Abstract
As a disaster-prone country with unique geographical features, snake biting is a major public health concern in Bangladesh. The primary reasons of mortality from snakebite include late presentation to the hospital, low efficacy of antivenom, and a lack of adequate management facilities. Because snake venom characteristics vary depending on geographical location, antivenom should be manufactured from snakes native to the region in which it would be administered. Bungarus caeruleus is a highly venomous snake contributing to the major snakebite issue in Bangladesh. Therefore, the neutralization efficacy of the antivenom against B. caeruleus venom was evaluated in the current study along with the characterization of venom. For biological characterization of venom, RP-HPLC and SDS-PAGE profiling, hemolytic activity, hemorrhagic activity, phospholipases A2 (PLA2) activity, edema inducing activity and histopathological observations were carried out following standard protocol. LD50 of the venom was calculated along with neutralization potency of Incepta antivenom through probit analysis. Results showed that venom possesses phospholipase A2 activity, hemolytic activity and edema inducing activity while hemorrhagic activity was absent in the skin of envenomed mice. Histopathological alterations including necrosis, congestion and infiltrations were observed in envenomed mice organs after hematoxylin and eosin staining. Neutralization study showed that Incepta polyvalent antivenom could neutralize (potency 0.53 mg/ml) the lethal effect in in vitro study on mice. Further investigation on snakebite epidemiology and clinical observations of the envenomed patients will help in combating the snakebite problem more efficiently.
Keywords: Antivenom, B. caeruleus, Histopathology, Snakebite, Venom
Graphical abstract
Highlights
-
•
Biological characterization of B. caeruleus venom was performed as well as the determination of LD50 and ED50.
-
•
Histopathological alterations including necrosis, congestion and infiltrations were observed in envenomed mice organs.
1. Introduction
Snakebite is a major public health issue in rural regions of tropical and subtropical countries with large number of envenoming and deaths (Chippaux, 1998, Soares et al., 2005)). Most snake envenoming occurs geographically in Asia and Africa, with India reporting the most snakebite deaths (McDiarmid et al., 1999). Though the rate of snake bite in Indian subcontinent has been the highest among the world, its treatment protocol is not well established. Due to lack of hospital facilities and proper medications in rural areas, people tend choose non-scientific traditional methods including herbal medications. Plants and their extracts have been used for the treatment of snake bite in most of the areas where venomous species are endemic. Herbal Antidote may be an alternative but information on this aspect is inadequate. Using antivenom is the specific treatment and most effective therapy available against Snakebite, first developed by Albert Chalmette in 1985 against the Indian cobra (Gomes et al., 2010). Snake venoms consist of a mixture of proteins, peptides and small organic compounds such as citrates, nucleosides and acetylcholine (Jiménez-Charris et al., 2014). The envenomation includes cytotoxic, hemorrhagic, hemolytic, neurotoxic and edema-forming activity (Shashidharamurthy et al., 2002). The study of snake venom and its biological effects is becoming increasingly crucial for the discovery of novel strategies to battle human illnesses, and this is in addition to the creation of antivenoms.
Snakes are the carnivorous vertebrates belonging to the order Squamata. Among 3000 known species of snake found worldwide, approximately 15% are considered dangerous to humans. The common krait (B. caeruleus) is one of the “big four” snake's species and it has been responsible for one of the most human deaths and injuries from snakebites in Bangladesh and India. Typically, victims report acute stomach pains that worsen over time, followed by gradual paralysis. The majority of its venom is neurotoxins which are potent enough to cause muscular paralysis in humans. Several factors contribute to the high morbidity rate due to Krait bite in Bangladesh apart from the additional fact that after bite incidence the victim is usually taken to the traditional healers or ‘ojha’ and village doctors before going to hospitals in majority of the cases. Usually, Krait bite incidence occurs at night, most often when the victim is asleep. Besides, there is no clinical or pathological confirmatory test for Krait bite in Bangladesh. This makes late admission at the hospital as well as delayed diagnosis and late administration of antivenom. Krait venom contains high amount of β-bungarotoxin, which is a pre-synaptic neurotoxin (Silva et al., 2016). The effect of this pre-synaptic neurotoxin is irreversible (Harris and Scott-Davey, 2013; Dixon and Harris, 1999). Once paralysis has developed, they respond poorly to delayed antivenom administration (Isbister, 2005) and ultimately becomes catastrophic. At the same time, the antivenom used in Bangladesh is produced in India against Indian Krait venom and so far, no data was available (before this study) regarding the efficacy of this antivenom against Kraits from Bangladeshi origin. Generally, death occurs between 4 and 8 h after untreated krait bite. Therefore, it is suggested to hospitalize the patient immediately after the snakebite occurrence. Untreated mortality is estimated by a clinical toxicological investigation to be between 70 and 80 percent in Bangladesh. There are at least 100 species of snakes in Bangladesh, but only some of them are venomous for humans. The common krait is responsible for more than half of all snakebite deaths in Bangladesh (Mondal et al., 2011).
Although the compositions and immunological profiles of snake venoms differ between species, diversity of venom composition have become increasingly recognized for their implications in fundamental research, and snakebite envenomation management (Casewell et al., 2014; Warrell et al., 2013; Augusto-de-Oliveira et al., 2016). The snake's geographic location has been considered as a key determinant in intraspecific venom diversity, possibly due to changes in diet between geographical groups (Tan et al., 2015a; Sunagar et al., 2016). As a result, even when the bites are inflicted by the same species, the toxic profile and clinical manifestations of snakebite envenomation might differ spatially (Huang et al., 2015). The phenomena are frequently accompanied with a varied neutralizing response to antivenom therapy, particularly when the antivenom is acquired from a manufacturer in another country and the venom immunogen applied came from a different geographical region (Tan et al., 2015b; Williams et al., 2011; Wong et al., 2016). World Health Organization (WHO) recommends that antivenom should be made from the snakes of the country where it will be used, because the venom properties of snakes vary with geographical locations. Due to inadequate facility for antivenom production, Bangladesh usually imports antivenom from India, and there are limited data regarding the efficacy of Indian antivenom against Bangladeshi snakes (Pla et al., 2019). Incepta Vaccine Ltd. recently claimed to start manufacturing or repackaging snake venom antiserum under the trade name ‘Antivenom’ with the similar formula of Indian polyvalent antivenom.
No previous study was found about the venom's characterization and the pre-clinical efficacy of antivenom used in Bangladesh against B. caeruleus snakebite treatment. Therefore, aim of the present study is to characterize the B. caeruleus venom from a Bangladeshi snake and to check the efficacy of the antivenom used for its treatment in Bangladesh.
2. Materials and methods
2.1. Collection of venom and antivenom
B. caeruleus venom was obtained from single individual snake from Rajshahi, Bangladesh (Fig. 1). The venom was lyophilized and stored at −20 °C. Antivenom (Snake Venom Antiserum BP) used in the present study is a polyvalent antivenom, and were manufactured by Incepta Vaccine Ltd, Dhaka, Bangladesh (Manufacture date - July 2020; Expiry date - Jun 2022; Batch no – 20006, product was used before expiry). Each vial contains lyophilized preparation of Snake Venom Antiserum BP. As per instruction 10 ml of water (provided in the box of the antivenom) is added to the total lyophilized content for reconstitution. As per the information on the pack of antivenom, each ml reconstituted Snake Venom Antiserum neutralizes not less than Cobra venom (Naja naja) 0.60 mg, Common Krait venom (Bungarus caeruleus) 0.45 mg, Russell's Viper venom (Vipera russelli) 0.60 mg, Saw-scaled Viper venom (Echis carinatus) 0.45 mg.
Fig. 1.
Bangladesh Map showing distribution of B. caeruleus. Location of the snake from which venom was collected and used in the current has been shown with red circle. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.2. Ethical and institutional approvals
The animals were handled according with the EU Directive 2010/63/EU for animal experiments. The study was approved by the Institutional Animal, Medical Ethics, Biosafety and Biosecurity committee (IAMEBBC) for Experimentations on animal, human, microbes and living nature sources (Memo no.- 225/320IAMEBBC/IBSc), Institute of Biological Sciences, University of Rajshahi, Bangladesh.
2.3. LD50 determination
Toxicity of the venom was analyzed according to the method described by Meier and Theakston with modifications (Meier and Theakston, 1986). The median lethal doses (LD50) were determined by intraperitoneal injection. Briefly, various amount of freshly dissolved venom in phosphate buffered saline (PBS) was injected intraperitoneally to Swiss albino mice (20 g, 10 mice per dose) in a final volume of 150 μl. Control mice received PBS only. They were monitored for 24 h and their survival ratio was recorded. Values of LD50 were calculated with the Probit analysis method (Hayes and Kruger, 2014).
2.4. Hemorrhagic activity assay
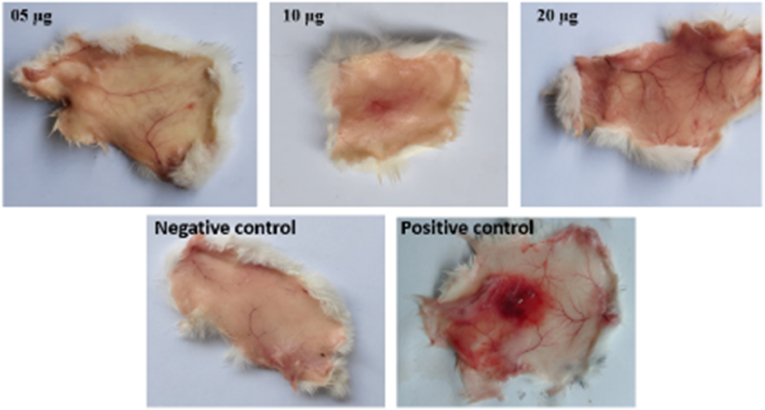
Hemorrhagic activity was assayed as described by Kondo et al. with slight modifications (Kondo et al., 1960). In brief, various amount of venom (5 μg, 10 μg, and 20 μg) in 50 μl saline were injected intra-dermally into mice and control mice injected equal volume of saline instead of venom. After 3 h, mice were euthanized. The dorsal surface of the skin was removed and the inner surface was analyzed for hemorrhagic damage. Daboia russelii venom (10 μg) was used as positive control.
2.5. Edema inducing activity
The edema forming activity was determined in mice as described in previous method (Das et al., 2013). A total 12 mice were divided into three groups of 4 mice in each (25 g). Various dose of venom dissolved in constant volume of 20 μl PBS were injected in right hind paw of mice. For control, 20 μl PBS were injected in left hind paw of each mice. Edema formation were observed after 3 h. Edema was quantified as the percentage increase in weight of the right foot compared to the left foot. The minimum edema dose (MED) was estimated as the amount of venom causing an edema ratio of 30% (Das et al., 2013).
2.6. Phospholipase A2 activity assay
PLA2 activity of B. caeruleus venom was detected using egg yolk agar-plate method with modifications (Gutiérrez et al., 1988; Menaldo et al., 2015). In brief, various concentrations of venoms dissolved in 15 μl PBS were added to 2.5 mm wells in gel plate. Plates were incubated for 18 h at 37 °C. Hydrolytic haloes diameter were measured using scale (Camey et al., 2002).
2.7. Hemolytic activity assay
Fresh 5 ml bovine blood was collected in a tube containing 0.11 M tri sodium citrate at 1:9 ratios (citrate: blood). 4 ml blood was taken in two 2 ml tube. The tubes were centrifuged at 3000 rpm for 14 min to separate the red blood cells (RBC) and platelet poor plasma. The RBC pellet was washed 3 times and re-suspended in 0.9% (w/v) saline to a final concentration of 10% (v/v). The direct hemolytic activity was measured by adding different concentration of venom to RBC. Venom stock solution (1 mg/ml) was prepared with water. The combination of different concentration of venom and RBC were prepared. The tubes were incubated for 60 min at 37 °C and centrifuged at 5000 rpm for 10 min. Subsequently, the supernatant was taken into sterilized cuvette. The reading of PBS was adjusted as blank and RBC suspension with 1% Triton X-100 was marked as positive controls. Absorbance of supernatant was measured at 540 nm in UV–Vis spectrophotometer. Hemolysis percentage was calculated by dividing sample's absorbance on positive control absorbance multiplied by 100 as described by Gould et al. (2000).
2.8. Histopathological studies
To assess the effect of B. caeruleus venom on organ, histopathological studies were carried out. Swiss albino mice were treated with ½ LD50 dose of venom (0.035 mg/kg) with 50 l PBS. Control mouse received PBS without venom. After 6 h mice were euthanized by injecting barbitone (30 mg/kg, i.p.) and organs were collected through autopsy. Heart, liver, kidney, lung, brain and intestine were preserved in 10% formaldehyde. Samples were dehydrated by ethanol, cleaned, embedded in wax, cut 5 μm sections with microtome and stained with hematoxylin and eosin (Lendrum, 1968). Tissue alterations were observed under a light microscope (Optika XDS-2 ERGO, Italy). Important areas in the slide were photographed using an OPTIkAM B1 - 4083.B1 camera.
2.9. SDS-PAGE profiling
The crude venom (15 μg) were dissolved in 20 μl PBS. Sample was reduced and applied to 18% SDS-PAGE gel. Electrophoresis was performed at 120 V for 2 h using Mini-PROTEAN Tetra Cell (Biorad, USA). Subsequently, the proteins were visualized using Coomassie Brilliant Blue R-250 staining (LAEMMLI, 1970).
2.10. Reverse-phase HPLC profiling
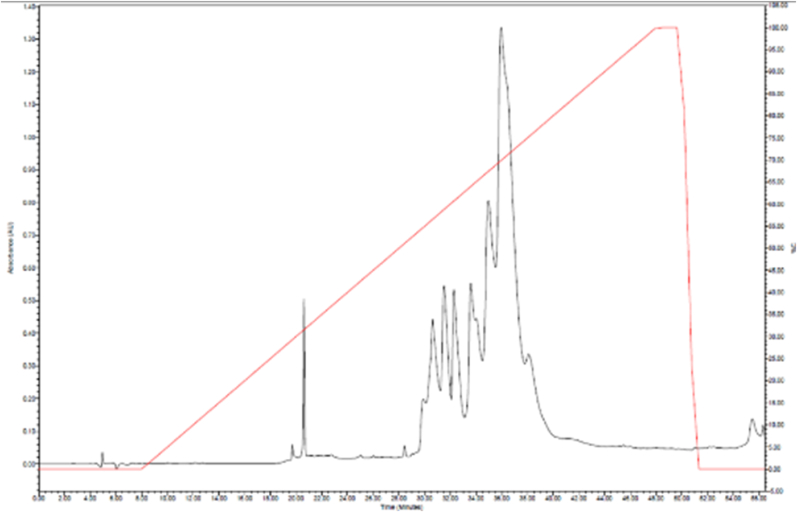
Lyophilized crude venom (3 mg) was dissolved in water and centrifuged at 10,000 g for 8 min. The supernatants were subjected to a Discovery™ BIO Wide Pore C18 (15cm × 2.1 mm, 5 μm) column, using Waters Alliance 2695 HPLC System. The column was pre-equilibrated with 0.1% trifluoroacetic acid in water. The elution was carried out in a gradient over 57 min at a flow rate of 0.5 ml/min with 0.1% TFA in water (Buffer A) and 0.1% TFA in 80% acetonitrile (Buffer B). The absorbance was measured at 215 nm.
2.11. Neutralization of B. caeruleus venom
For neutralization of venom lethality, antivenom preincubation method was followed (Tan et al., 2016). Briefly, a fixed dose of the venom comprising 3 LD50 was incubated at 37 °C for 30 min with various dilutions of the antivenom (μl). The mixture was subsequently injected intravenously into mice (24 g, n = 6 per dose). Venom (3 LD50) without antivenom incubated with PBS was injected to control group mice. Mice were allowed free access to water and food ad libitum. The number of survivals were recorded after 24 h. ED50 and potency were calculated using SPSS software. Neutralizing efficacy of the antivenom was expressed as median effective dose (ED50, the antivenom dose (μL) at which 50% of mice survived), estimated by probit analysis (Maduwage et al., 2016a). Neutralization capacity can also be expressed as Median effective ratio (ER50) which is the ratio of the amount of venom (mg) to the volume dose of antivenom (ml) at which 50% of mice survived (Faisal et al., 2021). The neutralization potency (P) of antivenom, defined as the amount of venom completely neutralized per unit volume of antivenom (mg/mL), was calculated accordingly as Morais V et al. (Morais et al., 2010)
3. Results
3.1. Biological characterization of venom
Biological activities of crude venom were investigated in the present study. After venom treatment, neurotoxic symptoms were observed such as difficulty in breathing and movement, venom induced edema formation in mice paw. Minimum edema dose (MED) was 0.12 μg. The venom did not induce hemorrhagic activity on mice skin up to 20 μg dose of B. caeruleus venom. Positive control showed hemorrhagic activity (Fig. 2). PLA2 activity was measured by agar plating method. Dose-dependent egg yolk in agar plate have revealed that the PLA2 enzyme activity increased with the increase of venom concentration. Negative control PBS represented no lysis zone formation while 2.5 μg venom induced 3 mm zone, 5 μg venom induced 6 mm zone and 10 μg venom induced 11 mm lysis zone (Supplementary Fig. 1). For direct hemolytic activity, 20%, 26.7%, 33.6%,46.7% and 53.3% hemolysis of RBC was observed for 6.25 μg/ml, 12.5 μg/ml, 25 μg/ml, 50 μg/ml and 100 μg/ml concentrations of venom respectively. Venom induced hemolysis, which implies that it has potent hemolytic activity. The LD50 of the venom in mice was 0.07 mg/kg when venom injected intraperitoneally.
Fig. 2.
Hemorrhagic activity of B. caeruleus venom. Hemorrhagic activity is absent in 5, 10 and 20 μg of venom treatments.
3.2. Histopathological effect of venom in mice organ
Histopathological changes in heart, liver, kidney, lung, brain and intestine tissues of mice were investigated through light microscopic examination. Histopathological changes in brain tissue of mice includes congestion of meningeal vessel and Inflammatory foci (Fig. 3B). The cardiac tissue samples from venom treated mice showed increased cytoplasmic vacuolization, infiltration and congestion of blood vessels. (Fig. 3D). Severe necrosis was evident in a broad area of the liver tissue. Infiltration of inflammatory cells was observed while hemorrhages was absent in liver (Fig. 3F). Renal tissue of mice kidney exhibited marked changes such as dilatation of renal capillary, diffuse glomeruli, tubular injury and vacuolization, congestion of capillaries and capsule membrane. The intestine tissue of control mice shows regular configuration while envenomed tissue showed mucosal necrosis, swelling and cellular inflammatory infiltration (Fig. 4B). Hemorrhage and severe congestion were observed in glomeruli and vessels (Fig. 4D). Infiltration of inflammatory cells into bronchiole lumen, congestion of capillaries and irregular capillary endothelium and focal anomalies in alveoli epithelium was observed in pulmonary tissue. Presence of infiltration of inflammatory cells in the inter-alveolar spaces and inside pulmonary alveoli, and alveolar hemorrhage was a major observation associated with lung tissue in envenomed mice (Fig. 4F).
Fig. 3.
Histopathological changes in Brain, Renal and Liver tissue of mice following 1/2LD50B. caeruleus venom treatment stained with hematoxylin and eosin magnified at 100×. (A) Control brain (B) Envenomed brain (C) Control kidney (D) Envenomed kidney (E) Control liver (F) Envenomed liver.
Fig. 4.
Histopathological changes in Intestine, Cardiac and Pulmonary tissue of mice following 1/2LD50B. caeruleus venom treatment stained with hematoxylin and eosin magnified at 100×. (A) Control intestine (B) Envenomed intestine (C) Control heart (D) Envenomed heart (E) Control lung (F) Envenomed lung.
3.3. SDS-PAGE of B. caeruleus
SDS-PAGE profile of the Bangladeshi B. caeruleus crude venom showed that venom consist of proteins of different molecular weight (Fig. 5). Thick and high intensity bands were observed in 1–14 kDa region. Another large band was found at around 20 kDa molecular weight. No protein band was observed within the range of 24–46 kDa. Multiple bands of proteins were found within 60–150 kDa range.
Fig. 5.

SDS-PAGE profile of B. caeruleus venom on 18% gel. Broad Range Protein Molecular Weight Marker, Promega, shown in Lane 1; protein bands for B. caeruleus venom in reduced condition shown in lane 2. Molecular weight of marker is shown in kDa.
3.4. Reverse-phase HPLC profile
The reverse-phase HPLC profile of B. caeruleus venom is shown in Fig. 6. A large pick was observed at 21 min and another broad spectrum of proteins were observed at 30–40 min.
Fig. 6.
C18 Reverse-phase HPLC profiles of 20 μg of B. caeruleus (Bangladesh) venom toxins detected by absorbance at 215 nm (black).
3.5. Neutralization of B. caeruleus venom
The neutralization of lethality was assessed with different doses (1:1, 1:10, 1:50, 1:70 and 1:100, w/w). At 1:1 and 1:10 concentrations, all of the venom treated mice were dead. However, at 1:50 and 1:70 concentrations some of the treated mice survived while severe edema and behavioral abnormality was observed among them. At 1:100 concentrations all of the mice survived. Calculated ED50 was 5.48 μl for fixed 3LD50 dose of venom. ER50 in the current study was 0.90 mg/ml. Potency was 0.53 mg/ml, defined as the amount of venom in mg neutralized per mL of antivenom.
4. Discussion
Snakebite envenomation was classified as Category A of the Neglected Tropical Diseases by the World Health Organization on June 9th, 2017 (Chippaux, 2017). B. caeruleus is a highly venomous snake that contributes to the major snakebite envenoming issue in South Asia. B. caeruleus envenoming can result in quick and severe neurotoxicity, with the patient succumbing to paralysis, respiratory failure, and death.
The determination of venom median lethal doses (LD50) is an important step in determining the toxic activity of specific venom, which indicate the amount of venom is needed to kill half of the test population. The LD50 of the B. caeruleus venom in mice was found to be 0.07 mg/kg in the current study when injected intraperitoneally. Previous experiment determined LD50 values of its venom as 0.325 μg/g subcutaneously, 0.169 μg/g intravenously, and 0.089 μg/g intraperitoneally in mice (Wolf-Eberhard and Fritz Jürgen, 1981). The estimated lethal dose for humans is 2–3 mg. LD50 of Pakistani B. caeruleus venom is 0.2828 μg/g for intravenous route calculated by Perveen et al. (Parveen et al., 2017). In another study, calculated LD50 was 0.16 μg/g (T. A, 1991). An experiment by Laxme RS et al. estimated an LD50 of 0.02 μg/g for B. sindanus, which was five times as potent as B. caeruleus (0.1 μg/g) and 56 times potent compared to B. fasciatus (1.12 μg/g), making it one of the most potent snake venoms in the world. (Laxme et al., 2019). At lethal dose most of the mice died within 4 h, suggesting the necessity of antivenom administration as soon as possible.
In the current study, the histopathological changes on different organs of mice (heart, liver, kidney, lung, brain and intestine) were studied following ½ LD50 dose injection of venom B. caeruleus venom (Fig. 3, Fig. 4). Severe necrosis was found in liver, renal and intestinal tissues. Inflammatory infiltration was seen in liver, lung and kidney. Slight changes were observed in brain tissue of mice including congestion of meningeal vessel and inflammatory foci. Cytoplasmic vacuolization, infiltration and congestion of blood vessels were major changes in cardiac tissue. The findings of current study are almost consistent with previous observations (Al-Mamun et al., 2015a; Kiran et al., 2004; Nanayakkara et al., 2009). However, in this study, clear and broad range images are presented for histopathological tissue sections of B. caeruleus envenomed mice. The severity of tissue changes is largely determined by the snake species, the composition of its venom, and the tissue's susceptibility to a specific venom component (Kamiguti et al., 2000). B. caeruleus venom major components are phospholipases which hydrolyze phospholipids in the cell membrane resulting in wide ranges of alterations on tissues. Toxins are primarily excreted through the kidney, which in higher concentrations may cause damage in renal tissue. The liver is the body's primary detoxifying organ, could be damaged by a variety of toxic components found in venom. More severe tissue damages are caused by elapid and viper (Al-Mamun et al., 2015b; Dissanayake et al., 2018) venoms.
Snake venom contains a combination of systemic toxins and hydrolytic enzymes (Dufton, 1993). Snake venom proteins resolve into multiple bands on electrophoresis, resulting in a protein-banding pattern that is unique to each species. RP- HPLC profile is also unique for a particular species and profiles can be studied to check the variation of venom composition from different geographical locations. The venoms of krait were also found to be dominated by neurotoxic 3FTxs and PLA2s. In the current study, bands of SDS-PAGE (Fig. 5) and the picks of reverse-phase HPLC profile (Fig. 6) of B. caeruleus venom revealed the presence of multiple proteins which is consistent with phospholipases A2 (PLA2) and three-fingers toxin (3FTx) proteins. They are predominantly found in the venoms of elapid and colubrid snakes, and they cause flaccid paralysis in snakebite patients by binding post-synaptically at the neuromuscular junctions (Barber et al., 2013). From the B. caeruleus SDS-PAGE bands, Manisha et al. identified twenty-three proteins from six different toxin families (Choudhury et al., 2017). They also identified 19 PLA2 proteins in B. caeruleus venoms. Patra et al. study showed similar SDS-PAGE banding pattern of venom from Indian origin where low molecular weight bands are predominant. However, some distinct bands are also observable (e.g. 25 and 100 kDa bands is absent in the Indian venom) which indicate the variation of proteins in venom composition (Patra et al., 2019). C18 Reverse-phase HPLC of Indian krait venoms from different geographical locations of India and Sri Lankan origin were studied which show some similar and some dissimilar picks in chromatogram (Oh et al., 2017).
Laxme RR et al. showed that, the krait venoms were found to be dominated by neurotoxic 3FTxs and PLA2s. Abundance of PLA2, 3FTxs, Acetylcholinesterases (AChEs), snake venom metalloproteinase (SVMP) and L-amino acid oxidase (LAAO) were 38.16%, 32.4%, 11.76%, 5.54% and 4.86% respectively for venoms of Indian origin (Laxme et al., 2019). In another venomics study, it was reported that krait venom of Sri Lankan origin is predominated with a diverse pool of phospholipases A2 comprising 68.2% of total proteins, in which at least 8.3% are β-bungarotoxins (β-BTx). Three-finger toxins are the second most prevalent (19.0%), accounting for 15.6 percent κ-bungarotoxins (κ-BTx), which are powerful post-synaptically acting neurotoxins (Oh et al., 2017). Three-finger toxins are non-enzymatic neurotoxins with a three-finger fold structure supported by disulfide bridges that range in size from 58 to 81 residues (V Osipov and Utkin, 2017; Kessler et al., 2017). Kraits venom contains 3 major types of neurotoxins, of which alpha-bungarotoxin binds to postsynaptic neuronal acetylcholine receptors (nAChR) at the neuromuscular junction, causes neuromuscular transmission failure (Mebs, 1991). The second is κ-bungarotoxin which binds to nAchR post-synaptically (Chiappinelli et al., 1996). The third pre-synaptically active beta-bungarotoxin induces long-term neuromuscular transmission failure by depleting synaptic vesicles at nerve terminals (Dixon and Harris, 1999; Chen and Lee, 1970; Prasarnpun et al., 2005). It also causes structural damage to motor nerve terminals and the damage is complete by 12–24 h (Chang et al., 1973; Abe et al., 1976). As a result, recovery is dependent on the regeneration of synaptic vesicles, which takes a long period and necessitates much more mechanical ventilation than in post-synaptic envenoming (Dixon and Harris, 1999).
Pathophysiological consequences of snake bites include the activity of various enzymes, proteins, and peptides, including phospholipases A2, metalloproteases, and other proteolytic enzymes (Markland, 1997). Toxins from snakes have a wide range of functions (Faisal et al., 2021). Phospholipases A2 are a broad superfamily of proteins that have hydrolytic activity against phospholipids and can cleave fatty acids in the second position specifically. PLA2 is one of the most common and potent muscle-damaging substances found in snake venom. PLA2 has a variety of pharmacological actions, including edema, platelet aggregation regulation, as well as neurotoxic, anticoagulant, and myotoxic effects (Six and Dennis, 2000; Kini, 2003). PLA2 enzymes produce fatty acids such as arachidonic acid, which is a precursor of pro-inflammatory eicosanoids, when they act on membrane phospholipids. The phospholipid bilayer can be destabilized as a result of this action (Mebs, 1991). Some reviews provide the most detailed explanation of structural characteristics, methods of action, localization, and significance for organisms of each variety of PLA2 (Dennis et al., 2011; Vasquez et al., 2018). Since the 1970s, test techniques for identifying PLA2 activity have been developed. The hydrolysis of lipids is monitored in all test methods for determining PLA2 activity. In our study, PLA2 activity was demonstrated by the production of hemolytic zones in egg yolk agarose gels induced by venom. The physicochemical features of egg yolk were studied following treatment with venom. Different concentration of venom was applied in different well and they showed variable hydrolytic activity. This is due to PLA2 activity which hydrolyze phospholipids. It also can lyse red blood cell membranes (Gutiérrez et al., 1988). We also carried out direct hemolytic activity test. Around 20–53.3% hemolysis was observed for different concentrations (6.25 μg–100 μg/ml) of venom dosage. After venom treatment in mice, neurotoxic symptoms were observed such as difficulty in breathing and movement. B. caeruleus venom induced edema formation after injecting venom in mice paw. Edema and inflammation are two of the most noticeable clinical manifestations of envenomation (Scharman and Noffsinger, 2001). Edema is a release of fluid into the interstitial space, causing inflammatory phenomenon. Excessive interstitial fluid deposition is generally regarded as detrimental to tissue function because edema formation increases the diffusion distance for oxygen and other nutrients, potentially impairing cellular metabolism. It also curtails the diffusional separation of potentially toxic byproducts of metabolic reactions. However, no hemorrhagic activity was observed in mice skin while injected intradermally in the present study (Fig. 1). The result is consistent with previous reports as the absent of hemorrhagic activities (Meenatchisundaram and Michael, 2010; Das et al., 2013). The hemorrhage is caused by metalloproteases, which are extensively found in viper venom (Mukherjee, 2008).
For decades, the choice of antivenom utilized in Bangladesh was based on the availability of the polyvalent antivenoms made in India. The Indian polyvalent products are developed against the venoms of the Big Four snakes (Naja naja, B. caeruleus, Daboia russelii, and Echis carinatus) of Indian origin. It was reported that Indian polyvalent antivenoms were less effective for Sri Lankan snakebite treatment (Keyler et al., 2013; De Silva et al., 2011; Theakston et al., 1990). When antivenoms developed from the venom of one geographic region are used to treat patients of another, the efficacy is greatly reduced (Shashidharamurthy and Kemparaju, 2007). However, some studies reported the positive results stating effective neutralization capability (Maduwage et al., 2016a; Leong et al., 2012) Incepta antivenom has recently been introduced in the drug market of Bangladesh. The efficacy of commercially available Incepta antivenom at neutralizing venom lethality were assessed in the current study by performing ED50 experiments. Calculated ED50 in the current study was 5.48 μl and ER50 was 0.90 mg/ml. Neutralization potency was.0.53 mg/ml. Thus, it is effective for neutralization of venom in mice. Previous study with venom of B. caeruleus from Sri Lanka, India and Pakistan showed neutralization potency as 0.44, 0.48 and 0.30 respectively while neutralizing with VINS polyvalent antivenom (Oh et al., 2017).
Venoms are natural toxins that serve as a crucial adaptive characteristic in evolved snakes (Lomonte et al., 2014). Snake venom toxicity vary by species and even within species, depending on geographical location, venom injection route, size etc. Therefore, it could have been better to work with a venom pool to have a better understanding of the neutralizing capacity of the antivenom against the Krait venom from different regions of Bangladesh. However, the focus of the manuscript is to evaluate the efficacy of the currently used antivenom in Bangladesh which is actually produced against the venom of Indian snakes. Therefore, the regional variation of venom within Bangladesh was not evaluated; moreover, during the study period we had venom sample from only one location of Bangladesh (Fig. 1). The results of the present study indicate that Incepta Antivenom is effective in neutralizing the lethality of B. caeruleus venom under in vitro condition. However, difficulty in breathing and movement was observed in survived mice. According to a study, procoagulant toxins of venom have different impacts on human and animal plasmas, making the efficacy of antivenom in rodent models difficult to interpret (Maduwage et al., 2016b). An epidemiological investigation on the treated patients is required to understand the actual scenario and the efficacy of the antivenom against human.
5. Conclusion
As venom composition of a snake species may vary considerably in different geographical location, it is important to decipher the venom properties and evaluate the efficacy of antivenom locally. The current study evaluated the biological properties of B. caeruleus venom and its effect on mice organs. In vitro neutralization of lethality test with Incepta Antivenom was found to be effective, but, with some physical abnormalities. For preparing a better and more effective antivenom, regional variation of the Krait venoms from different regions of Bangladesh should be taken into account and venom pool should be used for antivenom production. Further insights into clinical observations and assessment of the neutralizing potential of the antivenom against snakebite patients will help in better understanding the correlation with the laboratory experiments.
Credit author statement
Md. Jahangir Alam, Md. Mahmudul Hasan Maruf, Aniruddha Ghose and Md. Abu Reza conceptualized the whole project. Md. Jahangir Alam, Md. Mahmudul Hasan Maruf and Md. Asif Iqbal performed most of the experiments. Mahedi Hasan, Md. Sohanur Rahman Sohan, Md. Ragib Shahriar, Ibrahim Khalil Al Haidar and Mohammad Abdul Wahed Chowdhury were actively involved in different experiments. The first draft of the manuscript was written by Md. Jahangir Alam and Md. Mahmudul Hasan Maruff. All authors commented on previous versions of the manuscript. Kazi Md. Faisal Hoque and Md. Abu Reza supervised the whole work.
Ethical statement
On behalf of, and having obtained permission from all authors, I declare that:
-
(a)
the material has not been published in whole or in part elsewhere
-
(b)
the paper is not currently being considered for publication elsewhere
-
(c)
all authors have been personally and actively involved in the work. The authors have read the manuscript and agree to its publication in Toxicon X.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Current research project was directly and indirectly supported by different projects viz. Special Allocation for Science and Technology from the Ministry of Science and Technology, Bangladesh; Grant of Advanced Research in Education (GARE), Ministry of Education, Bangladesh; HPNSP research grants of Bangladesh Medical Research Council (BMRC) and The University of Rajshahi, Bangladesh. We cordially acknowledge and appreciate their support.
Handling Editor: Denise Tambourgi
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.toxcx.2023.100149.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Abe T., Limbrick A.R., Miledi R. Acute muscle denervation induced by β-bungarotoxin. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1976;194(1117):545–553. doi: 10.1098/rspb.1976.0093. [DOI] [PubMed] [Google Scholar]
- Al-Mamun M.A., Rahman M.A., Hasan R., Rahmann Z., Haque K.M.F. Histopathological alterations induced by common krait Bungarus caeruleus venom on hepatic, renal and cardiac tissues of albino mice. Digestion. 2015;6:10. [Google Scholar]
- Al-Mamun M., Hakim M., Zaman M., Hoque K., Ferdousi Z., Reza M. Histopathological alterations induced by Naja naja crude venom on renal, pulmonary and intestinal tissues of mice model. Br. Biotechnol. J. 2015;6(3):119–125. [Google Scholar]
- Augusto-de-Oliveira C., et al. Dynamic rearrangement in snake venom gland proteome: insights into Bothrops jararaca intraspecific venom variation. J. Proteome Res. 2016;15(10):3752–3762. doi: 10.1021/acs.jproteome.6b00561. [DOI] [PubMed] [Google Scholar]
- Barber C.M., Isbister G.K., Hodgson W.C. Alpha neurotoxins. Toxicon. 2013;66:47–58. doi: 10.1016/j.toxicon.2013.01.019. [DOI] [PubMed] [Google Scholar]
- Camey K.U., Velarde D.T., Sanchez E.F. Pharmacological characterization and neutralization of the venoms used in the production of Bothropic antivenom in Brazil. Toxicon. 2002;40(5):501–509. doi: 10.1016/s0041-0101(01)00245-8. [DOI] [PubMed] [Google Scholar]
- Casewell N.R., et al. Medically important differences in snake venom composition are dictated by distinct postgenomic mechanisms. Proc. Natl. Acad. Sci. USA. 2014;111(25):9205–9210. doi: 10.1073/pnas.1405484111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.C., Chen T.F., Lee C.Y. Studies of the presynaptic effect of β-bungarotoxin on neuromuscular transmission. J. Pharmacol. Exp. Therapeut. 1973;184(2):339–345. [PubMed] [Google Scholar]
- Chen I.-L., Lee C.Y. Ultrastructural changes in the motor nerve terminals caused by β-bungarotoxin. Virchows Arch. B. 1970;6(1):318–325. doi: 10.1007/BF02899133. [DOI] [PubMed] [Google Scholar]
- Chiappinelli V.A., Weaver W.R., McLane K.E., Conti-Fine B.M., Fiordalisi J.J., Grant G.A. Binding of native κ-neurotoxins and site-directed mutants to nicotinic acetylcholine receptors. Toxicon. 1996;34(11–12):1243–1256. doi: 10.1016/s0041-0101(96)00110-9. [DOI] [PubMed] [Google Scholar]
- Chippaux J.P. Snake-bites: appraisal of the global situation. Bull. World Health Organ. 1998;76(5):515. [PMC free article] [PubMed] [Google Scholar]
- Chippaux J.-P. Snakebite envenomation turns again into a neglected tropical disease. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017;23(1):1–2. doi: 10.1186/s40409-017-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury M., McCleary R.J.R., Kesherwani M., Kini R.M., Velmurugan D. Comparison of proteomic profiles of the venoms of two of the ‘Big Four’snakes of India, the Indian cobra (Naja naja) and the common krait (Bungarus caeruleus), and analyses of their toxins. Toxicon. 2017;135:33–42. doi: 10.1016/j.toxicon.2017.06.005. [DOI] [PubMed] [Google Scholar]
- Das D., Urs N., Hiremath V., Vishwanath B.S., Doley R. Biochemical and biological characterization of Naja kaouthia venom from North-East India and its neutralization by polyvalent antivenom. J. Venom Res. 2013;4:31. [PMC free article] [PubMed] [Google Scholar]
- De Silva H.J., Gunatilake S.B., Kularatne S.A.M., Sellahewa K.H. Anti-venom for snakebite in Sri Lanka. Ceylon Med. J. 2011;47(2) doi: 10.4038/cmj.v47i2.3449. [DOI] [PubMed] [Google Scholar]
- Dennis E.A., Cao J., Hsu Y.-H., Magrioti V., Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011;111(10):6130–6185. doi: 10.1021/cr200085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayake D.S.B., Thewarage L.D., Waduge R.N., Ranasinghe J.G.S., Kularatne S.A.M., Rajapakse R.P.V. The venom of spectacled cobra (Elapidae: Naja naja): in vitro study from distinct geographical origins in Sri Lanka. J. Toxicol. 2018 doi: 10.1155/2018/7358472. 2018:7358472. doi: 10.1155/2018/7358472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R.W., Harris J.B. Nerve terminal damage by β-bungarotoxin: its clinical significance. Am. J. Pathol. 1999;154(2):447–455. doi: 10.1016/s0002-9440(10)65291-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufton M.J. Kill and cure: the promising future for venom research. Endeavour. 1993;17(3):138–140. doi: 10.1016/0160-9327(93)90104-b. [DOI] [PubMed] [Google Scholar]
- Faisal T., Tan K.Y., Tan N.H., Sim S.M., Gnanathasan C.A., Tan C.H. Proteomics, toxicity and antivenom neutralization of Sri Lankan and Indian Russell's viper (Daboia russelii) venoms. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021;27 doi: 10.1590/1678-9199-JVATITD-2020-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes A., et al. 2010. Herbs and Herbal Constituents Active against Snake Bite. [PubMed] [Google Scholar]
- Gould L.A., Lansley A.B., Brown M.B., Forbes B., Martin G.P. Mitigation of surfactant erythrocyte toxicity by egg phosphatidylcholine. J. Pharm. Pharmacol. 2000;52(10):1203–1209. doi: 10.1211/0022357001777333. [DOI] [PubMed] [Google Scholar]
- Gutiérrez J., Avila C., Rojas E., Cerdas L. An alternative in vitro method for testing the potency of the polyvalent antivenom produced in Costa Rica. Toxicon. 1988;26(4):411–413. doi: 10.1016/0041-0101(88)90010-4. [DOI] [PubMed] [Google Scholar]
- Harris J.B., Scott-Davey T. Secreted phospholipases A2 of snake venoms: effects on the peripheral neuromuscular system with comments on the role of phospholipases A2 in disorders of the CNS and their uses in industry. Toxins. 2013;5(12):2533–2571. doi: 10.3390/toxins5122533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A.W., Kruger C.L. Crc Press; 2014. Hayes' Principles and Methods of Toxicology. [Google Scholar]
- Huang H.-W., et al. Cobra venom proteome and glycome determined from individual snakes of Naja atra reveal medically important dynamic range and systematic geographic variation. J. Proteonomics. 2015;128:92–104. doi: 10.1016/j.jprot.2015.07.015. [DOI] [PubMed] [Google Scholar]
- Isbister G.K. Snake antivenom research: the importance of case definition. Emerg. Med. J. 2005;22(6):399–400. doi: 10.1136/emj.2004.022251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-Charris E., et al. 2014. Proteomic and Functional Analyses of the Venom of. [Google Scholar]
- Kamiguti A.S., Theakston R.D.G., Sherman N., Fox J.W. Mass spectrophotometric evidence for P-III/P-IV metalloproteinases in the venom of the Boomslang (Dispholidus typus) Toxicon. 2000;38(11):1613–1620. doi: 10.1016/s0041-0101(00)00089-1. [DOI] [PubMed] [Google Scholar]
- Kessler P., Marchot P., Silva M., Servent D. The three‐finger toxin fold: a multifunctional structural scaffold able to modulate cholinergic functions. J. Neurochem. 2017;142:7–18. doi: 10.1111/jnc.13975. [DOI] [PubMed] [Google Scholar]
- Keyler D.E., Gawarammana I., Gutié rrez J.M., Sellahewa K.H., McWhorter K., Malleappah R. Antivenom for snakebite envenoming in Sri Lanka: the need for geographically specific antivenom and improved efficacy. Toxicon. 2013;69:90–97. doi: 10.1016/j.toxicon.2013.01.022. [DOI] [PubMed] [Google Scholar]
- Kini R.M. Excitement ahead: structure, function and mechanism of snake venom phospholipase A2 enzymes. Toxicon. 2003;42(8):827–840. doi: 10.1016/j.toxicon.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Kiran K.M., More S.S., Gadag J.R. Biochemical and clinicopathological changes induced by Bungarus coeruleus venom in a rat model. J. Basic Clin. Physiol. Pharmacol. 2004;15(3–4):277–288. doi: 10.1515/jbcpp.2004.15.3-4.277. [DOI] [PubMed] [Google Scholar]
- Kondo H., Kondo S., Ikezawa H., Murata R., Ohsaka A. Studies on the quantitative method for determination of hemorrhagic activity of Habu snake venom. Jpn. J. Med. Sci. Biol. 1960;13(1–2):43–51. doi: 10.7883/yoken1952.13.43. [DOI] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laxme R.R.S., et al. Beyond the ‘big four’: venom profiling of the medically important yet neglected Indian snakes reveals disturbing antivenom deficiencies. PLoS Neglected Trop. Dis. 2019;13(12) doi: 10.1371/journal.pntd.0007899. e0007899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lendrum A.C. Carleton's histological technique. J. Clin. Pathol. 1968;21(1):116. [Google Scholar]
- Leong P.K., Tan N.H., Fung S.Y., Sim S.M. Cross neutralisation of Southeast Asian cobra and krait venoms by Indian polyvalent antivenoms. Trans. R. Soc. Trop. Med. Hyg. 2012;106(12):731–737. doi: 10.1016/j.trstmh.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Lomonte B., et al. Venomics of New World pit vipers: genus-wide comparisons of venom proteomes across Agkistrodon. J. Proteonomics. 2014;96:103–116. doi: 10.1016/j.jprot.2013.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduwage K., Silva A., O'Leary M.A., Hodgson W.C., Isbister G.K. Efficacy of Indian polyvalent snake antivenoms against Sri Lankan snake venoms: lethality studies or clinically focussed in vitro studies. Sci. Rep. 2016;6(1):1–11. doi: 10.1038/srep26778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduwage K.P., Scorgie F.E., Lincz L.F., O'Leary M.A., Isbister G.K. Procoagulant snake venoms have differential effects in animal plasmas: implications for antivenom testing in animal models. Thromb. Res. 2016;137:174–177. doi: 10.1016/j.thromres.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Markland F.S. Snake venoms,” Drugs. 1997;54(3):1–10. doi: 10.2165/00003495-199700543-00003. [DOI] [PubMed] [Google Scholar]
- McDiarmid R.W., Campbell J.A., Touré T. 1999. Snake Species of the World: A Taxonomic and Geographic Reference. [Google Scholar]
- Mebs D. Amino acid sequences and toxicities of snake venom components. Snake toxins. 1991:425–447. [Google Scholar]
- Meenatchisundaram S., Michael A. Antitoxin activity of Mucuna pruriens aqueous extracts against Cobra and Krait venom by in vivo and in vitro methods. Int. J. PharmTech Res. 2010;2(1):870–874. [Google Scholar]
- Meier J., Theakston R.D.G. Approximate LD50 determinations of snake venoms using eight to ten experimental animals. Toxicon. 1986;24(4):395–401. doi: 10.1016/0041-0101(86)90199-6. [DOI] [PubMed] [Google Scholar]
- Menaldo D.L., Jacob-Ferreira A.L., Bernardes C.P., Cintra A.C.O., Sampaio S.V. Purification procedure for the isolation of a PI metalloprotease and an acidic phospholipase A 2 fromBothrops atrox snake venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015;21 doi: 10.1186/s40409-015-0027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal R.N., et al. Pattern of poisonous snake bite in rangpur medical college. RCMCJ. 2011;1:13–18. [Google Scholar]
- Morais V., Ifran S., Berasain P., Massaldi H. Antivenoms: potency or median effective dose, which to use? J. Venom. Anim. Toxins Incl. Trop. Dis. 2010;16(2):191–193. [Google Scholar]
- Mukherjee A.K. Characterization of a novel pro-coagulant metalloprotease (RVBCMP) possessing α-fibrinogenase and tissue haemorrhagic activity from venom of Daboia russelli russelli (Russell's viper): evidence of distinct coagulant and haemorrhagic sites in RVBCMP. Toxicon. 2008;51(5):923–933. doi: 10.1016/j.toxicon.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Nanayakkara D.P., Ratnayake R.M.P., Ranasinghe J.G. Histopathological changes in brain, kidney and liver of mice following intramuscular administration of krait venom. Ceylon J. Sci. Biol. Sci. 2009;38(1) [Google Scholar]
- Oh A.M.F., Tan C.H., Ariaranee G.C., Quraishi N., Tan N.H. Venomics of Bungarus caeruleus (Indian krait): comparable venom profiles, variable immunoreactivities among specimens from Sri Lanka, India and Pakistan. J. Proteonomics. 2017;164:1–18. doi: 10.1016/j.jprot.2017.04.018. [DOI] [PubMed] [Google Scholar]
- Parveen G., Khan M.F., Ali H., Ibrahim T., Shah R. Determination of lethal dose (LD50) of venom of four different poisonous snakes found in Pakistan. Biochem Mol Biol J. 2017;3(3):18. [Google Scholar]
- Patra A., Chanda A., Mukherjee A.K. Quantitative proteomic analysis of venom from Southern India common krait (Bungarus caeruleus) and identification of poorly immunogenic toxins by immune-profiling against commercial antivenom. Expert Rev. Proteomics. 2019;16(5):457–469. doi: 10.1080/14789450.2019.1609945. [DOI] [PubMed] [Google Scholar]
- Pla D., et al. Phylovenomics of Daboia russelii across the Indian subcontinent. Bioactivities and comparative in vivo neutralization and in vitro third-generation antivenomics of antivenoms against venoms from India, Bangladesh and Sri Lanka. J. Proteonomics. 2019;207 doi: 10.1016/j.jprot.2019.103443. [DOI] [PubMed] [Google Scholar]
- Prasarnpun S., Walsh J., Awad S.S., Harris J.B. Envenoming bites by kraits: the biological basis of treatment-resistant neuromuscular paralysis. Brain. 2005;128(12):2987–2996. doi: 10.1093/brain/awh642. [DOI] [PubMed] [Google Scholar]
- Scharman E.J., Noffsinger V.D. Copperhead snakebites: clinical severity of local effects. Ann. Emerg. Med. 2001;38(1):55–61. doi: 10.1067/mem.2001.116148. [DOI] [PubMed] [Google Scholar]
- Shashidharamurthy R., Kemparaju K. Region-specific neutralization of Indian cobra (Naja naja) venom by polyclonal antibody raised against the eastern regional venom: a comparative study of the venoms from three different geographical distributions. Int. Immunopharm. 2007;7(1):61–69. doi: 10.1016/j.intimp.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Shashidharamurthy R., Jagadeesha D.K., Girish K.S., Kemparaju K. Variation in biochemical and pharmacological properties of Indian cobra (Naja naja naja) venom due to geographical distribution. Mol. Cell. Biochem. 2002;229(1):93–101. doi: 10.1023/a:1017972511272. [DOI] [PubMed] [Google Scholar]
- Silva A., et al. Neuromuscular effects of common krait (Bungarus caeruleus) envenoming in Sri Lanka. PLoS Neglected Trop. Dis. 2016;10(2) doi: 10.1371/journal.pntd.0004368. e0004368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Six D.A., Dennis E.A. The expanding superfamily of phospholipase A2 enzymes: classification and characterization. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2000;1488(1–2):1–19. doi: 10.1016/s1388-1981(00)00105-0. [DOI] [PubMed] [Google Scholar]
- Soares A.M., et al. Medicinal plants with inhibitory properties against snake venoms. Curr. Med. Chem. 2005;12(22):2625–2641. doi: 10.2174/092986705774370655. [DOI] [PubMed] [Google Scholar]
- Sunagar K., Morgenstern D., Reitzel A.M., Moran Y. Ecological venomics: how genomics, transcriptomics and proteomics can shed new light on the ecology and evolution of venom. J. Proteonomics. 2016;135:62–72. doi: 10.1016/j.jprot.2015.09.015. [DOI] [PubMed] [Google Scholar]
- T. A . Crc Press; 1991. Handbook of Natural Toxins: Reptile Venoms and Toxins. [Google Scholar]
- Tan K.Y., Tan C.H., Fung S.Y., Tan N.H. Venomics, lethality and neutralization of Naja kaouthia (monocled cobra) venoms from three different geographical regions of Southeast Asia. J. Proteonomics. 2015;120:105–125. doi: 10.1016/j.jprot.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Tan C.H., Tan K.Y., Fung S.Y., Tan N.H. Venom-gland transcriptome and venom proteome of the Malaysian king cobra (Ophiophagus hannah) BMC Genom. 2015;16(1):1–21. doi: 10.1186/s12864-015-1828-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan C.H., Liew J.L., Tan K.Y., Tan N.H. Assessing SABU (Serum Anti Bisa Ular), the sole Indonesian antivenom: a proteomic analysis and neutralization efficacy study. Sci. Rep. 2016;6(1):1–10. doi: 10.1038/srep37299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theakston R.D.G., et al. Envenoming by the common krait (Bungarus caeruleus) and Sri Lankan cobra (Naja naja naja): efficacy and complications of therapy with Haffkine antivenom. Trans. R. Soc. Trop. Med. Hyg. 1990;84(2):301–308. doi: 10.1016/0035-9203(90)90297-r. [DOI] [PubMed] [Google Scholar]
- V Osipov A., Utkin Y.N., editors. Snake Venom Toxins Targeted at the Nervous System. Snake Venoms-Toxinology Springer; Dordr.: 2017. pp. 189–214. [Google Scholar]
- Vasquez A.M., Mouchlis V.D., Dennis E.A. Review of four major distinct types of human phospholipase A2. Adv. Biol. Regul. 2018;67:212–218. doi: 10.1016/j.jbior.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrell D.A., Gutiérrez J.M., Calvete J.J., Williams D. New approaches & technologies of venomics to meet the challenge of human envenoming by snakebites in India. Indian J. Med. Res. 2013;138(1):38. [PMC free article] [PubMed] [Google Scholar]
- Williams D.J., et al. Ending the drought: new strategies for improving the flow of affordable, effective antivenoms in Asia and Africa. J. Proteonomics. 2011;74(9):1735–1767. doi: 10.1016/j.jprot.2011.05.027. [DOI] [PubMed] [Google Scholar]
- Wolf-Eberhard E., Fritz Jürgen O. English ve; New York: 1981. Snakes: Biology, Behavior, and Relationship to Man. [Google Scholar]
- Wong K.Y., Tan C.H., Tan N.H. Venom and purified toxins of the spectacled cobra (Naja naja) from Pakistan: insights into toxicity and antivenom neutralization. Am. J. Trop. Med. Hyg. 2016;94(6):1392. doi: 10.4269/ajtmh.15-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.