Abstract
Objective
To assess the frequency of polyautoimmunity (PolyA) in a cohort of Colombian patients with systemic lupus erythematosus (SLE) and to identify associated factors.
Methods
This is an analytical cross-sectional study in a specialized center., a comprehensive review of the medical records of SLE patients was performed from 2015 to 2020 in order to obtain demographic, clinical data, laboratory, and treatment information. Associations between PolyA, demographic, and characteristics of the disease were explored.
Results
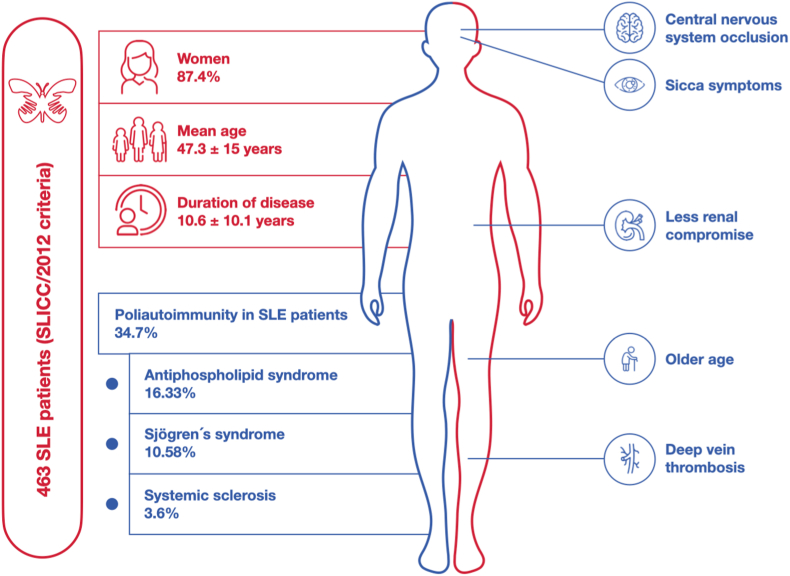
A total of 463 patients were included in the analysis. The average age was 47.3 ± 15 years. Most of this population were female (87.4%), whom were diagnosed with SLE in a long-term SLE (10.6 ± 10.1 years). Out of the total patients, 34.7% were diagnosed with PolyA. Among the most frequent clinical criteria for SLICC, arthritis (65%), kidney impairment (39.5%), and alopecia (34.8%) were found. The most frequent SLE-associated PolyA were antiphospholipid syndrome (APS) and Sjögren's syndrome (SS) (16.63% and 10.58%, respectively). PolyA-associated factors were age, xerophthalmia, central nervous system occlusion, and deep vein thrombosis (DVT). In contrast, renal impairment was significantly less frequent in PolyA patients after multivariate analysis.
Conclusion
The results have showed associated factors with PolyA like age, xerophthalmia, central nervous system occlusion, and deep vein thrombosis in this cohort. On the other hand, lupus nephritis was less frequent in patients with PolyA. This study provides a spotlight of a specific SLE population as real-life evidence for a better characterization of PolyA in the future.
Keywords: Systemic lupus erythematosus, Autoimmune diseases, Epidemiology, Polyautoimmunity
Abbreviations
- AD
Autoimmune diseases
- AITD
Autoimmune thyroid disease
- ANA
antinuclear antibodies
- anti-DFS70
Dense-Fine-Speckled
- Anti-dsDNA
Anti-double stranded
- Anti-Sm
Smith antibodies
- APS
antiphospholipid syndrome
- DVT
Deep vein thrombosis
- GLADEL
Grupo Latino-Americano de estudio del Lupus
- HLA
Human leukocyte antigen
- ICD-10
International Classification of Diseases-10th
- LUMINA
Systemic Lupus erythematosus in a multiethnic cohort
- OR
Odds Ratio
- PE
Pulmonary embolism
- PolyA
Polyautoimmunity
- RELESSER
Systemic lupus erythematosus of the Spanish Society of Rheumatology
- SLE
Systemic lupus erythematosus
- SLICC
Systemic Lupus International Collaborating Clinics
- SS
Sjögren's syndrome
- STROBE
Strengthening the Reporting of Observational studies in Epidemiology
- TLR
Toll Like receptor
1. Introduction
Autoimmune diseases (AD) represent one of the heterogeneous pathologies that affect specific organs or systems. AD manifestations and pathogenesis are diverse. The estimated global prevalence is 4.5%, affecting up to 10% of the population in developed countries. Compared to the general population these diseases affect the functionality and quality of life. Moreover, mortality rate has been estimated near to 14.6 per million population in some research, however this number maybe underestimated because the small number of the studies about this topic [1,2]. The mean age of onset is between 40 and 50 years of age. Evidence has shown that ADs share multiple clinical signs, physio-pathological mechanisms, environmental and genetic factors as a common origin. This evidence might explain the concept of polyautoimmunity (PolyA), as the presence of two or more (well-defined) AD in a single individual [3]. Female gender, joint involvement, history of familial autoimmunity, anti-Ro antibody positivity, and reduced exposure to antimalarial drugs have been described as risk factors for developing PolyA [4].
It has been suggested that multifactorial environmental triggers, immunological and hormonal factors, genetic susceptibility, and epigenetics are involved in the pathogenesis of PolyA, even though the nature of etiopathogenesis and the precise cause of AD remains unknown. The frequency of AD has increased significantly in recent decades, suggesting a more significant influence of environmental factors than genetics in developing AD [5].
The presence of PolyA may be associated with worse outcomes and higher morbidity, as recently demonstrated in a population-based study carried out involving more than 20 million people in UK [6]. An increased risk of cardiovascular disease with AD was seen for every individual cardiovascular disease and increased progressively with the number of AD present (i.e., Polyautoimmunity). Analyzing in depth the factors associated with PolyA and its possible outcomes, contributing to the context of the classical term called comorbidity which refers to the presence of additional diseases in relation to an index disease in one individual, in the present case PolyA. This is important in the framework of the construct that evaluates the multimorbidity (i.e., presence of multiple diseases in one individual) on the morbidity burden and patient's complexity as exposed by Valderas et al. [7].
The frequency of PolyA has been evaluated in various autoimmune pathologies, showing that it is frequently in different groups [8]. PolyA has been observed in approximately 40% of patients diagnosed with systemic lupus erythematosus (SLE) or Sjögren's syndrome (SS) and in 15–20% of patients diagnosed with rheumatoid arthritis [4]. Multiple studies to date have shown that race, gender, socioeconomic status, and educational level, among others, are associated with the development of AD, particularly SLE [9].
Studies in patients diagnosed with SLE and PolyA in different latitudes allowed us to evaluate the behavior in other populations, including Latin American patients. A multicenter cohort “Grupo Latino-Americano de estudio del Lupus” by its acronym in spanish (GLADEL) performed in Latin America, found significant differences between Latins, African Americans, mixed race or mestizos, and caucasian regarding kidney disease, pericarditis, polyadenopathies, severity of disease measured through disease scales, and different outcomes in patients with SLE [10,11].
Nowadays, clinical and immunological relevance of PolyA has not been thoroughly studied and some research findings suggest a common origin for the different AD [12]. Furthermore, differential pattern recognition in autoimmunity may allow the implementation of personalized strategies in order to manage these diseases [13]. Therefore, the present study intend to assess the frequency of PolyA in a cohort of Colombian patients with SLE and its associated factors in order to provide evidence for profiling patients with PolyA in the Latin American population.
2. Materials and methods
An analytical cross-sectional observational study was conducted in a specialized AD center in Bogotá, Colombia. The convenience sample size included all patients treated over 18 years of age with a diagnosis of SLE (according to the ICD-10 code), between January 2015 and December 2020, who met the SLICC/2012 criteria [14]. Patients whose clinical chart did not have the pre-defined variables were excluded. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement was followed be available at Appendix B [15].
PolyA, defined as the presence of one or more AD in addition to SLE, was assessed. Compliance with internationally validated classificatory criteria for the following diseases was verified: Antiphospholipid syndrome (APS) [16], autoimmune thyroid disease (AITD) [17], Sjögren Syndrome (SS) [18], rheumatoid arthritis [19], systemic sclerosis [20], autoimmune hepatitis [21], primary biliary cirrhosis [22], vitiligo [23], type 1 diabetes mellitus [24], myasthenia gravis [25], multiple sclerosis [26], and systemic vasculitis [[27], [28], [29]].
Data were extracted from electronic medical records by two expert physicians filling out a form previously established by the researchers using the REDCap tool [30]. Records were included consecutively in order to address the selection and data collection bias. As a result, information was obtained on sociodemographic variables, comorbidities, clinical and laboratory variables of the SLICC/2012 criteria [14], signs and symptoms of the disease, data on hematological, hepatic, renal involvement, and any other systemic level and the presence of autoantibodies such as rheumatoid factor (RA test), Anticardiolipin IgM, Anticardiolipin IgG, Lupus Anticoagulant, antinuclear antibodies (ANA), Anti-double stranded (Anti-dsDNA), Smith Antibodies (Anti-Sm) and Complement C3 and C4. Additionally, information regarding treatments received in the follow-up period from 2015 to 2020 was extracted.
Regarding statistical analysis, categorical variables are presented as frequencies and proportions and quantitative variables through central tendency and dispersion measures according to their distribution. The Chi-squared or Fisher test was used to compare patients with and without PolyA for categorical variables. T-test or the Mann-Whitney U test was used to compare quantitative variables according to their distribution. Finally, a simple logistic regression model and a multiple logistic regression model were performed by calculating the Odds Ratio (OR) measure of association and models were adjusted by confounding variables. The dependent variable was PolyA, and the independent variables were those statistically significant variables from the bivariate analysis and those biologically plausible. Statistical significance was defined with a p-value ≤0.05. Data were analyzed using the statistical program Stata 13®.
2.1. Ethics approval
This study was conducted following the principles of the Declaration of Helsinki and was approved by the Research Ethics Committee on Human Beings - Hospital de San José, Bogotá, Colombia (Record 0317–2021, June the 1st 2021) and by the Research committee of Biomab IPS (Record 006, May the 3rd 2022).
3. Results
480 medical records of patients with a diagnosis of SLE were evaluated fulfilling strict SLICC/2012 criteria. There were 17 patients with insufficient data in the clinical chart and were excluded, therefore, 463 were finally included. Out of the total included, 405 were women (87.4%). The mean age was 47.3 ± 15 years, and the mean disease duration was 10.6 ± 10.1 years. The most frequent comorbidities were arterial hypertension (25.5%), osteoarthritis (18.8%), and hypothyroidism (17.5%). Three percent of patients had familial autoimmunity (at least one first-degree relative with confirmed AD). The remaining comorbidities and clinical and baseline characteristics of the disease can be seen in Table 1.
Table 1.
Sociodemographic and clinical characteristics of the disease.
| Characteristics n (%) | No PolyA n = 302 (%) | PolyA n = 161 (%) | Total N = 463 (%) | P-value | |||
|---|---|---|---|---|---|---|---|
| Female sex | 270 | 89.4 | 135 | 83.9 | 405 | 87.5 | 0.086 |
| Age, mean (SD) | 45.4 | 15.7 | 50.9 | 13.7 | 47.3 | 15.3 | 0.000 |
| Duration of illness, mean (SD) | 10.0 | 10.7 | 11.7 | 8.7 | 10.6 | 10.1 | 0.007 |
| Age of onset, mean (SD) | 36.0 | 15.7 | 39.0 | 14.5 | 37.1 | 15.3 | 0.025 |
| Arterial hypertension | 84 | 27.8 | 34 | 21.1 | 118 | 25.5 | 0.115 |
| Diabetes mellitus | 17 | 5.6 | 7 | 4.3 | 24 | 5.2 | 0.554 |
| Cancer | 11 | 3.6 | 8 | 5.0 | 19 | 4.1 | 0.493 |
| Osteoporosis | 30 | 9.9 | 29 | 18.0 | 59 | 12.7 | 0.013 |
| Dyslipidemia | 2 | 0.7 | 3 | 1.9 | 5 | 1.1 | 0.234 |
| Infectionsa | 15 | 5.0 | 12 | 7.5 | 27 | 5.8 | 0.277 |
| Osteoarthritis | 52 | 17.2 | 35 | 21.7 | 87 | 18.8 | 0.236 |
| Hypothyroidism | 46 | 15.2 | 35 | 21.7 | 81 | 17.5 | 0.079 |
| Heart failure | 5 | 1.7 | 2 | 1.2 | 7 | 1.5 | 0.728 |
| Chronic kidney disease | 21 | 7.0 | 6 | 3.7 | 27 | 5.8 | 0.158 |
| Ischemic heart disease | 8 | 2.6 | 4 | 2.5 | 12 | 2.6 | 0.915 |
| Other conditionb | 97 | 34.2 | 66 | 45.5 | 163 | 38.0 | 0.022 |
| Non-SLE-APS thrombosis | 2 | 0.7 | 0 | 0.0 | 2 | 0.4 | 0.301 |
| Smoking | 3 | 1.0 | 2 | 1.2 | 5 | 1.1 | 0.805 |
| Familial autoimmunity | 7 | 2.7 | 5 | 3.5 | 12 | 3.0 | 0.641 |
| SLEDAI | 2.3 | 3.0 | 2.4 | 3.1 | 2.3 | 3.0 | 0.986 |
| SLICC Clinical criteria n (%) | |||||||
| Acute cutaneous lupus | 104 | 34.4 | 48 | 29.8 | 152 | 32.8 | 0.313 |
| Chronic cutaneous lupus | 12 | 4.0 | 2 | 1.2 | 14 | 3.0 | 0.102 |
| Oral or nasal ulcers | 70 | 23.2 | 38 | 23.6 | 108 | 23.3 | 0.918 |
| Non- scarring alopecia | 110 | 36.4 | 51 | 31.7 | 161 | 34.8 | 0.307 |
| Synovitis | 194 | 64.2 | 107 | 66.5 | 301 | 65.0 | 0.633 |
| Serositis | 22 | 7.3 | 15 | 9.3 | 37 | 8.0 | 0.443 |
| Renal | 137 | 45.4 | 46 | 28.6 | 183 | 39.5 | 0.000 |
| Neurological | 6 | 2.0 | 6 | 3.7 | 12 | 2.6 | 0.262 |
| SLICC immunological criteria n (%) | |||||||
| Hemolytic anemia | 34 | 11.3 | 25 | 15.5 | 59 | 12.7 | 0.189 |
| Lymphopenia | 145 | 48.0 | 100 | 62.1 | 245 | 52.9 | 0.004 |
| Thrombocytopenia | 40 | 13.2 | 45 | 28.0 | 85 | 18.4 | 0.000 |
| ANAs (n = 404) | 239/258 | 92.6 | 141/146 | 96.6 | 380/404 | 94.0 | 0.107 |
| Anti-dsDNA | 123 | 40.7 | 66 | 41.0 | 189 | 40.8 | 0.956 |
| Anti-sm | 56 | 18.5 | 27 | 16.8 | 83 | 17.9 | 0.636 |
| Antiphospholipid Antibodies | 56 | 18.5 | 72 | 44.7 | 128 | 27.6 | 0.000 |
| Low complement | 161 | 53.3 | 89 | 55.3 | 250 | 54.0 | 0.686 |
| Positive direct Coombs ‘test | 3 | 1.0 | 4 | 2.5 | 7 | 1.5 | 0.210 |
Chronic infections like Tuberculosis, Hepatitis virus, and others.
Other musculoskeletal conditions, ophthalmologic pathologies, gastroesophageal reflux and psychiatric or metabolic disorders not SLE related; PolyA: polyautoimmunity; SD: standard deviation; SLE: systemic lupus erythematosus; APS: antiphospholipid syndrome; SLEDAI: Systemic Lupus Erythematosus Disease Activity Index (Most recent value obtained from electronic medical records); SLICC: Systemic Lupus International Collaborating Clinics; ANA: Antinuclear Antibodies; Anti-dsDNA: Anti-double stranded DNA; Anti-sm: Smith Antibodies.
Among the most frequent SLICC (Systemic Lupus International Collaborating Clinics) clinical criteria, arthritis (65%), renal impairment (39.5%), and alopecia (34.8%) were found. The frequency of compliance with immunological and other clinical criteria can be seen in Table 1. Among the symptoms not contemplated in the SLICC criteria, the most frequent were fatigue (14.9%) and xerostomia (14%), among others that can be visualized in Appendix A.1. Regarding treatment, 76% received antimalarials followed by glucocorticoids in 65%, and azathioprine in 38.7%. Additional information on medications and laboratory findings can be found in Appendix A.2 and Appendix A.3.
From all SLE patients, 34.77% presented PolyA (n = 161), APS as being the most frequent AD (n = 77, 16.63%), followed by SS (n = 49, 10.58%), and systemic sclerosis (n = 16, 3.6%). Patients with PolyA were significantly older, experienced by symptoms and duration disease, presented more frequently osteoporosis, and had a higher frequency of comorbidities when compared to those without PolyA. Furthermore, patients with PolyA had a significantly higher frequency of dry symptoms, thrombotic manifestations (deep vein thrombosis, pulmonary embolism (PE), and central nervous system occlusion), higher frequency of thrombocytopenia, and lower frequency of edema and proteinuria. It was observed that patients with PolyA received a lower proportion of antimalarials when compared to those without PolyA in the same analysis. When analyzing the SLICC clinical criteria comparatively in the two groups, patients with PolyA had a lower frequency of renal involvement and a higher frequency of lymphopenia and thrombocytopenia. From an immunological criteria point of view, patients with PolyA had a significantly higher frequency of antiphospholipid antibodies (Table 2).
Table 2.
Simple logistic regression results in Polyautoimmunity cohort in SLE patients.
| OR | CI 95% | |
|---|---|---|
| Demographic and baseline characteristics of the disease | ||
| Age | 1,02 | 1,01 - 1,03 |
| Female sex | 0,62 | 0,35 - 1,07 |
| Duration of symptoms | 1,02 | 1,00 - 1,05 |
| Osteoporosis | 1,99 | 1,14 - 3,45 |
| Osteoarthritis | 1,33 | 0,83 - 2,16 |
| Chronic kidney disease | 0,52 | 0,20 - 1,31 |
| Ischemic heart disease | 0,93 | 0,27 - 3,15 |
| Familial autoimmunity | 1,31 | 0,41 - 4,23 |
| Symptoms and signs | ||
| Xerophthalmia | 3,92 | 2,06 - 7,48 |
| Proteinuria | 0,6 | 0,38 - 0,95 |
| Pulmonary embolism | 5,21 | 1,82 - 14,9 |
| Dry skin | 2,25 | 1,11 - 4,54 |
| Dry mouth | 3,39 | 1,97 - 5,82 |
| Oedema | 0,43 | 0,21 - 0,89 |
| Central nervous system occlusion | 11 | 2,40 - 50,26 |
| Peripheral arterial occlusion | 0,93 | 0,08 - 10,41 |
| Deep vein occlusion | 5,56 | 2,69 - 11,52 |
| Superficial venous occlusion | 1,88 | 0,11 - 30,27 |
| Treatment | ||
| Metotrexate | 1,37 | 0,81 - 2,33 |
| Antimalarials | 0,56 | 0,36 - 0,86 |
| Corticosteroids | 1,2 | 0,80 - 1,80 |
| Laboratory Results | ||
| Positive anti-dsDNA antibodies | 1,01 | 0,68 - 1,49 |
| Positive RF test | 4,25 | 1,59 - 11,29 |
| Positive aCL IgM | 2,33 | 1,22 - 4,43 |
| Positive aCL IgG | 6,67 | 2,88 - 15,46 |
| Positive Lupus anticoagulant testing | 2,78 | 1,42 - 5,41 |
| SLEDAI | 1,00 | 0,94 - 1,07 |
| SLICC Criteria | ||
| Acute cutaneous lupus | 0,80 | 0,53 - 1,22 |
| Chronic cutaneous lupus | 0,30 | 0,07 - 1,37 |
| Oral or nasal ulcers | 1,02 | 0,65 - 1,60 |
| Non- scarring alopecia | 0,80 | 0,53 - 1,21 |
| Synovitis | 1,10 | 0,73 - 1,65 |
| Serositis | 1,30 | 0,65 - 2,59 |
| Renal | 0,48 | 0,31 - 0,72 |
| Neurological | 1,90 | 0,60 - 6,01 |
| Hemolytic anemia | 1,44 | 0,83 - 2,52 |
| Lymphopenia | 1,77 | 1,20 - 2,62 |
| Thrombocytopenia | 2,54 | 1,07 - 3,20 |
| ANA positive | 2,24 | 0,82 - 6,13 |
| Anti-dsDNA | 1,01 | 0,68 - 1,49 |
| Anti-sm | 0,88 | 0,53 - 1,46 |
| Antiphospholipid Antibodies | 3,55 | 2,32 - 5,43 |
| Low complement | 1,08 | 0,73 - 1,58 |
| Positive direct Coombśtest | 2,53 | 0,56 - 1,48 |
aCL: anticardiolipin antibodies; ANA: Antinuclear Antibodies; Anti-dsDNA: Anti-double stranded DNA; Anti-SM: smith antibodies; APS: antiphospholipid syndrome; PolyA: polyautoimmunity; SD: standard deviation; RF: Rheumatoid factor; SLE: systemic lupus erythematosus; SLEDAI: Systemic Lupus Erythematosus Disease Activity Index; SLICC: Systemic Lupus International Collaborating Clinics.
It was found through multiple logistic regression, that a higher age, the presence of xerophthalmia, central nervous system occlusion, and deep vein thrombosis were associated factors with PolyA. On the contrary, renal impairment by SLICC was significantly less frequent in PolyA patients (Table 3 and Fig. 1).
Table 3.
Multiple logistic regression results.
| OR | CI 95% | |
|---|---|---|
| Male | 1,77 | 0,94–3,32 |
| Age | 1,01 | 1,00–1,03 |
| Xerophthalmia | 3,64 | 1,84–7,22 |
| CNS Occlusion | 10,23 | 2,11–49,46 |
| Deep vein occlusion | 5,98 | 2,79–12,8 |
| SLICC renal | 0,61 | 0,38–0,96 |
| Corticosteroids | 1,24 | 0,79–1,93 |
CNS: central nervous , system, SLICC: Systemic, Lupus, International Collaborating Clinics.
Fig. 1.
Associated factors with Polyautoimmunity in patients with Systemic Lupus Erythematosus.
Antiphospholipid syndrome was more prevalent in this cohort of patients. Patients with PolyA shows less renal compromise than patients without PolyA.
4. Discussion
When analyzed as a group, PolyA is frequently reported in AD catching up with one-third of patients [31,32]. This study, which compared the clinical and serological characteristics of patients with SLE, shows a prevalence of 34% of PolyA in this cohort of Colombian SLE patients, lower than a previous report that estimated 41% of PolyA in a similar population [32].
AD shows different clinical behavior but share phenotypes that demonstrate common susceptibility and characteristics [1,33]. SLE association with other AD such as SS, APS, or autoimmune thyroid disease is not uncommon [32]. In this study, up to 16.6% of patients with SLE had associated APS. However, the distinction between two coexisting AD in the same individual is considered a challenge in clinical practice due to the similarity of the various symptoms and signs in this group of diseases [8]. An example of this is the differentiation between lupus arthritis and rheumatoid arthritis. Only joint pain is present without systemic manifestations in early SLE stages, making it difficult for clinical differentiation between these two entities. For example, rheumatoid arthritis and SLE, association is called “Ruphus” [34]. In addition, patients may be up to 2 times more likely to present another autoimmune disease in the long term [34].
As studied through the years, the coexistence of APS as a primary AD, not as a superimposed syndrome secondary to SLE, is essential to characterize it as an AD that shares common clinical features and pathophysiologic mechanisms with other AD. Rivier Gilles et al. [35], since 1994, described the presence of SLE in a retrospective cohort of 23 patients with APS, as a co-morbidity and not as a secondary disease, as well as Cervera et al. [36], that in 2002 described a cohort of 1000 patients with APS, where 36.2% (362) had SLE as co-morbidity. Thus, the international consensus statement on updating classification criteria for APS in 2006 discourages the term referring to secondary APS and suggests referring to coexistence with SLE or other related AD [16].
This association between APS and SLE is imperative in the description of PolyA and the various clinical characteristics of the patients who are suffering from that kind of disease [[37], [38], [39], [40]]. In fact, as shown by Cervera et al. [41], where they analyzed a cohort for 10 years, they found that 36.2% of patients had associated APS with SLE. This contrasts with the results of this study, since only 16.63% were found with this association. The presence of these two diseases has been associated with an elevated cardiovascular risk and increased mortality from cardiovascular or thrombotic disease. The study by Cervera et al. [41], showed that the causes of death (mediated by thrombotic phenomena) were hemorrhagic events up to 22.2% and stroke in 11.1%. In our study we did not evaluate the association between mortality and PolyA due to the study type, given that we didn't have follow-up and temporality to evaluate that outcome.
In the present study, the frequency of SS in SLE patients was 10.5%, a very similar finding reported by Sieiro Santos et al. [42], who found a frequency of 11% in 453 SLE patients that were analyzed. This group of researchers highlights that patients with PolyA SLE-SS are a subgroup with distinct clinical and serologic characteristics, in addition to the fact that frequency of SLE-SS increases with age. Finally, they highlight that this group of patients have a higher frequency of oral ulcers, anti-Ro, and anti-La antibodies, and a lower frequency of renal disease, as described in the present study for the whole PolyA group. In assessing patients with SLE, it is crucial to consider all these characteristics, according to the opinion of other groups of investigators, within the taxonomic discussion to avoid the term secondary SS [43].
Given the morbidity associated with lupus nephritis, identifying patients with SLE who can potentially develop this organ involvement is critical. Different studies have concluded that patients carrying HLA-DR4 and DR11 alleles specifically are protected against lupus nephritis contrary to what happens with HLA-DR3 and DR15 carriers [44]. Although the genomic mechanisms of susceptibility or protection for developing lupus nephritis, including those specific to HLA, are not fully understood, they are considered part of the theory that would explain SLE onset and lupus nephritis [44].
Currently, there is little evidence to support a positive or negative association between the presence of PolyA and lupus nephritis. A case-control study of patients with PolyA SLE-SS compared to patients with SLE excluding PolyA showed greater renal failure and proteinuria onset in the latter (OR 0.44 CI95% 0.10–1.88 p = 0.27) [4,42]. This result agrees with the findings of the present study. Contrary to expected, an inverse association was demonstrated (OR 0.61 CI95% 0.38–0.96), with the presence of lupus nephritis being less frequent in those patients with PolyA. Similarly, in the registry of patients with SLE of the Spanish Society of Rheumatology (RELESSER), a higher frequency of renal impairment was reported in the group of patients with SLE without PolyA when compared to those with PolyA, a difference that was not significant when analyzed using binary logistic regression [4].
Although it was not the objective of the present study, in the literature it is found some plausible biological factors that may explain the lower frequency of lupus nephritis when PolyA is present in a SLE patient. Among them, the expression of Dense-Fine-Speckled-70 (anti-DFS70) antibodies that confer a protective role against renal injury in murine lupus nephritis [45,46]. These antibodies are present and increased in patients with thrombophilia [47,48], such as in APS case, the most frequent PolyA found in this study. Another possibility is the involvement of microRNA-16, which is involved in a variety of AD and has been shown to inactivate the TLR4 signaling pathway to inhibit lupus nephritis-induced kidney tissue hyperplasia and mesangial cell proliferation [49,50]. Its occurrence has been confirmed in patients with SS [51], another of the diseases found in PolyA with SLE in the present study. Notably, few studies have analyzed this association [52]. Therefore, further research is required concerning the search for protective factors for the development of lupus nephritis, such as gender, antimalarials, arterial hypertension, and the specific autoantibody profile, among others, involving the analysis of PolyA.
The therapy treatment course is a critical aspect in all AD and PolyA, which can only be determined based on knowledge of the pathogenesis. Antimalarials are one of the oldest groups of drugs used in treating SLE. Its effects are given by controlling disease activity, decreasing the risk of thrombosis, and improving long-term prognosis, among other effects. Regarding its use in individuals with PolyA, Mena-Vázquez et al. [4], indicate that patients with PolyA were approximately 50% less likely to receive antimalarial drugs than patients without PolyA. If they did receive them, it was for a shorter period. Similarly, in the study by Ordoñez-Cañizarez et al. [52], hydroxychloroquine was identified as a protective factor for PolyA. Findings showed agreement with the present study results, which suggest using antimalarials as a protective factor for PolyA (OR 0.56 CI95% 0.36–0.86). Notwithstanding this finding, the result was not confirmed in the multivariate analysis. Thus, further studies including this variable as an outcome are required to determine whether there is a protective association. Likewise, given the clinical subphenotype according to the diseases found in PolyA, consideration should be given to the type of treatment for patients with SLE.
Female gender and older age have been considered risk factors associated with PolyA. According to this study, 83.9% of patients with SLE and PolyA were women, agreeing with other research reports and patient registries [4,52,53]. In the study conducted by Ordoñez-Cañizares et al. [52], patients with SLE and PolyA were older than those without PolyA, as in the RELESSER cohort [4], where PolyA patients were older and had longer disease duration. These results match those found in the present study. Through a bivariate analysis, patients with SLE and PolyA were significantly older, had longer symptom and disease duration. In the multiple regression analysis, age was a factor associated with PolyA. Perhaps this factor is associated with an interval during which the patient is predisposed to new environmental interactions [52], according to what has been discussed from a tautology autoimmune point of view [1], allowing autoimmunity or aging progression, coupled with inflammaging (molecular aging of inflammatory origin) and the consequent failure to tolerance mechanisms [54]. Nonetheless, we did not analyze the time of appearance between one AD and the other, given the nature of our study design, as other groups have done. Such is the case of the study by Anaya et al. [55], where they found, in a group of patients with PolyA, that the most frequent first AD was SLE, and the time interval between the first and second AD, was 49 ± 68 months and between the second and third AD was 34 ± 48 months.
However, several weaknesses of the present study include its observational nature based on real world data, with biases inherent to this type of study. In addition, preference in selection is likely because we worked on prevalent cases. Nonetheless, as noted in the materials and methods, all patients were checked for strict compliance with SLICC/2012 classification criteria, as is applied at rheumatology center for SLE diagnosis. Although EULAR/ACR 2019 were not used, their sensibility has been shown to be similar as SLICC/2012 criteria [56,57] but there are differences on specificity, therefore could cause bias in the study. Also, the use of secondary sources of data collection leads to potential biases related to patient registries. On the other hand, routine antithyroid antibodies are not available in daily practice in Colombia. Thus, the frequency of autoimmune thyroid disease in PolyA in this group of SLE patients could not be accurately established.
Despite these limitations, this study relied on the availability of extensive data from a single specialized rheumatologic center. Based on this information, we could compare cohorts of patients whose results provide an overview of a specific population with SLE as real-life evidence for a better characterization of PolyA in the country. Associated factors with PolyA found in this and additional studies should be explored as part of future research to confirm the relationship and the role they play in developing PolyA.
Author contributions
Laura Villarreal coordinated resources project, administrated and reviewed the manuscript. Pedro Santos-Moreno and Adriana Rojas-Villarraga, were involved in all phases from planning the study, they contribute to the conceptualization and design of the work. Paula D. Nieto-Zambrano and Julian Arias-Aponte participated in Data curation, the formal analysis and writing original draft of the manuscript. Linda Ibatá; and Susan Martinez participated in the methodology, interpretation data and helped in the drafting process. Gabriel-Santiago Rodríguez-Vargas and Jaime-Andrés Rubio-Rubio helped with the investigation, Data curation and writing-review & editing the intellectual content of the manuscript. Pedro Rodríguez helped on the supervision and writing-review & editing the content of the manuscript. Pedro Santos-Moreno and Julian Arias-Aponte are the authors responsible for the overall content as the guarantors. All authors have contributed significantly to this study, they approval the final version to be published.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Submission declaration and verification
This study has not been published previously and is not under consideration for publication elsewhere. All authors and responsible authorities approved its publication. If accepted, it will not be published elsewhere in the same form in English or in any other language.
Declaration of competing interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Adriana Rojas-Villarraga reports a relationship with AbbVie Inc that includes: speaking and lecture fees. Adriana Rojas-Villarraga reports a relationship with Amgen Inc that includes: speaking and lecture fees. Adriana Rojas-Villarraga reports a relationship with Janssen Pharmaceuticals Inc that includes: speaking and lecture fees. Adriana Rojas-Villarraga reports a relationship with Pfizer that includes: speaking and lecture fees. Pedro Santos-Moreno reports a relationship with Janssen Pharmaceuticals Inc that includes: speaking and lecture fees. Pedro Santos-Moreno reports a relationship with AbbVie Inc that includes: speaking and lecture fees. Pedro Santos-Moreno reports a relationship with Biopass-UCB that includes: speaking and lecture fees. Pedro Santos-Moreno reports a relationship with Bristol Myers Squibb Co that includes: speaking and lecture fees. Pedro Santos-Moreno reports a relationship with Eli Lilly and Company that includes: speaking and lecture fees. Pedro Santos-Moreno reports a relationship with Pfizer that includes: speaking and lecture fees. Pedro Santos-Moreno reports a relationship with Roche that includes: speaking and lecture fees. Pedro Santos-Moreno reports a relationship with Tecnofarma that includes: speaking and lecture fees. Pedro Santos-Moreno reports a relationship with Sanofi that includes: speaking and lecture fees.
Acknowledgments
The authors would like to thank Elías Quintero-Muñoz for his participation and help in the data collection for this study. In addition, to Fernando Rodríguez-Florido, who help in the figures design.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtauto.2022.100187.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Appendix A.1 are signs and symptoms related to autoimmune diseases in the study population; Appendix A.2 presents current treatment for Systemic lupus erythematosus in the study population and Appendix A.3 are laboratory results of the study population. The supplementary data were available online. Appendix B is The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
Data availability
Data will be made available on request.
References
- 1.Anaya J.-M. The autoimmune tautology. A summary of evidence. Joint Bone Spine. 2017;84:251–253. doi: 10.1016/j.jbspin.2016.11.012. No 3 (May, 2017) ISSN: 1297-319X; EISSN: 1778-7254. [DOI] [PubMed] [Google Scholar]
- 2.Mitratza M., Klijs B., Hak A.E., Kardaun J.W.P.F., Kunst A.E. Systemic autoimmune disease as a cause of death: mortality burden and comorbidities. Rheumatology. 2021;60:1321–1330. doi: 10.1093/RHEUMATOLOGY/KEAA537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rojas-Villarraga A., Amaya-Amaya J., Rodriguez-Rodriguez A., Mantilla R.D., Anaya J.M. Introducing polyautoimmunity: secondary autoimmune diseases no longer exist. Autoimmune Dis. 2012;1 doi: 10.1155/2012/254319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mena-Vázquez N., Fernández-Nebro A., Pego-Reigosa J.M., Galindo M., Melissa-Anzola A., Uriarte-Isacelay E., et al. Hydroxychloroquine is associated with a lower risk of polyautoimmunity: data from the RELESSER Registry. Rheumatology. 2020;59:2043–2051. doi: 10.1093/rheumatology/kez562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lerner A., Jeremias P., Matthias T. The world incidence and prevalence of autoimmune diseases is increasing. Int. J. Celiac Dis. 2015;3:151–155. doi: 10.12691/ijcd-3-4-8. [DOI] [Google Scholar]
- 6.Conrad N., Verbeke G., Molenberghs G., Goetschalckx L., Callender T., Cambridge G., et al. Autoimmune diseases and cardiovascular risk: a population-based study on 19 autoimmune diseases and 12 cardiovascular diseases in 22 million individuals in the UK. Lancet. 2022;400:733–743. doi: 10.1016/S0140-6736(22)01349-6. [DOI] [PubMed] [Google Scholar]
- 7.Valderas J.M., Starfield B., Sibbald B., Salisbury C., Roland M. Defining comorbidity: implications for understanding health and health services. Ann. Fam. Med. 2009;7:357–363. doi: 10.1370/AFM.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rojas-Villarraga A., Amaya-Amaya J., Rodriguez-Rodriguez A., Mantilla R.D., Anaya J.M. Introducing polyautoimmunity: secondary autoimmune diseases no longer exist. Autoimmune Dis. 2012;1 doi: 10.1155/2012/254319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernández M., Calvo-Alén J., Alarcón G.S., Roseman J.M., Bastian H.M., Fessler B.J., et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA). XXI. Disease activity, damage accrual, and vascular events in pre- and postmenopausal women. Arthritis Rheum. 2005;52:1655–1664. doi: 10.1002/art.21048. [DOI] [PubMed] [Google Scholar]
- 10.Quintana R., Pons-Estel G.J., Roberts K., Sacnún M., Serrano R., Nieto R., et al. Clinical features, damage accrual, and survival in patients with familial systemic lupus erythematosus: data from a multi-ethnic, multinational Latin American lupus cohort. Lupus. 2020;29:1140–1145. doi: 10.1177/0961203320935184. [DOI] [PubMed] [Google Scholar]
- 11.Pons-Estel G.J., Catoggio L.J., Cardiel M.H., Bonfa E., Caeiro F., Sato E., et al. Lupus in Latin-American patients: Lessons from the GLADEL cohort. Lupus. 2015;24:536–545. doi: 10.1177/0961203314567753. [DOI] [PubMed] [Google Scholar]
- 12.Blank M., Gershwin M.E. Autoimmunity: from the mosaic to the kaleidoscope. J. Autoimmun. 2008;30:1–4. doi: 10.1016/j.jaut.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Rojas M., Ramírez-Santana C., Acosta-Ampudia Y., Monsalve D.M., Rodriguez-Jimenez M., Zapata E., et al. New insights into the taxonomy of autoimmune diseases based on polyautoimmunity. J. Autoimmun. 2022:126. doi: 10.1016/j.jaut.2021.102780. [DOI] [PubMed] [Google Scholar]
- 14.Petri M., Orbai A.M., Alarcõn G.S., Gordon C., Merrill J.T., Fortin P.R., et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677–2686. doi: 10.1002/art.34473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuschieri S. The STROBE guidelines. Saudi J. Anaesth. 2019;13:S31. doi: 10.4103/SJA.SJA_543_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miyakis Dj S., Cohen H., Asherson R.A., Cervera R., De Groot P.G., Erkan D., et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J. Thromb. Haemostasis. 2006;12:530–534. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 17.Jonklaas J., Bianco A.C., Bauer A.J., Burman K.D., Cappola A.R., Celi F.S., et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid. 2014;24:1670. doi: 10.1089/THY.2014.0028. 751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shiboski C.H., Shiboski S.C., Seror R., Criswell L.A., Labetoulle M., Lietman T.M., et al. American college of rheumatology/European league against rheumatism classification criteria for primary Sjögren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Arthritis Rheumatol. 2016;69:35–45. doi: 10.1002/ART.39859/ABSTRACT. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aletaha D., Neogi T., Silman A.J., Funovits J., Felson D.T., Bingham C.O., et al. Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/ART.27584. 2010. [DOI] [PubMed] [Google Scholar]
- 20.Van Den Hoogen F., Khanna D., Fransen J., Johnson S.R., Baron M., Tyndall A., et al. Classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013;72:1747–1755. doi: 10.1136/ANNRHEUMDIS-2013-204424. 2013. [DOI] [PubMed] [Google Scholar]
- 21.Manns M.P., Lohse A.W., Vergani D. Autoimmune hepatitis - update 2015. J. Hepatol. 2015;62:S100–S111. doi: 10.1016/j.jhep.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Reshetnyak V.I. vol. 21. 2015. p. 7683. (Primary biliary cirrhosis: Clinical and laboratory criteria for its diagnosis). 708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gawkrodger D.J., Ormerod A.D., Shaw L., Mauri-Sole I., Whitton M.E., Watts M.J., et al. Guideline for the diagnosis and management of vitiligo. Br. J. Dermatol. 2008;159:1051–1076. doi: 10.1111/J.1365-2133.2008.08881.X. [DOI] [PubMed] [Google Scholar]
- 24.Classification and diagnosis of diabetes: standards of medical care in diabetes-2022. 2021. [DOI]
- 25.Rousseff R.T. Diagnosis of myasthenia gravis. J. Clin. Med. 2021;10:1736. doi: 10.3390/JCM10081736. 1736 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.An update on immunopathogenesis, diagnosis, and treatment of multiple sclerosis, Profiles RNS, n.d. https://profiles.umassmed.edu/display/13364240 (accessed November 3 2022).
- 27.Suppiah R., Robson J.C., Grayson P.C., Ponte C., Craven A., Khalid S., et al. American college of rheumatology/European alliance of associations for rheumatology classification criteria for microscopic polyangiitis. Arthritis Rheumatol. 2022;74:400–406. doi: 10.1002/ART.41983. 2022. [DOI] [PubMed] [Google Scholar]
- 28.Robson J.C., Grayson P.C., Ponte C., Suppiah R., Craven A., Judge A., et al. American college of rheumatology/European alliance of associations for rheumatology classification criteria for granulomatosis with polyangiitis. Ann. Rheum. Dis. 2022;81:315–320. doi: 10.1136/ANNRHEUMDIS-2021-221795. 2022. [DOI] [PubMed] [Google Scholar]
- 29.Shoenfeld Y., Cervera R., Gershwin M.E. first ed. Humana Totowa; New Jersey: 2008. Diagnostic Criteria in Autoimmune Diseases. [Google Scholar]
- 30.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., Neal L.O., et al. Of software platform partners. J. Biomed. Inf. 2020:1–24. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atehortúa L., Rojas M., Vásquez G., Muñoz-Vahos C.H., Vanegas-García A., Posada-Duque R.A., et al. Endothelial activation and injury by microparticles in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res. Ther. 2019;21:1–15. doi: 10.1186/s13075-018-1796-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rojas-Villarraga A., Toro C.E., Espinosa G., Rodríguez-Velosa Y., Duarte-Rey C., Mantilla R.D., et al. Factors influencing polyautoimmunity in systemic lupus erythematosus. Autoimmun. Rev. 2010;9:229–232. doi: 10.1016/j.autrev.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Shoenfeld Y., Ehrenfeld M., Perry O. The kaleidoscope of autoimmunity – from genes to microbiome. Clin. Immunol. 2019;199:1–4. doi: 10.1016/j.clim.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Frade-Sosa B., Narváez J., Salman-Monte T.C., Castellanos-Moreira R., Ortiz-Santamaria V., Torrente-Segarra V., et al. A comparative study on clinical and serological characteristics between patients with rhupus and those with systemic lupus erythematosus and rheumatoid arthritis. Lupus. 2020;29:1216–1226. doi: 10.1177/0961203320938456. [DOI] [PubMed] [Google Scholar]
- 35.Rivier G., Herranz M.T., Khamashta M.A., Hughes GR v. Thrombosis and antiphospholipid syndrome: a preliminary assessment of three antithrombotic treatments. Lupus. 1994;3:85–90. doi: 10.1177/096120339400300205. [DOI] [PubMed] [Google Scholar]
- 36.Cervera R., Piette J.C., Font J., Khamashta M.A., Shoenfeld Y., Camps M.T., et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019–1027. doi: 10.1002/art.10187. [DOI] [PubMed] [Google Scholar]
- 37.Esdaile J.M., Abrahamowicz M., Grodzicky T., Li Y., Panaritis C., Du Berger R., et al. Traditional framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–2337. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 38.Rubin L.A., Urowitz M.B., Gladman D.D. Mortality in systemic lupus erythematosus: the bimodal pattern revisited. Q. J. Med. 1985;55:87–98. [PubMed] [Google Scholar]
- 39.Aguirre-Valencia D., Suárez-Avellaneda A., Ocampo-Piraquive V., Posso-Osorio I., Naranjo-Escobar J., Nieto-Aristizábal I., et al. Mortality in patients with systemic lupus erythematosus in Colombia: a case series. Clin. Rheumatol. 2019;38:1865–1871. doi: 10.1007/s10067-019-04546-w. [DOI] [PubMed] [Google Scholar]
- 40.Franco J.S., Molano-González N., Rodríguez-Jiménez M., Acosta-Ampudia Y., Mantilla R.D., Amaya-Amaya J., et al. The coexistence of antiphospholipid syndrome and systemic lupus erythematosus in Colombians. PLoS One. 2014;9 doi: 10.1371/journal.pone.0110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cervera R., Serrano R., Pons-Estel G.J., Ceberio-Hualde L., Shoenfeld Y., De Ramón E., et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: a multicentre prospective study of 1000 patients. Ann. Rheum. Dis. 2015;74:1011–1018. doi: 10.1136/annrheumdis-2013-204838. [DOI] [PubMed] [Google Scholar]
- 42.Sieiro Santos C., Moriano Morales C., Álvarez Castro C., Díez Alvarez E. Polyautoimmunity in systemic lupus erythematosus: secondary Sjogren syndrome. Z. Rheumatol. 2021 doi: 10.1007/s00393-021-01051-x. [DOI] [PubMed] [Google Scholar]
- 43.Mavragani C.P., Moutsopoulos H.M. Primary versus secondary sjögren syndrome: is it time to reconsider these terms? J. Rheumatol. 2019;46:665–666. doi: 10.3899/jrheum.180392. [DOI] [PubMed] [Google Scholar]
- 44.Almaani S., Meara A., Rovin B.H. Update on lupus nephritis. Clin. J. Am. Soc. Nephrol. 2017;12:825–835. doi: 10.2215/CJN.05780616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aljadeff G., Shemer A., Katz I., Andrade L.E.C., Gilburd B., Halpert G., et al. Infusion of anti-DFS70 antibodies prolonged survival of lupus-prone mice. Lupus. 2021;30:320–324. doi: 10.1177/0961203320969976. [DOI] [PubMed] [Google Scholar]
- 46.Choi M.Y., E Clarke A., Fritzler M.J. Do anti-DFS70 antibodies temper disease activity and progression in SLE? Lupus. 2021;30:852–853. doi: 10.1177/0961203321990439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Natorska12 J., Undas A., Natorska J. 2016. Prace Oryginalne Anti-DFS Antibodies Occur More Commonly in Patients Following Unprovoked Venous Thromboembolism; pp. 816–820. [PubMed] [Google Scholar]
- 48.Marlet J., Ankri A., Charuel J.L., Ghillani-Dalbin P., Perret A., Martin-Toutain I., et al. Thrombophilia associated with anti-DFS70 autoantibodies. PLoS One. 2015;10 doi: 10.1371/journal.pone.0138671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qi H., Cao Q., Liu Q. MicroRNA-16 directly binds to DEC2 and inactivates the TLR4 signaling pathway to inhibit lupus nephritis-induced kidney tissue hyperplasia and mesangial cell proliferation. Int. Immunopharm. 2020;88 doi: 10.1016/j.intimp.2020.106859. [DOI] [PubMed] [Google Scholar]
- 50.Yan L., Liang M., Hou X., Zhang Y., Zhang H., Guo Z., et al. The role of microRNA-16 in the pathogenesis of autoimmune diseases: a comprehensive review. Biomed. Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.01.044. [DOI] [PubMed] [Google Scholar]
- 51.Wang Y., Zhang G., Zhang L., Zhao M., Huang H. Decreased microRNA-181a and -16 expression levels in the labial salivary glands of Sjögren syndrome patients. Exp. Ther. Med. 2018;15:426–432. doi: 10.3892/etm.2017.5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ordoñez-Cañizares M.C., Mena-Vázquez N., Redondo-Rodriguez R., Manrique-Arija S., Jimenez-Núñez F.G., Ureña-Garnica I., et al. Frequency of polyautoimmunity in patients with rheumatoid arthritis and systemic lupus erythematosus. J. Clin. Rheumatol. 2022;28:E38–E43. doi: 10.1097/RHU.0000000000001574. [DOI] [PubMed] [Google Scholar]
- 53.Quintero O.L., Amador-Patarroyo M.J., Montoya-Ortiz G., Rojas-Villarraga A., Anaya J.M. Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity. J. Autoimmun. 2012;38 doi: 10.1016/j.jaut.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 54.Santos-Moreno P., Burgos-Angulo G., Martinez-Ceballos M.A., Pizano A., Echeverri D., Bautista-Niño P.K., et al. Inflammaging as a link between autoimmunity and cardiovascular disease: the case of rheumatoid arthritis. RMD Open. 2021;7 doi: 10.1136/rmdopen-2020-001470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anaya J.M., Castiblanco J., Rojas-Villarraga A., Pineda-Tamayo R., Levy R.A., Gómez-Puerta J., et al. The multiple autoimmune syndromes. A clue for the autoimmune tautology. Clin. Rev. Allergy Immunol. 2012;43:256–264. doi: 10.1007/S12016-012-8317-Z. [DOI] [PubMed] [Google Scholar]
- 56.Guavita-Navarro D., Gallego-Cardona L., Arredondo A.M., Cubides H., Cajamarca-Barón J., Ibáñez C., et al. Comparison of the sensitivity of the EULAR/ACR 2019 and SLICC 2012 classification criteria in a Colombian population with systemic lupus erythematosus. J. Transl. Autoimmun. 2021;4 doi: 10.1016/J.JTAUTO.2021.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu W., Tian F., Ma J., Zhong Y., Liu Z., Xue L. Diagnostic accuracy of the European League against rheumatism/American College of Rheumatology-2019 versus the Systemic Lupus International Collaborating Clinics-2012 versus the ACR-1997 classification criteria in adult systemic lupus erythematosus: a systematic review and meta-analysis. Front. Immunol. 2022:6178. doi: 10.3389/FIMMU.2022.1023451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.