Abstract
Further understanding of when to initiate therapies in pulmonary arterial hypertension (PAH) is important to improve long-term outcomes. Post hoc analyses of GRIPHON (NCT01106014) and exploratory analyses of TRITON (NCT02558231) suggested benefit of early selexipag initiation on long-term outcomes, despite no additional benefit versus initial double combination on haemodynamic and functional parameters in TRITON. Post hoc analyses investigated the effect of early selexipag initiation on disease progression and survival in a large, pooled PAH cohort. Data from newly diagnosed (≤6 months) PAH patients from GRIPHON and TRITON were pooled. Patients on active therapy with selexipag (pooled selexipag group) were compared with those on control therapy with placebo (pooled control group). Disease progression end-points were defined as per the individual studies. Hazard ratios (HR) and 95% CI for time to first disease progression event up to end of double-blind treatment (selexipag/placebo) +7 days and time to all-cause death up to end of study were estimated using Cox regression models. The pooled dataset comprised 649 patients, with 44% on double background therapy. Selexipag reduced the risk of disease progression by 52% versus control (HR: 0.48; 95% CI: 0.35–0.66). HR for risk of all-cause death was 0.70 (95% CI: 0.46–1.10) for the pooled selexipag versus control group. Sensitivity analyses accounting for the impact of PAH background therapy showed consistent results, confirming the appropriateness of data pooling. These post hoc, pooled analyses build on previous insights, further supporting selexipag use within 6 months of diagnosis, including as part of triple therapy, to delay disease progression.
Short abstract
This post hoc pooled analysis of GRIPHON and TRITON patients with a diagnosis of ≤6 months suggests that early targeting of the prostacyclin pathway with selexipag may be beneficial in delaying disease progression in a broad PAH population https://bit.ly/3CocEBe
Introduction
Pulmonary arterial hypertension (PAH) is a rare, progressive disorder [1, 2]. Several pathogenic pathways contribute to its progression, including the prostacyclin, endothelin and nitric oxide pathways, which can be targeted by medical treatment [1–5]. In clinical practice, drugs targeting the prostacyclin pathway, including the oral prostacyclin receptor (IP receptor) agonist selexipag, are typically initiated in PAH patients years after diagnosis [6, 7]. Selexipag is indicated to delay progression of PAH, including a reduced risk of hospitalisation. Recent analyses suggest that these effects might be optimised by early initiation of selexipag [8–10].
The TRITON randomised controlled trial assessed the impact of early use of selexipag as triple oral therapy (macitentan, tadalafil, selexipag) versus double oral therapy (macitentan, tadalafil, placebo) in newly diagnosed, treatment-naive patients with PAH, with a primary end-point of change in pulmonary vascular resistance at week 26 [8]. Though both treatment groups showed clinically meaningful improvements, no differences were observed between the treatment groups for either the primary end-point or for the secondary end-points of 6-min walk distance (6MWD) and World Health Organization functional class (WHO FC) assessed over a short-term period of 26 weeks. TRITON also evaluated PAH disease progression and survival in a blinded manner over the long-term (median follow-up duration was 77.6 and 75.8 weeks in the initial triple and initial double therapy groups, respectively). Assessment of these end-points suggested a potential benefit with initial triple versus initial double combination therapy. In the exploratory analysis of the secondary end-point of time to disease progression, the hazard ratio (HR) for initial triple versus initial double therapy for occurrence of an event was 0.59 (95% confidence intervals (Cl): 0.32–1.09) [8]. Observing an improvement in long-term outcomes without an effect on short-term parameters has been made in other disease areas but not in PAH [8]. It is therefore important to take the opportunity to further explore the potential for long-term benefits.
These findings regarding long-term outcomes in the newly diagnosed TRITON population were consistent with observations in the predominantly prevalent GRIPHON population (n=1156) [11]. In GRIPHON, selexipag reduced the risk of disease progression by 40% (composite primary end-point), irrespective of whether patients were receiving background PAH therapy. The effect of selexipag on 6MWD and WHO FC was more modest, particularly in patients receiving background therapy [11]. In a recent post hoc analysis from GRIPHON, selexipag treatment within 6 months of diagnosis reduced the risk of disease progression by 55% compared with placebo, including in a small proportion of patients on PAH background therapy, providing evidence supporting the benefits of early treatment with selexipag to delay the progression of PAH [9].
This analysis further investigated the signal observed in TRITON for improved long-term outcomes by pooling PAH patients from the GRIPHON and TRITON clinical trials and assessing the impact of initiating selexipag within 6 months of diagnosis on disease progression and survival.
Methods
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through Yale Open Data Access (YODA) Project site at http://yoda.yale.edu.
Study design
For this post hoc analysis, data from the GRIPHON (NCT01106014) and TRITON (NCT02558231) studies were pooled together, based on similarities between the two studies in rationale, objectives and study design (e.g., study treatments, observation time, end-points, adjudication of events). An overview of the individual study designs is shown in supplementary table S1. Censoring rules and follow-up between the two studies were aligned following previously described methodology [12].
Patient selection
This post hoc analysis included newly diagnosed patients from GRIPHON and TRITON, i.e., those with a diagnosis of PAH within the previous 6 months. The individual patient selection criteria by study are shown in supplementary table S1. Written informed consent was obtained for all patients before participation. Both studies adhered to the principles outlined in the amended Declaration of Helsinki and the study protocols were approved by the local institutional review board or independent ethics committee at each study site. Patients randomised to selexipag in each study were pooled to form the pooled selexipag group, and patients randomised to placebo in each study were pooled to form the pooled control group.
Analyses objectives and end-points
The main objective of this analysis was to investigate the effect of early initiation of selexipag on long-term outcomes. For the analysis of disease progression up to end of treatment period, the time to first disease progression event end-point was defined for patients with ≥1 event as the time from randomisation until the first event or, for patients with no event, until end of treatment +7 days in GRIPHON or until end of treatment +7 days or end of main observation period +7 days in TRITON (whichever was earliest). The individual components of each disease progression end-point for each study can be found in supplementary table S2. Other end-points were time to all-cause death (defined as time from randomisation to all-cause death up to study closure in GRIPHON or TRITON) and safety (treatment emergent adverse events (AEs), serious AEs and AEs leading to study treatment discontinuation).
Statistical analyses
The treatment effect of selexipag on time to disease progression up to end of treatment period or time to all-cause death up to end of study was estimated with HR and corresponding 95% CI. For the main analysis of time to disease progression or time to all-cause death, Cox proportional hazard regression was performed, using a model which included treatment, age, sex, race, PAH aetiology, region, WHO FC, 6MWD, N-terminal pro-brain natriuretic peptide (NT-proBNP) levels and study as covariates. Covariates were chosen based on their clinical importance in PAH. The Kaplan–Meier (KM) method was used to estimate event-free rates and their 95% CIs. KM curves in graphical presentations were truncated when <10% of patients remained, as per Pocock's Rule [13], and CIs were constructed using Greenwood's formula [14]. To explore potential differences between studies in the time to disease progression end-point, the censoring rules pattern and median follow-up for each study was summarised using reverse KM methodology [12, 15].
To explore the time-varying intervention effect, an additional sensitivity analysis was performed using the same model as the main analysis but adding the variable of concomitant (post-baseline) use of endothelin receptor antagonist (ERA) and phosphodiesterase type 5 inhibitor (PDE-5i) as a time-dependent covariate [16]. Owing to the relatively small number of events and patients, a subgroup analysis was performed in patients receiving an ERA and PDE-5i at baseline using a model that included only treatment, region, WHO FC and study as covariates. As this was not a randomised controlled trial and in order to explore potential differences and confounding factors between the pooled control and pooled selexipag groups, sensitivity analyses for time to disease progression using simple exact matching and propensity score weighting were performed (supplementary methods 1). Pooled safety data, collected until end of treatment +30 days for TRITON or end of study treatment +7 days for GRIPHON, were analysed descriptively as categorical variables.
Results
Patient disposition and characteristics of pooled dataset
The combined GRIPHON and TRITON dataset included 649 patients (329 in the pooled selexipag group and 320 in the pooled control group; figure 1). Overall, the demographics and baseline characteristics of both pooled treatment groups were well balanced (table 1). In both groups, most patients (78%) were female, and most commonly had idiopathic PAH (48% in the pooled selexipag and 54% in the pooled control group). The majority of patients were in WHO FC III/IV (57% in the pooled selexipag and 62% in the pooled control group) and received selexipag/placebo as part of combination therapy (77% in the pooled selexipag group and 74% in the pooled control group). In both groups, 44% of patients were already treated with double combination therapy with an ERA+PDE-5i at baseline (table 1). Based on previous data from the individual studies, ∼47%, 30% and 21% of patients received a high (1200–1600 µg), a medium (600–1000 µg) or a low (200–400 µg) dose, respectively, of selexipag [8, 9].
FIGURE 1.
Patient disposition. Disease progression end-points defined as in GRIPHON [11] and TRITON [8], respectively, up to end of double-blind treatment +7 days. Two patients were excluded from TRITON because their time since diagnosis was over 6 months (183 days). #: Placebo patients in TRITON received placebo, macitentan and tadalafil.
TABLE 1.
Demographics and baseline characteristics
| Characteristic | Pooled selexipag | Pooled control |
| Patients n | 329 | 320# |
| Female, n (%) | 256 (77.8) | 249 (77.8) |
| Age years, mean±sd | 47.3±15.1 | 47.0±15.7 |
| Time since PAH diagnosis days, median (Q1–Q3) | 24.4 (12.2–82.4) | 24.4 (9.2–67.1) |
| PAH aetiology, n (%) | ||
| Idiopathic | 158 (48.0) | 174 (54.4) |
| Associated with connective tissue disease | 109 (33.1) | 102 (31.9) |
| Associated with congenital heart disease | 26 (7.9) | 24 (7.5) |
| Drug- or toxin-induced | 18 (5.5) | 7 (2.2) |
| Heritable | 13 (4.0) | 8 (2.5) |
| Associated with HIV infection | 5 (1.5) | 5 (1.6) |
| Geographical region, n (%) | ||
| North America | 84 (25.5) | 82 (25.6) |
| Rest of the world | 245 (74.5) | 238 (74.4) |
| BMI kg·m−2, mean±sd | 26.9±5.8 | 26.2±5.9 |
| 6MWD m, mean±sd | 346.8±96.0 | 342.3±99.6 |
| NT-proBNP, n (%) ¶ | ||
| Low risk (<300 ng·L−1) | 104 (31.6) | 84 (26.3) |
| Medium risk (300–1400 ng·L−1) | 104 (31.6) | 110 (34.4) |
| High risk (>1400 ng·L−1) | 116 (35.3) | 122 (38.1) |
| WHO FC, n (%) | ||
| I/II | 141 (42.9) | 123 (38.4) |
| III/IV | 188 (57.1) | 197 (61.6) |
| Other PAH therapy+, n (%) | 254 (77.2) | 238 (74.4) |
| ERA+PDE-5i | 145 (44.1)§ | 140 (43.8) |
| PDE-5i | 88 (26.7) | 77 (24.1) |
| ERA | 21 (6.4) | 21 (6.6) |
PAH: pulmonary arterial hypertension; HIV: human immunodeficiency virus; BMI: body mass index; 6MWD: 6-min walk distance; NT-proBNP: N-terminal pro-brain natriuretic peptide; WHO FC: World Health Organization functional class; ERA: endothelin receptor antagonist; PDE-5i: phosphodiesterase-5 inhibitor. #: n=318 for 6MWD; n=319 for BMI; ¶: data were missing for five selexipag and four control patients. +: PAH therapy was started >3 months before randomisation, except for the 121 and 123 patients from TRITON who started ERA+PDE-5i therapy at randomisation. §: one TRITON patient received background PDE-5i therapy prior to randomisation.
Effect of selexipag on time to disease progression
In the pooled dataset, 67 (20%) patients in the pooled selexipag group experienced a first disease progression event versus 116 (36%) in the pooled control group (table 2). KM estimates (95% CI) for patients without an event in the pooled selexipag and pooled control groups, respectively, were 91.8% (88.0–94.4) and 84.3% (79.6–87.9) at month 6, 83.9% (79.1–87.7) and 73.0% (67.4–77.8) at month 12, and 74.3% (68.0–79.6) and 58.6% (51.7–64.9) at month 24.
TABLE 2.
Breakdown of disease progression events
| Pooled selexipag | Pooled control | |
| Patients n | 329 | 320 |
| First disease progression events up to end of treatment period#, n (%) | 67 (20.4) | 116 (36.3) |
| Hospitalisation for worsening of PAH | 29 (8.8) | 54 (16.9) |
| Clinical worsening of PAH¶ | 18 (5.5) | 45 (14.1) |
| Death | 14 (4.3) | 13 (4.1) |
| Initiation of prostacyclin for worsening of PAH | 6 (1.8) | 4 (1.3) |
| Deaths up to end of study, n (%) | 40 (12.2) | 55 (17.2) |
PAH: pulmonary arterial hypertension; 6MWD: 6-min walk distance; WHO FC: World Health Organization functional class. #: end of treatment period was defined as end of double-blind treatment +7 days in GRIPHON and end of main observation period +7 days or end of double-blind treatment +7 days in TRITON. ¶: disease progression in GRIPHON [11] defined as a decrease from baseline of ≥15% in 6MWD (confirmed by a second test on a different day) accompanied by a worsening in WHO FC (for the patients in WHO FC II or III at baseline) or the need for additional PAH therapy (for the patients in WHO FC III or IV at baseline); clinical worsening in TRITON [8] defined as a post-baseline decrease in 6MWD >15% from the highest 6MWD obtained at/after screening and WHO FC III/IV (both conditions confirmed at two consecutive post-baseline visits 1–21 days apart).
Selexipag reduced the risk of disease progression by 52% (HR: 0.48; 95% CI: 0.35–0.66) compared to control (figure 2a) up to end of treatment period. This difference was driven by hospitalisation for worsening of PAH and by clinical worsening of PAH (table 2). Similar results were also obtained in the subgroup analysis of patients receiving ERA and PDE-5i double combination therapy at randomisation (n=285). In these patients, a 48% reduction in the risk of disease progression was observed for selexipag versus control (HR: 0.52; 95% CI: 0.30–0.92; figure 2b).
FIGURE 2.
Time to disease progression up to end of treatment period in the pooled dataset: a) main analysis and b) subgroup of patients receiving ERA+PDE-5i combination therapy at randomisation. Kaplan–Meier curves illustrating time from randomisation to first disease progression event up to end of treatment period, defined as end of double-blind treatment +7 days in GRIPHON and end of main observation period +7 days or end of double-blind treatment +7 days in TRITON. Curves are cut when <10% of patients remain at risk (Pocock's rule) [13]. Kaplan–Meier estimates are shown at Months 6, 12 and 24. a) HR estimated using a Cox model which included treatment, age, sex, race, PAH aetiology, region, WHO FC, 6MWD, NT-proBNP and study as covariates. b) HR estimated using a Cox model which included treatment, region, WHO FC at baseline and study as covariates. 6MWD: 6-min walk distance; HR: hazard ratio; ERA: endothelin receptor antagonist; NT-proBNP: N-terminal pro-brain natriuretic peptide; PAH: pulmonary arterial hypertension; PDE-5i: phosphodiesterase-5 inhibitor; WHO FC: World Health Organization functional class.
In order to assess whether other PAH-specific therapies (initiated either before or after randomisation) impacted the results, ERA and PDE-5i therapy use was added as a time-dependent variable to the same model used for the main analysis. In this analysis, the HR (95% CI) for time to first disease progression event was 0.48 (0.35–0.66) for the pooled selexipag group versus the pooled control group.
Matching via simple exact matching or propensity score weighting methods was possible for almost all patients (supplementary table S3). The treatment effect of selexipag versus control on disease progression for both sensitivity analyses in matched populations was consistent with the control analysis (supplementary figure S1) and the main analysis.
Effect of selexipag on time to death
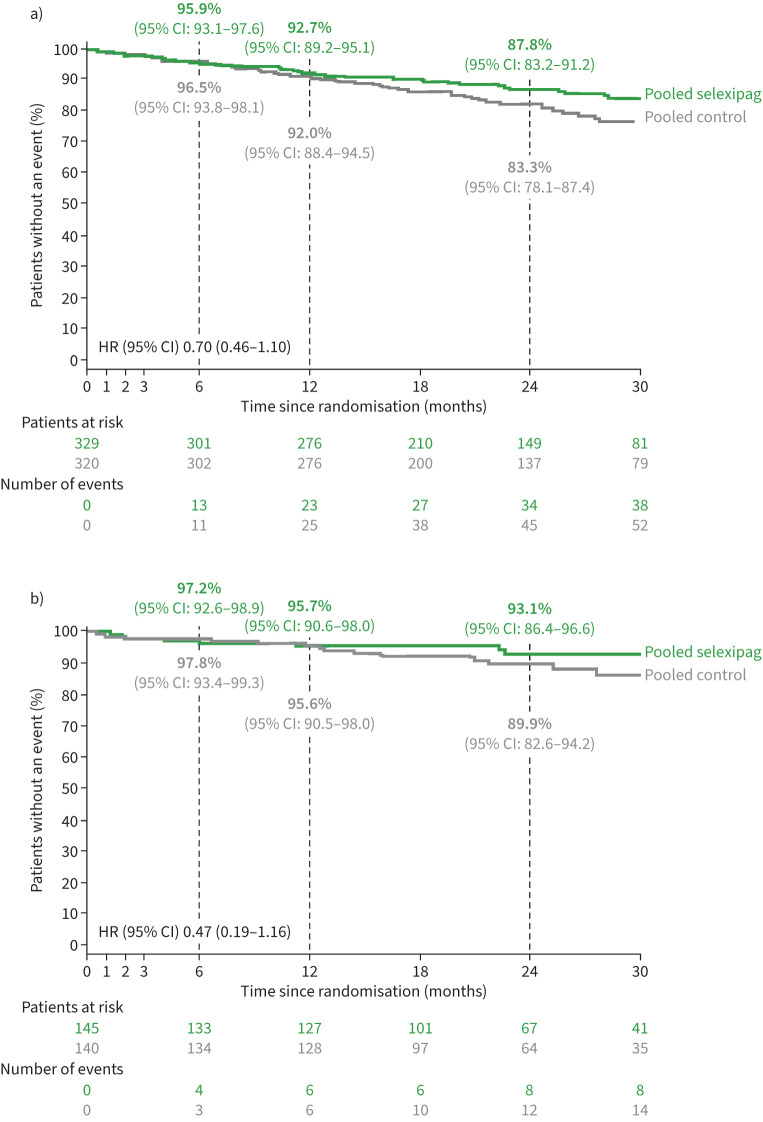
The median (Q1–Q3) follow-up time was 25.0 (16.3–31.4) months for the pooled selexipag group and 24.2 (16.8–32.7) months for the pooled control group. The median follow-up time according to each individual study is shown in supplementary table S4. There were 40 (12.2%) deaths in the pooled selexipag group and 55 (17.2%) deaths in the pooled control group. A breakdown of deaths by individual study is shown in supplementary table S5. KM estimates (95% CI) for survival in the pooled selexipag and pooled control groups, respectively, were 95.9% (93.1–97.6) and 96.5% (93.8–98.1) at month 6, 92.7% (89.2–95.1) and 92.0% (88.4–94.5) at month 12, and 87.8% (83.2–91.2) and 83.3% (78.1–87.4) at month 24. The HR for risk of all-cause death up to end of study was 0.70 (95% CI: 0.46–1.10) for the pooled selexipag versus the pooled control group (figure 3a). Results were consistent in the analysis that included ERA and PDE-5i therapy use as a time-dependent covariate (HR: 0.70; 95% CI: 0.46–1.08) and in the subgroup analysis of patients receiving background therapy with an ERA and PDE-5i (HR: 0.47; 95% CI: 0.19–1.16; figure 3b).
FIGURE 3.
Time to all-cause death up to end of study for the pooled dataset: a) main analysis and b) subgroup of patients receiving ERA+PDE-5i therapy at randomisation. Kaplan–Meier curves illustrating time from randomisation to all-cause death up to end of study. Curves are cut when <10% of patients remain at risk (Pocock's rule) [13]. Kaplan–Meier estimates are shown at Months 6, 12 and 24. a) HR estimated using a Cox model which included treatment, age, sex, race, PAH aetiology, region, WHO FC, 6MWD, NT-proBNP and study as covariates. b) HR estimated using a Cox model which included treatment, region, WHO FC at baseline and study as covariates. 6MWD: 6-min walk distance; HR: hazard ratio; ERA: endothelin receptor antagonist; NT-proBNP: N-terminal pro-brain natriuretic peptide; PAH: pulmonary arterial hypertension; PDE-5i: phosphodiesterase-5 inhibitor; WHO FC: World Health Organization functional class.
Safety
The median (min, max) exposure to study treatment was 16.7 (0.1, 42.0) and 13.4 (0.1, 43.2) months in the pooled selexipag and pooled control groups, respectively (table 3). The most common AEs are shown in table 3, with the most frequent being headache, diarrhoea, nausea and peripheral oedema. There were 85 (26%) patients in the pooled selexipag group and 100 (31%) in the pooled control group who discontinued treatment due to an AE (table 3).
TABLE 3.
Exposure and safety
| Pooled selexipag | Pooled control | |
| Patients n | 329 | 320 |
| Exposure to study treatment months, median (min, max) | 16.7 (0.1, 42.0) | 13.4 (0.1, 43.2) |
| AEs, n (%) | ||
| Patients with ≥1 AE | 323 (98.2) | 307 (95.9) |
| Patients with ≥1 serious AE | 140 (42.6) | 129 (40.3) |
| Patients with ≥1 AE leading to discontinuation of double-blind study treatment | 85 (25.8) | 100 (31.3) |
| Number of AEs | 2836 | 2259 |
| Most frequent AEs, n (%)# | ||
| Headache | 208 (7.3) | 126 (5.5) |
| Diarrhoea | 130 (4.6) | 64 (2.8) |
| Nausea | 105 (3.7) | 51 (2.2) |
| Peripheral oedema | 79 (2.8) | 76 (3.3) |
| Pain in jaw | 68 (2.4) | 20 (0.9) |
| Pain in extremity | 65 (2.3) | 24 (1.1) |
| Vomiting | 62 (2.2) | 29 (1.3) |
| PAH worsening | 53 (1.9) | 94 (4.1) |
| Dyspnoea | 49 (1.7) | 61 (2.7) |
| Myalgia | 48 (1.7) | 33 (1.5) |
| Arthralgia | 44 (1.5) | 29 (1.3) |
| Dizziness | 41 (1.4) | 47 (2.1) |
| Nasopharyngitis | 40 (1.4) | 34 (1.5) |
| Flushing | 38 (1.3) | 26 (1.1) |
| Cough | 34 (1.2) | 37 (1.6) |
| Upper respiratory tract infection | 33 (1.2) | 46 (2.0) |
| Fatigue | 32 (1.1) | 33 (1.5) |
| Dyspepsia | 32 (1.1) | 18 (0.8) |
| Anaemia | 31 (1.1) | 21 (0.9) |
| Back pain | 23 (0.8) | 24 (1.1) |
| Nasal congestion | 22 (0.8) | 24 (1.1) |
| Right ventricular failure | 18 (0.6) | 26 (1.1) |
| Gastroesophageal reflux disease | 16 (0.6) | 22 (1.0) |
AE: adverse event; PAH: pulmonary arterial hypertension. #: calculated based on the number of AEs; includes AEs with a frequency of ≥1% in either group.
Discussion
This post hoc analysis of pooled data from the GRIPHON and TRITON clinical trials showed that selexipag treatment initiated within 6 months of diagnosis reduced the risk of disease progression in a large population of PAH patients that included >600 patients, a rarity in PAH analyses, with many receiving double background therapy with an ERA and PDE-5i. Selexipag halved the risk of disease progression, and a consistent treatment effect was observed when selexipag was administered within 6 months of diagnosis as part of a triple therapy regimen. Similar to other studies in PAH that assess disease progression, this treatment effect was driven by PAH worsening and PAH-related hospitalisation [8, 11, 17, 18]. The nature and severity of the reported AEs reflect the safety profile of selexipag and the underlying morbidity and/or mortality of PAH populations.
PAH is a progressive disease with a current treatment paradigm recommending multiple therapies, often in combination [3–5]. In this context, it is important to consider the long-term management of patients with the goal to delay disease progression. In the GRIPHON study, selexipag significantly delayed disease progression, especially in newly diagnosed patients, and led to a moderate effect on 6MWD [9, 11]. In the TRITON study, there was no effect of selexipag compared to placebo on short-term end-points, such as haemodynamic status or 6MWD, but there was a signal that early selexipag treatment may reduce the risk of PAH disease progression. The current analysis complements this latter finding by demonstrating consistent results in a larger dataset with assessment of a greater number of events. In addition, the pooled survival data show a trend consistent with the data for time to disease progression. Previous data have indicated that the treatment effect of selexipag on disease progression is not impacted by use of other PAH therapies [9, 11] which is also demonstrated by the results reported here. Accounting for the potential impact of other PAH therapies did not affect the benefit of early selexipag initiation on disease progression observed in the pooled cohort, including in patients for whom selexipag was added on top of double oral combination therapy (i.e., ERA and PDE-5i combination therapy). These data support the signal observed in TRITON for a reduced risk of disease progression with initial triple versus initial double combination therapy. However, as short-term haemodynamic or functional parameters such as 6MWD were not investigated within this analysis, no additional insights could be gained into the discrepancy between the effect of triple versus double oral combination therapy on short-term haemodynamic and functional parameters versus long-term outcomes. These results are also important as they support the targeting of the three established pathogenic pathways in PAH [1] within 6 months of diagnosis and are in line with the recommendations for combination therapy in today's management of PAH patients [3–5]. However, as data were only available on the treatment effect of selexipag added to an ERA+PDE-5i, no conclusions could be drawn on what the outcomes on disease progression might have been with different treatment combinations.
Pooling data from GRIPHON and TRITON was considered appropriate as time to disease progression was assessed in a similar way in both studies, albeit as a primary end-point in GRIPHON and as a secondary end-point (in an exploratory analysis) in TRITON. These end-points were assessed over a comparable follow-up time and with similar censoring rules applied [8, 11]. Both studies allowed long-term analysis of time to disease progression and were similar in design and included similar study populations of newly diagnosed patients, further supporting the appropriateness to pool data from these two studies. Consistent results were also observed in additional analyses using time-dependent variables and sensitivity analyses that used patient matching methodologies to account for other differences between groups. Pooling data from clinical trials, taking into account the associated caveats, allows analyses in larger populations [19], which is specifically relevant in rare diseases, such as PAH. Here, it provided the opportunity to further investigate the efficacy of early initiation of selexipag on outcomes in a large population of >600 PAH patients.
This post hoc analysis of pooled clinical trial data is subject to limitations. No systematic review to identify additional studies in databases or registries was performed, and therefore results were not reported according to the PRISMA guidelines [20]. Pooling of the data from both clinical trials was performed at the patient level. For the analysis of the subgroup of patients receiving double combination therapy at randomisation fewer variables were included in the model as compared to that of the main model due to patient and event numbers. The confidence intervals for analyses in this subgroup were wide, especially for the time to all-cause death analysis, and the results should be interpreted with caution. Despite the strong similarities between the two studies, and although sensitivity analyses were performed to show that results were consistent across studies, some differences may still have impacted the current results. These include possible variations in clinical practice at the time of the studies (enrolment for GRIPHON was between 2009 and 2013 whereas screening for TRITON was between 2016 and 2018) and differences in the countries/sites present (GRIPHON was conducted worldwide while TRITON was conducted in North America and Europe).
Conclusions
This post hoc analysis of pooled data from the GRIPHON and TRITON clinical trials suggests that selexipag use within 6 months of diagnosis is beneficial in delaying disease progression in PAH, including as part of a combination therapy regimen.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00456-2022.SUPPLEMENT (545.6KB, pdf)
Acknowledgements
Biostatistical programming support was provided by Lorenzo Accarri of Valos (Genoa, Italy) and medical writing support was provided by Laura Corbett of eluSCIdate Ltd (Meggen, Switzerland). Both were funded by Actelion Pharmaceuticals Ltd, a Janssen Pharmaceutical Company of Johnson & Johnson.
Provenance: Submitted article, peer reviewed.
This article has an editorial commentary: https://doi.org/10.1183/23120541.00592-2022
Support statement: Study funded by Actelion Pharmaceuticals Ltd, a Janssen Pharmaceutical Company of Johnson & Johnson. Funding information for this article has been deposited with the Crossref Funder Registry.
Conflicts of interest: J.G. Coghlan has received grants, speaker fees and support for attending meetings from Janssen Pharmaceutical Companies of Johnson & Johnson; and has received consulting fees from Janssen Pharmaceutical Companies of Johnson & Johnson, Acceleron, Bayer and Vicore.
Conflicts of interest: S. Gaine has served as a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson; has received speaker fees from Janssen Pharmaceutical Companies of Johnson & Johnson, Bayer and AOP; and has served on advisory boards/data safety monitoring boards for Janssen Pharmaceutical Companies of Johnson & Johnson, United Therapeutics, Gossamer Bio and Altavant.
Conflicts of interest: R. Channick has served as a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson; has served on an advisory board for Janssen Pharmaceutical Companies of Johnson & Johnson and Bayer; has received consultancy fees from Bayer, Gossamer, Third Pole, Acceleron, Arena Pharmaceuticals and Janssen Pharmaceutical Companies of Johnson & Johnson; and has received speaker fees from Janssen Pharmaceutical Companies of Johnson & Johnson and Bayer.
Conflicts of interest: K.M. Chin has served as a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson; has received research grants from Janssen Pharmaceutical Companies of Johnson & Johnson, Altavant, Acceleron, United Therapeutics, Pfizer, Merck, Gossamer Bio; has received support for travel to meetings from Janssen Pharmaceutical Companies of Johnson & Johnson; and has received consultancy fees from Janssen Pharmaceutical Companies of Johnson & Johnson, Altavant, Acceleron, United Therapeutics and Gossamer Bio.
Conflicts of interest: C. du Roure is an employee of Janssen Pharmaceutical Companies of Johnson & Johnson.
Conflicts of interest: J.S.R. Gibbs has served as a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson, Complexa and Acceleron/Merck; has received consultancy fees from Acceleron/Merck; has served as a clinical endpoints committee member for Pfizer, Aerovate, Janssen Pharmaceutical Companies of Johnson & Johnson and United Therapeutics; and has served as a data and safety monitoring board member for Janssen Pharmaceutical Companies of Johnson, Merck Sharp & Dohme, Gossamer Bio and Bial.
Conflicts of interest: M.M. Hoeper has served as a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson; has received speaker and consultancy fees from Janssen Pharmaceutical Companies of Johnson & Johnson, Bayer, GlaxoSmithKline, Merck Sharp & Dohme, and Pfizer; and has received research grants from Janssen Pharmaceutical Companies of Johnson & Johnson.
Conflicts of interest: I.M. Lang has served as a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson; has received research grants from Janssen Pharmaceutical Companies of Johnson & Johnson and AOP Orphan Pharmaceuticals; has received consultancy fees from Janssen Pharmaceutical Companies of Johnson & Johnson, AOP Health, Bayer, Ferrer and United Therapeutics; has received speaker fees from Janssen Pharmaceutical Companies of Johnson & Johnson, AOP Health and Bayer; has received support for attending meetings from Janssen Pharmaceutical Companies of Johnson & Johnson, AOP Health, Bayer, Ferrer and Medtronic.
Conflicts of interest: S.C. Mathai has served as a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson; has received consultancy fees from Acceleron, Bayer, United Therapeutics and Janssen Pharmaceutical Companies of Johnson & Johnson; has received speaker fees from Practice Point CME; has participated on an advisory board for United Therapeutics; and has had a leadership role on the PCORI, Rare Disease Advisory Panel and served as ACCP PVD Network Chair.
Conflicts of interest: V.V. McLaughlin has served as a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson; reports grant support from Aerovate, Altavant, Gossamer-Bio, Janssen Pharmaceutical Companies of Johnson & Johnson, Merck and Sonovie; and has received consultancy fees from Aerami, Aerovate, Altavant, Bayer, Caremark, L.L.C., Corvista, Gossamer Bio, Janssen Pharmaceutical Companies of Johnson & Johnson, Merck and United Therapeutics.
Conflicts of interest: L. Mitchell is an employee of Janssen Pharmaceutical Companies of Johnson & Johnson.
Conflicts of interest: G. Simonneau has served as a steering committee member for and received research grants from Janssen Pharmaceutical Companies of Johnson & Johnson; and has received speaker and consultancy fees from Janssen Pharmaceutical Companies of Johnson & Johnson, Bayer, GlaxoSmithKline, Merck Sharp & Dohme and Pfizer.
Conflicts of interest: O. Sitbon has served as a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson and Gossamer Bio; has received research grants from Janssen Pharmaceutical Companies of Johnson & Johnson, Acceleron Pharmaceuticals, GlaxoSmithKline, and Merck Sharp & Dohme; has received consultancy fees from Janssen Pharmaceutical Companies of Johnson & Johnson, AOP Orphan, Ferrer Gossamer Bio and Merck Sharp & Dohme and United Therapeutics; has received speaker fees from Janssen Pharmaceutical Companies of Johnson & Johnson, AOP Orphan, Ferrer and Merck Sharp & Dohme; and has served on an advisory board for Acceleron Pharmaceuticals, Altavant Pharmaceuticals, Gossamer Bio, Janssen Pharmaceutical Companies of Johnson & Johnson and Merck Sharp & Dohme.
Conflicts of interest: V.F. Tapson has served as a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson, Bayer and United Therapeutics; has received consultancy fees from Janssen Pharmaceutical Companies of Johnson & Johnson, Arena Pharmaceuticals, Bayer, Daiichi-Sankyo, EKOS/BTG and United Therapeutics; has received research grants from Arena Pharmaceuticals, Arena, and Bayer; has received speaker fees from Janssen Pharmaceutical Companies of Johnson & Johnson; and serves as Vice President of Medical Affairs at Inari Medical.
Conflicts of interest: N. Galiè is a steering committee member for Janssen Pharmaceutical Companies of Johnson & Johnson; has received grant support from Janssen Pharmaceutical Companies of Johnson & Johnson; has received consulting fees and speaker fees from Janssen Pharmaceutical Companies of Johnson & Johnson, Ferrer and Pfizer.
References
- 1.Hemnes AR, Humbert M. Pathobiology of pulmonary arterial hypertension: understanding the roads less travelled. Eur Respir Rev 2017; 26: 170093. doi: 10.1183/16000617.0093-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humbert M, Guignabert C, Bonnet S, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J 2019; 53: 1801887. doi: 10.1183/13993003.01887-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galiè N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019; 53: 1801889. doi: 10.1183/13993003.01889-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2022; in press [ 10.1183/13993003.00879-2022]. [DOI] [PubMed] [Google Scholar]
- 5.Humbert M, Kovacs G, Hoeper MM, et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Heart J 2022; 43: 3618–3731. doi: 10.1093/eurheartj/ehv317 [DOI] [PubMed] [Google Scholar]
- 6.Del Pozo R, Hernandez Gonzalez I, Escribano-Subías P. The prostacyclin pathway in pulmonary arterial hypertension: a clinical review. Expert Rev Respir Med 2017; 11: 491–503. doi: 10.1080/17476348.2017.1317599 [DOI] [PubMed] [Google Scholar]
- 7.Farber HW, Miller DP, Meltzer LA, et al. Treatment of patients with pulmonary arterial hypertension at the time of death or deterioration to functional class IV: insights from the REVEAL Registry. J Heart Lung Transplant 2013; 32: 1114–1122. doi: 10.1016/j.healun.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 8.Chin KM, Sitbon O, Doelberg M, et al. Three- versus two-drug therapy for patients with newly diagnosed pulmonary arterial hypertension. J Am Coll Cardiol 2021; 78: 1393–1403. doi: 10.1016/j.jacc.2021.07.057 [DOI] [PubMed] [Google Scholar]
- 9.Gaine S, Sitbon O, Channick RN, et al. Relationship between time from diagnosis and morbidity/mortality in pulmonary arterial hypertension: results from the phase III GRIPHON study. Chest 2021; 160: 277–286. doi: 10.1016/j.chest.2021.01.066 [DOI] [PubMed] [Google Scholar]
- 10.Sitbon O, Jaïs X, Savale L, et al. Upfront triple combination therapy in pulmonary arterial hypertension: a pilot study. Eur Respir J 2014; 43: 1691–1697. doi: 10.1183/09031936.00116313 [DOI] [PubMed] [Google Scholar]
- 11.Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015; 373: 2522–2533. doi: 10.1056/NEJMoa1503184 [DOI] [PubMed] [Google Scholar]
- 12.de Jong VMT, Moons KGM, Riley RD, et al. Individual participant data meta-analysis of intervention studies with time-to-event outcomes: a review of the methodology and an applied example. Res Synth Methods 2020; 11: 148–168. doi: 10.1002/jrsm.1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pocock SJ, Clayton TC, Altman DG. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet 2002; 359: 1686–1689. doi: 10.1016/S0140-6736(02)08594-X [DOI] [PubMed] [Google Scholar]
- 14.Collett D. Modelling Survival Data in Medical Research. Chapman & Hall/CRC Texts in Statistical Science series. Boca Raton, Chapman & Hall/CRC, 2015. [Google Scholar]
- 15.Sathish N, Chia-Ling WA. Let's Flip: An Approach to Understand Median Follow-up by the Reverse Kaplan-Meier Estimator from a Statistical Programmer's Perspective. www.pharmasug.org/proceedings/2019/ST/PharmaSUG-2019-ST-081.pdf Date last accessed: 6 April 2022.
- 16.Powel TM, Bagnell ME. . Your “Survival” guide to using time-dependent covariates. SAS Global Forum 2012: 168–2012. [Google Scholar]
- 17.Galiè N, Barbera JA, Frost AE, et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844. doi: 10.1056/NEJMoa1413687 [DOI] [PubMed] [Google Scholar]
- 18.Pulido T, Adzerikho I, Channick R, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013; 369: 809–818. doi: 10.1056/NEJMoa1213917 [DOI] [PubMed] [Google Scholar]
- 19.Bangdiwala SI, Bhargava A, O'Connor DP, et al. Statistical methodologies to pool across multiple intervention studies. Transl Behav Med 2016; 6: 228–235. doi: 10.1007/s13142-016-0386-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00456-2022.SUPPLEMENT (545.6KB, pdf)