Abstract
Background and purpose
Reduction of respiratory tumour motion is important in liver stereotactic body radiation therapy (SBRT) to reduce side effects and improve tumour control probability. We have assessed the distribution of use of voluntary exhale breath hold (EBH), abdominal compression (AC), free breathing gating (gating) and free breathing (FB), and the impact of these on treatment time.
Materials and Methods
We assessed all patients treated in a single institution with liver SBRT between September 2017 and September 2021. Data from pre-simulation motion management assessment using fluoroscopic assessment of liver dome position in repeat breath holds, and motion with and without AC, was reviewed to determine liver dome position consistency in EBH and the impact of AC on motion. Treatment time was assessed for all fractions as time from first image acquisition to last treatment beam off.
Results
Of 136 patients treated with 145 courses of liver SBRT, 68 % were treated in EBH, 20 % with AC, 7 % in gating and 5 % in FB. AC resulted in motion reduction < 1 mm in 9/26 patients assessed. Median treatment time was higher using EBH (39 min) or gating (42 min) compared with AC (30 min) or FB (24 min) treatments.
Conclusions
Motion management in liver SBRT needs to be assessed per-patient to ensure appropriate techniques are applied. Motion management significantly impacts treatment time therefore patient comfort must also be taken into account when selecting the technique for each patient.
Keywords: Liver, Stereotactic body radiation therapy, Motion management, exhale breath hold. Stereotactic ablative body radiotherapy, Abdominal compression
Abbreviations: AC, Abdominal Compression; BED, Biologically Effective Dose; CBCT, Cone Beam Computed Tomography; EBH, Exhale Breath Hold; FB, Free Breathing; FFF, Flattening Filter Free; GTV, Gross Tumor Volume; IMRT, Intensity Modulated Radiation Therapy; ITV, Internal Target Volume; PTV, Planning Target Volume; SBRT, Stereotactic body radiation thearpy; VMAT, Volumetric Modulated Arc Therapy
1. Introduction
Stereotactic body radiation therapy (SBRT) for cancer in the liver has emerged as a promising local treatment, showing excellent local control for both primary liver cancer, and metastases from other histologies [1], [2]. Improved local control rate is associated with higher SBRT doses (BED10 ≥ 100 Gy) and smaller tumor volumes (<40 cm3) [2], [3]. This however does come at the risk of side effects, which includes liver toxicity, typically a biochemical endpoint that contributes to decline in liver function [4]. For this reason, isotoxic prescription regimes are typically employed which aim to deliver as high tumour dose as possible while keeping to liver constraints; if liver constraints are exceeded, the tumour dose is typically reduced until liver tolerance is met. In this context, motion management is a critical component of successful liver SBRT [5]. The liver moves predominantly in the superior-inferior and anterior-posterior directions with respiration. Reduction of liver respiratory motion reduces the treatment margins, not only reducing dose to surrounding liver and adjacent critical organs, but facilitating tumour dose increase [6]. Increased tumour control probability has been demonstrated in liver metastases when using active respiratory motion management [1], [7]. Motion management options include dynamic tracking, breath hold, abdominal compression and free breathing gating. The optimal motion management depends on available technique and technology, staff training, and patient related factors such as magnitude of respiratory motion, comfort and compliance with the motion management technique. Consequently, there is a clinical need to examine what can be the best approach in terms of individualised treatment setup and motion management, an upstream issue for treatment simulation, planning and delivery, thus critical to achieve intended clinical outcome from liver SBRT.
In this work, we aim to determine the proportion of patients who can achieve active motion management such as voluntary breath hold or free-breathing gating, and the impact of motion management on treatment time.
2. Materials and methods
This project was approved by the Institutional Ethics Committee. Between September 2017 and December 2021, 136 patients with liver tumours were treated with 145 courses of SBRT in our institution. All participants were assessed clinically and deemed eligible for liver SBRT based on an institutional clinical protocol. Participants had either primary liver cancer (hepatocellular carcinoma or cholangiocarcinoma) or liver metastasis, adequate volume of uninvolved liver (≥700 cc), Child-Pugh class A/B7 liver function and adequate liver function. All participants were informed by the radiation oncologists about the importance of motion management and subsequently given two appointments: a motion management education and assessment session followed by a simulation session. The first appointment consisted of education about the requirement of motion management, and guidance of patient through various motion management options and assessment of compliance with a given technique. Motion management assessment was performed for 140 courses prior to simulation; the remaining patients were not assessed due to patient performance status (4) or knowledge of patient capabilities from previous liver SBRT course motion management (1).
2.1. Motion management assessment
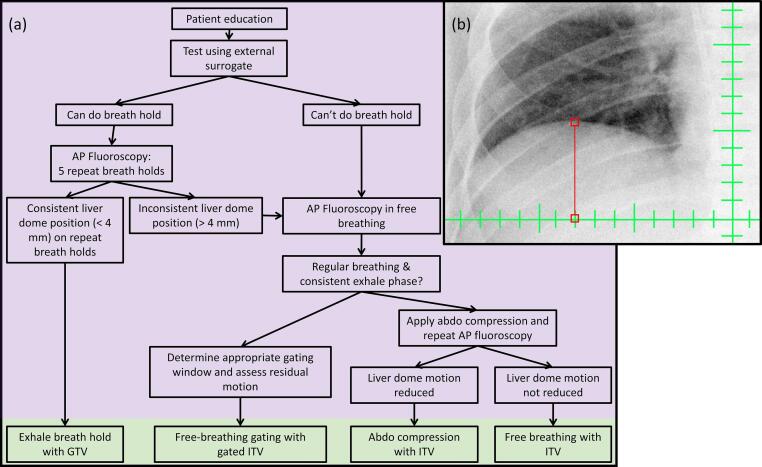
The motion management workflow shown in Fig. 1 was implemented using a Varian TrueBeam STx linear accelerator (v2.0–2.7, Varian Medical Systems, Palo Alto, USA). The workflow was split into two sessions; the motion management assessment as highlighted in purple in Fig. 1 was performed in the afternoon, and the simulation imaging highlighted in green in Fig. 1 was performed the following morning. The sessions were on separate days to minimise patient fatigue and discomfort, in particular since the patient was typically required to be fasting for the simulation imaging. The first was at the linac, at which the patient was educated about the requirement of motion management. The patient was then set up on the treatment couch in an approximation of the treatment position and was guided through attempts at relaxed exhale breath hold (EBH). This step included determining breathing instructions customised to each patient. Breath hold consistency was monitored using the respiratory gating interface (Varian Medical Systems, Palo Alto, USA). If desired by the patient, the respiratory trace display was projected to an iPad device so that the patients could see the breathing trace in the bunker. If the patient was able to achieve repeat, stable relaxed EBH of more than 15 s, imaging evaluation was performed. In this, the patient was instructed to enter relaxed breath hold, during which fluoroscopy (using typically 80 kV, 5 mAs and 7 frames per second) was acquired of the liver dome while in breath hold. The position of the liver dome was compared between each breath hold to ensure that the liver position was consistent between repeat breath holds. A criteria of 4 mm was applied as the maximum difference between repeat breath holds. In the case where the patient was not able to achieve consistent exhale breath hold based on either the external surrogate monitoring, or fluoroscopy assessment, the patient’s breathing trace was assessed for reproducibility and a consistent exhale phase based on the respiratory gating interface. If deemed consistent, and a gating window could be applied which resulted in < 4 mm liver dome motion and a suitable duty cycle (> 25 %), respiratory gating in free breathing was selected. Free breathing gating was introduced in January 2020 following a software upgrade, allowing cone beam CT (CBCT) in free breathing respiratory gating mode to be performed. If EBH or free breathing gating were not appropriate, the patient underwent assessment for abdominal compression. In this assessment, the liver dome superior-inferior motion was first measured in free breathing using fluoroscopy, the Abdominal Compression (AC) device was then applied (Orfit Industries, Belgium) and superior-inferior motion again measured. If AC reduced the liver dome motion, and the patient could tolerate AC, this was used at simulation and treatment, otherwise the patient was simulated and treated in free breathing.
Fig. 1.
Motion management assessment workflow. The steps in purple were performed on the linear accelerator, and in green was at CT simulation. Note the free breathing gating arm was added in January 2020. Inset shows an example fluoroscopy measurement of the liver dome relative to the isocentre, performed on the linear accelerator.
The motion management selection was taken forward to the simulation session. Participants able to achieve EBH would undergo a 3DCT followed by arterial and portal venous phase contrast images, both in EBH. For the remaining patients, a 4DCT was acquired, followed by breath hold arterial and portal venous phase contrast images. In these patients, the breath hold for the contrast CTs was at any respiratory state achievable for the patient.
For EBH, isotropic PTV margins of 5 mm were used if the inter-breath hold reproducibility was ≤ 2 mm, otherwise 8 mm superior-inferior, and 5 mm left–right, anterior-posterior were used. For free breathing gating, a gated internal target volume (ITV) was created using the phases contained in the gating window, and a 5 mm isotropic PTV margin was used. For abdominal compression and free breathing, an ITV was created using all phases of the 4DCT, and a 5 mm isotropic PTV margin was used.
2.2. Treatment delivery
Patients were treated with 1–5 fractions of 6–20 Gy per fraction. Treatment was delivered on a TrueBeam or TrueBeam STx linear accelerator (Varian Medical Systems, Palo Alto, USA). Treatment was performed with 6 MV or 10 MV with or without flattening filter free (FFF) delivery at 600 MU/min, 1400 MU/min or 2400 MU/min. Image guidance was performed with a kV-kV pair, matching to bone anatomy, followed by a CBCT to match to the target or surrounding soft tissue. No fiducial markers were used for matching, however when available, other surrogates such as surgical clips, remnant lipiodol were used to assist with image guidance. CBCT acquisition was matched to the motion management; free breathing CBCT was used for FB and AC deliveries, and for gated or EBH deliveries gated CBCT in amplitude or breath hold gating modes was used respectively. If the shift exceeded 2 mm in any direction, the CBCT was repeated. In general, a mid-treatment CBCTs, halfway through the beams was acquired. The respiratory signal from the Varian Respiratory Gating interface was used for all respiratory triggering and gating.
2.3. Data collection and statistical analysis
The consistency of the liver dome position in repeat EBHs was measured in each assessment session. The superior-inferior distance between the superior most position of the liver dome and the isocentre was recorded for each successive EBH. The difference in superior-inferior liver dome motion between FB and AC was also recorded at time of this assessment. Patient arm position for simulation was recorded.
Treatment time was defined as the time from the first setup imaging (kV-kV pair) to the time of last beam off. This data was extracted from the record and verify system (Mosaiq, Elekta, Stockholm, Sweden) using SQL queries.
Normality of the distributions was tested using the Shapiro-Wilk test. Non-parametric comparisons between distributions was performed using the Mann Whitney U test, with p < 0.05 used for the level of statistical significance.
3. Results
Of the total 145 courses, 98 (67.6 %) were simulated in EBH, 10 (6.9 %) were simulated with gating, 29 (20.0 %) were simulated with AC and 8 (5.5 %) were simulated with FB. In the majority of courses the patients were simulated and treated with both arms up (87.3 %), with the remainder one (10.7 %) or both arms down (2.0 %).
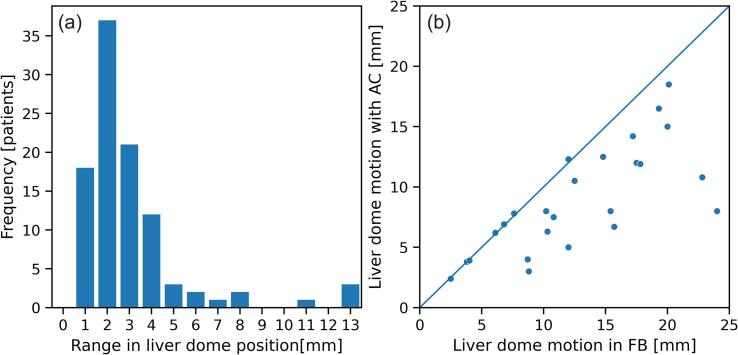
Of 140 tumours assessed, data from measurement of EBH reproducibility or impact of AC on motion with fluoroscopy was available for 100 patients; the remainder could either not do EBH based on assessment prior to fluoroscopy (25) or assessment data was not recorded or missing (15). The range in superior-inferior liver dome position from repeat breath holds as measured from fluoroscopy is shown in Fig. 2(a). These were all patients that had consistent EBH based on the external surrogate. The vast majority of patients could achieve liver dome position within 4 mm, however a subset (12) had variation in liver dome position greater than 4 mm, despite consistency in the external surrogate. Three patients who were deemed suitable for EBH at the mock up session could not reproducibly perform EBH at time of first fraction treatment. Of these, one was treated in free breathing, and one in abdominal compression and one with gating. A further patient treated with gating could not achieve reproducible breathing in their fourth fraction; this patient had one field treated in that session, and the subsequent field treated the next day. Measurement of motion with and without AC was available for 26 patients. The liver dome motion in the superior-inferior direction when AC was applied as a function of the motion in FB is shown in Fig. 2(b). AC reduced the liver dome motion by an average of 3.5 mm (range −0.3 – 16 mm); there were 9/26 patients in whom the change in motion between AC and FB was < 1 mm.
Fig. 2.
(a) Range in liver dome superior-inferior position between repeat exhale breath holds and (b) liver dome superior-inferior motion with abdominal compression (AC) as a function of liver dome motion in free breathing (FB).
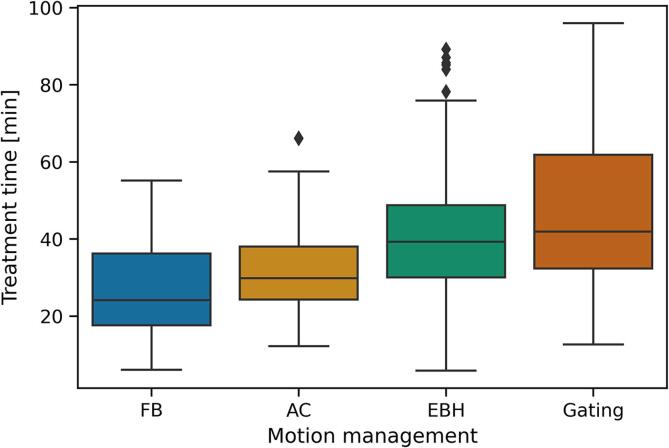
From the 145 treatment courses, there were 613 fractions available for treatment time analysis. Data was not normally distributed. The total treatment time per fraction varied by motion management technique (Fig. 3). Treatment time (median [range]) was larger for EBH treatments (39 [5 – 89] min), compared with AC (30 [12–66] min, p ≪ 0.001) and FB (24 [6 – 55] min, p ≪ 0.001). Treatment times for gated treatments were comparable with EBH (42 [12 – 96] min, p = 0.15), and higher than AC (p ≪ 0.001), and FB (p ≪ 0.001), despite the substantially reduced number of gated treatments in the sample. The treatment time for the first fraction in EBH (43 [16 – 89] min) was longer than subsequent fractions (38 [6 – 85] min, p < 0.01), whereas for AC, FB and gating there was no difference in treatment time between fractions.
Fig. 3.
Box plot of the treatment time per fraction (time from first image acquisition to last beam off) as a function of the motion management used.
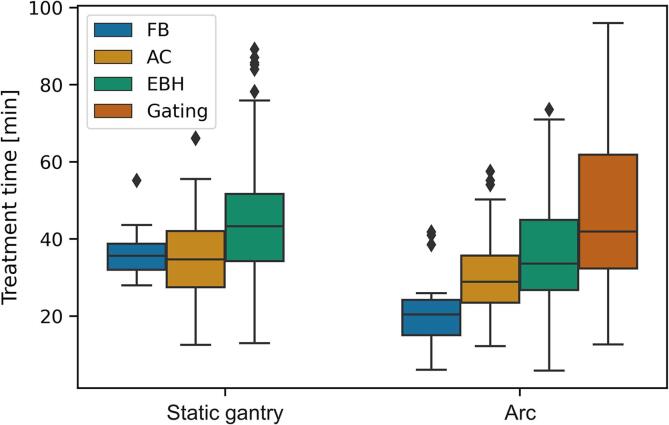
Arc based treatments were quicker than static gantry treatments (32 [6 – 96] vs 41 [13 – 89] min, p ≪ 0.001) (Fig. 4). This held for all motion management techniques; FB (20 [6 – 42] min vs 36 [28 – 55] min, p < 0.01), AC (29 [12 – 58] min vs 35 [13 – 66] min, p < 0.01) and EBH (34 [6 – 74] min vs 43 [13 – 89] min, p ≪ 0.001). Treatment time was not shorter when using 2400 MU/min compared with 1400 MU/min or 600 MU/min (p >> 0.05 and p >> 0.05 respectively). Treatment time did not depend on fraction size.
Fig. 4.
Box plots of the treatment time per fraction (time from first image acquisition to last beam off) as a function of beam delivery techniques (static gantry vs arc).
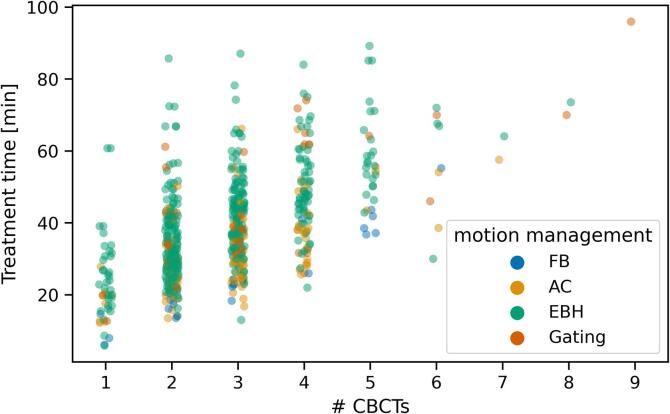
The treatment time per fraction increased with the number of CBCTs acquired in that fraction (Fig. 5). A linear fit to the data from 1 to 5 CBCTs per fraction (97.5 % of all fractions) yielded a slope of 6.9 min / CBCT, and an intercept of 18.4 min. The data was further categorised into fractions where the CBCT acquisition interrupted by the breathing (EBH and gating) or not (FB and AC). For EBH and gated fractions the slope was 7.2 min / CBCT with an intercept of 20.4 min, and for FB and AC fractions the slope was 6.6 min / CBCT with an intercept of 11.0 min. The increased intercept with fractions where the CBCT acquisition is activated by the patient breathing demonstrates both an increased time for CBCT acquisition, but also in beam delivery time. Increased treatment time for the first fraction in EBH treatments was not a result of increased CBCTs in that fraction (3 [1], [2], [3], [4], [5], [6], [7], [8] CBCTs in fraction 1, 3 [1], [2], [3], [4], [5], [6], [7], [8], [9] CBCTs per fraction in subsequent fractions, p = 0.54).
Fig. 5.
Treatment time per fraction (time from first image acquisition to last beam off) as a function of the number of CBCTs acquired in that fraction.
4. Discussion
We have reviewed feasibility of active motion management in 136 patients receiving SBRT for tumours in the liver, and demonstrated the majority of patients can be treated in voluntary exhale breath hold. A significant proportion (32 %) of patients however could not achieve reproducible exhale breath hold and required alternative methods to reduce respiratory motion. The importance of motion management for local control of liver metastases has been demonstrated by Stera et. al. [7]. The availability of multiple motion management approaches is thus beneficial; in this cohort, only 5 % of patients were treated in free breathing with the full range of respiratory motion.
Eccles et. al. demonstrated that 62 % of patients were suitable for exhale breath hold with the Active Breathing Control (ABC) device; suitability was based on breath hold greater than 10 s and diaphragm motion < 3 mm during breath hold [8]. The liver dome position variation was within 3 mm for 86 % of repeat breath holds. The current study observed similar numbers; 68 % achieved reproducible voluntary exhale breath hold, and of those assessed for breath hold consistency with fluoroscopy, liver dome motion was within 3 mm for 76 % of patients. Slightly lower consistency may be due to the voluntary aspect of the breath hold in the current study compared with that achieved with ABC. Mast et. al. investigated the use of inhale breath hold (IBH) using the ABC, showing 95 % of patients could tolerate IBH [9]. Reproducibility was assessed through CT scans of 10 repeat IBHs which were subsequently used to generate PTV margins and compared with margins generated based on free-breathing 4DCTs and an ITV. In all cases, the PTV with IBH was less than that based on an ITV, regardless of the IBH reproducibility. Similarly, Lu et. al. measured superior-inferior position between three repeat IBH with the ABC device, and showed 14 % of patients had variation greater than 5 mm, highlighting the potential large irreproducibility for some patients [10]. The dosimetric impact of this was clear; given standardised PTV margin of 5 mm, a lack of breath hold consistency results in a high probability of geographic miss and reduced tumour control probability [10]. The data presented here, and in the discussed papers, highlight that there is a subset of patients who cannot perform reproducible breath holds; in our practice, we elected to employ other motion management approaches for these patients, however it may beneficial to instead quantify the variation in liver position between breath holds, and include this in a margin. As shown by Mast et al., this will likely still result in a reduction in the total treated volume compared with a PTV based on an ITV [9].
Despite ability to maintain consistent breath hold in pre-simulation sessions, a small number of patients in our cohort were unable to achieve this at time of treatment. This may be due to patient stress or anxiety, or changes in the performance status of patients. These patients present a challenging scenario and typically require re-simulation and re-planning, with a different motion management approach. By performing an assessment prior to simulation, instances such as these may be minimised. The effectiveness of the abdominal compression band was inconsistent, with reduction in liver motion not observed for all patients. This is similar to results from Van Gelder et. al., who used a similar compression band, and measured the respiratory motion with and without compression using 4DCTs [11].
The use of active motion management however comes at a significant cost in terms of treatment time. Both EBH (39 min) and free breathing gating (42 min) resulted in increased median treatment time compared with treatment in free breathing (24 min) or with abdominal compression (30 min). This increase in treatment time is an important consideration in the context of patient comfort and compliance; anecdotally we have observed reductions in breath hold compliance or respiratory cycle consistency when patients are in pain or discomfort. Optimatization of pain control prior to simulation and treatment may improve patient compliance and should be explored [12]. However, narcotic analgesia may impair participants' ability to follow instruction when breath hold is the motion management of choice. For a subset of patients, comfort was improved with one or both arms down; in some cases this resulted in the patient being able to be treated with EBH or gating, which may not have otherwise been an option due to discomfort and pain.
Similar to previous work previous work in single fraction stereotactic ablative body radiation therapy treatments, the use of arc therapy reduced total treatment time [13]. We however did not see any impact of use of FFF beams on treatment time, likely due to the very small sample size of non-FFF in our cohort. In the current study, the increased treatment time comes not only due to reduced treatment beam duty cycle (between repeat breath holds, or between individual breaths), but due to the acquisition of CBCTs using motion management. We observed approximately 7 min increase in treatment time per CBCT acquired. This is similar to that found by Gaudreault et. al., where each CBCT added approximately 6 min to the treatment time [13]. In our image guidance protocols, CBCTs are acquired prior to treatment. In the majority of patients, we also performed mid-treatment CBCTs acquired between the two treatment arcs in the case of VMAT or DCAT, or halfway through the fields in the case of IMRT or 3DCRT. Both pre- and mid-treatment CBCTs are repeated if the online correction is ≥ 2 mm in any direction. As a result, in these treatments we would expect in most treatment fractions at least 2–3 CBCTs; in our data, the majority of fractions (92 %) are delivered with 4 CBCTs or fewer. Thus the often large numbers of CBCTs acquired as shown in Fig. 5, reflect not only general patient positioning instability, but likely demonstrate irreproducibility of respiratory phase such as exhale breath hold or a consistent exhale phase in free breathing at the time of image guidance and treatment. Reduction of CBCT acquisitions, and the associated increased treatment time and radiation dose, may be facilitated by use of real-time tumour tracking technology such as with implanted electromagnetic beacons, intrafraction CBCT, or kilovoltage imaging of implanted fiducial markers [14], [15], [16].
The variation in consistency of exhale breath hold and abdominal compression, and large impacts on treatment time when using breath hold or gating approaches highlights the need for patient specific assessment by a multi-disciplinary team prior to or at time of simulation for liver SBRT. Assessment of the appropriate motion management that minimises respiratory tumour motion, but is reproducible and takes into account patient positioning and comfort is a critical aspect of liver SBRT. The small subset of patients who required changing of motion management approach after simulation demonstrated the effectiveness of pre-simulation assessment of motion management.
A limitation of this study is the lack of monitoring the breath hold position during the treatment beam delivery. The reproducibility of the breath hold however was assessed prior to and halfway through treatment delivery through repeat CBCTs acquired in breath hold. As such, the consistency of the breath hold could still be assessed and in three cases resulted in patients being deemed unsuitable for EBH and re-simulated and treated with an alternative motion management method. Further, in a minority of cases, the consistency the breath hold was assessed during treatment beam delivery via kV imaging of the liver dome or other surrogates such as surgical clips or lipiodol [17]. Lastly the treatment time was defined as the period between first image acquisition to last beam off. All online images were matched by a radiation oncologist, so the treatment time includes the waiting period if any for the radiation oncologist, and time incurred for image registration and couch shifts. Further reduction in total treatment time may be achieved through radiation therapists performing the image guidance.
The majority of patients (68 %) treated with SBRT for liver tumours can achieve reproducible exhale breath hold, minimising the treated volume and increasing the probability of local control. Alternative motion management options should however be available and assessed on a per-patient basis to determine the appropriate motion management option. Median treatment time for exhale breath hold and gated treatments was substantially higher compared with free-breathing and abdominal compression methods.
Declaration of Competing Interest
Nicholas Hardcastle and Mathieu Gaudreault receive research grant funding from Varian Medical Systems for kidney SBRT research, and from Reflexion Medical for biologically guided radiation therapy research.
References
- 1.Andratschke N., Alheid H., Allgauer M., Becker G., Blanck O., Boda-Heggemann J., et al. The SBRT database initiative of the German Society for Radiation Oncology (DEGRO): patterns of care and outcome analysis of stereotactic body radiotherapy (SBRT) for liver oligometastases in 474 patients with 623 metastases. BMC Cancer. 2018;18:283. doi: 10.1186/s12885-018-4191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohri N., Tome W.A., Mendez Romero A., Miften M., Ten Haken R.K., Dawson L.A., et al. Local Control After Stereotactic Body Radiation Therapy for Liver Tumors. Int J Radiat Oncol Biol Phys. 2021;110:188–195. doi: 10.1016/j.ijrobp.2017.12.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahadevan A., Blanck O., Lanciano R., Peddada A., Sundararaman S., D'Ambrosio D., et al. Stereotactic Body Radiotherapy (SBRT) for liver metastasis - clinical outcomes from the international multi-institutional RSSearch(R) Patient Registry. Radiat Oncol. 2018;13:26. doi: 10.1186/s13014-018-0969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miften M., Vinogradskiy Y., Moiseenko V., Grimm J., Yorke E., Jackson A., et al. Radiation Dose-Volume Effects for Liver SBRT. Int J Radiat Oncol Biol Phys. 2021;110:196–205. doi: 10.1016/j.ijrobp.2017.12.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brock K.K. Imaging and image-guided radiation therapy in liver cancer. Semin Radiat Oncol. 2011;21:247–255. doi: 10.1016/j.semradonc.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gargett M., Haddad C., Kneebone A., Booth J.T., Hardcastle N. Clinical impact of removing respiratory motion during liver SABR. Radiat Oncol. 2019;14:93. doi: 10.1186/s13014-019-1300-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stera S., Miebach G., Buergy D., Dreher C., Lohr F., Wurster S., et al. Liver SBRT with active motion-compensation results in excellent local control for liver oligometastases: An outcome analysis of a pooled multi-platform patient cohort. Radiother Oncol. 2021;158:230–236. doi: 10.1016/j.radonc.2021.02.036. [DOI] [PubMed] [Google Scholar]
- 8.Eccles C., Brock K.K., Bissonnette J.P., Hawkins M., Dawson L.A. Reproducibility of liver position using active breathing coordinator for liver cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2006;64:751–759. doi: 10.1016/j.ijrobp.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 9.Mast M., Kouwenhoven E., Roos J., van Geen S., van Egmond J., van Santvoort J., et al. Two years' experience with inspiration breath-hold in liver SBRT. Tech Innov Patient Support Radiat Oncol. 2018;7:1–5. doi: 10.1016/j.tipsro.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu L, Ouyang Z, Lin S, Mastroianni A, Stephans KL, and Xia P. Dosimetric assessment of patient-specific breath-hold reproducibility on liver motion for SBRT planning. Journal of Applied Clinical Medical Physics. 20201-7. 10.1002/acm2.12887. [DOI] [PMC free article] [PubMed]
- 11.Van Gelder R., Wong S., Le A., Podreka A., Briggs A., Haddad C., et al. Experience with an abdominal compression band for radiotherapy of upper abdominal tumours. J Med Radiat Sci. 2018;65:48–54. doi: 10.1002/jmrs.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsang D.S., Voncken F.E., Tse R.V., Sykes J., Wong R.K., Dinniwell R.E., et al. A randomized controlled trial of lorazepam to reduce liver motion in patients receiving upper abdominal radiation therapy. Int J Radiat Oncol Biol Phys. 2013;87:881–887. doi: 10.1016/j.ijrobp.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 13.Gaudreault M., Yeo A., Kron T., Hanna G.G., Siva S., Hardcastle N. Treatment Time Optimization in Single Fraction Stereotactic Ablative Radiation Therapy: A 10-Year Institutional Experience. Adv Radiat Oncol. 2022 doi: 10.1016/j.adro.2021.100829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee Y.Y.D., Nguyen D.T., Moodie T., O'Brien R., McMaster A., Hickey A., et al. Study protocol of the LARK (TROG 17.03) clinical trial: a phase II trial investigating the dosimetric impact of Liver Ablative Radiotherapy using Kilovoltage intrafraction monitoring. BMC Cancer. 2021;21:494. doi: 10.1186/s12885-021-08184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Worm E.S., Hoyer M., Hansen R., Larsen L.P., Weber B., Grau C., et al. A Prospective Cohort Study of Gated Stereotactic Liver Radiation Therapy Using Continuous Internal Electromagnetic Motion Monitoring. Int J Radiat Oncol Biol Phys. 2018;101:366–375. doi: 10.1016/j.ijrobp.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Brown E., Muscat E., O'Connor P., Liu H., Lee Y.Y., Pryor D. Intrafraction cone beam computed tomography verification of breath hold during liver stereotactic radiation therapy. J Med Radiat Sci. 2021;68:52–59. doi: 10.1002/jmrs.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beaton L., Daly M., Tregidgo H.F., Grimes H., Moinuddin S., Stacey C., et al. Radiopaque drug-eluting embolisation beads as fiducial markers for stereotactic liver radiotherapy. Br J Radiol. 2022;95:20210594. doi: 10.1259/bjr.20210594. [DOI] [PMC free article] [PubMed] [Google Scholar]