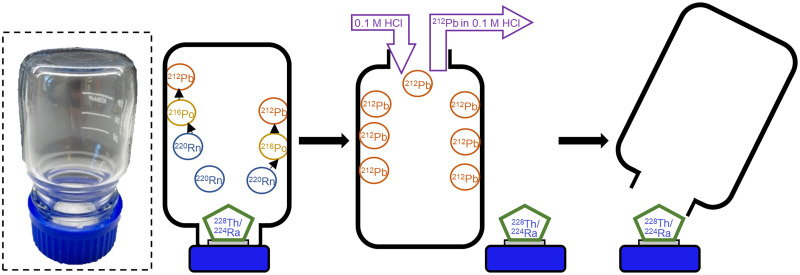
Visual Abstract
Keywords: lead-212, 212Pb, 220Rn, 212Pb generator, radionuclide production
Abstract
The feasibility, performance, and radiation safety of an experimental generator were evaluated to efficiently produce 212Pb intended for radiopharmaceuticals. Methods: The generator consisted of a flask with a removable cap containing a source of 224Ra or 228Th absorbed on quartz wool. Gaseous 220Rn emanated from the decaying source, which subsequently decayed to 212Pb, which was adsorbed on the flask’s interior surface. The 212Pb was collected by washing the flask with 0.5–1 mL of 0.1 M HCl. Results: The generator collector flask trapped 62%–68% of the 212Pb, of which more than 87% (tested up to 26 MBq) could be harvested. The obtained 212Pb solution had a high purity (>99.98%) and could be used for the preparation of radioconjugates with more than 97% radiochemical purity. Future designs of the generator should aim to further reduce the risk of radon and γ-energy exposure to operators. Conclusion: The presented technology is a promising method for easy and convenient 212Pb production.
Lead-212 (212Pb; half-life, 10.6 h), a β-emitter itself, is an in vivo generator of α-particles through the α-emitting progenies 212Bi and 212Po. Convenient chelation chemistry makes 212Pb suitable for targeted α-therapy (1). However, the radiolabeling of targeting agents should preferably be performed on-site because of the short half-life of 212Pb. Rapid and efficient processes are required to ensure sufficient 212Pb availability for end users.
As a member of the thorium series (Fig. 1), 212Pb can be obtained from generators that contain the longer-lived mother nuclides 228Th (half-life, 1.9 y) or 224Ra (half-life, 3.6 d). Current generators are based on isolating 212Pb from 224Ra or 228Th through several purification steps. 224Ra has become the preferred radionuclide source over 228Th to minimize radiation hazards (1). A generator used to supply 212Pb for clinical trials by Orano Med is based on 224Ra immobilized on a cation-exchange column from which 212Pb can be eluted (2–4). The eluate is then evaporated and treated several times with concentrated acid before the final solution is ready for radiolabeling (3). A similar generator purchased from Oak Ridge National Laboratory was integrated into an automated synthesis module, where 212Pb was eluted in dilute HCl for labeling of peptides (5). An alternative method that avoids purification of 212Pb from the generator source material uses a solution of 224Ra/212Pb in equilibrium directly for the radiolabeling process (6,7). However, this procedure still requires a final purification step to remove 224Ra and unconjugated daughters.
FIGURE 1.
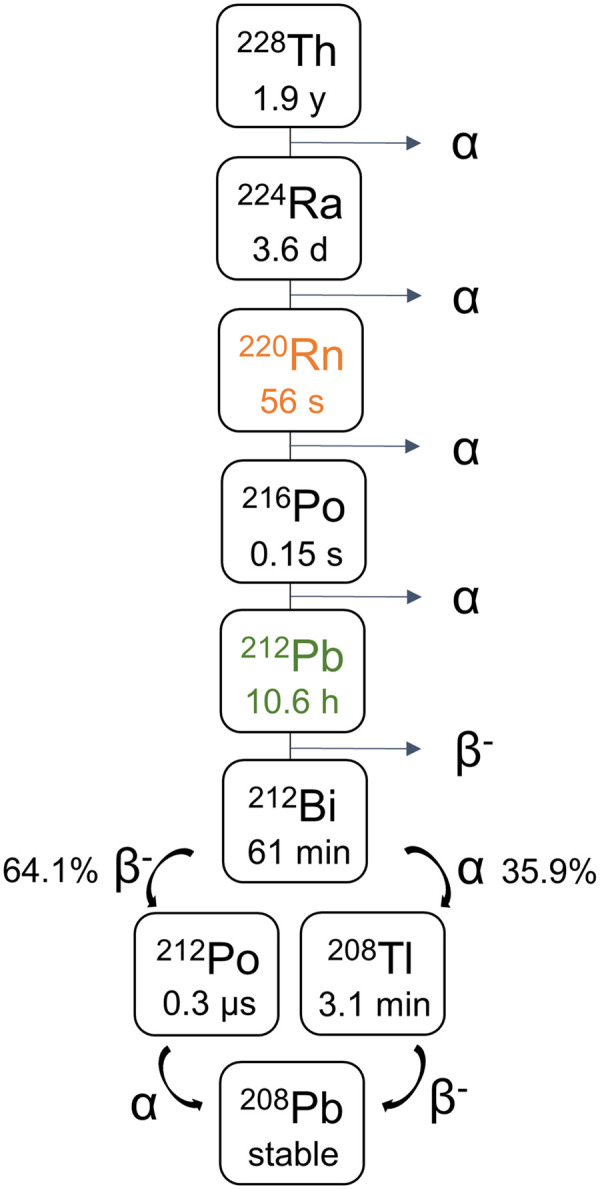
228Th series, with 220Rn and 212Pb highlighted.
A second approach is based on radon emanation, which involves obtaining 212Pb from gaseous 220Rn (half-life, 55.6 s) emanated from the decaying (228Th/)224Ra parent. Thus, 212Pb can be isolated from parent nuclides without the need for dedicated equipment for the separation process. Hassfjell and Hoff reported a generator comprising a 228Th source distributed within barium stearate and stored in a housing chamber connected to a vacuum pump (8). The source could be slid into the collection chamber via a gate valve. The generator experienced a relatively poor yield (11%–50%) because of radiation damage of the source when 40–50 MBq of 228Th were used. Other examples are based on 2-compartment systems in which 220Rn is transferred from a source chamber with parent nuclides into a collector chamber by airflow (9–11). These generators require significant effort and advanced equipment and have been tested in only small-scale production (≤2 MBq). Another drawback of such 2-compartment systems is that 220Rn may decay before reaching the collector chamber, potentially resulting in low 212Pb yields (9).
Here, we report a novel single-chamber generator based on 220Rn emanation from decaying 224Ra or 228Th to produce high yields of 212Pb for radiolabeling of ligands and monoclonal antibodies (mAbs). The generator is compact and user-friendly—key considerations for a shippable device that can be operated by the staff at a nuclear medicine facility.
MATERIALS AND METHODS
The 228Th/224Ra/212Pb Generator
An earlier generation of the generator was previously reported (12), but the recent version was optimized to increase output capacity and reduce the risk of cross-contamination. The generator, consisting of a 100-mL glass flask standing upside down, with the radionuclide source contained in the screw cap (Fig. 2) (13), was kept at room temperature the entire time. 228Th (Eckert and Ziegler or Oak Ridge National Laboratory) or 224Ra (prepared as previously described (7)) in 100–200 μL of 0.1–1 M HCl was applied to approximately 0.2 g of porous quartz wool (ProQuarz GmbH). The quartz wool was placed on a small plastic cap covered in aluminum foil to minimize 220Rn retention and secured inside the screw cap (Fig. 2). During 228Th/224Ra decay, the short-lived 220Rn emanated from the quartz wool, followed by adsorption of the longer-lived 212Pb daughter onto the interior surfaces of the flask. After approximately 2 d, the flask was carefully replaced with a clean flask to harvest 212Pb and reuse the generator, ensuring no cross-contamination from the source. To extract the 212Pb, 0.5–1 mL of 0.1 M HCl solution was added, and the flask was carefully swirled to cover the inner surface for about 5 min before the solution was collected.
FIGURE 2.
Single-chamber 212Pb generator consisting of glass flask and removable cap that contains 228Th or 224Ra source fixed onto porous quartz wool.
Radioactivity Measurements
A pure source of 224Ra reaches transient equilibrium with 212Pb after 2 d. We evaluated the yield of the 224Ra-based generator when 212Pb was harvested after 2–3 d, or as the average yield for the 228Th-based generator when 1 generator was used multiple times with at least a 2-d interval. The yield was defined as the percentage of 212Pb activity adsorbed to the flask relative to parent 224Ra or 228Th. The yield was also evaluated for generators that were milked for the second time. Radioactivity was quantified by a radioisotope dose calibrator (CRC-25R; Capintec Inc.) (12).
The breakthrough of 224Ra or 228Th in the washout solution at harvesting was quantified indirectly through the 212Pb activity of decayed samples—activity that was measured in the 60- to 110-keV window on a γ-counter (automatic γ-counter; Hidex Oy) (12). The details of the measurements and calculations are described in Supplemental Section 1 (supplemental materials are available at http://jnm.snmjournals.org).
220Rn emanation from the generator and the dose rate resulting from x-rays and γ-rays were evaluated for radiation safety purposes as described in detail in Supplemental Section 2.
Radiolabeling
To evaluate the quality of the extracted 212Pb, the tumor-targeting ligand NG001 (PSMA617-TCMC TFA; MedKoo Biosciences Inc.) and the S-2-(4-Isothiocyanatobenzyl)-1,4,7,10-tetraaza-1,4,7,10-tetra(2-carbamoylmethyl)cyclododecane (TCMC)–conjugated cetuximab (Erbitux; Merck Group) and rituximab (MabThera; Roche) were radiolabeled and the radiochemical purity was measured as described in Supplemental Section 3.
RESULTS
Generator Yield, Performance, and Feasibility
The single-chamber 212Pb generator was easy to use and handle. The 212Pb solution could be extracted at regular intervals, and the generator cap could be transferred to a clean flask each time for reuse. Its small size allowed measurement in a standard ionization chamber dose calibrator. The yield was approximately 62% for the tested 224Ra/212Pb generators of 2–22 MBq, of which 87%–91% of the deposited 212Pb could be extracted with 0.5–1 mL of 0.1 M HCl (Table 1; Supplemental Table 1). The 228Th-based generator of approximately 3.5 MBq had a stable yield of 67%–70% (Table 1; Supplemental Table 1). Hence, approximately 262 MBq of 224Ra and 163 MBq of 228Th are necessary initially per 100 MBq of 212Pb to be obtained after 2 d.
TABLE 1.
Data on Performance of Generators Based on 220Rn Emanation from Source of 224Ra or 228Th in Equilibrium with Its Daughters
| Type of generator | Yield | Available 212Pb in washout | Radioactivity breakthrough of parent source nuclide |
|---|---|---|---|
| 224Ra source | 62% (56%*) | 91% | 0.02%† (0.0004%–0.14%) |
| 228Th source | 68%‡ | 87% | 0.0001%–0.005%¶ |
Second-time use of generator (n = 3).
Average of 9.
Average of multiple uses of single generator (n = 8).
In 6/8 samples, measured radioactivity was below quantification limit after >2 mo (<0.0015% breakthrough; Supplemental Section 1).
The breakthroughs of 224Ra and 228Th were attributed to cross-contamination from the source. The radioactivity of 224Ra and 228Th was 0.0004%–0.14% and 0.0001%–0.005% relative to 212Pb, respectively, at the time of harvesting 212Pb (Table 1; Supplemental Table 1). In 6 of 8 samples (65–269 kBq of 212Pb initially) from the 228Th-based generator, the measured radioactivity was below the quantification limit of the instrument.
Radiation Safety Aspects
Our evaluation of generator integrity did not indicate any escape of 220Rn when the generator was closed. However, radon exposure from the generator is a potential radiation safety concern when the generator is opened, because the half-life of 220Rn is long enough for the gas to reach its surroundings. In the experimental setup in which a 1-MBq 224Ra-based generator was opened inside a sealed bag for 10 s, approximately 11% of the available 220Rn escaped (Supplemental Section 2).
Exposure to x-rays and γ-rays is another potential safety concern. The measurements on the surface of a 2-cm lead shield showed an average dose rate of 20 μSv/h per MBq of 228Th. The dose rate was considerably reduced to 2.3 μSv/h per MBq for a 5-cm lead shield and to 0.7 μSv/h per MBq for a 7-cm lead shield.
Radiochemical Purity of Radioconjugates
The 212Pb extracted from 224Ra-based generators was used to radiolabel TCMC-conjugated ligands and mAbs with a high and reproducible radiochemical purity for all tested compounds (Table 2).
TABLE 2.
Radiochemical Purity of Various Radioconjugates After Radiolabeling with 212Pb
| Radiolabeled substance | Radiochemical purity (average ± SD) |
|---|---|
| 212Pb-NG001 (n = 11) | 97% ± 2% |
| 212Pb-TCMC-cetuximab (n = 7) | 99% ± 1% |
| 212Pb-TCMC-rituximab (n = 6) | 99% ± 1% |
DISCUSSION
Here, we present an experimental 212Pb generator that is compact, easy to use, and operable without advanced equipment or hazardous chemicals. These considerations are important for the convenient and efficient routine production of 212Pb in clinical applications. To our knowledge, there are no existing 212Pb generators that meet these criteria entirely (1,14,15). The 228Th-based generator bypasses the 224Ra separation step from 228Th while being a longer-lived device that facilitates upscaled production of 212Pb at an industrial scale. Results show that a single 228Th-based generator could be milked every 2–5 d to routinely supply high-purity 212Pb for research and development. Radiopharmacies and hospitals must consider the exemption limit of 228Th—which is a tenth of that of 224Ra and 212Pb in the European Union and United States—when applying for permits for certified use. No well-defined criteria for an acceptable level of 228Th impurity in a radiopharmaceutical exist, but for 7 of 8 samples, the values were below the acceptance limit (<0.002%) for the impurity level of another therapeutic radiopharmaceutical that is described in the European Pharmacopoeia (16). Assuming a 100-MBq patient dose, the value is comparable to the effective dose-derived annual limit of intake of 228Th (17). The breakthrough of 224Ra from the 224Ra-based generator was comparable to the current state of the art (14). Hence, a clinically relevant purity is achievable with the presented technology.
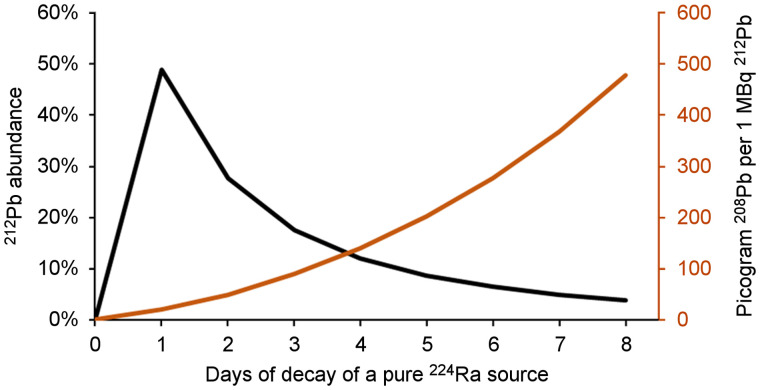
Decay of 228Th/224Ra results in an increasing accumulation of the stable daughter nuclide 208Pb in the generator, which potentially competes with 212Pb in radiolabeling procedures. The cumulative amount of 208Pb in the extracted 212Pb can be estimated on the basis of the generator yield (Fig. 3; Supplemental Section 4). In terms of mAb binding, these fractions may not influence radiochemical purity, as only 1 in about 2,000 mAbs needs to be bound by a 212Pb atom for a clinically relevant specific activity (18).
FIGURE 3.
Relationship between radioactive 212Pb and stable daughter nuclide 208Pb as function of time, shown as abundance of 212Pb relative to total amount of lead (black curve) and mass concentration of 208Pb per 1 MBq of extracted 212Pb (red curve), assuming generator yield of 62%.
The current generator is a prototype from which a limited number of 212Pb extractions have been performed. Along with upscaling, the radiation safety and yield may be areas for improvement in future studies. Both issues can be addressed by design considerations. The source-holding material, its size and volume, and the inner surface area of the generator can be optimized to increase the levels of 212Pb depositing onto the surface. It should be verified that the holding material is not affected when one is working with higher radioactivity levels. A closed system with an integrated shielding unit for the source in which the source or the shielding unit is movable (e.g., by a plunger) would facilitate operation without exposing the source during 212Pb extraction. Handling the generator inside hot cells or inside glove boxes or bags, or the use of tongs or similar equipment to protect the operator, is an important measure when working with clinically relevant activity levels (e.g., 100 MBq). Automation of the extraction process is considered feasible given that it entails only a surface-washing step and subsequent recovery of the solution.
CONCLUSION
220Rn emanation can be exploited to create a simple and effective generator that produces high-purity 212Pb without the need for advanced equipment, labor-intensive steps, or hazardous chemicals. Future versions of the presented technology should include simple modifications to shield the source during extraction of the 212Pb. The generator represents a promising method for efficient 212Pb production.
DISCLOSURE
Sciencons AS, owned by Roy Larsen, holds intellectual property rights for the presented technology under a patent application. Ruth Li and Vilde Stenberg were industrial PhD students financially supported by the Norwegian Research Council (grants 291228 and 290639) at the time of contributing to the article, at which Vilde Stenberg was also a shareholder at ArtBio AS. Ruth Li is employed at Oncoinvent AS, Vilde Stenberg is employed at ArtBio AS, and Roy Larsen is the chairman of the board of both companies, which use the presented technology for research-and-development projects. Roy Larsen owns stock directly or indirectly in Sciencons AS, Oncoinvent AS, and ArtBio AS. No other potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENT
We thank Marion Masitsa Malenge for the experimental work concerning the radiolabeling of antibodies.
KEY POINTS
QUESTION: Can 212Pb, intended for radiopharmaceuticals, be produced by a simple generator based on 220Rn emanation from a 228Th or 224Ra source?
PERTINENT FINDINGS: The proposed generator was easy to handle and could routinely be used to produce 212Pb of high purity, suitable for radiolabeling of antibodies and ligands.
IMPLICATIONS FOR PATIENT CARE: Rapid and efficient production methods such as the one proposed are important for 212Pb to be available for patients with metastatic cancer.
REFERENCES
- 1. Yong K, Brechbiel M. Application of 212Pb for targeted α-particle therapy (TAT): pre-clinical and mechanistic understanding through to clinical translation. AIMS Med Sci. 2015;2:228–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atcher RW, Friedman AM, Hines JJ. An improved generator for the production of 212Pb and 212Bi from 224Ra. Int J Rad Appl Instrum [A]. 1988;39:283–286. [DOI] [PubMed] [Google Scholar]
- 3. Baidoo KE, Milenic DE, Brechbiel MW. Methodology for labeling proteins and peptides with lead-212 (212Pb). Nucl Med Biol. 2013;40:592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Torgue J, Maquaire P, Yong J, et al., inventors; Orano Med SAS, assignee. Method and apparatus for the production of lead 212 for medical use. U.S. Patent US11037690B2. June 15, 2021.
- 5. Li M, Zhang X, Quinn TP, et al. Automated cassette-based production of high specific activity [(203/212Pb)Pb]peptide-based theranostic radiopharmaceuticals for image-guided radionuclide therapy for cancer. Appl Radiat Isot. 2017;127:52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stenberg VY, Juzeniene A, Bruland ØS, et al. In situ generated 212Pb-PSMA ligand in a 224Ra-solution for dual targeting of prostate cancer sclerotic stroma and PSMA-positive cells. Curr Radiopharm. 2020;13:130–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Westrøm S, Generalov R, Bonsdorff TB, et al. Preparation of 212Pb-labeled monoclonal antibody using a novel 224Ra-based generator solution. Nucl Med Biol. 2017;51:1–9. [DOI] [PubMed] [Google Scholar]
- 8. Hassfjell SP, Hoff P. A generator for production of 212Pb and 212Bi. Appl Radiat Isot. 1994;45:1021–1025. [Google Scholar]
- 9. Boldyrev PP, Egorova BV, Kokov KV, et al. Physical and chemical processes on the 212 Pb radionuclide production for nuclear medicine. J Phys Conf Ser. 2018;1099:012003. [Google Scholar]
- 10. Hassfjell S. 212Pb generator based on a 228Th source. Appl Radiat Isot. 2001;55:433–439. [DOI] [PubMed] [Google Scholar]
- 11. O’Hara MJ, inventor; Battelle Memorial Institute, assignee. System, emanation generator, and process for production of high-purity therapeutic radioisotopes. U.S. patent US2018/0047474A1. February 15, 2018.
- 12. Napoli E, Stenberg VY, Juzeniene A, et al. Calibration of sodium iodide detectors and reentrant ionization chambers for 212Pb activity in different geometries by HPGe activity determined samples. Appl Radiat Isot. 2020;166:109362. [DOI] [PubMed] [Google Scholar]
- 13. Napoli E. Quantification Methods of 224Ra and 212Pb Activity Applied to Characterize Therapeutic Radiopharmaceuticals. Thesis. University of Oslo; 2021.
- 14. Kokov K, Egorova B, German M, et al. 212Pb: production approaches and targeted therapy applications. Pharmaceutics. 2022;14:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Radchenko V, Morgenstern A, Jalilian AR, et al. Production and supply of α-particle-emitting radionuclides for targeted α-therapy. J Nucl Med. 2021;62:1495–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yttrium (90Y) chloride solution for radiolabeling. In: European Pharmacopoeia 9.7. 2019:2803.
- 17. Carbaugh EH. New Stochastic Annual Limits on Intake for Selected Radionuclides. National Technical Information Service; 2009. [Google Scholar]
- 18. Meredith RF, Torgue J, Azure MT, et al. Pharmacokinetics and imaging of 212Pb-TCMC-trastuzumab after intraperitoneal administration in ovarian cancer patients. Cancer Biother Radiopharm. 2014;29:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]