Abstract
Background
There is increasing evidence that 12-lead electrocardiograms (ECG) can be used to predict biological age, which is associated with cardiovascular events. However, the utility of artificial intelligence (AI)-predicted age using ECGs remains unclear.
Methods
Using a single-center database, we developed an AI-enabled ECG using 17 042 sinus rhythm ECGs (SR-ECG) to predict chronological age (CA) with a convolutional neural network that yields AI-predicted age. Using the 5-fold cross validation method, AI-predicted age deriving from the test dataset was yielded for all ECGs. The incidence by AgeDiff and the areas under the curve by receiver operating characteristic curve with AI-predicted age for cardiovascular events were analyzed.
Results
During the mean follow-up period of 460.1 days, there were 543 cardiovascular events. The annualized incidence of cardiovascular events was 2.24 %, 2.44 %, and 3.01 %/year for patients with AgeDiff < −6, −6 to ≤6, and >6 years, respectively. The areas under the curve for cardiovascular events with CA and AI-predicted age, respectively, were 0.673 and 0.679 (Delong’s test, P = 0.388) for all patients; 0.642 and 0.700 (P = 0.003) for younger patients (CA < 60 years); and 0.584 and 0.570 (P = 0.268) for older patients (CA ≥ 60 years).
Conclusions
AI-predicted age using 12-lead ECGs showed superiority in predicting cardiovascular events compared with CA in younger patients, but not in older patients.
Keywords: Electrocardiogram, Biological age, Cardiovascular event, Artificial intelligence
1. Introduction
Aging is a crucial factor associated with mortality and cardiovascular disease. Heart and vascular aging can cause organ dysfunction, especially in the heart, brain, and kidneys. Although chronological age (CA) has been used as a marker for decision-making regarding invasive treatment[1], [2], CA refers only to the time since birth. Aging can be heterogeneous, with a balance between exposure to damaging properties and resiliency[3], [4]. The gap between biological age and CA have been thought to reflect acceleration of epigenetic age due to the associations with a higher risk of all-cause mortality[5], [6], cardiovascular disease[7], [8], and cross-sectionally with obesity[9], earlier menopause[10], and frailty[11]. The concept of biological age was developed to represent the actual status of individual aging. However, the process of calculating biological age is complex[12], and a simple, non-invasive, and cost-effective method for estimating biological age is required for its practical use.
Electrocardiography (ECG) is widely used to detect or evaluate the risk of cardiac diseases. ECG can be performed readily and repeatedly and with low cost. Patient conditions, such as those in the circulatory and respiratory systems, can affect the ECG waveform, and aging is also a key factor that causes electrophysiological and electroanatomical changes in the ECG. Several studies have used ECG to predict biological age or “heart age”[13], [14], [15], [16], some of which reported models that examined only a small number of representative ECG parameters using a linear regression model[14], [17].
Recently, we reported a method of calculating biological age that incorporates hundreds of automatically-measured ECG parameters using the principal component analysis algorithm (PCA) and Klemera and Doubal’s method (KDM). Although this method of calculating biological age showed relatively good predictive capability for all-cause mortality (area under the curve [AUC] 0.657 to 0.731) in patients without structural heart disease[12], the ranges of the predicted age were too wide (min/max −5.31/132.97 years for PCA, −66.21/202.17 years and −44.94/178.58 for two methods by KDM), suggesting the possible limitation in using these methodologies or in using a large number of ECG parameters.
Recently, several reports have used artificial intelligence (AI) modeling to predict biological age[13], [16], [18]. This methodology uses the digital raw ECG waveform data, and the ranges of the predicted age was mostly similar to the actual age. One study reported that AI-predicted age was a good predictor of mortality; however, no studies have investigated the association between AI-predicted age and cardiovascular (CV) events. In this report, we constructed an age-prediction model using an AI algorithm derived from 12-lead ECG waveforms using a single hospital’s database, and evaluated the model’s predictive capability for CV events.
2. Methods
2.1. Ethics and informed consent
This study was performed in accordance with the Declaration of Helsinki (revised in 2013) and the Ethical Guidelines for Medical and Health Research Involving Human Subjects (Public Notice of the Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health, Labour and Welfare, Japan, issued in 2017). Written informed consent was obtained from all participants. The study protocol was approved by the Institutional Review Board of the Cardiovascular Institute.
2.2. Total study population
The Shinken Database [19] comprises all new patients visiting the Cardiovascular Institute, a specialized cardiology hospital in an urban area of Tokyo, Japan, excluding foreign travelers and patients with active cancer. This single hospital-based database was established in June 2004 to investigate the prevalence and prognosis of various types of CV diseases (CVDs). To investigate the new appearance of CVDs, patients who visited our hospital but who were not diagnosed with CVDs at baseline were also included in the cohort. The details of this database have been described elsewhere[19]. All attending physicians were cardiologists or cardiothoracic surgeons.
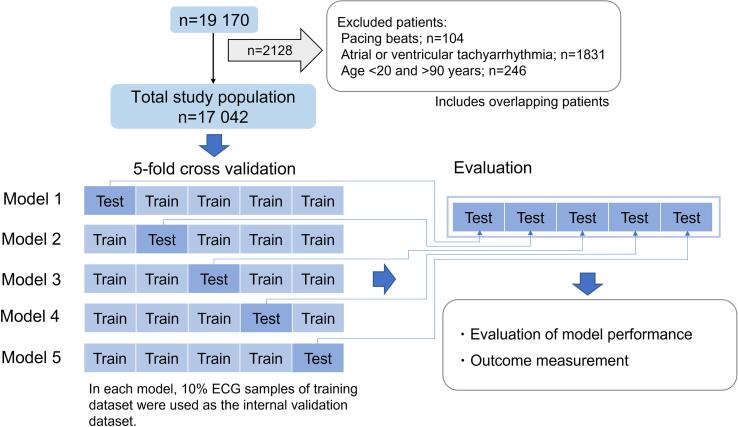
In the present study, data for 19 170 patients registered between February 2010 and March 2018 were extracted from the Shinken database because a computerized electrocardiogram database has been available since February 2010. After excluding patients aged < 20 years or > 90 years (n = 246), and those with pacing beats (n = 104), or atrial or ventricular tachyarrhythmia (n = 1831), 17 042 patients comprised the total study population (Fig. 1).
Fig. 1.
Study flow chart. CNN, convolutional neural network.
2.3. Development of the AI-enabled ECG to predict CA
2.3.1. Data sampling
A 12-lead ECG was recorded for 10 s in the supine position using an ECG machine (GE CardioSoft V6.71 and MAC 5500 HD; GE Healthcare, Chicago, IL, USA) at a sampling rate of 500 Hz. The raw data in the digital records were stored using the MUSE data management system (GE Healthcare).
Before we performed whole analyses in the present study, we have compared the performance of two models: a model with rhythm waveform and a model with median waveform, where the former used all records included in the 10-second ECG record and the latter extracted a median waveform (consisted of 500 dimensions) out of the 10-second ECG record. To construct the model, we used the waveforms of 8 leads (I, II, and V1–V6). Finally, we employed the median waveform because the model with median waveform showed better performance than that with rhythm waveform.
2.3.2. Dataset management
By ordinary, the total dataset is divided into training, internal validation, and testing datasets. But in this method, the number of data that can be used in the testing dataset is limited. In the present study, given the relatively small number of the CV event, as the labeled training data, we employed the fivefold cross validation method to enable all data to be included in the test dataset[20].
Dataset management with the fivefold cross validation method was performed as follows (Fig. 1). First, the dataset was randomly divided into five groups. Second, one of the five groups was set as the testing dataset, and the others were set as the training dataset, in which 10 % were used as the internal validation dataset. Third, the model was run 5 times using different combinations of training and testing datasets. Accordingly, model output was obtained from 5 testing datasets out of 5 different models, in which all data were included in the testing dataset once per each data.
2.3.3. CNN modeling
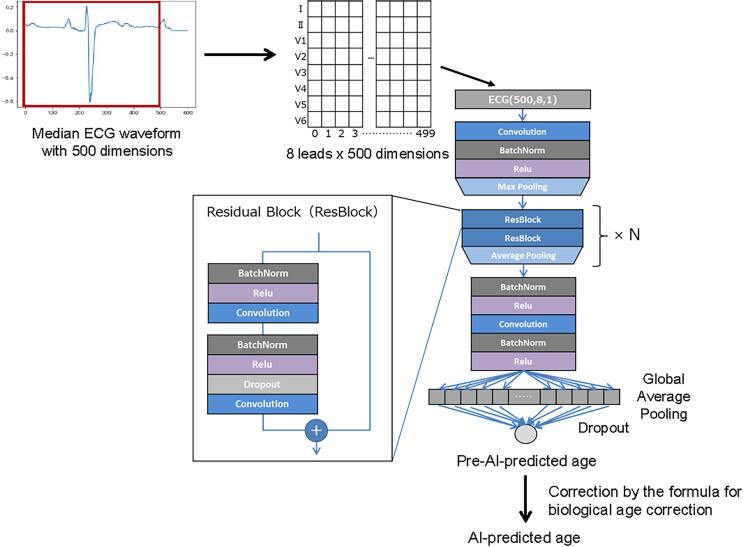
We constructed a CNN using the Keras Framework with a Tensorflow (Google; Mountain View, CA, USA) backend and Python (Python Software Foundation, Beaverton, OR, USA). The architecture of the CNN in the present study was inspired by previous studies[21], [22]. The CNN model had layers for a temporal axis and a lead axis[21]. The layers for the temporal axis comprised a convolution part and a residual part. The convolution part comprised a convolution layer, batch-normalization layer, a layer for non-linear Rectified Linear Unit (ReLU) activation, and a maximum pooling layer.[23] The residual part comprised a combination of two residual blocks based on Residual Network (ResNet; https://resnet.unl.edu/)[24] and average pooling, which was repeated N times, and the value of N was tuned to obtain the best performance (the method is outlined below). The layers for the lead axis comprised a paired batch-normalization layer and a layer for non-linear ReLU activation, followed by a convolution layer. Subsequently, a second paired batch-normalization layer and a layer for non-linear ReLU activation were added. Finally, the data were fed to a dropout layer with global average pooling and to the final output as a continuous value, which generated the pre-AI-predicted age. Subsequently, the AI-predicted age was obtained by correcting the pre-AI-predicted age using the following formula, which is generally applied for biological age[12], [25]: [AI-predicted age] = [pre-AI-predicted age] + (CA − average[CA])(1 − β), where β indicates the standardized coefficient in the univariate linear regression analysis in which pre-AI-predicted age and CA are the dependent and independent variables, respectively. The architecture of the model is shown in Fig. 2.
Fig. 2.
Convolutional neural network (CNN) model. The CNN algorithm was developed using the median waveform of 10-second ECG samples. ECG, electrocardiogram.
The model was trained on a computer with 192-GB RAM and a single Quadro P-2200 (NVIDIA, Santa Clara, CA, USA) graphics processing unit running Keras.
2.4. Evaluation of the predictive capability of AI-predicted age for CV event
2.4.1. Calculating the gap between AI-predicted age and CA
We defined AgeDiff as the gap between AI-predicted age and CA, which was calculated as follows: AgeDiff = AI-predicted age − CA. AgeDiff was categorized as < −X, −X to ≤ X, and > X years, where “X” is the mean absolute error between the AI-predicted age and CA.
2.4.2. Outcomes measurement
The primary outcome in the present study was the predicting capability of AI-predicted age for CV events, which comprised CV-related death, heart failure, acute coronary syndrome, ischemic stroke, aortic disease, and intracranial hemorrhage. The secondary outcome was the predicting capability of AI-predicted age for comorbidities related to CV events, such as hypertension, diabetes, heart failure, ischemic heart disease, valvular heart disease, cardiomyopathy, and atrial fibrillation.
2.5. Statistical analysis
First, the patient characteristics were summarized, with categorical and continuous data presented as number (%) and mean with standard deviation (SD), respectively. Second, as the primary outcome measure, the predicting capability of AI-predicted age for the incidence of CV events was evaluated as follows: i) the annualized overall incidence rate of CV events and the incidence for the three AgeDiff categories were described; ii) the cumulative incidence of CV events was depicted by Kaplan–Meier curves, and the statistical difference based on the AgeDiff categories was tested by the log rank test; iii) receiver operating characteristic analysis was performed to evaluate the AUC to predict CV events by AI-predicted age and by CA, and the statistical difference between the two ages was tested by the Delong test; and iv) the analyses of i)–iii) were also performed separately in younger and older populations (aged < 60 and ≥ 60 years, respectively). Third, as the secondary outcome measure, the predicting capability of AI-predicted age for the coexistence of comorbidities closely associated with CV events (CV-event-related comorbidities) was evaluated as follows: i) the overall prevalence of CV-event-related comorbidities and the prevalences in the three AgeDiff categories were described; ii) receiver operating characteristic analysis was performed to evaluate the AUC to predict each comorbidity by AI-predicted age and by CA, and the statistical difference between the ages was tested by the Delong test; and iii) the analyses of i) and ii) were also performed separately in younger and older populations. Statistical significance was set at two-sided P < 0.05. Statistical analyses were performed using SPSS version 28.0 (IBM Corp., Armonk, NY, USA), R version 4.0.3 (The RFoundation, Vienna, Austria), and Python version 3.7.6 (Python Software Foundation, DE, USA).
3. Results
3.1. Patient characteristics
The patients’ characteristics are shown in Table 1. The patients comprised 9983 men (58.6 %) and 7059 women (41.4 %), and the mean age was 57.7 (SD: 15.0) years. During the mean follow-up period of 460.1 (SD: 492.5) days, 543 CV events occurred, namely 50 CV-related deaths, 182 heart failure events, 148 acute coronary syndrome events, 57 ischemic strokes, 96 vascular disease-related events, and 34 intracranial hemorrhage events. The mean ages of the patients without and with CV events were 57.4 (SD: 15.0) years and 66.3 (SD: 12.7) years, respectively.
Table 1.
Patients' characteristics.
| Total n = 17 042 |
No CV events n = 16 499 |
CV events n = 543 |
|
|---|---|---|---|
| Age, years | 57.7 ± 15 | 57.4 ± 15 | 66.3 ± 12.7 |
| Male, n (%) | 9983 (58.6) | 9590 (58.1) | 393 (72.4) |
| Height, cm | 163.8 ± 9.4 | 163.8 ± 9.4 | 163.5 ± 9.3 |
| Weight, kg | 63.0 ± 15.5 | 63.0 ± 15.5 | 64.7 ± 15.4 |
| BMI, kg/m2 | 23.5 ± 13.3 | 23.4 ± 13.5 | 24.1 ± 4.7 |
| SBP, mmHg | 127.4 ± 19.2 | 127.3 ± 19.1 | 129.3 ± 22.8 |
| DBP, mmHg | 75.5 ± 14.7 | 75.5 ± 14.7 | 75.1 ± 14.2 |
| Hypertension, n (%) | 7333 (43.0) | 6940 (42.1) | 393 (72.4) |
| Dyslipidemia, n (%) | 5028 (29.5) | 4783 (29.0) | 245 (45.1) |
| Diabetes, n (%) | 2152 (12.6) | 1976 (12.0) | 176 (32.4) |
| Hyperuricemia, n (%) | 2516 (14.8) | 2361 (14.3) | 155 (28.5) |
| CKD, n (%) | 2584 (15.2) | 2346 (14.2) | 238 (43.8) |
| Anemia, n (%) | 447 (2.6) | 384 (2.3) | 63 (11.6) |
| eGFR, ml/min/1.73 cm2 | 71.9 ± 19.3 | 72.4 ± 19.0 | 61.3 ± 23.3 |
| LVEF, % | 66.3 ± 9.6 | 66.6 ± 9.1 | 57.6 ± 17.7 |
| Atrial fibrillation, n (%) | 1572 (9.2) | 1464 (8.9) | 108 (19.9) |
| Heart disease, n (%) | 4306 (25.3) | 3912 (23.7) | 394 (72.6) |
Data are presented as mean ± standard deviation unless otherwise stated.
CV, cardiovascular; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction.
3.2. Development of the algorithm for AI-predicted age
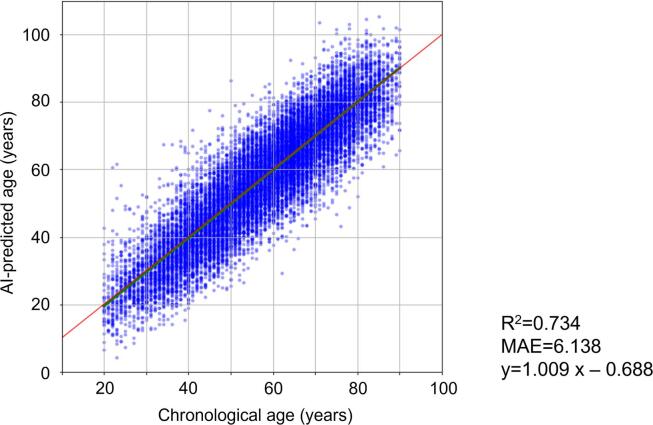
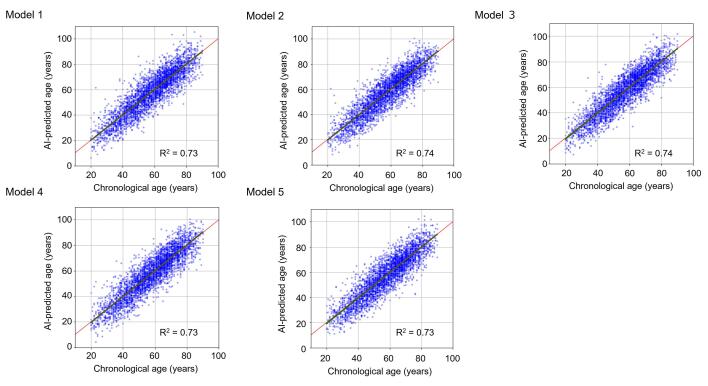
The algorithm for the AI-predicted age using CNN was developed in five different models (Fig. 1). The coefficient of determination (R2) for the prediction of CA using AI-predicted age in five models ranged between 0.73 and 0.74, and the mean absolute error between CA and AI-predicted age ranged between 6.03 and 6.20 (Supplementary Table 1, Supplementary Fig. 1). When five models were combined, R2 and mean absolute error were 0.73 (SD, 0.01) and 6.14 (SD, 0.07), respectively (Fig. 3).
Fig. 3.
AI-predicted age and chronological age. The association between AI-predicted age and chronological age was shown. AI, artificial intelligence; MAE, mean absolute error.
3.3. Primary outcome
3.3.1. Incidence of CV events according to AgeDiff
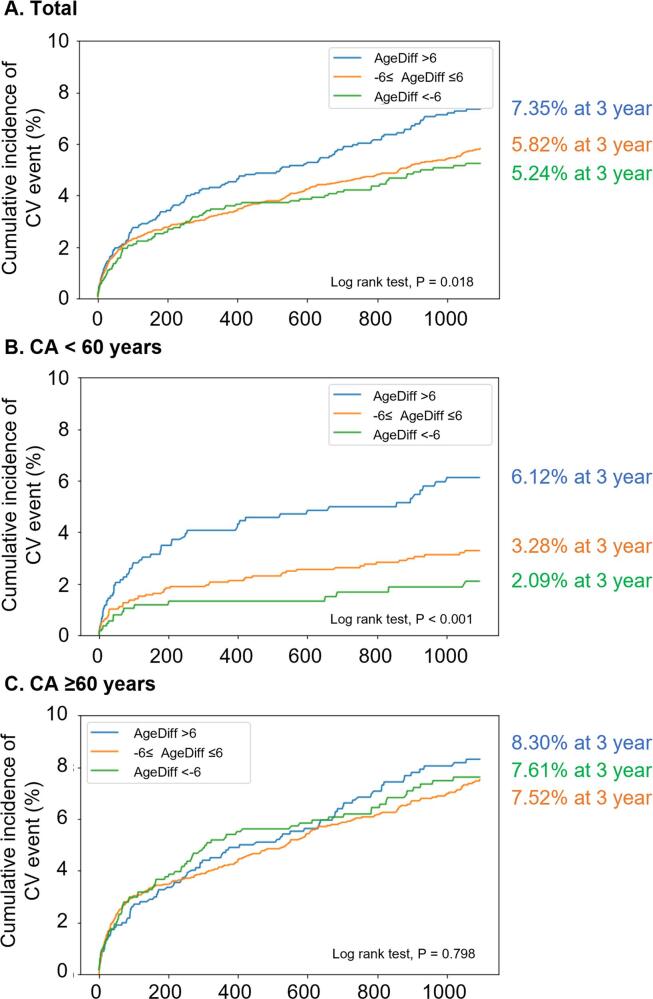
The annualized incidence of CV events was 2.53 %/y for the entire population, and 2.24 %/y, 2.44 %/y, and 3.01 %/y for patients with AgeDiff < −6 years, −6 to ≤ 6 years, and > 6 years, respectively (Table 2). There was a statistically significant difference between the AgeDiff categories (log rank test; P = 0.018; Fig. 4A), and the association was linear (Table 2).
Table 2.
Annualized incidence of CV events by AgeDiff category.
| Events (n) | Person-years | Annualized incidence of CV events (%/year) | 95 % CI | ||
|---|---|---|---|---|---|
| Total population | 543 | 21 483.25 | 2.53 | 2.32–2.75 | |
| By AgeDiff category | AgeDiff < −6 | 98 | 4378.62 | 2.24 | 1.84–2.73 |
| AgeDiff − 6 to ≤ 6 | 298 | 12 216.99 | 2.44 | 2.18–2.73 | |
| AgeDiff > 6 | 147 | 4887.63 | 3.01 | 2.56–3.53 | |
| CA < 60 years | 149 | 8814.53 | 1.69 | 1.44–1.98 | |
| By AgeDiff category | AgeDiff < −6 | 18 | 1831.59 | 0.98 | 0.62–1.55 |
| AgeDiff − 6 to ≤ 6 | 73 | 4805.86 | 1.52 | 1.21–1.91 | |
| AgeDiff > 6 | 58 | 2177.09 | 2.66 | 2.06–3.44 | |
| CA ≥ 60 years | 394 | 12 668.71 | 3.11 | 2.82–3.43 | |
| By AgeDiff category | AgeDiff < −6 | 80 | 2547.03 | 3.14 | 2.52–3.91 |
| AgeDiff − 6 to ≤ 6 | 225 | 7411.14 | 3.04 | 2.66–3.46 | |
| AgeDiff > 6 | 89 | 2710.55 | 3.28 | 2.67–4.04 |
CV, cardiovascular; CA, chronological age; AgeDiff, difference between AI-predicted age and CA; CI, confidence interval.
Fig. 4.
Incidence of CV events by AgeDiff category. The cumulative incidence of CV events in the entire population (A), patients with CA < 60 years (B), and patients with CA ≥ 60 years (C). CV, cardiovascular; AgeDiff, difference between AI-predicted age and CA; AI, artificial intelligence; CA, chronological age.
In patients with CA < 60 years, the annualized incidence of CV events was 1.69 %/y for all patients, and 0.98 %/y, 1.52 %/y, and 2.66 %/y for patients with AgeDiff < −6 years, −6 to ≤ 6 years, and > 6 years, respectively (Table 2). There was a statistically significant difference between the AgeDiff categories (log rank test; P < 0.001; Fig. 4B), and the association was linear (Table 2).
In patients with CA ≥ 60 years, the annualized incidence of CV events was 3.11 % for all patients, and 3.14 %, 3.04 %, and 3.28 % for patients with AgeDiff < −6 years, −6 to ≤ 6 years, and > 6 years, respectively (Table 2). There was no statistically significant difference between the AgeDiff categories (log rank test; P = 0.798; Fig. 4C, Table 2).
3.3.2. Predictive capability for CV events by AI-predicted age and CA
The AUCs for predicting CV events by AI-predicted age and CA are shown in Table 3. In total patients, the AUC for CV events was 0.673 (95 % CI: 0.651–0.694) for CA and 0.679 (95 % CI: 0.658–0.699) for AI-predicted age, with no statistically significant difference (Delong’s test; P = 0.388). In patients with CA < 60 years, the AUCs of CA and AI-predicted age for CV events, respectively, were 0.642 (95 % CI: 0.598–0.686) and 0.700 (95 % CI: 0.660–0.739), with a statistically significant difference (Delong’s test, P = 0.003). In patients with CA ≥ 60 years, the AUCs of CA and AI-predicted age, respectively, for CV events were 0.584 (95 % CI: 0.554–0.613) and 0.570 (95 % CI: 0.541–0.599), with no statistically significant difference (Delong’s test; P = 0.268).
Table 3.
AUCs for predicting CV event by AI-predicted age and CA.
| CA |
AI-predicted Age |
P-value CA vs AI-predicted age |
|||
|---|---|---|---|---|---|
| AUC | 95 % CI | AUC | 95 % CI | ||
| CV events | |||||
| Total population | 0.673 | 0.651–0.694 | 0.679 | 0.658–0.699 | 0.388 |
| CA < 60 years | 0.642 | 0.598–0.686 | 0.700 | 0.660–0.739 | 0.003 |
| CA ≥ 60 years | 0.584 | 0.554–0.613 | 0.570 | 0.541–0.599 | 0.268 |
CA, chronological age; AUC, area under the curve; CI, confidence interval; AI, artificial intelligence; CV, cardiovascular.
3.4. Secondary outcome
3.4.1. Prevalence of comorbidities according to AgeDiff category
The prevalences of CV-event-related comorbidities based on the AgeDiff category are shown in Table 4. The prevalences in patients with AgeDiff < −6, −6 to ≤ 6, and > 6 years were 34.2 %, 43.2 %, and 52.0 % for hypertension (P < 0.001); 9.0 %, 12.9 %, and 15.8 % for diabetes (P < 0.001); and 10.4 %, 14.4 %, and 17.9 % for ischemic heart disease (P < 0.001), respectively (Table 4).
Table 4.
Prevalence of comorbidities by AgeDiff category.
| Population | AgeDiff < −6 | AgeDiff − 6 to ≤ 6 | AgeDiff > 6 | P-value | |
|---|---|---|---|---|---|
| Hypertension, n (%) | Total | 2496 (34.2) | 5529 (43.2) | 1823 (52.0) | <0.001 |
| CA < 60 years | 1648 (21.5) | 3342 (31.3) | 989 (44.6) | <0.001 | |
| CA ≥ 60 years | 848 (50.0) | 2685 (55.1) | 1027 (59.6) | <0.001 | |
| Diabetes, n (%) | Total | 3454 (9.0) | 8484 (12.9) | 2952 (15.8) | <0.001 |
| CA < 60 years | 2021 (3.8) | 4550 (6.5) | 1599 (10.4) | <0.001 | |
| CA ≥ 60 years | 1433 (15.5) | 3934 (19.3) | 1353 (21.4) | <0.001 | |
| Heart failure, n (%) | Total | 3597 (5.2) | 9191 (5.6) | 3201 (8.7) | <0.001 |
| CA < 60 years | 2066 (1.6) | 4715 (3.1) | 1667 (6.6) | <0.001 | |
| CA ≥ 60 years | 1531 (9.7) | 4476 (8.1) | 1534 (10.9) | 0.001 | |
| Ischemic heart disease, n (%) | Total | 3399 (10.4) | 8335 (14.4) | 2878 (17.9) | <0.001 |
| CA < 60 years | 2012 (4.2) | 4483 (7.9) | 1550 (13.2) | <0.001 | |
| CA ≥ 60 years | 1387 (18.2) | 3852 (20.9) | 1328 (22.9) | 0.003 | |
| Valvular heart disease, n (%) | Total | 3510 (7.5) | 9047 (7.1) | 3155 (10.0) | <0.001 |
| CA < 60 years | 2039 (2.9) | 4714 (3.2) | 1662 (6.9) | <0.001 | |
| CA ≥ 60 years | 1471 (13.2) | 4333 (11.1) | 1493 (13.3) | 0.010 | |
| Cardiomyopathy, n (%) | Total | 3634 (4.2) | 9206 (5.5) | 3192 (9.0) | <0.001 |
| CA < 60 years | 2053 (2.2) | 4663 (4.2) | 1608 (9.9) | <0.001 | |
| CA ≥ 60 years | 1581 (6.7) | 4543 (6.8) | 1584 (8.0) | 0.213 | |
| Paroxysmal atrial fibrillation, n (%) | Total | 3508 (7.6) | 8868 (9.0) | 3154 (10.1) | 0.001 |
| CA < 60 years | 2000 (4.8) | 4576 (6.0) | 1632 (8.6) | <0.001 | |
| CA ≥ 60 years | 1508 (11.0) | 4292 (11.9) | 1522 (11.6) | 0.639 |
AgeDiff, difference between AI-predicted age and CA; CA, chronological age.
In patients with CA < 60 years, the prevalences in patients with AgeDiff < −6, −6 to ≤ 6, and > 6 years were 21.5 %, 31.3 %, and 44.6 % for hypertension (P < 0.001); 3.8 %, 6.5 %, and 10.4 % for diabetes (P < 0.001); and 4.2 %, 7.9 %, and 13.2 % for ischemic heart disease (P < 0.001), respectively. Similarly, in patients with CA ≥ 60 years, the prevalences in patients with AgeDiff < −6, −6 to ≤ 6, and > 6 years were 50.0 %, 55.1 %, and 59.6 % for hypertension (P < 0.001); 15.5 %, 19.3 %, and 21.4 % for diabetes (P < 0.001); and 18.2 %, 20.9 %, and 22.9 % for ischemic heart disease (P = 0.003), respectively. Thus, the prevalence increased with each increment of AgeDiff for most of the CV-event-related comorbidities (Table 4).
However, heart failure and valvular heart disease showed different tendency. The prevalences of heart failure in patients with AgeDiff < −6, −6 to ≤ 6, and > 6 years were 5.2 %, 5.6 %, and 8.7 % for heart failure for total patients (P < 0.001); 1.6 %, 3.1 %, and 6.6 % for patients with CA < 60 years (P < 0.001); and 9.7 %, 8.1 %, and 10.9 % for patients with CA ≥ 60 years (P = 0.001). Thus, a U-shaped association was observed between AgeDiff and the prevalence of heart failure and valvular heart disease in patients with CA ≥ 60 years. The prevalences of valvular heart disease in patients with AgeDiff < −6, −6 to ≤ 6, and > 6 years were 7.5 %, 7.1 %, and 10.0 % for total patients (P < 0.001); 2.9 %, 3.2 %, and 6.9 % for patients with CA < 60 years (P < 0.001); and 13.2 %, 11.1 %, and 13.3 % for patients with CA ≥ 60 years (P = 0.010). Thus, a U-shaped association was observed between AgeDiff and the prevalence of valvular heart disease in patients with CA ≥ 60 years.
More precise prevalences of the CV-related diseases in each 6-year age group are shown in Supplementary Fig. 2. A linear association between the AgeDiff and the prevalence was constantly observed across age categories in hypertension, diabetes, and ischemic heart disease, which was diminished in patients aged ≥ 78 years. Meanwhile, U-shaped association between the AgeDiff and the prevalence was observed for heart failure in patients aged ≥ 60 years and for valvular heart disease in patients aged ≥ 54 years.
3.4.2. Predictive capability for each comorbidity by AI-predicted age and CA
The AUCs for each comorbidity by CA and AI-predicted age are shown in Table 5. The AUC of CA and AI-predicted age for hypertension was 0.636 (95 % CI: 0.623–0.648) and 0.672 (95 % CI: 0.660–0.684), respectively, in patients with CA < 60 years (Delong’s test; P < 0.001), and 0.558 (95 % CI: 0.546–0.571) and 0.574 (95 % CI: 0.562–0.587), respectively, in patients with CA ≥ 60 years (Delong’s test; P = 0.002). On the other hand, the AUC of CA and AI-predicted age for heart failure was 0.632 (95 % CI: 0.603–0.662) and 0.701 (95 % CI: 0.674–0.728), respectively, in patients with CA < 60 years (Delong’s test; P < 0.001), while that was 0.595 (95 % CI: 0.573–0.617) and 0.580 (95 % CI: 0.559–0.602), respectively, in patients with CA ≥ 60 years (Delong’s test; P = 0.137). For valvular heart disease, the AUC of CA and AI-predicted age was 0.570 (95 % CI: 0.537–0.603) and 0.621 (95 % CI: 0.590–0.652), respectively, in patients with CA < 60 years (Delong’s test; P < 0.001), while that was 0.651 (95 % CI: 0.633–0.670) and 0.615 (95 % CI: 0.596–0.635), respectively, in patients with CA ≥ 60 years (Delong’s test; P < 0.001).
Table 5.
AUCs for each comorbidity by CA and AI-predicted age.
| CA |
AI-predicted age |
P-value CA vs AI-predicted age |
|||
|---|---|---|---|---|---|
| AUC | 95 % CI | AUC | 95 % CI | ||
| Hypertension | |||||
| Total population | 0.664 | 0.656–0.672 | 0.679 | 0.672–0.687 | <0.001 |
| CA < 60 years | 0.636 | 0.623–0.648 | 0.672 | 0.660–0.684 | <0.001 |
| CA ≥ 60 years | 0.558 | 0.546–0.571 | 0.574 | 0.562–0.587 | 0.002 |
| Diabetes | |||||
| Total population | 0.680 | 0.669–0.690 | 0.686 | 0.675–0.696 | 0.059 |
| CA < 60 years | 0.702 | 0.683–0.721 | 0.722 | 0.704–0.740 | 0.024 |
| CA ≥ 60 years | 0.532 | 0.517–0.548 | 0.547 | 0.531–0.562 | 0.031 |
| Heart failure | |||||
| Total population | 0.672 | 0.656–0.688 | 0.680 | 0.665–0.695 | 0.096 |
| CA < 60 years | 0.632 | 0.603–0.662 | 0.701 | 0.674–0.728 | <0.001 |
| CA ≥ 60 years | 0.595 | 0.573–0.617 | 0.580 | 0.559–0.602 | 0.137 |
| Ischemic heart disease | |||||
| Total population | 0.674 | 0.664–0.684 | 0.685 | 0.675–0.695 | <0.001 |
| CA < 60 years | 0.698 | 0.681–0.715 | 0.733 | 0.716–0.750 | <0.001 |
| CA ≥ 60 years | 0.539 | 0.524–0.554 | 0.550 | 0.535–0.566 | 0.085 |
| Valvular heart disease | |||||
| Total population | 0.703 | 0.688–0.718 | 0.695 | 0.681–0.710 | 0.078 |
| CA < 60 years | 0.570 | 0.537–0.603 | 0.621 | 0.590–0.652 | <0.001 |
| CA ≥ 60 years | 0.651 | 0.633–0.670 | 0.615 | 0.596–0.635 | <0.001 |
| Cardiomyopathy | |||||
| Total population | 0.578 | 0.561–0.595 | 0.611 | 0.595–0.627 | <0.001 |
| CA < 60 years | 0.597 | 0.572–0.622 | 0.686 | 0.663–0.710 | <0.001 |
| CA ≥ 60 years | 0.533 | 0.509–0.557 | 0.542 | 0.518–0.566 | 0.371 |
| Paroxysmal atrial fibrillation | |||||
| Total population | 0.613 | 0.599–0.626 | 0.616 | 0.602–0.630 | 0.351 |
| CA < 60 years | 0.601 | 0.578–0.624 | 0.621 | 0.598–0.644 | 0.019 |
| CA ≥ 60 years | 0.531 | 0.512–0.550 | 0.529 | 0.510–0.547 | 0.775 |
AUC, area under the curve; CA, chronological age; AI, artificial intelligence; CI, confidence interval.
4. Discussion
4.1. Major findings
We constructed a CNN prediction model for AI-predicted age using 12-lead ECG data and evaluated the predictive capability of the model for CV events. The association between AgeDiff and CV events was linear for the entire population and patients with CA < 60 years, however, that association was not observed in patients with CA ≥ 60 years. Accordingly, the superiority of the AI-predictive capability for age by AUC for CV events was statistically significant for the entire population, clearly observed in the younger population (≤60 years), and not detected in the older population (≥60 years). Regarding the prevalence of comorbidities related to CV events, a linear association with AgeDiff was clearly observed for hypertension, diabetes, and ischemic heart disease in both the younger and older populations. For the prevalence of valvular heart disease, the association with AgeDiff was linear in the younger population, but U-shaped in the older population.
4.2. AI-predicted age in the younger population
Biological age can be affected by an individual’s lifestyle, environmental and hereditary factors, acquired conditions, and diseases[26]. Accelerated biological age indicates a decline in tissue/organ function at a faster rate compared with the average. To date, biological age has been estimated by complex equations that involve PCA and KDM. Irrespective of the difficulty in calculation, biological age by PCA and KDM predicts mortality or CV events better than that with CA[12]. AI-predicted age by ECG was first reported by Attia et al[13]. In that report, the gap between AI-predicted age and CA was clearly associated with the increased prevalence of various cardiovascular diseases and, of note, the mean age of the patients was relatively young (58.6 years). Our data confirmed these findings in the younger population, and furthermore, suggested that the gap between AI-predicted age and CA is linearly associated with the incidence of CV events. Although some analyses have found that AI-predicted age by ECG is a better predictor of death compared with CA[16], to our knowledge, no analyses have evaluated the predictive capabilities of AI-predicted age for CV events.
4.3. AI-predicted age in the older population
In the older population in the present study, the association between AgeDiff and CV events was U-shaped, which was unexpected because it means that AgeDiff directed to younger age was associated with increased CV events.
When we analyzed the prevalence of CV-event-related comorbidities by AgeDiff category, two patterns were observed in the older population. First, the prevalence of hypertension, diabetes, and ischemic heart disease increased linearly by AgeDiff category in the older population, which was similar to that in the younger population and in line with the findings in the report by Attia et al[13]. This suggests that AgeDiff primarily represents atherosclerotic change in the cardiovascular system mainly caused by lifestyle-related illness. Second, the prevalence of heart failure and valvular heart disease showed a U-shaped association with AgeDiff in the older population, which differed from that in the younger population. This discrepancy between the two patterns requires discussion.
The phenomenon that AgeDiff directed to younger age was associated with increased CV event or increased prevalence of CV disease, especially heart failure and valvular heart disease, may derive from the similarity between the ECG change by age and by left ventricular overload. Left ventricular overload is reflected in ECG as a large amplitude of R wave or T wave in left precordial leads (V4-6)[27], which are also the representative characteristics of young persons[28], where the electrical distance is close between electrical leads and the heart in view of thin fat and not too swelling lung. Therefore, in heart diseases with left ventricular overload, such as heart failure or valvular heart disease, the ECG characteristics with disease burden may be mistakenly regarded as that with young age in the age-prediction model by CNN.
These points would be the critical limitations of AI-predicted age especially when applied to patients with older age. But these limitations may be specifically enhanced in our cohort which was derived from a cardiovascular-specialized hospital. Therefore, the clinical significance of AgeDiff in patients older than 60 years should be re-evaluated in different cohorts, such as multi-center cohorts or the general population.
5. Limitations
There were several limitations in this study. First, this was a single-center study, and all participants were patients who visited a cardiovascular hospital in an urban area. Second, although we separated data for the entire cohort into a training dataset and testing dataset to develop the models for internal validation, our model was not validated in an external cohort. Therefore, our findings should be validated in other populations from different hospitals or in the general population. Third, we chose patients with sinus rhythm because we put priority on existence of P wave in the age-prediction model. Therefore, our model cannot be applied to patients with atrial fibrillation on ECG, and we would analyze it in a future study. Finally, our model was developed using ECG data only, and patients’ characteristics, such as cardiac anatomical information, comorbidities, concomitant medications, and frailty were not included.
6. Conclusion
AI-predicted age using 12-lead ECG showed better predictive capability for cardiovascular events compared with CA in younger patients (≤60 years), presumably representing the progression of atherosclerotic change. In patients aged > 60 years, AI-predicted age was not predictive for cardiovascular events, possibly due to a weakened impact of AgeDiff and/or a confliction with ECG characteristics of left ventricular overload.
CRediT authorship contribution statement
Naomi Hirota: Conceptualization, Validation, Writing – original draft. Shinya Suzuki: Conceptualization, Validation, Writing – review & editing. Jun Motogi: Conceptualization, Validation. Hiroshi Nakai: Conceptualization, Data curation, Validation. Wataru Matsuzawa: Data curation, Formal analysis. Tsuneo Takayanagi: Data curation, Formal analysis. Takuya Umemoto: Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Akira Hyodo: Formal analysis, Validation. Keiichi Satoh: Formal analysis, Validation. Takuto Arita: Writing – review & editing. Naoharu Yagi: Writing – review & editing. Takayuki Otsuka: Writing – review & editing. Takeshi Yamashita: Writing – review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr Suzuki received research funding from Mitsubishi Tanabe Pharm and Daiichi Sankyo. Dr Yamashita received research funds and/or lecture fees from Daiichi Sankyo, Bayer Yakuhin, Bristol Myers Squibb, Pfizer, Nippon Boehringer Ingelheim, Eisai, Mitsubishi Tanabe Pharm, Ono Pharmaceutical, and Toa Eiyo. The remaining authors have no conflicts of interest to declare.
Acknowledgments
Acknowledgements
We thank Jane Charbonneau, DVM, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Authorship
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.
Grant support
None.
Sources of funding
None.
Registration number of clinical studies
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2023.101172.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
AI-predicted age and chronological age in 5-fold cross validated models. AI, artificial intelligence.
Supplementary figure 2.
(A) Incidence of CV event, and prevalences of (B) hypertension, (C) diabetes, (D) ischemic heart disease, (E) heart failure, (F) valvular heart disease, (G) cardiomyopathy, and (H) atrial fibrillation by AgeDiff category stratified by CA category. AgeDiff, difference between AI-predicted age and CA; AI, artificial intelligence; CA, chronological age; CV event; cardiovascular event.
References
- 1.Liebherr M., Schiebener J., Averbeck H., Brand M. Decision Making under Ambiguity and Objective Risk in Higher Age - A Review on Cognitive and Emotional Contributions. Front. Psychol. 2017;8:2128. doi: 10.3389/fpsyg.2017.02128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tranvag E.J., Norheim O.F., Ottersen T. Clinical decision making in cancer care: a review of current and future roles of patient age. BMC Cancer. 2018;18:546. doi: 10.1186/s12885-018-4456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine M.E. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:667–674. doi: 10.1093/gerona/gls233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arbeev K.G., Ukraintseva S.V., Yashin A.I. Dynamics of biomarkers in relation to aging and mortality. Mech. Ageing Dev. 2016;156:42–54. doi: 10.1016/j.mad.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen B.H., Marioni R.E., Colicino E., Peters M.J., Ward-Caviness C.K., Tsai P.C., Roetker N.S., Just A.C., Demerath E.W., Guan W., Bressler J., Fornage M., Studenski S., Vandiver A.R., Moore A.Z., Tanaka T., Kiel D.P., Liang L., Vokonas P., Schwartz J., Lunetta K.L., Murabito J.M., Bandinelli S., Hernandez D.G., Melzer D., Nalls M., Pilling L.C., Price T.R., Singleton A.B., Gieger C., Holle R., Kretschmer A., Kronenberg F., Kunze S., Linseisen J., Meisinger C., Rathmann W., Waldenberger M., Visscher P.M., Shah S., Wray N.R., McRae A.F., Franco O.H., Hofman A., Uitterlinden A.G., Absher D., Assimes T., Levine M.E., Lu A.T., Tsao P.S., Hou L., Manson J.E., Carty C.L., LaCroix A.Z., Reiner A.P., Spector T.D., Feinberg A.P., Levy D., Baccarelli A., van Meurs J., Bell J.T., Peters A., Deary I.J., Pankow J.S., Ferrucci L., Horvath S. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY) 2016;8:1844–1865. doi: 10.18632/aging.101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marioni R.E., Shah S., McRae A.F., Chen B.H., Colicino E., Harris S.E., Gibson J., Henders A.K., Redmond P., Cox S.R., Pattie A., Corley J., Murphy L., Martin N.G., Montgomery G.W., Feinberg A.P., Fallin M.D., Multhaup M.L., Jaffe A.E., Joehanes R., Schwartz J., Just A.C., Lunetta K.L., Murabito J.M., Starr J.M., Horvath S., Baccarelli A.A., Levy D., Visscher P.M., Wray N.R., Deary I.J. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015;16:25. doi: 10.1186/s13059-015-0584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roetker N.S., Pankow J.S., Bressler J., Morrison A.C., Boerwinkle E. Prospective Study of Epigenetic Age Acceleration and Incidence of Cardiovascular Disease Outcomes in the ARIC Study (Atherosclerosis Risk in Communities) Circ. Genom. Precis. Med. 2018;11:e001937. doi: 10.1161/CIRCGEN.117.001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perna L., Zhang Y., Mons U., Holleczek B., Saum K.U., Brenner H. Epigenetic age acceleration predicts cancer, cardiovascular, and all-cause mortality in a German case cohort. Clin. Epigenetics. 2016;8:64. doi: 10.1186/s13148-016-0228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horvath S., Erhart W., Brosch M., Ammerpohl O., von Schonfels W., Ahrens M., Heits N., Bell J.T., Tsai P.C., Spector T.D., Deloukas P., Siebert R., Sipos B., Becker T., Rocken C., Schafmayer C., Hampe J. Obesity accelerates epigenetic aging of human liver. PNAS. 2014;111:15538–15543. doi: 10.1073/pnas.1412759111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine M.E., Lu A.T., Chen B.H., Hernandez D.G., Singleton A.B., Ferrucci L., Bandinelli S., Salfati E., Manson J.E., Quach A., Kusters C.D., Kuh D., Wong A., Teschendorff A.E., Widschwendter M., Ritz B.R., Absher D., Assimes T.L., Horvath S. Menopause accelerates biological aging. PNAS. 2016;113:9327–9332. doi: 10.1073/pnas.1604558113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breitling L.P., Saum K.U., Perna L., Schottker B., Holleczek B., Brenner H. Frailty is associated with the epigenetic clock but not with telomere length in a German cohort. Clin. Epigenetics. 2016;8:21. doi: 10.1186/s13148-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirota N., Suzuki S., Arita T., Yagi N., Otsuka T., Yamashita T. Prediction of biological age and all-cause mortality by 12-lead electrocardiogram in patients without structural heart disease. BMC Geriatr. 2021;21:460. doi: 10.1186/s12877-021-02391-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Attia Z.I., Friedman P.A., Noseworthy P.A., Lopez-Jimenez F., Ladewig D.J., Satam G., Pellikka P.A., Munger T.M., Asirvatham S.J., Scott C.G., Carter R.E., Kapa S. Age and Sex Estimation Using Artificial Intelligence From Standard 12-Lead ECGs. Circ. Arrhythm. Electrophysiol. 2019;12:e007284. doi: 10.1161/CIRCEP.119.007284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ball R.L., Feiveson A.H., Schlegel T.T., Starc V., Dabney A.R. Predicting “heart age” using electrocardiography. J. Pers. Med. 2014;4:65–78. doi: 10.3390/jpm4010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch J.R., Waits G., Li Y., Soliman E.Z. Racial Differences in Heart Age and Impact on Mortality. J. Natl Med. Assoc. 2018;110:169–175. doi: 10.1016/j.jnma.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Lima E.M., Ribeiro A.H., Paixao G.M.M., Ribeiro M.H., Pinto-Filho M.M., Gomes P.R., Oliveira D.M., Sabino E.C., Duncan B.B., Giatti L., Barreto S.M., Meira W., Jr., Schon T.B., Ribeiro A.L.P. Deep neural network-estimated electrocardiographic age as a mortality predictor. Nat. Commun. 2021;12:5117. doi: 10.1038/s41467-021-25351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khane R.S., Surdi A.D., Bhatkar R.S. Changes in ECG pattern with advancing age. J. Basic Clin. Physiol. Pharmacol. 2011;22:97–101. doi: 10.1515/JBCPP.2011.017. [DOI] [PubMed] [Google Scholar]
- 18.Toya T., Ahmad A., Attia Z., Cohen-Shelly M., Ozcan I., Noseworthy P.A., Lopez-Jimenez F., Kapa S., Lerman L.O., Friedman P.A., Lerman A. Vascular Aging Detected by Peripheral Endothelial Dysfunction Is Associated With ECG-Derived Physiological Aging. J. Am. Heart Assoc. 2021;10:e018656. doi: 10.1161/JAHA.120.018656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suzuki S., Otsuka T., Sagara K., Semba H., Kano H., Matsuno S., Takai H., Kato Y., Uejima T., Oikawa Y., Nagashima K., Kirigaya H., Kunihara T., Yajima J., Sawada H., Aizawa T., Yamashita T. Nine-Year Trend of Anticoagulation Use, Thromboembolic Events, and Major Bleeding in Patients With Non-Valvular Atrial Fibrillation- Shinken Database Analysis. Circ. J. 2016;80:639–649. doi: 10.1253/circj.CJ-15-1237. [DOI] [PubMed] [Google Scholar]
- 20.Baecker L., Garcia-Dias R., Vieira S., Scarpazza C., Mechelli A. Machine learning for brain age prediction: Introduction to methods and clinical applications. EBioMedicine. 2021;72 doi: 10.1016/j.ebiom.2021.103600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Attia Z.I., Noseworthy P.A., Lopez-Jimenez F., Asirvatham S.J., Deshmukh A.J., Gersh B.J., Carter R.E., Yao X., Rabinstein A.A., Erickson B.J., Kapa S., Friedman P.A. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: a retrospective analysis of outcome prediction. Lancet. 2019;394:861–867. doi: 10.1016/S0140-6736(19)31721-0. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki S., Motogi J., Nakai H., Matsuzawa W., Takayanagi T., Umemoto T., Hirota N., Hyodo A., Satoh K., Otsuka T., Arita T., Yagi N., Yamashita T. Identifying patients with atrial fibrillation during sinus rhythm on ECG: Significance of the labeling in the artificial intelligence algorithm. Int. J. Cardiol. Heart Vasc. 2022;38 doi: 10.1016/j.ijcha.2022.100954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.J. Nagi, F. Ducatelle, A. Di Caro, D. Ciresan, U. Meier, A. Giusti, F. Nagi, J. Schmidhuber, M. Gambardella, Max-Pooling Convolutional Neural Networks for Vision-based Hand Gesture Recognition, in: IEEE International Conference on Signal and Image Processing Applicationss; Kuala Lumpur; Nov 16–18, 2011, pp. 342–347.
- 24.K. He, X. Zhang, S. Ren, J. Sun, Deep Residual Learning for Image Recognition, arXiv pre-print server, 2015, None arxiv:1512.03385.
- 25.Jia L., Zhang W., Jia R., Zhang H., Chen X. Construction Formula of Biological Age Using the Principal Component Analysis. Biomed Res. Int. 2016;2016:4697017. doi: 10.1155/2016/4697017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamczyk M.R., Nevado R.M., Barettino A., Fuster V., Andres V. Biological Versus Chronological Aging: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020;75:919–930. doi: 10.1016/j.jacc.2019.11.062. [DOI] [PubMed] [Google Scholar]
- 27.Strauss D.G., Schocken D.D. thirteenth ed. Wolters Kluwer; 2021. Marriot’s Practical Electrocardiography. [Google Scholar]
- 28.Simova I., Bortolan G., Christov I. ECG attenuation phenomenon with advancing age. J. Electrocardiol. 2018;51:1029–1034. doi: 10.1016/j.jelectrocard.2018.08.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.