.Kufoy E A, Fox K, Fox A, Parks C, Pakalnis V. Modulation of the blood-aqueous barrier by gram positive and gram negative bacterial cell wall components in the rat and rabbit. Exp Eye Res. 1990;50:189–195. doi: 10.1016/0014-4835(90)90230-r. [DOI] [PubMed] [Google Scholar]
- 28.Ladesic B, Tomasic J, Kveder S, Hrsak I. The metabolic fate of 14C-labeled immunoadjuvant peptidoglycan monomer. II. In vitro studies. Biochim Biophys Acta. 1981;678:12–17. doi: 10.1016/0304-4165(81)90042-8. [DOI] [PubMed] [Google Scholar]
- 29.Lehman T J A, Allen J B, Plotz P, Wilder R L. Polyarthritis in rats following the systemic injection of Lactobacillus casei cell walls in aqueous suspension. Arthr Rheum. 1983;26:1259–1265. doi: 10.1002/art.1780261013. [DOI] [PubMed] [Google Scholar]
- 30.Lehtonen L, Kortekangas P, Oksman P, Eerola E, Aro H, Toivanen A. Synovial fluid muramic acid in acute inflammatory arthritis. Br J Rheumatol. 1994;33:1127–1130. doi: 10.1093/rheumatology/33.12.1127. [DOI] [PubMed] [Google Scholar]
- 31.Lemaire L C, van Lanschot J J, Stoutenbeek C P, van Deventer S J, Wells C L, Gouma D J. Bacterial translocation in multiple organ failure: cause or epiphenomenon still unproven. Br J Surg. 1997;84:1340–1350. [PubMed] [Google Scholar]
- 32.Majcherczyk P A, Langen H, Heumann D, Fountoulakis M, Glauser M P, Moreillon P. Digestion of Streptococcus pneumoniae cell walls with its major peptidoglycan hydrolase releases branched stem peptides carrying proinflammatory activity. J Biol Chem. 1999;274:12537–12543. doi: 10.1074/jbc.274.18.12537. [DOI] [PubMed] [Google Scholar]
- 33.Noer H R. An experimental epidemic of Reiter's syndrome. JAMA. 1966;197:693–698. [PubMed] [Google Scholar]
- 34.Pappenheimer J R, Koski G, Fencl V, Karnovsky M L, Krueger J. Extraction of sleep-promoting factor S from cerebrospinal fluid and from brains of sleep-deprived animals. J Neurophysiol. 1975;38:1299–1311. doi: 10.1152/jn.1975.38.6.1299. [DOI] [PubMed] [Google Scholar]
- 35.Parant M, Parant F, Chedid L, Yapo A, Petit J F, Lederer E. Fate of the synthetic immunoadjuvant, muramyl dipeptide (14C-labelled) in the mouse. Int J Immunopharmacol. 1979;1:35–41. doi: 10.1016/0192-0561(79)90028-6. [DOI] [PubMed] [Google Scholar]
- 36.Riesenfeld-Orn I, Wolpe S, Garcia-Bustos J F, Hoffmann M K, Tuomanen E. Production of interleukin-1 but not tumor necrosis factor by human monocytes stimulated with pneumococcal cell surface components. Infect Immun. 1989;57:1890–1893. doi: 10.1128/iai.57.7.1890-1893.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider O, Michel U, Zysk G, Dubuis O, Nau R. Clinical outcome in pneumococcal meningitis correlates with CSF lipoteichoic acid concentrations. Neurology. 1999;53:1584–1587. doi: 10.1212/wnl.53.7.1584. [DOI] [PubMed] [Google Scholar]
- 38.Schrijver I A, Melief M-J, Eulderink F, Hazenberg M P, Laman J. Bacterial peptidoglycan polysaccharides in sterile human spleen induce proinflammatory cytokine production by human blood cells. J Infect Dis. 1999;179:1459–1468. doi: 10.1086/314761. [DOI] [PubMed] [Google Scholar]
- 39.Sen Z, Karnovsky M L. Qualitative detection of muramic acid in normal mammalian tissues. Infect Immun. 1984;43:937–941. doi: 10.1128/iai.43.3.937-941.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Severijnen A J, Hazenberg M P, van de Merwe L. Induction of chronic arthritis in rats by cell wall fragments of anaerobic coccoid rods isolated from the faecal flora of patients with Crohn's disease. Digestion. 1988;39:118–125. doi: 10.1159/000199614. [DOI] [PubMed] [Google Scholar]
- 41.Severijnen A J, van Kleef R, Hazenberg M P, van de Merwe J P. Cell wall fragments from major residents of the human intestinal flora induce chronic arthritis in rats. J Rheumatol. 1989;16:1061–1068. [PubMed] [Google Scholar]
- 42.Simelyte E, Rimpilainen M, Rantakokko K, Lehtonen L, Zhang X, Aho H, Isomaki P, Toivanen P. Tissue distribution and persistence of arthritogenic and non-arthritogenic Eubacterium cell walls. Clin Exp Rheumatol. 1999;17:281–288. [PubMed] [Google Scholar]
- 43.Spellerberg B, Rosenow C, Sha W, Tuomanen E I. Pneumococcal cell wall activates NF-kB in human monocytes: aspects distinct from endotoxin. Microb Pathog. 1996;20:309–317. doi: 10.1006/mpat.1996.0029. [DOI] [PubMed] [Google Scholar]
- 44.Stimpson S A, Brown R R, Anderle S K, Klapper D G, Clark R L, Cromartie W J, Schwab J H. Arthropathic properties of cell wall polymers from normal flora bacteria. Infect Immun. 1986;51:240–249. doi: 10.1128/iai.51.1.240-249.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomasic J, Ladesic B, Valinger Z, Hrsak I. The metabolic fate of 14C-labeled peptidoglycan monomer in mice. I. Identification of the monomer and the corresponding pentapeptide in urine. Biochim Biophys Acta. 1980;629:77–82. doi: 10.1016/0304-4165(80)90266-4. [DOI] [PubMed] [Google Scholar]
- 46.Zidek Z, Masek K, Jiricka Z. Arthritogenic activity of a synthetic immunoadjuvant, muramyl dipeptide. Infect Immun. 1982;35:674–679. doi: 10.1128/iai.35.2.674-679.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]