Abstract
Staphylococcus aureus is a prominent human pathogen. Here we report that intact S. aureus bacteria activate the contact system in human plasma in vitro, resulting in a massive release of the potent proinflammatory and vasoactive peptide bradykinin. In contrast, no such effect was recorded with Streptococcus pneumoniae. In the activation of the contact system, blood coagulation factor XII and plasma kallikrein play central roles, and a specific inhibitor of these serine proteinases inhibited the release of bradykinin by S. aureus in human plasma. Furthermore, fragments of the cofactor H-kininogen of the contact system efficiently blocked bradykinin release. The results suggest that activation of the contact system at the surface of S. aureus and the subsequent release of bradykinin could contribute to the hypovolemic hypotension seen in patients with severe S. aureus sepsis. The data also suggest that the contact system could be used as a target in the treatment of S. aureus infections.
Gram-positive bacteria are currently as common as gram-negative bacteria in causing sepsis, and Staphylococcus aureus and Streptococcus pneumoniae are the most frequently isolated pathogens in gram-positive sepsis (5, 6, 16). These species can give rise to septic shock, a condition with a high mortality rate despite antibiotic treatment and improvements in intensive care. The pathogenesis of sepsis is not fully understood. However, there appears to be a common pathway by which both gram-negative and gram-positive bacteria induce the production of different inflammatory mediators, such as factors of the complement, coagulation, and contact systems, which act together with cytokines to form a complex inflammatory network (5, 9).
The contact system consists of three enzymatic factors, factor XI (FXI), FXII, and plasma prekallikrein (PK), and the nonenzymatic cofactor H-kininogen (HK) (24). Activation of FXII is the initial step leading to the formation of kallikrein and activated FXI. As a result, bradykinin (BK), a nonapeptide, is released from HK. BK induces vasodilatation and increased microvascular permeability, effects that in part are mediated by the secondary release of other mediators (for instance, nitric oxide and platelet activating factor) via activation of BK receptors of the vascular endothelium. The contact system can also be activated directly by endotoxin and microbial proteinases (11, 15, 18).
When injected into animals, BK reduces peripheral vascular resistance, leading to hypotension and elevated cardiac output (26), and several animal studies have shown that activation of the contact system correlates with irreversible hypotension during sepsis (25, 26). Investigations of humans have revealed that factors of the contact system are consumed in plasma of patients with severe sepsis and, especially, that persistently low levels of FXII are a bad prognostic sign (14, 21, 27, 28). A pathogenic role for the contact system is also suggested by observations that it can be activated by Streptococcus pyogenes, Salmonella, and Escherichia coli through interactions between contact factors and bacterial surface proteins (2, 3,13). In the present work, we demonstrate that S. aureus, but not S. pneumoniae, activates the contact system and releases BK. Moreover, a specific inhibitor of FXII and fragments of HK were found to efficiently block S. aureus-induced BK release in vitro.
MATERIALS AND METHODS
Strains of bacteria.
Clinical isolates of S. aureus (strain 5120) and S. pneumoniae (strain 1508) derived from patients with septic shock were grown in brain heart infusion (Difco, Detroit, Mich.) at 37°C overnight. Prior to plasma incubation, bacteria were washed three times, resuspended in 15 mM HEPES (ICN Biomedicals, Inc., Aurora, Ohio) containing 135 mM NaCl and 50 μM ZnCl2 (pH 7.4) (HEPES buffer), and diluted to a final concentration of 2 × 1010 CFU/ml.
Plasma sources.
Fresh frozen plasma samples from healthy individuals were obtained from the blood bank at Lund University Hospital, Lund, Sweden, and kept frozen at −20°C until use. Plasma depleted of FXI, FXII, PK, HK, and fibrinogen was purchased from George King Bio-Medical, Inc. (Overland Park, Kans.). To collect plasma from patients with suspected sepsis, blood was drawn in a sterile tube containing sodium citrate directly after blood culture sampling. The blood was subsequently transferred directly to a plastic tube and centrifuged at 3,000 × g. The plasma was stored at −70°C until the BK content was determined.
Incubation of bacteria in plasma.
Bacteria were prepared as described above. Five hundred microliters of bacterial suspensions (2 × 1010 CFU/ml) was incubated with an equal volume of normal human plasma or depleted plasma on a rotator at room temperature for 15 min unless indicated otherwise. After incubation, bacteria were washed twice and resuspended in 300 μl of HEPES buffer. The suspensions were allowed to stand at room temperature for 15 min, followed by centrifugation at 10,000 rpm. Supernatants were collected and kept at −20°C until the BK content was analyzed. Inhibition of the production of BK induced by bacteria in plasma was analyzed with various inhibitors added at different amounts to the bacterial suspensions, followed by plasma incubation as described above.
Protein and peptide sources.
FXII-PK inhibitor (H-D-Pro-Phe-Arg-chloromethylketone [CMK]) was obtained from Bachem, Feinchemikalien AG, Bubendorf, Switzerland. Peptides HKH20 and GHG19 were synthesized in the Proteinchemisches Zentrallabor of the Johannes Gutenberg University (Mainz, Germany) (10, 12). Cysteine proteinase inhibitor E64:trans-epoxysuccinyl-l-leucylamideo(4-guanidino)-butane was obtained from Sigma.
Production and purification of recombinant domain D5 of HK.
For expression of rD5, a modified pET25b expression vector (Novagen, Inc., Madison, Wis.) was used. The primers DK3 (5′-GCA GCA GTC ATG ACT GTA AGT CCA CCC CAC ACT TCC-3′) and DK4 (5′-GCA GCA GGA TCC ACT GTC TTC AGA AGA GCT TGC-3′) were used to amplify a fragment encoding the D5 domain and part of the D6 domain. The fragment was cleaved with BspHI and BamHI and ligated to the vector, which was cleaved with NcoI and BamHI. The protein was expressed in E. coli strain BL21(DE3). Protein production was induced by addition of 1 mM isopropyl-β-d-thiogalactopyranoside to exponentially growing bacteria. After 3 h of incubation, bacteria were harvested by centrifugation. The pellet was resuspended in buffer A (50 mM phosphate, 300 mM NaCl). The bacteria were subsequently lysed by repeated cycles of freeze-thawing. The lysate was then centrifuged at 29,000 × g for 30 min. The supernatant was mixed with 2 ml of Ni nitrilotriacetic acid-Sepharose (Qiagen, GmbH, Hilden, Germany) and incubated with rotation for 1 h. The Sepharose was loaded into a column and washed with the following combinations of buffer A: 10 ml of buffer A with 0.1% (vol/vol) Triton X-100, 10 ml of buffer A alone, 5 ml of buffer A with 1 M NaCl, 5 ml of buffer A alone, 10 ml of 20% ethanol, 10 ml of buffer A containing 5 mM imidazole, and 10 ml of buffer A containing 30 mM imidazole. The protein was eluted with buffer A containing 500 mM imidazole. The identity of D5 was verified by Western blot analysis and amino-terminal sequencing (data not shown).
BK assays.
BK contents were determined with an enzyme-linked immunosorbent assay (ELISA) (MARKIT-M-Bradykinin; Dainippon Pharmaceutical Co., Ltd., Osaka, Japan). BK was also measured indirectly with a bioassay (4) or by analysis of HK degradation products by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting.
ELISA.
BK in a sample and peroxidase-labeled BK were allowed to react competitively with anti-BK antibodies (rabbit) captured in microstrip wells coated with goat anti-rabbit immunoglobulin G (IgG) antibodies. The BK concentration was determined from the enzyme activity of peroxidase-labeled BK bound to the anti-BK-antibodies (detection limit, 4.9 pg/well). A standard curve was prepared with five BK concentrations (4.9, 19.6, 78, 313, and 1,250 pg/well). Samples were analyzed in duplicate, and these results are expressed as picomolar concentrations.
Cellular assay.
CHO-K1 cells overexpressing B2 receptor (kindly provided by A. Breit, Institute for Physiological Chemistry, University Mainz, Mainz, Germany) were grown to confluence in 24-well plates and subsequently labeled with myo-[2-3H]inositol with a 1-μCi/ml concentration of buffer (116 mM NaCl, 5.3 mM KCl, 0.81 mM MgSO4 · 7H2O, 1 mM CaCl2, 5 mM α-glucose, 20 mM HEPES, 1× minimum essential medium amino acids [pH 7.4]) for 20 h. Cells were then incubated with 10 mM LiCl at 37°C for 15 min and subsequently stimulated with BK standard solutions (Dainippon Pharmaceutical Co., Ltd., Osaka, Japan) or supernatants from bacterium-plasma incubations for 10 min. In experiments that included the B2 receptor inhibitor HOE 140 (a kind gift from Hoechst, Frankfurt, Germany), the cells were preincubated with HOE 140 (final concentration, 8 μM) for 10 min before LiCl was added. The reaction was stopped by aspiration of the medium followed by the addition of 1 ml of ice-cold 10 mM formic acid (pH 3). After incubation at 4°C for 2 h, the supernatants were added to 3 ml of 50 mM ammonium solution (pH >9). The suspensions were applied to anion-exchange columns prepared from 0.3 to 0.5 g of Dowex AG:1–8x, in which the hydroxide form had been exchanged to the formate form as described by the manufacturer (Bio-Rad). Columns were eluted with 10 ml of H2O to remove free inositol, followed by elution with 2× 2 ml of 2 M ammonium formate acid (pH 5.2) to elute the total amount of inositol phosphate. The radioactivity present in the eluates was quantitated in a liquid scintillation counter (LS 6000 TA; Beckman) and is expressed as disintegrations per minute.
Electrophoresis and Western blot analysis.
SDS-PAGE was performed as described by Neville et al. (20). Proteins in the supernatants from bacterium-plasma incubations were separated on gels of 10% total acrylamide with 3% bisacrylamide. Plasma samples diluted 1/300, untreated or treated with kaolin (Diagnostica Stago, Asmiers, France) for 1 min, served as controls. Before loading, samples were boiled in sample buffer containing 2% SDS and 5% β-mercaptoethanol for 10 min. For Western blot analyses, proteins were transferred to nitrocellulose membranes (Immobilon; Millipore), as described previously (30), with a Trans Blot semidry transfer cell (Bio-Rad). Subsequently, the polyvinylidene difluoride membranes were blocked in phosphate-buffered saline–Tween (PBST) containing 5% (wt/vol) nonfat dry milk at 37°C for 20 min, washed three times with PBST for 5 min, and incubated with sheep antibodies against HK and its degradation products (1:6,000 in the blocking buffer) at 37°C for 30 min (29). After being washed, the sheets were incubated with peroxidase-conjugated secondary donkey antibodies against goat IgG at 37°C for 30 min. Secondary antibodies were detected by the chemiluminescence method (19). Autoradiography was performed at room temperature for 1 to 2 min with Kodak X-Omat S films and Cronex Xtra Plus intensifying screens.
RESULTS
BK in plasma from patients with S. aureus and S. pneumoniae sepsis.
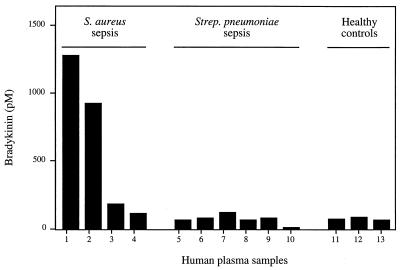
Plasma samples from patients with suspected sepsis were collected at admittance to the hospital in order to investigate whether the BK level in plasma was elevated compared to those in plasma from healthy individuals. Samples from four patients with S. aureus sepsis (patients1 to 4) and six patients with S. pneumoniae sepsis (patients 5 to 10) were tested for BK content by ELISA (Fig. 1). Three healthy volunteers served as controls (patients 11 to 13). Patient 1 was undergoing treatment with an angiotensin-converting enzyme (ACE) inhibitor (captopril) at admittance to the hospital. He was in septic shock when the plasma sample was collected and subsequently developed severe organ failure. Patient 2 had multiple abscesses in the gluteal region, but was not in shock. BK levels in the plasma of these patients were 1,272 and 921 pM, respectively. The other two patients, patients 3 and 4, with S. aureus sepsis showed slightly elevated BK levels (178 and 108 pM, respectively) compared to the controls (mean value, 69 ± 9 pM). In one of the S. pneumoniae sepsis patients, patient 7, a minor increase in plasma BK (115 pM) was observed. The patients with S. pneumoniae sepsis were in stable circulatory condition, except for patient 5, who was in septic shock at admittance to the hospital. Despite being in septic shock, patient 5 showed no elevated level of BK.
FIG. 1.
BK levels in human plasma samples. Samples 1 to 4 are from patients with S. aureus sepsis, 5 to 10 are from patients with S. pneumoniae sepsis, and 11 to 13 are from healthy controls. The BK levels were determined with an ELISA. Values are means of two different determinations. The variation was <15%.
Degradation in vitro of HK bound to S. aureus following plasma absorption.
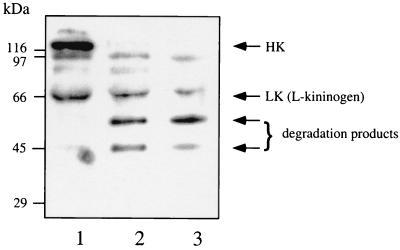
BK is released by proteolytic cleavage of kininogens. To analyze in vitro whether kininogens associated with the surface of S. aureus are also degraded, S. aureus 5120 bacteria were incubated with plasma for 15 min, washed, and resuspended in buffer. After an additional incubation of 15 min, the bacteria were spun down, and the proteins in the supernatant were separated by SDS-PAGE, transferred to nitrocellulose, and immunostained with antibodies against HK and its degradation products. Plasma alone or plasma treated with kaolin served as controls. There are two molecular mass forms of kininogens in plasma, which result from differential splicing of a single gene transcript. Antibodies to HK will therefore also identify the low-molecular-mass form, l-kininogen (Fig. 2, lane 1). Kaolin activates the contact system, and in kaolin-treated plasma, HK (120 kDa) was cleaved to degradation products with molecular masses of 55 and 46 kDa, respectively (Fig. 2, lane 2). The same degradation pattern, typical of BK release, was also obtained following incubation of S. aureus with plasma (Fig. 2, lane 3), indicating that the absorbed HK is cleaved to release BK (plasma kallikrein does not cleave l-kininogen). In contrast, and as previously reported (3), HK does not interact with S. pneumoniae (data not shown).
FIG. 2.
Cleavage of HK at the surface of S. aureus. S. aureus (1010 CFU/ml) was incubated with human plasma (final concentration, 50%) for 15 min. Subsequently, bacteria were washed, resuspended in buffer, incubated for another 15 min, and spun down. The resulting supernatant and plasma (nontreated or kaolin treated) were then subjected to SDS-PAGE (10% polyacrylamide gel), and the separated proteins were transferred to Immobilon filters and probed with antibodies to HK. Lanes: 1, normal plasma; 2, kaolin-treated plasma; 3, proteins absorbed from plasma by S. aureus.
S. aureus induces BK release in vitro.
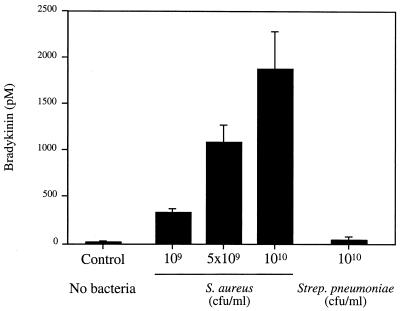
To investigate more directly whether BK release was induced by S. aureus following plasma incubation, different numbers of S. aureus 5120 and S. pneumoniae B1508 bacteria, isolated from patients with septic shock (patients 1 and 5, respectively, in Fig.1) were incubated for 15 min with a constant volume of 50% human plasma. Following washing, the bacteria were resuspended and incubated with buffer. After an additional incubation of 15 min, the release of BK was quantitated by ELISA. Figure 3 shows that BK release was induced by S. aureus strain 5120 in a concentration-dependent manner. When the washing of the bacteria following plasma incubation was excluded, the amounts of BK increased more than 10-fold. Similar results were obtained with other isolates of S. aureus (data not shown). S. pneumoniae B1508 and other clinical and laboratory strains of S. pneumoniae did not induce BK release in this assay. Also when a constant number of S. aureus 5120 bacteria was preincubated with different dilutions of plasma, BK was induced in a concentration-dependent fashion (Table 1). As shown in Table 1, an initial incubation with plasma diluted to 6% was sufficient to load the bacteria with contact factors resulting in a significant BK release.
FIG. 3.
S. aureus induces BK release in human plasma. Various numbers of S. aureus or S. pneumoniae bacteria were incubated with human plasma (final concentration of 50%) for 15 min. Bacteria were washed and resuspended in buffer. Following another incubation period of 15 min, bacteria were pelleted, and the BK content of the resulting supernatants was determined with an ELISA. Plasma alone was the negative control. Values are means ± standard deviations (n = 3).
TABLE 1.
Induction of BK release by S. aureus (1010 CFU/ml) in various dilutions of human plasma
| Plasma concn (%) | BK concn (pM)a |
|---|---|
| 3 | 100 ± 30 |
| 6 | 421 ± 195 |
| 12 | 1,859 ± 731 |
| 25 | 3,408 ± 576 |
| 50 | 4,200 ± 602 |
BK concentration was determined with an ELISA. Values are means ± standard deviations (n = 3).
Kinetics of S. aureus-induced BK release
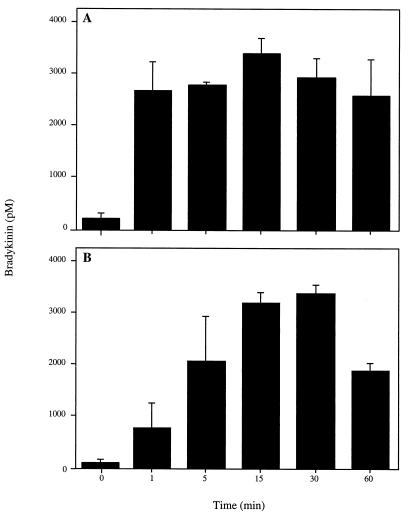
To study the kinetics of contact system assembly, 1010 CFU/ml were preincubated with 50% plasma (final concentration) for different time periods. Following washing and 15 min of incubation in buffer, bacteria were spun down, and the amount of BK in the supernatants was determined. The results demonstrate very rapid assembly at the surface of S. aureus. Already after 1 min of preincubation with plasma, the level of BK released reached a plateau (Fig. 4A). In a second set of experiments, plasma and bacteria were incubated for 15 min and washed. Bacteria were then left in buffer for different periods of time, before the supernatants were collected and analyzed. In this case, the concentration of BK peaked at 15 to 30 min. (Fig. 4B). The data show that the contact system following a rapid initial assembly is activated at the bacterial surface over a sustained time period.
FIG. 4.
Kinetics of BK release induced by S. aureus in human plasma. (A) Bacteria were incubated (1010 CFU/ml) with 50% human plasma for different time periods. After washing, bacteria were resuspended in buffer and incubated for 15 min. (B) Bacteria (1010 CFU/ml) were incubated with 50% plasma for 15 min, washed, resuspended in buffer, and incubated for different time periods. In both panels A and B, bacteria were spun down and the BK content in supernatants was measured with an ELISA. Values are means ± standard deviations (n = 3).
BK released in plasma following incubation with S. aureus is biologically active.
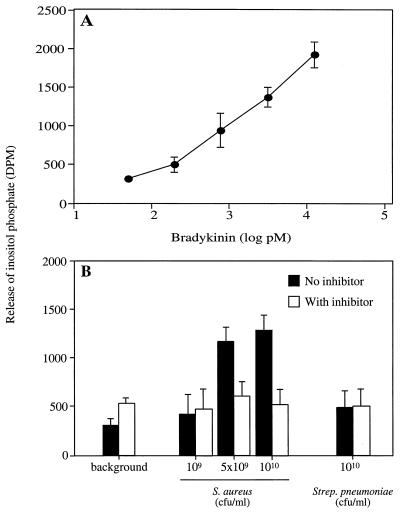
CHO-K1 cells overexpressing BK B2 receptors were used to analyze whether the BK released as a result of incubation of S. aureus with plasma was functional. In this assay, binding of BK to the B2 receptors induces the release of inositol phosphate (4), and Fig. 5A shows a typical standard curve obtained when different amounts of BK were added to the transfected cells. In Fig. 5B, bacteria were preincubated with plasma, and the effect of bacterially absorbed plasma proteins on the release of inositol phosphate by the CHO-K1 cells was measured in the presence or absence of the B2 receptor inhibitor HOE 140. In the presence of HOE 140, none of the samples induced inositol phosphate release above the background level, whereas in the absence of HOE 140, S. aureus, but not S. pneumoniae, caused a significantly increased release. In contrast, when nontransfected CHO-K1 cells were used in identical experiments, the S. aureus samples induced inositol phosphate release at the background level only (data not shown). In summary, the results show that BK released at the surface of S. aureus following plasma incubation is biologically active.
FIG. 5.
Incubation of S. aureus in plasma induces the release of functional BK. (A) Different amounts of BK were added to CHO-K1 cells, and the release of 3H-labeled inositol phosphate was expressed as disintegrations per minute (DPM). (B) Samples from preincubations of plasma with buffer (1:1 [background]) or from preincubations of the indicated numbers of bacteria with plasma (final concentration of 50%) were added to CHO-K1 cells in the presence or absence of the BK B2 receptor inhibitor HOE 140 (8 μM). The release of inositol phosphate was measured. Values are means ± standard deviations (n = 3).
BK release in contact factor-depleted plasma.
The involvement of different contact factors in S. aureus-induced BK release was investigated with plasma depleted of contact factors. In contrast to normal plasma, S. aureus preincubated with plasma lacking FXII, PK, or HK did not trigger the release of BK (Table 2). FXI activation occurs after the activation of FXII and PK, and preincubation with FXI-depleted plasma did result in BK release. The reason why this release was higher than with normal plasma is unclear. The amount of BK induced by preincubation with fibrinogen-depleted plasma was the same as that for normal plasma.
TABLE 2.
Induction of BK release by S. aureus (1010 CFU/ml) in protein-deficient plasma
| Plasma | BK concn (pM)a | % of control concn |
|---|---|---|
| Normal (control) | 2,272 ± 739 | 100 |
| FXII depleted | 114 ± 52 | 5 |
| PK depleted | 68 ± 40 | 3 |
| HK depleted | 136 ± 48 | 6 |
| FXI depleted | 4,589 ± 737 | 202 |
| Fibrinogen depleted | 2,022 ± 638 | 89 |
BK content of the supernatants was measured with an ELISA. Values are means ± standard deviations (n = 3).
Inhibition of S. aureus-induced BK release.
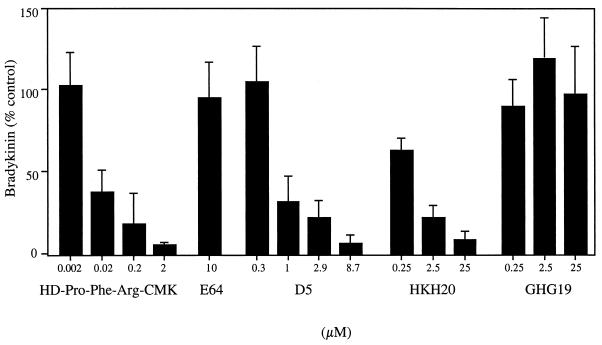
In the activation of the contact system, FXII activation is an early and crucial step. An inhibitor (HD-Pro-Phe-Arg-CMK) of the serine proteinases FXII and PK was therefore added to the mixture of plasma and bacteria. This reagent efficiently blocked the induction of BK in a concentration-dependent manner, whereas the negative control, the specific cysteine proteinase inhibitor E64, did not influence BK generation (Fig. 6). The nonenzymatic cofactor HK is composed of a heavy chain and a light chain, including domains D1 to D4 and D5 and D6, respectively. Domain D5 contains binding sites for zinc and for cellular and negatively charged surfaces (8, 10). D5 and its peptide fragments HKH20 (comprising the cell binding region) and GHG19 (the zinc binding region) were separately incubated together with S. aureus and human plasma. D5 and HKH20 both inhibited BK release in a concentration-dependent manner, whereas GHG19 had no effect (Fig. 6). The results suggest that the inhibitory effect of D5 and HKH20 is due to competition with HK binding to the bacterial surface.
FIG. 6.
Inhibition of S. aureus-induced BK release. S. aureus bacteria (1010 CFU/ml) were incubated for 15 min with plasma in the absence (100% of control) or presence of a peptide inhibitor of FXII-PK (HD-Pro-Phe-Arg-CMK), domain D5 of HK, or peptides derived from D5 (HKH20 and GHG19). E64, a specific cysteine proteinase inhibitor, served as a negative control. After washing and incubation with buffer (15 min), the bacteria were spun down, and the BK content in the supernatant was determined. Values are means ± standard deviations (n = 3).
DISCUSSION
Several previous investigations have demonstrated that activation of the contact system contributes to the pathophysiology of sepsis (24). It has also been shown that staphylococcal cell wall preparations in a mixture of purified FXII, PK, and HK activate PK into kallikrein, whereas intact S. aureus bacteria did not have this effect (15). However, with a different approach, more recent studies have established that the contact system can be assembled and activated at the surface of significant human pathogens, such as E. coli, Salmonella, and Streptococcus pyogenes (2, 3,13). The present investigation also shows that intact S. aureus can induce BK release in the plasma environment in vitro in a time- and concentration-dependent manner. In contrast, BK release was not induced by S. pneumoniae in vitro. Because of the short half-life of BK (see below), it is not possible to exclude that BK is released in vivo during S. pneumoniae sepsis. Sepsis is a multifactorial condition, and other mechanisms are probably more important in S. pneumoniae-caused sepsis.
Most infections with S. aureus and other bacteria inducing BK release are uncomplicated, suggesting that the release of BK normally is local and without systemic effects. A local release of BK leading to increased vascular permeability and leakage of plasma into the site of infection will provide growing bacteria with nutrients and should also promote the dissemination of the infection. Host-microbe relationships are generally well balanced and based on a multitude of molecular interactions. A given mechanism can sometimes appear advantageous to both parts, and the increase in vascular permeability by BK could be beneficial to the host as well by facilitating the recruitment of granulocytes to the infectious focus.
The half-life of BK is short (measured in seconds) in vivo, and the bacterial concentrations in the blood during sepsis are far from those of the in vitro experiment performed in this study. Still, two out of four patients with S. aureus sepsis had significantly increased levels of plasma BK compared to healthy controls or patients with S. pneumoniae sepsis. BK is degraded by kininases such as ACE, and it is interesting that patient 1 (Fig. 1), who developed a severe septic shock with multiorgan dysfunction, was being treated with captopril, an ACE inhibitor, when admitted to the hospital. The other patient with high levels of plasma BK (patient 2, Fig. 1) had multiple abscesses in the gluteal region, and samples from the abscesses also showed high BK concentrations (600 pM). The notion that local release of BK could promote spreading of an infection is supported by a recent publication that shows that in experimental infections in mice, BK release at the focus of Vibrio vulnificus infections facilitated intravascular dissemination (17). The aim of analyzing BK in patient plasma was to investigate whether BK levels were increased in sepsis patients, since earlier studies mainly have analyzed the consumption of contact factors. In order to draw any conclusions, plasma samples from a larger group of patients must be collected, and a series of plasma samples taken at different time points from the same patient should be analyzed.
In Salmonella and E. coli surface-associated fibrous proteins, thin aggregative fimbriae (7) and curli (22), respectively, are responsible for the assembly and activation of the contact system (3, 13), whereas in Streptococcus pyogenes, the M proteins, a family of α-helical, coiled-coil, fibrous surface proteins (23), play a similar role (1, 2). Current studies in our laboratory suggest that in the case of S. aureus, surface proteins do not seem to contribute to the interactions with contact factors. Thus, the release of BK in plasma was rather higher when surface protein-rich strains of S. aureus were pretreated with proteolytic enzymes (E. Mattsson et al., unpublished observations). It could be that surface proteins of S. aureus, in contrast to those of Salmonella, E. coli, and Streptococcus pyogenes, do not provide the negatively charged surfaces that are required for interactions with contact factors, especially HK and FXII, but rather mask other structures interacting with the contact system. For instance, S. aureus has a high negative net surface charge related to teichoic and lipoteichoic acid (31). The focus of ongoing investigations is to reveal the nature of the surface structure or structures of S. aureus responsible for the activation of the contact system.
The experiments performed with plasma deficient in various contact-phase proteins show that the BK release induced by S. aureus results from activation of the contact system (Table 2) and not from a direct cleavage of HK by staphylococcal proteinase V8 (18). Previous studies have demonstrated that consumption of FXII is a bad prognostic sign in sepsis (14, 21, 27, 28), and in a baboon model of E. coli sepsis, antibodies to FXII inhibited irreversible hypotension and prolonged survival (25). A major finding of this work is that an FXII inhibitor and fragments of HK block the generation of BK. The inhibition of BK release by the D5 and HKH20 peptides is probably due to competition at the bacterial surface with HK-binding structures. Such inhibitors and inhibitors of FXII could hypothetically be used to treat infections in which the contact system is activated.
ACKNOWLEDGMENTS
This work was supported by the Swedish Medical Research Council (grant 7480); the foundations of Lars Hierta, Kock, Knut, and Alice Wallenberg; the foundation of Österlund; the Göran Gustafsson Foundation for Research in Natural Sciences and Medicine; and the Medical Faculty, Lund University.
REFERENCES
- 1.Ben Nasr A, Herwald H, Muller-Esterl W, Björck L. Human kininogens interact with M protein, a bacterial surface protein and virulence determinant. Biochem J. 1995;305:173–180. doi: 10.1042/bj3050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ben Nasr A, Herwald H, Sjöbring U, Renne T, Muller-Esterl W, Björck L. Absorption of kininogen from human plasma by Streptococcus pyogenes is followed by the release of bradykinin. Biochem J. 1997;326:657–660. [PMC free article] [PubMed] [Google Scholar]
- 3.Ben Nasr A, Olsen A, Sjöbring U, Muller-Esterl W, Björck L. Assembly of human contact phase proteins and release of bradykinin at the surface of curli-expressing Escherichia coli. Mol Microbiol. 1996;20:927–935. doi: 10.1111/j.1365-2958.1996.tb02534.x. [DOI] [PubMed] [Google Scholar]
- 4.Berridge M J, Dawson R C, Downes C P, Heslop J P, Irvine R F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983;212:473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bone R C. How Gram-positive organisms cause sepsis. J Crit Care. 1993;8:51–59. doi: 10.1016/0883-9441(93)90033-h. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J, Abraham E. Microbiologic findings and correlations with serum tumor necrosis factor-α in patients with severe sepsis and septic shock. J Infect Dis. 1999;180:116–121. doi: 10.1086/314839. [DOI] [PubMed] [Google Scholar]
- 7.Collinson S K, Emödy L, Muller K-H, Trust T J, Kay W W. Purification and characterization of thin, aggregative fimbriae from Salmonella enteritidis. J Bacteriol. 1991;173:4773–4781. doi: 10.1128/jb.173.15.4773-4781.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeLa Cadena R A, Colman R W. The sequence HGLGHGHEQQ-HGLGHGH in the light chain of high molecular weight kininogen serves as a primary structural feature for zinc-dependent binding to an anionic surface. Protein Sci. 1992;1:151–160. doi: 10.1002/pro.5560010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glauser M P, Zanetti G, Baumgartner J D, Cohen J. Septic shock: pathogenesis. Lancet. 1991;338:732–736. doi: 10.1016/0140-6736(91)91452-z. [DOI] [PubMed] [Google Scholar]
- 10.Hasan A H, Cines D B, Herwald H, Schmaier A H, Muller-Esterl W. Mapping the cell binding site on high molecular weight kininogen domain 5. J Biol Chem. 1995;270:19256–19261. doi: 10.1074/jbc.270.33.19256. [DOI] [PubMed] [Google Scholar]
- 11.Herwald H, Collin M, Muller-Esterl W, Björck L. Streptococcal cysteine proteinase releases kinins: a novel virulence mechanism. J Exp Med. 1996;184:665–673. doi: 10.1084/jem.184.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herwald H, Hasan AH, Godovac-Zimmermann J, Schmaier A H, Muller-Esterl W. Identification of an endothelial cell binding site on kininogen domain D3. J Biol Chem. 1995;270:14634–14642. [PubMed] [Google Scholar]
- 13.Herwald H, Mörgelin M, Olsen A, Rehn M, Dahlbäck B, Muller-Esterl W, Björck L. Activation of the contact-phase system on bacterial surfaces—a clue to serious complications in infectious diseases. Nat Med. 1998;4:298–302. doi: 10.1038/nm0398-298. [DOI] [PubMed] [Google Scholar]
- 14.Kalter E S, Daha M R, ten Cate J W, Verhoef J, Bouma B. Activation and inhibition of Hageman factor-dependent pathways and the complement system in uncomplicated bacteremia or bacterial shock. J Infect Dis. 1985;151:1019–1027. doi: 10.1093/infdis/151.6.1019. [DOI] [PubMed] [Google Scholar]
- 15.Kalter E S, van Dijk W, Timmerman A, Verhoef J, Bouma B. Activation of purified human plasma prekallikrein triggered by cell wall fractions of Escherichia coli and Staphylococcus aureus. J Infect Dis. 1983;148:682–691. doi: 10.1093/infdis/148.4.682. [DOI] [PubMed] [Google Scholar]
- 16.Kieft H, Hoepelman A, Zhou W, Rozenberg-Arska M, Struyvenberg A, Verhoef J. The sepsis syndrome in a Dutch university hospital. Arch Intern Med. 1993;153:2241–2247. [PubMed] [Google Scholar]
- 17.Maruo K, Akaike T, Ono T, Maeda H. Involvement of bradykinin generation in intravascular dissemination of Vibrio vulnificus and prevention of invasion by a bradykinin antagonist. Infect Immun. 1998;66:866–869. doi: 10.1128/iai.66.2.866-869.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Molla A, Yamamoto T, Akaike T, Miyoshi S, Maeda H. Activation of Hageman factor and prekallikrein and generation of kinin by various microbial proteinases. J Biol Chem. 1989;264:10589–10594. [PubMed] [Google Scholar]
- 19.Nesbitt S A, Horton M A. A non-radioactive biochemical characterization of membrane proteins using enhanced chemiluminescence. Anal Biochem. 1992;206:267–272. doi: 10.1016/0003-2697(92)90365-e. [DOI] [PubMed] [Google Scholar]
- 20.Neville D M J. Molecular weight determination of protein-dodecyl sulphate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971;246:6328–6334. [PubMed] [Google Scholar]
- 21.O'Donell T F, Cloves G H, Talamo R C, Colman R W. Kinin activation in the blood of patients with sepsis. Surg Gynecol Obstet. 1976;143:539–545. [PubMed] [Google Scholar]
- 22.Olsen A, Jonson A, Normark S. Fibronectin binding mediated by a novel class of surface organelles on Escherichia coli. Nature. 1989;338:652–655. doi: 10.1038/338652a0. [DOI] [PubMed] [Google Scholar]
- 23.Phillips G N, Flicker P F, Cohen C, Manjula B N, Fischetti V A. Streptococcal M protein: alpha-helical coiled structure and arrangement on the cell surface. Proc Natl Acad Sci USA. 1981;78:4689–4693. doi: 10.1073/pnas.78.8.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pixley R A, Colman R W. The Kallikrein-kinin system in sepsis syndrome. In: Farmer SG, editor. Handbook of immunopharmacology—the kinin system. New York, N.Y: Academic Press; 1997. pp. 173–186. [Google Scholar]
- 25.Pixley R A, DeLa Cadena R, Page J, Kaufman N, Wyshock E, Chang A, Taylor F, Colman R. The contact system contributes to hypotension but not disseminated intravascular coagulation in lethal bacteremia. J Clin Investig. 1993;91:61–68. doi: 10.1172/JCI116201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pixley R A, DeLa Cadena R, Page J, Kaufman N, Wyshock E, Colman R, Chang A, Taylor F. Activation of the contact system in lethal hypotensive bacteremia in a baboon model. Am J Pathol. 1992;140:897–906. [PMC free article] [PubMed] [Google Scholar]
- 27.Pixley R A, Zellis S, Bankes P, DeLa Cadena R, Page J, Scott C, Kappelmayer J, Wyshock E, Kelly J, Colman R. Prognostic value of assessing contact system activation and factor V in systemic inflammatory response syndrome. Crit Care Med. 1995;23:41–51. doi: 10.1097/00003246-199501000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Smit-Erichsen N, Aasen A O, Gallimore M J, Amundsen E. Studies of components of the coagulation systems in normal individuals and septic shock patients. Circ Shock. 1982;9:491–497. [PubMed] [Google Scholar]
- 29.Timmons T M, Dunbar S D. Protein blotting and immunodetection. Methods Enzymol. 1990;182:679–688. doi: 10.1016/0076-6879(90)82053-5. [DOI] [PubMed] [Google Scholar]
- 30.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wadström T. Hydrophobic characteristics of staphylococci: role of surface structures and role in adhesion and host colonization. In: Doyle R J, Rosenberg M, editors. Microbial cell surface hydrophobicity. Washington, D.C.: American Society for Microbiology; 1990. pp. 315–333. [Google Scholar]