Highlights
-
•
US-assisted solubilization of hydrolysis lignin in mild alkaline conditions.
-
•
US effect up to 30 % in mild conditions with 20 kHz.
-
•
Induces the inclusion of high MW fractions into the solution.
-
•
Involves the mechanoacoustical and sonochemical effects of US.
-
•
NMR shows increased OH groups in the obtained fractions.
Keywords: Forest biorefinery, Hydrolysis lignin, Power ultrasound, Solubilization
Abstract
In the forest biorefinery, hydrolysis lignin (HL) is often dissolved with high concentration NaOH solution, followed by acid precipitation to obtain purified HL. For the first time, this study evaluates the effect of ultrasound (US) on the dissolution of industrially produced HL in aqueous NaOH solutions and the acid precipitation yield of HL. The solubility of HL in mild aqueous NaOH solutions was studied with and without US treatment at 20 kHz concerning the solid-to-liquid ratio, molecular weight of dissolved fractions and structural changes in dissolved HL. Results showed that the solubility of HL at 25 °C was strongly dependent on NaOH concentration. However, the US treatment significantly improved the solubility of HL, reaching a solubility plateau at 0.1 NaOH/HL ratio. US treatment enhanced the solubilization of HL molecules with higher MW compared to conventional mixing. The increase of HL solubility was up to 30 % and the recovery yield of purified lignin with acid precipitation was 37 % higher in dilute NaOH solution. A significant result was that the Mw of dissolved HL in homogeneous alkali solutions decreased with US treatment. SEC, HSQC and 31P NMR analyses of dissolved HL characteristics showed that both, the mechanoacoustic and sonochemical solubilization pathways contribute to the dissolution process. However, US does not cause major changes in the HL structure compared to the native lignin. Indeed, US technology has the potential to advance the dissolution and purification of HL in biorefineries by reducing the amount of chemicals required; thus, more controlled and environmentally friendly conditions can be used in HL valorization.
1. Introduction
The modern chemical industry, stimulated by political and environmental concerns, is intensively looking for alternatives to petroleum resources. One such contender in this field is lignocellulosic forestry biomass, which is mainly comprised of three major polymers: cellulose (40–50 %), hemicellulose (20–30 %) and lignin (10–30 %) [[1], [2], [3], [4], [5]]. There are some approaches, where the main products are either cellulose or C5-C6 sugars and lignin is treated as a recalcitrant contaminant or cheap energy resource. In contrast, the major upside for the biorefinery concept is the intent to create value-added chemicals from all three polymers, not only from the saccharide-based ones. This creates abundant possibilities for producing biofuels, chemicals, bio-based polymers and many more [[6], [7]]. However, these biorefineries are financially unviable in the long term unless all streams are optimally valorized [8]. Here the improvement of lignin processing is crucial, where major innovations are yet to be discovered [5].
Lignin is a complex, highly branched polyphenolic macromolecule formed in plants by connecting aromatic monomers [9]. The principal building blocks for lignin are guaiacyl- (G), syringyl- (S) and hydroxyphenyl- (H) propane units and these are connected via β-O-4, β-β, β-5 (Fig. 1) and many other types of linkages [10]. This results in a recalcitrant heterogeneous polymer, the most abundant, one-of-a-kind, potentially renewable source for aromatic compounds [11]. Many different technical lignin types are available, including lignin from pulping streams such as Kraft lignin, soda lignin or lignosulfonates and sugar-based biorefinery lignin such as wood hydrolysis lignin (HL) [[5], [12]].
Fig. 1.
Visualization of the lignin subunits guaiacyl (G), syringyl (S) and hydroxyphenyl (H) as well as the primary linkage types present in lignin: β-O-4, β-β, β-5.
Biorefinery wood HL has a more native structure, higher MW (molecular weight) and is sulfur-free, in contrast to many types of lignin from pulping, with highly condensed structure and chemical contamination [[13], [14]]. HL is obtained by using mild physicochemical conditions with an enzymatic treatment to hydrolyze polysaccharides into C5 and C6 sugars, resulting in crude HL [15]. The production of HL is estimated to rise and could become the major contributor in the lignin market [5]. However, many valorization avenues require HL dissolution or purification, which remains challenging.
Solubilization of lignin makes it more conducive to chemical and biochemical reactions [[9], [16]]. Most types of wood lignin, poorly soluble in water and organic solvents, are soluble in alkaline aqueous solvents [[17], [18]]. The use of a high base concentration can lead to unwanted changes in the structure of lignin, cause polycondensation and pollution of the material. In addition, it affects the cost efficiency, thus reducing opportunities for further valorization. The solubility of lignin in alkaline conditions is well established in the industry, however only recently, a comprehensive study about Kraft lignin solubility in various alkali was published [19].
Therefore, a sustainable, cost-effective and low chemical load choice is critical for the dissolution of HL. Based on the experience of our research group, we proposed that application of US energy shows promise for the valorization of HL during solubilization [[20], [21]]. In-depth reviews have recently been published with great perspective on US use in biomass pretreatments, lignin degradation and many successful biomass conversions into value-added products. These comprehensive reviews highlight different US physical and chemical effects that have yielded beneficial results in biomass valorization [[22], [23], [24]].
These are mainly acoustic cavitation-related physical effects such as shock waves, high-speed microjets, microbubble implosion and hotspots that can increase mass transfer, homogenization and turbulence. Cavitation effects can impact the characteristics of the biomass, such as cellulose crystallinity, accessibility, the solubility of biomass components, particle size and surface area [[22], [25], [26]]. Besides these, sonochemical effects are also integral and are associated with forming highly reactive radical species due to the implosion of the cavitation bubbles [[22], [27], [28], [29]].
The central theme of the studies available in the literature is about the non-wood herbal plants and agricultural biomass pretreatment with US, often focusing on the lignin removal in the separation process of lignocellulosic materials, not on the valorization of lignin [[30], [26], [31], [32]]. To obtain better results for the delignification of corn straw, pretreatments using US have also shown the requirement for additional energy input, e.g. microwave. This highlights the difficulties in the implementation of US during the pretreatment phase, where a great deal of energy is used for heating and dispersion [26]. In the earliest work, milled wood was treated with US in a neutral solvent, with higher yields reported [33]. However, some studies have focused on wood-based lignin valorization with US assistance. It has been postulated that US could cleave the ether bonds in lignin, which leads to depolymerization or polycondensation reactions. For example, treatment of Kraft lignin with US resulted in high MW lignin fractions [34]. Other examples include methods for lignin nanoparticle formation or depolymerization at 250 °C (300 W and 35 kHz) for 6 h [[35], [36], [37]]. Recently, the efficiency of Kraft lignin dissolution in protic ionic liquids was improved, with US achieving saturation in less than 4 h compared with a required 8 h using mechanical agitation [38].
On an industrial scale US is found in environmental remediation, synthesis, extraction and the food industry [39]. However, the US technology has not yet been applied in forest biorefinery due to the low efficiency and high energy consumption required to overcome the intrinsic recalcitrant nature of wood biomass. Modern forest biorefineries use various efficient methods for pretreatment of wood chips, such as nitrogen or steam explosion, supercritical conditions and/or thermo-mechanical-chemical treatment, after which new avenues for US treatment open [[2], [5], [40], [41], [42], [43]].
To the best of the authors knowledge, no studies have been presented dealing with alkali solubilization and purification of industrially produced wood HL and the effect of US to promote the processes of wood biorefineries.
In this article, the US effects on the solubility of industrially produced birch HL at various mild alkaline conditions and HL contents were investigated. The resulting solution concentrations and the molecular weight distributions of dissolved HL fractions were analyzed. The HL was isolated via acid precipitation and a higher purity product was obtained. The HL structure was characterized by size exclusion chromatography (SEC) and two different NMR methods: HSQC and 31P to elucidate the US effects on HL linkages and hydroxyl groups.
2. Materials and methods
2.1. Materials
Fibenol OÜ provided the birch HL, this is produced by using SunburstTM technology and the resulting slurry is processed with cellulases. Composition of the HL: lignin content of 86.3 ± 1.75 %, 1.15 % ash, 4.7 % glucose, 2.1 % xylose, 1.3 % galactose, 0.6 % arabinose, 1.4 % mannose.
DMSO‑d6, CDCl3 and TMDP were purchased from Sigma-Aldrich. Concentrated H2SO4 was purchased from Honeywell and NaOH from Lachner.
2.2. Hydrolysis lignin solubilization and recovery
All solubilization experiments were carried out in Sonopuls cooling vessel KG5 with 60 ml of solution at 25 ± 1 °C. 0.00, 0.50, 0.75, 1.00 and 2.00 wt% aqueous NaOH solutions were used with 100 S/L and 150 S/L, solid-to-liquid ratio (S/L) in grams of HL per liters of NaOH solution. The NaOH solution was added to the dried HL powder and continually mixed on a magnetic stirrer (800 rpm) for a total of 90 min. Temperature in the reaction vessel was controlled and recorded with a Refrigerated Heating Circulator Bath (Ministat 125, Peter Huber Kältemaschinenbau GmbH). Soluble and insoluble fractions were separated by centrifugation with Eppendorf 5804, FA-45–6-30 rotor at 11 000 rpm for 10 min and the solution was filtered. The lignin concentration in solutions was measured by UV–vis spectroscopy and calculated via a calibration curve at 280 nm and from the insoluble residue gravimetrically. All of the solubility experiments were done in at least triplicate and the standard deviation from separate experiments were calculated. Dissolved lignin was isolated from solution by acid precipitation with 3 M H2SO4 with adjusting the pH to 2. The pH value was determined using Metrohm 744 pH meter. Precipitated lignin was washed three times with Milli-Q H2O to remove any residual salts and other water-soluble contaminants. The yield of dried precipitated lignin was determined gravimetrically.
2.3. Ultrasound-assisted solubilization of hydrolysis lignin
US-assisted solubilization was carried out under the same conditions as described in 2.2. However, dried HL powder was first mixed in corresponding NaOH solution on magnetic stirrer for 30 min and was continued when US was switched on. Then the mixture was sonicated for 60 min at 25 ± 1 °C 30 % power by Bandelin Sonopuls HD 4200 (20 kHz, the maximum power of the ultrasonic transmitter was 200 W). The energy output was 34 W in water, estimated calorimetrically in the same reaction vessel with the TT 213 titanium tip, apparatus shown in the Supplementary Fig. 2S. The solubility measurements as well as the acid precipitated lignin were obtained as described above.
2.4. Ultrasound treatment of homogeneous hydrolysis lignin-alkali solution
The HL was first solubilized as described in 2.2. and after the filtration of the homogenous alkaline HL solution (60 ml) was treated with US for 60 min at 25 ± 1 °C. After every 10 min 200 μl of sample was taken: analyzed with SEC and UV–vis. After US treatment the lignin was recovered by acid precipitation as described above.
2.5. Molecular weight determination of dissolved hydrolysis lignin fractions
The relative molecular weight distributions of the soluble lignin were measured by SEC. The analysis was done using a Shimadzu Prominence system with a UV detector at 280 nm with size exclusion columns MCX 1000 Å 5 µm, 8 mm × 300 mm and MCX, 8 × 300 mm, 10 000 Å, 5 µm with pre-column MCX 5 µm, 8 mm × 50 mm (Polymer Standards Service). Isocratic flow with 0.1 M NaOH solution at a flow rate of 0.6 ml/min, samples were run for 50 min, with an injection volume of 10 ul. The relative molecular weight of lignin was determined using a polystyrene sulfonate sodium salt standard-based calibration curve in the range of 246 Da to 100 000 Da. The obtained chromatograms from the samples were analyzed with Lab Solutions GPC software.
2.6. Structural analysis of dissolved hydrolysis lignin fractions
2.6.1. Two-dimensional heteronuclear single quantum coherence NMR spectroscopy
The measurement method was adapted from the method used by Zikeli et al. [35] and the interpretations were based on literature [[35], [37], [44]]. The two-dimensional heteronuclear single quantum coherence (HSQC) NMR method is commonly used to quantify the aromatic subunits and aliphatic linkages in lignin structure.
HSQC (1H–13C) spectra were recorded by the dissolution of 120 mg of dry lignin sample in 0.5 ml DMSO‑d6. The material was solubilized overnight and measured using Bruker Avance-III 700 MHz. The spectra were obtained using Bruker TopSpin pulse program hsqcedetgpsisp2.3, with 16 scans, 0.08 s acquisition time and 3 s relaxation delay. The resulting signals were integrated as seen in Fig. 1S. The monomeric composition is calculated via C2,6-H2,6 and the inter-unit linkages are calculated via Cα-Hα signals.
2.6.2. Hydroxyl group quantification by 31P NMR
The number of hydroxyl groups was determined with 31P NMR spectroscopy using the procedure by Meng et al. [45].
30 mg of dry lignin sample was dissolved in 0.5 ml solvent mixture of pyridine/deuterated chloroform (1.6/1 v/v). To the dissolved lignin sample 0.1 ml internal standard solution was added, containing 5 mg/ml chromium(III)2,4-pentanedionate (Cr(acac)3) and 18 mg/ml N-hydroxy-5-norbornene-2,3-dicarboxylic acid imide (NHND), as the relaxation agent and internal standard respectively. Followed by the addition of 0.1 ml 2-chloro-4,4,5,5- tetramethyl-1,2,3-dioxaphospholane (TMDP). The obtained solution was mixed and measured immediately. Bruker Avance-III 700 MHz spectrometer was used to obtain the spectra. The inverse gated decoupling pulse (zgig) program was used with a spectral width of 100 ppm, the acquisition time of 0.8 s, the center spectrum of 140 ppm and 256 scans. The resulting spectra were processed and hydroxyl groups were calculated as previously described in literature.
3. Results and discussion
3.1. Solubilization of hydrolysis lignin in mild alkaline conditions assisted by ultrasound
It is important to note that industrial HL from the carbohydrate-based biorefinery contains large amounts of impurities, up to 20–50 %, including free saccharides (from cellulose and hemicellulose hydrolysis), mineral salts, recalcitrant polysaccharides as well as some cellulases [[5], [10], [14]]. In this study, very high purity industrial HL was used, about 86 % of the total weight accounts for native lignin. However, when evaluating the HL dissolution results, it should be noted that mineral salts and free saccharides are soluble in NaOH aqueous solutions in addition to lignin. About 10 % of HL is purely water-soluble.
The solubilization results of HL in the NaOH aqueous solutions without and with US treatment are listed in Table 1, Table 2. Two solid-to-liquid ratios (S/L) in grams of HL per liters of NaOH solution were studied: 100 and 150 S/L.
Table 1.
HL solubility (g/l) and the soluble fraction (%) obtained from the experiments, with and without US at 100 S/L at 25 °C.
| 100 S/L | ||||||
|---|---|---|---|---|---|---|
| NaOH (wt%) |
NaOH/HL ratio |
non-US |
US |
|||
| Solubility (g/l) |
Soluble fraction (%) |
Solubility (g/l) |
Soluble fraction (%) |
|||
| 0.00 | 0.00 | 10.2 ± 0.8 | 10.1 ± 0.7 | 10.0 ± 0.8 | 10.0 ± 0.8 | |
| 0.50 | 0.50 | 53.9 ± 0.7 | 54.5 ± 0.8 | 65.4 ± 1.2 | 65.2 ± 1.2 | |
| 0.75 | 0.08 | 68.2 ± 0.4 | 68.8 ± 0.3 | 78.1 ± 0.9 | 77.9 ± 1.0 | |
| 1.00 | 0.10 | 77.8 ± 0.4 | 78.4 ± 0.5 | 87.4 ± 0.6 | 87.6 ± 0.8 | |
| 2.00 | 0.20 | 79.5 ± 0.7 | 79.5 ± 0.7 | 88.9 ± 1.7 | 89.2 ± 1.5 | |
Table 2.
HL solubility (g/l) and the soluble fraction (%) obtained from the experiments, with and without US at 150 S/L at 25 °C.
| 150 S/L | ||||||
|---|---|---|---|---|---|---|
| NaOH (wt%) |
NaOH/HL ratio |
non-US |
US |
|||
| Solubility (g/l) |
Soluble fraction (%) |
Solubility (g/l) |
Soluble fraction (%) |
|||
| 0.00 | 0.00 | 13.4 ± 0.8 | 8.8 ± 0.5 | 14.2 ± 1.1 | 9.5 ± 0.7 | |
| 0.50 | 0.03 | 63.5 ± 2.4 | 42.3 ± 1.5 | 78.2 ± 1.5 | 52.0 ± 0.9 | |
| 0.75 | 0.05 | 83.9 ± 3.0 | 55.7 ± 2.0 | 98.9 ± 0.9 | 65.9 ± 0.7 | |
| 1.00 | 0.07 | 100.6 ± 2.2 | 67.1 ± 1.3 | 115.9 ± 2.7 | 77.2 ± 1.6 | |
| 2.00 | 0.13 | 119.5 ± 3.0 | 79.6 ± 1.9 | 133.7 ± 2.0 | 89.0 ± 1.1 | |
It was found that the solubility of HL in pure water is 10.2 g/l at 100 S/L and 13.4 g/l at 150 S/L, most likely due to the increase in the amount of water soluble salts, monosaccharides and water soluble lignin fragments. The addition of NaOH increased the solubility of HL from 53.9 g/l in 0.5 wt% NaOH solution to the maximum solubility of 79.5 g/l in 2 wt% NaOH solution at 100 S/L.
However, the relative solubility (%) in water remained lower at 150 S/L than at 100 S/L, 8.8 % vs 10.1 %, respectively (Table 1, Table 2). This could be due to the salting- and sugaring-out effect [46], which could reduce the solubility of lignin molecules in water. The absolute solubility difference (g/l) suggests that the saturation point of the solvent had not yet been reached. Similarly, the solubility increases with the amount of NaOH and the maximal solubility of 119.5 g/l is reached at 2 wt% NaOH solution at 150 S/L.
The results in Table 1 and Table 2 showed a correlation between the ratio of solid NaOH (g) to solid HL (g) and the relative solubility of HL. The dissolved HL fraction yield (%) at both S/L ratios vs NaOH/HL were plotted (Fig. 2a). It was found that the soluble fraction yield is higher with an increase of NaOH/HL ratio and reaches a maximum solubility plateau at a ratio of 0.1, as seen in Fig. 2.
Fig. 2.
Soluble fraction of HL (%) in correlation to NaOH/HL (g/g) ratio. a) non-US, b) US treatment at 20 kHz. Filled black and empty red circles represent the 100 and 150 S/L respectively.
Recently a systematic analysis of Kraft lignin dissolution in alkaline-based aqueous solvents was performed. Among the many conclusions, it was found that increasing the alkali concentration directly improves the solubility of Kraft lignin [19]. This has also been supported by a previous study, which concludes that the lignin solubility depends not only on the relative concentration of the base in the solution but also on its absolute concentration [47]. Similar behavior of HL can be seen in this study up to 0.1 NaOH/HL ratio, however at higher ratios, a fraction of HL remains insoluble, thus seemingly independent of NaOH concentration.
While US-assisted solubility of HL shows the same tendency as without US, there was an increase in the maximal HL solubility, up to 90 %, it should be noted such solubility is not achieved under extended conventional mixing time or at higher temperatures. It can be seen from Table 1 that, at 100 S/L the solubility of HL at 1 wt% NaOH solution without US is equal to the solubility at 0.75 wt% NaOH with US, 77.8 and 78.1 g/l respectively, thus reducing the amount of NaOH by a quarter. Interestingly, the absolute solubility of HL under US increases on average by the same amount, 10.4 and 15.0 g/l in 100 and 150 S/L, regardless of the increase in NaOH concentration. This seems to suggest that US affects mainly a certain fraction of HL.
3.2. Dissolution dynamics under ultrasonic treatment
To better understand the US effect on HL dissolution process, the solubility enhancement during the irradiation was determined. Samples were taken at time intervals from HL – NaOH solution mixtures at 100 and 150 S/L and the corresponding concentrations of HL (g/l) in solutions with US (CUS) and without sonication (Cnon) were determined by UV–vis spectroscopy. The results are shown in Table 2S and 3S. The ratio of the respective concentrations (CUS/Cnon) was considered as the effect of US on solubility enhancement during sonication (Fig. 3; Fig. 3S and 4S). Numerical values of CUS/Cnon can be found in Table 4S.
Fig. 3.
Improvement of HL solubilization (CUS/Cnon) in NaOH solutions at 0.5, 0.75, 1 and 2 wt% with 60 min of sonication (150 S/L ratio at 25 °C).
As can be seen in Fig. 3 the CUS/Cnon is irradiation time-dependent. In all NaOH solutions a significant increase in HL dissolution is observed after 10 min sonication. The maximum CUS/Cnon at 2 wt% NaOH is reached at 30 min, no significant increase occurs during further processing. However, in dilute NaOH solutions, lignin continues to dissolve uniformly over time with sonication. It could be concluded that a fraction of HL, which is insoluble even at a high NaOH concentration (2 wt%), is dissolved in all NaOH solutions within a short period of US irradiation. It is essential to note that this did not happen in pure water. Nevertheless, in dilute NaOH solutions, solubility of HL increases under further sonication. The CUS/Cnon reaches 30 % in 0.5 wt% NaOH solution after irradiation for 60 min.
3.3. Lignin recovery from NaOH solution – Acid precipitation
The acid precipitation of lignin with H2SO4 is a standard method for lignin recovery from black liquor in the Kraft process [[48], [49], [50]]. For optimal precipitation, H2SO4 is added until the pH is 2, which allows solid lignin to be separated from the solution without other impurities.
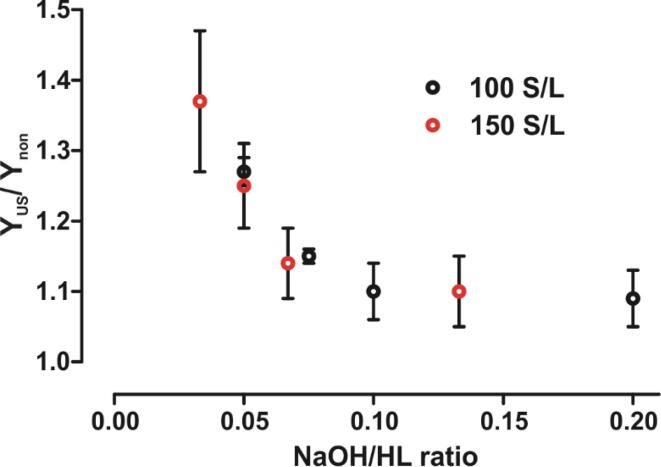
Acid-precipitated lignin (APL) was yielded from dissolved HL solutions obtained with and without US treatment. APL yields (mass of recovered APL from corresponding solution, g/l) from various NaOH solutions at 100 and 150 S/L are given in detail in Table 1S. In Fig. 4 the effect of US on the APL yield (YUS/ Ynon), as a ratio of corresponding yields from solutions treated with (YUS, g/l) and without US (Ynon, g/l), are plotted against the initial NaOH/HL ratio.
Fig. 4.
The US effect on enhancement of APL yield (YUS/ Ynon) vs the NaOH/HL ratio in solubilization of HL at 25 °C (60 min of sonication). Filled black circles indicate data from 100 S/L and empty red circles for 150 S/L.
As seen in Fig. 4, YUS is considerably higher compared to Ynon. The YUS/ Ynon increased up to 37 % at 0.5 wt% NaOH and at 150 S/L. The Y corresponds to the solubility results obtained in the dissolution process by US. It can be concluded that US primarily affects the dissolution of lignin molecules rather than cellulose or other polymeric impurities. Even with 2 wt% NaOH solution, the addition of US during solubilization leads to a 10 % increase in YUS/Ynon. The added H2SO4 for lignin precipitation is in proportion to the NaOH used in the dissolution, so the US treatment could significantly reduce the consumption of chemicals.
3.4. The mode of ultrasound affecting HL solubility – Size exclusion chromatography
Lignin, like other polymers, can be characterized as a combination of its average molar masses (number-average, Mn and weight-average, Mw) and polydispersity (Mn/Mw; PDI), which can provide information on the molar mass distribution of lignin, where the molecules have various degrees of polymerization [51]. SEC has been used for the characterization of different technical lignins [51], soluble and insoluble lignin fractions from solvent fractionation [52] and the effects of US on Kraft lignin in solutions [34].
The average values of the Mn, Mw and PDI of the dissolved HL fractions in NaOH solutions with and without US treatment are given in the Table 5S (100 S/L) and 6S (150 S/L). In order to better understand the HL dissolution process with and without US, Mn, Mw and PDI of the dissolved HL fractions were plotted as a function of the ratio of NaOH/HL (Fig. 5). As can be seen in Fig. 5a, Mn of dissolved HL fractions without US treatment increases linearly from 8.1 to 12.2 kDa with an increase of NaOH/HL ratio. However, Fig. 5b shows that Mw seems to increase, ranging from 77.2 to 88 kDa, up to 0.1 NaOH/HL ratio, but does not increase further. These data are consistent with the maximum solubility data illustrated in Fig. 2. Consequently, the PDI decreases progressively with increasing NaOH concentration in the mixture, which decreases from approximately 10 to 7.2, even after the maximum dissolution has been achieved at 0.1 of NaOH/HL ratio, as shown in Fig. 5c. A lower PDI means a narrower molecular size distribution and therefore a more homogeneous material. Lignin molecules in NaOH solution are assumed to be dissolved in a colloid-like form, where the weakly acidic phenolic OH groups are dissociated and the negatively charged lignin molecules are associated with sodium as the counter ion [53]. Thus, extra dissociation of OH groups caused by increased NaOH concentration could lead to the dissolution of larger HL molecules. The lignin dissolution with strong bases, like KOH and NaOH, can also involve its degradation [9]. However, no depolymerization of HL can be seen during solubilization.
Fig. 5.
The values of Mn, Mw and PDI of dissolved HL fractions with and without US treatment as a function of NaOH/HL ratio. Orange rectangles denote US-treated, whereas black circles are for non-US. a) Mn; b) Mw; c) PDI (Mw/Mn).
A significant increase in Mn and Mw of dissolved HL fractions obtained with US compared to conventional mixing, Fig. 5 (a; b). Mn of dissolved fractions with US increases almost linearly from 12.4 to 15.2 kDa and corresponding Mn of fractions is averagely 30 % higher compared to non-US samples. The most considerable change in Mw of dissolved HL is at low NaOH concentrations, ranging from about 80 kDa non-US to 100 kDa with US. The Mw varies insignificantly in different NaOH solutions under US. At higher NaOH/HL ratios, of course, the solubility of HL has already reached its maximum. However, it can be seen that the shift in the Mw of dissolved HL under sonication is in the same direction as the effect of US on solubility at different NaOH/HL ratios, seen in Fig. 4.
The molecular weight distribution (MWD) of 150 S/L fractions from 0.5 wt% and 2 wt% NaOH solutions are illustrated in Fig. 6a, the distribution is shifted towards higher MW with increased NaOH concentration. Ultrasonic treatment introduces a certain fraction of lignin molecules with a very high MW to the solution, illustrated in Fig. 6b. This applies to all NaOH/HL ratios obtained with sonication. Considering that at lower NaOH/HL ratio, the average soluble HL fraction consists mainly of the lower MW molecules and US significantly increases the solubility of relatively large molecules. This fraction solubilizes with 10 min of sonication, which may explain the jump in solubility at the same irradiation time as seen in Fig. 3.
Fig. 6.
Molecular weight distributions (MWD) of dissolved HL fractions treated with and without US at solid-to-liquid ratios 150 S/L: normalized signal at 280 nm in correlation to the molecular weight on a logarithmic scale in Da. a) HL fractions dissolved in 0.5 and 2 wt% of NaOH solutions without US b) MWD of HL fractions dissolved in 0.5 wt% of NaOH solution treated with and without US.
Previous reports have demonstrated that the mechanoacoustic effects dominate delignification of biomass at lower US frequencies [[23], [54]]. In the present work, the increased lignin solubility can be attributed to physical US effects - as a result of improved erosion and accessibility of insoluble residual lignocellulose, by presumably breaking down lignin-cellulose or lignin-lignin complexes [[23], [26], [54]].
However, increased molecular weight of dissolved HL in solutions imply the possibility of condensation reactions. Different studies have found that during US pretreatment of lignocellulose, lignin condensation reactions can occur [55] and for example the molecular weight of dissolved Kraft lignin increased in solution during US treatment [34].
Thus, homogeneous HL solutions with various NaOH concentrations, produced by conventional stirring and removal of the insoluble fraction, were treated with US for 60 min. As seen in Fig. 7, no increase in Mw was detected. In contrast, HL degradation occurs during sonication as Mw drops on average from about 80 to 65 kDa in all NaOH solutions. While there is a decrease in the Mn, it is relatively insignificant (Fig. 5S). This could indicate that radically induced homolytic cleavage of linkages occurs in solution, as observed previously [[37], [56]]. Indeed, the degradation of lignin in the solution could affect the overall solubility, but at the same time, in the dissolution process, the heterogeneous insoluble solid can adsorb a part of the US energy.
Fig. 7.

Decrease in Mw of HL in homogeneous 0.5, 0.75, 1 and 2 % of NaOH solutions during 60 min sonication at 20 kHz and at 25° C.
The results of SEC analysis suggest the presence of different US effects in the solubilization process of HL. Firstly, the mechanoacoustic effects dramatically increases the dissolution of larger molecules through microjet formation for example. Secondly, sonochemical degradation of lignin continues throughout the dissolution process in the corresponding solution (compare Fig. 3, Fig. 5 and Fig. 7).
3.5. Structural characterization of dissolved hydrolysis lignin
3.5.1. Determination of structural changes in dissolved hydrolysis lignin via 2D-HSQC NMR
The possible structural changes of the dissolved HL fractions resulting from NaOH solutions with and without ultrasonic irradiation were analyzed with state-of-the-art NMR methods. From the selected conditions, the corresponding APL was analyzed with HSQC as well as 31P NMR [[35], [37], [44], [45]].
The distribution of the main cross-signals in the 2D-HSQC NMR spectrum of APL can be found in Fig. 1S. The HSQC NMR of the HL used in this study shows a typical bias of hardwood lignin [57] towards the S units and a high S/G ratio (subunit structure shown on Fig. 1). Here S-units are highly present at 78–81 % and G units at 15–18 %, while H-units are found only in low amounts 3–4 % (Table 3). Although the changes in the structure of APL are minimal at different NaOH contents and with sonication, however a particular trend can be observed. Alkali input affects the solubility and the monomeric composition of APL without US, where the S/G ratio is 6 and 5, at 0.5 wt% and 2 wt% NaOH respectively. Thus, it can be concluded that a fraction of HL rich in G subunits is solubilized with higher NaOH concentration. A similar phenomenon occurs with the US-treated 0.5 wt% NaOH mixtures, where the increase in solubility is comparable to the high NaOH solution without US and G units are more represented in APL. As the content of G units increases from 15 % to 18 % with US, the S/G ratio is shifted from 6 to 4 (Table 3). At 2 wt% NaOH, the US treatment shows no measurable change in the S/G ratio; however, the solubility has already reached its maximum under the action of NaOH, thus US has little effect.
Table 3.
Monomeric ratios and ratios of inter-unit linkages of the APL isolated from HL dissolved at 150 S/L ratio in of NaOH solutions (0.5 and 2 %) treated with and without US (60 min), estimated on the basis of cross-peak signal integrals of the respective 2D-HSQC NMR spectra. Monomeric compositions are calculated via C2,6-H2,6 and the inter-unit linkages via Cα-Hα.
| NaOH (wt%) |
Monomeric ratio (%) | S/G ratio |
Inter-unit linkages (%) | |||||
|---|---|---|---|---|---|---|---|---|
| G | S | H | β-O-4 | β-5 | β-β | |||
| 0.50 | Non-US | 15 | 81 | 4 | 6 | 62 | 6 | 32 |
| US | 18 | 78 | 4 | 4 | 61 | 6 | 33 | |
| 2.00 | Non-US | 17 | 80 | 3 | 5 | 52 | 7 | 41 |
| US | 17 | 80 | 3 | 5 | 52 | 7 | 41 | |
The inter-unit linkages in dissolved HL with and without US in alkali mixtures are shown in Table 3 and are illustrated in Fig. 1S. The β-O-4 bonds are the most present linkage type in dissolved HL from 62 % to 52 %, at 0.5 wt% and 2 wt% NaOH respectively. The difference here suggests that the HL soluble at lower NaOH has higher abundance of the polar β-O-4 bonds and the fraction soluble at higher NaOH contains more β-β bonds. The possibility of β-O-4 cleavage via NaOH catalysis is also present at higher alkali concentrations, which could lead to the formation of new β-β via condensation reactions [[58], [59]]. Presumably, the depolymerization of HL enhances the dissolution and polycondensation causes precipitation. However, as shown above, the relative Mw increased with higher NaOH content (Fig. 4), as well as the solubility of HL (Fig. 1) and achieved equilibrium at a ratio of 0.1 NaOH/HL.
Surprisingly, US has an insignificant effect on the amount of β-O-4 and β-β linkages of dissolved HL, although solubility increases compared to the non-US samples (Table 1, Table 2). Still, in NaOH solutions, the more HL is solubilized and thus affects the abundance of different linkages. However, irradiation of homogenous HL solutions (Fig. 6), there was a noticeable decrease in β-O-4 bonds from 55 % to 50 % (Table 7S). This decrease in β-O-4 bonds can explain the decrease in Mw of HL in solutions under US treatment (Fig. 6), which reflects the depolymerization of HL. The effect of US on degradation of HL in homogeneous solutions will be discussed in more depth in our following article.
3.5.2. Determination of hydroxyl group content in hydrolysis lignin fractions by 31P NMR
The solubility of Kraft lignin in water is determined by the abundance of OH groups enhancing the hydrophilicity [60] and its dissolution in alkali bases on the acid-base properties of the dissolved fractions determined by the phenolic groups [47]. The 31P NMR spectral shifts allow the differentiation of aromatic and aliphatic hydroxyl groups and carboxylic acid groups [45].
Quantitative 31P NMR experiments were performed with phosphitylated crude HL (washed with water) and APL samples precipitated from 0.5, 1 and 2 wt% NaOH solutions of 150 S/L with mechanical stirring and treated with US. Crude HL was washed with water to avoid overlapping OH groups of free sugars. However, the washed crude HL was only partially soluble (only the soluble fraction was used) in the phosphitylation reaction, so the complete determination of all the OH groups is restricted. All APL samples were fully soluble in the phosphitylation process. These data are summarized in Table 4 and more detailed results are given in Table 8S. As can be seen in Table 4, crude HL should be more similar to the native lignin structure, exhibit lower phenolic OH and higher aliphatic OH content and likely contain more aryl alkyl ethers (Fig. 1).
Table 4.
Hydroxyl content in HL fractions dissolved in selected NaOH solutions with and without US treatment at 150 S/L ratio as determined by quantitative 31P NMR.
| Functional groups |
Non-US NaOH (wt%) |
US NaOH (wt%) |
|||||
|---|---|---|---|---|---|---|---|
| (OH, mmol/g) | HL | 0.50 | 1.00 | 2.00 | 0.50 | 1.00 | 2.00 |
| Aliphatic | 1.44 | 2.08 | 1.95 | 1.74 | 2.04 | 2.12 | 1.92 |
| Phenolic | 1.25 | 2.37 | 2.30 | 1.90 | 2.48 | 2.49 | 2.11 |
| Carboxylic acid | 0.06 | 0.11 | 0.07 | 0.07 | 0.10 | 0.11 | 0.10 |
| Total | 2.75 | 4.56 | 4.32 | 3.71 | 4.62 | 4.72 | 4.13 |
It was found that lower NaOH concentration selectively dissolves smaller and hydrophilic HL molecules (Fig. 6a) rich in OH groups. However, an increase in the alkali concentration leads to the dissolution of the larger molecules with the lower total content of OH groups, as seen in Table 4. The aliphatic OH groups decrease from 2.08 mmol/g at 0.5 wt% NaOH to 1.74 mmol/g at 2 wt% NaOH. The same tendency occurs with the phenolic groups, from 2.37 mmol/g to 1.90 mmol/g, respectively.
US treatment shows relatively little change in the linkages of dissolved HL (Table 3). However, compared to the conventional mixing the OH groups in dissolved HL are drastically increased. There is an overall increase in the amount of OH groups, up to 0.42 mmol/g (ca 10 % increase). The increase could be due to the mechanoacoustic disruption of possible lignin-cellulose complexes, where the high MW lignin molecules, rich in OH groups, are hydrogen bonded to the cellulose residues or molecularly integrated with the cellulose residue.
Another aspect is the cleavage of β-O-4 in solution via the sonochemical reactions with cavitation induced radicals, yielding phenolic or aliphatic OH groups. The concentration of aliphatic and aromatic OH groups increased with US treatment of homogeneous solution (Table 9S). Of course, OH• radicals produced sonolytically from water can also react directly with the aromatic rings of HL. However, the highest US solubility effect is found at low NaOH/HL ratios, but here the abundance of OH groups remains similar for US and non-US fractions (Table 4). This could indicate the dominance of mechanoacoustic effects on HL solubility at low NaOH/HL ratios. Still it can be concluded that higher abundance of OH groups in lignin is generally behind the higher solubility.
4. Conclusions
In order to achieve maximal solubility of HL in water, strong alkali conditions are commonly used in the industry, followed by acid precipitation to obtain purified HL. For the first time, this work describes the use of US (20 kHz) to enhance the solubility and purification of industrially produced birch HL under alkaline conditions. Dissolution of HL was correlated to the increase of NaOH concentration in the solution and the maximum solubility of HL was slightly below 80 wt% at 0.1 NaOH/HL ratio. However, US treatment enhanced the maximum solubility of HL up to 90 wt%. It was shown that the sonication effect on the solubility of HL was up to 30 wt% higher at low ratios of NaOH/HL (0.03–0.1) compared to conventional mixing. In addition, the lignin recovery yield with acid precipitation was found to be 37 % higher with US treatment at low ratio of NaOH/HL. Thus, leading to novel HL valorization approaches through lower chemical load and milder conditions for processing. Equal lignin yield can be achieved with US by using 25 % less NaOH during the solubilization process, while also reducing the required amount of acid for purified lignin precipitation.
The soluble HL fractions obtained with US treatment in all NaOH solutions, were characterized by increased Mw, due to larger lignin molecules from the solid phase being released into the solution, which was attributed to the mechanical effect of US. No polycondensation occurred under these conditions, in contrast sonication of homogeneous HL alkali solution results in the decrease of Mw. This was attributed to radically induced homolytic cleavage of HL inter-unit linkages, as evidenced by the reduction of β-O-4 bonds. The generation of oxidizing free radicals in solution caused by cavitation was supported by the increase of OH groups in the soluble lignin structure. Thus, both mechanical and sonochemical solubilization pathways contribute to the dissolution of HL in aqueous alkali solutions.
US technology offers high potential in combination with novel pretreatment technologies to enhance the biorefinery processes sustainability and introduce the new bioproducts in multiple ways in the valorization of forestry biomass.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by ERDF and the Estonian Research Council via project RESTA6 and RESTA22, as well as by LignoCOST (European Cooperation in Science and Technology).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2022.106288.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Garlapati V.K., Chandel A.K., Kumar S.P.J., Sharma S., Sevda S., Ingle A.P., Pant D. Circular economy aspects of lignin: Towards a lignocellulose biorefinery. Renew. Sustain. Energy Rev. 2020;130 doi: 10.1016/j.rser.2020.109977. [DOI] [Google Scholar]
- 2.Matsakas L., Nitsos C., Raghavendran V., Yakimenko O., Persson G., Olsson E., Rova U., Olsson L., Christakopoulos P. A novel hybrid organosolv: Steam explosion method for the efficient fractionation and pretreatment of birch biomass. Biotechnol. Biofuels. 2018;11:1–14. doi: 10.1186/s13068-018-1163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Padilha C.E.d.A., Nogueira C.d.C., Alencar B.R.A., de Abreu Í.B.S., Dutra E.D., Ruiz J.A.C., Souza D.F.d.S., dos Santos E.S. Production and Application of Lignin-Based Chemicals and Materials in the Cellulosic Ethanol Production: An Overview on Lignin Closed-Loop Biorefinery Approaches. Waste Biomass Valorizat. 2021;12(12):6309–6337. [Google Scholar]
- 4.Cao L., Yu I.K.M., Liu Y., Ruan X., Tsang D.C.W., Hunt A.J., Ok Y.S., Song H., Zhang S. Lignin valorization for the production of renewable chemicals: State-of-the-art review and future prospects. Bioresour. Technol. 2018;269:465–475. doi: 10.1016/j.biortech.2018.08.065. [DOI] [PubMed] [Google Scholar]
- 5.Balakshin M.Y., Capanema E.A., Sulaeva I., Schlee P., Huang Z., Feng M., Borghei M., Rojas O.J., Potthast A., Rosenau T. New Opportunities in the Valorization of Technical Lignins. ChemSusChem. 2021;14:1016–1036. doi: 10.1002/cssc.202002553. [DOI] [PubMed] [Google Scholar]
- 6.Lee S.Y., Kim H.U., Chae T.U., Cho J.S., Kim J.W., Shin J.H., Kim D.I., Ko Y.S., Jang W.D., Jang Y.S. A comprehensive metabolic map for production of bio-based chemicals. Nat. Catal. 2019;2:18–33. doi: 10.1038/s41929-018-0212-4. [DOI] [Google Scholar]
- 7.Ahvazi B., Cloutier É., Wojciechowicz O., Ngo T.D. Lignin Profiling: A Guide for Selecting Appropriate Lignins as Precursors in Biomaterials Development. ACS Sustain. Chem. Eng. 2016;4:5090–5105. doi: 10.1021/acssuschemeng.6b00873. [DOI] [Google Scholar]
- 8.Poveda-Giraldo J.A., Solarte-Toro J.C., Cardona Alzate C.A. The potential use of lignin as a platform product in biorefineries: A review. Renew. Sustain. Energy Rev. 2021;138 doi: 10.1016/j.rser.2020.110688. [DOI] [Google Scholar]
- 9.Chio C., Sain M., Qin W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019;107:232–249. doi: 10.1016/j.rser.2019.03.008. [DOI] [Google Scholar]
- 10.Ragauskas A.J., Beckham G.T., Biddy M.J., Chandra R., Chen F., Davis M.F., Davison B.H., Dixon R.A., Gilna P., Keller M., Langan P., Naskar A.K., Saddler J.N., Tschaplinski T.J., Tuskan G.A., Wyman C.E. Lignin valorization: Improving lignin processing in the biorefinery. Science. 2014;80:344. doi: 10.1126/science.1246843. [DOI] [PubMed] [Google Scholar]
- 11.Tuck C.O., Pérez E., Horváth I.T., Sheldon R.A., Poliakoff M. Valorization of Biomass: Deriving More Value from Waste. Science. 2012;337(6095):695–699. doi: 10.1126/science.1218930. [DOI] [PubMed] [Google Scholar]
- 12.Li T., Takkellapati S. The current and emerging sources of technical lignins and their applications, Biofuels. Bioprod. Biorefining. 2018;12:756–787. doi: 10.1002/bbb.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Svensson I., Roncal T., De Winter K., Van Canneyt A., Tamminen T., Mikkelson A., Barrio A. Valorisation of Hydrolysis Lignin Rest From Bioethanol Pilot Plant: Process Development and Upscaling. Ind. Crops Prod. 2020;156 doi: 10.1016/j.indcrop.2020.112869. [DOI] [Google Scholar]
- 14.A. Vishtal, A. Kraslawski, Challenges in industrial applications of technical lignins, BioResources. 6 (2011) 3547–3568. https://doi.org/10.15376/BIORES.6.3.3547-3568.
- 15.Cho J., Chu S., Dauenhauer P.J., Huber G.W. Kinetics and reaction chemistry for slow pyrolysis of enzymatic hydrolysis lignin and organosolv extracted lignin derived from maplewood. Green Chem. 2012;14:428–439. doi: 10.1039/c1gc16222e. [DOI] [Google Scholar]
- 16.Xu C.C., Dessbesell L., Zhang Y., Yuan Z. Lignin valorization beyond energy use: has lignin’s time finally come? Biofuels Bioprod. Biorefining. 2021;15:32–36. doi: 10.1002/bbb.2172. [DOI] [Google Scholar]
- 17.Rashid T., Sher F., Rasheed T., Zafar F., Zhang S., Murugesan T. Evaluation of current and future solvents for selective lignin dissolution–A review. J. Mol. Liq. 2021;321 doi: 10.1016/j.molliq.2020.114577. [DOI] [Google Scholar]
- 18.García A., Erdocia X., González Alriols M., Labidi J. Effect of ultrasound treatment on the physicochemical properties of alkaline lignin. Chem. Eng. Process. Process Intensif. 2012;62:150–158. doi: 10.1016/j.cep.2012.07.011. [DOI] [Google Scholar]
- 19.Melro E., Filipe A., Sousa D., Valente A.J.M., Romano A., Antunes F.E., Medronho B. Dissolution of kraft lignin in alkaline solutions. Int. J. Biol. Macromol. 2020;148:688–695. doi: 10.1016/j.ijbiomac.2020.01.153. [DOI] [PubMed] [Google Scholar]
- 20.Salmar S., Cravotto G., Tuulmets A., Hagu H. Effect of Ultrasound on the Base-Catalyzed Hydrolysis of 4-Nitrophenyl Acetate in Aqueous Ethanol. J. Phys. Chem. B. 2006;110(11):5817–5821. doi: 10.1021/jp057405w. [DOI] [PubMed] [Google Scholar]
- 21.A. Tuulmets, S. Salmar, J. Järv, Sonochemistry in Water Organic Solutions, Novinka/Nova Science Publishers, 2010. https://books.google.com/books/about/Sonochemistry_in_Water_Organic_Solutions.html?hl=et&id=evvOcQAACAAJ (vaadatud 29. september 2022).
- 22.Flores E.M.M., Cravotto G., Bizzi C.A., Santos D., Iop G.D. Ultrasound-assisted biomass valorization to industrial interesting products: state-of-the-art, perspectives and challenges. Ultrason. Sonochem. 2021;72 doi: 10.1016/j.ultsonch.2020.105455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bussemaker M.J., Zhang D. Effect of ultrasound on lignocellulosic biomass as a pretreatment for biorefinery and biofuel applications. Ind. Eng. Chem. Res. 2013;52:3563–3580. doi: 10.1021/ie3022785. [DOI] [Google Scholar]
- 24.Ranjan A., Singh S., Malani R.S., Moholkar V.S. Ultrasound-assisted bioalcohol synthesis: Review and analysis. RSC Adv. 2016;6:65541–65562. doi: 10.1039/c6ra11580b. [DOI] [Google Scholar]
- 25.Cui R., Zhu F. Ultrasound modified polysaccharides: A review of structure, physicochemical properties, biological activities and food applications. Trends Food Sci. Technol. 2021;107:491–508. doi: 10.1016/j.tifs.2020.11.018. [DOI] [Google Scholar]
- 26.Yan D., Ji Q., Yu X., Li M.o., Abiola Fakayode O., Yagoub A.E.A., Chen L.i., Zhou C. Multimode-ultrasound and microwave assisted natural ternary deep eutectic solvent sequential pretreatments for corn straw biomass deconstruction under mild conditions. Ultrason. Sonochem. 2021;72:105414. doi: 10.1016/j.ultsonch.2020.105414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bundhoo Z.M.A., Mohee R. Ultrasound-assisted biological conversion of biomass and waste materials to biofuels: A review. Ultrason. Sonochem. 2018;40:298–313. doi: 10.1016/j.ultsonch.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 28.Mason T.J. Ultrasound in synthetic organic chemistry. Chem. Soc. Rev. 1997;26:443–451. doi: 10.1039/cs9972600443. [DOI] [Google Scholar]
- 29.Athanassiadis A.G., Ma Z., Moreno-Gomez N., Melde K., Choi E., Goyal R., Fischer P. Ultrasound-Responsive Systems as Components for Smart Materials. Chem. Rev. 2022;122(5):5165–5208. doi: 10.1021/acs.chemrev.1c00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koutsianitis D., Mitani C., Giagli K., Tsalagkas D., Halász K., Kolonics O., Gallis C., Csóka L. Properties of ultrasound extracted bicomponent lignocellulose thin films. Ultrason. Sonochem. 2015;23:148–155. doi: 10.1016/j.ultsonch.2014.10.014. [DOI] [PubMed] [Google Scholar]
- 31.Li M.F., Sun S.N., Xu F., Sun R.C. Ultrasound-enhanced extraction of lignin from bamboo (Neosinocalamus affinis): Characterization of the ethanol-soluble fractions. Ultrason. Sonochem. 2012;19:243–249. doi: 10.1016/j.ultsonch.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 32.García A., Alriols M.G., Llano-Ponte R., Labidi J. Ultrasound-assisted fractionation of the lignocellulosic material. Bioresour. Technol. 2011;102:6326–6330. doi: 10.1016/j.biortech.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 33.G. Wegener, D. Fengel, Rapid ultrasonic isolation of milled wood lignins. fractionation and degradation experiments., tappi. 62 (1979) 97–100.
- 34.Wells T., Kosa M., Ragauskas A.J. Polymerization of Kraft lignin via ultrasonication for high-molecular- weight applications. Ultrason. Sonochem. 2013;20(6):1463–1469. doi: 10.1016/j.ultsonch.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Zikeli F., Vinciguerra V., D’Annibale A., Capitani D., Romagnoli M., Scarascia Mugnozza G. Preparation of lignin nanoparticles from wood waste for wood surface treatment. Nanomaterials. 2019;9(2):281. doi: 10.3390/nano9020281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gilca I.A., Popa V.I., Crestini C. Obtaining lignin nanoparticles by sonication. Ultrason. Sonochem. 2015;23:369–375. doi: 10.1016/j.ultsonch.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 37.Liu B., Du B., Sun Y., Zhu M., Yang Y., Wang X., Zhou J. Ultrasound acoustic cavitation enhances depolymerization of organosolv lignin to phenolic monomers and low molecular weight lignin bio-oils. Fuel Process. Technol. 2020;203 doi: 10.1016/j.fuproc.2020.106387. [DOI] [Google Scholar]
- 38.Dias R.M., Petrin L.C.G., Sosa F.H.B., da Costa Lopes A.M., Coutinho J.A.P., da Costa M.C. Investigation of Kraft Lignin Solubility in Protic Ionic Liquids and Their Aqueous Solutions. Ind. Eng. Chem. Res. 2020;59:18193–18202. doi: 10.1021/acs.iecr.0c02605. [DOI] [Google Scholar]
- 39.D. Meroni, R. Djellabi, M. Ashokkumar, C.L. Bianchi, D.C. Bo, Sonoprocessing : From Concepts to Large-Scale Reactors, (s.a.). Doi: 10.1021/acs.chemrev.1c00438. [DOI] [PubMed]
- 40.He Q., Ziegler-Devin I., Chrusciel L., Obame S.N., Hong L., Lu X., Brosse N. Lignin-First Integrated Steam Explosion Process for Green Wood Adhesive Application. ACS Sustain. Chem. Eng. 2020;8:5380–5392. doi: 10.1021/acssuschemeng.0c01065. [DOI] [Google Scholar]
- 41.Raud M., Krennhuber K., Jäger A., Kikas T. Nitrogen explosive decompression pre-treatment: An alternative to steam explosion. Energy. 2019;177:175–182. doi: 10.1016/j.energy.2019.04.071. [DOI] [Google Scholar]
- 42.Escobar E.L.N., da Silva T.A., Pirich C.L., Corazza M.L., Pereira Ramos L. Supercritical Fluids: A Promising Technique for Biomass Pretreatment and Fractionation. Front. Bioeng. Biotechnol. 2020;8:1–18. doi: 10.3389/fbioe.2020.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramos L.P., Suota M.J., Pavaneli G., Corazza M.L. The role of biomass pretreatment for sustainable biorefineries. Bulg. Chem. Commun. 2019;51:70–76. doi: 10.34049/bcc.51.B.015. [DOI] [Google Scholar]
- 44.Agarwal A., Jo Y.T., Park J.H. Hybrid microwave-ultrasound assisted catalyst-free depolymerization of Kraft lignin to bio-oil. Ind. Crops Prod. 2021;162 doi: 10.1016/j.indcrop.2021.113300. [DOI] [Google Scholar]
- 45.Meng X., Crestini C., Ben H., Hao N., Pu Y., Ragauskas A.J., Argyropoulos D.S. Determination of hydroxyl groups in biorefinery resources via quantitative 31P NMR spectroscopy. Nat. Protoc. 2019;14:2627–2647. doi: 10.1038/s41596-019-0191-1. [DOI] [PubMed] [Google Scholar]
- 46.Fu C., Li Z., Sun Z., Xie S. A review of salting-out effect and sugaring-out effect: driving forces for novel liquid-liquid extraction of biofuels and biochemicals. Front. Chem. Sci. Eng. 2021;15:854–871. doi: 10.1007/s11705-020-1980-3. [DOI] [Google Scholar]
- 47.Evstigneev E.I. Factors affecting lignin solubility. Russ. J. Appl. Chem. 2011;84:1040–1045. doi: 10.1134/S1070427211060243. [DOI] [Google Scholar]
- 48.Zhu W., Theliander H. Precipitation of lignin from softwood black liquor: An investigation of the equilibrium and molecular properties of lignin. BioResources. 2015;10:1696–1714. doi: 10.15376/biores.10.1.1696-1715. [DOI] [Google Scholar]
- 49.Koljonen K., Ӧsterberg M., Kleen M., Fuhrmann A., Stenius P. Precipitation of lignin and extractives on kraft pulp: Effect on surface chemistry, surface morphology and paper strength. Cellulose. 2004;11:209–224. doi: 10.1023/B:CELL.0000025424.90845.c3. [DOI] [Google Scholar]
- 50.Olivares M., Guzmάn J.A., Natho A., Saavedra A. Kraft lignin utilization in adhesives. Wood Sci.Technol. 1988;22(2):157–165. [Google Scholar]
- 51.Constant S., Wienk H.L.J., Frissen A.E., De Peinder P., Boelens R., Van Es D.S., Grisel R.J.H., Weckhuysen B.M., Huijgen W.J.J., Gosselink R.J.A., Bruijnincx P.C.A. New insights into the structure and composition of technical lignins: A comparative characterisation study. Green Chem. 2016;18:2651–2665. doi: 10.1039/c5gc03043a. [DOI] [Google Scholar]
- 52.Goldmann W.M., Ahola J., Mikola M., Tanskanen J. Solubility and fractionation of Indulin AT kraft lignin in ethanol-water media. Sep. Purif. Technol. 2019;209:826–832. doi: 10.1016/j.seppur.2018.06.054. [DOI] [Google Scholar]
- 53.Kienberger M., Maitz S., Pichler T., Demmelmayer P. Systematic review on isolation processes for technical lignin. Processes. 2021;9(5):804. [Google Scholar]
- 54.Bussemaker M.J., Xu F., Zhang D. Manipulation of ultrasonic effects on lignocellulose by varying the frequency, particle size, loading and stirring. Bioresour. Technol. 2013;148:15–23. doi: 10.1016/j.biortech.2013.08.106. [DOI] [PubMed] [Google Scholar]
- 55.Niu M., Zhao G.-J., Alma M.H. Polycondensation reaction and its mechanism during lignocellulosic liquefaction by an acid catalyst: A review. For. Stud. China. 2011;13(1):71–79. [Google Scholar]
- 56.Yoshioka A., Seino T., Tabata M., Takai M. Homolytic scission of interunitary bonds in lignin induced by ultrasonic irradiation of MWL dissolved in dimethylsulfoxide. Holzforschung. 2000;54:357–364. doi: 10.1515/HF.2000.062. [DOI] [Google Scholar]
- 57.Balakshin M.Y., Capanema E.A. Comprehensive structural analysis of biorefinery lignins with a quantitative 13C NMR approach. RSC Adv. 2015;5:87187–87199. doi: 10.1039/c5ra16649g. [DOI] [Google Scholar]
- 58.Roberts V.M., Stein V., Reiner T., Lemonidou A., Li X., Lercher J.A. Towards quantitative catalytic lignin depolymerization. Chem. - A Eur. J. 2011;17:5939–5948. doi: 10.1002/chem.201002438. [DOI] [PubMed] [Google Scholar]
- 59.Ahmad Z., Mahmood N., Yuan Z., Paleologou M., Xu C. Effects of process parameters on hydrolytic treatment of black liquor for the production of low-molecular-weight depolymerized kraft lignin. Molecules. 2018;23(10):2464. doi: 10.3390/molecules23102464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hansen C.M., Björkman A. The Ultrastructure of Wood from a Solubility Parameter Point of View. Holzforschung. 1998;52:335–344. doi: 10.1515/hfsg.1998.52.4.335. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.