Abstract
Hepatic vasculature can exhibit a wide variety of variants, some of which may resemble pathologic findings. In this case, a 53-year-old man presenting for staging of biochemically recurrent prostatic adenocarcinoma was found to have focally increased prostate-specific membrane antigen (PSMA) tracer uptake on positron emission tomography (PET) imaging in hepatic segment IV. This finding was initially concerning for hepatic metastasis of the patient's primary prostate adenocarcinoma. However, the area of radiotracer uptake was not associated with a discrete lesion on CT, and the geographic morphology of the uptake raised the possibility of a vascular etiology. Magnetic resonance imaging (MRI) of the liver showed no hepatic metastases and confirmed the presence of an aberrant right gastric vein directly perfusing the corresponding portion of hepatic segment IV. This case highlights PSMA uptake in the liver secondary to vascular variants as a potential mimic for metastatic disease on PSMA-PET/CT.
Keywords: Vascular variants, Hepatic vasculature, Prostate adenocarcinoma, PSMA-PET/CT
Introduction
The hepatic vasculature can display a wide spectrum of normal variants. The “third inflow” variants involve a source of blood supply to the liver not derived from branches of the portal vein or hepatic artery. Aberrant gastric veins are one type of third inflow. Though often clinically insignificant, this variant can produce focal areas of fatty sparing or steatosis, provide a direct route for the spread of gastric cancer, or serve as a source of alternate hepatic blood supply in the setting of portal vein thrombosis [1,2]. Importantly, third inflow variants can result in interpretive errors on imaging studies due to areas of differential enhancement or tracer activity. Proper identification of hepatic vascular variants and their associated radiographic findings is essential, as resulting abnormalities in enhancement or tracer activity can be confused for areas of true pathology.
Case report
A 53-year-old man was initially diagnosed with prostate adenocarcinoma in 2016 after he was incidentally noted to have an elevated serum prostate-specific antigen (PSA) of 6.5 ng/mL. He underwent prostate core biopsy with 4/12 positive cores showing Gleason 4 + 3 = 7, 3 + 4 = 7, and 3 + 3 = 6 on pathology. He was treated with external beam radiation therapy and subsequently monitored via serum PSA levels, with a post-radiation PSA nadir of 1.29 ng/mL.
In October 2018, his PSA values were noted to be elevated to 7.7 ng/mL, which prompted an MRI of the pelvis and a bone scan for evaluation of disease recurrence. The MRI revealed a PI-RADS 4 lesion in the left prostatic transitional zone, and the bone scan showed no metastatic disease. A prostate biopsy was positive for persistent prostate adenocarcinoma with treatment changes. His PSA continued to rise to a peak of 17.5 ng/mL in February 2019. A 18F-fluciclovine (FACBC)-PET/CT at that time showed an 11 mm nodule in the middle lobe of the right lung without substantial tracer uptake, as well as 2 tracer-avid right hilar lymph nodes measuring up to 2.5 cm. A CT-guided biopsy of the right middle lobe lung nodule revealed metastatic prostate adenocarcinoma. His metastatic disease was then treated with leuprolide, abiraterone, and prednisone, with serum PSA levels dropping to 0.2 ng/mL. A repeat chest CT after several months of treatment showed complete resolution of the right middle lobe nodule. From that point onwards, he was monitored for biochemical recurrence via monthly serum PSA levels.
In 2022, he again presented with gradually increasing serum PSA levels to 0.54 ng/mL. The patient underwent PET/CT utilizing 18F-piflufolastat, a tracer targeting the prostate-specific membrane antigen (PSMA), to restage his biochemically recurrent prostate cancer and aid in treatment planning.
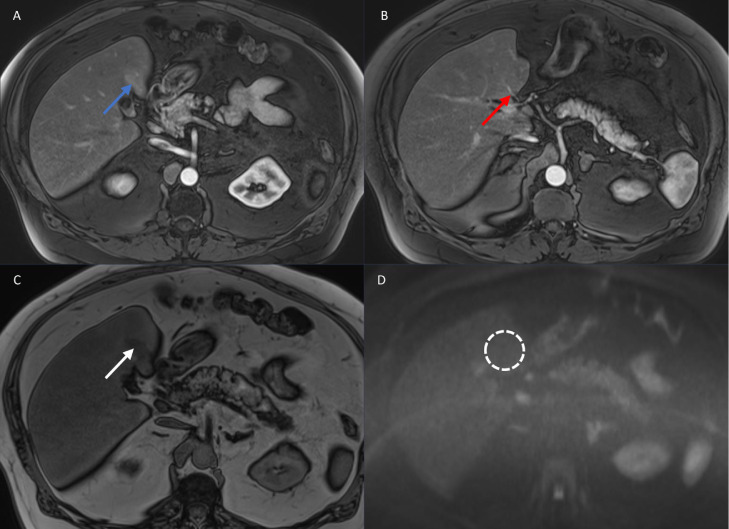
The PSMA-PET/CT revealed foci of intense radiotracer uptake in the T4 vertebral body (not shown) and left fourth rib (Fig. 1, images B1-3, blue arrows), consistent with sites of metastatic prostate adenocarcinoma. There was also a focus of tracer uptake in the right lung hilum that likely represented residual nodal metastatic disease at the site seen on the FACBC-PET/CT in 2019. Finally, there was an ill-defined focus of moderate tracer uptake in hepatic segment IV that corresponded to a vague area of hypodensity on CT (Fig. 1, images A1-3, red arrows). This finding was initially concerning for metastatic spread into the liver. However, the somewhat geographic distribution of this activity raised the alternate possibility of a hepatic vascular inflow variant. More specifically, the location in the posterior aspect of hepatic segment IV was suggestive of an aberrant right gastric vein. A subsequent liver MRI showed no evidence of hepatic metastatic disease but confirmed the presence of an aberrant right gastric vein (Fig. 2, image B, red arrow).
Fig. 1.
PSMA-PET/CT showed ill-defined moderate tracer uptake in posterior hepatic segment IV (red arrows, images A1-A3), corresponding to a vague area of hypodensity without a definite focal lesion (red arrow, image A1). There was also intense tracer uptake in the T4 vertebral body (not shown) and left fourth rib (blue arrows, images B1-B3), compatible with sites of osseous metastatic disease.
Fig. 2.
MRI of the liver including T1-weighted arterial phase post-contrast images (images A and B), T1-weighted opposed-phase images (image C), and diffusion-weighted images (image D) was performed. In the area in question on the PSMA-PET/CT, there was vague arterial phase hyperenhancement (blue arrow) associated with focal fat deposition (white arrow). An anomalous vessel (red arrow) was noted to enter the liver directly through the capsule at this site, corresponding to an aberrant right gastric vein. There was no restricted diffusion (dashed circle) to indicate a metastasis at this location or elsewhere in the liver.
Discussion
This case of an aberrant right gastric vein complicating prostate cancer staging is an important illustration of the potential clinical impacts of hepatic vascular variants. These normal variants can involve the portal veins, hepatic arteries, or hepatic veins, as well as sites of venous “third inflow” into the liver. Aberrant gastric veins are among the more common third inflow variants, though prevalence ranges widely across studies. Variants involving the gastric venous drainage are thought to be more common on the right than on the left. Some studies estimate the prevalence of aberrant right gastric veins to be between 1.5% and 34%, although one study of 100 patients undergoing angiography reported a prevalence of 49% [3], [4], [5]. In our experience, findings related to aberrant right gastric venous drainage are relatively uncommon on cross-sectional imaging studies. Left gastric venous variants are exceedingly rare, with prevalence estimated to be 0.8% in one cadaveric study [6]. Aberrant right gastric veins often drain directly into the posterior aspect of hepatic segment IV, whereas aberrant left hepatic veins often drain into the posterior margins of hepatic segments II and III.
This additional vascular supply to portions of the hepatic parenchyma may result in differential enhancement patterns on dynamic post-contrast imaging studies [1]. On emission imaging studies (ie, performed with tracers), the associated perfusion anomalies may result in focally higher or lower tracer activity, depending on the tracer injection site. On our PSMA-PET/CT study, an aberrant right gastric vein resulted in focally greater tracer activity in the differentially perfused portions of liver parenchyma. Similarly, in the setting of superior vena cava obstruction, areas of focally increased tracer activity in hepatic segment IV related to venous collateralization via third inflow pathways have been reported on PET studies performed with 18F-fluorodeoxyglucose (FDG) and pulmonary perfusion scintigraphy studies performed with 99mTc-macroaggregated albumin [7,8].
This case of increased tracer uptake on PSMA-PET/CT secondary to an aberrant right gastric vein represents an important consideration when assessing for metastatic disease. Although there may initially appear to be a hepatic metastasis, the characteristic location of differential tracer activity and the absence of a discrete lesion on anatomic imaging should suggest aberrant right gastric venous drainage. Hepatic vascular variants should be considered when a hepatic metastasis is suspected on PSMA-PET/CT, especially when the patient's disease is not otherwise widely metastatic, as the liver is a relatively uncommon site for prostate cancer metastases (particularly in isolation). Awareness of hepatic vascular variants and their imaging features can prepare radiologists to distinguish between benign anatomic variants and true pathologic conditions.
Conclusion
The hepatic vasculature can display a range of normal vascular variants, including aberrant gastric veins. The radiologic features produced by variants in hepatic vasculature can sometimes be mistaken for more serious pathologies, such as metastatic disease. Familiarity with the characteristic radiologic findings associated with these variants is essential for accurate diagnosis and staging. This case report of an aberrant right gastric vein mimicking hepatic metastatic disease on PSMA-PET/CT illustrates the importance of recognizing these variants, as misdiagnosis could have altered this patient's oncologic treatment.
Patient consent
All images and information were published with patient consent.
Footnotes
Competing Interests: The authors have no potential conflicts of interest to disclose.
References
- 1.Elsayes KM, Shaaban AM, Rothan SM, Javadi S, Madrazo BL, Castillo RP, et al. A comprehensive approach to hepatic vascular disease. Radiographics. 2017;37(3):822. doi: 10.1148/rg.2017160161. [DOI] [PubMed] [Google Scholar]
- 2.Unal E, Ozmen MN, Akata D, Karcaaltincaba M. Imaging of aberrant left gastric vein and associated pseudolesions of segments II and III of the liver and mimickers. Diagn Interv Radiol. 2015;21(2):105–110. doi: 10.5152/dir.2014.14360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seong NJ, Chung JW, Kim HC, Park JH, Jae HJ, An SB, et al. Right gastric venous drainage: angiographic analysis in 100 patients. Korean J Radiol. 2012;13(1):53–60. doi: 10.3348/kjr.2012.13.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deneve E, Caty L, Fontaine C, Guillem P. Simultaneous aberrant left and right gastric veins draining directly into the liver. Ann Anat. 2003;185(3):263–266. doi: 10.1016/S0940-9602(03)80037-7. [DOI] [PubMed] [Google Scholar]
- 5.Takayasu K, Aoki K, Ichikawa T, Ohmura T, Sekiguchi R, Terauchi T, et al. Aberrant right gastric vein directly communicating with left portal vein system. Incidence and implications. Acta Radiol. 1990;31:575–577. [PubMed] [Google Scholar]
- 6.Miyaki T, Yamada M, Kumaki K. Aberrant course of the left gastric vein in the human. Possibility of a persistent left portal vein. Acta Anat (Basel) 1987;130:275–279. doi: 10.1159/000146456. [DOI] [PubMed] [Google Scholar]
- 7.Jundt MC, Broski SM, Binkovitz LA. 18F-FDG PET/CT equivalent of the hepatic hot spot sign with CT correlation. Clin Nucl Med. 2018;43(5):e147–e148. doi: 10.1097/RLU.0000000000002022. [DOI] [PubMed] [Google Scholar]
- 8.Kapur S, Paik E, Rezaei A, Vu DN. Where there is blood, there is a way: unusual collateral vessels in superior and inferior vena cava obstruction. Radiographics. 2010;30(1):67–78. doi: 10.1148/rg.301095724. [DOI] [PubMed] [Google Scholar]