Abstract
This report describes a case of Sphingomonas paucimobilis bacteremia and infective endocarditis with a mycotic aneurysm in a young patient with Crohn’s disease. Our patient reported prolonged intermittent fever followed by sudden hemiparesis and a tonic clonic convulsion. His blood cultures showed growth of Sphingomonas paucimobilis, and further cardiac imaging revealed the presence of a vegetation with severe valvular regurgitation. Cerebral angiography showed middle cerebral artery occlusion and aneurysm. The patient was treated with meropenem for 6 weeks, coupled with aortic valve replacement, and coiling of the aneurysm. Our patient recovered with good results. S. paucimobilis is an opportunistic gram-negative pathogen of growing importance in the clinical setting.
Keywords: Sphingomonas paucimobilis, Bacteremia, Infective endocarditis, Mycotic cerebral aneurysm, Ischemic cerebrovascular event
Highlights
-
•
Sphingomonas paucimobilis is present in hospital settings, especially around water sources.
-
•
Implicated in a variety of nosocomial and community-acquired infections of various sites and severity.
-
•
Immunosuppression, malignancy, and diabetes mellitus are its associated comorbidities.
-
•
It is rarely the causative organism of infective endocarditis with a favorable prognosis.
Introduction
Sphingomonas paucimobilis is a fairly uncommon opportunistic pathogen causing infections in humans. It is typified as a yellow pigmented strictly aerobic non-fermenting gram-negative bacterium, with weak motility due to the presence of a single polar flagellum [1]. Since its initial isolation in 1977, case reports of a wide variety of infections are being increasingly published. We report a case of native valve infective endocarditis with mycotic aneurysm and ischemic stroke in a young patient with Crohn’s disease on immunosuppressive therapy. To our knowledge, this is the first reported case of this bacterium causing infective endocarditis in Lebanon.
Case presentation
A 28-year-old male patient, smoker, with a six-year history of Crohn’s disease on treatment by adalimumab injections was first admitted for epigastric abdominal pain, and intermittent fever and chills for 4 months, for which he has recently taken an unknown antibiotic without improvement. He had no significant medical or surgical history. He works as a local driver with no history of travel. Physical exam was nonrevealing. Inflammatory markers were elevated, and an extensive workup, shown in Table 1, only revealed flare up of Crohn’s disease of the small intestine by colonoscopy and biopsy. All of blood, urine, and stool cultures showed no growth. Fever only lasted three days, then resolved without in-hospital antibiotics.
Table 1.
Workup done during the first admission.
| Lab test | Parameter | Result | Reference Range |
|---|---|---|---|
| Blood cell count and differential | White blood cells | 11,400/mm³ | 4000–11000 |
| Neutrophils (%) | 74.4 | 40–65 | |
| Lymphocytes (%) | 20.7 | 25–40 | |
| Hemoglobin (g/dl) | 11.6 | 13–18 (male) | |
| Platelets | 355 × 1000/mm³ | 150–400 | |
| Inflammatory markers | C-reactive Protein (mg/l) | 150 | 0–5 |
| Erythrocyte Sedimentation Rate | 33 mm/hr | 0–20 | |
| Kidney Function | Blood urea nitrogen (mg/dl) | 8 | 7–28 |
| Creatinine (mg/dl) | 0.66 | 0.6–1.3 | |
| Liver Function tests | SGOT (IU/l) | 28 | 40 |
| SGPT (IU/l) | 44 | 40 | |
| GGT (IU/l) | 49 | 8–37 | |
| Alkaline phosphatase (IU/l) | 77 | 35–120 | |
| Total Bilirubin (mg/dl) | 0.33 | 0.1–1.2 | |
| Direct Bilirubin (mg/dl) | 0.09 | 0–0.2 | |
| Lipase (IU/l) | 17 | 0–160 | |
| Other | LDH (IU/l) | 231 | 91–180 |
| Viral Panel | CMV IgG (AU/ml) | 246.9 | Positive |
| CMV IgM (AU/ml) | 0.33 | Not reactive | |
| CMV PCR (Serum) | Not Detected | ||
| EBV IgM | 0.02 | Negative | |
| EBV IgG | 3.21 | Positive | |
| Brucella Titers | Direct & Indirect Agglutinins | < 1/40 | Negative |
| Salmonella Agglutinins (Widal Test) | TO; TH; AH; BH | < 1/40 | Negative |
| Stool | Analysis | No Ova and parasites, few WBC | |
| Culture | No salmonella, No shigella | ||
| Urine | Analysis | 6–10 WBC | |
| Culture | No Growth | ||
| Blood Cultures | Culture (x6 bottles) | No Growth |
CMV: cytomegalovirus, EBV: Epstein-Barr Virus, GGT: gamma-glutamyltransferase, LDH: lactate dehydrogenase, SGOT: serum glutamic oxaloacetic transaminase, SGPT: serum glutamate-pyruvate transaminase, WBC: white blood cells.
Two months later, the patient presented for right sided upper and lower limbs weakness, right sided facial weakness with left mouth deviation, and slurred speech that started early in the morning of the visit, followed by a witnessed tonic clonic convulsion. He reported intermittent fever since his discharge, and denied having headaches, confusion, and any change in personality. He was febrile (38.9 °C) at presentation, heart rate 95 bpm, blood pressure130/90 mmHg, oxygen saturation 95% on room air, and glucose was 122 mg/dl. Glasgow coma scale was 15/15. He had slurred speech, right sided sensory and motor weakness in the lower two-thirds of the face, with left sided mouth deviation, as well as right upper and lower limbs motor weakness (motor power was 2/5 in both affected extremities vs. 5/5 in the left extremities), as well as sensory deficits in the aforementioned limbs. There was no neck stiffness. Examination of his oral cavity revealed oropharyngeal candidiasis. Auscultation of the heart showed regular heart sounds, with no audible murmur. Physical exam was otherwise nonrevealing.
ECG showed sinus tachycardia. Urgent nonenhanced brain CT did not show any signs of ischemia or space occupying lesions. Three sets of blood cultures were drawn, spaced thirty minutes apart, from three different sites. Lumbar puncture (LP) was done in the emergency department to rule out meningitis. Awaiting the results of the LP, infectious diseases consultation was obtained, and the patient was started on broad spectrum empirical antibiotics. The differential diagnosis included chronic fungal meningitis or endocarditis with septic embolization, due to the nature of the presentation. Therefore, he was started on meropenem 1 g every 8 h intravenously, vancomycin 1.5 g loading dose then 1 g every 12 h intravenously, fluconazole at a loading dose of 800 mg followed by maintenance dose of 400 mg once daily intravenously, and acyclovir 750 mg every 8 h intravenously. The CSF analysis showed the presence of 10 white blood cells, with 65% polymorphonuclear cells and 35% lymphocytic cells. CSF protein was 0.36 (reference range 0.15–0.45 g/l), CSF glucose was 71 (reference range 40–70 mg/dl), and CSF gram stain showed no organisms. He was also started on aspirin 100 mg orally once daily, enoxaparin 40 mg subcutaneously twice daily, and atorvastatin 40 mg orally once daily for the acute ischemic event. His initial labs showed leucocytosis WBC 16,000/mm³ (normal range 4,000- 11,000) with 83% neutrophils (normal range 40–65%). CRP and ESR were elevated, 87 mg/l (normal range <5 mg/l), and 91 mm/h respectively. Full workup is shown in Table 2.
Table 2.
workup done during the second admission.
| Lab test | Parameter | Result | Reference Range |
|---|---|---|---|
| Blood cell count and differential | White blood cells | 16,000/mm³ | 4000–11000 |
| Neutrophils (%) | 83.7 | 40–65 | |
| Lymphocytes (%) | 11.1 | 25–40 | |
| Hemoglobin (g/dl) | 11.6 | 13–18 (male) | |
| Platelets | 312 × 1000/mm³ | 150–400 | |
| Inflammatory markers | C-reactive Protein (mg/l) | 87.1 | 0–5 |
| Erythrocyte Sedimentation Rate | 91 mm/hr | 0–20 | |
| Procalcitonin | 0.049 | ||
| Kidney Function | Blood urea nitrogen (mg/dl) | 11 | 7–28 |
| Creatinine (mg/dl) | 0.81 | 0.6–1.3 | |
| Liver Function tests | SGOT (IU/l) | 19 | 40 |
| SGPT (IU/l) | 25 | 40 | |
| GGT (IU/l) | 31 | 8–37 | |
| Total Bilirubin (mg/dl) | 0.43 | 0.1–1.2 | |
| Direct Bilirubin (mg/dl) | 0.10 | 0–0.2 | |
| Other | LDH (IU/l) | 210 | 91–180 |
| Homocysteine (umol/l) | 16.36 | 5.46–16.2 | |
| CSF | WBC | 10/mm³ | |
| RBC | None seen | ||
| PMN | 65% | ||
| Lymphocytes | 35% | ||
| Protein (g/l) | 0.36 | 0.15–0.45 | |
| Glucose (mg/dl) | 71 | 40–70 | |
| Gram stain and culture | Negative | ||
| Viral CSF Panel (neuro9 Multiplex) | HSV1, HSV2, VZV, EBV, CMV, HHV6, HHV7, Adenovirus, Human Parechovirus, Human Enterovirus, Mumps virus, & Parvovirus B19 | Not detected | |
| Lipid Panel | Cholesterol (mg/dl) | 233 | < 200 |
| Triglycerides (mg/dl) | 120 | 36–160 | |
| HDL (mg/dl) | 39 | 29–71 | |
| LDL-c (mg/dl) | 170 | 130 | |
| Chol/HDL ratio | 6 | ||
| Endocrinology | TSH (mIU/ml) | 0.215 | 0.27–4.2 |
| HbA1c (%) | 5.8 | ||
| Coagulation | Prothrombin time activity (%) | 91 | |
| INR | 1.07 | ||
| Bleeding time (min/sec) | 5.2 | 1–9 | |
| Coagulation Time (min/sec) | 4 | 4–8 | |
| Urine | Analysis | 3–5 WBC | |
| Culture | No Growth | ||
| Autoimmunity | Factor V Leiden (G1691A) PCR | Not detected | |
| Anticardiolipin Antibodies (IgA, G, M) (RU/ml) | 10.84 | < 12 | |
| Anti-B2 Glycoprotein (IgA,M,G) (RU/ml) | 21.21 | < 20 | |
| Electroencephalogram (EEG) | No epileptic activity | ||
| Ultrasound Doppler Carotids | Normal caliber and flow | ||
Chol: cholesterol, GGT: gamma-glutamyltransferase, HDL: high-density lipoprotein, LDH: lactate dehydrogenase, PCR: polymerase chain reaction, SGOT: serum glutamic oxaloacetic transaminase, SGPT: serum glutamate-pyruvate transaminase, TSH: thyroid-stimulating hormone, WBC: white blood cells.
An MRI/MRA of the brain revealed an acute ischemic stroke in the left lentiform nucleus, part of the left caudate nucleus, and the left external capsule, with thrombosis of the left middle cerebral artery (Fig. 1). CSF culture showed no growth. A neuro9 PCR panel performed on the CSF was negative for viral etiology. Thus, with these findings on neuroimaging, a diagnosis of central nervous system infection was ruled out, and the pleocytosis found in the CSF was considered to be related to the seizure. Furthermore, the blood cultures drawn initially grew Sphingomonas paucimobilis, but unfortunately an antibiogram for this bacterium was unavailable at the hospital. On the third day of admission, a transthoracic echocardiogram (TTE) revealed a bicuspid aortic valve with a very mobile 0.9 × 0.8 cm echodense mass in the left ventricular outflow tract (anterior mitral leaflet and aortic valve) along with mild eccentric aortic regurgitation, and a preserved ejection fraction. Thus, a diagnosis of infective endocarditis was established, and further workup was continued to rule out a mycotic aneurysm. At this point, the patient was continued on meropenem, as the possibility of a resistance could not be ruled out, and the patient showed clinical and biochemical improvement on this regimen. Acyclovir was stopped, but fluconazole was continued for the oropharyngeal candida infection.
Fig. 1.
: MRI Brain on day 1 (above a to d) and day 21 (below A to D) showing acute ischemia at the level of the left lentiform nucleus, part of the left caudate nucleus, and the left external capsule (arrow heads); (e): thrombosis of the left middle cerebral artery (star); (E): Recanalization of the middle cerebral artery with suspicion of a 4 mm aneurysm (arrow).
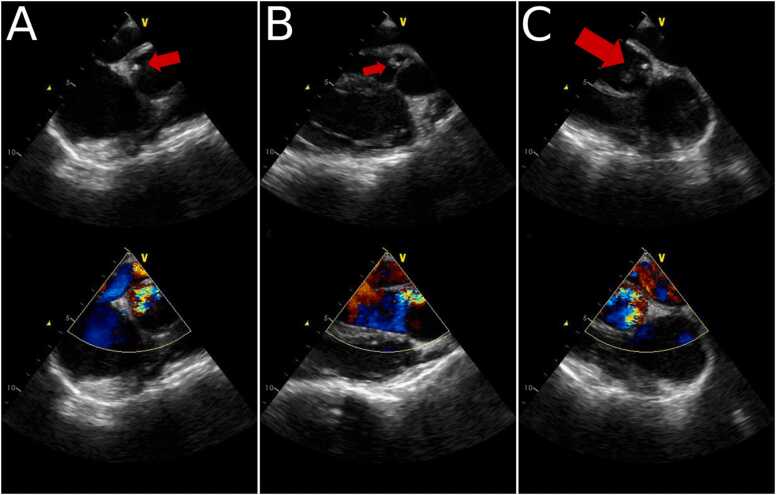
All of the subsequent blood cultures were negative for bacterial growth. Cerebral arteriography showed left MCA bifurcation aneurysm, left M1 branch stenosis, and left M2 superior division occlusion. A follow up transthoracic echocardiogram, done 20 days after the first TTE, showed a slight decrease in the size of the vegetation 0.6 × 0.6 cm, with significant aortic regurgitation. Transesophageal echocardiogram was done at day 27 of admission. It showed the mobile echodense mass attached to the aortomitral fibrosa, of 0.4 × 0.4 cm in size, with severe aortic valve regurgitation, and a preserved ejection fraction (Fig. 2).
Fig. 2.
Transesophageal echocardiography reveals the presence of a vegetation at the aortomitral fibrosa (arrows) with aortic valve regurgitation.
During this period the patient was afebrile, and clinically improving. There was significant amelioration in his neurological deficits. The inflammatory markers decreased. The size of the vegetation was decreasing, however, the aortic valve regurgitation was increasing in severity, so the patient was referred to a major cardiac center for surgical management, where he underwent coiling of the aneurysm, and aortic valve replacement. Serial follow ups up to a year and a half after his surgery were done, the patient had no complications, nor residual neurological deficits.
Discussion
Sphingomonas paucimobilis is the type species of the genus sphingomonas, which comprises at least 95 species, of whom only Sphingomonas paucimobilis is a known human pathogen [2]. Isolated in 1977, it was initially called seudomonas paucimobilis [1], but the name was later changed to Sphingomonas paucimobilis in 1990, due to the presence of sphingoglycolipids in the cell membrane [3]. Occurring broadly in soil and water, S. paucimobilis has been repeatedly isolated from various nosocomial settings [4]. These include hospital water sources and various medical equipment such as faucet aerators, showerheads, oxygen flow meters, temperature probes, distilled water containers [5], and waterpipes and hemodialysis machines [6]. Beside these sites, a case series study reporting an outbreak of S. paucimobilis in a haematology/oncology unit has shown that this bacterium can even be found on the hands of hospital workers and staff [7]. Furthermore, it has been demonstrated to have the ability to form biofilms in water systems [8]. Due to this ubiquitous environmental and nosocomial nature, this bacterium has been implicated in a wide variety of both community and hospital-acquired infections [4]. Diabetes mellitus and alcoholism have been demonstrated to be significant risk factors [9], and case series analysis have shown that the most common comorbidities associated with S. paucimobilis are malignancy, immunosuppressant use, and diabetes mellites [10]. Infections caused by S. paucimobilis are rarely fatal, and the bacterium is considered to be of low virulence due to the lack of lipid A in its outer membrane [11]. A retrospective review by Toh et al. found a low overall mortality rate of 5.5% (3 of 55 patients) [9]. Despite being a fairly-uncommon pathogen, a large array of infections is being increasingly reported, with the most common being bacteremia. Other infections include peritonitis, urinary tract infections, lower respiratory tract infections, skin and soft tissue infections, endophthalmitis, diarrheal diseases, septic arthritis, osteomyelitis, cervical adenitis, catheter-related blood stream infections, biliary tract infections, and meningitis [10], [12].
The patient in our case report was first admitted for a flare-up of Crohn’s disease, with negative blood cultures and a fever that resolved as the flare up was contained. He, then, started having new febrile episodes after his discharge that were not investigated. The patient’s clinical improvement during the first hospitalization that paralleled the improvement of his gastrointestinal symptoms without in-hospital antibiotics, pointed our team to consider the possible nosocomial acquisition of this bacterium, considering its widespread colonization of the hospital environment and instruments. As our patient was not a consumer of alcohol, and is not a known diabetic, his only suggested risk factors, therefore, were immunosuppression and the frequent exposure to healthcare settings. Upon his second admission, the patient presented with fever alongside neurological symptoms compatible with cerebral ischemia which later proved to be a mycotic aneurysm. He’s had positive bacterial growth of S. paucimobilis from his initial blood cultures, and subsequent blood cultures showed no growth. Eventually cardiac imaging demonstrated the presence of a mobile mass causing progressive valvular insufficiency. According to the modified Duke’s criteria, a definitive diagnosis of infective endocarditis can be fulfilled if two major criteria, one major criterion and three minor, or five minor criteria are met [13]. The patient in our case report has one major criterion (echocardiogram positive for infective endocarditis), and three minor criteria (fever, blood culture not meeting major criteria, and mycotic aneurysm), thus establishing a diagnosis of infective endocarditis.
The susceptibility patterns for S. paucimobilis have been inconsistent among published case reports and series, and treatment has been individualized to each susceptibility profile. Due to the production of chromosomally encoded beta-lactamases, S. paucimobilis is usually resistant to penicillins and first generation cephalosporins [14]. Previous studies have proposed that the antibiotics of choice for treatment of this bacterium are third-generation cephalosporins or aminoglycosides, however a review by Cheong et al. has shown a higher resistance pattern to these two classes of antibiotics than previously published [15]. A study conducted by Toh et al. has illustrated good susceptibility profiles to aminoglycosides, carbapenems, and TMP-SMX, with variable resistance patterns to third-generation cephalosporins and fluoroquinolones [9]. A case series by Lin et al. has shown that the most effective antimicrobials were fluoroquinolones, β-lactam/β-lactamase inhibitor combinations, carbapenems, and aminoglycosides [10]. One recently published case report of early prosthetic valve endocarditis presented a multidrug-resistant S. paucimobilis that was only susceptible to tigecycline [16]. Our patient was treated with a 6-week meropenem regimen in the lack of an antibiogram to this germ. Taking into consideration his immunosuppression and recent hospitalization, it was preferred to select a broad-spectrum agent with fewer resistance profiles.
Reviewing the current literature, we found four case reports of native valve endocarditis caused by Sphingomonas paucimobilis, and one early prosthetic valve endocarditis. None of the cases report a mycotic aneurysm or neurological symptoms. The first case presented by Rognrud et al. from USA reports an immunocompetent patient with contact to refrigerators and cooling systems, which were assumed to be the source of acquisition of the bacterium, who developed prolonged fever similar to our patient, and found to have S. paucimobilis endocarditis involving the mitral valve. He underwent cardiovascular surgery and was treated successfully with meropenem for 6 weeks [17]. Another case report published in Mexico in 2006 reported a case of native aortic valve bacterial endocarditis caused by S. paucimobilis [18]. A retrospective study analysing the clinical features and mortality in patients with infective endocarditis in Taiwan found one case of S. paucimobilis as the etiological agent [19]. Comparably, another retrospective study addressing the epidemiology and the prognosis of healthcare-associated endocarditis in China included a single case of infective endocarditis caused by S. paucimobilis [20]. And finally, Saboe et al. has recently published a fatal case of MDR S. paucimobilis causing an early prosthetic valve endocarditis with multivalvular involvement and perivalvular abscess, that was only sensitive to tigecycline [16].
Conclusion
In conclusion we report a case of infective endocarditis with mycotic aneurysm caused by a low virulence gram negative bacterium that is being reported increasingly throughout the literature. Immunosuppression has been suggested as a risk factor, as the case with our patient. More standardized data need to be published regarding its treatment, as it may have deleterious complications on the patients. This bacterium needs to be recognized as an important nosocomial pathogen that is widely distributed around the hospital setting with the potential to cause various types of infections.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Farah Assi: conceptualization, writing (original draft), data analysis. Ramzi Hammoud: writing (review and editing). Ahmad Ezzedine: data collection. Hasan Rahal: project supervision.
Conflicts of interest
None.
Ethical approval
Ethical approval was waived based on the observational nature of the report.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Contributor Information
Farah Assi, Email: f.assi-md@outlook.com.
Ramzi Hammoud, Email: hammoudramzi.md@hotmail.com.
Ahmad Ezzedine, Email: ahmad.m.ezzedine@gmail.com.
Hasan Rahal, Email: dr_hrahal@hotmail.com.
References
- 1.Holmes B., Owen R.J., Evans A., Malnick H., Willcox W.R. Pseudomonas paucimobilis, a new species isolated from human clinical specimens, the hospital environment, and other sources. Int J Syst Bacteriol. 1977;27(2) 133–46. [Google Scholar]
- 2.Chaudhary D.K., Kim J. Sphingomonas naphthae sp. nov., isolated from oil-contaminated soil. Int J Syst Evol Microbiol. 2016;66(11):4621–4627. doi: 10.1099/ijsem.0.001400. [DOI] [PubMed] [Google Scholar]
- 3.Yabuuchi E., Yano I., Oyaizu H., Hashimoto Y., Ezaki T., Yamamoto H. Proposals of Sphingomonas paucimobilis gen. nov. and comb. nov., Sphingomonas parapaucimobilis sp. nov., Sphingomonas yanoikuyae sp. nov., Sphingomonas adhaesiva sp. nov., Sphingomonas capsulata comb. nov., and Two Genospecies of the Genus Sphingomonas. Microbiol Immunol. 1990;34(2):99–119. doi: 10.1111/j.1348-0421.1990.tb00996.x. [DOI] [PubMed] [Google Scholar]
- 4.Hsueh P.R., Teng L.J., Yang P.C., Chen Y.C., Pan H.J., Ho S.W., et al. Nosocomial infections caused by Sphingomonas paucimobilis: clinical features and microbiological characteristics. Clin Infect Dis. 1998;26(3):676–681. doi: 10.1086/514595. [DOI] [PubMed] [Google Scholar]
- 5.Meric M., Willke A., Kolayli F., Yavuz S., Vahaboglu H. Water-borne Sphingomonas paucimobilis epidemic in an intensive care unit. J Infect. 2009;58(3):253–255. doi: 10.1016/j.jinf.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 6.Bavaro D.F., Mariani M.F., Stea E.D., Gesualdo L., Angarano G., Carbonara S. Sphingomonas paucimobilis outbreak in a dialysis room: case report and literature review of an emerging healthcare associated infection. Am J Infect Control. 2020;48(10):1267–1269. doi: 10.1016/j.ajic.2020.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Kilic A., Senses Z., Kurekci A.E., Aydogan H., Sener K., Kismet E., Basustaoglu A.C. Nosocomial outbreak of Sphingomonas paucimobilis bacteremia in a hemato/oncology unit. Jpn J Infect Dis. 2007;60(6):394–396. [PubMed] [Google Scholar]
- 8.Gulati P., Ghosh M. Biofilm forming ability of Sphingomonas paucimobilis isolated from community drinking water systems on plumbing materials used in water distribution. J Water Health. 2017;15(6) doi: 10.2166/wh.2017.294. 942–54. [DOI] [PubMed] [Google Scholar]
- 9.Toh H.S., Tay H.T., Kuar W.K., Weng T.C., Tang H.J., Tan C.K. Risk factors associated with Sphingomonas paucimobilis infection. J Microbiol Immunol Infect. 2011;44(4):289–295. doi: 10.1016/j.jmii.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 10.Lin J.N., Lai C.H., Chen Y.H., Lin H.L., Huang C.K., Chen W.F., et al. Sphingomonas paucimobilis bacteremia in humans: 16 case reports and a literature review. J Microbiol Immunol Infect. 2010;43(1):35–42. doi: 10.1016/S1684-1182(10)60005-9. [DOI] [PubMed] [Google Scholar]
- 11.Kawasaki S., Moriguchi R., Sekiya K., Nakai T., Ono E., Kume K., et al. The cell envelope structure of the lipopolysaccharide-lacking gram- negative bacterium Sphingomonas paucimobilis. J Bacteriol. 1994;176(2):284–290. doi: 10.1128/jb.176.2.284-290.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan M.P., Adley C.C. Sphingomonas paucimobilis: a persistent Gram-negative nosocomial infectious organism. J Hosp Infect. 2010;75(3):153–157. doi: 10.1016/j.jhin.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 13.Baddour L.M., Wilson W.R., Bayer A.S., Fowler V.G.J., Tleyjeh I.M., Rybak M.J., et al. Infective endocarditis in adults: Diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 14.Corkill J.E., Hart C.A., McLennan A.G., Aspinall S. Characterization of a β-lactamase produced by Pseudomonas paucimobilis. J Gen Microbiol. 1991;137(6):1425–1429. doi: 10.1099/00221287-137-6-1425. [DOI] [PubMed] [Google Scholar]
- 15.Cheong H.S., Wi Y.M., Moon S.Y., Kang C.-I., Son J.S., Ko K.S., et al. Clinical features and treatment outcomes of infections caused by Sphingomonas paucimobilis. Infect Control Hosp Epidemiol. 2008;29(10):990–992. doi: 10.1086/591091. [DOI] [PubMed] [Google Scholar]
- 16.Saboe A., Adrian Y., Widyatmoko L., Hasan M., Cool C.J., Hartantri Y., et al. A fatal case of early prosthetic valve endocarditis caused by multidrug-resistant (MDR) – Sphingomonas paucimobilis. IDCases. 2021;24 doi: 10.1016/j.idcr.2021.e01152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rognrud K., Diaz A.M., Hill C., Kershaw M.A. Bacterial endocarditis caused by sphingomonas paucimobilis: a case report and literature review. Case Rep Infect Dis. 2020;2020 doi: 10.1155/2020/7185834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alvarado R.S., Mancilla N.J.F., Garcia F.M.T. Endocarditis bacteriana por sphingomonas paucimobilis en valvula nativa aortica: reporta de un caso y revision de la literatura. Med Interna De Mex. 2006;vol. 22(2):46. [Google Scholar]
- 19.Lin Y.C., Zheng J.F., Lin Y.H. Clinical features and risks associated with in-hospital mortality in patients with infective endocarditis at a medical center in Central Taiwan from 2012 to 2016 - a retrospective analysis. J Intern Med Taiwan. 2018;29(5) 309–16. [Google Scholar]
- 20.Yang F., Zhang B., Yu J., Shao L., Zhou P., Zhu L., et al. Epidemiology and the prognosis of healthcare-associated infective endocarditis in China: The significance of non-nosocomial acquisition. Emerg Microbes Infect. 2015;4:7. doi: 10.1038/emi.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]