Abstract
Global interest in the non‐intoxicating cannabis constituent, cannabidiol (CBD), is increasing with claims of therapeutic effects across a diversity of health conditions. At present, there is sufficient clinical trial evidence to support the use of high oral doses of CBD (e.g., 10–50 mg/kg) in treating intractable childhood epilepsies. However, a question remains as to whether “low‐dose” CBD products confer any therapeutic benefits. This is an important question to answer, as low‐dose CBD products are widely available in many countries, often as nutraceutical formulations. The present review therefore evaluated the efficacy and safety of low oral doses of CBD. The review includes interventional studies that measured the clinical efficacy in any health condition and/or safety and tolerability of oral CBD dosed at less than or equal to 400 mg per day in adult populations (i.e., ≥18 years of age). Studies were excluded if the product administered had a Δ9‐tetrahydrocannabinol content greater than 2.0%. Therapeutic benefits of CBD became more clearly evident at doses greater than or equal to 300 mg. Increased dosing from 60 to 400 mg/day did not appear to be associated with an increased frequency of adverse effects. At doses of 300–400 mg, there is evidence of efficacy with respect to reduced anxiety, as well as anti‐addiction effects in drug‐dependent individuals. More marginal and less consistent therapeutic effects on insomnia, neurological disorders, and chronic pain were also apparent. Larger more robust clinical trials are needed to confirm the therapeutic potential of lower (i.e., <300 mg/day) oral doses of CBD.
INTRODUCTION
Cannabidiol
The Cannabis sativa plant has been used with therapeutic intent since antiquity and recent times have seen a significant resurgence in the use of cannabis and its constituents as medicines. Amidst this renaissance, there have been significant advances in the scientific evidence base supporting the use of cannabis‐derived products in treating epilepsy, chronic pain, and various neurological and mental health conditions. 1 , 2 , 3 , 4 The medical profession remains cautious about medicinal cannabis, reflecting traditional concerns around the adverse effects of recreational cannabis use and an evidence base around medicinal cannabis that is still evolving. 5 , 6 , 7
Cannabis plant material contains a complex mixture of approximately 140 different compounds termed cannabinoids. Of these, only two have been well‐studied scientifically: Δ9 ‐tetrahydrocannabinol (THC), which is responsible for the distinctive intoxicated state associated with cannabis consumption; and cannabidiol (CBD), a non‐intoxicating component that has a range of therapeutic properties. Unlike THC, CBD does not act as a cannabinoid receptor type 1 (CB1R) agonist, thus explaining the absence of intoxication with its use. 8 , 9 Rather, CBD has a diverse suite of pharmacological actions, including effects on 5‐HT1A, GABAA, PPAR, and TRPV1 receptors, and a purported ability to increase endocannabinoid tone via the inhibition of anandamide breakdown. 8 , 10
Different cannabis cultivars contain varying ratios of THC and CBD: cannabis grown for non‐medical (“recreational”) purposes tends to have very high concentrations of THC and negligible CBD content, whereas “hemp” strains that are primarily grown for seed and fiber tend to be enriched in CBD but with very low THC concentrations. With such minimal presence of THC, hemp‐derived products can be consumed with little risk of intoxication but with the possibility of beneficial therapeutic effects.
An array of preclinical and clinical studies have indicated analgesic, anti‐inflammatory, anxiolytic, antipsychotic, and anticonvulsant effects of CBD. 1 , 2 , 3 , 4 , 11 However, there is only strong evidence for therapeutic efficacy based on phase III randomized controlled clinical trials in which relatively high oral doses of ~600–3000 mg CBD (or 10–50 mg/kg in a 62 kg person) were used to reduce seizures in patients with Dravet syndrome, Lennox Gastaut syndrome, and tuberous sclerosis complex. 4 , 12 Indeed, CBD (Epidiolex) is now a registered medicine in many jurisdictions around the world for the treatment of the intractable epilepsies, including in the United States, Europe, and Australia. 1 , 13 Overall, CBD has an excellent safety profile, even with relatively high doses. 1 , 9 , 13 , 14 , 15 , 16 For example, in a phase I trial, CBD was well‐tolerated when administered up to 6000 mg. 17
Over‐the‐counter access to CBD products
Many countries, including the United States, Canada, the United Kingdom, Australia, and most European Union member countries, now allow consumer access to a range of CBD products without a prescription. These products can be obtained over‐the‐counter (OTC) at pharmacies, health food stores, single purpose CBD stores, or from online sources. There is a diversity of regulatory approaches for access to OTC CBD products around the world. In some countries, there is little to no regulation, whereas others have tighter regulations. For example, a tighter regulatory framework exists in Australia where OTC CBD products must be dosed at less than or equal to 150 mg CBD per day and have a total cannabinoid content greater than or equal to 98% CBD. This OTC pathway is specifically for products that treat conditions that do not require medical oversight. However, clinical evidence and a formal drug regulatory registration process are still required before these products can be sold. At the time of writing, there are still no “low‐dose” CBD products available at pharmacies in Australia.
Non‐prescription CBD products in the United States and the European Union typically involve relatively low daily doses of CBD (<1 mg/kg) obtained from products such as capsules containing 10–50 mg CBD, or from orally administered oils containing 15–240 mg/ml CBD (typically dosed with a few drops i.e., 0.1–0.5 ml per day). Recommended daily oral dosing of such products tends to be less than 100 mg CBD/day and often in the range of 10–25 mg per day, an order of magnitude lower than the doses that have demonstrated efficacy in clinical trials (i.e., 600–3000 mg). 12 This widespread availability of OTC CBD products is consistent with there being few safety concerns around this cannabinoid. For example, in 2019, the World Health Organization (WHO) proposed changes to the United Nations through its Expert Committee of Drug Dependence to exclude CBD from international drug control. This was on the basis that CBD does not intoxicate and has little potential for abuse or dependence. 18 , 19
Evidence gaps with low‐dose CBD products
Previous analyses suggest an absence of high‐quality evidence for benefits of “low dose” CBD. 4 , 12 , 16 This is surprising given the enormous worldwide use of low‐dose CBD products as health supplements, “wellness” products, and nutraceuticals, with claims that one in seven American citizens currently use CBD, and that CBD products will have a global market worth $17 billion by the year 2026. A recent study compared access to CBD products across nine different countries. 12 One concern was that the labeling of the OTC products was sometimes found to be inaccurate with regard to CBD and THC content. The analysis also highlighted the ambiguity of current legislative and regulatory frameworks covering CBD products, particularly in the United States, the United Kingdom, and the European Union. 12 Analysis of the products available OTC, primarily oils and capsules, concluded that consumers were unlikely to be using more than 150 mg CBD/day when products were dosed as recommended.
Aim of the current review
We conducted a review of the current scientific literature to evaluate the efficacy and safety of oral CBD products at lower dose ranges (≤400 mg/day). It is worth noting that a 400 mg limit, which represents ~ 6–7 mg/kg of CBD in a human of average body weight (62 kg), is still a relatively low dose given that a recent review of dosing in clinical trials suggested that evidence of therapeutic effects to‐date is primarily centered around oral CBD doses of 10–23 mg/kg. 4 , 20
METHODS
The current narrative review aims to identify all interventional studies that measured the clinical efficacy (i.e., in any health condition) and/or safety and tolerability of oral CBD dosed at less than or equal to 400 mg per day in adult populations (i.e., ≥18 years of age). Studies were excluded if the treatment had a THC content greater than 2.0%. The Australian Therapeutic Goods Administration (TGA) had originally proposed low‐dose CBD products be regulated in Australia with products containing 98% CBD and less than or equal to 2.0% of other cannabinoids including THC (percentage of total cannabinoid content). Further, the low‐dose CBD products allowed under the regulation were to be limited to a maximum oral daily dose of CBD of 60 mg. 16 We thus wished to examine interventional studies that dosed in the very low‐dose range of less than or equal to 60 mg. There is no clear consensus on what constitutes a low CBD dose range, although doses administered in clinical studies of intractable epilepsies are generally considered high doses (e.g., 620–3100 mg/day). 4 , 12 , 14 Initial analyses of low‐dose CBD products suggest that most products are administered at less than 200 mg/day, 12 , 14 and that in clinical trials doses as low as less than 62 mg/day have been administered. 4 Our cross‐country analysis of low‐dose CBD products, found that most products administered less than 150 mg/day, although there were some products available that made it conceivable that higher doses were being used. 12 We thus chose 400 mg as the upper limit of what could be considered a low dose and be plausibly administered using current OTC CBD products. Accordingly, the results are described by using five different daily dose categories: ≤60 mg, >60–100 mg (i.e., studies that dosed between 61 mg and 100 mg), >100–200 mg, >200–300 mg, and >300–400 mg.
We used a two‐pronged approach to identify relevant studies. The search, screening, and extraction of the studies was conducted by one researcher. First, all major reviews published covering the literature up to and including 2018 2 , 4 , 9 , 14 , 15 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 were systematically screened to identify eligible studies. These reviews were identified by searching the online databases Scopus and PubMed (MEDLINE) from conception to 2018 (inclusive) using the Boolean expression: (TITLE [cannabidiol] OR ABSTRACT [cannabidiol]). The search was then refined by “Document Type” (review, only), “Language” (English, only), and “Species” (human, only), if permitted by the database. This search identified a total of 64 relevant citations. Second, more recent studies were identified by searching the online databases Scopus and PubMed (MEDLINE) from 2018 until April 5, 2022, using the Boolean expression: (TITLE [cannabidiol] OR ABSTRACT [cannabidiol] AND NOT TITLE [review]). The search was then refined by “Document Type” (article, only), “Language” (English, only), and “Species” (human, only), if permitted by the database. This search identified a total of 2434 unique records. Each record was screened against the eligibility criteria, first by title and abstract, and subsequently by full text, to identify relevant studies. The study selection process is summarized in Figure 1. The two‐pronged approach was used because the post‐2018 search strategy would have retrieved a very large number of records with many ineligible for inclusion; in other words, the breadth of the review which included all interventional studies of “low‐dose” CBD, irrespective of population and treatment indication, made it difficult to devise an appropriate search strategy.
FIGURE 1.
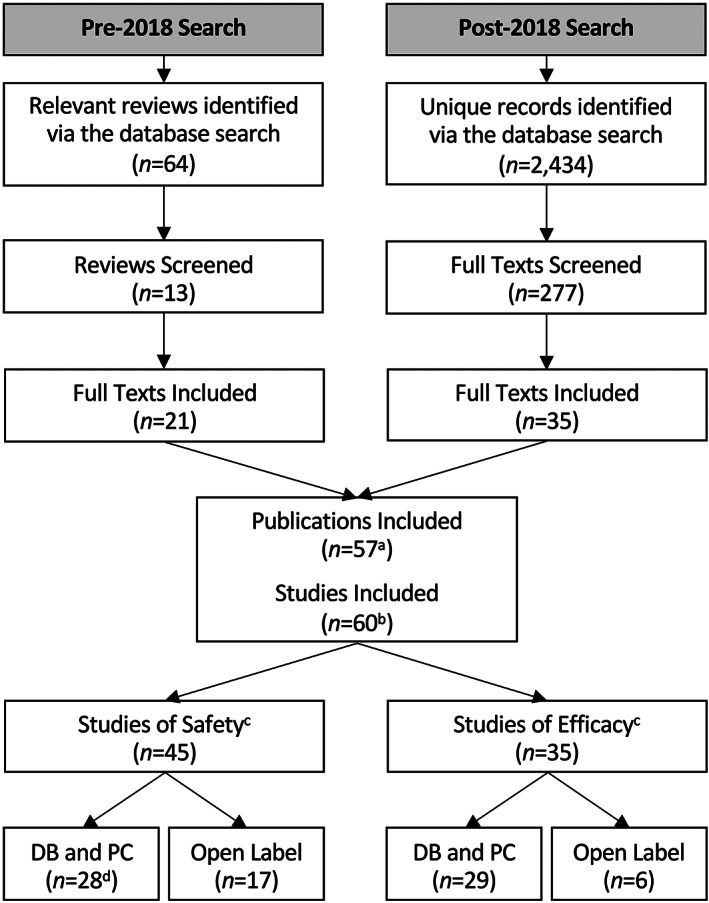
The two‐pronged study selection process. (a) Includes one publication found elsewhere; (b) one publication 29 described three eligible studies; (c) some studies (N = 14 DB and PC TRIALS and N = 6 OPEN LABEL TRIALS) reported on both safety and efficacy and are therefore included in both groups; (d) includes two studies that used “blinded” and PC designs (i.e., the type of blind employed was not specified). DB, double‐blind; PC, placebo‐controlled.
A total of 57 eligible publications describing a total of 60 interventional studies were identified (Figure 1). The characteristics and results of these studies are summarized (by dose and type) in Tables 1 and 2. Although all interventional study designs were included in the review, conclusions around efficacy were primarily based on evidence from double‐blind, placebo‐controlled trials (n = 43). In each section below, however, we also summarize evidence from relevant open‐label trials (n = 17). Evidence from these sources is given a lesser weighting in arriving at conclusions, particularly around efficacy.
TABLE 1.
Interventional studies investigating the efficacy (and safety) of low‐dose CBD
| Citation | Study design | Participant population | CBD dose (mg) | Primary outcome(s) | Effect | Side effects (n incidence) |
|---|---|---|---|---|---|---|
| Studies involving doses of ≤60 mg CBD | ||||||
| Naftali et al. 30 | DB (PC); BSD |
20 (12 M); 18–50 years Crohn's disease |
10 mg/day; 8 weeks | Crohn's disease symptoms | No effect | Did not differ from placebo |
| Notcutt et al. 31 | “N‐of‐1” DB (PC); WSD |
34 (11 M); 47 ± 10 years chronic non‐cancer pain |
≤15 mg/day; 2 × 1‐week periods | Pain | No effect | NS |
| Lopez et al. 32 | DB (PC); BSD | 65 (33 M); 18–55 years overweight healthy volunteers | 15 mg/day; 6‐weeks | Multiple metabolic, QOL, and sleep‐related outcomes | Increased HDL | Did not differ from placebo |
| Vela et al. 33 | DB (PC); BSD |
136 (48 M); ~62 years hand osteoarthritis or psoriatic arthritis |
20–30 mg/day; 12‐weeks | Pain | No effect | CBD group: ductal carcinoma (1) and lipotymia (1). Placebo group: Shoulder fracture (1) and malignant hypertension (1) |
| Hobbs et al. 34 | Open label (without controls) |
5 (2 M); 34 ± 13 years Healthy volunteers |
30 mg; SD (water soluble) | LPS‐induced inflammation in PBMCs | Positive | None reported. No effect of either CBD formulation on blood pressure. |
|
5 (2 M); 27 ± 5 years Healthy volunteers |
30 mg; SD (lipid soluble) | |||||
| Carlini and Cunha 29 (experiment 6) | DB (PC); WSD |
15 (NS) poor sleepers |
40 mg; SD | Sleep quality (subjective) | No effect | NS |
| Isenmann et al. 35 | DB (PC); WSD |
16 (NS); 24 ± 3 years Healthy volunteers |
60 mg; SD | Exercise‐induced muscle damage | Positive | NS |
| Studies involving doses of >60–100 mg CBD | ||||||
| Chagas et al. 36 | DB (PC); BSD |
21 (15 M); 64 ± 10 years Parkinson's disease |
75 mg/day; 6‐weeks | Parkinson's disease symptoms | No effect | None reported |
| Carlini and Cunha 29 (experiment 6) | DB (PC); WSD |
15 (NS) Poor sleepers |
80 mg; SD | Sleep quality (subjective) | No effect | NS |
| Zuardi et al. 37 | DB (PC); BSD |
35 (17 M); ~22 years Healthy volunteers |
100 mg; SD | Anxiety | No effect | NS |
| Studies involving doses of >100–200 mg CBD | ||||||
| Linares et al. 38 | DB (PC); BSD |
45 M; ~24 years Healthy volunteers |
150 mg; SD | Anxiety | No effect | NS |
| Crippa et al. 39 | DB (PC); BSD |
45 M; 18–35 years Healthy volunteers |
150 mg; SD (powder) | Anxiety and facial emotion recognition | No effect | NS |
| 150 mg; SD (corn oil) | ||||||
| Cochrane‐Snyman et al. 40 | DB (PC); WSD |
13 M; 22 ± 3 years Healthy volunteers |
150 mg/day; 3‐days | Exercise‐induced muscle damage | No effect | NS |
| Carlini and Cunha 29 (experiment 6) | DB (PC); WSD |
15 (NS) Poor sleepers |
160 mg; SD | Sleep quality (subjective) | Positive | NS |
| Arout et al. 41 | DB (PC); WSD |
17 (8 M); 32 ± 8 years Healthy volunteers |
200 mg; SD | Experimentally induced pain | Positive | Did not differ from placebo |
| Freeman et al. 42 | DB (PC); BSD |
59 (43 M); ~26 years Cannabis use disorder |
200 mg/day; 4‐weeks | Cannabis abstinence | No effect | Did not differ from placebo |
| Solowij et al. 43 | Open label (without controls) |
20 (16 M); ~25 years Regular cannabis users |
200 mg/day; 10‐weeks | Psychological symptoms | Positive | None reported |
| Jadoon et al. 44 | DB (PC); BSD |
13 (10 M); 57 ± 10 years Type 2 diabetes |
200 mg/day; 13‐weeks | Serum HDL and cholesterol | No effect | No SAEs |
| Studies involving doses of >200–300 mg CBD | ||||||
| de Faria et al. 45 | DB (PC); WSD |
24 (22 M); 64 ± 10 years Parkinson's disease |
300 mg; SD | Anxiety and tremors | Positive | None reported |
| Linares et al. 38 | DB (PC); BSD |
45 M; ~24 years Healthy volunteers |
300 mg; SD | Anxiety | Positive | NS |
| Zuardi et al. 37 | DB (PC); BSD |
35 (17 M); ~22 years Healthy volunteers |
300 mg; SD | Anxiety | Positive | NS |
| Zuardi et al. 46 | DB (PC); BSD |
20 (NS) Healthy volunteers |
300 mg; SD | Anxiety | Positive | NS |
| Sahinovic et al. 47 | DB (PC); WSD |
9 M; ~33 years Healthy volunteers |
300 mg; SD | Exercise physiology and enjoyment | Positive a | None reported |
| Bolsoni et al. 48 | DB (PC); BSD |
33 (8 M); ~33 years PTSD |
300 mg; SD | PTSD symptoms | No effect | NS |
| Bolsoni et al. 49 | DB (PC); BSD |
33 (8 M); ~33 years PTSD |
300 mg; SD | PTSD symptoms | Positive | NS |
| Hallak et al. 50 | DB (PC); BSD |
28 (18 M); NS Schizophrenia |
300 mg; SD | Selective attention | No effect | NS |
| Linares et al. 51 | DB (PC); WSD |
26 (12 M); 29 ± 9 years Healthy volunteers |
300 mg; SD | Sleep quality (objective) | No effect | NS |
| Arndt and de Wit 52 | DB (PC); WSD |
38 (19 M); 24 ± 4 years Healthy volunteers |
300 mg; SD | Response to negative emotional stimuli | No effect | None reported |
| de Alencar et al. 53 | DB (PC); WSD |
19 (10 M); ~63 years Essential tremor |
300 mg; SD | Upper limb tremors and motor function | No effect | None reported |
| Masataka 54 | DB (PC); BSD |
37 (26 M); 18–19 years Social anxiety disorder |
300 mg/day; 4‐weeks | Anxiety | Positive | NS |
| Crippa et al. 55 | Open label (with controls) |
118 (39 M); ~33 years Healthcare workers during COVID pandemic |
300 mg/day; 4‐weeks | Emotional exhaustion and burnout | Positive | Increased appetite on CBD (n = 5) vs. control (n = 14) |
| Yeshurun et al. 56 | Open label (historical controls) |
48 (31 M); ~56 years Patients receiving alloHCT |
300 mg/day; 37‐days | Incidence of GVHD | Positive | “CBD was well tolerated, and no SAEs were attributed to its consumption.” |
| Chagas et al. 36 | DB (PC); BSD |
21 (15 M); 64 ± 10 years Parkinson's disease |
300 mg/day; 6‐weeks | Parkinson's disease symptoms | Positive | None reported |
| de Meneses‐Gaya et al. 57 | DB (PC); BSD |
31 M; ~33 years Crack‐cocaine dependence |
300 mg/day; 10‐days | Crack‐cocaine withdrawal symptoms | No effect | Did not differ from placebo |
| Studies involving oral of >300 to 400 mg CBD | ||||||
| Pacheco, Souza et al. 58 | Open label (without controls) |
13 (6 M); 33 ± 7 years Healthcare workers during COVID pandemic |
330 mg/day; 4‐weeks | ‘Burnout’, depression, anxiety and insomnia | Positive | Nausea (1) and gastric discomfort (1) |
| Zuardi et al. 59 | Open label (without controls) |
6 (4 M); 59 ± 15 years Parkinson's disease and psychosis |
150–400 mg/day; 4‐weeks | Psychotic symptoms | Positive | None reported |
| Crippa et al. 60 | DB (PC); WSD |
10 M; 24 ± 4 years Social anxiety disorder |
400 mg; SD | Anxiety | Positive | NS |
| Crippa et al. 61 | DB (PC); BSD |
10 M; 30 ± 5 years Healthy volunteers |
400 mg; SD | Anxiety | Positive | NS |
| Bebee et al. 62 | DB (PC); BSD |
100 (56 M); ~47 years Lower back pain |
400 mg; SD | Pain | No effect | No differences in AEs: CBD (n = 35) vs. placebo (n = 42) |
| Arout et al. 41 | DB (PC); WSD |
17 (8 M); 32 ± 8 years Healthy volunteers |
400 mg; SD | Experimentally induced pain | No effect | Stomach upset on CBD (n = 4) vs. placebo (n = 1) |
| Hurd et al. 63 | DB (PC); WSD |
14 (12 M); 52 ± 8 Heroin use disorder |
400 mg/day; 3‐days | Opioid craving and anxiety | Positive | CBD group: Chest pain (1), headache (1), eye irritation (1), and mild diarrhea (2). No SAEs reported |
| Freeman et al. 42 | DB (PC); BSD |
59 (43 M); ~26 years Cannabis use disorder |
400 mg/day; 4‐weeks | Cannabis abstinence | Positive | Mild AEs: CBD (n = 96) vs. placebo (n = 65). Moderate AEs: CBD (n = 8) vs. placebo (n = 9). No SAEs reported |
Note: Those that were administered CBD only (i.e., no placebo) were considered “open label” regardless of the design used, as participants were aware that they would be receiving CBD. In the side‐effect column “None reported” refers to studies in which potential adverse events were addressed in the study but there were no adverse effects of CBD found. “Not specified” or NS, refers to studies in which potential adverse effects were not addressed by the study.
Abbreviations: AE, adverse event; alloHCT, allogeneic hematopoietic cell transplantation; BSD, between‐subjects design; CBD, cannabidiol; COVID, coronavirus disease; DB, double‐blind; GVHD, graft versus host disease; HDL, high density lipoproteins; LPS, bacterial lipopolysaccharide; M, male; NS, not specified; PBMC, peripheral blood mononuclear cells; PC, placebo‐controlled; PTSD, post‐traumatic stress disorder; QOL, quality of life; SAEs, serious adverse events; SD, single dose; WSD, within subjects design; y, years.
Statistical analyses were not performed; however, the study found sufficient evidence to suggest the effects of CBD on exercise enjoyment were worthy of further investigation.
TABLE 2.
Interventional studies investigating the safety (only) of low‐dose CBD
| Citation | Study design | Participant population | CBD dose (mg) | Primary outcome(s) | Side effects (n Incidence) |
|---|---|---|---|---|---|
| Studies involving doses of ≤60 mg CBD | |||||
| Atsmon et al. 64 | Open label (without controls) |
15 M; ~31 years Healthy volunteers |
10 mg; SD | Pharmacokinetic | Headache (reported in 14–24% participants). No SAEs reported. |
| Carlini and Cunha 29 (experiment 4) | DB (PC); BSD |
4 M; NS Healthy volunteers |
10 mg/day; 20‐days | Safety | CBD group: Somnolence (2) |
| Škopek et al. 65 | “Blind” (PC); WSD |
16 M; 23 ± 1 years Healthy volunteers |
11 mg; SD | Cognitive | None reported. No effect of CBD on simple or choice reaction time. |
| Knaub et al. 66 | Open label (without controls) |
16 (8 M); ~28 years Healthy volunteers |
25 mg; SD (2 different formulations) |
Pharmacokinetic | Diarrhea (1). No SAEs reported. |
| Bird et al. 67 | DB (PC); WSD |
161 (122 M); ~21 years Healthy volunteers |
320 μg/kg (~21 mg); SD |
Cognitive | None reported. No effect of CBD on cognitive performance. |
| Belgrave et al. 68 | DB (PC); WSD |
15 (11 M); ~21 years Healthy volunteers |
320 μg/kg (~21 mg); SD |
Cognitive | None reported. No effect of CBD on cognitive performance. |
| Williams et al. 69 | Open label (without controls) |
15 (9 M); 29 ± 11 years Healthy volunteers |
30 mg; SD (5 different formulations) |
Pharmacokinetic | None reported. No effect of the CBD formulations on heart rate variability, heart rate or blood pressure |
| Carlini and Cunha 29 (experiment 1) | Open label (without controls) |
2 (NS) Healthy volunteers |
10 mg; SD | Safety | None reported |
|
2 (NS) Healthy volunteers |
40 mg; SD | ||||
| Patrician et al. 70 | DB (PC); WSD |
12 M; 24 ± 4 years Healthy volunteers |
45 mg; SD (2 different formulations) |
Pharmacokinetic | None reported |
| Devinsky et al. 71 | Open label (without controls) |
11 (9 M); 36 ± 8 years Healthy volunteers |
50 mg; SD | Pharmacokinetic | No “treatment‐emergent” AEs reported |
| Karniol et al. 72 | DB (PC); BSD |
20 M; 21–42 years Healthy volunteers |
15 mg; SD | Subjective effects | None reported. No effect of CBD at any dose on heart rate or time estimation. Low grade psychological impact of CBD on 1 of 5 participants at both 15 and 30 mg |
| 30 mg; SD | |||||
| 60 mg; SD | |||||
| Abbotts et al. 73 | “Blind” (PC); WSD |
14 M; 20–51 years Healthy volunteers |
30 mg; (5 different formulations) |
Pharmacokinetic | None reported. No effect on HR or BP. No effect on liver enzymes. Food increased plasma CBD concentrations |
| Studies involving doses of >60–100 mg CBD | |||||
| Carlini and Cunha 29 (experiment 1) | Open label (without controls) | 2 (NS) | 80 mg; SD | Safety | None reported |
| Izgelov et al. 74 | Open label (without controls) |
12 M; 27–35 years Healthy volunteers |
90 mg; SD (3 different formulations) |
Pharmacokinetic | Somnolence (6) and abdominal pain (2) |
| Patrician et al. 70 | DB (PC); WSD |
12 M; 24 ± 4 years Healthy volunteers |
90 mg; SD (2 different formulations) |
Pharmacokinetic | None reported |
| Spindle, Cone, Goffi et al. 75 | DB (PC); WSD |
19 (8 M); 31 ± 6 years Healthy volunteers |
100 mg; SD | Pharmacokinetic and cognitive | No SAEs reported |
| Spindle et al. 76 | DB (PC); WSD |
6 (3 M); 31 ± 6 years Healthy volunteers |
100 mg; SD | Pharmacokinetic | None reported |
| Atsmon et al. 64 | Open label (without controls) |
15 M; ~31 years Healthy volunteers |
100 mg; SD | Pharmacokinetic | Headache (reported in 14–24% participants). No SAEs reported. |
| Studies involving doses of >100–200 mg CBD | |||||
| Carlini and Cunha 29 (experiment 1) | Open label (without controls) |
2 (NS) Healthy volunteers |
160 mg; SD | Safety | None reported |
| Cunha et al. 77 | DB (PC); BSD |
16 (11 M); 22–35 years Healthy volunteers |
3 mg/kg/day; 30 days (~195 mg/day a ) |
Safety | CBD group: Somnolence (2). No SAEs reported. |
| Carlini and Cunha 29 (experiment 3) | DB (PC); WSD |
10 (6 M); NS Healthy volunteers |
200 mg; SD | Cognitive | No effect of CBD on the cancellation test, differential aptitude test or finger tap test. |
| Tayo et al. 78 | Open label (without controls) |
8 (5 M); 62 ± 11 years Mild renal impairment |
200 mg; SD | Pharmacokinetic | Visual disturbance (1), nausea (1) and drowsiness (1) |
|
8 (5 M); 59 ± 12 years Moderate renal impairment |
200 mg; SD | Pharmacokinetic | None reported | ||
|
8 (3 M); 65 ± 11 years Severe renal impairment |
200 mg; SD | Pharmacokinetic | None reported | ||
|
8 (3 M); 60 ± 12 years Healthy volunteers |
200 mg; SD | Pharmacokinetic | Back pain (1) and hip pain (1) | ||
| Taylor et al. 79 | Open label (without controls) |
8 (4 M); 62 ± 11 years Mild hepatic impairment |
200 mg; SD | Pharmacokinetic | Diarrhea (3) and dizziness (1) |
|
8 (5 M); 59 ± 12 years Moderate hepatic impairment |
200 mg; SD | Pharmacokinetic | Low platelet count (1) | ||
|
8 (3 M); 65 ± 11 years Severe hepatic impairment |
200 mg; SD | Pharmacokinetic | None reported | ||
|
8 (4 M); 60 ± 12 years Healthy volunteers |
200 mg; SD | Pharmacokinetic | None reported | ||
| Studies involving doses of >200–300 mg CBD | |||||
| Crippa et al. 80 | Open label (without controls) |
120 (NS) Healthy volunteers |
300 mg; SD | Pharmacokinetic | None reported |
| Birnbaum et al. 81 | Open label (without controls) |
8 (6 M); ~49 years Localization‐related or intractable epilepsy |
300 mg; SD (fed and fasted) | Pharmacokinetic | None reported |
| Good et al. 82 | Open label (without controls) |
16 (14 M); 58 ± 12 years Advanced cancer |
Median 300 mg/day; 28 days | Tolerability | “…generally well‐tolerated, the major adverse effect being drowsiness that seemed dose‐related and improved with dose reduction” |
| Studies involving oral of >300–400 mg CBD | |||||
| Perkins et al. 83 | DB (PC); BSD |
12 (7 M); ~28 years Healthy volunteers |
5 mg/kg; SD (~310 mg/kg b ) | Pharmacokinetics | CBD group: Hunger (1), dizziness (1), headache (1) and drug eruption (1) |
| Manini et al. 84 | DB (PC); WSD |
17 (9 M); 39 ± 2 years Healthy volunteers |
400 mg; SD (with fentanyl) | Safety and pharmacokinetics of fentanyl | CBD group: Gastrointestinal discomfort (6), dizziness/drowsiness (5), itching/rash (3), headache (2). No SAEs. |
| Haney et al. 85 | DB (PC); WSD |
31 (17 M); 29 ± 9 years Regular cannabis users |
400 mg; SD (with cannabis) | Subjective and physiological effects of cannabis | CBD group: Headache (2), gastrointestinal (1) and blurred vision (1) |
Note: Those that were administered CBD only (i.e., no placebo) were considered “open label” regardless of the design used as participants were aware that they would be receiving CBD.
Abbreviations: AE, adverse event; BP, blood pressure; BSD, between‐subjects design; CBD, cannabidiol; DB, double‐blind; HR, heart rate; M, male; NS, not specified; PC, placebo‐controlled; SAEs, serious adverse events; SD, single dose; WSD, within‐subjects design.
At a reported average body weight of 65 kg.
Assuming an average body weight of 62 kg.
RESULTS
The characteristics and results of the included studies are summarized in Tables 1 and 2 and detailed below.
Studies involving oral CBD doses of less than or equal to 60 mg
Double blind, placebo‐controlled trials of efficacy
This review identified six double‐blind, placebo‐controlled trials investigating the efficacy of ≤60 mg oral CBD (Table 1).
Naftali et al. 30 did not observe a beneficial effect of 8 weeks of oral CBD treatment (10 mg/day CBD) on symptoms of Crohn's disease, as assessed via the Crohn's Disease Activity Index.
Notcutt et al. 31 conducted 34 “n of 1” double‐blind placebo‐controlled trials of patients suffering from various forms of chronic non‐cancer pain that were administered CBD. The CBD was delivered in a sublingual spray (2.5 mg/spray) that could be used up to six times per day (i.e., up to 15 mg/day) over two 1‐week intervals. CBD did not reduce pain compared to placebo – although significant improvements in self‐reported sleep quality were observed, albeit in the absence of a change in sleep duration.
Lopez et al. 32 reported that 6 weeks of oral CBD treatment (15 mg/day) increased blood concentrations of high‐density lipoproteins (HDL; sometimes known as “good cholesterol”) in overweight but otherwise healthy individuals. Self‐reported improvements in sleep quality and quantity were also measured relative to baseline within the CBD‐treated group; however, no statistically significant differences between the CBD and placebo groups were detected. CBD treatment did significantly reduce blood aspartate transaminase concentrations compared to placebo; however, the clinical significance of this is unclear. The study found that CBD was well‐tolerated with no effects on body composition, nutritional intake, various hematological and metabolic markers, perceived recovery (from exercise), executive function, heart rate variability, blood pressure, or heart rate. Rates of adverse events also did not differ between groups.
Vela et al. 33 investigated the effects of 12 weeks of oral CBD treatment (20–30 mg/day) on pain intensity in 136 individuals with hand osteoarthritis or psoriatic arthritis. CBD did not reduce pain compared to placebo or affect sleep quality or ratings of depression and anxiety. CBD was well‐tolerated with no statistically significant increase in adverse events compared to placebo.
In a small study, Carlini and Cunha 29 observed no effect of 40 mg oral CBD on self‐reported sleep duration or quality in individuals with symptoms of insomnia.
Isenmann et al. 35 investigated the effect of 60 mg oral CBD on biochemical and functional measures of exercise‐induced muscle damage in healthy, strength‐trained individuals. Blood concentrations of creatine kinase (CK) and myoglobin (Mb) increased from baseline at 24‐, 48‐, and 72‐h post‐exercise suggesting that the exercise protocol induced some degree of muscle damage; back squat performance was also reduced 24‐h post‐exercise. CBD attenuated these effects, significantly reducing blood CK and Mb concentrations, and improving back squat performance, 72‐h post‐exercise compared to placebo.
Open label trials of efficacy
Hobbs et al. 34 conducted an open label trial in which healthy participants were administered 30 mg oral CBD in two different formulations: one water soluble and the other lipid soluble. Peripheral blood mononuclear cells (PBMCs) were collected 90‐min post‐treatment, cultured, and treated with bacterial lipopolysaccharide to induce an inflammatory response. CBD significantly reduced concentrations of the inflammatory cytokine, TNF‐α, in cultured PBMCs. CBD was also well‐tolerated with no adverse events reported in the trial. CBD had no effect on blood pressure.
Interventional studies of safety (only)
Eleven additional studies investigating the safety of ≤60 mg oral CBD were identified (Table 2). Most administered a single dose of CBD to healthy participants. Consistent with a report from the TGA, the major drug regulator in Australia, 16 CBD dosed orally at this level appears safe and well‐tolerated in humans.
In an early phase I safety study, Carlini and Cunha 29 examined oral CBD (10 and 40 mg) in healthy participants (two participants per dose) and found no abnormal effects on electrocardiogram (ECG) and electroencephalogram (EEG) recordings (referred to as experiment 1). Blood and urine biochemistry tests were also normal and patients did not report any untoward effects of CBD. In an associated study (experiment 4), two healthy participants received 10 mg CBD daily for 20 days. Another participant received 10 mg CBD daily for 10 days. None of the participants reported adverse effects, although two participants complained of somnolence on days 3 and 4 which had dissipated by day 15 of dosing. No abnormal effects were observed on ECG, EEG, or blood or urine biochemistry tests.
Atsmon et al., 64 Williams et al. 69 and Knaub et al. 66 conducted open‐label pharmacokinetic investigations of different oral CBD formulations dosed at 10, 30, and 25 mg, respectively. None of these studies reported any serious adverse events. In addition, CBD did not affect heart rate, heart rate variability, or blood pressure. 69 Six of the 16 participants in one such trial 66 (37.3%) reported experiencing headache; however, without a placebo control, this is not readily attributable to CBD.
Belgrave et al. 68 and Bird et al. 67 showed, in two double‐blind, placebo‐controlled trials involving a combined total of 176 healthy participants, that ~21 mg oral CBD did not affect various performance measures, including reaction time, standing steadiness, and psychomotor function. A small placebo‐controlled study of 16 participants also found that 11 mg oral CBD did not affect performance in simple or complex reaction time tasks. 65
More recently, Patrician et al. 70 conducted a double‐blind, placebo‐controlled pharmacokinetic investigation of two oral CBD formulations dosed at 45 mg: TurboCBD (also containing 600 mg American ginseng, 240 mg ginkgo biloba, and 150 mg organic hemp oil) and CBD encapsulated in 150 mg of organic hemp oil. Neither formulation affected cognitive function, gastrointestinal function, anxiety, blood pressure, whole blood cell counts, or blood concentrations of C‐reactive protein, insulin, or glucose.
Karniol et al. 72 conducted a double‐blind placebo‐controlled trial investigating the effects of oral CBD, dosed at 15, 30, and 60 mg, on heart rate, time perception, and psychological function. None of the doses affected any of these measures, although one of the five participants in both the 15 and 30 mg CBD groups reported experiencing low‐grade psychological effects.
Devinsky et al. 71 compared the pharmacokinetics of 50 mg oral CBD to 2.1 mg CBD delivered using dry powder inhaler technology. As expected, pulmonary delivery accelerated absorption and increased bioavailability of CBD compared to oral administration. Oral CBD did not produce any adverse events, nor influence subjective sleep quality. One participant that received pulmonary CBD had liver enzyme abnormalities that were attributed to recent vigorous exercise rather than CBD.
Abbotts et al. 73 examined the pharmacokinetics of five different oral CBD formulations each dosed at 30 mg in healthy participants. The water‐soluble CBD formulations containing various excipients (sorbitol, gum arabic, or maltodextrin) attained much greater plasma concentrations of CBD and metabolites than standard formulations, such as medium chain triglyceride oil or CBD as a crystalline powder. This did not translate into any increase in liver enzymes (e.g., alanine aminotransferase), although there were some modest changes in bilirubin, albumin, and total protein. It was concluded the effects were modest and within clinical thresholds. The study also reported that food consumption increased plasma concentrations of CBD, and CBD reduced food consumption‐induced increases in plasma insulin and triglyceride concentrations. CBD did not affect heart rate or blood pressure.
Studies involving oral CBD doses of greater than 60–100 mg
Double blind, placebo‐controlled trials of efficacy
This review identified three double‐blind, placebo‐controlled trials (and no open‐label trials) investigating the efficacy of >60–100 mg oral CBD (Table 1). None of these three studies observed significant therapeutic effects.
Chagas et al. 36 examined the effects of 6 weeks of oral CBD treatment (75 mg/day) on symptoms of Parkinson's disease. This dose had no effect on motor or general symptoms, wellbeing, or quality of life. No effects were observed on blood concentrations of brain‐derived neurotrophic factor or N‐acetyl‐aspartate to creatine ratios, both markers of neuroprotective effects. There were no significant differences between placebo and CBD with regard to adverse events recorded in this trial.
Zuardi et al. 37 investigated the effects of 100 mg oral CBD on anxiety during a simulated public speaking test. This dose did not influence anxiety or blood pressure compared to placebo.
In a small study, Carlini and Cunha 29 observed no effect of 80 mg oral CBD on self‐reported sleep duration or quality in individuals with symptoms of insomnia.
Interventional studies of safety (only)
Six additional studies investigating the safety of >60–100 mg oral CBD were identified (Table 2).
Carlini and Cunha 29 examined oral CBD (80 mg) in two healthy participants and found no abnormal effects on ECG and EEG recordings nor on blood and urine biochemistry tests.
Izgelov et al. 74 conducted an open label pharmacokinetic trial of three oral CBD formulations dosed at 90 mg: one in powder form (no excipient) and the others in sesame oil and a self‐nano‐emulsifying drug delivery system (SNEDDS). Although no statistical analyses were performed, mild–moderate somnolence (3/12 participants) and mild abdominal pain (1/12 participants), was observed with both the sesame oil and SNEDDS formulation. No somnolence or abdominal pain was reported in the CBD powder group (although peak plasma CBD concentrations and overall CBD exposure were lower on this treatment).
In a double‐blind placebo‐controlled trial, Patrician et al. 70 reported that two oral CBD formulations dosed at 90 mg: TurboCBD and CBD encapsulated in organic hemp oil, did not have any adverse effects.
Spindle et al. 75 administered 100 mg oral CBD in a double‐blind placebo‐controlled trial involving infrequent cannabis users. CBD did not differ from placebo in various measures of subjective drug effects, or in cognitive performance as assessed via the digit symbol substitution, divided attention, and paced serial addition tasks. The 100 mg CBD dose did not affect heart rate or blood pressure. Spindle et al. 76 further examined the urinary pharmacokinetics of 100 mg oral CBD in six infrequent cannabis users: no adverse events were observed with the treatment.
Atsmon et al. 64 conducted an open‐label pharmacokinetic investigation of a novel oral CBD formulation dosed at 100 mg: no adverse events were observed with the treatment.
Studies involving oral CBD doses of greater than 100–200 mg
Double blind, placebo‐controlled trials of efficacy
This review identified seven double‐blind, placebo‐controlled trials investigating the efficacy of >100–200 mg oral CBD (Table 1).
Linares et al. 38 investigated the effects of 150 mg oral CBD on anxiety during a simulated public speaking anxiety test. This dose did not offer any advantage over placebo in reducing anxiety and there were no effects observed on systolic or diastolic blood pressure.
Crippa et al. 39 examined the effects of 150 mg oral CBD in two formulations; one as a powder and the other as CBD dissolved in corn oil. CBD did not affect anxiety, sedation, cognitive impairment, or discomfort scales. There was no effect on systolic or diastolic blood pressure. CBD also did not affect facial emotion recognition. The CBD in oil formulation attained much higher plasma CBD exposures than the powder formulation.
Cochrane‐Snyman et al. 40 investigated the effects of 3 days of oral CBD treatment (150 mg/day) on noninvasive measures of exercise‐induced muscle damage in healthy, untrained men. Whereas two measures: perceived muscle soreness and hanging joint angle, demonstrated time effects, suggesting that the exercise protocol induced some degree of muscle damage, CBD did not counteract these effects or influence any other parameters.
In a small study, Carlini and Cunha 29 found that 160 mg oral CBD increased self‐reported sleep duration in individuals with symptoms of insomnia. Time to sleep onset, number of sleep interruptions, and the likelihood of experiencing “good sleep” were, however, unchanged.
Arout et al. 41 investigated the effects of 200 mg oral CBD on experimental pain (cold pressor task), subjective appraisal of pain and drug liking, as well as measures of blood pressure and heart rate. It is noteworthy that the CBD formulation was the (+) isomer, not the (−) isomer which is found in the cannabis plant. CBD was found to increase pain thresholds (i.e., latency to report pain on the cold pressor task) compared to placebo suggesting an analgesic effect. However, it also yielded higher subjective ratings of painfulness. In addition, cardiovascular measures were affected: CBD decreased systolic blood pressure and increased heart rate during the cold pressor task and decreased systolic blood pressure at rest. CBD also yielded lower ratings of “good drug effect” compared to placebo. CBD did not increase the frequency of adverse events compared to placebo.
Freeman et al. 42 showed that 4 weeks of oral CBD treatment (200 mg/day) did not affect cannabis use (number of days abstinent) or urine THC‐COOH: creatinine ratios (a biomarker of cannabis use) in cannabis‐dependent individuals. No severe adverse events were reported. CBD did not increase the frequency of mild or moderate adverse events compared to placebo.
Jadoon et al. 44 examined effects of 13 weeks of oral CBD treatment (200 mg/day) in patients with type 2 diabetes. There were no effects on the primary end point: blood HDL‐cholesterol concentrations. CBD did decrease plasma concentrations of adipokine resistin and increased plasma concentrations of the gut hormone glucose‐dependent insulinotropic peptide. However, these effects did not improve glycemic control. CBD did not affect blood pressure, heart rate, or depressive symptoms assessed by the Beck Depression Inventory (BDI). Adverse events did not differ between groups.
Open label trials of efficacy
Solowij et al. 43 conducted an open‐label trial examining the effects of 10 weeks of oral CBD treatment (200 mg/day) in regular cannabis users who continued to use cannabis (Table 1). CBD was found to reduce the subjective intoxicating effects of cannabis as well as depression and psychosis‐related symptoms compared to baseline. It also improved attention, verbal learning, and memory function, although the lack of a placebo control group means that such results must be treated with caution. CBD was well‐tolerated with no adverse effects reported.
Interventional studies of safety (only)
Five additional studies investigating the safety of >100–200 mg oral CBD were identified (Table 2). However, one involved only two participants and will not be discussed further (Table 2). 29
Cunha et al. 77 investigated the effects of 30 days of oral CBD treatment (~195 mg/day) in a double‐blind placebo‐controlled trial. CBD did not elicit any psychoactive effects. Two of the eight participants receiving CBD reported experiencing somnolence; however, the number experiencing somnolence on the placebo was not reported. No differences between placebo and CBD were observed during neurological and clinical examinations, EEG and ECG recordings, and on blood and urine biochemical tests (e.g., hematocrit, blood counts, bilirubin, osmolarity, pH, etc.).
In a second double‐blind placebo‐controlled trial, Carlini and Cunha 29 found that 200 mg of oral CBD did not affect cognitive performance as assessed via the cancellation, differential aptitude, and finger tap tasks.
Tayo 78 and Taylor et al. 79 conducted open label pharmacokinetic investigations of 200 mg oral CBD (Epidiolex) on patients with mild to severe renal impairment or hepatic impairment. Renal impairment did not affect the pharmacokinetics or metabolism of CBD compared with healthy controls. 78 Hepatic impairment increased plasma CBD and metabolite exposure (6‐OH‐CBD and 7‐OH‐CBD), particularly in participants with moderate to severe impairment compared with healthy controls. 79 CBD dose modification might therefore be required in patients with hepatic impairment. CBD was well‐tolerated in all patient groups, with only mild adverse events reported and no effect of CBD on vital signs or ECG.
Studies involving oral CBD doses of greater than 200–300 mg
Double blind, placebo‐controlled trials of efficacy
This review identified 14 double‐blind, placebo‐controlled trials investigating the efficacy of >200–300 mg oral CBD (Table 1). Five of these trials showed that oral CBD 300 mg reduced anxiety compared to placebo, either in healthy volunteers exposed to “stress‐inducing” conditions or patients.
de Faria et al. 45 subjected patients with Parkinson's disease to a simulated public speaking task and found that 300 mg oral CBD reduced self‐reported anxiety compared to placebo. Patients with Parkinson's disease were chosen in this study because this disease is often comorbid with anxiety disorders. CBD also reduced tremor associated with anticipatory and post‐stress anxiety. No adverse events were observed.
The studies of Zuardi et al. 46 Zuardi et al., 37 and Linares et al. 38 involve three demonstrations of the efficacy of 300 mg oral CBD on public speaking‐induced anxiety. Collectively, 100 participants were assessed in these double‐blind placebo‐controlled trials. Whereas two 38 , 46 assessed anxiety during a simulated public speaking exercise, the third 37 conducted the public speaking test under real‐world conditions with an audience present. CBD did not affect heart rate or blood pressure in any of these studies.
The anxiolytic effects of CBD translated to patients with social anxiety disorder and avoidant personality disorder in a study by Masataka, 54 who reported that 4 weeks of oral CBD treatment (300 mg/day) reduced anxiety compared to placebo. The magnitude of benefit was comparable to the effects of paroxetine, a standard treatment for this disorder. 86
Further data supporting the view that 300 mg may be a threshold dose for efficacy against therapeutic end points comes from Chagas et al. 36 who reported improved quality of life in patients with Parkinson's disease receiving 300 mg oral CBD including improved activities in daily life and reduced self‐stigma, with trending improvements in emotional well‐being and mobility. CBD did not cause any adverse effects.
In addition, Sahinovic et al. 47 recently conducted a small pilot trial investigating the effect of 300 mg oral CBD on physiological and subjective responses to submaximal and exhaustive running exercise. Formal statistical analyses were not performed as the study was underpowered to assess “effect.” However, CBD was found to increase feelings of pleasure during submaximal exercise, increase maximal oxygen consumption, and decrease exercise‐induced inflammation (i.e., serum interleukin‐1β concentrations) to a level the authors deemed “worthy of further investigation” (i.e., the 85% confidence interval around Cohen's dz included ±0.5 but not zero). No serious adverse events were observed.
Bolsoni et al. 48 examined the effect of 300 mg oral CBD on traumatic event recall‐related symptoms in patients with post‐traumatic stress disorder (PTSD). CBD did not affect traumatic event recall‐induced increases in anxiety, alertness, discomfort, and systolic blood pressure. However, CBD did slightly but significantly reduce cognitive impairment associated with traumatic recall compared to placebo. The effects of CBD on this measure endured for 1 week. In a more recent study, Bolsoni et al. 49 reported that acute 300 mg CBD reduced traumatic recall‐induced anxiety and cognitive impairment in patients with PTSD whose traumatic event was nonsexual but not sexual in nature.
Other studies involving 300 mg oral CBD have, however, reported less impressive outcomes. de Alencar et al., 53 for example, found no effect on upper limb tremor or motor performance in patients with essential tremor. Hallak et al. 50 found no effect on cognitive function or positive and negative symptoms in patients with schizophrenia. Linares et al. 51 found no effect on polysomnography measures of sleep quality in healthy participants. Arndt and de Wit 52 found no effect on responses to negative emotional stimuli in healthy participants, and de Meneses‐Gaya et al. 57 found no effect on symptoms of crack‐cocaine withdrawal, including effects on craving, anxiety, depression, and sleep.
Open label trials of efficacy
In an open label trial, Crippa et al. 55 assessed the effects of 4 weeks of oral CBD treatment (300 mg/day) on emotional exhaustion and burnout in frontline healthcare workers during the coronavirus disease 2019 (COVID‐19) pandemic. CBD treatment plus standard care reduced emotional exhaustion scores on a subscale of the Maslach Burnout Inventory at 14, 21, and 28 days compared to standard care alone. CBD treatment plus standard care also reduced scores on depression and anxiety scales at days 7, 14, 21, and 28 compared to standard care. Of concern, four of the 59 participants in the CBD group experienced the severe adverse event of elevated blood concentrations of liver transaminase enzymes (one critical and three mild); one also experienced a skin reaction (pharmacodermia). All participants recovered fully after CBD was discontinued.
Yeshurun et al. 56 examined the effects 37 days of oral CBD treatment (300 mg/day) on the incidence of graft‐versus‐host disease (GVHD) in patients undergoing allogeneic hematopoietic cell transplantation. The patients received CBD 7 days prior to, and 30 days following, transplantation. They also received prophylactic cyclosporine and methotrexate treatment (standard care in this population). Acute GVHD was not observed in any of the patients while receiving CBD. Longer‐term follow‐up showed the incidence of GVHD was lower among CBD‐treated patients than historical controls. The median latency to acute GVHD was also increased in the CBD‐treated patients compared with controls (60 vs. 20 days). The authors concluded that 300 mg oral CBD was safe and effective for GVHD.
Interventional studies of safety (only)
Three additional studies investigating the safety of >200–300 mg oral CBD were identified; all utilized open label designs (Table 2).
Crippa et al. 80 administered 300 mg oral CBD to 120 participants to examine whether CBD converted to THC in vivo. Participant were tested under both “fed” and “fasted” conditions. No evidence for the bioconversion of CBD into THC was found and no adverse psychotropic effects of CBD were observed.
Birnbaum et al. 81 examined the effect of food (a high‐fat meal) versus fasting on the pharmacokinetics of 300 mg oral CBD in participants with refractory epilepsy. Being in a fed state significantly increased plasma CBD exposure compared to the fasted state. No clinically significant adverse effects of CBD were observed.
Good et al. 82 examined the tolerability of 28 days of oral CBD treatment (median maximum tolerated dose of 300 mg/day) in patients with advanced cancer. The most common side effect of CBD was drowsiness, although this may not have been directly related to CBD, given that that the patients were receiving many other medications. Some patients appeared to display a marked improvement in total symptom distress.
Studies involving oral CBD doses of greater than 300 mg to 400 mg
Double blind, placebo‐controlled trials of efficacy
This review identified six double‐blind, placebo‐controlled trials investigating the efficacy of >300–400 mg oral CBD (Table 1).
Crippa et al. 61 and Crippa et al. 60 reported anxiolytic effects of 400 mg oral CBD both in small cohorts of participants (10 participants in each study). One study 60 showed that this dose reduced self‐reported anxiety in patients with social anxiety disorder. Patients also underwent single photon emission computed tomography (SPECT) imaging, which revealed that CBD altered cerebral blood flow in the posterior cingulate gyrus and parahippocampal gyrus compared to placebo. CBD did not cause physical or mental sedation in this participant population. The other study 61 then exploited the anxiogenic nature of brain imaging procedures, showing that 400 mg oral CBD significantly reduced self‐reported anxiety in healthy participants undergoing brain imaging. Unlike in patients with social anxiety disorder, CBD significantly increased mental, but not physical, sedation in this participant population. Brain imaging results showed that CBD significantly modulated cerebral blood flow in limbic and paralimbic cortical areas of the brain that are implicated in anxiety.
Bebee et al. 62 found that 400 mg oral CBD did not affect pain scores in patients presenting to an emergency department with lower back pain. The length of time spent in the emergency department did not differ between CBD and placebo. CBD did not affect the doses of other drugs received, such as oxycodone, paracetamol, or ibuprofen. CBD was well‐tolerated and there was no difference between CBD and placebo on any of the side effects reported.
Arout et al. 41 administered 400 mg oral CBD (albeit the non‐plant derived [+] isomer). CBD did not significantly affect pain tolerance or thresholds on the cold pressor task – but did increase subjective ratings of painfulness. It also increased ratings of “bad drug effect” and “good drug effect,” as well as “take again,” consistent with abuse liability. Cardiovascular measures were affected as follows: CBD increased heart rate during the cold pressor task and decreased systolic and diastolic blood pressure at rest. CBD also appeared to increase the incidence of stomach upset compared to placebo.
Hurd et al. 63 reported that 3 days of oral CBD treatment (400 mg/day) significantly reduced opioid craving and anxiety in heroin‐dependent individuals. CBD also reduced heroin cue‐induced increases in craving, anxiety, heart rate, and salivary cortisol compared to placebo. This was achieved following an acute dose and after three repeated doses of CBD, with protracted improvements observed 4 days after the last dose of CBD. The CBD was generally well‐tolerated with no serious adverse events.
Reinforcing the notion that CBD has anti‐craving activity in drug‐dependent individuals, Freeman et al. 42 reported that 4 weeks of oral CBD treatment (400 mg/day) significantly decreased cannabis use (number of days abstinent) and urine THC‐COOH: creatinine ratios (a biomarker of cannabis use) in cannabis‐dependent individuals. No severe adverse events were reported. CBD caused significantly more mild adverse events than placebo (96 vs. 65) but did not differ from placebo in the number of moderate adverse events (8 vs. 9).
Open label trials of efficacy
A recent study by Pacheco et al. 58 found that 4 weeks of oral CBD treatment (330 mg/day) significantly reduced burn out, anxiety, depression, and insomnia compared to baseline in healthcare workers in Brazil during the COVID‐19 pandemic. No adverse events were reported.
Zuardi et al. 59 examined the effects of 4 weeks of oral CBD treatment (increasing from 150 to 400 mg/day) on treatment‐induced psychosis in six patients with Parkinson's disease. The patients had been treated with L‐DOPA which elevates brain dopamine concentrations sometimes causing psychosis (i.e., hallucinations and delusions). There was a significant improvement in scores on the Brief Psychiatric Scale (BPRS), including reduced anxiety and thinking disorder, as well as significantly reduced scores on the Parkinson's Psychosis Questionnaire (PPQ) compared to baseline. CBD also improved Clinical Global Impression (CGI) scores and tended to improve Unified Parkinson's Disease Ratings Scale (UPDRS) scores on daily living and motor function, although the latter effect was not statistically significant. No adverse events were observed.
Interventional studies of safety (only)
Three additional studies investigating the safety of >300–400 mg oral CBD were identified; all utilized double‐blind, placebo‐controlled designs (Table 2).
Perkins et al. 83 conducted a phase I CBD dose‐escalation study (5, 10, and 20 mg/kg) in 12 fed healthy participants. CBD was well‐tolerated at all dose levels following a single oral dose. The safety profiles of placebo and CBD treatment groups were similar. There were no serious adverse events.
Manini et al. 84 co‐administered 400 mg oral CBD with intravenous fentanyl (a potent opioid). CBD did not influence the toxicity of fentanyl, with no respiratory or cardiovascular complications observed, and was well‐tolerated. There was no correlation between CBD plasma concentrations and the occurrence of adverse events.
Haney et al. 85 examined the effects of 400 mg oral CBD on the behavioral and cardiovascular effects of inhaled cannabis. CBD alone did not have psychoactive or cardiovascular effects. In addition, CBD did not influence the rewarding or cardiovascular effects of cannabis consumption. CBD was well‐tolerated, and its adverse effects did not differ greatly from placebo.
DISCUSSION
This review identified a total of 29 double‐blind, placebo‐controlled trials and six open label studies investigating the efficacy of “low” oral doses of CBD (i.e., ≤400 mg/day; Table 1). Forty‐five studies also examined the safety and pharmacokinetics of CBD (Tables 1 and 2). Most interventional studies had relatively small sample sizes and many involved healthy volunteers rather than clinical populations. High‐quality consistent evidence from multiple large double‐blind, placebo‐controlled trials, as is seen, for example, in the literature around CBD and pediatric epilepsy, was not present for any condition considered in the current review. The largest trial had 136 participants and found no effect of ~20–30 mg CBD per day on pain. 33
As such, few definitive conclusions in terms of efficacy can be reached from this review, other than the general observations that: (1) clinically relevant CBD effects tend to become more robust as dosage is increased (up to 400 mg); (2) CBD appears exceptionally safe, with very few concerns even at the highest dose range considered (>300–400 mg); and (3) further high‐quality clinical trials involving lower oral doses of CBD are urgently needed to clarify therapeutic actions.
In discussing the results obtained, we will focus on five different therapeutic domains of relevance: anxiety, insomnia, addiction‐related disorders, chronic pain, and other conditions.
Anxiety
The most replicable results emerging from our analysis related to the ability of CBD, primarily at doses of 300–400 mg, to ameliorate anxiety. These studies have often involved placing healthy volunteers treated with CBD in situations that elicit anxiety (e.g., simulated public speaking tests, 37 , 38 , 46 and a brain scanner 61 ). Under these conditions, CBD seems clearly anxiolytic at doses of 300–400 mg, but not at lower doses. Perhaps of greater interest are the anxiolytic effects observed at oral doses of 300–400 mg in small double‐blind, placebo‐controlled trials involving patients with social anxiety disorder 54 , 60 or Parkinson's disease. 45 It would be premature to conclude that very low doses of CBD (<300 mg) do not have anxiolytic effects in patients given the paucity of data. However, higher dose ranges, specifically 300–400 mg, appear more assured of efficacy based on the existing evidence base. CBD might have more generalized “anti‐stress” effects, as supported by recent open label trials reporting that ~300 mg CBD per day reduced emotional exhaustion and burnout in healthcare workers during the COVID pandemic. 55 , 58
Insomnia
Relative to anxiety, the findings relating to insomnia and sleep quality are less robust. The very low dose of CBD (15 mg) in healthy overweight participants produced marginally significant improvements in sleep quality and quantity over 6 weeks of dosing compared to baseline, but not compared to placebo. 32 Sleep quality improvements were also evident in patients with insomnia in a study, 29 although only with 160 mg CBD and not with doses of 40 or 80 mg. On the other hand, sleep quality and sleep architecture were not improved in healthy volunteers with 300 mg CBD in the more recent study, which used polysomnography. 51 Clearly, larger scale dose‐ranging double‐blind, placebo‐controlled trials in sleep disordered patient populations using objective measures of sleep quality are required to better assess the effects of CBD on sleep. Some recent case reports hint at possible effects of CBD on specific sleep disorders; CBD (75 mg) had beneficial effects on rapid eye movement (REM) sleep behavior disorder (RBD) 87 ; and, 4.6 mg/day CBD appeared to promote remarkable improvements in sleep in autistic children. 88 Both these observations are worthy of follow‐up with larger trials. Medicinal cannabis companies in Australia are currently mounting trials to test whether CBD doses less than or equal to 150 mg have soporific effects in participants with subclinical sleep disruption to provide data to support registration of OTC low‐dose CBD products.
Addiction‐related disorders
There is emerging interest in CBD as an “anti‐addiction” therapeutic, as has been discussed in recent reviews, 27 , 89 and informed by recent preclinical research results with animal models of addiction. 90 , 91 There are also a significant number of ongoing clinical trials with CBD in the addiction medicine area. CBD efficacy in this domain at oral doses of 400 mg was evident in one reasonable quality double‐blind, placebo‐controlled trial relating to cannabis use disorder 42 and also in a laboratory‐based study of opiate‐dependent individuals. 63 As with anxiety, it appears that CBD might have dose‐dependent effects as one double‐blind placebo‐controlled trial 42 showed that 200 mg CBD was ineffective in ameliorating cannabis use disorder. On the other hand, 200 mg CBD appeared to reduce depression and psychosis‐like symptoms in regular cannabis users in an open label trial. 43
Pain
Chronic pain is by far the most common indication for which medicinal cannabis products are prescribed. 92 , 93 However, there is little compelling evidence that CBD administered at any dose up to 400 mg reduces pain in humans. Consistent with this, there were no double‐blind, placebo‐controlled trials of CBD for chronic pain identified in the current review. One placebo‐controlled trial 62 showed that CBD 400 mg was ineffective in reducing lower back pain in an emergency department. An “n of 1” placebo‐controlled trial 31 failed to provide evidence of benefits in 34 patients repeatedly treated with a sublingual CBD spray at ~15 mg/day. It is clear that placebo‐controlled clinical trials of CBD in the specific area of chronic pain are urgently required.
Other conditions
The current review also found intriguing but not necessarily compelling evidence for low‐dose CBD efficacy in a range of other conditions. This includes the protective effects against GVHD (300 mg CBD) 56 ; positive effects (300 mg CBD) on Parkinsonian tremor 45 ; improved quality of life and reduced psychotic symptoms in patients with Parkinson's disease (300 mg CBD) 36 , 59 ; and beneficial effects (15 mg CBD) on HDL (cholesterol), 32 although this was not repeated with higher doses of CBD (200 mg CBD). 44
Safety
Several high‐quality systematic reviews and meta‐analyses of CBD safety have recently been conducted 9 , 14 , 15 , 21 and these generally conclude that CBD has a remarkably safe profile. Phase I studies show that CBD is generally well‐tolerated at doses up to 6000 mg in single doses or 1500 mg in multiple doses. 17 The 6000 mg dose represents 15 times the maximal 400 mg dose threshold we have set in the current review. Likewise, Epidiolex is dosed up to 50 mg/kg/day, equating to ~3000 mg per day for a 62 kg adult, which is 7.5‐fold higher than the highest 400 mg dose considered here. A recent meta‐analysis suggests few serious adverse events in clinical trials involving high doses of CBD but also that non‐serious adverse events (e.g., somnolence, decreased appetite, and gastrointestinal upset) are significantly less likely at lower CBD doses ranges. 14 Outside of clinical trials involving epilepsy, the only adverse event more prominent with CBD over placebo, even at high doses, was diarrhea. 14
The current review found few concerns around safety across the 45 studies analyzed. Where side effects were reported they were typically minor, and often in studies that lacked a placebo control, and therefore could not be unambiguously attributed to CBD itself. Few adverse events were reported in any of the studies considered even at the 300–400 mg dose range where efficacy was most often reported. The only minor concerns around safety were altered metabolism of CBD in hepatically impaired patients 79 and reduced tolerability in patients with advanced cancer who were medicated with many other drugs. 82
An open label, phase I study recently reported that a high oral CBD dose (1500 mg/day) was associated with elevated serum alanine aminotransferase concentrations consistent with drug‐induced liver injury. 94 Pharmacovigilance of liver enzymes may be required even at low CBD doses, as we identified one study in which elevated liver enzymes were observed in four out of 59 participants following repeated oral dosing with 300 mg CBD. 55 However, in a more recent study, a single dose of 30 mg CBD did not affect liver enzymes, 73 highlighting lower doses may be of less concern. Future studies are needed to assess liver function following repeated lower doses.
It is notable that feeding can increase peak plasma concentrations of CBD. 17 , 81 Birnbaum et al. 81 showed that a high‐fat meal increased plasma concentrations of CBD following an oral dose of 300 mg CBD. More recently, prior food consumption was shown to elevate plasma concentrations of CBD and its metabolites following an oral dose of 30 mg CBD. 73 It is conceivable that this “food effect” could have implications for therapeutic and adverse effects but more research is needed.
Pharmacodynamic and pharmacokinetic interactions between CBD and prescription medications remain possible, 95 , 96 but the likelihood of such interactions at the lower CBD doses considered here remains to be established. There is little information available on long‐term effects of CBD consumption outside of the 1–12 week time interval involved in the clinical trials considered here and elsewhere. 14 Obviously, then, there remains the possibility that hitherto unrecognized and problematic side effects may emerge in the future when patients using CBD over many months or years are studied.
CONCLUSION
The current review has identified little by way of high‐quality evidence to support the efficacy of CBD at lower doses (up to 400 mg). The currently sparse evidence base around low doses of CBD may be improved by future clinical trials that better validate efficacy at this dose range. The current evidence suggests CBD at doses of 300–400 mg has promise, especially as an anxiolytic and anti‐addiction agent, and larger randomized, double‐blind, placebo‐controlled trials are required to reinforce these data. Of course, actual plasma and tissue exposure to CBD needs to incorporated into our thinking, as advances in drug delivery and pharmaceutics may lead to improved delivery of CBD, a drug with notoriously low oral bioavailability. Given the current intensity of worldwide research activity around CBD, and associated commercial potential, it would appear that advances in our knowledge are just around the corner.
FUNDING INFORMATION
I.S.M., D.M., A.S., and J.C.A. receive salary support from the Lambert Initiative for Cannabinoid Therapeutics, a philanthropically funded center for medicinal cannabis research at the University of Sydney. An earlier version of the article was funded by the Medicinal Cannabis Industry Australia (MCIA) to assist in deliberations on CBD being scheduled as an over‐the‐counter medication in Australia.
CONFLICT OF INTEREST
J.C.A. has served as an expert witness in various medicolegal cases involving cannabis and cannabinoids and served as a temporary advisor to the World Health Organization (WHO) in their review of cannabis and the cannabinoids. He has received consulting fees from Creo Inc. I.S.M. acts as a consultant to Kinoxis Therapeutics and has received honoraria from Janssen. He has also served as an expert witness in various medicolegal cases involving cannabis and cannabinoids. J.C.A. and I.S.M. hold patents on cannabinoid therapies (PCT/AU2018/05089 and PCT/AU2019/050554). J.C.A., I.S.M., D.M. and A.S. have received consulting fees from the Medicinal Cannabis Industry Australia (MCIA).
ACKNOWLEDGMENTS
This study was supported by the Lambert Initiative for Cannabinoid Therapeutics, a philanthropically funded center for medicinal cannabis research at the University of Sydney and the Australian National Health and Medical Research Council (NHMRC). The authors gratefully acknowledge Barry and Joy Lambert for their continued support of the Lambert Initiative for Cannabinoid Therapeutics.
Arnold JC, McCartney D, Suraev A, McGregor IS. The safety and efficacy of low oral doses of cannabidiol: An evaluation of the evidence. Clin Transl Sci. 2023;16:10‐30. doi: 10.1111/cts.13425
Contributor Information
Jonathon C. Arnold, Email: jonathon.arnold@sydney.edu.au.
Iain S. McGregor, Email: iain.mcgregor@sydney.edu.au.
REFERENCES
- 1. Arzimanoglou A, Brandl U, Cross JH, et al. Epilepsy and cannabidiol: a guide to treatment. Epileptic Disord. 2020;22:1‐14. [DOI] [PubMed] [Google Scholar]
- 2. Bonaccorso S, Ricciardi A, Zangani C, Chiappini S, Schifano F. Cannabidiol (CBD) use in psychiatric disorders: a systematic review. Neurotoxicology. 2019;74:282‐298. [DOI] [PubMed] [Google Scholar]
- 3. McGuire P, Robson P, Cubala WJ, et al. Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry. 2018;175:225‐231. [DOI] [PubMed] [Google Scholar]
- 4. Millar SA, Stone N, Bellman Z, et al. A systematic review of cannabidiol dosing in clinical populations. Br J Clin Pharmacol. 2019;85:1888‐1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benson MJ, Abelev SV, Corte CJ, Connor SJ, McGregor IS. Attitudes and knowledge of Australian gastroenterologists around use of medicinal cannabis for inflammatory bowel disease. Crohn's Colitis 360. 2020;2(2):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Karanges EA, Suraev A, Elias N, Manocha R, McGregor IS. Knowledge and attitudes of Australian general practitioners towards medicinal cannabis: a cross‐sectional survey. BMJ Open. 2018;8:e022101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arnold JC. A primer on medicinal cannabis safety and potential adverse effects. Australian J General Pract. 2021;50:345‐350. [DOI] [PubMed] [Google Scholar]
- 8. Ibeas Bih C, Chen T, Nunn AV, et al. Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics. 2015;12:699‐730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Larsen C, Shahinas J. Dosage, efficacy and safety of cannabidiol administration in adults: a systematic review of human trials. J Clin Med Res. 2020;12:129‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Elmes MW, Kaczocha M, Berger WT, et al. Fatty acid‐binding proteins (FABPs) are intracellular carriers for Delta9‐tetrahydrocannabinol (THC) and cannabidiol (CBD). J Biol Chem. 2015;290:8711‐8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gulbransen G, Xu W, Arroll B. Cannabidiol prescription in clinical practice: an audit on the first 400 patients in New Zealand. BJGP Open. 2020;4:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McGregor IS, Cairns EA, Abelev S, et al. Access to cannabidiol without a prescription: a cross‐country comparison and analysis. Int J Drug Policy. 2020;85:102935. [DOI] [PubMed] [Google Scholar]
- 13. Morano A, Fanella M, Albini M, et al. Cannabinoids in the treatment of epilepsy: current status and future prospects. Neuropsychiatr Dis Treat. 2020;16:381‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chesney E, Oliver D, Green A, et al. Adverse effects of cannabidiol: a systematic review and meta‐analysis of randomized clinical trials. Neuropsychopharmacology. 2020;45(11):1799‐1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iffland K, Grotenhermen F. An update on safety and side effects of cannabidiol: a review of clinical data and relevant animal studies. Cannabis Cannabinoid Res. 2017;2:139‐154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Therapeutic Goods Administration . Safety of low dose cannabidiol. Therapeutic Goods Administration; 2020:20. [Google Scholar]
- 17. Taylor L, Gidal B, Blakey G, Tayo B, Morrison G. A phase I, randomized, double‐blind, placebo‐controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs. 2018;32:1053‐1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organisation . Cannabidiol (CBD): World Health Organisation Expert Committee on Drug Dependence Thirty‐ninth Meeting. World Health Organisation; 2017:41‐43. [Google Scholar]
- 19. World Health Organisation . Annex 1 – Extract from the Report of the 41st Expert Committee on Drug Dependence: Cannabis and cannabis‐related substances. WHO; 2019. [Google Scholar]
- 20. Walpole SC, Prieto‐Merino D, Edwards P, Cleland J, Stevens G, Roberts I. The weight of nations: an estimation of adult human biomass. BMC Public Health. 2012;12:439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dos Santos RG, Guimarães FS, Crippa JAS, et al. Serious adverse effects of cannabidiol (CBD): a review of randomized controlled trials. Expert Opin Drug Metab Toxicol. 2020;16:517‐526. [DOI] [PubMed] [Google Scholar]
- 22. Elsaid S, Kloiber S, Le Foll B. Effects of cannabidiol (CBD) in neuropsychiatric disorders: a review of pre‐clinical and clinical findings. Prog Mol Biol Transl Sci. 2019;165:25‐75. [DOI] [PubMed] [Google Scholar]
- 23. Hindocha C, Cousijn J, Rall M, Bloomfield M. The effectiveness of cannabinoids in the treatment of posttraumatic stress disorder (PTSD): a systematic review. J Dual Diagn. 2020;16:120‐139. [DOI] [PubMed] [Google Scholar]
- 24. Millar SA, Stone NL, Yates AS, O'Sullivan SE. A systematic review on the pharmacokinetics of cannabidiol in humans. Front Pharmacol. 2018;9:1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sarris J, Sinclair J, Karamacoska D, Davidson M, Firth J. Medicinal cannabis for psychiatric disorders: a clinically‐focused systematic review. BMC Psychiatry. 2020;20:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sholler DJ, Schoene L, Spindle TR. Therapeutic efficacy of cannabidiol (CBD): a review of the evidence from clinical trials and human laboratory studies. Curr Addict Rep. 2020;7(3):405‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Turna J, Syan SK, Frey BN, et al. Cannabidiol as a novel candidate alcohol use disorder pharmacotherapy: a systematic review. Alcohol Clin Exp Res. 2019;43:550‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. White CM. A review of human studies assessing cannabidiol's (CBD) therapeutic actions and potential. J Clin Pharmacol. 2019;59:923‐934. [DOI] [PubMed] [Google Scholar]
- 29. Carlini EA, Cunha JM. Hypnotic and antiepileptic effects of cannabidiol. J Clin Pharmacol. 1981;21:417S‐427S. [DOI] [PubMed] [Google Scholar]
- 30. Naftali T, Mechulam R, Marii A, et al. Low‐dose cannabidiol is safe but not effective in the treatment for Crohn's disease, a randomized controlled trial. Dig Dis Sci. 2017;62:1615‐1620. [DOI] [PubMed] [Google Scholar]
- 31. Notcutt W, Price M, Miller R, et al. Initial experiences with medicinal extracts of cannabis for chronic pain: results from 34 'N of 1′ studies. Anaesthesia. 2004;59:440‐452. [DOI] [PubMed] [Google Scholar]
- 32. Lopez HL, Cesareo KR, Raub B, et al. Effects of hemp extract on markers of wellness, stress resilience, recovery and clinical biomarkers of safety in overweight, but otherwise healthy subjects. J Dietary Suppl. 2020;17:561‐586. [DOI] [PubMed] [Google Scholar]
- 33. Vela J, Dreyer L, Petersen KK, et al. Cannabidiol treatment in hand osteoarthritis and psoriatic arthritis: a randomized, double‐blind placebo‐controlled trial. Pain. 2021;163(6):1206‐1214. [DOI] [PubMed] [Google Scholar]
- 34. Hobbs JM, Vazquez AR, Remijan ND, et al. Evaluation of pharmacokinetics and acute anti‐inflammatory potential of two oral cannabidiol preparations in healthy adults. Phytother Res. 2020;34:1696‐1703. [DOI] [PubMed] [Google Scholar]
- 35. Isenmann E, Veit S, Starke L, Flenker U, Diel P. Effects of cannabidiol supplementation on skeletal muscle regeneration after intensive resistance training. Nutrients. 2021;13(9):3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chagas MHN, Zuardi AW, Tumas V, et al. Effects of cannabidiol in the treatment of patients with Parkinson's disease: an exploratory double‐blind trial. J Psychopharmacol. 2014;28:1088‐1098. [DOI] [PubMed] [Google Scholar]
- 37. Zuardi AW, Rodrigues NP, Silva AL, et al. Inverted U‐shaped dose‐response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Front Pharmacol. 2017;8:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Linares IM, Zuardi AW, Pereira LC, et al. Cannabidiol presents an inverted U‐shaped dose‐response curve in a simulated public speaking test. Rev Bras Psiquiatr. 2019;41:9‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crippa JA, Pereira Junior LC, Pereira LC, et al. Effect of two oral formulations of cannabidiol on responses to emotional stimuli in healthy human volunteers: Pharmaceutical vehicle matters. Brazilian J Psychiatry. 2022;44:15‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cochrane‐Snyman KC, Cruz C, Morales J, Coles M. The effects of cannabidiol oil on noninvasive measures of muscle damage in men. Med Sci Sports Exerc. 2021;53:1460‐1472. [DOI] [PubMed] [Google Scholar]
- 41. Arout CA, Haney M, Herrmann ES, Bedi G, Cooper ZD. A placebo‐controlled investigation of the analgesic effects, abuse liability, safety and tolerability of a range of oral cannabidiol doses in healthy humans. Br J Clin Pharmacol. 2021;88:347‐355. [DOI] [PubMed] [Google Scholar]
- 42. Freeman TP, Hindocha C, Baio G, et al. Cannabidiol for the treatment of cannabis use disorder: a phase 2a, double‐blind, placebo‐controlled, randomised, adaptive Bayesian trial. Lancet Psychiatry. 2020;7:865‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Solowij N, Broyd SJ, Beale C, et al. Therapeutic effects of prolonged cannabidiol treatment on psychological symptoms and cognitive function in regular cannabis users: a pragmatic open‐label clinical trial. Cannabis Cannabinoid Res. 2018;3:21‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jadoon KA, Ratcliffe SH, Barrett DA, et al. Efficacy and safety of cannabidiol and tetrahydrocannabivarin on glycemic and lipid parameters in patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled, parallel group pilot study. Diabetes Care. 2016;39:1777‐1786. [DOI] [PubMed] [Google Scholar]
- 45. de Faria SM, de Morais Fabrício D, Tumas V, et al. Effects of acute cannabidiol administration on anxiety and tremors induced by a Simulated Public Speaking Test in patients with Parkinson's disease. J Psychopharmacol. 2020;34:189‐196. [DOI] [PubMed] [Google Scholar]
- 46. Zuardi AW, Cosme R, Graeff F, Guimarães F. Effects of ipsapirone and cannabidiol on human experimental anxiety. J Psychopharmacol. 1993;7:82‐88. [DOI] [PubMed] [Google Scholar]
- 47. Sahinovic A, Irwin C, Doohan PT, et al. Effects of cannabidiol on exercise physiology and bioenergetics: a randomised controlled pilot trial. Sports Medicine ‐ Open. 2022;8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bolsoni LM, Crippa JAS, Hallak JEC, Guimarães FS, Zuardi AW. Effects of cannabidiol on symptoms induced by the recall of traumatic events in patients with posttraumatic stress disorder. Psychopharmacology. 2022;239:1499‐1507. [DOI] [PubMed] [Google Scholar]
- 49. Bolsoni LM, Crippa JAS, Hallak JEC, Guimarães FS, Zuardi AW. The anxiolytic effect of cannabidiol depends on the nature of the trauma when patients with post‐traumatic stress disorder recall their trigger event. Brazilian Journal of Psychiatry. 2022;44:298‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hallak JE, Machado‐de‐Sousa JP, Crippa JAS, et al. Performance of schizophrenic patients in the Stroop Color Word Test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Brazilian Journal of Psychiatry. 2010;32:56‐61. [DOI] [PubMed] [Google Scholar]
- 51. Linares IMP, Guimaraes FS, Eckeli A, et al. No acute effects of cannabidiol on the sleep‐wake cycle of healthy subjects: A randomized, double‐blind, placebo‐controlled, crossover study. Front Pharmacol. 2018;9:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Arndt DL, de Wit H. Cannabidiol does not dampen responses to emotional stimuli in healthy adults. Cannabis Cannabinoid Res. 2017;2:105‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. de Alencar SS, Crippa JAS, Brito MC, et al. A single oral dose of cannabidiol did not reduce upper limb tremor in patients with essential tremor. Parkinsonism Relat Disord. 2021;83:37‐40. [DOI] [PubMed] [Google Scholar]
- 54. Masataka N. Anxiolytic effects of repeated cannabidiol treatment in teenagers with social anxiety disorders. Front Psychol. 2019;10:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Crippa JAS, Zuardi AW, Guimarães FS, et al. Efficacy and safety of cannabidiol plus standard care vs standard care alone for the treatment of emotional exhaustion and burnout among frontline health care workers during the COVID‐19 pandemic: a randomized clinical trial. JAMA Netw Open. 2021;4:e2120603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yeshurun M, Shpilberg O, Herscovici C, et al. Cannabidiol for the prevention of graft‐versus‐host‐disease after allogeneic hematopoietic cell transplantation: results of a phase II study. Biol Blood Marrow Transplant. 2015;21:1770‐1775. [DOI] [PubMed] [Google Scholar]
- 57. de Meneses‐Gaya C, Crippa JA, Hallak JE, et al. Cannabidiol for the treatment of crack‐cocaine craving: an exploratory double‐blind study. Brazilian J Psychiatry. 2021;43:467‐476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pacheco JC, Souza JDS, Hallak JEC, et al. Cannabidiol as a treatment for mental health outcomes among health care workers during the coronavirus disease pandemic. J Clin Psychopharmacol. 2021;41:327‐329. [DOI] [PubMed] [Google Scholar]
- 59. Zuardi AW, Crippa J, Hallak J, et al. Cannabidiol for the treatment of psychosis in Parkinson's disease. J Psychopharmacol. 2009;23:979‐983. [DOI] [PubMed] [Google Scholar]
- 60. Crippa JAS, Derenusson GN, Ferrari TB, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J Psychopharmacol. 2011;25:121‐130. [DOI] [PubMed] [Google Scholar]
- 61. Crippa JA, Zuardi AW, Garrido GE, et al. Effects of cannabidiol (CBD) on regional cerebral blood flow. Neuropsychopharmacology. 2004;29:417‐426. [DOI] [PubMed] [Google Scholar]
- 62. Bebee B, Taylor DM, Bourke E, et al. The CANBACK trial: a randomised, controlled clinical trial of oral cannabidiol for people presenting to the emergency department with acute low back pain. Med J Aust. 2021;214:370‐375. [DOI] [PubMed] [Google Scholar]
- 63. Hurd YL, Spriggs S, Alishayev J, et al. Cannabidiol for the reduction of cue‐induced craving and anxiety in drug‐abstinent individuals with heroin use disorder: a double‐blind randomized placebo‐controlled trial. Am J Psychiatry. 2019;176:911‐922. [DOI] [PubMed] [Google Scholar]
- 64. Atsmon J, Heffetz D, Deutsch L, Deutsch F, Sacks H. Single‐dose pharmacokinetics of oral cannabidiol following administration of PTL101: a new formulation based on gelatin matrix pellets technology. Clin Pharmacol Drug Dev. 2018;7:751‐758. [DOI] [PubMed] [Google Scholar]
- 65. Škopek M, Heidler J, Hnizdil J, Šulc J. The effect of cannabidiol (Cbd) on simple and complex reaction times. Trends Sport Sci. 2021;28:147‐151. [Google Scholar]
- 66. Knaub K, Sartorius T, Dharsono T, Wacker R, Wilhelm M, Schön C. A novel self‐emulsifying drug delivery system (SEDDS) based on Vesisorb® formulation technology improving the oral bioavailability of cannabidiol in healthy subjects. Molecules. 2019;24(16):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bird K, Boleyn T, Chesher G, et al. Intercannabinoid and cannabinoid‐ethanol interactions and their effects on human performance. Psychopharmacology. 1980;71:181‐188. [DOI] [PubMed] [Google Scholar]
- 68. Belgrave B, Bird K, Chesher G, et al. The effect of cannabidiol, alone and in combination with ethanol, on human performance. Psychopharmacology. 1979;64:243‐246. [DOI] [PubMed] [Google Scholar]
- 69. Williams NNB, Ewell TR, Abbotts KSS, et al. Comparison of five oral cannabidiol preparations in adult humans: pharmacokinetics, body composition, and heart rate variability. Pharmaceuticals. 2021;14:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Patrician A, Versic‐Bratincevic M, Mijacika T, et al. Examination of a new delivery approach for oral cannabidiol in healthy subjects: a randomized, double‐blinded. Placebo‐Control Pharmacokinetics Study Adv Therapy. 2019;36:3196‐3210. [DOI] [PubMed] [Google Scholar]
- 71. Devinsky O, Kraft K, Rusch L, Fein M, Leone‐Bay A. Improved bioavailability with dry powder cannabidiol inhalation: a phase 1 clinical study. J Pharm Sci. 2021;110:3946‐3952. [DOI] [PubMed] [Google Scholar]
- 72. Karniol IG, Shirakawa I, Kasinski N, Pfeferman A, Carlini EA. Cannabidiol interferes with the effects of delta 9 ‐ tetrahydrocannabinol in man. Eur J Pharmacol. 1974;28:172‐177. [DOI] [PubMed] [Google Scholar]
- 73. Abbotts KSS, Ewell TR, Butterklee HM, et al. Cannabidiol and cannabidiol metabolites: pharmacokinetics, interaction with food, and influence on liver function. Nutrients. 2022;14:2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Izgelov D, Davidson E, Barasch D, Regev A, Domb AJ, Hoffman A. Pharmacokinetic investigation of synthetic cannabidiol oral formulations in healthy volunteers. Eur J Pharm Biopharm. 2020;154:108‐115. [DOI] [PubMed] [Google Scholar]
- 75. Spindle TR, Cone EJ, Goffi E, et al. Pharmacodynamic effects of vaporized and oral cannabidiol (CBD) and vaporized CBD‐dominant cannabis in infrequent cannabis users. Drug Alcohol Depend. 2020;211:107937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Spindle TR, Cone EJ, Kuntz D, et al. Urinary pharmacokinetic profile of cannabinoids following administration of vaporized and oral cannabidiol and vaporized CBD‐dominant cannabis. J Anal Toxicol. 2020;44:109‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cunha JM, Carlini E, Pereira AE, et al. Chronic administration of cannabidiol to healthy volunteers and epileptic patients. Pharmacology. 1980;21:175‐185. [DOI] [PubMed] [Google Scholar]
- 78. Tayo B, Taylor L, Sahebkar F, Morrison G. A phase i, open‐label, parallel‐group, single‐dose trial of the pharmacokinetics, safety, and tolerability of cannabidiol in subjects with mild to severe renal impairment. Clin Pharmacokinet. 2020;59:747‐755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Taylor L, Crockett J, Tayo B, Morrison G. A phase 1, open‐label, parallel‐group, single‐dose trial of the pharmacokinetics and safety of cannabidiol (CBD) in subjects with mild to severe hepatic impairment. J Clin Pharmacol. 2019;59:1110‐1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Crippa JAS, Zuardi AW, Hallak JEC, et al. Oral cannabidiol does not convert to Δ8‐THC or Δ9‐THC in humans: a pharmacokinetic study in healthy subjects. Cannabis Cannabinoid Res. 2020;5:89‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Birnbaum AK, Karanam A, Marino SE, et al. Food effect on pharmacokinetics of cannabidiol oral capsules in adult patients with refractory epilepsy. Epilepsia. 2019;60:1586‐1592. [DOI] [PubMed] [Google Scholar]
- 82. Good PD, Greer RM, Huggett GE, Hardy JR. An open‐label pilot study testing the feasibility of assessing total symptom burden in trials of cannabinoid medications in palliative care. J Palliat Med. 2020;23:650‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Perkins D, Butler J, Ong K, et al. Randomised, placebo‐controlled, dose escalation study to investigate the safety, tolerability and pharmacokinetics of cannabidiol in fed healthy volunteers. Eur J Drug Metab Pharmacokinet. 2020;45:575‐586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Manini AF, Yiannoulos G, Bergamaschi MM, et al. Safety and pharmacokinetics of oral cannabidiol when administered concomitantly with intravenous fentanyl in humans. J Addict Med. 2015;9(204):204‐210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Haney M, Malcolm RJ, Babalonis S, et al. Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology. 2016;41:1974‐1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Nordahl HM, Vogel PA, Morken G, Stiles TC, Sandvik P, Wells A. Paroxetine, cognitive therapy or their combination in the treatment of social anxiety disorder with and without avoidant personality disorder: a randomized clinical trial. Psychother Psychosom. 2016;85:346‐356. [DOI] [PubMed] [Google Scholar]
- 87. Chagas MH, Eckeli A, Zuardi AW, et al. Cannabidiol can improve complex sleep‐related behaviours associated with rapid eye movement sleep behaviour disorder in Parkinson's disease patients: a case series. J Clin Pharm Ther. 2014;39:564‐566. [DOI] [PubMed] [Google Scholar]
- 88. Fleury‐Teixeira P, Caixeta FV, da Silva LCR, Brasil‐Neto JP, Malcher‐Lopes R. Effects of CBD‐enriched cannabis sativa extract on autism spectrum disorder symptoms: an observational study of 18 participants undergoing compassionate use. Front Neurol. 2019;10:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chye Y, Christensen E, Solowij N, Yucel M. The endocannabinoid system and cannabidiol's promise for the treatment of substance use disorder. Front Psych. 2019;10:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Gonzalez‐Cuevas G, Martin‐Fardon R, Kerr TM, et al. Unique treatment potential of cannabidiol for the prevention of relapse to drug use: preclinical proof of principle. Neuropsychopharmacology. 2018;43:2036‐2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hay GL, Baracz SJ, Everett NA, et al. Cannabidiol treatment reduces the motivation to self‐administer methamphetamine and methamphetamine‐primed relapse in rats. J Psychopharmacol. 2018;32:1369‐1378. [DOI] [PubMed] [Google Scholar]
- 92. Arnold JC, Nation T, McGregor IS. Prescribing medicinal cannabis. Aust Prescr. 2020;43:152‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. MacPhail SL, Bedoya‐Pérez MA, Cohen R, Kotsirilos V, McGregor IS, Cairns EA. Medicinal cannabis prescribing in Australia: an analysis of trends over the first five years. Front Pharmacol. 2022;13:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Watkins PB, Church RJ, Li J, Knappertz V. Cannabidiol and abnormal liver chemistries in healthy adults: results of a phase I clinical trial. Clin Pharmacol Ther. 2021;109:1224‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Morrison G, Crockett J, Blakey G, Sommerville K. A phase 1, open‐label, pharmacokinetic trial to investigate possible drug‐drug interactions between clobazam, stiripentol, or valproate and cannabidiol in healthy subjects. Clin Pharmacol Drug Dev. 2019;8:1009‐1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Qian Y, Gurley BJ, Markowitz JS. The potential for pharmacokinetic interactions between cannabis products and conventional medications. J Clin Psychopharmacol. 2019;39:462‐471. [DOI] [PubMed] [Google Scholar]


