Abstract
Chicken chaphamaparvovirus (CkChpV) is a novel parvovirus species that belongs to the Chaphamaparvovirus genus and is frequently detected in different vertebrates exhibiting diarrhea symptoms. In this study, screening tests were performed on samples from 478 chickens, including 357 with diarrhea and 121 healthy, collected from 25 farms in China to investigate CkChpV infection in China. CkChpV, avian nephritis virus, rotavirus, chicken parvovirus, Newcastle disease virus, infectious bronchitis virus, chicken proventricular necrosis virus, and chicken circovirus were all detected in the samples at a positivity rate of 32%, 9%, 6%, 2%, 2%, 1%, 0%, and 0%, respectively. Statistical analyses suggested a correlation between the infection by the virus and diarrhea (P < 0.05). The genome of 9 strains from the CkChpV-positive samples, whose length was 4,432 nucleotides, have been completely sequenced. The strains shared 97.2 to 98.7% genomic similarity, 98.1 to 99.1%, and 98.2 to 99.2% amino acid similarity, respectively, for NS1 and VP1 compared with CkChpV strain RS/BR/15/2S in GenBank. The genetic relationship between these strains and CkChpV was established through phylogenetic analysis. These findings indicated the infection existence of CkChpV in China, which enriches our understanding of the diversity of the chaphamaparvoviruses and its host spectrum.
Key words: chicken chaphamaparvovirus, complete-genome sequencing, evolution analysis, diarrhea symptoms
INTRODUCTION
Parvovirus is envelope-free, icosahedral, single-stranded DNA (ssDNA) virus, that belongs to the Parvoviridae family (Kisary et al., 1985). Historically, the Parvoviridae family has been divided into Parvoviridae and Densovirinae subfamilies, which infect vertebrates and invertebrates, respectively (Cotmore et al., 2014; Kursa et al., 2019). Recently, according to the reorganization by the International Committee on Taxonomy of Viruses, the novel subfamily Hamaparvovirinae, which contains the genera Penstyldensovirus, Brevihamaparvovirus, Hepandensovirus, Ichthamaparvovirus, and Chapparvovirus has been regrouped (Pénzes et al., 2020). The genome of Parvovirus is approximately 3.9 to 6.3kbp in length, contains 2 major open reading frames (ORFs), and has a relatively conserved overall genomic structure: a nonstructural gene (NS) located at the 3′ end and a structural viral protein gene at the 5′ end (Day and Zsak, 2010; Pénzes et al., 2020). Additionally, both genomic termini have hairpin-like DNA secondary structures (Pénzes et al., 2019).
Numerous novel parvoviruses have been discovered so far owing to the development of sequencing technology and the demand for the study of functional genomics (Siqueira et al., 2017). Among these identified novel parvoviruses, the chaphamaparvovirus (ChPVs) was first detected in a fruit bat (Eidolon helvum) in Africa (Baker et al., 2013). According to reports on its phylogenetic analysis conducted in previous reports, ChPVs is closely related to other members of the Hamaparvovirinae subfamily, which have been identified in several arthropods. This relationship suggests that the virus has spread between vertebrates as well as invertebrates during the past 10 yr (Souza et al., 2017). Since then, ChPVs have also been found in rats (Yang et al., 2016), bats (Souza et al., 2017), pigs (Palinski et al., 2016), turkeys, and chickens (Reuter et al., 2014; Lima et al., 2019). Furthermore, ChPVs have also been detected in dogs and cats, possibly in conjunction with gastroenteric symptoms (Fahsbender et al., 2019; Li et al., 2020a; Di Profio et al., 2022). Unlike chicken parvovirus, which has been associated with the runting-stunting syndrome (RSS), the infection and pathogenesis of the chicken chaphamaparvovirus (CkChpV) remains unknown (Kisary et al., 1984; Nuñez et al., 2016). Therefore, we aimed to understand the CkChpV infection mechanism better. In this study, we collected healthy chickens and chickens with diarrhea to screen for and molecularly characterize CkChpV.
MATERIALS AND METHODS
Sample Collection and Ethics Statement
In this study, permission was obtained from by farm owners to collect cloacal swabs or intestine tissue samples from healthy and sick chickens with diarrhea symptoms. Birds that died naturally were autopsied in strict accordance with the recommendations of the guidelines for the care and use of experimental animals from the South China Agricultural University Committee for Animal Experiments (approval ID: SYXK 2019-0136).
Nucleic Acid Extraction and Virus Detection
Intestine and fecal samples were collected from 478 (357 with diarrhea and 121 healthy) chickens from the Henan, Hubei, Jiangsu, and Anhui Provinces from January 2020 to May 2022. A set of nested polymerase chain reaction (PCR) primers was designed to screen for CkChpV in the collected samples. The first and second round of primer pairs included CkChpV-OF/CkChpV-OR and CkChpV-IF/CkChpV-IR. The reaction conditions are as follows: 95°C for 5 min, 35 cycles of 95°C for 30 s, 50°C for the first round and 52°C for the second round of primers, 72°C for 40 s, followed by a final extension at 72°C for 10 min. Gel electrophoresis and Sanger sequencing were performed to confirm the screening results. In addition to CkChpV, the samples were screened for other gastroenteritis-associated viruses, including avian nephritis virus (ANV), rotavirus (RTV), chicken parvovirus (CkPV), Newcastle disease virus (NDV), infectious bronchitis virus (IBV), chicken proventricular necrosis virus (CPNV), and chicken circovirus (CCV), as previously reported using PCR/RT-PCR. The DNA and RNA in the collected tissues were extracted using the Biospin Virus DNA/RNA Extraction Kit (Hangzhou Bioer Technology Co., Ltd., China) and preserved at −80°C. The primer sequences used for all screening assays are listed in Table 1.
Table 1.
Primers used in this study for detection of the viruses.
| Primer name | Primer Sequence (5′–3′) | Reference |
|---|---|---|
| CkChpV-OF | TGTATAATTCCACGTCAATGGG | This study for nested PCR |
| CkChpV-OR | TGTAGAATATGCAGCTAACCAA | |
| CkChpV-IF | CTGCTTTCAACAATTGCACGTA | |
| CkChpV-IR | TTTTCCAGCTCGCAATTCACC | |
| CkChpV-F1 | CTAGGGTATAAGTATGAGTAAGT | This study for genome amplification |
| CkChpV-R1 | AGAATCCTGGTCTATGTAAA | |
| CkChpV-F2 | AAAGCACCAGTTTGGATAATGTCG | |
| CkChpV-R2 | CACATCCTCTGGCACTATCGG | |
| CkChpV-F3 | CGTCTACTTCTGGCATCCCAAC | |
| CkChpV-R3 | TCCCAAAATACACCATTCGGTA | |
| CkChpV-F4 | CACCAACACGTTATCAATGGC | |
| CkChpV-R4 | ATCTGTATCACGAGACCACGTT | |
| CkChpV-F5 | TACGATGCAGGAAATAATCCAA | |
| CkChpV-R5 | GTACGTGGCTGCCTGCATGT | |
| ANV-F | GYTGGGCGCYTCYTTTGAYAC | Day et al., 2007 |
| ANV-R | CRTTTGCCCKRTARTCTTTRT | |
| ARV-F | GGTGCGACTGCTGTA TTTGGTAAC | Day et al., 2007 |
| ARV-R | AA TGGAACGA TAGCGTGTGGG | |
| RTV-F | GTGCGGAAAGATGGAGAAC | Day et al., 2007 |
| RTV-R | GTTGGGGTACCAGGGATTAA | |
| FAdV-1-F | CAARTTCAGRCAGACGGT | Meulemans et al., 2001 |
| FAdV-1-R | TAGTGATGMCGSGACATCAT | |
| CkPV-F | TTCTAATAACGATATCACT | Zsak et al., 2009 |
| CkPV-R | TTTGCGCTTGCGGTGAAGTCTGGCTCG | |
| NDV-F | GGAGGATGTTGGCAGCATT | Pang et al., 2002 |
| NDV-R | GTCAACATATACACCTCATC | |
| IBV-F | ATGTCTATCGCCAGGGAAATGTC | Cavanagh et al., 2002 |
| IBV-R | GGGCGTCCAAGTGCTGTACCC | |
| CPNV-F | CGTAGACCTCGTCCTTCTGC | Guy et al., 2011 |
| CPNV-R | GGGCGGTAACCA TTCAGA TA | |
| CCV-F | CATCTCCTTATGCTCCTG | Li et al., 2020 |
| CCV-R | GTGGTACTTATGCCGATT |
Complete Genome Sequencing
Five specific primer pairs for overlapping amplification of the complete CkChpV genome were designed based on the chicken chapparvovirus sequence (Chicken chapparvovirus 2s [accession no.: MG846442]), which covers the 2 major ORFs encoding NS1 and VP1. Sequence amplification was performed under the following cyclic conditions: Initial denaturation at 95°C for 3 min, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 53.5°C for 30 s, and extension at 72°C for 1 min 20 s, with a final extension at 72°C and then cooled to 4°C. The obtained amplicons were then cloned into a pMD18-T vector (TaKaRa Biotechnology Co., Ltd., Dalian, China) for further sequencing (Sangon Biotech, Zhengzhou, China). All sequence amplification and sequencing steps were repeated 3 times.
Viral Isolation
Three cell lines, DF-1, LMH, and MDCC-MSB1, were cocultured with CkChpV-positive sample supernatants for 4 d and blindly passaged to the fifth generation. The samples were subjected to PCR assay as described above.
Sequence Identification, Phylogeny, and Genotype
The 26 parvovirus complete genome data (including ChPV strains and other parvovirus members) were retrieved from GenBank (http://blast.ncbi.nlm.nih.gov/Blast.cgi) to determine the phylogenetic relationship between the 9 CkChpV strains obtained in this study with these reference strains. Pair-wise sequence alignment was performed using the Clustal W model in MegAlign program of Lasergene 7.0 software (DNASTAR Inc., Madison, WI). A phylogenetic tree based on the nucleotides (nt) of the whole genome and deduced amino acids (aa) of NS1 and VP1 for evolution analysis was constructed via the maximum-likelihood method with LG+G+F model and 1,000 bootstrap replicates in MEGA X, respectively (Kumar et al., 2018).
Moreover, sequence alignment was implemented and visualized by ChiPlot (https://www.chiplot.online/). The SMART database (http://smart.embl.de/) was used for the protein structure prediction of NS1 and VP1 of the CkChpVs and IBS (Illustrator for Biological Sequences) software was used for the visualization (Liu et al., 2015). Finally, the virus proportion was visualized using the Wayne diagram (http://jvenn.toulouse.inra.fr/app/example.html) and UpSet diagram created using TBtools 1.09876 (Bardou et al., 2014; Chen et al., 2020).
Recombination Analysis
The recombination events in these 9 CkChpVs strains were predicted using RDP4.36. The default parameters used for this step comprised RDP, Maxchi, and GENECONV (Martin et al., 2015). Therefore, the results obtained were confirmed using SimPlot 3.5.1 (Samson et al., 2022).
Prediction of Protein Structure for NS1 and VP1 and Antigenic Epitopes
The VP1 structure of protein CkChpV-CHN210413 was predicted and constructed using SWISS-MODEL (https://swissmodel.expasy.org/) and PyMol 2.5 based on different aa sites (Biasini et al., 2014; Waterhouse et al., 2018). The antigenic epitopes of VP1 on CkChpV were predicted using SOPMA (https://prabi.ibcp.fr/htm/site/web/home) and SVMTtiP (Rigsby and Parker, 2016).
Statistical Analysis
The chi-square test was used to compare the frequency of CkChpV in healthy and diarrheic chickens and those with diarrhea. All statistical analyses were performed using GraphPad Prism 8.0 (San Diego, CA). A P value of <0.05 was considered statistically significant.
RESULTS
Clinical History and Virus Screening
After screening for viruses, 7 of the 121 healthy chickens and 116 of the 357 diarrhea chickens were positive for CkChpVs as determined by nested PCR. The positive rates for ANV, RTV, CkPV, NDV, IBV, CPNV, and CCV were 9% (42/357), 6% (27/357), 2% (9/357), 2% (9/357), 1% (5/357), 0% (0/37), and 0% (0/357), respectively (Figure 1). Most CkChpV-positive samples (94.3%, 116/123) were detected in chickens with diarrhea symptoms. Statistical analysis revealed a relationship between CkChpV infection and the clinical symptoms (P < 0.05).
Figure 1.
CKChPV distribution in China and the positive rates of CkChpV, ANV, RTV, CkPV, NDV, and IBV. Illustrates the combination of the six-pathogen co-infection in the form a Venn diagram and UpSet plot. The UpSet plot presents the distribution of different viruses in the samples. The bar chart above represents the number of genes contained in each type of group, and left represents the number of positive numbers included in each type of pathogen. The dotted line at the bottom right presents the types of events contained in the group.
Virus Cultivation
Until the fifth generation, no specific cytopathic effect (CPE) was observed in cultured cells, and DNA of CkChpVs was not detected by the nested PCR described above.
Sequence Identities and Phylogenetic Analyses
The complete genome of the 9 strains was 4,432 nt in length; all 9 sequences were deposited in GenBank (GenBank accession numbers: OP172522-OP172530). The obtained CkChpV genome had 2 ORFs, one encoding a 674-aa nonstructural protein 1 (NS1) and other a 558-aa capsid protein (VP1) (Figure 2). Genomic identity of the ChPVs from Henan and Hubei Provinces were 96.7% to 98.8%, and aa identity was 95.9 to 98.0% for NS1 and 97.8 to 99.2% for VP1 (Figure 3). The aa identity of the 9 CkChpVs strains were 17.7 to 99.4% for NS1 and 13.5 to 99.3% for VP1 compared with other ChPV members obtained from GenBank. NS1 and VP1 shared the highest aa identity with the chicken chapparvovirus 2s strain (MG846442) belonging to Galliform chaphamaparvovirus 2 compared with viral strains detected in other hosts. The detailed data are shown in Table 2.
Figure 2.
Genome organization of CkChpV. The nucleotide position of ORFs in the genome is also indicated.
Figure 3.
Sequence identity of CkChpV and other ChpV strains.
Table 2.
Sequence identities of the study strains of nine CkChpV with the members of the CHPV.
| Virus strains | Accession no. | Sequence identity (%) |
Genetic distance of VP1 | ||
|---|---|---|---|---|---|
| Genome (nt) | NS1(aa) ORF1b (aa) | VP1(aa) | |||
| Turkey parvovirus-TP1 | KF925531 | 46.8–47.0 | 44.3–45.6 | 28.0–28.1 | 0.910–0.915 |
| Tasmanian devil-6 | MK513533 | 45.1–45.5 | 43.7–44.6 99.2–100 |
40.3–40.7 | 0.728–0.732 |
| Tasmanian devil-2 | MK513529 | 48.2–48.4 | 39.0–39.5 | 48.1–48.8 | 0.644–0.650 |
| Tasmanian devil-1 | MK513528 | 41.7–41.9 | 32.3–33.1 | 40.3–40.7 | 0.715–0.723 |
| Simian parvo-cg5864 | KT961660 | 31.6–31.8 | 31.1–31.5 | 27.1–27.2 | 0.918–0.924 |
| Rat parvovirus 2 | KX272741 | 45.8–46.0 | 38.7–39.6 | 45.0–45.4 | 0.661–0.665 |
| Porcine parvovirus 7 | KU563733 | 43.9–44.1 | 37.5–37.8 | 45.9–46.4 | 0.660–0.653 |
| Parvoviridae yc-9 | KY312548 | 50.3–50.7 | 42.6–44.1 | 46.7–47.1 | 0.677–0.683 |
| Murine chapparvovirus | MF175078 | 45.0–45.3 | 38.9–39.2 64.7–65.2 |
48.1–48.5 | 0.647–0.651 |
| TuPV 1090 | KM598420 | 34.4–34.8 | 17.7–18.4 | 13.5–13.9 | 0.926–0.930 |
| TuPV 1030 | KM598418 | 34.7–35.0 | 17.8–18.2 | 13.7–14.1 | 0.924–0.928 |
| Feline IDEXX-1 | MN396757 | 46.5-46.7 | 39.5-40.2 | 45.4–45.4 | 0.562–0.569 |
| Eidolon helvum-2 | JX885610 | 27.7–27.9 | 37.2–37.5 | NA | NA |
| Desmodus rotundus | NC032097 | 47.2–47.4 | 39.0–39.7 | 46.8-47.2 | 0.663–0.669 |
| Chicken-ADL120035 | KJ486490 | 33.4–33.6 | 17.7–18.2 | 13.8–13.9 | 0.924–0.926 |
| Chicken-ADL120019 | KJ486489 | 33.4–33.7 | 17.7–18.2 | 13.8–13.9 | 0.924–0.926 |
| Chicken-HK | KM254174 | 64.7–65.2 | 78.9–79.1 | NA | NA |
| Chicken-RS/BR/15/5S | MG846443 | 92.9–95.1 | 95.5–98.2 | 95.6–96.2 | 0.027–0.331 |
| Chicken-RS/BR/15/2S | MG846442 | 96.6–98.8 | 95.2–99.2 | 98.5–99.3 | 0.008–0.030 |
| Chicken-RS/BR/15/6S | MG846441 | 43.6–43.8 | 71.9–73.0 | NA | NA |
| Chapparvovirus-BDchPV4181 | MT732119 | 45.7–45.9 | 33.9–34.4 | 35.7–35.9 | 0.733–0.736 |
| ChPV/PB32-SII33 | OM469072 | 94.7–96.2 | 95.4–99.4 | 97.5–98.7 | 0.017–0.030 |
| Carnivore-VRI 849 | MN794869 | 47.9–48.1 | 39.7–40.3 | 45.1–45.4 | 0.654–0.658 |
| Cachavirus-1B | MK448316 | 47.2–47.5 | 38.7–39.0 60.6–61.2 |
45.5–46.0 | 0.666–0.671 |
| Cachavirus-1A | MH893826 | 47.9–48.2 | 38.9–39.1 | 47.2–47.3 | 0.672–0.674 |
| Bat parvovirus | MG693107 | 47.7–47.8 | 39.2–39.8 | 47.9–48.9 | 0.651–0.659 |
NA: not available.
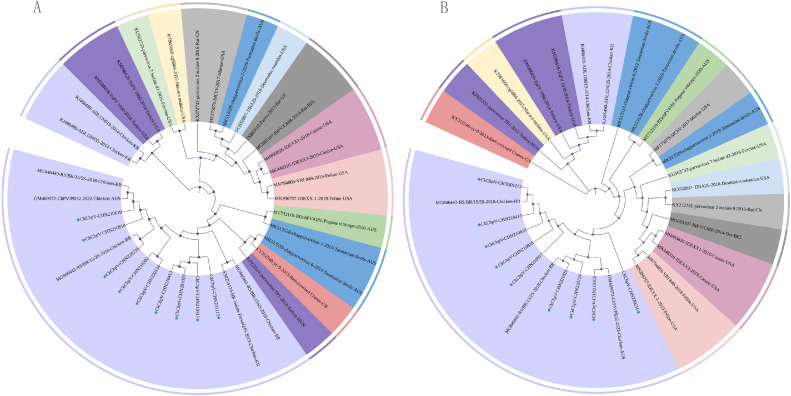
Phylogenetic trees were constructed for the NS1 (aa) (Figure 4A) and VP1 (aa) (Figure 4B) of CkChpVs. By analyzing the phylogenetic tree based on NS1 and VP1, the 9 strains from chicken were found to be closely related to the chicken chapparvovirus 2s strain and clustered into one major branch. They are distantly related with other parvovirus members, including canine and feline ChPVs.
Figure 4.
Phylogenetic tree of the NS1 and VP1 genes in CkChpV. The nine CKChpV detects in this report were indicated by blue solid star. The same color is used to identify the species. (A) Phylogenetic tree of NS1; (B) phylogenetic tree of VP1.
Recombination Analysis and Mutation of CkChpV
Recombination events in the CkChpV strains predicted by RDP and SimPlot are listed in Table 3. As per recombination analysis, 15 of the 35 strains showed recombination events, and the regions in some strains were similar to those of the recombinant event in the CkChpV-CHN210818, CkChpV-CHN201029, CkChpV-CHN220124, and CkChpV-CHN210619 strains (Figure 5).
Table 3.
Recombination analysis of the CkChpV strains included in this study.
| Recombination strains | Breakpoint positions | Minor parent | Major parent | P-value | |||
|---|---|---|---|---|---|---|---|
| Start | End | Similarity | Similarity | ||||
| CkChpV-CN210818 | 2,413 | 2,672 | CkChpV-CN220216 | 98.6% | OM469072-ChPV/PB32 | 98.2% | 2.41E-07 |
| CkChpV-CN201029 | 2,199 | 2,473 | CkChpV-CN210619 | 98.9% | CkChpV-CN210413 | 99.2% | 1.25E-12 |
| CkChpV-CN220124 | 942 | 1,103 | CkChpV-CN201029 | 99.2% | MG846443-RS/BR/15/5S | 99.6% | 1.28E-06 |
| CkChpV-CN210619 | 885 | 1,024 | CkChpV-CN210917 | 97.7% | OM469072-ChPV/PB32 | 99.6% | 5.73E-17 |
Figure 5.
Representative recombined strains and predicted recombination events.
The chicken chapparvovirus 2s strain was used to as reference to observe the mutation sites of the 9 Chinese strains. NS1 showed several mutations at sites 132 to 142 in strains CkChpV-CHN220216 and CkChpV-CHN220124 and at sites 577 to 618 in strains CkChpV-CHN201029, CkChpV-CHN210818, CkChpV-CHN201113, and CkChpV-CHN210413. The mutation sites in other strains were relatively consistent. Additionally, some aa sites showed high mutation rates, such as Ile31Val, Val147Thr, Ser155Thr, and Ser290Asn in VP1. The other mutation sites in NS1 and VP1 are shown in Table 4.
Table 4.
(a). Analyses of the main amino acid mutation sites in the NS1 capsid protein of CkChpV in Chinese strains (“CHN”; this study) and reference strains. (b). Analyses of the main amino acid mutation sites in the VP1 capsid protein of CkChpV in Chinese strains (“CHN”; this study) and reference strains.
| (a) NS1 Position | CHN210917 | CkChpV-CHN220124 | CkChpV-CHN220216 | CkChpV-CHN210619 | CkChpV-CHN210302 | CkChpV-CHN201029 | CkChpV-CHN210818 | CkChpV-CHN201113 | CkChpV-CHN210413 | ChPV-RS/BR/15/2S |
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | I | V | V | V | V | V | V | V | V | V |
| 8 | S | S | S | S | P | P | S | S | P | S |
| 12 | I | V | V | V | V | I | V | V | I | V |
| 16 | L | F | F | L | F | L | F | L | L | F |
| 36 | S | T | T | T | T | T | T | T | S | T |
| 49 | V | V | V | V | V | A | V | V | A | V |
| 85 | F | L | L | L | L | L | L | L | L | L |
| 94 | A | A | A | P | A | A | A | P | A | A |
| 132 | S | K | K | S | S | T | S | S | T | S |
| 139 | R | R | R | K | K | R | K | K | R | K |
| 142 | T | I | I | I | I | T | I | T | T | I |
| 149 | N | N | N | H | N | N | N | Q | N | N |
| 151 | P | S | S | S | S | S | S | S | S | S |
| 167 | L | L | F | L | L | L | L | L | L | L |
| 178 | L | L | M | M | L | L | M | L | L | M |
| 193 | T | A | V | I | A | T | V | T | T | V |
| 201 | K | K | K | K | K | K | K | R | K | K |
| 203 | S | S | S | S | S | T | S | S | T | S |
| 221 | I | I | I | I | I | I | I | I | I | L |
| 235 | E | E | D | E | E | E | E | E | E | D |
| 261 | L | L | L | F | L | L | L | L | L | F |
| 289 | I | I | I | I | I | I | I | V | V | I |
| 299 | H | H | H | H | Y | Y | H | H | H | H |
| 302 | Y | F | Y | Y | Y | Y | Y | Y | Y | Y |
| 317 | C | R | C | C | C | C | C | C | C | C |
| 333 | S | A | S | S | S | S | S | S | S | S |
| 355 | L | L | F | L | L | L | L | L | L | L |
| 379 | V | I | I | V | V | V | V | V | V | V |
| 387 | I | I | I | I | I | I | V | I | I | I |
| 397 | L | F | L | L | L | L | L | L | L | L |
| 412 | D | E | D | D | D | D | D | D | D | D |
| 436 | D | D | H | D | D | D | D | D | D | D |
| 466 | P | P | P | P | P | S | P | P | P | P |
| 469 | S | S | S | S | P | P | S | S | S | S |
| 477 | S | S | P | S | S | S | S | S | S | S |
| 517 | E | E | E | E | E | E | E | K | E | E |
| 524 | A | A | A | A | T | T | T | A | A | A |
| 534 | D | D | A | D | D | D | D | D | D | D |
| 577 | K | K | K | Q | Q | Q | K | Q | Q | K |
| 606 | V | V | M | M | V | M | V | V | V | M |
| 618 | I | V | I | V | I | I | I | I | I | V |
| 619 | H | H | H | H | H | P | H | H | H | H |
| (b) VP1 Position | CkChpV-CHN210917 | CkChpV-CHN220124 | CkChpV-CHN220216 | CkChpV-CHN210619 | CkChpV-CHN210302 | CkChpV-CHN201029 | CkChpV-CHN210818 | CkChpV-CHN201113 | CkChpV-CHN210413 | ChPV-RS/BR/15/2S |
| 31 | I | V | V | I | V | I | V | V | V | I |
| 147 | T | I | I | T | I | T | V | V | V | T |
| 150 | Q | P | Q | Q | P | Q | T | T | T | Q |
| 155 | T | T | T | T | T | T | T | T | S | T |
| 174 | D | D | D | D | D | G | D | D | D | D |
| 263 | E | E | E | E | E | E | E | E | K | E |
| 281 | S | A | S | S | S | A | S | S | S | A |
| 290 | N | S | N | S | S | S | N | N | N | S |
| 295 | S | S | S | S | G | A | S | S | S | S |
| 329 | V | V | V | V | I | V | V | V | V | V |
| 391 | F | F | F | F | F | L | F | F | F | F |
| 455 | G | G | G | G | G | G | S | S | S | G |
| 457 | T | N | S | N | N | S | T | T | T | S |
| 482 | I | I | I | V | I | V | V | V | V | V |
| 500 | T | T | T | T | S | T | S | S | S | T |
| 506 | T | T | T | T | T | T | S | S | S | T |
| 514 | K | K | K | K | K | K | K | K | E | K |
| 535 | K | E | E | E | E | E | E | E | E | E |
| 553 | L | L | L | L | L | I | L | L | L | L |
Tertiary Structure and Antigenic Epitope of VP1
Refer to the Chicken chapparvovirus 2s strain, NS1 and VP1 models of CkChpV strains were predicted by SWISS-MODEL, Figure 6 showed the predicted structural changes brought on by the mutant sites.
Figure 6.
Predicted tertiary structural model of CkChpV in NS1 and VP1 (A) Tertiary structural model of NS1; (B) tertiary structural model of VP1, “←” and letter indicate specific amino acid sequences with specific colors.
Based on CkChpV-CHN210413, the antigenic epitopes of VP1 were using ABSpred, SOPMA and SVMTriP are shown in Table 5. Nine common epitope regions for these strains were obtained, with a reliability of 0.748 to 0.99.
Table 5.
VP1 epitope location and sequences.
| Epitopes | Residues (amino acid) | Epitope sequences |
|---|---|---|
| A | 3–22 | ETIKFQNVYMTYIDNNPYQY |
| B | 122–141 | PWNTLERKKQLHLSQREGLV |
| C | 186–205 | GVYDTDATTSLADSHQAIPN |
| D | 238–257 | DANKFFNLDWLAAYSTWTVD |
| E | 344–363 | TWFKKPDKYWSGTEYEACTY |
DISCUSSION
For a long time, the specificity of vertebrate or invertebrate hosts has been the standard for the classification of Parvoviridae and has been supported by phylogenetic inference. With the development of virus classification protocols, most researchers have concluded that the traditional classification is no longer applicable and that the classification of the Parvoviridae family should be more precise (Pénzes et al., 2019).
Diarrhea usually leads to malnutrition and growth stagnation in chickens, leading to economic losses to the poultry industry. For all of the collected samples in this study, the positive rate of CkChpV was 32% (116/357) in chickens with diarrhea symptoms. Moreover, statistical analysis revealed a correlation between the presence of the virus and diarrhea symptoms (P < 0.05). However, it is difficult to prove their potential pathogenic mechanism in chickens as the virus cannot be cultivated on cell lines for animal experiments.
ChPVs have been detected in various animals, but the pathogenicity of these viruses remains unclear. To date, only mouse kidney parvovirus, a parvovirus causing severe chronic interstitial nephropathy and renal failure, belonging to the Chaphamaparvovirus genus has been isolated from laboratory and wild mouse populations (Roediger et al., 2018; Edmondson et al., 2020) Additionally, a high prevalence of murine ChPV in murine liver tissue suggests that it is a gastrointestinal agent (Williams et al., 2018). The pathogenesis of CkChpV needs to be further investigated, which calls for extensive clinical animal experiments.
According to the phylogenetic evolutionary tree constructed in this study, all 9 strains belonged to the CkChpV group. The phylogenetic tree based on genomic sequences revealed that 9 strains from China were clustered at the same branch that included chicken chapparvovirus 2 strains and shared high similarity (96.6–98.8% genomic similarity, 95.2–99.2% aa similarity in NS1, and 98.5–99.3% aa similarity in VP1). The evolutionary trees also indicated that the 9 newly strains were different from the chicken chapparvovirus 1 strain (MG846441) and chicken chapparvovirus HK (KM254174), which shared 43.6 to 65.2% genomic similarity and 71.9 to 79.1% in NS1 this indicates the relationship and genetic distance between these nine CkChpV and other chicken chapparvovirus strains. The nine CkChpVs strains, however, shared a low identity with the representative chicken parvovirus (Chicken-ADL120035 and Chicken-ADL120019) in terms of the whole genome sequence (33.45–33.7%) and aa sequence (17.7–18.2% for NS1 and 13.8–13.9% for VP1), which demonstrated the stark differences between CkChpV and chicken parvovirus. The nine CkChpVs strains shared the highest identity with the Parvoviridae sp. strain yc-9 (KY312548) with a 50.7% genomic similarity and 44.1% and 47.1% aa similarity for NS1 and VP1, respectively. More research is needed to determine whether the relatively high similarity was because of cross-host transmission or other reasons.
In this study, we found that CkChpV shares various traits, including genomic length, encoding a relatively large NS1 (673 aa) and a comparatively short VP1 (557 aa). The NS1 protein contains the Parvo domain, a helicase domain representing the parvovirus NS1 protein that contains the ATP/GTP binding site motif A (P-loop) that is necessary for viral DNA replication (Nüesch and Tattersall, 1993; Iseki et al., 2005). The VP1 proteins of CkChpV are definitely related to those of other ChPVs. However, CkChpVs and parvovirus differ in some ways. The PLA2 domains, which are thought to be crucial for most parvovirus infections, were not predicted for any of the 9 CkChpV strains, which is consistent with protein domain predictions (Pénzes et al., 2019). Additionally, aa sites of these 9 strains from chicken showed some mutations that affected the tertiary structure modeling of the NS1 and VP1 proteins compared with the chicken chapparvovirus 2s strain. Although the NS1 structural protein (multidomain protein) showed certain mutations, they are less noticeable than the structural alterations caused by the VP1 mutation because NS1 has a complete coding area and comprises substantial nonstructural proteins that are crucial for virus replication, control, and immunity (Nüesch and Tattersall, 1993). From the structure prediction of VP1, our strain had changed compared with the reference strain, which possibly indicates that the changes at Val31Glu, Val147Thr, 120 to 124 WFPWN, and 141 to 143 VWT led to the changes in its structure. As a capsid protein, VP1 is responsible for translating the structural proteins and protecting viral nucleic acids (Pénzes et al., 2020). However, site mutations may alter its function and pathogenesis. Further research is needed in this regard. Further, antigenic epitopes were predicted, and it was suggested that a large number of mutations are caused by virus evolution under antibody pressure, further indicating the high prevalence of CkChpVs in China. Recombination analysis indicated that the existence of reorganization event, predicted recombination events that not only happened with chicken chapparvovirus 2s strain in a foreign environment but also occurred in these 9 strains. These results showed that a reorganization event occurred along with the gradual spread of the virus.
In conclusion, this study firstly reported the presence of the novel chaphamaparvovirus (CkChpV) in Chinese chickens. These findings provide a reference for studying the transmission and evolution of ChPVs. The relatively high prevalence of CkChpV in diarrhea chickens should be given more attention. Large-scale studies are warranted to confirm the pathogenicity of this virus.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Grant nos. 31802185 and 31870917), the Program for Science & Technology Innovation Talents in University of Henan Province (Grant no. 22HASTIT042), the Scientific and Technological Project of Henan province (Grant no. 182102310077), the Key Scientific and Technological Project of the Education Department of Henan province (Grant no. 18A230011), the program for Innovative Research Team of Science and Technology in University of Henan Province (Grant no. 20IRTSTHN024), the Guangdong Basic and Applied Basic Research Foundation (Grant no. 2019A1515012006), and the Technological Project of Nanyang Normal University (Grant no. 2022STP002).
Ethical Statement: The autopsy and sampling protocols for dead birds were approved by the South China Agricultural University Committee for Animal Experiments (approval ID: SYXK-2014-0136).
Data Availability Statement: All data generated or analyzed during this study are included in this article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contribution Statement: JJ and LGY conceived and design the research; XX and KM performed the sampling and data collection; HC performed the clinical investigations and molecular tests; SSP performed the molecular genetic studies; JJ and YCK analyzed data and conducted statistical analyses; HC and SSP wrote the manuscript; LGY, YZB and QMX revised the manuscript critically. All authors read and approved the manuscript.
DISCLOSURES
The authors have no competing interests to declare.
REFERENCES
- Baker K.S., Leggett R.M., Bexfield N.H., Alston M., Daly G., Todd S., Tachedjian M., Holmes C.E., Crameri S., Wang L.F., Heeney J.L., Suu-Ire R., Kellam P., Cunningham A.A., Wood J.L., Caccamoand M., Murcia P.R. Metagenomic study of the viruses of African straw-coloured fruit bats: detection of a chiropteran poxvirus and isolation of a novel adenovirus. Virology. 2013;441:95–106. doi: 10.1016/j.virol.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardou P., Mariette J., Escudié F., Djemieland C., Klopp C. jvenn: an interactive Venn diagram viewer. BMC Bioinform. 2014;15:293. doi: 10.1186/1471-2105-15-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biasini M., Bienert S., Waterhouse A., Arnold K., Studer G., Schmidt T., Kiefer F., Gallo Cassarino T., Bertoni M., Bordoliand L., Schwede T. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014;42:W252–W258. doi: 10.1093/nar/gku340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D., Mawditt K., Welchman Dde B., Brittonand P., Gough R.E. Coronaviruses from pheasants (Phasianus colchicus) are genetically closely related to coronaviruses of domestic fowl (infectious bronchitis virus) and turkeys. Avian Pathol. 2002;31:81–93. doi: 10.1080/03079450120106651. [DOI] [PubMed] [Google Scholar]
- Chen C., Chen H., Zhang Y., Thomas H.R., Frank M.H., Heand Y., Xia R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;13:1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- Cotmore S.F., Agbandje-McKenna M., Chiorini J.A., Mukha D.V., Pintel D.J., Qiu J., Soderlund-Venermo M., Tattersall P., Tijssen P., Gatherer D., Davison A.J. The family Parvoviridae. Arch Virol. 2014;159:1239–1247. doi: 10.1007/s00705-013-1914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day J.M., Spackmanand E., Pantin-Jackwood M. A multiplex RT-PCR test for the differential identification of turkey astrovirus type 1, turkey astrovirus type 2, chicken astrovirus, avian nephritis virus, and avian rotavirus. Avian Dis. 2007;51:681–684. doi: 10.1637/0005-2086(2007)51[681:AMRTFT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Day J.M., Zsak L. Determination and analysis of the full-length chicken parvovirus genome. Virology. 2010;399:59–64. doi: 10.1016/j.virol.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Profio F., Sarchese V., Palombieri A., Fruci P., Massirio I., Martella V., Fulvioand M., Di Martino B. Feline chaphamaparvovirus in cats with enteritis and upper respiratory tract disease. Transbound. Emerg. Dis. 2022;69:660–668. doi: 10.1111/tbed.14032. [DOI] [PubMed] [Google Scholar]
- Edmondson E.F., Hsieh W.T., Kramer J.A., Breed M.W., Roelke-Parker M.E., Stephens-Devalle J., Pate N.M., Bassel L.L., Hollingshead M.G., Karim B.O., Butcher D.O., Warner A.C., Nagashimaand K., Gulani J. Naturally acquired mouse kidney parvovirus infection produces a persistent interstitial nephritis in immunocompetent laboratory mice. Vet. Pathol. 2020;57:915–925. doi: 10.1177/0300985820953500. [DOI] [PubMed] [Google Scholar]
- Fahsbender E., Altan E., Seguin M.A., Young P., Estrada M., Leuteneggerand C., Delwart E. Chapparvovirus DNA found in 4% of dogs with diarrhea. Viruses. 2019;11:398. doi: 10.3390/v11050398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J.S., Westand A.M., Fuller F.J. Physical and genomic characteristics identify chicken proventricular necrosis virus (R11/3 virus) as a novel birnavirus. Avian Dis. 2011;55:2–7. doi: 10.1637/9504-081610-reg.1. [DOI] [PubMed] [Google Scholar]
- Iseki H., Shimizukawa R., Sugiyama F., Kunita S., Iwama A., Onodera M., Nakauchiand H., Yagami K. Parvovirus nonstructural proteins induce an epigenetic modification through histone acetylation in host genes and revert tumor malignancy to benignancy. J. Virol. 2005;79:8886–8893. doi: 10.1128/JVI.79.14.8886-8893.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisary J., Avalosse B., Miller-Faures A., Rommelaere J. The genome structure of a new chicken virus identifies it as a parvovirus. J. Gen. Virol. 1985;66(Pt 10):2259–2263. doi: 10.1099/0022-1317-66-10-2259. [DOI] [PubMed] [Google Scholar]
- Kisary J., Nagyand B., Bitay Z. Presence of parvoviruses in the intestine of chickens showing stunting syndrome. Avian Pathol. 1984;13:339–343. doi: 10.1080/03079458408418536. [DOI] [PubMed] [Google Scholar]
- Kumar S., Stecher G., Li M., Knyazand C., Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kursa O., Tomczyk G., Sawicka A. Prevalence and phylogenetic analysis of mycoplasma synoviae strains isolated from polish chicken layer flocks. J. Vet. Res. 2019;63:41–49. doi: 10.2478/jvetres-2019-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Yuan S., Yan T., Shanand H., Cheng Z. Identification and characterization of chicken circovirus from commercial broiler chickens in China. Transbound. Emerg. Dis. 2020;67:6–10. doi: 10.1111/tbed.13331. [DOI] [PubMed] [Google Scholar]
- Lima D.A., Cibulski S.P., Tochetto C., Varela A., Finkler F., Teixeira T.F., Loiko M.R., Cerva C., Junqueira D.M., Mayerand F.Q., Roehe P.M. The intestinal virome of malabsorption syndrome-affected and unaffected broilers through shotgun metagenomics. Virus Res. 2019;261:9–20. doi: 10.1016/j.virusres.2018.12.005. [DOI] [PubMed] [Google Scholar]
- Liu W., Xie Y., Ma J., Luo X., Nie P., Zuo Z., Lahrmann U., Zhao Q., Zheng Y., Zhao Y., Xueand Y., Ren J. IBS: an illustrator for the presentation and visualization of biological sequences. Bioinformatics. 2015;31:3359–3361. doi: 10.1093/bioinformatics/btv362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D.P., Murrell B., Golden M., Khoosal A., Muhire B. RDP4: detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015;1 doi: 10.1093/ve/vev003. vev003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulemans G., Boschmans M., Bergand T.P., Decaesstecker M. Polymerase chain reaction combined with restriction enzyme analysis for detection and differentiation of fowl adenoviruses. Avian Pathol. 2001;30:655–660. doi: 10.1080/03079450120092143. [DOI] [PubMed] [Google Scholar]
- Nuñez L.F., Sá L.R., Parra S.H., Astolfi-Ferreira C.S., Carranzaand C., Ferreira A.J. Molecular detection of chicken parvovirus in broilers with enteric disorders presenting curving of duodenal loop, pancreatic atrophy, and mesenteritis. Poult. Sci. 2016;95:802–810. doi: 10.3382/ps/pev439. [DOI] [PubMed] [Google Scholar]
- Nüesch J.P., Tattersall P. Nuclear targeting of the parvoviral replicator molecule NS1: evidence for self-association prior to nuclear transport. Virology. 1993;196:637–651. doi: 10.1006/viro.1993.1520. [DOI] [PubMed] [Google Scholar]
- Palinski R.M., Mitraand N., Hause B.M. Discovery of a novel Parvovirinae virus, porcine parvovirus 7, by metagenomic sequencing of porcine rectal swabs. Virus Genes. 2016;52:564–567. doi: 10.1007/s11262-016-1322-1. [DOI] [PubMed] [Google Scholar]
- Pang Y., Wang H., Girshick T., Xieand Z., Khan M.I. Development and application of a multiplex polymerase chain reaction for avian respiratory agents. Avian Dis. 2002;46:691–699. doi: 10.1637/0005-2086(2002)046[0691:DAAOAM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Pénzes J.J., de Souza W.M., Agbandje-McKennaand M., Gifford R.J. An ancient lineage of highly divergent Parvoviruses Infects both vertebrate and invertebrate hosts. Viruses. 2019;11:525. doi: 10.3390/v11060525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pénzes J.J., Söderlund-Venermo M., Canuti M., Eis-Hübinger A.M., Hughes J., Cotmore S.F., Harrach B. Reorganizing the family Parvoviridae: a revised taxonomy independent of the canonical approach based on host association. Arch Virol. 2020;165:2133–2146. doi: 10.1007/s00705-020-04632-4. [DOI] [PubMed] [Google Scholar]
- Reuter G., Boros Á., Delwartand E., Pankovics P. Novel circular single-stranded DNA virus from turkey faeces. Arch. Virol. 2014;159:2161–2164. doi: 10.1007/s00705-014-2025-3. [DOI] [PubMed] [Google Scholar]
- Rigsby R.E., Parker A.B. Using the PyMOL application to reinforce visual understanding of protein structure. Biochem. Mol. Biol. Educ. 2016;44:433–437. doi: 10.1002/bmb.20966. [DOI] [PubMed] [Google Scholar]
- Roediger B., Lee Q., Tikoo S., Cobbin J., Henderson J.M., Jormakka M., Rourke M.B., Padula M.P., Pinello N., Henry M., Wynne M., Santagostino S.F., Brayton C.F., Rasmussen L., Lisowski L., Tay S.S., Harris D.C., Bertram J.F., Dowling J.P., Bertolino P., Lai J.H., Wu W., Bachovchin W.W., Wong J.J., Gorrell M.D., Shaban B., Holmes E.C., Jolly C.J., Monetteand S., Weninger W. An atypical parvovirus drives chronic tubulointerstitial nephropathy and kidney fibrosis. Cell. 2018;175:530–543. doi: 10.1016/j.cell.2018.08.013. e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson S., Lord É., Makarenkov V. SimPlot ++: a Python application for representing sequence similarity and detecting recombination. Bioinformatics. 2022;38:3118–3120. doi: 10.1093/bioinformatics/btac287. [DOI] [PubMed] [Google Scholar]
- Siqueira J.D., Ng T.F., Miller M., Li L., Deng X., Dodd E., Batac F., Delwart E. Endemic infection of stranded southern sea otters (enhydra lutris nereis) with novel parvovirus, polyomavirus, and adenovirus. J. Wildl. Dis. 2017;53:532–542. doi: 10.7589/2016-04-082. [DOI] [PubMed] [Google Scholar]
- Souza W.M., Romeiro M.F., Fumagalli M.J., Modha S., de Araujo J., Queiroz L.H., Durigon E.L., Figueiredo L., Murciaand P.R., Gifford R.J. Chapparvoviruses occur in at least three vertebrate classes and have a broad biogeographic distribution. J. Gen. Virol. 2017;98:225–229. doi: 10.1099/jgv.0.000671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse A., Bertoni M., Bienert S., Studer G., Tauriello G., Gumienny R., Heer F.T., de Beer T., Rempfer C., Bordoli L., Leporeand R., Schwede T. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S.H., Che X., Garcia J.A., Klena J.D., Lee B., Muller D., Ulrich W., Corrigan R.M., Nichol S., Jainand K., Lipkin W.I. Viral diversity of house mice in New York City. mBio. 2018;9 doi: 10.1128/mBio.01354-17. e01354-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Liu Z., Wang Y., Li W., Fu X., Lin Y., Shen Q., Wang X., Wangand H., Zhang W. A novel rodent Chapparvovirus in feces of wild rats. Virol. J. 2016;13:133. doi: 10.1186/s12985-016-0589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsak L., Strotherand K.O., Day J.M. Development of a polymerase chain reaction procedure for detection of chicken and turkey parvoviruses. Avian Dis. 2009;53:83–88. doi: 10.1637/8464-090308-Reg.1. [DOI] [PubMed] [Google Scholar]