Abstract
Dual-specificity tyrosine phosphorylation-regulated kinase 1 A (DYRK1A) has been proposed as a novel regulator of adaptive immune homeostasis through modulating T cell polarization. Thus, DYRK1A could present a potential target in autoimmune disorders. Here, we identify FRTX-02 as a novel compound exhibiting potent and selective inhibition of DYRK1A. FRTX-02 induced transcriptional activity of the DYRK1A substrate NFAT in T cell lines. Correspondingly, FRTX-02 promoted ex vivo CD4+ polarization into anti-inflammatory Tregs and reduced their polarization into pro-inflammatory Th1 or Th17 cells. We show that FRTX-02 could also limit innate immune responses through negative regulation of the MyD88/IRAK4–NF-κB axis in a mast cell line. Finally, in mouse models of psoriasis and atopic dermatitis, both oral and topical formulations of FRTX-02 reduced inflammation and disease biomarkers in a dose-dependent manner. These results support further studies of DYRK1A inhibitors, including FRTX-02, as potential therapies for chronic inflammatory and autoimmune conditions.
Keywords: Inflammation, Autoimmunity, T-cell polarization, NFAT, MyD88, Innate immunity, Adaptive immunity, DYRK1A
Highlights
-
•
Novel DYRK1A inhibitor FRTX-02 attenuates inflammation in mouse models of atopic dermatitis and psoriasis.
-
•
FRTX-02 promotes Treg differentiation and suppresses pro-inflammatory Th17 and Th1 differentiation in vitro.
-
•
FRTX-02 induces alternative splicing of MyD88 that results in a reduction of IRAK4-mediated NF-κB activation.
-
•
FRTX-02 has the potential to restore immune homeostasis by regulating innate and adaptive inflammatory immune responses.
1. Introduction
DYRK1A (dual-specificity tyrosine-phosphorylation-regulated kinase 1 A) is a serine/threonine kinase encoded on chromosome 21 in humans. DYRK1A plays a critical role in embryonic neurogenesis [1]. It has been particularly extensively studied in the context of Down Syndrome (DS), which is characterized by the presence of an extra copy of chromosome 21 or its parts [2,3]. However, both DYRK1A's plethora of diverse candidate substrates and its ubiquitous expression point to roles beyond neurodevelopment. In this regard, DYRK1A has also been implicated in the pathogenesis of neurodegenerative disorders, type 1 diabetes, and cancer [[4], [5], [6], [7]].
Consistent with this notion, DS also comprises numerous co-occurring conditions beyond perturbed neurodevelopment, including increased predisposition to autoimmune diseases and increased incidence of specific hematologic malignancies [[8], [9], [10], [11]]. For example, people with DS exhibit a higher prevalence of psoriasis (8% vs. 2–3% in the overall population), type 1 diabetes (4.5-fold increased risk), and juvenile arthritis (20/1000 vs. 1/1000) [12,13]. These point to pro-inflammatory immune dysregulation in people with DS. Inflammatory homeostasis is critically regulated by balanced differentiation of naïve CD4+ T cells into pro-inflammatory T helper (Th) subsets, such as Th17 and Th1, versus anti-inflammatory T regulatory cells (Tregs). The notion that this pathway is dysregulated in people with DS is supported by observations of an increased proportion of Th1, Th17, and Th17/1 cells [14,15] in people with DS. Other Th axis defects associated with DS include impaired Treg function [16] and impaired response to Tregs [14].
One mechanism by which DYRK1A may regulate pro-inflammatory T cell potential may be by regulating the nuclear factor of activated T cells (NFAT), a key regulator of multiple aspects of T cell biology [17]. DYRK1A phosphorylates NFAT, promoting nuclear exclusion of NFAT that, in turn, impacts its ability to promote expression of target genes [18]. Conversely, harmine and other DYRK1A inhibitors lead to NFAT accumulation in the nucleus [2,[19], [20], [21]]. Inhibition of DYRK1A exerts potent anti-inflammatory effects on T cell differentiation, inhibiting Th17 and promoting Treg differentiation [20]. Therefore, DYRK1A inhibition could present a novel approach to regulating immune homeostasis in chronic inflammatory and autoimmune diseases.
Despite its therapeutic potential in various conditions, few DYRK1A inhibitors have progressed into clinical development to this date. In this study, we report identification of FRTX-02 (formerly VRN024219), a novel compound exhibiting selective inhibition of DYRK1A. We explore FRTX-02 effects on adaptive immune responses, specifically NFAT signaling in T cell lines, as well as polarization of naïve CD4+ T cells. Additionally, we provide new evidence supporting the potential involvement of DYRK1A in innate immune responses, specifically interleukin-1 family of receptor signaling. We confirm FRTX-02 therapeutic potential in vivo in the animal model of psoriasis – inflammatory skin condition with the prominent role of the adaptive immune system. Finally, we propose the utility of FRTX-02 for the treatment of atopic dermatitis based on the in vivo efficacy of FRTX-02 in animal models of this disease on par with currently available therapies and biomarker data supporting FRTX-02 clinical development in this indication.
2. Materials and methods
2.1. Kinase selectivity and inhibitory activity
The kinase selectivity of FRTX-02 was assessed in vitro by ScanMax KINOMEscan™ (Eurofins, France) performed by Discover X (Fremont, CA, USA) using 1 μM FRTX-02, supplied by Voronoi. The results were plotted on a TREEspot™ interaction map. Inhibitory activity of FRTX-02 against selected kinases was evaluated by Reaction Biology (Malvern, PA, USA) using radiometric HotSpot™ kinase assay in the presence of 10 μM ATP.
2.2. NFAT reporter assay and IL-2 mRNA expression
For studies of NFAT-inducible luciferase, Jurkat-Lucia NFAT cells were purchased from InvivoGen (San Diego, CA) and grown in Iscoves's Modified Dulbecco's Medium (IMDM) (Hyclone, Logan, UT) containing 10% fetal bovine serum (FBS), 1% penicillin-streptomycin (Welgene), and 100 μg/mL zeocin added to the growth medium every other passage. For assays, cells were collected into 50 mL conical tubes and centrifuged at 1000 rpm for 5 min. The supernatant was removed, and cells were resuspended and placed in test wells at 5 × 105 cells/mL in IMDM containing 10% FBS without zeocin. Cells were treated for 24 h with 50 ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma Aldrich), 500 ng/mL ionomycin (Sigma Aldrich), and FRTX-02 or INDY (both in 0.05% v/v dimethylsulfoxide [DMSO]) at 0.5, 1, 2, or 4 μM or vehicle (0.05% v/v DMSO). At the end of the incubation period, 20 μL of media per well was transferred to wells of clear-bottom white polystyrene microplates (Corning, Corning NY) and mixed with 20 μL QUANTI-Luc™ (InvivoGen). Luminescence was measured by Synergy Neo 2 HTS Multi-Mode Microplate reader (BioTek, Winooski, VT).
For studies of IL-2 mRNA expression, Jurkat cells were purchased from the American Type Culture Collection (ATCC, Manassas VA) and cultured on 12-well plates coated with anti-CD3 (Invitrogen, Waltham, MA) and anti-CD28 (Invitrogen) antibodies (coating was performed using 1 μg/mL and 0.5 μg/mL, respectively, in PBS at 4° C overnight). The culture medium was (RPMI-1640, Hyclone) containing 10% FBS (Hyclone). Cells were treated with FRTX-02 (0.5, 1, or 3 μM) for three days. Total mRNA was isolated from the cells using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) following the manufacturer's instructions. The total mRNA was reverse-transcribed to cDNA using the PrimeScript ™ RT reagent kit (Takara Bio, Kusatsu, Shiga, Japan) following the manufacturer's instructions. cDNA purity was analyzed by spectrophotometric profiling using the Nano-Drop 2000 spectrophotometer (Thermo-Fisher Scientific, Waltham, MA). Real-time PCR was performed in a 96-well plate format using the StepOnePlus™ Real-Time PCR system instrument (Applied Biosystems, Waltham, MA) and the SYBR™ Green PCR Master Mix (Applied Biosystems) following the manufacturer's instructions. Each reaction (2 μL) contained 200 ng cDNA template (see Table 1) and was performed in duplicate. Absolute gene expression data were normalized to the reference gene GAPDH.
Table 1.
Primer sequences.
| Target genes | Direction | Sequence | Experiment |
|---|---|---|---|
| Human IL-2 | Forward | 5′-GCACTAAGTCTTGCACTTGTCA-3′ | qPCR |
| Human IL-2 | Reverse | 5′-AATGCTCCAGTTGTAGCTGTG-3′ | qPCR |
| Human GAPDH | Forward | 5′-GGCTCTCCAGAACATCATC-3′ | qPCR |
| Human GAPDH | Reverse | 5′-TCTTCCTCTTGTGCTCTT G-3′ | qPCR |
| Human MyD88 | Forward | 5′-TCTGGAAGTCACATTCTTTGC-3′ | RT-PCR |
| Human MyD88 | Reverse | 5′-CGGCAACTGGAGACACAAG-3′ | RT-PCR |
| Human GAPDH | Forward | 5′-GAAGGTGAAGGTCGGAGTC-3′ | RT-PCR |
| Human GAPDH | Reverse | 5′-GAAGATGGTGATGGGATTTC-3′ | RT-PCR |
2.3. MyD88/IRAK/NF-κB signaling in HMC1.2 cells
HMC1.2 cells were purchased from Merck, USA, and maintained in IMDM media supplemented with 10% FBS and 1% P/S. Cells seeded in a 6-well plate were pretreated with FRTX-02 (0.01, 0.1, 1, or 10 μM) for 4 h, then stimulated with IL-33 (50 ng/mL; Peprotech) for 30 min. Cells were harvested, and lysates were prepared with RIPA buffer, followed by running on bio-tris/Tris-Glycine gels with 30 μg protein per lane, including one lane employing PageRuler™ prestained protein ladder 26,616 (Thermo-Fisher). SDS-PAGE gels were transferred to nitrocellulose membranes with the semi-dry method and followed by immunoblotting with indicated antibodies (NF-κB, p–NF–κB, MyD88 (D80F5), IRAK4, p-IRAK4 (Thr345/Ser 346), IKK-αp-IKK α/β, p-SF3B1) were purchased from Cell Signaling Technologies (Danvers, MA).
mRNA was prepared from cell lysate using a prep kit from Qiagen. After quantification using NanoDrop™ (ThermoFisher Scientific), 1 μg mRNA, 100 pmol primer sets for MyD88, which can yield 520 bp product for the L form and 400 bp for the S form, and GAPDH, which can yield 172 bp, were used for cDNA synthesis. RT-PCR was performed using PrimeScript™ RT reagent Kit (TAKARA). PCR products were analyzed by 1% agarose gel. Primer sequences for RT PCR are indicated in Table 1.
2.4. Isolation, culture, and polarization of CD4+ T cells from mouse spleen and/or lymph node
C57BL/6 mice were purchased from Jackson Laboratory and housed under specific pathogen-free conditions. Naïve CD4+ T cells from the spleen and/or lymph nodes of 8–12 weeks C57BL/6 mice were purified by positive selection using CD4-conjugated magnetic beads (MiltenyiBiotec). Purified CD4+ T cells (2 × 105/well) were cultured in vitro for four days (37° C, 5% CO2) on flat-bottom plates in the presence of polarizing conditions as described below.
For Th17 polarization, cell culture plates were coated with mouse anti-CD3 antibody (clone 145-2C11, Biolegend). Isolated CD4+ T cells were suspended (2 × 105/mL) in RPMI containing 10% FBS, 1% penicillin-streptomycin, and TGF-β1 (1 ng/mL, R&D system), IL-6 (10 ng/mL, R&D system), anti-IL-4 (5 μg/mL, Biolegend) and anti- IFN-γ (5 μg/mL, Biolegend). In addition, FRTX-02 was added to the suspension at various different concentrations indicated, respectively. The cells were plated and cultured for four days in a humidified incubator at 37° C, 5% CO2.
For Treg polarization, the procedure was similar to above, except the cells were suspended at 2 × 105/mL and the differentiation factors added to the culture medium were: recombinant mouse IL-2 (10 ng/mL, R&D Systems), recombinant human TGF-β1 (1.5 ng/mL, R&D Systems), mouse anti-IL-12/IL-23 (2 μg/mL, Biolegend) and mouse anti–IFN–γ (2 μg/mL, Biolegend). Cells were cultured for three days.
For Th1 polarization, the differentiation factors added to the culture media were: IL-12 (10 ng/mL; Biolegend) and anti-IL-4 (5 μg/mL, Biolegend) as a blocking antibody. Cells were cultured for 3 days.
2.5. Flow cytometry
Cultured cells were harvested and resuspended in culture medium without FBS and with the addition of 2 μM GolgiStop (BD Biosciences), 1 μM ionomycin, 10 ng/mL phorbol-myristate acetate and incubated for 4 h. Cells were then centrifuged and resuspended in staining buffer containing 5% normal mouse/rat plasma to reduce non-specific staining. Cell-surface antigens were stained using fluorochrome-conjugated monoclonal antibodies. The cells were then washed and centrifuged (10,000 RPM for 1 min), resuspended, fixed, and permeabilized (BD Cytofix/Cytoperm kit [BD Biosciences] for IL-17 A and IFN-γ intracellular staining; Foxp3/transcription factor staining buffer [eBioscience] for Foxp3 intracellular staining) according to the manufacturer instructions. Antibodies used for staining were PerCP-Cy™5.5 Rat Anti-Mouse CD3 Molecular Complex (BD Biosciences), PE-Cy™7 Rat Anti-Mouse CD4 (BD Biosciences), Alexa Fluor® 647 Rat anti-Mouse IL-17 A (BD Biosciences), PE Rat anti–IFN–γ (XMG1.2, Biolegend), APC Rat Anti-Mouse CD25 (BD Biosciences, cat # 557,192), FOXP3 Monoclonal Antibody (150D/E4), PE-conjugated (eBioscience). Cell counts were then obtained by flow cytometry (BD FACS Verse).
2.6. Mouse pharmacokinetics
ICR (Institute of Cancer Research) mice (purchased from Sino-British SIPPR/BK Lab Animal, Ltd, Shanghai, China) were housed and treated in accordance with Guidelines for the Care and Use of Laboratory Animals. On day 0, animals were orally administered 20 or 30 mg/kg FRTX-02 in vehicle (10% polyethylene glycol 400 in distilled water) using sonde. Blood samples (250 μL per sample) were obtained from the jugular vein after 6 and 12 h and on days 1, 2, 4, 8, and 10, 24 h after the last administration. Samples were placed in tubes containing heparin sodium and centrifuged at 6800 g for 6 min at 2–8° C. The resulting plasma was transferred to labeled tubes and stored at −80° C until analysis. Samples were analyzed by liquid chromatography with tandem mass spectrometry (LC-MS/MS). Pharmacokinetic parameters were calculated using Phoenix WinNonlin® 7.0.
2.7. BALB/c mouse-imiquimod model of psoriasis
BALB/c male mice were purchased from BioLasco Taiwan (under license from Charles River Laboratories) and maintained in a controlled environment. Animals were housed and treated in accordance with the Guidelines for Care and Use of Laboratory Animals. Once daily for nine consecutive days, 15 mg imiquimod cream (5%, Aldara, 3 M Pharmaceuticals) was applied to right ear to induce psoriasis-like inflammation. For studies of oral therapy, each animal was administered by oral gavage 1 h before each imiquimod induction: vehicle (0.5% citric acid, 20% HP-b-CD in double-distilled water), methotrexate (2 mg/kg), tofacitinib (5 mg/kg) or one of two doses of FRTX-02 (45 mg/kg or 60 mg/kg) in vehicle. For studies of topical therapy, the right ear of each animal was treated with 20 μL of vehicle (5% DMSO, 75% PEG400, 30% ethanol), dexamethasone (0.15%) or one of three concentrations of FRTX-02 (0.3%, 1%, 3%) in vehicle. Ear thickness was measured by an investigator blinded to treatment assignment using a dial thickness micrometer gauge on day 0, 30 min before dosing on days 2, 4, 6, and 8, and on day 10 (24 h after the last dose).
2.8. NC/Nga mouse model of atopic dermatitis
NC/Nga TndCrlj mice (7-week-old) were purchased from Charles River Laboratories (Kanagawa, Japan) and maintained in a controlled environment. Animals were housed and treated in accordance with the Guidelines for the Care and Use of Laboratory Animals. Studies were conducted on 10-week-old mice. Mice were shaved on the back and auricle, and 150 μL of 4% sodium dodecyl sulfate (SDS) was spread on the shaved area using the back of a plastic spoon. After drying for approximately 1–2 h, approximately 0.1 g of Biostir AD was spread over the shaved area to induce dermatitis. Dermatitis induction was performed twice weekly for three weeks. Biostir AD (Biostir, Inc) consists of a hydrophilic petrolatum base containing house dust mite (Dermatophagoides farina) allergens. At 3 weeks, animals with a dermatitis severity score (DSS) of 6–9 were grouped to ensure mean body weight and mean DSS were consistent across groups. Animals were then treated orally with upadacitinib (6 mg/kg) or one of two doses of FRTX-02 (20 mg/kg or 30 mg/kg) in vehicle (0.5% citric acid in double-distilled water). Each treatment was administered twice daily for 14 days. DSS was evaluated on days 0, 3, 7, 10, and 14. DSS evaluates 4 categories: erythema/hemorrhage, edema, scale/dryness/and excoriation/erosion, each on a scale of 0: none, 1: mild, 2: moderate, or 4: severe. Scores for each category were summed to obtain an overall DSS score. On treatment day 14, blood samples were drawn from the vena in the abdomen and stored at −80° C. Then, skin samples were excised from the back and cut into two equal parts. One was stored in 10% formalin solution, and the other half was stored at −80° C. IgE and cytokine levels in blood and skin samples were determined by Biostir, Inc (Osaka, Japan).
For studies of topical FRTX-02, the same procedure as above was followed for dermatitis induction and scoring. During the treatment phase, the affected areas were treated with a topical ointment containing dexamethasone (0.15%) or FRTX-02 (1.5% or 3%) once daily for 14 days.
3. Results
3.1. FRTX-02 is a selective and potent inhibitor of DYRK1A
We selected FRTX-02 as a promising DYRK1A inhibitor from a proprietary small-molecule library. At a concentration of 1 μM, FRTX-02-unbound DYRK1A was 0.35% of control (seen in Fig. 1A). Apart from DYRK1A, FRTX-02 (at 1 μM) interacted with only six of the 468 kinases assessed, with FRTX-02-unbound values between 1 and 8% of control (Fig. 1A). FRTX-02 inhibited DYRK1A with a half-maximal inhibitory concentration (IC50) of 2.9 nM (Fig. 1B).
Fig. 1.
Kinase selectivity and in vitro IC50 of FRTX-02. (A) TREEspot™ plot of the 403 non-mutant kinases included in the scanMAX Kinomescan® assay of FRTX-02. Red circles depict kinases that are <10% drug-unbound (%Ctrl <10) in the presence of 1 μM FRTX-02. DYRK1A is indicated as a blue circle with the highest FRTX-02 binding (0.35% drug-unbound vs. control). Apart from DYRK1A, FRTX-02 at 1 μM concentration exhibited binding interactions (drug-unbound below 10% of control) with CLK1, DYRK1B, ALK(C1156Y), CLK2, ALK, and MYLK (drug-unbound 1.1%, 1.8%, 2.5%, 3.3%, 3.5%, and 7.8% respectively). (B) DYRK1A activity according to Reaction Biology in vitro kinase assay in the presence of 10 μM ATP. TREEspot images generated using TREEspot™ Software Tool and reprinted with permission from KINOMEscan®, a division of DiscoveRx Corporation, © DISCOVERX CORPORATION 2010.
We characterized plasma pharmacokinetics of orally administered FRTX-02 to support its preclinical and clinical development. FRTX-02 administered by oral gavage to ICR mice at 20 mg/kg or 30 mg/kg showed maximal plasma concentrations of 2.2 μM and 3.0 μM, respectively, and plasma half-life of 1.53 h and 1.94 h, respectively. These results support the use of these doses to achieve plasma levels approximately 10-times the determined IC50. Overall, FRTX-02 is a selective and potent DYRK1A inhibitor with favorable pharmacokinetic properties.
3.2. FRTX-02 promotes NFAT transcriptional activity in T cell lines
We next sought to validate that FRTX-02 enhances NFAT-driven transcription. We used Jurkat-Lucia NFAT cells, which contain an NFAT-inducible luciferase reporter [22]. Stimulation with phorbol myristate acetate and ionomycin drove the production of luciferase, which was enhanced by co-incubation with FRTX-02 in a dose-dependent manner (Fig. 2A).
Fig. 2.
Effects of FRTX-02 on NFAT-mediated gene expression in Jurkat cells. (A) Luciferase activity in the supernatant of Jurkat-Lucia NFAT cells after 24-h incubation with phorbol myristate acetate, ionomycin, and the indicated concentrations of FRTX-02. (B) Relative mRNA expression levels (normalized to GAPDH expression) in Jurkat cells pre-stimulated with anti-CD3 and anti-CD-28 antibodies and incubated for 3 days with the indicated concentrations of FRTX-02. All values are normalized to vehicle control and represent the mean ± SD of 3 experiments. P values versus vehicle control by ordinary one-way ANOVA followed by Turkey's multiple comparisons test; *, p < 0.05; ***, p < 0.001; ****, p < 0.0001.
We also examined the expression of the endogenous Il2 gene, which has been shown to be regulated by NFAT by binding to the interleukin-2 (IL-2) promoter [[23], [24], [25]]. Stimulation of Jurkat T cells with anti-CD3 and anti-CD28 induced IL-2 gene expression, which was further increased by FRTX-02 (Fig. 2B; p = 0.003). Together, these findings support the notion that FRTX-02-mediated DYRK1A inhibition promotes NFAT-mediated gene expression in T cell lines, including the expression of Il2, which is essential for the differentiation, function, and survival of Tregs [26].
3.3. FRTX-02 modulates the differentiation of naïve CD4+ T cells ex vivo
To understand how FRTX-02 impacts adaptive immune responses, we evaluated the effect of FRTX-02 on T cell differentiation in vitro. Naïve murine CD4+ T cells were cultured in pro-Treg, -Th17, or -Th1 conditions in the absence or presence of FRTX-02 and T cell differentiation was assessed by flow cytometry. In pro-Treg conditions, FRTX-02 significantly enhanced Treg differentiation in a dose-dependent manner. In contrast, FRTX-02 significantly inhibited Th17 differentiation in pro-Th17 conditions (Fig. 3). These data demonstrate that FRTX-02 exerts similar effects on Treg/Th17 differentiation as previously observed with earlier-generation DYRK1A inhibitors [20]. Moreover, we also found that FRTX-02 inhibits Th1 differentiation (Fig. 3B). Overall, these results support the notion that FRTX-02 exerts anti-inflammatory effects on adaptive immunity by enhancing the differentiation of anti-inflammatory Tregs while suppressing the differentiation of pro-inflammatory Th17 and Th1 cells.
Fig. 3.
Effects of FRTX-02 on differentiation of naïve CD4+ T cells from C57BL/6 mice. Isolated cells in culture were exposed to the indicated polarizing conditions (see METHODS) and doses of FRTX-02. Negative control indicates no polarizing reagents added to the culture medium. Positive control indicates polarizing conditions and FRTX-02 vehicle. (A) Percentage of CD4+ cells (isolated from spleen and treated for 4 days) expressing Treg (CD25 and Foxp3; left panel) or Th17 (IL-17 A; right panel) markers by flow cytometry. P values versus positive control by unpaired t-test; *, p < 0.05; ***, p < 0.001. (B) Percentage of CD4+ cells (isolated from spleen and lymph nodes and treated for 3 days) expressing Treg (CD25 and Foxp3; left panel), Th17 (IL-17 A; middle panel), or Th1 (interferon-γ; right panel) markers by flow cytometry. P values versus positive control by ordinary one-way ANOVA followed by Turkey's multiple comparisons test; *, p < 0.05; **, p < 0.01; ***, p < 0.001. All values represent the mean ± SEM of 3 experiments.
3.4. FRTX-02 suppresses innate immune responses in vitro
Given that DYRK1A is expressed in both innate and adaptive immune cells, we next explored the possibility that DYRK1A may also regulate innate immune responses. We focused on signaling through IL-33, an alarmin of increasing interest in many immune disorders, including autoimmunity and allergy [[27], [28], [29]]. As previously reported [30], stimulation of the HMC1.2 human mast cell line with IL-33 drove phosphorylation of IL-1 receptor-activated kinase 4 (IRAK4), and the downstream signaling effectors IKKα and NF-κB (Fig. 4A, lane 2). These downstream effects of IL-33 stimulation were inhibited by preincubation with FRTX-02 in a dose-dependent manner (Fig. 3A, lanes 3–6).
Fig. 4.
MyD88/IRAK4/NF-κB signaling in response to IL-33 and BI-02. (A) Immunoblot of MyD88/IRAK4/NF-κB signaling components after 30-min incubation of HMC1.2 cells with IL-33 (50 ng/mL) and preincubation (4 h) with the indicated doses of FRTX-02. (B) RT-PCR of MyD88 splicing variants from the same experiment.
One of the most proximal signaling molecules engaged by the IL-33 receptor is the adapter protein MyD88 [[31], [32], [33]]. Interestingly, reports show that MyD88 signaling can be regulated by alternative splicing, which can in turn be regulated by DYRK1A. These studies report two splice variants of MyD88; the canonical, NF-κB-activating isoform (‘MyD88 long’, MyD88L), and a truncated isoform (‘MyD88 short’, MyD88S) that lacks the ability to induce IRAK phosphorylation and NF-κB-activation and acts as a dominant-negative regulator of MyD88 signaling [[34], [35], [36]]. Remarkably, we observed that FRTX-02 altered the ratio of these isoforms, increasing the proportion of the inactive MyD88S isoform relative to MyD88L in a dose-dependent manner (Fig. 4B).
Previous reports suggested that DYRK1A phosphorylates the splicing factor SF3B1 [37]. In turn, SF3B1 was shown to regulate innate immune responses, at least in part, by controlling alternative splicing of MyD88 [35]. Consistent with these findings, we showed that FRTX-02 inhibits phosphorylation of SF3B1 in a dose-dependent manner (Fig. 4A) [37]. These data support a framework that FRTX-02 can suppress innate immune responses by inhibiting a DYRK1A/MyD88/IRAK/NF-κB signaling pathway.
3.5. FRTX-02 alleviates inflammation in a mouse model of psoriasis
Psoriasis is a chronic immune-mediated skin disorder characterized by the pathogenic inflammation driven by Th1 and Th17 lymphocytes that is inadequately controlled by Tregs [38]. Systemic therapies for severe psoriasis include immune suppressants like methotrexate, although potential severe side effects highlight the need for additional approaches. We evaluated whether FRTX-02 might be of benefit in the well-characterized imiquimod-induced psoriasis model [39]. Daily application of imiquimod cream to the right ear of BALB/c mice drove local inflammation that was quantified by measuring ear thickness. This inflammation was significantly attenuated by methotrexate or tofacitinib, administered twice daily by oral gavage. Notably, orally administered twice daily, FRTX-02 significantly attenuated ear inflammation by day 8 to at least the same extent as methotrexate and tofacitinib; this benefit persisted at day 10 (Fig. 5A).
Fig. 5.
Effects of twice-daily oral or once-daily topical FRTX-02 and comparators in the imiquimod-BALB/C mouse model of psoriasis. (A) Results of oral treatment. Left panel: Right ear thickness at baseline (day 0) and 1 h before on indicated days. Right panel: Exploded view of right ear thickness on day 8. Untreated ears in the same animals (left ears) and sham-treated animals showed no consistent changes in ear thickness, except for the methotrexate group, which had a significant loss of mean body weight (∼10%) during the study. (B) Results of topical treatment. Left panel: Right ear thickness at baseline (day 0) and 1 h before topical treatment on indicated days. Right panel: Exploded view of right ear thickness on day 8. Untreated ears (left ears) showed no consistent changes except for the dexamethasone group, which had decreases in left ear thickness. All values are mean ± SEM of 8 animals. P values vs. vehicle by two-way ANOVA followed by Bonferroni's multiple comparisons test; *, p < 0.05.
Although we did not observe adverse effects of systemic FRTX-02 therapy, we recognized that topical treatment modalities could help achieve therapeutic efficacy while minimizing adverse effects from systemic exposure to the drug. Using the same imiquimod model, we found that once daily topical administration of FRTX-02 significantly attenuated ear inflammation in a dose-dependent manner. These results highlight that FRTX-02 can attenuate inflammation in animal models of psoriasis.
3.6. Oral or topical FRTX-02 alleviates inflammation in a mouse model of atopic dermatitis
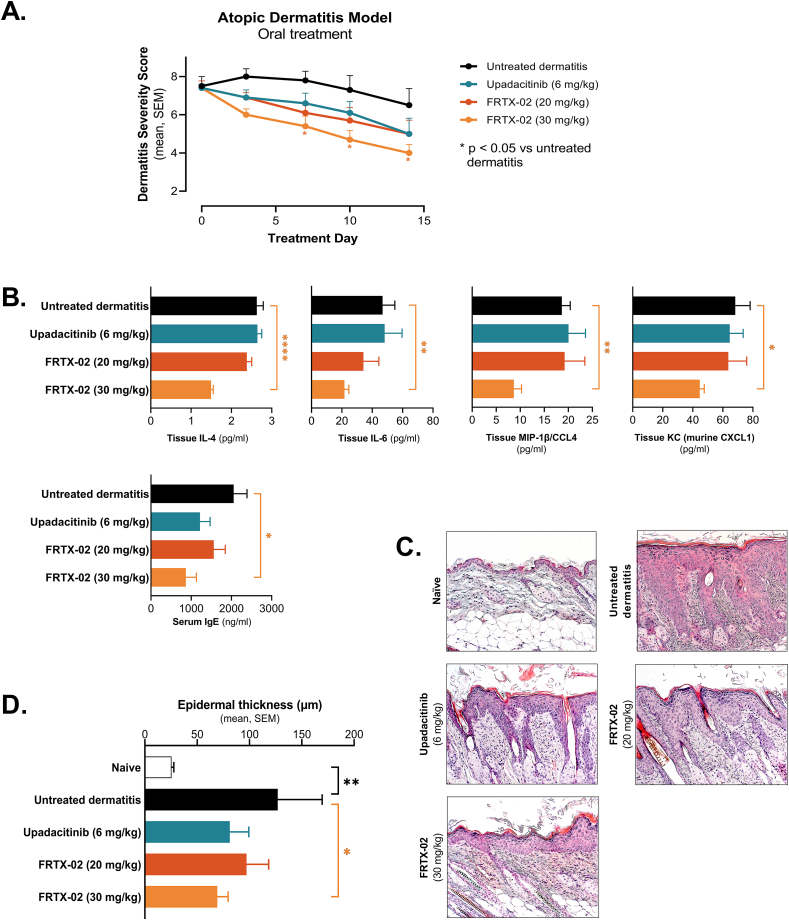
Atopic dermatitis is another common recurrent inflammatory skin disorder associated with defects in both adaptive and innate immunity. While thought to be primarily Th2-driven, reports suggesting contribution of (i) Th1 and Th17 cells [[40], [41], [42], [43], [44], [45]] to atopic dermatitis, and (ii) IL-33 in driving Th2 responses [[46], [47], [48]] suggest utility of FRTX-02. We used the well-characterized animal model of allergen-caused atopic dermatitis, which includes induction with topical 4% SDS and house dust mite (HDM) allergens, followed by continued topical treatment with HDM allergens; dermatitis was scored using well-established methods [49]. After dermatitis induction, animals with dermatitis severity scores (DSS; see METHODS; the sum of scores from back and auricle) of 6–9 were allocated to treatment groups and monitored for up to 14 days. Oral administration of 30 mg/kg FRTX-02 twice daily significantly attenuated inflammation as measured by DSS by day 7, with continued benefit through day 14 (Fig. 6A). This was further verified by histological analyses, including quantification of skin thickness from the back biopsy samples (Fig. 6B and C; p < 0.05). Further, treatment with FRTX-02 also attenuated levels of serum IgE (Fig. 6D) and key inflammatory cytokines in treated skin (Fig. 6E). In all these studies, FRTX-02 (30 mg/kg) performed at least as well as the JAK inhibitor upadacitinib, supporting the notion that systemic FRTX-02 may be of utility in attenuating inflammation related to atopic dermatitis.
Fig. 6.
Effects of twice-daily oral treatment with FRTX-02 or positive control (upadacitinib) in the Nc/Nga mouse model of house dust mite-induced AD. (A) Dermatitis severity score (DSS; the sum of scores on back and auricle) during the treatment period. Control animals, in which no dermatitis was induced (not shown), had DSS of zero at all time points. P values vs. untreated dermatitis by two-way ANOVA followed by Dunnett's multiple comparisons test; *, p < 0.05. (B) Photomicrographs of representative hematoxylin and eosin-stained skin sections demonstrating epidermal thickness. Magnification x20. Scale bar, 100 μm. (C) Epidermal thickness in back skin biopsy samples collected on treatment day 14. Values are based on averages of 5 randomly selected microscopic fields per sample independently measured by two blinded researchers. P values versus untreated dermatitis by unpaired t-test; *, p < 0.05; **, p < 0.01. (D and E) Serum IgE levels (D) and selected cytokine levels in biopsy specimens taken from the back (E) at the end of the 14-day treatment period. Untreated dermatitis and vehicle groups, n = 4 per group; upadacitinib and FRTX-02 groups, n = 7 per group. P values versus untreated dermatitis by unpaired t-test; *, p < 0.05; **, p < 0.01; ****, p < 0.0001.
We also used the same model to test the efficacy of topically formulated FRTX-02. FRTX-02 attenuated inflammation in a dose-dependent manner. Notably, the application of high-dose FRTX-02 once daily significantly attenuated inflammation to at least the same extent as the positive control, dexamethasone, as quantitated by both DSS and by histological analysis (Fig. 7). Taken together, these preclinical findings support that different formulations of FRTX-02 may be therapeutically useful in atopic dermatitis.
Fig. 7.
Effects of once-daily treatment with a topical formulation of FRTX-02, dexamethasone, or vehicle in the Nc/Nga mouse model of house dust mite-induced dermatitis. (A) DSS during the 14-day treatment period. N = 5 animals per group. P values vs. vehicle by two-way ANOVA followed by Dunnett's multiple comparisons test; *, p < 0.05. (B) Photomicrographs of representative skin sections from back biopsy samples.
4. Discussion
FRTX-02 is an orally available, selective, small-molecule inhibitor of DYRK1A, with an IC50 in cell-free assays in the low nM range (2.9 nM). In whole-cell assays in cells derived from both adaptive and innate immune precursors, FRTX-02 inhibited canonical DYRK1A signaling pathways involved in immune activation. Specifically, FRTX-02 promoted NFAT-mediated gene transcription in T-cell lines; and exerted anti-inflammatory effects on CD4+ T cell differentiation (enhanced differentiation of anti-inflammatory Tregs and inhibited differentiation of pro-inflammatory Th1 and Th17 cells), largely corroborating and extending previous DYRK1A research [20]. MyD88 is a key transducer of many innate immune receptors regulated in part by alternative splicing [36]. FRTX-02 also promoted alternative splicing of a dominant-negative isoform of MyD88 (MyD88S) in human mast cells, potentially by regulating DYRK1A-mediated phosphorylation of the splicing factor SF3b1, limiting innate immune responses [34,35,50,51]. These findings point to under-studied anti-inflammatory effects of DYRK1A inhibition on innate immune cells.
Further, FRTX-02 demonstrated pre-clinical therapeutic effects in two animal models of skin inflammation: psoriasis and atopic dermatitis. Psoriasis is a systemic immune disorder driven at least in part by excessive contribution by Th1 and/or Th17 cells [[52], [53], [54], [55]]. Our study extends previous findings pointing to DYRK1A as a druggable regulator of Th differentiation and suggests a broader role for DYRK1A inhibitors in restoring adaptive immune homeostasis [20]. Atopic dermatitis exemplifies an autoinflammatory condition that may include significant contribution of innate immune dysregulation. Therapeutic efficacy of FRTX-02 in an experimental model of atopic dermatitis supports the notion that FRTX-02 may additionally be of benefit in driving anti-inflammatory innate immune responses and suggesting broad utility in various autoimmune and autoinflammatory conditions.
Notably, FRTX-02 showed tolerability in both oral and topical formulations in animal experiments detailed above. Additionally, FRTX-02 suggested therapeutic effects potentially comparable to currently approved compounds, supporting additional study. Taken together, our results demonstrate that FRTX-02 is an innovative selective and potent DYRK1A inhibitor with the potential to shift both adaptive and innate immune system pathways toward immune equilibrium. These properties may be invaluable in the treatment of chronic inflammatory and autoimmune disorders and warrant further study.
Authors contribution statement
Sunghwan Kim and Daekwon Kim conceived the project. Soochan Kim performed experiments, including mouse work. Soochan Kim and Sunghwan Kim analyzed data. Monica Luchi contributed to data analysis and writing the manuscript. All authors directly provided contributions, reviewed, and approved the final manuscript.
Funding
Voronoi, Inc. Funded this study. Fresh Tracks Therapeutics, Inc. Supported the development of this manuscript and paid for medical writing support.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Yeonsil Kim, Dongwoon Shin, and Hyun-Kyung Kim for helping with experiments and sample processing. We also thank the rest of the Voronoi R&D center members for useful discussions. Medical writing support for parts of the Results and Discussion was provided by Ken Scholz, PhD, in affiliation with Innovative BioPharma. In addition, we are grateful to Kai-Hsin Chan, Sofya Polyanskaya, and Sarah Oelsner (Scitaris GmbH) for their support in developing and critically reviewing this manuscript.
Handling Editor: Y Renaudineau
Data availability
Data will be made available on request.
References
- 1.Hämmerle B., Elizalde C., Tejedor F.J. The spatio-temporal and subcellular expression of the candidate Down syndrome gene Mnb/Dyrk1A in the developing mouse brain suggests distinct sequential roles in neuronal development. Eur. J. Neurosci. 2008;27:1061–1074. doi: 10.1111/j.1460-9568.2008.06092.x. [DOI] [PubMed] [Google Scholar]
- 2.Ogawa Y., Nonaka Y., Goto T., Ohnishi E., Hiramatsu T., Kii I., Yoshida M., Ikura T., Onogi H., Shibuya H., Hosoya T., Ito N., Hagiwara M. Development of a novel selective inhibitor of the Down syndrome-related kinase Dyrk1A. Nat. Commun. 2010;1:86. doi: 10.1038/ncomms1090. [DOI] [PubMed] [Google Scholar]
- 3.Duchon A., Herault Y. DYRK1A, a dosage-sensitive gene involved in neurodevelopmental disorders, is a target for drug development in Down syndrome. Front. Behav. Neurosci. 2016;10:104. doi: 10.3389/fnbeh.2016.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wegiel J., Dowjat K., Kaczmarski W., Kuchna I., Nowicki K., Frackowiak J., Mazur Kolecka B., Wegiel J., Silverman W.P., Reisberg B., de Leon M., Wisniewski T., Gong C.X., Liu F., Adayev T., Chen-Hwang M.C., Hwang Y.W. The role of overexpressed DYRK1A protein in the early onset of neurofibrillary degeneration in Down syndrome. Acta Neuropathol. 2008;116:391–407. doi: 10.1007/S00401-008-0419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arbones M.L., Thomazeau A., Nakano-Kobayashi A., Hagiwara M., Delabar J.M. DYRK1A and cognition: a lifelong relationship. Pharmacol. Ther. 2019;194:199–221. doi: 10.1016/J.PHARMTHERA.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Kumar K., Suebsuwong C., Wang P., Garcia-Ocana A., Stewart A.F., DeVita R.J. DYRK1A inhibitors as potential therapeutics for β-cell regeneration for diabetes. J. Med. Chem. 2021;64:2901–2922. doi: 10.1021/acs.jmedchem.0c02050. [DOI] [PubMed] [Google Scholar]
- 7.Rammohan M., Harris E., Bhansali R.S., Zhao E., Li L.S., Crispino J.D. The chromosome 21 kinase DYRK1A: emerging roles in cancer biology and potential as a therapeutic target. Oncogene. 2022;41:2003–2011. doi: 10.1038/S41388-022-02245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonarakis S.E., Skotko B.G., Rafii M.S., Strydom A., Pape S.E., Bianchi D.W., Sherman S.L., Reeves R.H. Down syndrome. Nat. Rev. Dis. Prim. 2020;6 doi: 10.1038/S41572-019-0143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bull M.J. Down syndrome. N. Engl. J. Med. 2020;382:2344–2352. doi: 10.1056/NEJMRA1706537. [DOI] [PubMed] [Google Scholar]
- 10.Hasle H., Friedman J.M., Olsen J.H., Rasmussen S.A. Low risk of solid tumors in persons with Down syndrome. Genet. Med. 2016;18:1151–1157. doi: 10.1038/GIM.2016.23. [DOI] [PubMed] [Google Scholar]
- 11.O'Leary L., Hughes-McCormack L., Dunn K., Cooper S.A. Early death and causes of death of people with Down syndrome: a systematic review. J. Appl. Res. Intellect. Disabil. 2018;31:687–708. doi: 10.1111/JAR.12446. [DOI] [PubMed] [Google Scholar]
- 12.Madani A., Almuhaideb Q. Adalimumab therapy in a patient with psoriasis, Down syndrome, and concomitant hepatitis B virus infection. Biologics. 2021;15:375–378. doi: 10.2147/btt.S317888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foley C.M., Deely D.A., MacDermott E.J., Killeen O.G. Arthropathy of Down syndrome: an under-diagnosed inflammatory joint disease that warrants a name change. RMD Open. 2019;5 doi: 10.1136/rmdopen-2018-000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araya P., Waugh K.A., Sullivan K.D., Núñez N.G., Roselli E., Smith K.P., Granrath R.E., Rachubinski A.L., Enriquez Estrada B., Butcher E.T., Minter R., Tuttle K.D., Bruno T.C., Maccioni M., Espinosa J.M. Trisomy 21 dysregulates T cell lineages toward an autoimmunity-prone state associated with interferon hyperactivity. Proc. Natl. Acad. Sci. U. S. A. 2019;116:24231–24241. doi: 10.1073/pnas.1908129116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambert K., Moo K.G., Arnett A., Goel G., Hu A., Flynn K.J., Speake C., Wiedeman A.E., Gersuk V.H., Linsley P.S., Greenbaum C.J., Long S.A., Partridge R., Buckner J.H., Khor B. Deep immune phenotyping reveals similarities between aging, Down syndrome, and autoimmunity. Sci. Transl. Med. 2022;14 doi: 10.1126/SCITRANSLMED.ABI4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pellegrini F.P., Marinoni M., Frangione V., Tedeschi A., Gandini V., Ciglia F., Mortara L., Accolla R.S., Nespoli L. Down syndrome, autoimmunity and T regulatory cells. Clin. Exp. Immunol. 2012;169:238–243. doi: 10.1111/j.1365-2249.2012.04610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macian F. NFAT proteins: key regulators of T-cell development and function. Nat. Rev. Immunol. 2005;5:472–484. doi: 10.1038/nri1632. [DOI] [PubMed] [Google Scholar]
- 18.Gwack Y., Sharma S., Nardone J., Tanasa B., Iuga A., Srikanth S., Okamura H., Bolton D., Feske S., Hogan P.G., Rao A. A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature. 2006;441:646–650. doi: 10.1038/nature04631. [DOI] [PubMed] [Google Scholar]
- 19.Jarhad D.B., Mashelkar K.K., Kim H.R., Noh M., Jeong L.S. Dual-specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) inhibitors as potential therapeutics. J. Med. Chem. 2018;61:9791–9810. doi: 10.1021/acs.jmedchem.8b00185. [DOI] [PubMed] [Google Scholar]
- 20.Khor B., Gagnon J.D., Goel G., Roche M.I., Conway K.L., Tran K., Aldrich L.N., Sundberg T.B., Paterson A.M., Mordecai S., Dombkowski D., Schirmer M., Tan P.H., Bhan A.K., Roychoudhuri R., Restifo N.P., O'Shea J.J., Medoff B.D., Shamji A.F., Schreiber S.L., Sharpe A.H., Shaw S.Y., Xavier R.J. The kinase DYRK1A reciprocally regulates the differentiation of Th17 and regulatory T cells. Elife. 2015;4 doi: 10.7554/eLife.05920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw S.J., Goff D.A., Lin N., Singh R., Li W., McLaughlin J., Baltgalvis K.A., Payan D.G., Kinsella T.M. Developing DYRK inhibitors derived from the meridianins as a means of increasing levels of NFAT in the nucleus. Bioorg Med Chem Lett. 2017;27:2617–2621. doi: 10.1016/j.bmcl.2017.03.037. [DOI] [PubMed] [Google Scholar]
- 22.Shaw J.P., Utz P.J., Durand D.B., Toole J.J., Emmel E.A., Crabtree G.R. Identification of a putative regulator of early T cell activation genes. Science. 1988;241:4972–4975. doi: 10.1126/SCIENCE.3260404. [DOI] [PubMed] [Google Scholar]
- 23.Chow C.W., Rincón M., Davis R.J. Requirement for transcription factor NFAT in interleukin-2 expression. Mol. Cell Biol. 1999;19:2300–2307. doi: 10.1128/mcb.19.3.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dienz O., Eaton S.M., Krahl T.J., Diehl S., Charland C., Dodge J., Swain S.L., Budd R.C., Haynes L., Rincon M. Accumulation of NFAT mediates IL-2 expression in memory, but not naïve, CD4+ T cells. Proc. Natl. Acad. Sci. U. S. A. 2007;104:7175–7180. doi: 10.1073/pnas.0610442104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rooney J.W., Sun Y.L., Glimcher L.H., Hoey T. Novel NFAT sites that mediate activation of the interleukin-2 promoter in response to T-cell receptor stimulation. Mol. Cell Biol. 1995;15:6299–6310. doi: 10.1128/mcb.15.11.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbas A.K., Trotta E., Simeonov R.D., Marson A., Bluestone J.A. Revisiting IL-2: biology and therapeutic prospects. Sci Immunol. 2018;3 doi: 10.1126/sciimmunol.aat1482. [DOI] [PubMed] [Google Scholar]
- 27.Pinto S.M., Subbannayya Y., Rex D.A.B., Raju R., Chatterjee O., Advani J., Radhakrishnan A., Keshava Prasad T.S., Wani M.R., Pandey A. A network map of IL-33 signaling pathway. J Cell Commun Signal. 2018;12:615–624. doi: 10.1007/s12079-018-0464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oboki K., Ohno T., Kajiwara N., Arae K., Morita H., Ishii A., Nambu A., Abe T., Kiyonari H., Matsumoto K., Sudo K., Okumura K., Saito H., Nakae S. IL-33 is a crucial amplifier of innate rather than acquired immunity. Proc. Natl. Acad. Sci. U. S. A. 2010;107:18581–18586. doi: 10.1073/PNAS.1003059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong Y., Zhong J., Dong L. IL-33 in rheumatic diseases. Front. Med. 2021;8 doi: 10.3389/FMED.2021.739489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Espinosa-Riquer Z.P., Segura-Villalobos D., Ramírez-Moreno I.G., Pérez Rodríguez M.J., Lamas M., Gonzalez-Espinosa C. Signal transduction pathways activated by innate immunity in mast cells: translating sensing of changes into specific responses. Cells. 2020;9 doi: 10.3390/CELLS9112411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adachi O., Kawai T., Takeda K., Matsumoto M., Tsutsui H., Sakagami M., Nakanishi K., Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 32.Griesenauer B., Paczesny S. The ST2/IL-33 Axis in immune cells during inflammatory diseases. Front. Immunol. 2017;8 doi: 10.3389/FIMMU.2017.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pei C., Barbour M., Fairlie-Clarke K.J., Allan D., Mu R., Jiang H.R. Emerging role of interleukin-33 in autoimmune diseases. Immunology. 2014;141:9–17. doi: 10.1111/IMM.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janssens S., Burns K., Tschopp J., Beyaert R. Regulation of interleukin-1- and lipopolysaccharide-induced NF-kappaB activation by alternative splicing of MyD88. Curr. Biol. 2002;12:467–471. doi: 10.1016/s0960-9822(02)00712-1. [DOI] [PubMed] [Google Scholar]
- 35.de Arras L., Alper S. Limiting of the innate immune response by SF3A-dependent control of MyD88 alternative mRNA splicing. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saikh K.U. MyD88 and beyond: a perspective on MyD88-targeted therapeutic approach for modulation of host immunity. Immunol. Res. 2021;69:117–128. doi: 10.1007/s12026-021-09188-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Graaf K., Czajkowska H., Rottmann S., Packman L.C., Lilischkis R., Lüscher B., Becker W. The protein kinase DYRK1A phosphorylates the splicing factor SF3b1/SAP155 at Thr434, a novel in vivo phosphorylation site. BMC Biochem. 2006;7:7. doi: 10.1186/1471-2091-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu P., Wang M., Gao H., Zheng A., Li J., Mu D., Tong J. The role of helper T cells in psoriasis. Front. Immunol. 2021;12 doi: 10.3389/FIMMU.2021.788940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Fits L., Mourits S., Voerman J.S.A., Kant M., Boon L., Laman J.D., Cornelissen F., Mus A.-M., Florencia E., Prens E.P., Lubberts E. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009;182:5836–5845. doi: 10.4049/JIMMUNOL.0802999. [DOI] [PubMed] [Google Scholar]
- 40.Suárez-Fariñas M., Dhingra N., Gittler J., Shemer A., Cardinale I., de Guzman Strong C., Krueger J.G., Guttman-Yassky E. Intrinsic atopic dermatitis shows similar TH2 and higher TH17 immune activation compared with extrinsic atopic dermatitis. J. Allergy Clin. Immunol. 2013;132:361–370. doi: 10.1016/J.JACI.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martel B.C., Litman T., Hald A., Norsgaard H., Lovato P., Dyring-Andersen B., Skov L., Thestrup-Pedersen K., Skov S., Skak K., Poulsen L.K. Distinct molecular signatures of mild extrinsic and intrinsic atopic dermatitis. Exp. Dermatol. 2016;25:453–459. doi: 10.1111/EXD.12967. [DOI] [PubMed] [Google Scholar]
- 42.Noda S., Suárez-Fariñas M., Ungar B., Kim S.J., de Guzman Strong C., Xu H., Peng X., Estrada Y.D., Nakajima S., Honda T., Shin J.U., Lee H., Krueger J.G., Lee K.H., Kabashima K., Guttman-Yassky E. The Asian atopic dermatitis phenotype combines features of atopic dermatitis and psoriasis with increased TH17 polarization. J. Allergy Clin. Immunol. 2015;136:1254–1264. doi: 10.1016/J.JACI.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 43.Sanyal R.D., Pavel A.B., Glickman J., Chan T.C., Zheng X., Zhang N., Cueto I., Peng X., Estrada Y., Fuentes-Duculan J., Alexis A.F., Krueger J.G., Guttman-Yassky E. Atopic dermatitis in African American patients is T H 2/T H 22-skewed with T H 1/T H 17 attenuation. Ann. Allergy Asthma Immunol. 2019;122:99–110. doi: 10.1016/J.ANAI.2018.08.024. e6. [DOI] [PubMed] [Google Scholar]
- 44.Brunner P.M., Israel A., Zhang N., Leonard A., Wen H.C., Huynh T., Tran G., Lyon S., Rodriguez G., Immaneni S., Wagner A., Zheng X., Estrada Y.D., Xu H., Krueger J.G., Paller A.S., Guttman-Yassky E. Early-onset pediatric atopic dermatitis is characterized by T H 2/T H 17/T H 22-centered inflammation and lipid alterations. J. Allergy Clin. Immunol. 2018;141:2094–2106. doi: 10.1016/J.JACI.2018.02.040. [DOI] [PubMed] [Google Scholar]
- 45.Tsoi L.C., Rodriguez E., Stölzl D., Wehkamp U., Sun J., Gerdes S., Sarkar M.K., Hübenthal M., Zeng C., Uppala R., Xing X., Thielking F., Billi A.C., Swindell W.R., Shefler A., Chen J., Patrick M.T., Harms P.W., Kahlenberg J.M., Perez White B.E., Maverakis E., Gudjonsson J.E., Weidinger S. Progression of acute-to-chronic atopic dermatitis is associated with quantitative rather than qualitative changes in cytokine responses. J. Allergy Clin. Immunol. 2020;145:1406–1415. doi: 10.1016/J.JACI.2019.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Savinko T., Matikainen S., Saarialho-Kere U., Lehto M., Wang G., Lehtimäki S., Karisola P., Reunala T., Wolff H., Lauerma A., Alenius H. IL-33 and ST2 in atopic dermatitis: expression profiles and modulation by triggering factors. J. Invest. Dermatol. 2012;132:1392–1400. doi: 10.1038/jid.2011.446. [DOI] [PubMed] [Google Scholar]
- 47.Li C., Maillet I., Mackowiak C., Viala C., di Padova F., Li M., Togbe D., Quesniaux V., Lai Y., Ryffel B. Experimental atopic dermatitis depends on IL-33R signaling via MyD88 in dendritic cells. Cell Death Dis. 2017;8 doi: 10.1038/cddis.2017.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen Y.L., Gutowska-Owsiak D., Hardman C.S., Westmoreland M., MacKenzie T., Cifuentes L., Waithe D., Lloyd-Lavery A., Marquette A., Londei M., Ogg G. Proof-of-concept clinical trial of etokimab shows a key role for IL-33 in atopic dermatitis pathogenesis. Sci. Transl. Med. 2019;11 doi: 10.1126/SCITRANSLMED.AAX2945. [DOI] [PubMed] [Google Scholar]
- 49.Suto H., Matsuda H., Mitsuishi K., Hira K., Uchida T., Unno T., Ogawa H., Ra C. NC/Nga mice: a mouse model for atopic dermatitis. Int. Arch. Allergy Immunol. 1999;120(Suppl 1):70–75. doi: 10.1159/000053599. [DOI] [PubMed] [Google Scholar]
- 50.Burns K., Janssens S., Brissoni B., Olivos N., Beyaert R., Tschopp J. Inhibition of interleukin 1 receptor/Toll-like receptor signaling through the alternatively spliced, short form of MyD88 is due to its failure to recruit IRAK-4. J. Exp. Med. 2003;197:263–268. doi: 10.1084/jem.20021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee F.F., Davidson K., Harris C., McClendon J., Janssen W.J., Alper S. NF-κB mediates lipopolysaccharide-induced alternative pre-mRNA splicing of MyD88 in mouse macrophages. J. Biol. Chem. 2020;295:6236–6248. doi: 10.1074/jbc.RA119.011495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Campanati A., Marani A., Martina E., Diotallevi F., Radi G., Offidani A. Psoriasis as an immune-mediated and inflammatory systemic disease: from pathophysiology to novel therapeutic approaches. Biomedicines. 2021;9 doi: 10.3390/biomedicines9111511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tesmer L.A., Lundy S.K., Sarkar S., Fox D.A. Th17 cells in human disease. Immunol. Rev. 2008;223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marson J.W., Snyder M.L., Lebwohl M.G. Newer therapies in psoriasis. Med. Clin. 2021;105:627–641. doi: 10.1016/j.mcna.2021.04.004. [DOI] [PubMed] [Google Scholar]
- 55.Horwitz D.A., Fahmy T.M., Piccirillo C.A., la Cava A. Rebalancing immune homeostasis to treat autoimmune diseases. Trends Immunol. 2019;40:888–908. doi: 10.1016/j.it.2019.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.