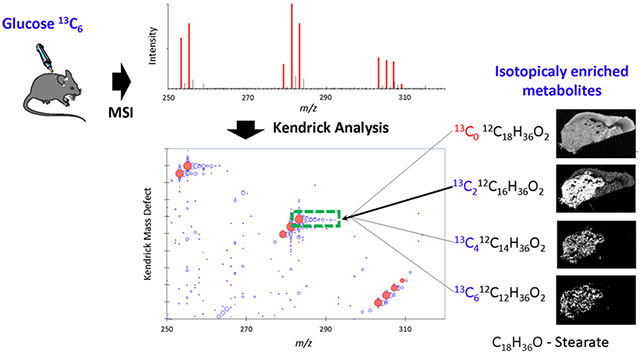
Abstract
Besides many other applications, isotopic labeling is commonly used to decipher the metabolism of living biological systems. By giving a stable isotopically labeled compound as a substrate, the biological system will use this labeled nutrient as it would with a regular substrate and incorporate stable heavy atoms into new metabolites. Utilizing mass spectrometry, by comparing heavy atom enriched isotopic profiles and naturally occurring ones, it is possible to identify these metabolites and deduce valuable information about metabolism and biochemical pathways. The coupling of this approach with mass spectrometry imaging (MSI) allows one then to obtain 2D maps of metabolisms used by living specimens. As metabolic networks are convoluted, a global overview of the isotopically labeled data set to detect unexpected metabolites is crucial. Unfortunately, due to the complexity of MSI spectra, such untargeted processing approaches are difficult to decipher. In this technical note, we demonstrate the potential of a variation around the Kendrick analysis concept to detect the incorporation of stable heavy atoms into metabolites. The Kendrick analysis uses as a base unit the difference between the mass of the most abundant isotope and the mass of the corresponding stable isotopic tracer (namely, 12C and 13C). The resulting Kendrick plot offers an alternative method to process the MSI data set with a new perspective allowing for the rapid detection of the 13C-enriched metabolites and separating unrelated compounds. This processing method of MS data could therefore be a useful tool to decipher isotopic labeling and study metabolic networks, especially as it does not require advanced computational capabilities.
Graphical Abstract
INTRODUCTION
Isotopic labeling can be used to decipher the metabolisms of living biological systems. Tracing of stable isotopes such as 13C carbon or 15N nitrogen enables analyses that provide dynamic information on the flow of matter in a biological system.1 By giving a stable isotopically labeled compound as a substrate, such as 13C-glucose2 in energetic metabolism studies or 15NH4 in proteomic analyses,3 the biological system will use this labeled nutrient as it would any naturally occurring compound and incorporate stable heavy isotopes into new metabolites. Mass spectrometry (MS) is a versatile and well-established technology able to provide the isotopic profile of these metabolites. By comparing the signal intensity of the naturally occurring isotopic profile with the one of the specimen enriched by heavy-labeled tracers,4 valuable information can be deduced about metabolism and biochemical pathways employed by the living specimen.2 These types of strategies have been used and reviewed in various fields such as cancer research,2,5,6 infectious disease,7 pharmaceutical production,8 and industrial cell factories.9 To date, various software, such as isosearch,10 Isotopo,11 MIDcor,12 X13CMS,13 and 13CFLUX2,14 have been designed for liquid chromatography (LC)-MS data analysis of isotopomer tracing in targeted and nontargeted approaches.
In recent years, mass spectrometry imaging (MSI) has become a powerful tool to map the distributions of multiple molecules such as proteins, carbohydrates, glycans, lipids, metabolites, or drugs and address various scientific questions in the biomedical field15. Coupling MSI and isotopic labeling has been successfully applied to study and map the metabolism of different lipid classes, including fatty acid (FA) lipogenesis in brain metastases,16 cholesterol and phospholipids in atherosclerosis,17 and phosphatidylcholines in lung.18 However, due to the complexity of MSI spectra, combined with the lack of LC separation and with combination MSI-ion mobility still in its infancy, isotopic tracing strategies are limited to targeted approaches.
In this technical note, we demonstrate the potential of a variation around the classic Kendrick mass defect (KMD) method to visualize MSI data as intuitive maps that easily decipher unnatural isotopic profiles highlighting metabolites generated from stable isotopic tracers. Briefly, KMD is a 60 year old analysis technique19 resulting in a two-dimensional map to visualize a mass spectrum, where eq 1 is used to transform the base atomic mass unit. Since the Dalton (Da) is defined as 1/12 of the mass of an unbound neutral atom of carbon-12 (12C), adding a 12C to any elemental composition increases its mass by exactly 12 Da. Edward Kendrick proposed to use the methylene group, –CH2–, as a new base unit (instead of 12C), with R in eq 1 being the accurate mass of –CH2– or 14.01565 Da. With this transformation, adding a –CH2– to any elemental composition increased its rescaled mass (Kendrick Mass, KM) by exactly 14, leaving the fractional part (number after decimal point or the KMD) unchanged. Consequently, molecules with similar substructures (such as polar head groups) but different lipid chain lengths (made of –CH2– group) will have the same KMD. For a given MS spectrum, by plotting the KMD of each peak as a function of mass to charge ratio (m/z), molecules with the same KMD will be aligned in a horizontal line, grouping together molecules with related substructures. This procedure, named “Kendrick analysis”, commonly used to analyze polymer data sets of repeating backbones,20,21 has also been applied to interpret MSI maps of lipids in biological samples.22,23 KMD analysis was extensively reviewed by Thierry Fouquet.24
Equations for calculation of Kendrick mass defect variation:
| (1) |
In stable isotopic labeling, the substrate allows the incorporation of multiple heavy atoms into metabolites. To trace and decipher 13C incorporations in biological samples, we implemented a variation of the KMD analysis proposed in 2019 by Nakamura et al.,25 using the mass difference between 13C and 12C, or 1.0033548378 (eq 2) as the new base unit to generate rescaled “Kendrick” mass sets. The mass spectrum from Figure 1A can then be turned into a Kendrick plot (Figure 1B) in which 13C isotopes of given molecules present the same KMD and are aligned horizontally. Therefore, isotopically labeled 13C accumulation is easily identified (Figure 1D) compared to the conventional MS spectra of the same data (Figure 1C).
Figure 1.

Deciphering 13C incorporation from stable isotope tracer by using a KMD variation. (A) Negative MS spectrum of a brain tumor from a mouse labeled with 13C-glucose isotopic trace. (B) Zoom into fatty mass range; annotations have been made by using MS exact mass and the LipidMaps database. (C) Kendrick plot of panel A spectrum. (D) Zoom into green rectangle. In KMD maps, sizes of spots are proportional to MS peak relative intensities. Yellow arrows indicate species presenting a non-natural isotopic profile with accumulation of 13C.
13C incorporation as repeated backbone:
| (2) |
EXPERIMENTAL SECTION
To test and validate our KMD variation approach, we used a previously published isotopically labeled mouse model MSI data set.16 In this brain metastasis study, 13C6-glucose was injected to mice with brain metastases (Figure S1). Mouse brains were analyzed by MSI in negative ion mode to map metabolite distribution and decipher the metabolic processes underlying brain tumor cell lipogenesis. Due to the complexity of the data set, the published analysis focused on fatty acids and not on the mass range of larger lipids, like phospholipids. The Kendrick approach was developed to optimize and expand the interpretation of the complex data set, allowing for the quick detection of all metabolites enriched in 13C detected in the spectra.
Mouse Experiment Labeling with 13C6-Glucose.
A detailed protocol of the mouse experiment is given in Ferraro et al.16 and in all Supporting Information files. All animal procedures were performed according to the guidelines of the Public Health Service Policy on Human Care of Laboratory Animals and in accordance with a protocol approved by the Institutional Animal Care and Use Committee of the Massachusetts General Hospital and Massachusetts Institute of Technology. Briefly, three tumor-bearing mice received daily bolus injections of 13C6-glucose over 4 days, while three naïve mice were used as control. Tissues from mice euthanized on day 4 were collected, flash frozen, and stored at −80 °C for subsequent analysis.
MSI Acquisition and Preprocessing.
Sample preparation and MSI acquisition protocols are given in Ferraro et al.16 Briefly, MALDI-MSI acquisition was performed in negative ion mode using a MALDI LTQ Orbitrap XL mass spectrometer (ThermoFisher Scientific, USA) with the resolution of 60,000 at m/z 400 (full width at half-maximum) in centroid mode. Raw MSI data were exported to an imzML26 file format using ImageQuest (ThermoFisher Scientific, USA), and two-dimensional MS ion images were generated using the free and open-source MSiReader.27 To annotate, average MS spectra from tumor regions were obtained with Excalibur Qual Browser (ThermoFisher Scientific, USA), and peak lists were exported to Excel (Microsoft, USA). The MS data from the different mouse experiments were recalibrated and annotated with the LipidMaps (https://lipidmaps.org/) database.28 Data sets for the described experiments are available on META-SPACE2020 at https://metaspace2020.eu/project/Brain_Metastases_MSI_Isotopic_labeling.
Kendrick Analysis and Data Processing.
MSI spectra from the tumor area of one control and one 13C labeled mouse were averaged (Figure S1 for tumor area). The m/z peak list was extracted, and Kendrick analysis was performed following eq 1 with R = 1.0033548378. By plotting the KMD as a function of m/z, the control and 13C labeled Kendrick plots were obtained (Figure 2A and Figure 3A). Zooming into the different mass ranges of Kendrick plots allows for visualization of the isotopic profiles of multiple molecules (see SI Spreadsheet 1 for all Kendrick plots).
Figure 2.

Deciphering 13C incorporation into fatty acid by using a KMD variation. (A) Kendrick mass defect map of tumor area from 13C-glucose labeled and unlabeled mouse. Size of spots is proportional to MS peaks relative intensity. Annotations have been made by using MS exact mass and LipidMaps database. Yellow arrows and rectangles indicate species presenting a non-natural isotopic profile with accumulation of 13C. (B) Isotopic profile of stearate and arachidonate in brain tumor area for three 13C-glucose labeled mouse and three unlabeled. Round spots correspond to average relative abundances in tumor areas for each mouse; error bars represent standard deviation. Crosses correspond to theoretical natural relative abundance of each isotope. (C) MSI maps for different isotopes of m/z 283.264, [FA 18:0-H]− stearate; m/z 303.233, [FA20:4-H]− arachidonate.
Figure 3.

Deciphering 13C incorporation into phospholipids by using a KMD variation. (A) Kendrick mass defect map of tumor area from 13C-glucose labeled and unlabeled mouse. Sizes of spots are proportional to MS peak relative intensities. Annotations have been made by using MS exact mass and LipidMaps database. Yellow arrows and rectangles indicate species presenting a non-natural isotopic profile with accumulation of 13C. (B) Isotopic abundances of PI 34:1 and PI 34:2 in brain tumor area for three 13C-glucose labeled mouse and three unlabeled. Round spots correspond to average relative abundances in tumor areas for each mouse; error bars represent standard deviation. Crosses correspond to theoretical natural relative abundance of each isotope. (C) MSI maps of different isotopes of m/z 835.534, [PI 34:1-H]−; m/z 833.5187, [PI 34:2-H]−.
For isotopic profiles (Figure 2B and Figure 3B), regions of interest (ROI) of the tumor area were defined in MSiReader for the three control and three 13C labeled mice. The abundance of each metabolite isotope (Table S1) was normalized by the abundance of the metabolite containing only 12C for each pixel. For a given isotope, the mean relative abundance and standard deviation are calculated by averaging all pixels of the ROI. Plots are generated with GraphPad Prism (GraphPad Software Inc., USA). For MS images of isotopic profiles (Figure 2C and Figure 3C), m/z values from Table S1 were used and mapped with MSiReader with a mass tolerance of 5 ppm and TIC normalization.
RESULTS AND DISCUSSION
To further leverage our prior study,16 the data sets were reprocessed with the KMD analysis. The brain tumor area in the control (Figure S1B) and in 13C6-glucose labeled mice (Figure S1D) were defined as ROI, and the average MS spectra exported and plotted in KMD maps (SI Spreadsheet 1).
By zooming on the fatty acid mass range in the KMD plots of control and 13C labeled tissues, we detected fatty acids that presented an enriched isotopic profile, visible by a horizontal cluster of dots at the right of the 12C monoisotopic peak (Figure 2A) in agreement with the published targeted strategy.16 We observed a nonconventional 13C isotopic profile for [FA(16:0)-H]− palmitate, [FA(16:1)-H]− palmitoleate, [FA(18:0)-H]− stearate, and [FA(18:1)-H]− oleate, indicating that they were generated from 13C6-glucose, whereas the more unsaturated or longer chain fatty acids, such as linoleate or arachidonate, did not. The isotopic profiles (Figure 2B) confirmed the Kendrick plot deductions. This trend was consistently observed in the three labeled mouse tissues, while the isotopic profiles of the control mice followed the anticipated natural profile. As predicted by the KMD analysis, stearate but not arachidonate presents a non-natural accumulation of 13C (up to 8 13C atoms) in brain tumor, not seen in the control mice (Figure 2C), indicating it may have been generated from glucose 13C6.
Using a variation of the KMD approach, we have developed a platform to visualize the incorporation of 13C (from 13C6-glucose tracers) in biologically relevant lipids that play roles in brain metastasis. Only saturated and monounsaturated fatty acids with 16 and 18 carbon atoms accumulated 13C (Figure 1D and Figure 2A), consistent with the fatty acids being synthesized from glucose through acetyl-CoA.30 Moreover, no 13C accumulation was observed in essential fatty acids not synthesized by mammalian cells but instead obtained through the diet, such as linoleate FA(18:2) and its derivatives (FA(20:2), FA(20:3), FA(20:4)).
Interestingly, Kendrick plots of both conditions in Figure 2A reveal spectral artifacts in the MSI datatset, likely “ringing” associated with the Fourier transform of the Orbitrap signal.29 This manifests as vertical alignments of low intensity data points on either side of intense peaks in the KMD analysis. Therefore, this method highlights that, for this data set, caution has to be taken when interpreting peaks from 13C isotopes that could overlap with ringing artifact peaks from more saturated ions (such as ringing peaks from FA(18:0) overlapping with 13C2–FA(18:1) at m/z 283.256). This could lead to a missed peak assignment. However, and despite this instrumental artifact, we are able to detect incorporation of up to eight carbons 13C. Such large mass shifts are not in the range of these ringing artifacts, and we can assess that fatty acids that have been labeled by glucose 13C trough lipogenesis.
The KMD plot also enabled the detection of 13C incorporation in the crowded phospholipid mass range where MS spectra were highly complex, such as phosphatidylinositol [PI(34:1)-H]− and [PI(36:1)-H]− (see Figure 3A and Table S2 for all predicted 13C enriched metabolited). Using ROI analysis of MSI maps, we confirmed the unnatural isotopic profile with strong accumulation of 13C compared to natural abundance in these phospholipids (Figure 3B). MSI confirmed accumulation of these 13C-enriched metabolites in the tumor area (Figure 3C).
However, thanks to the Kendrick plot, it is important to notice that for many phospholipids only the M+1 isotope is detected, whereas the detection of further 13C isotopes, like M+2 or M+3, could be expected even without 13C6-glucose labeling. This probably highlights a limitation of this MSI data set due to a lack MS resolution. Typically, at 60,000 MS resolution, the error of the measured m/z is 16.6 ppm. The error for a singly charged phospholipid (around m/z 800) is typically m/z 0.0133, whereas the mass difference between incorporation of two carbon 13C (m/z 2.0067) or the loss of a double bond (m/z 2.0156) is around m/z 0.0089. With the mass resolution used with our instrument, the M+2 13C isotope of an unsaturated phospholipid could overlap with the M+0 isotope of a less unsaturated form, and centroiding postprocessing will promote the most abundant form. For the most saturated phospholipids of each series, as there are no more saturated species sufficiently abundant to overlap with, like PI(34:1) or PI(36:1), the 13C enrichment in their isotopic profiles can be detected. However, one cannot tell if the 13C labeling is limited on the more saturated species or also present in other levels of unsaturation.
Consequently, we observed 13C accumulation in phospholipids indicating the probable incorporation of newly synthesized fatty acids from lipogenesis into phospholipids, but a new MSI acquisition with a higher MS resolution is required to decipher the labeling into the different unsaturated forms of each phospholipid species.
CONCLUSIONS
Isotopic labeling, coupled with MSI, enables the study of flux materials in living models and obtaining maps of cell metabolisms in their environments like tumor cells in brain. Unfortunately, the untargeted identification of isotopically enriched metabolites can be convoluted. In this technical note, we have demonstrated the potential of a variation of the Kendrick analysis concept to decipher the incorporation of stable heavy atoms into metabolites by using an R variable equal to the difference between the mass of the most abundant isotope and the mass of the corresponding stable isotope tracer.
Moreover, as T. Fouquet. explained in his review, “A Kendrick plot contains the same information as a mass spectrum but it is visualized from a different point of view or perspective”.24 So Kendrick plots offer also a way to visualize and evaluate artifacts or limitations of the studied MSI data set. Consequently, users can properly consider such limitations while they are investigating a data set or making conclusions.
This intuitive method to visualize isotopically labeled MS data facilitates the analysis of complex mass spectra produced by MSI, especially as it does not require advanced computational capabilities. It is applicable to a variety of tracers, where the choice of the base unit (the R variable) will depend on the chosen isotopic tracer: R = 1.0033548378 for 13C, R = 1.006276746 for 2H, or R = 0.997034893 for 15N. Moreover, this KMD variation could also be applied to other kinds of MS data sets like LC-MS or ion mobility data, adding new perspective for visualization.
Supplementary Material
Table S1: Mass-to-charge ratio used to quantify the relative abundance of the different isotopes (PDF)
Figure S1: DAPI and MS images of brain metastasis from 13C-glucose labeled and unlabeled mouse (PDF)
Table S2: List of detected 13C-enriched metabolites (PDF)
Spreadsheet 1: Kendrick plots, Kendrick analysis, and peak list of associated spectra (XLSX)
ACKNOWLEDGMENTS
L.B. is supported by a grant from the “Fondation pour la Recherche Médicale” (FRM, ARF201809007123). This work was financially supported by the Agence National de la Recherche (France, Grant ANR-19-CE29-0010601 “Multi-RaMaS”).
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acs.analchem.1c03916
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.analchem.1c03916.
MSI dataset: https://metaspace2020.eu/project/Brain_Metastases_MSI_Isotopic_labeling
The authors declare no competing financial interest.
Contributor Information
Landry Blanc, Univ. Bordeaux, CNRS, CBMN, UMR 5248, F-33600 Pessac, France.
Gino B. Ferraro, Edwin L. Steele Laboratories, Department of Radiation Oncology, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts 02114, United States
Michael Tuck, Univ. Bordeaux, CNRS, CBMN, UMR 5248, F-33600 Pessac, France.
Brendan Prideaux, Department of Neuroscience, Cell Biology, and Anatomy, University of Texas Medical Branch (UTMB), Galveston, Texas 77555, United States.
Véronique Dartois, Center for Discovery and Innovation, Hackensack Meridian School of Medicine, Department of Medical Sciences, Hackensack Meridian Health, Nutley, New Jersey 07601, United States.
Rakesh K. Jain, Edwin L. Steele Laboratories, Department of Radiation Oncology, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts 02114, United States
Nicolas Desbenoit, Univ. Bordeaux, CNRS, CBMN, UMR 5248, F-33600 Pessac, France.
REFERENCES
- (1).Crown SB; Antoniewicz MR Metab. Eng 2013, 20, 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Gkiouli; Biechl; Eisenreich; Otto. Cells 2019, 8 (10), 1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Taubert M; von Bergen M; Seifert J Anal. Bioanal. Chem 2013, 405 (12), 3989–3996. [DOI] [PubMed] [Google Scholar]
- (4).Lee W-NP; Byerley LO; Bergner EA; Edmond J Biol. Mass Spectrom 1991, 20 (8), 451–458. [DOI] [PubMed] [Google Scholar]
- (5).Lagziel S; Lee WD; Shlomi T BMC Biol. 2019, 17 (1), 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Bayram S; Fürst S; Forbes M; Kempa S Mol. Metab 2020, 33, 38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Cronan MRMR; Matty MAMA; Rosenberg AFAF; Blanc L; Pyle CJCJ; Espenschied STST; Rawls JFJF; Dartois V; Tobin DMDM Nat. Methods 2018, 15 (12), 1098–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Boghigian BA; Seth G; Kiss R; Pfeifer BA Metab. Eng 2010, 12 (2), 81–95. [DOI] [PubMed] [Google Scholar]
- (9).Schwechheimer SK; Becker J; Wittmann C Curr. Opin. Biotechnol 2018, 54, 128–137. [DOI] [PubMed] [Google Scholar]
- (10).Huang H; Yuan M; Seitzer P; Ludwigsen S; Asara JM Methods Protoc. 2020, 3 (3), 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Ahmed Z; Zeeshan S; Huber C; Hensel M; Schomburg D; Munch R; Eylert E; Eisenreich W; Dandekar T Database 2014, 2014, bau077–bau077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Selivanov VA; Benito A; Miranda A; Aguilar E; Polat IH; Centelles JJ; Jayaraman A; Lee PWN; Marin S; Cascante M BMC Bioinf. 2017, 18 (1), 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Huang X; Chen Y-J; Cho K; Nikolskiy I; Crawford PA; Patti GJ Anal. Chem 2014, 86 (3), 1632–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Weitzel M; Nöh K; Dalman T; Niedenführ S; Stute B; Wiechert W Bioinformatics 2013, 29 (1), 143–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Tuck M; Blanc L; Touti R; Patterson NH; Van Nuffel S; Villette S; Taveau J-C; Rompp A; Brunelle A; Lecomte S; Desbenoit N Anal. Chem 2021, 93, 445. [DOI] [PubMed] [Google Scholar]
- (16).Ferraro GB; Ali A; Luengo A; Kodack DP; Deik A; Abbott KL; Bezwada D; Blanc L; Prideaux B; Jin X; Posada JM; Chen J; Chin CR; Amoozgar Z; Ferreira R; Chen IX; Naxerova K; Ng C; Westermark AM; Duquette M; Roberge S; Lindeman NI; Lyssiotis CA; Nielsen J; Housman DE; Duda DG; Brachtel E; Golub TR; Cantley LC; Asara JM; Davidson SM; Fukumura D; Dartois VA; Clish CB; Jain RK; Vander Heiden MG Nat. Cancer 2021, 2 (4), 414–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Ellis SR; Hall E; Panchal M; Flinders B; Madsen J; Koster G; Heeren RMA; Clark HW; Postle AD J. Lipid Res 2021, 62, 100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Castro-Perez J; Hatcher N; Kofi Karikari N; Wang S-P; Mendoza V; Shion H; Millar A; Shockcor J; Towers M; McLaren D; Shah V; Previs S; Akinsanya K; Cleary M; Roddy TP; Johns DG Rapid Commun. Mass Spectrom 2014, 28 (22), 2471–2479. [DOI] [PubMed] [Google Scholar]
- (19).Kendrick E. Anal. Chem 1963, 35 (13), 2146–2154. [Google Scholar]
- (20).Cody RB; Fouquet T J. Am. Soc. Mass Spectrom 2018, 29 (10), 2110–2113. [DOI] [PubMed] [Google Scholar]
- (21).Zheng Q; Morimoto M; Sato H; Fouquet T Fuel 2019, 235, 944–953. [Google Scholar]
- (22).Kune C; McCann A; Raphaël LR; Arias AA; Tiquet M; Van Kruining D; Martinez PM; Ongena M; Eppe G; Quinton L; Far J; De Pauw E Anal. Chem 2019, 91 (20), 13112–13118. [DOI] [PubMed] [Google Scholar]
- (23).Müller WH; Verdin A; Kune C; Far J; De Pauw E; Malherbe C; Eppe G Anal. Bioanal. Chem 2021, 413 (10), 2821–2830. [DOI] [PubMed] [Google Scholar]
- (24).Fouquet TNJ J. Mass Spectrom 2019, 54 (12), 933–947. [DOI] [PubMed] [Google Scholar]
- (25).Nakamura S; Cody RB; Sato H; Fouquet T Anal. Chem 2019, 91 (3), 2004–2012. [DOI] [PubMed] [Google Scholar]
- (26).Römpp A; Schramm T; Hester A; Klinkert I; Both J-PP; Heeren RMAA; Stöckli M; Spengler B Methods Mol. Biol 2011, 696, 205–224. [DOI] [PubMed] [Google Scholar]
- (27).Bokhart MT; Nazari M; Garrard KP; Muddiman DC J. Am. Soc. Mass Spectrom 2018, 29 (1), 8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Sud M; Fahy E; Cotter D; Brown A; Dennis EA; Glass CK; Merrill AH; Murphy RC; Raetz CRH; Russell DW; Subramaniam S Nucleic Acids Res. 2007, 35 (Database), D527–D532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Mitchell JM; Flight RM; Wang QJ; Higashi RM; Fan TW-M; Lane AN; Moseley HNB Metabolomics 2018, 14 (10), 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Kamphorst JJ; Chung MK; Fan J; Rabinowitz JD Cancer Metab. 2014, 2 (1), 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Mass-to-charge ratio used to quantify the relative abundance of the different isotopes (PDF)
Figure S1: DAPI and MS images of brain metastasis from 13C-glucose labeled and unlabeled mouse (PDF)
Table S2: List of detected 13C-enriched metabolites (PDF)
Spreadsheet 1: Kendrick plots, Kendrick analysis, and peak list of associated spectra (XLSX)


