Key points
-
•
Early PEG-asparaginase discontinuation is common in young adults with ALL treated with pediatric-inspired regimens
-
•
Early PEG-asparaginase discontinuation may lead to inferior survival in young adults with standard-risk ALL
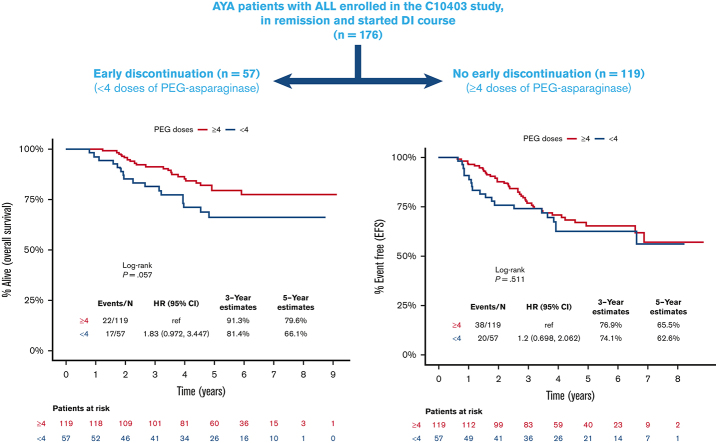
Visual Abstract
Abstract
Asparaginase is a key component of pediatric-inspired regimens in young adults with acute lymphoblastic leukemia (ALL). Truncation of asparaginase therapy is linked to inferior outcomes in children with ALL. However, a similar correlation in adults is lacking. Here, we studied the prevalence and risk factors associated with pegylated (PEG)-asparaginase discontinuation in young adults with ALL treated on the US intergroup Cancer and Leukemia Group B (CALGB) 10403 study and examined the prognostic impact of early discontinuation (ED) (defined as <4 of 5 or 6 planned doses) on survival outcomes. The analysis included 176 patients who achieved complete remission and initiated the delayed intensification (DI) cycle. The median number of PEG-asparaginase doses administered before DI was 5 (range, 1-6), with 57 (32%) patients with ED. The ED patients were older (median, 26 vs 23 years; P = .023). Survival was apparently lower for ED patients compared with those receiving ≥4 doses, but this finding was not statistically significant (hazard ratio [HR], 1.82; 95% confidence interval [CI], 0.97-3.43; P = .06), with corresponding 5-year overall survival (OS) rates of 66% and 80%, respectively. In patients with standard-risk ALL, the ED of PEG-asparaginase adversely influenced OS (HR, 2.3; 95% CI, 1.02-5.22; P = .04) with a trend toward inferior event-free survival (EFS) (HR, 1.84; 95% CI, 0.92-3.67; P = .08). In contrast, there was no impact of early PEG-asparaginase discontinuation on OS (P = .64) or EFS (P = .32) in patients with high-risk disease based on the presence of high-risk cytogenetics, Ph-like genotype, and/or high white blood cell count at presentation. In conclusion, early PEG-asparaginase discontinuation is common in young adults with ALL and may adversely impact survival of patients with standard-risk ALL.
Introduction
Asparaginase is a key chemotherapeutic agent used in most contemporary regimens for children and young adults with acute lymphoblastic leukemia (ALL).1 The inclusion and more intensive use of asparaginase therapy in pediatric ALL regimens have led to superior outcomes compared with counterpart regimens with less intense or without asparaginase.2, 3, 4 Asparagine is a nonessential amino acid, which lymphoblasts depend upon for survival to produce required proteins and nutrients but are unable to synthesize. Administering asparaginase depletes serum asparagine, which impairs the survivability of lymphoblasts, resulting in starvation and death of cells.
Studies have linked sustained depletion of serum asparagine after asparaginase therapy to improved outcomes in patients with ALL.5, 6, 7, 8 Asparaginase activity assays are available commercially, and monitoring is an important practice to reveal silent hypersensitivity, which could compromise treatment efficacy.1,8,9 The Dana-Farber Cancer Institute ALL Consortium (DFCI 00-01) study demonstrated superior relapse-free survival (RFS) in children with ALL who were randomized to individualized dosing of asparaginase based on the enzymatic activity level as compared with children who received fixed dosing of asparaginase.6 Furthermore, early asparaginase discontinuation due to toxicity or hypersensitivity has been correlated with inferior RFS in children, especially if E. coli asparaginase is not replaced with an Erwinia-based formulation. This was illustrated in the Nordic Society of Pediatric Hematology and Oncology (NOPHO), Children’s Oncology Group (COG), and DFCI 91-01 analyses.5,10,11
As pediatric studies have underscored the negative effect of truncated asparaginase therapy on ALL outcomes, studies reproducing similar findings in adults have been mostly lacking. Asparaginase toxicities occur more frequently in adults compared to children;12 thus, adults are at a higher probability of stopping the drug prematurely. It is imperative to establish the prognostic implication of early asparaginase discontinuation in adults with ALL as treating oncologists encounter this situation far more often. Early discontinuation (ED) of asparaginase therapy may impact clinicians’ decisions to pursue more intensive approaches for post remission therapy including allogeneic hematopoietic cell transplantation (HCT) in first complete remission (CR1) as an alternative or transitioning to less intensive, and potentially less effective, combination chemotherapy.
In this study, we examine the patterns of pegylated (PEG)-asparaginase administration in young adults treated on the US intergroup Cancer and Leukemia Group B (CALGB) 10403 study, one of the largest published multicenter prospective studies using a pediatric intensive regimen in adults with ALL conducted in the United States13 We analyze the prevalence and risk factors associated with early PEG-asparaginase discontinuation and its impact on ALL outcomes in this young adult population.
Methods
Study population
This is a post hoc analysis of the CALGB 10403 study, a multicenter prospective phase 2 study that was published previously.13 The CALGB 10403 study was conducted by US adult cancer cooperative groups to test the feasibility, safety, and efficacy of delivering an intensive pediatric ALL regimen to newly diagnosed adolescents and young adults (AYAs), ages 17 to 39 years, in the adult cancer treatment setting. The study adopted one of the arms of the COG AALL0232 study for high-risk childhood ALL.14 The treatment course incorporates 7 doses of PEG-asparaginase: 1 dose during remission induction, 2 doses during remission consolidation, 2 doses during interim maintenance, and 2 doses during delayed intensification (DI). For patients who required an extended remission induction cycle, an extra dose of PEG-asparaginase was given. The prescribed dose of PEG-asparaginase in the CALGB 10403 regimen was 2500 IU/m2.
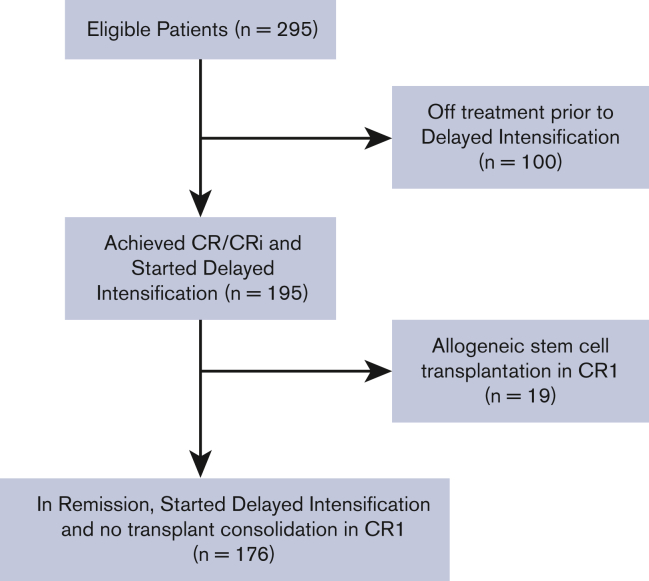
This analysis includes patients who were enrolled in the CALGB 10403 study and achieved a complete remission (CR) or CR with incomplete count recovery (CRi). We restricted this analysis to patients who completed induction with or without extension, consolidation, and interim maintenance cycles, and started therapy on the DI cycle while remaining in remission. We excluded patients who were taken off the study before DI and patients who proceeded to allogeneic HCT consolidation in CR1 after DI. The reason for excluding these patients was to provide a better understanding of the prognostic impact of inadequate asparaginase therapy in young adults with ALL who remained on the C10403 regimen backbone without switching or intensifying therapy.
The study did not record the toxicity that led to drug discontinuation; however, reasons for drug discontinuation were assigned by the treating physician as either hypersensitivity, adverse event, patient refusal, or others. The study did not record switching of PEG-asparaginase to Erwinia-based asparaginase formulation in the event of drug hypersensitivity, therefore, this information is missing. Furthermore, asparaginase activity was not monitored centrally during the study; thus, we could not account for truncated PEG-asparaginase therapy based on silent hypersensitivity.
Statistical analysis
The objectives of the study were to demonstrate the rate of and risk factors associated with early PEG-asparaginase discontinuation in young adults with ALL and to correlate the prognostic impact of ED with leukemia outcomes among patients who remained on the regimen backbone without undergoing subsequent consolidation with allogenic HCT. Subanalyses were performed to identify subgroups that are more likely to experience inferior outcomes as a result of receiving insufficient PEG-asparaginase therapy based on age, body mass index (BMI), measurable residual disease (MRD) status, and disease risk. We defined early PEG-asparaginase discontinuation as receiving <4 doses (out of a planned 5 or 6) during the treatment course by the time of initiating DI. The definition of ED was arbitrary, and the cut off was chosen to allow the study of the prognostic impact in patients at most risk with limited exposure to PEG-asparaginase during therapy. Additional analyses were performed to study the effect of receiving <5 or ≥5 doses of PEG-asparaginase in this cohort. Subanalyses were performed to study the impact of early PEG-asparaginase doses on outcome based on disease risk, age, post induction MRD status, and BMI. We defined high-risk disease as having either unfavorable cytogenetics, high white blood cell count (WBC), and/or a Ph-like genotype. In the absence of the previously mentioned high-risk features or if features were unknown, the patient was deemed to have a standard-risk ALL.
We compared the clinical characteristics and survival of patients who received <4 doses with those of patients who received ≥4 doses of PEG-asparaginase during the treatment course. We summarized categorical data as frequency counts and percentages and continuous measures as means, standard deviations, medians, and ranges. Categorical variables were compared using the chi-square test or Fisher exact test. Continuous variables were compared using the one-way ANOVA or Kruskal–Wallis test. Overall survival (OS) was defined as time from diagnosis to death from any cause. Event-free survival (EFS) was defined as time from diagnosis to the earliest occurrence of any of the following: death, relapse at any site, or development of second malignant disease. The distributions of time-to-event outcomes were estimated using the Kaplan-Meier methods and compared between patients who received <4 doses and those who received ≥4 doses, using the log-rank test. Cumulative incidences of relapse were estimated by the Aalen-Johansen estimator. Cox proportional hazards models were used to estimate hazard ratios (HRs). HRs and 95% confidence intervals (CIs) were estimated using a multivariate Cox model to evaluate the effect of PEG-asparaginase dose as a continuous variable, while adjusting for confounding effects from age, WBC, BMI, cytogenetics, Ph-like, and MRD. The distribution of time-to-event outcome was estimated using the Kaplan-Meier methods and compared between the 2 dose groups (dose ≥4 vs dose <4) after removing patients who had modified, discontinued, or held their PEG-asparaginase dose for hypersensitivity/allergic reactions. Two-sided values of P < .05 were considered statistically significant. Statistical analyses were performed using R version 4.0.3.
Results
Between November 2007 and September 2012, 318 AYA patients with newly diagnosed B-cell or T-cell Philadelphia (Ph) chromosome-negative ALL were enrolled in the study, of whom 295 were evaluable. There were 247 patients who achieved CR/CRi before the DI cycle. One hundred patients were taken off the study before initiating the DI cycle for various reasons, including induction failure, relapsed disease, having received off-protocol treatment, and death in remission or due to disease progression. Of the 195 patients who started DI in CR/CRi, 176 patients did not undergo subsequent allogeneic HCT while in CR1 and, therefore, were included in this analysis. Figure 1 illustrates the patients’ enrollment in a consort diagram.
Figure 1.
Consortdiagram for study population.
At the time of starting DI, the patient should have received either 5 or 6 doses of PEG-asparaginase; however, this depended on whether or not an extended induction cycle was administered. The median number of PEG-asparaginase doses administered before DI for this study cohort was 5 (range, 1-5). The number of patients who discontinued treatment with PEG-asparaginase after 1, 2, 3, and 4 doses were 13 (7.4%), 23 (13.1%), 21 (11.9%), and 17 (9.7%), respectively.
In total, 57 (32%) patients discontinued PEG-asparaginase treatment early with <4 administered doses by the initiation of DI, with a median of 2 (range, 1-3) administered doses. The remaining 119 (68%) patients received ≥4 doses of PEG-asparaginase by the initiation of DI with a median of 5 (range, 4-6) administered doses.
Notably, the median age was higher for patients who discontinued PEG-asparaginase early as opposed to patients who received ≥4 doses of PEG-asparaginase (26 vs 23 years; P = .023). However, there were no significant differences in patient or disease characteristics among these subgroups with regard to weight (P = .20) or BMI (P = .22), leukemia lineage (B cell vs T cell; P = .34), race (P = .16), ethnicity (P = .63), elevated WBC at diagnosis (P = .34), cytogenetic risk (P = .086), Ph-like status (P = 1.00), sex (P = .87), MRD response (P = .77), receipt of extended induction (P = .148), or presence of high-risk disease (P = 1.00). Table 1 shows patient and disease characteristics.
Table 1.
Patient and disease characteristics
| < 4 (N = 57) | ≥ 4 (N = 119) | Total (N = 176) | P value | |
|---|---|---|---|---|
| PEG doses | < .001 | |||
| N | 57 | 119 | 176 | |
| Median | 2.000 | 5.000 | 5.000 | |
| Range | 1.000 - 3.000 | 4.000 - 6.000 | 1.000 - 6.000 | |
| Extended induction status | .148 | |||
| Did not receive extended induction | 55 (96.5%) | 106 (89.1%) | 161 (91.5%) | |
| Received extended induction | 2 (3.5%) | 13 (10.9%) | 15 (8.5%) | |
| BMI (grouped) | .482 | |||
| Underweight | 1 (1.8%) | 2 (1.7%) | 3 (1.7%) | |
| Normal | 19 (33.3%) | 53 (44.5%) | 72 (40.9%) | |
| Overweight | 17 (29.8%) | 32 (26.9%) | 49 (27.8%) | |
| Obese | 20 (35.1%) | 32 (26.9%) | 52 (29.5%) | |
| Median BMI | 26.265 | 25.712 | 26.051 | .215 |
| Race | .164 | |||
| White | 52 (91.2%) | 85 (71.4%) | 137 (77.8%) | |
| Black | 2 (3.5%) | 11 (9.2%) | 13 (7.4%) | |
| Asian | 1 (1.8%) | 4 (3.4%) | 5 (2.8%) | |
| Native Hawaiian/Pacific Islander | 0 (0.0%) | 1 (0.8%) | 1 (0.6%) | |
| American Indian/Alaska native | 0 (0.0%) | 3 (2.5%) | 3 (1.7%) | |
| Unknown/not reported | 2 (3.5%) | 15 (12.6%) | 17 (9.7%) | |
| Ethnicity | ||||
| Hispanic | 6 (10.5%) | 20 (16.8%) | 26 (14.8%) | |
| Non-Hispanic | 44 (77.2%) | 81 (68.1%) | 125 (71.0%) | |
| Unknown/not reported | 7 (12.3%) | 18 (15.1%) | 25 (14.2%) | |
| CRLF-2 | .396 | |||
| N-tested cases | 31 | 59 | 90 | |
| No | 19 (73.1%) | 49 (81.7%) | 68 (79.1%) | |
| Yes | 7 (26.9%) | 11 (18.3%) | 18 (20.9%) | |
| Immunophenotype | .339 | |||
| B cell | 47 (82.5%) | 90 (75.6%) | 137 (77.8%) | |
| T cell | 10 (17.5%) | 29 (24.4%) | 39 (22.2%) | |
| Cytogenetics | .086 | |||
| N-missing | 35 | 51 | 86 | |
| Favorable | 0 (0.0%) | 8 (11.8%) | 8 (8.9%) | |
| Intermediate | 19 (86.4%) | 57 (83.8%) | 76 (84.4%) | |
| Unfavorable | 3 (13.6%) | 3 (4.4%) | 6 (6.7%) | |
| WBC | .335 | |||
| N-missing | 0 | 3 | 3 | |
| ≤30 | 47 (82.5%) | 87 (75.0%) | 134 (77.5%) | |
| >30 | 10 (17.5%) | 29 (25.0%) | 39 (22.5%) | |
| Ph-like | 1.000 | |||
| N-Tested cases | 31 | 59 | 90 | |
| No | 18 (69.2%) | 41 (68.3%) | 59 (68.6%) | |
| Yes | 8 (30.8%) | 19 (31.7%) | 27 (31.4%) | |
| Sex | .868 | |||
| Male | 37 (64.9%) | 75 (63.0%) | 112 (63.6%) | |
| Female | 20 (35.1%) | 44 (37.0%) | 64 (36.4%) | |
| Weight | .195 | |||
| Median | 82.000 | 77.000 | 78.500 | |
| Grouped age (y) | .097 | |||
| 15-19 | 8 (14.0%) | 36 (30.3%) | 44 (25.0%) | |
| 20-24 | 19 (33.3%) | 36 (30.3%) | 55 (31.2%) | |
| 25-29 | 13 (22.8%) | 27 (22.7%) | 40 (22.7%) | |
| 30-34 | 10 (17.5%) | 13 (10.9%) | 23 (13.1%) | |
| 35-39 | 7 (12.3%) | 7 (5.9%) | 14 (8.0%) | |
| Age (y) | .023 | |||
| Median | 26 | 23 | 24 | |
| Range | 18 - 39 | 17 - 38 | 17 - 39 | |
| Cause of death | .246 | |||
| N/A (Alive) | 40 (70.2%) | 97 (81.5%) | 137 (77.8%) | |
| Not related to protocol treatment or protocol disease | 4 (7.0%) | 3 (2.5%) | 7 (4.0%) | |
| Protocol disease-related | 12 (21.1%) | 18 (15.1%) | 30 (17.0%) | |
| Protocol treatment-related | 1 (1.8%) | 1 (0.8%) | 2 (1.1%) | |
| Risk group | 1.000 | |||
| High risk | 20 (35.1%) | 41 (34.5%) | 61 (34.7%) | |
| Standard risk | 37 (64.9%) | 78 (65.5%) | 115 (65.3%) |
Survival analysis
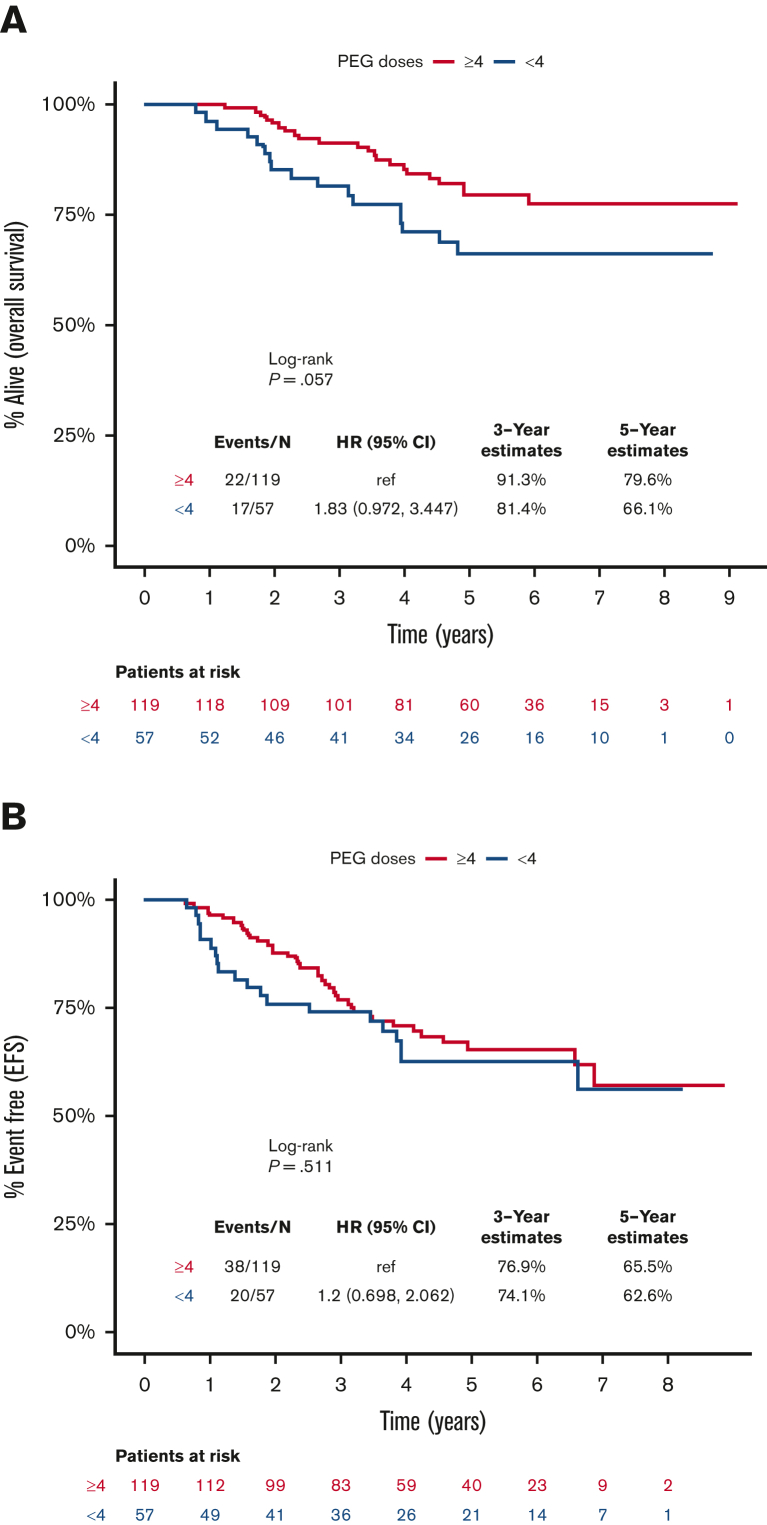
The median time of follow-up for the included patients was 59.6 months (range, 6.7-109.5). The 5-year EFS and OS rates were 64.5% and 75.3%, respectively, for all patients included in this analysis. Survival was apparently lower for patients who received <4 doses of PEG-asparaginase compared with those receiving ≥4 doses, but this finding was not statistically significant (HR, 1.83; 95% CI, 0.97-3.45; P = .06), with corresponding 5-year OS rates of 66.1% and 79.6%, respectively (Figure 2A). Nonetheless, receiving <4 doses of PEG-asparaginase did not influence EFS (HR, 1.2; 95% CI, 0.70-2.06; P = .51; Figure 2B). Furthermore, there was no observable difference in cumulative incidence of relapse between the 2 subgroups (P = .51; supplemental Figure 1). There was also no difference in the cause of death among patients who received <4 or ≥4 doses of PEG-asparaginase, and disease-related deaths accounted for 71% and 82% of deceased patients (P = .25), respectively.
Figure 2.
Kaplan-Meier curves for survival outcomes, starting from day 1 of the DI course, for patients who received <4 (blue) or ≥4 (red) doses of PEG-asparaginase. (A) OS, (B) EFS.
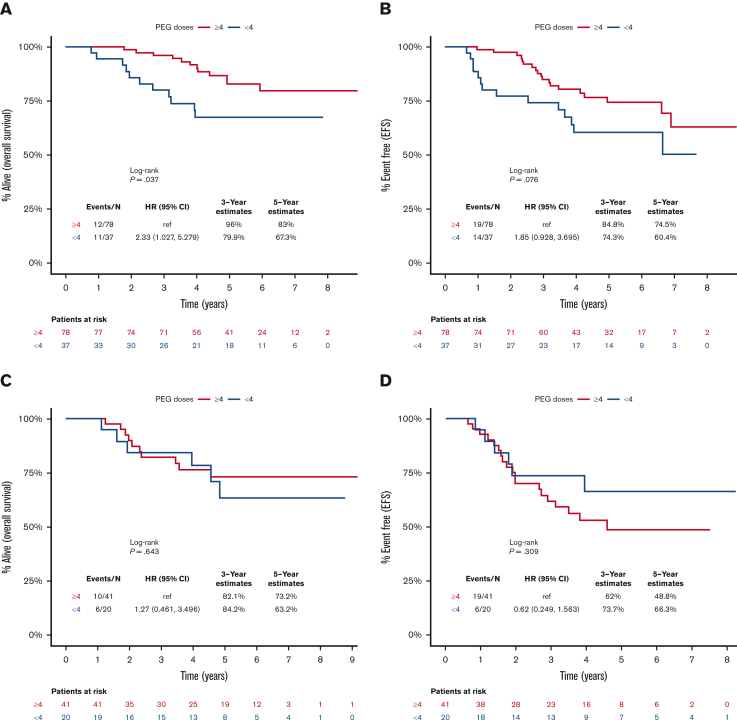
Early PEG-asparaginase discontinuation negatively influenced OS (HR, 2.3; 95% CI, 1.03-5.28; P = .04), with a trend toward inferior EFS (HR, 1.85; 95% CI, 0.93-3.70; P = .08) in patients with standard-risk ALL (Figure 3A and 3B). In contrast, there was no prognostic impact for early PEG-asparaginase discontinuation on OS (P = .64) or EFS (P = .31) in patients who met the definition of high-risk disease (Figure 3C and 3D). Furthermore, ED of PEG-asparaginase did not influence survival outcomes when we stratified patients based on age (<25 or ≥25 years) or BMI (low/normal or high; supplemental Figures 2A-2D and 3A-3D). Our cohort included 60 patients with known MRD status post induction. Early PEG-asparaginase discontinuation was associated with a trend toward inferior 5-year OS in patients with detectable MRD (47% vs 78%; P = .06) but it did not influence survival in patients who achieved undetectable MRD (83% vs 83%; P = .83). Refer to supplemental Figure 4A-D.
Figure 3.
Kaplan-Meier curves for survival outcomes, starting from day 1 of the DI course, for patients who received <4 (blue) or ≥4 (red) doses of PEG-asparaginase. (A) OS for patients with standard-risk ALL, (B) EFS for patients with standard-risk ALL, (C) OS for patients with high-risk ALL, and (D) EFS for patients with high-risk ALL.
We constructed a multivariate model analysis including PEG-asparaginase dosing as a continuous variable, age, cytogenetics, Ph-like status, WBC at diagnosis, BMI, and post induction MRD. Only having Ph-like genotype predicted inferior OS (HR, 2.81; 95% CI, 1.04-7.54; P = .04) and EFS (HR, 3.42; 95% CI, 1.52-7.72; P = .003). PEG-asparaginase dose as a continuous variable did not impact OS (P = .27) or EFS (P = .78). Refer to supplemental Table 1 and 2.
To account for the missing data on Erwinia-based replacement for hypersensitivity, we compared outcomes of patients who received <4 (n = 35) or ≥4 (n = 117) doses of PEG-asparaginase after excluding patients who discontinued PEG-asparaginase due to hypersensitivity. Early PEG-asparaginase discontinuation was associated with a trend toward lower OS (HR, 1.97; 95% CI, 0.96-4.07; P = .06), with 5-year OS of 65.7% and 79.1%, respectively, however, EFS was comparable between groups (P = .88). Refer to supplemental Figure 5A and B.
When 5 doses of PEG-asparaginase was selected as a cut off, patients who received <5 doses (n = 74; 42%) had similar OS (5-year rates, 72.8% vs 77.2%; P =.50) and EFS (5-year rates, 68.6% vs 61.1%; P= .67) to patients receiving ≥5 doses before initiating DI cycle.
Discussion
Herein, we have shown that ED of PEG-asparaginase is common in young adults with ALL, and approximately a third of responders who continued treatment on the CALGB 10403 study had stopped PEG-asparaginase therapy prior to the initiation of DI while continuing the intended regimen’s backbone. However, the actual rate for PEG-asparaginase discontinuation is underestimated in this analysis as we excluded high-risk patients who came off the study early before starting DI for various reasons, including asparaginase toxicity. The rate of PEG-asparaginase discontinuation appears higher in adults than in children with ALL (12%-25%),5,10,11 notwithstanding the fewer doses of PEG-asparaginase that were administered to the adults in this study compared with those administered in other pediatric regimens.5,10 Furthermore, monitoring for asparaginase activity was not conducted to uncover silent hypersensitivity; thus, these patients could not be included in the truncated asparaginase therapy subgroup analysis. As most patients enrolled on the CALGB 10403 study were treated at academic and tertiary leukemia centers, we predict that the rate of PEG-asparaginase discontinuation would be even higher in adults treated in the community setting. There, oncologists are less familiar with recognition and management of the multiple asparaginase toxicities. This unfamiliarity may lead to higher rates of premature discontinuation of therapy, even though many of the biochemical toxicities associated with PEG-asparaginase administration are frequently reversible and not life-threatening. Hence, inadequate asparaginase exposure represents a substantial problem in young adults with ALL who are treated with curative intent.
Patient age is a well-established risk factor for increased PEG-asparaginase toxicity.12,15, 16, 17 Here, we have observed that patients who stopped PEG-asparaginase therapy early were older than patients who received at least 4 doses. Unexpectedly, the median BMI for patients who continued or discontinued PEG-asparaginase was comparable, despite the fact that a high BMI is a well-recognized risk factor for asparaginase-induced hepatotoxicity and thrombosis.15, 16, 17, 18, 19 This may be related to the fact that asparaginase-induced hepatotoxicity and thrombosis are manageable and do not necessarily mandate permanent drug discontinuation;1 thereby, asparaginase can be safely resumed with adequate precautions.15,17,20 Alternately, certain toxicities (pancreatitis and hypersensitivity) that require permanent asparaginase discontinuation do not correlate with the patient’s weight.12,21 Nonetheless, the lack of significant difference in median BMI could be related to the insufficient power of the analysis as a result of the relatively small sample size.
Here, and with the limitation of missing data on Erwinia-based switching in patients who developed hypersensitivity, we demonstrated the deleterious effect of receiving fewer doses of PEG-asparaginase on survival in responders who completed most of the CALGB 10403 regimen before starting maintenance therapy with a 13% absolute difference in 5-year OS rates. This absolute difference in survival remained the same even after we excluded patients who developed PEG-asparaginase hypersensitivity from both subgroups to account for the missing Erwinia-based replacement effect. It is interesting to note that the adverse impact of early PEG-asparaginase discontinuation on survival was predominantly observed in patients with standard-risk disease, in whom asparaginase therapy truncation led to significantly worse outcomes. We postulate that adults with standard-risk ALL benefit the most from chemotherapy treatment as they likely have more chemo-sensitive disease and the inadequate dosing of a key drug such as PEG-asparaginase jeopardizes the regimen’s curative potential. Our observation is in contrast to those of the COG ALL0232 and ALL0331 studies where the discontinuation of asparaginase was associated with inferior disease free survival in children with high-risk but not in those with standard-risk ALL.10 This discrepancy between the COG and the CALGB 10403 studies, in regard to which patient population is most vulnerable to not receiving adequate asparaginase exposure, could be related to the different definitions of high-risk disease. Older age in children is a high-risk feature in the COG study, but age was not part of the high-risk definition in young adult patients enrolled on the CALGB 10403 study. Otherwise, all patients would have been considered high risk, according to the COG definition. Notably, our results should be interpreted with caution as we acknowledge that many high-risk patients were excluded from our analysis as a result of not reaching the DI cycle, either due to relapse or transplant consolidation.
A pertinent question related to our findings is “how should inadequate asparaginase therapy influence our approach to consolidation therapy in young adults with ALL?” While allogeneic HCT has an established role in reducing the relapse rate in high-risk ALL, it carries nontrivial risks for morbidity and mortality.22 Therefore, it is vital to accurately define the term “inadequate therapy with asparaginase” that would provide a justified rationale to recommend allogeneic HCT in patients who have achieved CR1. This definition is not clear based on our analysis, as adult patients with <4 doses of asparaginase therapy still had 5-year OS and EFS rates of 66% and 63%, respectively. These relatively favorable outcomes in adults are not sufficiently inferior to recommend altering the treatment approach based solely on lack of additional asparaginase exposure. This is particularly true today with the advent of new immune and targeted therapies that result in high rates of subsequent CR in the majority of relapsed patients with ALL and can serve as a bridge for subsequent definitive transplant.23, 24, 25 Nevertheless, additional analyses for particular subsets of the early asparaginase discontinuation subgroups in a larger cohort could successfully segregate patients with inadequate asparaginase therapy and identify subgroups who would benefit further from allogeneic HCT consolidation.
An alternative approach to consolidating patients with inadequate asparaginase therapy is to compensate for the missing therapy by integrating novel agents in the treatment regimen, such as blinatumomab, inotuzumab, or chimeric antigen receptor T-cell therapy. These therapies have nonoverlapping toxicity with asparaginase and are generally safer than allogeneic HCT. This would be an intriguing approach to investigate in adults with ALL who are treated with curative intent but unable to complete an entire PEG-asparaginase course due to its toxicities. Finally, it is possible that lower doses of PEG-asparaginase than the 2500 IU/m2 used in the CALGB 10403 regimen might result in lower rates of discontinuation. Several studies of both pediatric and adult ALL use only 1000 IU/m2 of PEG-asparaginase, which may be better tolerated and still result in therapeutic levels of asparaginase activity and potentially less toxicity.16,26, 27, 28
Our study has several limitations. It was a post hoc analysis of a prospective clinical trial and one of the largest studies to investigate the impact of truncated asparaginase therapy on outcomes of AYAs with ALL. However, it was limited by insufficient power for additional in-depth subanalyses and no documentation if the patient was switched to an alternate asparaginase formulation. Missing data on Erwinia-based asparaginase use are a major limitation here, as prior studies have shown that replacement with Erwinia-based asparaginase for hypersensitivity overcomes the adverse effect of asparaginase therapy truncation in children with ALL. Furthermore, central monitoring of asparaginase activity throughout the study was not employed to identify patients with silent hypersensitivity. Because of the subsequent loss of enzymatic activity, these patients should have been assigned to the ED subgroup, especially if their therapy was not switched to Erwinia-based asparaginase. Furthermore, our results are limited by assigning disease risk group based on missing cytogenetic and molecular data in a large proportion of patients in this analysis, and this assignment was not prespecified.
In conclusion, we showed that young adults with ALL are at considerable risk for ED of PEG-asparaginase therapy in their treatment course, and that adults with standard-risk disease who received fewer doses of PEG-asparaginase had inferior survival outcomes compared to those who received a higher number of doses. However, the long-term outcomes of adults who discontinued PEG-asparaginase therapy early were not sufficiently inferior to recommend treatment abandonment for allogeneic HCT in CR1 if no other high-risk features mandated its use. Because of the analysis design and limitations, some of our findings lack resolution, making it difficult to provide clear conclusions. Therefore, it is important to perform a larger prospective study with prespecified aims addressing the effect of inadequate asparaginase therapy in adults with ALL.
Conflict-of-interest disclosure: I.A. serves on advisory boards for Amgen, Kite pharmaceuticals, AbbVie, and Agios Pharmaceuticals, is a consultant for Pfizer, Autolus Therapeutics, and Amgen, and receives research support from MacroGenics and AbbVie. M.S.T. serves on advisory boards for AbbVie, Daiichi Sankyo, Orsenix, KAHR, Oncolyze, Jazz Pharma, Roche, Biosight, Novartis, Innate Pharmaceuticals, Kura, Syros Pharmaceuticals, and Ipsen Biopharmaceuticals, receives research funding from AbbVie, Orsenix, Biosight, Glycomimetics, Rafael Pharmaceuticals, and Amgen, and receives royalties from UpToDate. R.A.L. has acted as a consultant or adviser to Amgen, Ariad/Takeda, Astellas, Celgene/BMS, CVS/Caremark, Epizyme, Immunogen, MedPace, MorphoSys, Novartis, and Servier, and has received clinical research support to his institution from Astellas, Celgene, Cellectis, Daiichi Sankyo, Forty Seven/Gilead, Novartis, and Rafael Pharmaceuticals, and royalties from UpToDate. A.S.A. has acted as a consultant, received honoraria, and served on advisory boards for Jazz Pharmaceuticals, Kite, Amgen, Pfizer, Beam, Taiho, Glycomimetics, and Kura, and has received research support from Pfizer, Amgen, Incyte, Immunogen, Kite, Servier, Glycomimetics, Seattle Genetics, Macrogenics, and OBI. W.S. serves on advisory boards for Agios, Amgen, Astra-Zeneca, Beam, Deciphira, GlaxoSmithKline, Jazz, Kite, Kronos, Kura, MorphoSys, Newave, Pfizer, Pluristem, Servier, and Syndax, and has received honoraria from UpToDate, Jazz, Pfizer, and Research to Practice. S.M.L. has received honoraria from Daiichi Sankyo, Jazz, Bristol Myers Squibb, Acceleron, and Agios and research funding from Biosight, Celgene, Hoffmann-La Roche, Kura, Onconova, and Ariad. The remaining authors declare no competing financial interests.
Acknowledgments
This work was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: I.A., J.Y., A.W., A.A., W.S., and S.L. designed the study; J.Y. and A.W. performed the analyses; I.A., W.S., S.L., and J.Y. wrote the paper; and all authors edited the paper.
Footnotes
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Aldoss I, Douer D. How I treat the toxicities of pegasparaginase in adults with acute lymphoblastic leukemia. Blood. 2020;135(13):987–995. doi: 10.1182/blood.2019002477. [DOI] [PubMed] [Google Scholar]
- 2.Nachman JB, Sather HN, Sensel MG, et al. Augmented post-induction therapy for children with high-risk acute lymphoblastic leukemia and a slow response to initial therapy. N Engl J Med. 1998;338(23):1663–1671. doi: 10.1056/NEJM199806043382304. [DOI] [PubMed] [Google Scholar]
- 3.Amylon MD, Shuster J, Pullen J, et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: a Pediatric Oncology Group study. Leukemia. 1999;13(3):335–342. doi: 10.1038/sj.leu.2401310. [DOI] [PubMed] [Google Scholar]
- 4.Pession A, Valsecchi MG, Masera G, et al. Long-term results of a randomized trial on extended use of high dose L-asparaginase for standard risk childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23(28):7161–7167. doi: 10.1200/JCO.2005.11.411. [DOI] [PubMed] [Google Scholar]
- 5.Gottschalk Hojfeldt S, Grell K, Abrahamsson J, et al. Relapse risk following truncation of pegylated asparaginase in childhood acute lymphoblastic leukemia. Blood. 2021;137(17):2373–2382. doi: 10.1182/blood.2020006583. [DOI] [PubMed] [Google Scholar]
- 6.Vrooman LM, Stevenson KE, Supko JG, et al. Postinduction dexamethasone and individualized dosing of Escherichia Coli L-asparaginase each improve outcome of children and adolescents with newly diagnosed acute lymphoblastic leukemia: results from a randomized study--Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. J Clin Oncol. 2013;31(9):1202–1210. doi: 10.1200/JCO.2012.43.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong WH, Pieters R, Kaspers GJ, et al. A prospective study on drug monitoring of PEGasparaginase and Erwinia asparaginase and asparaginase antibodies in pediatric acute lymphoblastic leukemia. Blood. 2014;123(13):2026–2033. doi: 10.1182/blood-2013-10-534347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Sluis IM, Vrooman LM, Pieters R, et al. Consensus expert recommendations for identification and management of asparaginase hypersensitivity and silent inactivation. Haematologica. 2016;101(3):279–285. doi: 10.3324/haematol.2015.137380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wetzler M, Sanford BL, Kurtzberg J, et al. Effective asparagine depletion with pegylated asparaginase results in improved outcomes in adult acute lymphoblastic leukemia: Cancer and Leukemia Group B Study 9511. Blood. 2007;109(10):4164–4167. doi: 10.1182/blood-2006-09-045351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta S, Wang C, Raetz EA, et al. Impact of Asparaginase Discontinuation on Outcome in Childhood Acute Lymphoblastic Leukemia: A Report From the Children's Oncology Group. J Clin Oncol. 2020;38(17):1897–1905. doi: 10.1200/JCO.19.03024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood. 2001;97(5):1211–1218. doi: 10.1182/blood.v97.5.1211. [DOI] [PubMed] [Google Scholar]
- 12.Advani AS, Larsen E, Laumann K, et al. Comparison of CALGB 10403 (Alliance) and COG AALL0232 toxicity results in young adults with acute lymphoblastic leukemia. Blood Adv. 2021;5(2):504–512. doi: 10.1182/bloodadvances.2020002439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stock W, Luger SM, Advani AS, et al. A pediatric regimen for older adolescents and young adults with acute lymphoblastic leukemia: results of CALGB 10403. Blood. 2019;133(14):1548–1559. doi: 10.1182/blood-2018-10-881961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larsen EC, Devidas M, Chen S, et al. Dexamethasone and High-Dose Methotrexate Improve Outcome for Children and Young Adults With High-Risk B-Acute Lymphoblastic Leukemia: A Report From Children’s Oncology Group Study AALL0232. J Clin Oncol. 2016;34(20):2380–2388. doi: 10.1200/JCO.2015.62.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aldoss I, Douer D, Behrendt CE, et al. Toxicity profile of repeated doses of PEG-asparaginase incorporated into a pediatric-type regimen for adult acute lymphoblastic leukemia. Eur J Haematol. 2016;96(4):375–380. doi: 10.1111/ejh.12600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel B, Kirkwood AA, Dey A, et al. Pegylated-asparaginase during induction therapy for adult acute lymphoblastic leukaemia: toxicity data from the UKALL14 trial. Leukemia. 2017;31(1):58–64. doi: 10.1038/leu.2016.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orvain C, Balsat M, Tavernier E, et al. Thromboembolism prophylaxis in adult patients with acute lymphoblastic leukemia treated in the GRAALL-2005 study. Blood. 2020;136(3):328–338. doi: 10.1182/blood.2020004919. [DOI] [PubMed] [Google Scholar]
- 18.Rausch CR, Marini BL, Benitez LL, et al. PEGging down risk factors for peg-asparaginase hepatotoxicity in patients with acute lymphoblastic leukemia (dagger) Leuk Lymphoma. 2018;59(3):617–624. doi: 10.1080/10428194.2017.1349902. [DOI] [PubMed] [Google Scholar]
- 19.Schulte R, Hinson A, Huynh V, et al. Levocarnitine for pegaspargase-induced hepatotoxicity in older children and young adults with acute lymphoblastic leukemia. Cancer Med. 2021;10(21):7551–7560. doi: 10.1002/cam4.4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke PW, Aldoss I, Lunning MA, et al. Pegaspargase-related high-grade hepatotoxicity in a pediatric-inspired adult acute lymphoblastic leukemia regimen does not predict recurrent hepatotoxicity with subsequent doses. Leuk Res. 2018;66:49–56. doi: 10.1016/j.leukres.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Rank CU, Wolthers BO, Grell K, et al. Asparaginase-Associated Pancreatitis in Acute Lymphoblastic Leukemia: Results From the NOPHO ALL2008 Treatment of Patients 1-45 Years of Age. J Clin Oncol. 2020;38(2):145–154. doi: 10.1200/JCO.19.02208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wieduwilt MJ, Stock W, Advani A, et al. Superior survival with pediatric-style chemotherapy compared to myeloablative allogeneic hematopoietic cell transplantation in older adolescents and young adults with Ph-negative acute lymphoblastic leukemia in first complete remission: analysis from CALGB 10403 and the CIBMTR. Leukemia. 2021;35(7):2076–2085. doi: 10.1038/s41375-021-01213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N Engl J Med. 2016;375(8):740–753. doi: 10.1056/NEJMoa1509277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kantarjian H, Stein A, Gokbuget N, et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N Engl J Med. 2017;376(9):836–847. doi: 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah BD, Ghobadi A, Oluwole OO, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021;398(10299):491–502. doi: 10.1016/S0140-6736(21)01222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Derman BA, Streck M, Wynne J, et al. Efficacy and toxicity of reduced vs. standard dose pegylated asparaginase in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia. Leuk Lymphoma. 2020;61(3):614–622. doi: 10.1080/10428194.2019.1680839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albertsen BK, Grell K, Abrahamsson J, et al. Intermittent Versus Continuous PEG-Asparaginase to Reduce Asparaginase-Associated Toxicities: A NOPHO ALL2008 Randomized Study. J Clin Oncol. 2019;37(19):1638–1646. doi: 10.1200/JCO.18.01877. [DOI] [PubMed] [Google Scholar]
- 28.Lanvers-Kaminsky C, Niemann A, Eveslage M, et al. Asparaginase activities during intensified treatment with pegylated E. coli asparaginase in adults with newly-diagnosed acute lymphoblastic leukemia. Leuk Lymphoma. 2019;61:1–8. doi: 10.1080/10428194.2019.1658099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.