Abstract
The growth performance and histochemical characteristics of breast muscle fibers were used to estimate the standardized ileal digestible (SID) Lys requirements for 10- to 21-d-old male broilers. Three hundred and sixty 10-d-old Ross 308 broilers (290 ± 16.6 g) were allocated to 6 diets in a randomized complete block design with 6 replicate cages per treatment and 10 birds per cage. The 6 experimental diets were formulated to contain equally spaced increasing levels of SID Lys from 0.86% to 1.36%. The data were analyzed using the MIXED procedure of SAS. The Lys requirements were estimated by the NLIN procedure of SAS. An increase in dietary SID Lys from 0.86% to 1.36% resulted in a quadratic increase (P < 0.05) in body weight gain (BWG), gain to feed ratio (G:F), breast weight, muscle cross-sectional area (MCSA), and fiber area. The SID Lys requirements based on the one-slope broken-line, quadratic line, the first intercept between the plateau of the one-slope broken-line and quadratic-line models and 95% of the upper asymptote of the quadratic-line model were estimated to be 1.01%, 1.19%, 1.08%, and 1.13% for BWG, 1.06%, 1.22%, 1.11%, and 1.16% for G:F, 1.10%, 1.29%, 1.19%, and 1.22% for breast weight, 1.06%, 1.22%, 1.12%, and 1.16% for MCSA, and 1.14%, 1.22%, 1.16%, and 1.16% for breast muscle fiber area, respectively. It was concluded that the SID Lys requirements for broilers at the age of 10 to 21 d depended on the response variables used for estimation, and that histochemical characteristics of breast muscle fibers could be good indicators for estimating SID Lys requirements.
Keywords: Requirement, Lysine, Growth performance, Muscle fiber characteristics, Broiler chicken
1. Introduction
Lysine (Lys) is the second limiting amino acid (AA) when broilers are fed on corn and soybean meal (SBM)-based diets as major protein sources (Kidd et al., 2000). Lysine has been commonly used as a reference AA of ideal protein for modern broilers because it is used only for protein accretion and maintenance and not as a precursor for other AA (Emmert and Baker, 1997; Lee et al., 2020). Thus, the supply of Lys can influence the muscle development of birds and the supply of other AA for optimal growth of birds.
Growth responses, including body weight gain (BWG) and gain to feed ratio (G:F) have been widely used to evaluate Lys requirements in broiler chickens (Lee et al., 2018). However, experimental data with additional variables, including carcass parts yield, may give a comprehensive understanding of Lys utilization for efficient protein synthesis in broilers (Pesti, 2009). For this reason, from an economical perspective, recent research reported that the response variables for estimating Lys requirements should consider growth and diverse variables, such as breast meat yield and mass, carcass protein, or fat composition (Kidd and Tillman, 2016).
Ultimate muscle mass is mainly determined by muscle fiber hyperplasia (increased fiber number) and fiber hypertrophy (increased fiber size; Choi et al., 2013). In addition, muscle fiber growth can be affected by dietary AA, especially Lys concentration, as Lys is found in abundance in skeletal muscle (Munks et al., 1945). Changes in morphological characteristics of muscle fibers due to dietary Lys concentrations can affect the economic responses of poultry production. Therefore, the fiber characteristics may be useful in evaluating the Lys requirements of modern broilers. However, no studies have determined the standardized ileal digestible (SID) Lys requirements based on muscle histochemical characteristics. Therefore, this study evaluated and compared the SID Lys requirement estimates based on growth performance and histochemical characteristics of the pectoralis major muscle in broilers.
2. Materials and methods
2.1. Animal ethics
All the experimental procedures applied in this study were reviewed and approved by the Institutional Animal Care and Use Committee at Kyungpook National University (KNU 2019-0125).
2.2. Birds, diets, and management
Birds were fed a commercial diet from post-hatch to 10 d, followed by experimental diets provided for 11 d. Lysine requirements were estimated by using 360 male Ross 308 broilers at 10 d old. All birds (290 ± 16.6 g) individually tagged for identification and used in this experiment were allocated to 6 treatments using a randomized complete block design. Each treatment included 10 birds per cage and 6 cages per treatment. The 6 experimental diets were formulated based on corn and SBM. Except for Lys, all experimental diets met or exceeded the requirements of the broilers, as recommended by the NRC (1994), between 0 and 3 wk old for all nutrients (Table 1). A diet based on corn and SBM was formulated according to the ideal proteins for the starter phase based on SID AA (Hoehler et al., 2006). The SID AA content in the experimental diet, corn, and SBM were calculated using the equations as described by Kong and Adeola (2014). The levels of all indispensable AA, except Lys, were 110% of the level suggested by Hoehler et al. (2006) to avoid deficiency in AA other than Lys. All experimental diets were provided in a mashed form. The feed and water were offered ad libitum. The experimental environment was controlled with continuous light, and the temperatures were applied to birds by gradually reducing the temperature from 33 to 25 °C.
Table 1.
Ingredient and chemical composition of experimental diets (as-fed basis, %).
| Item | Standardized ileal digestible lysine concentrations, % |
|||||
|---|---|---|---|---|---|---|
| 0.86 | 0.96 | 1.06 | 1.16 | 1.26 | 1.36 | |
| Ingredients | ||||||
| Corn | 36.50 | 36.50 | 36.50 | 36.50 | 36.50 | 36.50 |
| Soybean meal | 28.00 | 28.00 | 28.00 | 28.00 | 28.00 | 28.00 |
| Corn-starch | 22.40 | 22.47 | 22.55 | 22.62 | 22.69 | 22.77 |
| Glutamic acid | 3.70 | 3.50 | 3.30 | 3.10 | 2.90 | 2.70 |
| Soybean oil | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 | 2.50 |
| L-Arginine | 0.26 | 0.26 | 0.26 | 0.26 | 0.26 | 0.26 |
| L-Isoleucine | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 |
| L-Leucine | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| L-Lysine·HCl | 0.05 | 0.18 | 0.30 | 0.43 | 0.56 | 0.68 |
| DL-Methionine | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 | 0.31 |
| L-Cysteine | 0.24 | 0.24 | 0.24 | 0.24 | 0.24 | 0.24 |
| L-Phenylalanine | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 |
| L-Threonine | 0.26 | 0.26 | 0.26 | 0.26 | 0.26 | 0.26 |
| L-Tryptophan | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| L-Valine | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 | 0.30 |
| Limestone | 1.30 | 1.30 | 1.30 | 1.30 | 1.30 | 1.30 |
| Dicalcium phosphate | 1.92 | 1.92 | 1.92 | 1.92 | 1.92 | 1.92 |
| Salt | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 |
| Calcium carbonate | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| Vitamin-mineral premix1 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Total | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Calculated composition, % | ||||||
| Crude protein | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Calcium | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Non-phytate phosphorus | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 |
| SID amino acids, % | ||||||
| Arginine | 1.28 | 1.28 | 1.28 | 1.28 | 1.28 | 1.28 |
| Histidine | 0.39 | 0.39 | 0.39 | 0.39 | 0.39 | 0.39 |
| Isoleucine | 0.86 | 0.86 | 0.86 | 0.86 | 0.86 | 0.86 |
| Leucine | 1.31 | 1.31 | 1.31 | 1.31 | 1.31 | 1.31 |
| Lysine | 0.86 | 0.96 | 1.06 | 1.16 | 1.26 | 1.36 |
| Methionine | 0.54 | 0.54 | 0.54 | 0.54 | 0.54 | 0.54 |
| Cysteine | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 |
| Phenylalanine | 0.79 | 0.79 | 0.79 | 0.79 | 0.79 | 0.79 |
| Threonine | 0.79 | 0.79 | 0.79 | 0.79 | 0.79 | 0.79 |
| Tryptophan | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
| Valine | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 | 0.98 |
SID = standardized ileal digestible.
Vitamin-mineral premix supplied per kilogram of diet: vitamin A, 5,484 IU; vitamin D3, 2,643 IU; vitamin E, 11 IU; menadione sodium bisulfite, 4.38 mg; thiamine mononitrate, 2.2 mg; riboflavin, 5.49 mg; D-pantothenic acid, 11 mg; niacin, 44.1 mg; choline chloride, 771 mg; vitamin B12, 13.2 μg; biotin, 55.2 μg; folic acid, 990 μg; pyridoxine hydrochloride, 3.3 mg; I, 1.11 mg as potassium iodide; Mn, 66.1 mg as manganese oxide; Cu, 4.44 mg as copper sulfate; Fe, 44.1 mg as iron sulfate; Zn, 44.1 mg as zinc oxide; Se, 0.3 mg as sodium selenite.
2.3. Performance measurements and chemical analysis
On d 10 and 21, the BW of individual birds and group feed intake were recorded to calculate BWG, feed intake, and G:F. The mortality rate for the experimental periods was 0.83%. The experimental diets and ingredients were ground and analyzed for dry matter (930.15, AOAC International, 2006) and AA composition (Method 982.30 E [a and b], AOAC International, 2006). The analyzed AA composition of the experimental diets shown in Table 2.
Table 2.
Analyzed amino acid composition of the experimental diets containing 6 concentrations of standardized ileal digestible lysine (as-fed basis, %).
| Item | Standardized ileal digestible lysine concentrations, % |
|||||
|---|---|---|---|---|---|---|
| 0.86 | 0.96 | 1.06 | 1.16 | 1.26 | 1.36 | |
| Indispensable amino acid | ||||||
| Arginine | 1.25 | 1.22 | 1.31 | 1.33 | 1.23 | 1.28 |
| Histidine | 0.42 | 0.43 | 0.43 | 0.43 | 0.39 | 0.42 |
| Isoleucine | 0.89 | 0.85 | 0.89 | 0.91 | 0.83 | 0.85 |
| Leucine | 1.44 | 1.39 | 1.48 | 1.48 | 1.34 | 1.41 |
| Lysine | 0.92 | 0.99 | 1.14 | 1.23 | 1.27 | 1.36 |
| Methionine | 0.53 | 0.55 | 0.57 | 0.56 | 0.51 | 0.51 |
| Phenylalanine | 0.86 | 0.84 | 0.89 | 0.89 | 0.81 | 0.86 |
| Threonine | 0.88 | 0.86 | 0.90 | 0.92 | 0.85 | 0.87 |
| Valine | 1.05 | 1.03 | 1.05 | 1.08 | 0.99 | 1.02 |
| Dispensable amino acid | ||||||
| Alanine | 0.75 | 0.75 | 0.79 | 0.80 | 0.71 | 0.76 |
| Aspartic acid | 1.65 | 1.59 | 1.72 | 1.72 | 1.56 | 1.63 |
| Cysteine | 0.46 | 0.44 | 0.45 | 0.45 | 0.47 | 0.44 |
| Glutamic acid | 6.38 | 5.88 | 5.94 | 5.95 | 5.43 | 5.35 |
| Glycine | 0.66 | 0.64 | 0.68 | 0.68 | 0.62 | 0.66 |
| Proline | 0.93 | 0.84 | 1.01 | 0.89 | 0.89 | 0.98 |
| Serine | 0.79 | 0.77 | 0.83 | 0.83 | 0.75 | 0.79 |
| Tyrosine | 0.59 | 0.56 | 0.59 | 0.60 | 0.54 | 0.58 |
2.4. Breast muscle sampling
The muscle growth characteristics of chicks were determined using 36 birds at 21 d of age. The bird in each cage was selected based on their average BW. Following standard procedures, individual birds were euthanized by CO2 asphyxiation (FASS, 1999). The breast muscle was exposed, and the entire right and left pectoralis major muscles were excised and weighed. Muscle cross-sectional area (MCSA) was measured at the diagonal line of a point 1/2 in the left pectoralis major muscle (Scheuermann et al., 2004; Choi et al., 2014) using an image analysis program (Image-Pro Plus, Media Cybernetics, Rockville, MD, USA). Muscle samples were also extracted from each of these regions. They were then cut parallel to the longitudinal direction of the muscle fiber, immediately frozen in isopentane cooled by liquid nitrogen, and stored at 80 °C for histochemical analysis.
2.5. Histochemical characteristics
Transverse muscle sections (10 μM) were obtained from each sample using a cryostat (CM1510S, Leica, Nussloch, Germany) at 25 °C. The hematoxylin and eosin staining method (Cardiff et al., 2014) was used to evaluate muscle fiber characteristics. All stained sections were collected using an optical microscope (DM500, Leica Microsystem, Germany) equipped with a high-definition digital camera (ICC50, Leica Microsystem, England) linked to Leica software (LAS EZ, Switzerland) and a standard work station computer that controlled the image analysis system (Image-Pro Plus, Media Cybernetics, Silver Spring, MD, USA). At least 500 fibers were measured for each sample. The mean area and total number of muscle fibers were measured to evaluate the characteristics of the muscle fibers. The mean fiber area was determined by dividing the total area measured by the total fiber count. Muscle fiber density was calculated from the average number of fibers per square millimeter. The total number of muscle fibers was calculated by multiplying fiber density by the MCSA. The histochemical analysis was performed at 100 × magnification.
2.6. Statistical analyses
The data for BWG and G:F were analyzed using a MIXED procedure of SAS (SAS Inst. Inc., Cary, NC, USA). Experimental diets were regarded as a fixed effect and block based on body weight per cage was a random effect. Orthogonoal polynomial contrast coefficients were used to test the linear and quadratic effects of increasing Lys in diets. The SID Lys requirements were estimated for a one-slope broken-line and quadratic model using the nonlinear regression analysis (NLIN) of SAS based on the study by Robbins et al. (2006). Statistical significance was set at 0.05.
3. Results
3.1. Growth performance
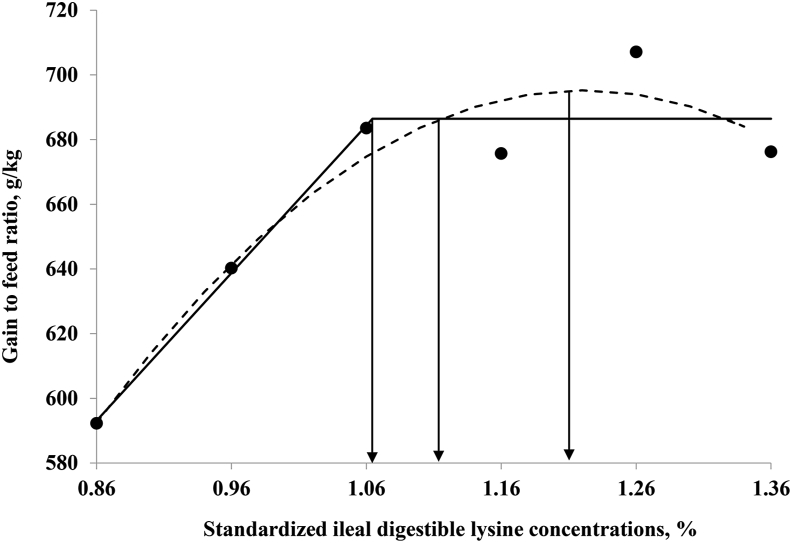
There were linear (P < 0.01) and quadratic (P < 0.01) increases in final BW, BWG, feed intake, and G:F with graded levels of dietary SID Lys in the experimental diets (Table 3). The estimated Lys requirements for BWG based on the broken-line model, the first intercept between the plateau of the one-slope broken-line and quadratic models, the quadratic line model, and the 95% upper asymptote of the quadratic line were 1.01%, 1.08%, 1.19%, and 1.13% of the diet, respectively (Fig. 1). The estimated Lys requirements for G:F based on the respective estimation methods were 1.06%, 1.11%, 1.22%, and 1.16% of the diet (Fig. 2).
Table 3.
Growth performance of 10- to 21-d-old male broilers fed diets with graded levels of standardized ileal digestible lysine1.
| Item | Standardized ileal digestible lysine concentrations, % |
SEM2 |
P-values |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.86 | 0.96 | 1.06 | 1.16 | 1.26 | 1.36 | Linear | Quadratic | ||||
| Initial body weight, g | 290 | 290 | 290 | 290 | 290 | 290 | 7.4 | 0.983 | 0.986 | ||
| Final body weight, g | 734 | 836 | 898 | 893 | 892 | 875 | 18.6 | <0.01 | <0.01 | ||
| Weight gain, g/bird | 444 | 546 | 609 | 603 | 602 | 586 | 14.7 | <0.01 | <0.01 | ||
| Feed intake, g/bird | 750 | 853 | 890 | 893 | 851 | 866 | 20.4 | <0.01 | <0.01 | ||
| Gain to feed, g/kg | 593 | 640 | 686 | 677 | 708 | 677 | 17.6 | <0.01 | 0.01 | ||
Data are the means of 6 cages of 10 male broilers (Ross 308) from d 10 to 21 post-hatch.
SEM = standard error of the mean.
Fig. 1.
Fitted broken (solid) and quadratic line (dot) of growth performance in weight gain as a function of standardized ileal digestible (SID) lysine in the diet. Data points express the means of 6 cages per treatment. The one-slope broken-line model shows that the SID lysine requirement is 1.01%. Y = 599.9 + 1,017.7 × (X − 1.01), (X < 1.01); r2 = 0.985; P < 0.01. The SID lysine requirement with the first intercept between the plateau of the one-slope broken-line and quadratic models is 1.08%. The quadratic line model shows that the SID lysine requirement is 1.19%. Y = 619.3 – 1,513.2 × (1.19 − X)2, (X < 1.19); r2 = 0.961; P < 0.01; and 95% of the upper asymptotic value of the quadratic model indicates that the SID lysine requirement is 1.13%.
Fig. 2.
Fitted broken (solid) and quadratic line (dot) of growth performance in the gain to feed ratio as a function of standardized ileal digestible (SID) lysine in the diet. Data points express the means of 6 cages per treatment. The one-slope broken-line model shows that the SID lysine requirement is 1.06%. Y = 686.4 + 456.5 × (X − 1.06), (X < 1.06); r2 = 0.921; P = 0.02. The SID lysine requirement with the first intercept between the plateau of the one-slope broken-line and quadratic models is 1.11%. The quadratic line model shows that the SID lysine requirement is 1.22%. Y = 695.2 − 789.0 × (1.22 − X)2, (X < 1.22); r2 = 0.934; P = 0.02; and 95% of the upper asymptotic value of the quadratic model indicates that the SID lysine requirement is 1.16%.
3.2. Muscle fiber characteristics
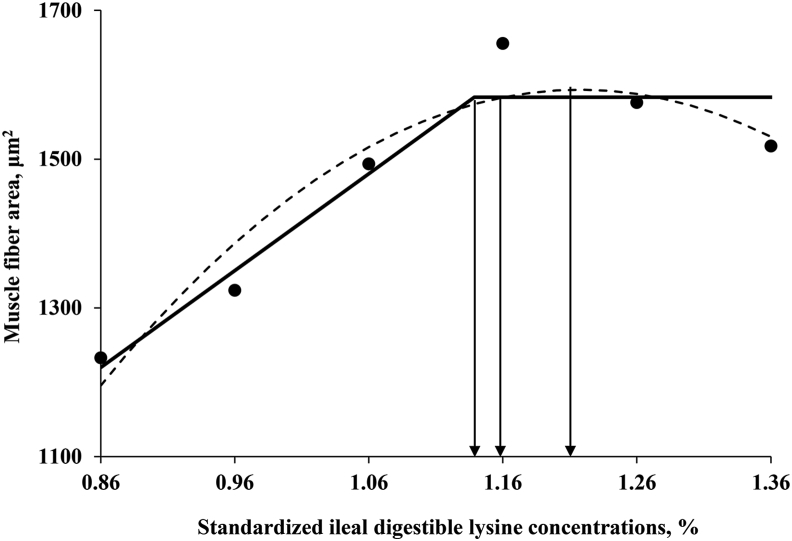
Breast weight and MCSA linearly (P < 0.01) and quadratically (P < 0.01) increased as the dietary SID Lys increased from 0.86% to 1.16% (Table 4). There were only linear (P < 0.01) effects of dietary SID Lys concentrations on the muscle fiber area, whereas dietary Lys concentrations did not affect the total fiber number. The estimated SID Lys requirements for breast weight based on the broken-line model, the first intercept between the plateau of the one-slope broken-line and quadratic models, the quadratic line model, and the 95% upper asymptote of the quadratic line model were 1.10%, 1.19%, 1.29%, and 1.22% of the diet, respectively (Fig. 3). The respective requirements for MCSA were 1.06%, 1.12%, 1.22%, and 1.16% of the diet (Fig. 4), and for muscle fiber area were 1.14%, 1.16%, 1.22%, and 1.16% of the diet, respectively (Fig. 5).
Table 4.
Breast weight, histochemical characteristics of pectoralis major muscle at 21-d-old male broilers fed diets with graded levels of standardized ileal digestible lysine1.
| Item | Standardized ileal digestible lysine concentrations, % |
SEM3 |
P-values |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.86 | 0.96 | 1.06 | 1.16 | 1.26 | 1.36 | Linear | Quadratic | |||
| Breast weight, g | 80.3 | 100.5 | 113.2 | 121.0 | 118.3 | 125.1 | 4.36 | <0.01 | <0.01 | |
| MCSA2, cm2 | 7.9 | 8.7 | 11.2 | 11.3 | 10.6 | 10.9 | 0.62 | <0.01 | <0.01 | |
| Muscle fiber area, μm2 | 1,233 | 1,324 | 1,494 | 1,656 | 1,576 | 1,517 | 94.3 | <0.01 | 0.053 | |
| Total fiber number, 1,000 | 643 | 661 | 752 | 690 | 725 | 787 | 50.4 | 0.054 | 0.994 | |
Data are the means of 6 cages of one male broiler (Ross 308) at 21 d post–hatch.
MCSA = muscle cross-sectional area.
SEM = standard error of the mean.
Fig. 3.
Fitted broken (solid) and quadratic line (dot) of muscle characteristics in the breast meat as a function of standardized ileal digestible (SID) lysine in the diet. Data points express the means of 6 cages per treatment. The one-slope broken-line model shows that the SID lysine requirement is 1.10%. Y = 121.5 + 164.7 × (X − 1.10), (X < 1.10); r2 = 0.976; P = 0.004. The SID lysine requirement with the first intercept between the plateau of the one-slope broken-line and quadratic models is 1.19%. The quadratic line model shows that the SID lysine requirement is 1.29%. Y = 123.7 − 229.7 × (1.29 − X)2 , (X < 1.29); r2 = 0.983; P = 0.002], and 95% of the upper asymptotic value of the quadratic model indicates that the SID lysine requirement is 1.22%.
Fig. 4.
Fitted broken (solid) and quadratic line (dot) of muscle characteristics in the muscle cross-sectional area (MCSA) as a function of standardized ileal digestible (SID) lysine in the diet. Data points express the means of 6 cages per treatment. The one-slope broken-line model shows that the SID lysine requirement is 1.06%. Y = 10.9 − 16.6 × (X − 1.06), (X < 1.06); r2 = 0.928; P = 0.02. The SID lysine requirement with the first intercept between the plateau of the one-slope broken-line and quadratic models is 1.12%. The quadratic line model shows that the SID lysine requirement is 1.22%. Y = 11.2 − 28.0 × (1.22 − X)2, (X < 1.22); r2 = 0.869; P = 0.04, and 95% of the upper asymptotic value of the quadratic model indicates that the SID lysine requirement is 1.16%.
Fig. 5.
Fitted broken (solid) and quadratic line (dot) of muscle characteristics in the fiber area as a function of standardized ileal digestible (SID) lysine in the diet. Data points express the means of 6 cages per treatment. The one-slope broken-line model shows that the SID lysine requirement is 1.14%. Y = 1,583 – 1,303.1 × (X − 1.14), (X < 1.14); r2 = 0.917; P = 0.02. The SID lysine requirement with the first intercept between the plateau of the one-slope broken-line and quadratic models is 1.16%. The quadratic line model shows that the SID lysine requirement is 1.22%. Y = 1,593.3 − 3,113.3 × (1.22 − X)2 , (X < 1.22); r2 = 0.909; P = 0.04, and 95% of the upper asymptotic value of the quadratic model indicates that the SID lysine requirement is 1.16%.
4. Discussion
Supplemented Lys in the diet is involved in protein accretion of tissue, which can help improve growth. Sufficient supplementation of dietary Lys concentration supports optimal growth and ensures maximum meat yield from broilers. Numerous studies have observed the advanced digestive efficiency, body composition, and utilization of metabolizable energy efficiency of modern broilers (Jackson and Diamond, 1996; Zuidhof et al., 1978; Aggrey et al., 2010). Genetic changes derived from the artificial selection of broilers led to a rapid growth rate and reduced the required feeding length to achieve the target slaughter weight (Tallentire et al., 2016) and, consequently, increased dietary nutrient requirements. Therefore, providing updated information on the growth performance of broilers in response to dietary Lys concentrations may be useful to formulate the feeds for modern broilers.
The optimum dietary quantity of specific nutrients represents the amount of a dietary nutrient that supports the maximization of the response of broiler chickens to certain growth performance. Changes in protein and fat accretion in the carcass in response to dietary Lys concentrations may also be an additional criterion for measuring the optimal Lys demands to maximize productivity. This is because the contribution of breast muscle to the total edible meat has been increased, and the growth rate of breast muscle in modern broilers has been improved (Schmidt et al., 2009). Thus, improvement in breast meat yield is considered a profitable variable of poultry production. In the present study, growth response and breast meat yield and muscle protein histochemical characteristics were employed to determine the SID Lys requirement of broilers.
Our findings are consistent with other investigators who have reported increased BWG and protein mass along with breast meat as dietary Lys increased from deficient to sufficient level (Leclercq, 1998; Si et al., 2001; Tesseraud et al., 2001). In this study, deficient or excessive dietary SID Lys showed a negative effect on growth, which the AA imbalance can explain. Mahdavi et al. (2012) also reported that the supply of digestible Lys above the requirement resulted in decreases in the growth and feed efficiency of the broilers due to the reduced efficiency of AA for protein synthesis.
In the present study, the requirement for feed efficiency was higher than that for weight gain. When the dietary SID Lys concentration increased to the requirement, both the BWG and feed efficiency increased, but after that, it appeared that the BWG was maintained, but the feed efficiency decreased as the SID Lys increased. The present result agrees with Vazquez and Pesti (1997) and Bernal et al. (2014). Vazquez and Pesti (1997) who predicted the Lys requirements for both sexes (3 and 7 wk of age) using the quadratic line model. Predicted Lys requirements for maximum weight gain and feed efficiency were 1.21% and 1.32%, respectively. Bernal et al. (2014) also reported that the digestible Lys requirement for feed conversion ratio was higher than that for weight gain in males and females. However, the previous study conducted in our laboratory indicated that the SID Lys requirement of broilers at the age of 0 to 10 d for BWG was greater than for feed efficiency (Lee et al., 2018). The clear reason for this discrepancy is unknown, but it may be attributed to the difference in the age of the birds used in the studies. Baker (1986) indicated that changes to more fat and less protein deposition as birds mature and criteria such as maximal carcass leanness or feed efficiency result in a greater requirement than that predicted for maximal BWG.
Muscle growth and ultimate mass are tightly associated with muscle fiber hyperplasia and hypertrophy (Petracci et al., 2015). In mammals and birds, the number of muscle fibers is fixed before the postnatal period. Therefore, ultimate muscle mass depends on the fiber numbers formed prenatally and an increase in the size of these existing fibers after the postnatal period (Choi et al., 2014). For instance, the increased pectoralis major muscle weight of broilers at 6 wk old was attributed to an increase in muscle fiber area and no difference in fiber number at the same age of broilers (Berri et al., 2007). Also, there was no significant change in the fiber number after birth in other animals, such as mice (Nimmo and Snow, 1983), cattle (Wegner et al., 2000), and chickens (Smith, 1963). Meanwhile, muscle fiber characteristics can be associated with dietary Lys concentrations, which play a key role in protein synthesis and breakdown (Munks et al., 1945). Thus, the development of the breast muscle of broilers could be affected by the dietary Lys concentrations (Cemin et al., 2017). There was no difference in the fiber number in the present study regardless of dietary SID Lys concentrations. However, graded levels of dietary SID Lys affected the fiber size of breast meat and breast muscle weight, which indicates that the fiber size could be used to estimate the SID Lys requirement of broiler chickens. The SID Lys requirements for the breast muscle weight and fiber size were estimated to be greater than that for BWG. Cemin et al. (2017) estimated digestible Lys requirements of broilers from 28 to 42 d as 0.879% for BWG, and 0.891% for breast meat yield using a linear broken-line model, which is comparable to the results in the present study. The greater requirement for breast muscle weight compared with BWG may be attributed to the body composition change to more body fat contents as birds aged (Baker, 1986; Wecke et al., 2018).
5. Conclusion
In conclusion, the dietary SID Lys concentration affected breast muscle, growth performance and histochemical characteristics, except the fiber number, of breast muscle in broilers. The SID Lys requirements of male broilers from d 10 to 21 were estimated to be 1.01% and 1.19% for BWG, 1.06% and 1.22% for G:F, and 1.10% and 1.29% for breast weight based on broken-line and quadratic line models, respectively. In addition, determined SID Lys requirements considering histochemical characteristics of breast muscle were 1.06% and 1.22% for MCSA and 1.14% and 1.22% for fiber area based on broken-line and quadratic line models, respectively.
Author contributions
Su Hyun An: Data curation, Formal analysis, Methodology, Investigation, Writing – original draft, preparation. Boin Lee: Data curation, Formal analysis, Investigation, Writing – original draft, preparation. Young Min Choi: Conceptualization, Investigation, Methodology, Writing – review & editing. Changsu Kong: Conceptualization, Data curation, Project administration, Writing – review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was carried out with the support of the “Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ013571)” Rural Development Administration, Republic of Korea.
Footnotes
Peer review under responsibility of Chinese Association of Animal Science and Veterinary Medicine.
References
- Aggrey S.E., Karnuah A.B., Sebastian B., Anthony N.B. Genetic properties of feed efficiency parameters in meat-type chickens. Genet Sel Evol. 2010;42:1–5. doi: 10.1186/1297-9686-42-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoac International . 18th ed. Association of Official Analytical Chemist International; Gaithersburg, MD, USA: 2006. Official methods of analysis. [Google Scholar]
- Baker D.H. Problems and pitfalls in animal experiments designed to establish dietary requirements for essential nutrients. J Nutr. 1986;116:2339–2349. doi: 10.1093/jn/116.12.2339. [DOI] [PubMed] [Google Scholar]
- Bernal L.E.P., Tavernari F.C., Rostagno H.S., Albino L.F.T. Digestible lysine requirements of broilers. Rev Bras Ciência Avícola. 2014;16:49–54. [Google Scholar]
- Berri C., Le Bihan-Duval E., Debut M., Sante-Lhoutellier V., Baeza E., Gigaud V., Jego Y., Duclos M.J. Consequence of muscle hypertrophy on characteristics of Pectoralis major muscle and breast meat quality of broiler chickens. J Anim Sci. 2007;85:2005–2011. doi: 10.2527/jas.2006-398. [DOI] [PubMed] [Google Scholar]
- Cardiff R.D., Miller C.H., Munn R.J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Protoc. 2014;2014:655–658. doi: 10.1101/pdb.prot073411. [DOI] [PubMed] [Google Scholar]
- Cemin H.S., Vieira S.L., Stefanello C., Kipper M., Kindlein L., Helmbrecht A. Digestible lysine requirements of male broilers from 1 to 42 days of age reassessed. PLoS One. 2017;12 doi: 10.1371/journal.pone.0179665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y.M., Sarah D., Shin S., Wick M.R., Kim B.C., Lee K. Comparative growth performance in different Japanese quail lines: the effect of muscle DNA content and fiber morphology. Poultry Sci. 2013;92:1870–1877. doi: 10.3382/ps.2012-02892. [DOI] [PubMed] [Google Scholar]
- Choi Y.M., Suh Y., Shin S., Lee K. Skeletal muscle characterization of Japanese quail line selectively bred for lower body weight as an avian model of delayed muscle growth with hypoplasia. PLoS One. 2014;9 doi: 10.1371/journal.pone.0095932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmert J.L., Baker D.H. Use of the ideal protein concept for precision formulation of amino acid levels in broilers diets. J Appl Poult Sci. 1997;6:462–470. [Google Scholar]
- FASS . 1st rev. Federation of Animal Science Societies; Champaign, IL, USA: 1999. Guide for the care and use of agricultural animals in agricultural research and teaching. [Google Scholar]
- Hoehler D., Lemme A., Ravindran V., Bryden W.L., Rostagno H.H. Proceedings of advances in poultry nutrition, VIII. International symposium on aquatic nutrition. UANL, Monterrey, New Lion; Mexico: 2006. Feed formulation in broiler chickens based on standardized ileal amino acid digestibility. 2006; pp. 197–212. [Google Scholar]
- Jackson S., Diamond J. Metabolic and digestive responses to artificial selection in chickens. Evolution. 1996;50:1638–1650. doi: 10.1111/j.1558-5646.1996.tb03936.x. [DOI] [PubMed] [Google Scholar]
- Kidd M.T., Tillman P.B. Key principles concerning dietary amino acid responses in broilers. Anim Feed Sci Technol. 2016;221:314–322. [Google Scholar]
- Kidd M.T., Kerr B.J., Allard J.P., Rao S.K., Halley J.T. Limiting amino acid responses in commercial broilers. J Appl Poult Sci. 2000;9:223–233. [Google Scholar]
- Kong C., Adeola O. Evaluation of amino acid and energy utilization in feedstuff for swine and poultry diets. Asian-Australas J Anim Sci. 2014;27:917–925. doi: 10.5713/ajas.2014.r.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq B. Lysine: specific effects of lysine on broiler production: comparison with threonine and valine. Poultry Sci. 1998;77:118–123. doi: 10.1093/ps/77.1.118. [DOI] [PubMed] [Google Scholar]
- Lee J., Sung Y., Kong C. Ideal ratios of standardized ileal digestible methionine, threonine, and tryptophan relative to lysine for male broilers at the age of 1 to 10 days. Anim Feed Sci Technol. 2020;262 [Google Scholar]
- Lee J., Sung Y., Kong C. Standardized ileal digestible lysine requirement of male broilers at the age of 0-10 days. Anim Feed Sci Technol. 2018;241:55–62. [Google Scholar]
- Mahdavi A., Shivazad M., Alemi F., Zaghari M., Moravej H., Darabighane B. Digestible lysine requirement of broilers based on practical diet. Ital J Anim Sci. 2012;11:e13. [Google Scholar]
- Munks B., Robinson A., Beach E.F., Williams H.H. Amino acids in the production of chicken egg and muscle. Poultry Sci. 1945;24:459–464. [Google Scholar]
- Nimmo M.A., Snow D.H. The effect of ageing on skeletal muscle fiber characteristics in two inbred strains of mice. J Physiol. 1983;340:24–25. [Google Scholar]
- NRC . Nutrient requirements of poultry. 9th ed. National Academy Press; Washington, DC, USA: 1994. [Google Scholar]
- Pesti G.M. Impact of dietary amino acid and crude protein levels in broiler feeds on biological performance. J Appl Poultry Res. 2009;18:477–486. [Google Scholar]
- Petracci M., Mudalal S., Soglia F., Cavani C. Meat quality in fast-growing broiler chickens. World’s Poult Sci J. 2015;71:363–373. [Google Scholar]
- Robbins K.R., Saxton A.M., Southern L.L. Estimation of nutrient requirements using broken-line regression analysis. J Anim Sci. 2006;84:E155–E165. doi: 10.2527/2006.8413_supple155x. [DOI] [PubMed] [Google Scholar]
- Scheuermann G.N., Bilgili S.F., Tuzun S., Mulvaney D.R. Comparison of chicken genotypes: myofiber number in pectoralis muscle and myostatin ontogeny. Poultry Sci. 2004;83:1404–1412. doi: 10.1093/ps/83.8.1404. [DOI] [PubMed] [Google Scholar]
- Schmidt C.J., Persia M.E., Feierstein E., Kingham B., Saylor W.W. Comparison of a modern broiler line and a heritage line unselected since the 1950s. Poultry Sci. 2009;88:2610–2619. doi: 10.3382/ps.2009-00055. [DOI] [PubMed] [Google Scholar]
- Si J., Fritts C.A., Burnham D.J., Waldroup P.W. Relationship of dietary lysine level to the concentration of all essential amino acids in broiler diets. Poultry Sci. 2001;80:1472–1479. doi: 10.1093/ps/80.10.1472. [DOI] [PubMed] [Google Scholar]
- Smith J.H. Relation of body size to muscle cell size and number in the chicken. Poultry Sci. 1963;42:283–290. [Google Scholar]
- Tallentire C.W., Leinonen I., Kyriazakis I. Breeding for efficiency in the broiler chicken: a review. Agron Sustain Dev. 2016;36:66. [Google Scholar]
- Tesseraud S., Temim S., Le Bihan-Duval E., Chagneau A.M. Increased responsiveness to dietary lysine deficiency of pectoralis major muscle protein turnover in broilers selected on breast development. J Anim Sci. 2001;79:927–933. doi: 10.2527/2001.794927x. [DOI] [PubMed] [Google Scholar]
- Vazquez M., Pesti G.M. Estimation of the lysine requirement of broiler chicks for maximum body gain and feed efficiency. J Appl Poultry Res. 1997;6:241–246. [Google Scholar]
- Wecke C., Khan D.R., Sünder A., Liebert F. Age and gender dependent amino acid concentrations in the feather, feather-free and whole empty body protein of fast growing meat-type chickens. Open J Anim Sci. 2018;8:223–238. [Google Scholar]
- Wegner J., Albrecht E., Fiedler I., Teuscher F., Papstein H.J., Ender K. Growth and breed-related changes of muscle fiber characteristics in cattle. J Anim Sci. 2000;78:1485–1496. doi: 10.2527/2000.7861485x. [DOI] [PubMed] [Google Scholar]
- Zuidhof M.J., Schneider B.L., Carney V.L., Korver D.R., Robinson F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poultry Sci. 1978;93:2970–2982. doi: 10.3382/ps.2014-04291. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]