Abstract
Several lines of evidence indicate that platelets protect against endovascular infections such as infective endocarditis (IE). It is highly likely that a principal mechanism of this platelet host defense role is the release of platelet microbicidal proteins (PMPs) in response to agonists generated at sites of endovascular infection. We studied the ability of platelets to limit the colonization and proliferation of Staphylococcus aureus in an in vitro model of IE. Three isogenic S. aureus strains, differing in their in vitro susceptibility to thrombin-induced platelet microbicidal protein-1 (tPMP), were used: ISP479C (parental strain; highly susceptible to tPMP [tPMPs]); ISP479R (transposon mutant derived from ISP479; tPMP resistant [tPMPr]); or 757-5 (tPMPr transductant of the ISP479R genotype in the ISP479 parental background). Time-kill assays and in vitro IE models were used to examine the temporal relationship between thrombin-induced platelet activation and S. aureus killing. In time-kill studies, early platelet activation (30 min prior to bacterial exposure) correlated with a significant bactericidal effect against tPMPs ISP479C (r2 > 0.90, P < 0.02) but not against tPMPr strains, ISP479R or 757-5. In the IE model, thrombin activation significantly inhibited proliferation of ISP479C within simulated vegetations compared to strains ISP479R or 757-5 (P < 0.05). The latter differences were observed despite there being no detectable differences among the three S. aureus strains in initial colonization of simulated vegetations. Collectively, these data indicate that platelets limit intravegetation proliferation of tPMPs but not tPMPr S. aureus. These findings underscore the likelihood that platelets play an important antimicrobial host defense role in preventing and/or limiting endovascular infections due to tPMPs pathogens.
The pathogenesis of endovascular infections, such as infective endocarditis (IE), is multifaceted, involving interactions among microorganisms (such as Staphylococcus aureus), host plasma proteins, endothelial cells, and platelets (23). The outcome of these interactions represents a pivotal step in either the limitation or progression of IE (3, 5, 9, 12, 23). However, the exact role of platelets in this regard has been enigmatic (3, 5, 9, 10, 12, 23–25, 28, 32). Recent evidence from several laboratories suggests that platelets serve an important host defense function against the induction and evolution of endovascular infection (7, 24–37; R. C. Mercier, M. R. Yeaman, A. S. Bayer, and M. J. Rybak, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother. [ICAAC], abstr. B-61, 1997).
The antimicrobial functions of mammalian platelets have been recently linked to their release of a family of small, cationic peptides termed platelet microbicidal proteins (PMPs) (32, 37; Y. Q. Tang, M. R. Yeaman, and M. E. Selsted, Blood 86:910, abstr. 3626, and Blood 86:556, abstr. 2212, 1995; J. Krijgsveld, S. A. Zaat, A. J. Kuijpers, G. H. Engbers, J. Feijen, and J. Dankert, Abstr. 38th ICAAC, abstr. F-179, 1998). For example, Yeaman et al. isolated and characterized seven antimicrobial peptides from quiescent and thrombin-stimulated rabbit platelets (37). Furthermore, Tang et al. discovered that human platelets likewise contain a group of peptides that exert potent and synergistic antimicrobial activities, including platelet factor-4, platelet basic peptide (PBP), connective tissue activating peptide-III (CTAP-III), and neutrophil activating peptide-2 (NAP-2; both CTAP-III and NAP-2 are derivatives of PBP) (Tang et al., abstr. 3626). Subsequently, Krijgsveld et al. confirmed that C-terminal modifications of human CTAP-III and NAP-2 also have direct antimicrobial properties (so-called “thrombocidins”) (Krijgsveld et al., 38th ICAAC). Moreover, both thrombin (generated at sites of damaged or infected endothelium) and infecting pathogens themselves (N. Azizi, C. Li, A. J. Shen, A. S. Bayer, and M. R. Yeaman, Abstr. 36th ICAAC, abstr. G-54, 1996) are potent agonists which stimulate platelets to release PMPs (2, 32, 37).
Both in vitro and in vivo evidence now exists substantiating the role of platelets and PMPs in antimicrobial host defense. In vitro, PMPs exert rapid and potent (at nanomolar to micromolar concentrations) microbicidal activities against pathogens that commonly gain access to the bloodstream, such as S. aureus, coagulase-negative staphylococci, viridans group streptococci, and Candida albicans (26, 29, 37). PMPs achieve these effects in microenvironmental conditions relevant to the vascular compartment, such as physiologic ionicity and pH, as well as in the presence of serum (19). PMPs have also been shown to interfere with platelet adherence and aggregation due to S. aureus and C. albicans in vitro and potentiate the microbicidal and postexposure growth-inhibitory effects of conventional antibiotics in vitro (3, 30, 34–36).
In vivo data also support the role of platelets in host defense. For example, Sullam et al. showed that cardiac vegetations from thrombocytopenic rabbits infected with PMP-susceptible viridans group streptococci had significantly higher bacterial densities compared to controls with normal platelet counts (25). Related studies have shown that in vitro PMP susceptibility correlates with a decreased propensity of clinical bloodstream isolates to cause IE (1, 26). Furthermore, in experimental IE, PMP susceptibility in S. aureus or C. albicans correlates with reduced microbial proliferation of these pathogens in vegetations and the spleen compared to isogenic PMP-resistant counterpart strains (8, 9, 33). Taken together, these in vitro and in vivo observations provide evidence that platelets play important roles in antimicrobial host defense, probably through localized elaboration of PMPs.
Recently, an in vitro pharmacokinetic and pharmacodynamic model has been developed to simulate IE (4, 6, 15, 17, 20, 22); R. C. Mercier, and M. J. Rybak, Abstr. 8th Eur. Congr. Clin. Microbiol. Infect. Dis. abstr. P395, 1997; Mercier et al., 37th ICAAC). This model has been shown to closely parallel key events in IE pathogenesis that are observed in the well-characterized rabbit model (e.g., colonization via initial adherence and proliferation of organisms to high densities within simulated IE vegetations) (16, 20, 21; G. W. Kaatz, S. M. Seo, J. R. Aeschlimann, H. H. Houlihan, R. C. Mercier, and M. J. Rybak, Abstr. 37th ICAAC, abstr. F-11, 1997; G. W. Kaatz, S. M. Seo, J. R. Aeschlimann, H. H. Houlihan, R. C. Mercier, and M. J. Rybak, Abstr. 37th ICAAC, abstr. B-25, 1997). Like the in vivo model, the in vitro model poses a rigorous challenge to antimicrobial agent efficacy, due to the presence of high organism densities in the setting of a complex biomatrix comprising the simulated vegetation. Additionally, this model minimizes the use of experimental animals, is cost- and time-effective, and allows precise control of key experimental variables (such as bacterial densities, platelet counts, and specific platelet agonists). With the above considerations in mind, the objective of the present study was to examine the influence of platelets on the survival of isogenic S. aureus strains differing in PMP susceptibility profiles within simulated vegetations in an in vitro model of IE.
MATERIALS AND METHODS
Organisms.
Three genetically related S. aureus strains differing in their in vitro susceptibilities to thrombin-induced PMP (tPMP) were used in these investigations: ISP479C (intrinsically tPMP susceptible [tPMPs]), ISP479R (tPMP resistant [tPMPr]), and 757-5 (tPMPr transductant of ISP479R [see below]). These isogenic strains have been previously characterized in detail and were found to have no other detectable differences microbiologically, biochemically, immunologically, or genotypically (9). In brief, S. aureus ISP479C is a spontaneous plasmid-cured variant of parental strain ISP479 (9). Strain ISP479R is an isogenic mutant of parental ISP479 derived by transposon mutagenesis (Tn551) (8, 9). Strain 757-5 (formerly called transductant-A [9]) was obtained by phage transduction of the ISP479R strain into the ISP479C parental genotypic background (9). For selection purposes, both strains ISP479R and 757-5 have erythromycin resistance determinants resulting from the use of the Tn551 transposon construct. In addition, ISP479R exhibits a stable tPMPr phenotype in vitro after serial passage in the absence of tPMP (mean percent survival of >50% versus <10% for parental strain ISP479C) or through experimental animals (8, 9). Likewise, the 757-5 mutant demonstrates a stable tPMPr phenotype compared with parental strain ISP479C that is equivalent to ISP479R (9). Previous studies have shown that the genetic elements that confer tPMP resistance to S. aureus are functionally consistent with the genetic context in an isogenic background (8, 9).
Media.
Organisms were prepared for experimentation by culture to logarithmic phase in cation-supplemented Mueller-Hinton broth (Difco Laboratories, Detroit, Mich.) immediately prior to use. For strains ISP479R or 757-5, this medium contained 15 μg of erythromycin per ml (Sigma Chemical Co., St. Louis, Mo.) (8, 9). Eagle minimal essential medium (MEM; Sigma) was used in both the time-kill assays and the simulated IE model. Brain heart infusion (BHI) agar (Difco, Detroit, Mich.) was used for quantitative culture of aliquots in all experiments and was supplemented with 15 μg of erythromycin per ml in studies involving strains ISP479R or 757-5.
Bacterial inocula.
S. aureus strains were washed in phosphate-buffered saline and resuspended in normal saline (NS) to achieve 0.5 McFarland units (108 CFU/ml; Vitek-Colorimeter; Biomerieux Vitek, Inc., Hazelwood, Mo.). In the time-kill experiments, an inoculum of 103 CFU/ml (in an 8-ml volume) was used for every experiment. Three different final inocula were used for preliminary testing in the in vitro IE model experiments: 103, 104, or 106 CFU/ml. These inocula represent readily achievable S. aureus densities consistent with those observed in early, developing, or mature IE vegetations, respectively (2, 8, 9).
Preparation of human tPMP.
For use in time-kill assays, tPMP was prepared as previously described (32) from fresh citrated human platelets (Tang et al., abstr. 3626). In brief, blood from a healthy human donor was collected into citrated, siliconized tubes. Low-speed centrifugation (75 × g) of blood samples produced a loose erythrocyte pellet and an upper, platelet-rich plasma supernatant. Collection of the upper fraction of the platelet-rich plasma supernatant routinely yielded platelets with <1% leukocyte contamination. The platelet-rich plasma was transferred to citrated, siliconized tubes and centrifuged for 10 min at 2,000 × g to pellet the platelets. The resulting platelet pellet was washed in Tyrode salts solution (0.08 mM NaCl, 3.8 mM K2HPO4, 4.9 mM Na2HPO4, 2.8 mM glucose, 16.6 mM citric acid, 34 mM sodium citrate [pH 6.8]; Sigma), and then resuspended in Eagle MEM to a concentration of 108 platelets per ml as determined by spectrophotometry (optical density, λ = 600 nm; Stasar; Gilford Instrument Laboratories, Inc., Palo Alto, Calif.). Supernatants rich in tPMP were then produced by thrombin stimulation (10 U of bovine thrombin per 108 platelets [GenTrac, Inc., Middleton, Wis.] in the presence of 10 μl of 0.2 M CaCl2 and 10 μl of homologous platelet-poor plasma per ml of washed platelet suspension; 30 min, 37°C). The resulting suspension was then centrifuged to remove residual platelets, and the supernatant (containing tPMP) was collected. The antimicrobial activity of supernatants from human platelets stimulated as described above was predominantly attributable to tPMPs as determined by acid-urea polyacrylamide gel electrophoresis (AU-PAGE) and reversed-phase high-pressure liquid chromatography (RP-HPLC) as previously described (Tang et al., abstr. 3626). Furthermore, the tPMP preparations were tested as previously described to exclude the possibility that their bactericidal activity was attributable to platelet-derived lysozyme (data not shown) (32).
Determination of tPMP bactericidal activity.
The bactericidal activity of the human tPMP preparations was determined as previously described (32). In brief, bioassays were performed using Bacillus subtilis ATCC 6633, an indicator organism highly susceptible to the bactericidal action of tPMPs (32). A B. subtilis inoculum of 104 CFU/ml was added to microtiter wells containing a range of dilutions of the tPMP-rich preparation to achieve a final inoculum of 103 CFU/ml per well and a final range of PMP dilutions of from 1:1 to 1:1,024. After incubation, the reaction was ceased by 0.01% sodium polyanetholsulfonate (SPS; an anionic compound used to inhibit further cationic tPMP-induced killing [32]), and the mixture was then quantitatively cultured onto 6.6% sheep blood agar. After incubation at 37°C for 24 h, the bactericidal activity of the tPMP preparation was quantified as the inverse of the highest dilution that retained ≥95% killing of the B. subtilis inoculum within 30 min (32).
Time-kill studies. (i) Controls.
For the time-kill assays, S. aureus strains were exposed to nonstimulated platelets, platelet-free plasma, thrombin, or CaCl2 to control for the possible antistaphylococcal effects of these individual components in vitro versus the S. aureus study strains. Bacterial inocula and platelets were prepared as described above. The final inoculum of S. aureus strains used in each study was 103 CFU/ml in a final volume of 8 ml following the addition of each of the respective components. The concentrations of plasma, thrombin, or CaCl2 tested were identical to those used in the in vitro IE model (see below). After incubation (37°C) for 0, 0.5, 1, 2, 4, or 8 h, the content of each assay tube was sonicated prior to quantitative culture of 10-μl aliquots onto BHI agar with or without erythromycin as described above. When present, nonstimulated platelets were removed by low-speed centrifugation prior to sonication and quantitative culture to avoid release of tPMPs by mechanical disruption of platelets. Plates were then incubated for 24 h, and surviving bacterial colonies determined as the CFU per milliliter. A minimum of two independent experiments was performed for each control.
(ii) Temporal relationship between platelet activation and S. aureus killing.
The time at which platelets were activated with respect to microbial attachment to, or colonization of, a vascular surface may be an important factor in antimicrobial host defense. Thus, pilot experiments were conducted in this regard. Thrombin-induced platelet activation was studied at three different time points in the macrovolume time-kill assays: 30 min prior to adding the 103-CFU/ml bacterial inoculum, simultaneous with the addition of the bacterial inoculum, or 30 min after addition of the bacterial inoculation. Platelets were activated as described above with thrombin, CaCl2, and homologous platelet-free plasma. The time at which maximal platelet antimicrobial effects were observed was then used to guide subsequent in vitro IE model studies (see below).
In vitro model of IE.
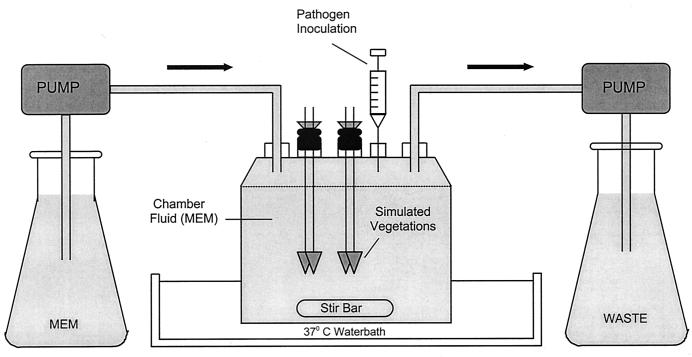
The in vitro model of IE, previously described by Kang et al., was used in these experiments (Fig. 1) (17). One-compartment infection models (250 ml) were used independently a minimum of two times, with duplicate samples processed at each time point. The models contained a liquid phase of MEM, and both reservoirs contained MEM to supplement the central MEM compartment. Simulated IE vegetations were suspended from sealed sampling ports (two clots per port). The models were placed in water baths and maintained at 37°C.
FIG. 1.
In vitro endocarditis model.
Preparation of simulated IE vegetations.
Simulated endocardial vegetations were prepared as previously described (22). Sterile fibrin clots were created in siliconized 1.5-ml tubes by combining 0.9 ml of human cryoprecipitate from volunteer donors (American National Red Cross, Detroit, Mich.), 0.05 ml of aprotinin solution, and 0.05 ml of washed platelet-rich plasma suspension (∼108 platelets/ml diluted in 0.9% NaCl to provide approximately 250,000 to 300,000 platelets per g of vegetation mass). A sterile monofilament line was inserted into the 1.5-ml tubes containing cryoprecipitate, aprotinin, and platelets, and 0.1 ml of bovine thrombin (1,000 U/ml) was added to the mixture. The resultant thrombus was removed from the 1.5-ml tube using a sterile 21-gauge needle and then used in the in vitro IE model (Fig. 1).
Colonization of sterile vegetations.
In pilot studies, we determined the minimal initial bacterial inocula required to achieve bacterial densities of 500 to 1,000 CFU per g of vegetation after 2 h of exposure to S. aureus in the absence of circulating platelets. These S. aureus densities mirror those which colonize sterile vegetations in experimental endocarditis early post-intravenous challenge (9). Three different bacterial inocula were prepared as described above and tested: 103, 104, or 106 CFU/ml. Two sterile vegetations were placed in the model for each time point, and each experiment was performed in duplicate. Peristaltic pumps supplied fresh MEM and removed spent MEM from the model at a half-life equal to 8 h. At this rate, the removal of S. aureus from the model did not significantly alter the total number of bacteria in the system (18). A magnetic stir bar mixed the suspension in the central compartment of the models, which were conducted over 8 h. It was anticipated that bacteria would rapidly adhere to simulated platelet vegetations; thus, frequent samples of both vegetations and chamber fluid were removed and quantitatively cultured early in the experiment (0, 0.5, 1, 2, 4, and 8 h). Therefore, at each time point, two vegetations were removed from each model and then weighed and placed in a 2-ml sterile capped vial preloaded with sterile 3-mm-diameter glass beads and 1.0 ml of sterile 1.25% trypsin solution (1:250 powder; Difco). In addition, 50 μl of 0.1% SPS was added to prevent further tPMP-mediated killing of S. aureus (32, 37). To homogenize the vegetation, each vial was placed in a mini-bead beater mixer (Biospec Products, Bartlesville, Okla.) for 3 min. Sterile, cold (4°C) 0.9% saline (i.e., NS) was used to dilute the homogenized vegetation, and 20 μl of this homogenate were plated in triplicate onto BHI with or without erythromycin and incubated for 24 h at 37°C; the colonies were then counted. Mean CFUs of the four samples at each time point were plotted to determine the log10 CFU/gram of vegetation over time. Two samples from the MEM suspension of the central compartment of each model were also quantitatively cultured in parallel. The mean CFUs of the chamber fluid phase from the two models were plotted as log10 CFU/milliliter versus time. The results from these pilot studies dictated the use of the following conditions in all subsequent models: 103 CFU staphylococci were inoculated into the model 30 min after thrombin stimulation of circulating platelets (as indicated below).
Influence of activated platelets on the evolution of simulated S. aureus IE.
In parallel with the above investigations, we conducted a series of studies to examine the effects of platelets on S. aureus colonization of simulated vegetations in the in vitro IE model. Simulated vegetations were prepared as described above, and a final concentration of 108 platelets/ml (representing ∼15 μg/ml of total tPMP activity) (37; Tang et al., abstr. 3626) was added and circulated throughout the model for 30 min. Thrombin was then added to the model fluid as described above to activate the platelets. Thirty minutes after thrombin was added, an inoculum of 103 CFU of S. aureus per ml was introduced into the model. A magnetic bar continuously stirred the contents of the central compartment. The models were held in static phase for 1 h before the peristaltic pump was activated to mimic an elimination half-life of 8 h. The colonization phase of the model was defined as the period from the time of the initial inoculation to the 1 h time point. At this 1 h time point, two vegetations from each model were removed, weighed, and processed for quantitative culture as above. The proliferation phase of the model was defined as the period of time beyond the 1 h time point. The log10 CFU/gram for all time points at which samples were collected (0, 0.5, 1, 2, 4, and 8 h) was determined by quantitative culture as described above and then compared to those obtained in the platelet-free models and plotted against time for the tPMPs or tPMPr S. aureus strains. Samples of the liquid phase from the central compartment were also taken at the same time points and quantitatively cultured, and the log10 CFU/milliliter values were plotted against time.
tPMP release in the IE model.
To assess tPMP release from thrombin-stimulated platelets into IE model chamber fluid, RP-HPLC and gel electrophoresis were conducted as previously described (37). RP-HPLC was first performed on a 0.46-by-25-cm C18 column (Vydac; The Separations Group, Hesperia, Calif.) developed with water-acetonitrile gradients containing 0.1% trifluoroacetic acid (TFA). Eluent was continuously monitored at 220 and 280 nm. When secondary RP-HPLC purification was needed, a 0.46-by-25-cm C4 column (Vydac) was developed with water-acetonitrile gradients containing 0.13% heptafluorobutyric acid (HFBA). The homogeneity of purified peptides was assessed by analytical RP-HPLC in a similar manner. Model chamber fluid, and peptides purified therefrom by RP-HPLC, were analyzed using AU- and/or sodium dodecyl sulfate-polyacrylamide gel electrophoresis to corroborate HPLC data in their identification as human tPMPs (37).
Statistical analysis.
The bacterial densities in simulated vegetations (log10 CFU/gram) or within model chamber fluid (log10 CFU/milliliter) at different sampling times were compared among treatment groups and growth controls using analysis of variance with Tukey's test for multiple comparisons. A P value of ≤0.05 was considered significant in these analyses. The time required to achieve 99.9% killing was determined by linear regression analysis of the time-kill curves and compared using Kaplan-Meier analysis. A linear correlation coefficient (r2) value of >0.5 was considered significant in these analyses.
RESULTS
Time-kill assays. (i) Controls.
No antistaphylococcal effects were observed following exposure of the three study strains to thrombin, platelet-free plasma, or CaCl2 alone over an 8-h period (data not shown).
(ii) Platelet activation.
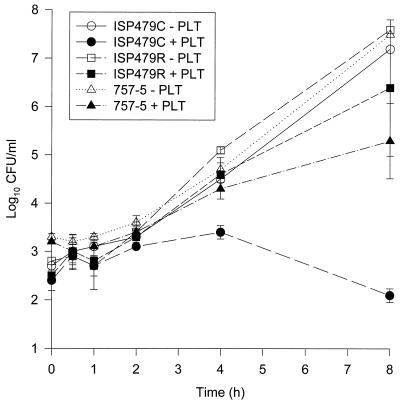
In the presence of platelets activated by thrombin 30 min prior to the introduction of bacteria, there was a significant inhibition in proliferation of the tPMPs strain, ISP479C, at 4 and 8 h compared to the growth control in the absence of activated platelets (Fig. 2; P < 0.001). The same effect was seen when platelets were activated simultaneously with bacterial inoculation (data not shown). In contrast, platelets activated 30 min after bacterial inoculation did not significantly inhibit or reduce the growth of strain ISP479C (P > 0.05). Platelet activation 30 min prior to or simultaneous with bacterial inoculation corresponded with maximal antibacterial activity at 4 or 8 h. For S. aureus PMPr strains ISP479R or 757-5, irrespective of the time of platelet activation, there were no significant reductions in CFU/milliliter in the presence of activated platelets compared to the respective growth control (Fig. 2; P > 0.05).
FIG. 2.
Results summarizing macrobroth time-kill studies. Platelets were activated 30 min prior to introducing S. aureus ISP479C, ISP479R, or 757-5. Note the reduction in CFU/milliliter of the PMP-susceptible strain ISP479C in the presence of activated platelets compared to its growth control or either of the tPMPr strains, ISP479R or 757-5, in the presence or absence of activated platelets.
In vitro model of IE. (i) Influence of platelets on S. aureus colonization in simulated IE.
Models conducted over 8 to 24 h enabled the evaluation of colonization of simulated IE vegetations by the three S. aureus strains over time; the following endpoints were obtained: (i) comparative binding of tPMPs and tPMPr strains to simulated vegetations within 1 h (colonization phase) and (ii) the influence of inoculum on this colonization process. We determined that the minimal initial bacterial inoculum required within the model chamber fluid to achieve 500 to 1,000 CFU/g of simulated vegetation after 1 h was 103 CFU/ml (data not shown). Therefore, an inoculum of 103 was used as the inoculum for all strains in all subsequent studies. No significant differences were found among the colonization properties of the three S. aureus strains studied at 0.5 and 1 h (P > 0.05 for each comparison).
(ii) Influence of platelets on S. aureus proliferation in simulated IE.
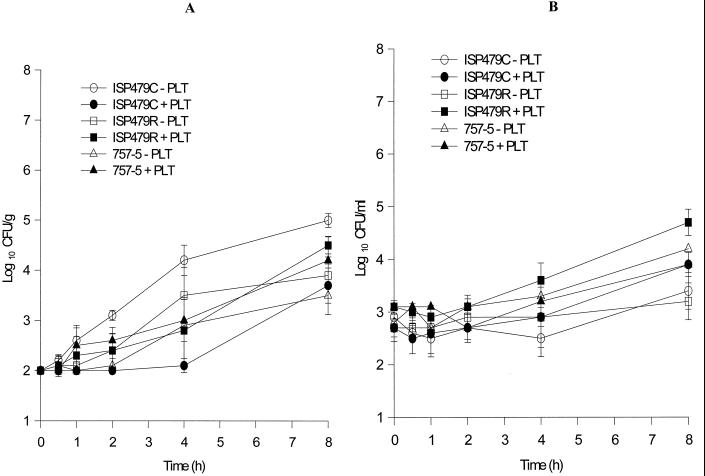
Subsequent to the colonization phase (1 h), we examined the influence of activated platelets on proliferation of the three S. aureus study strains in the IE model. The addition and activation of exogenous platelets significantly inhibited the proliferation of the PMPs strain, ISP479C, within simulated vegetations, compared to its ability to proliferate in simulated vegetations in the absence of platelets (Fig. 3A; P = 0.001). The anti-ISP479C activity of activated platelets was statistically significant at 2, 4, and 8 h after platelet stimulation compared with respective controls (P < 0.05). This effect was maximal 4 h after exposure to activated platelets (Fig. 3A). In contrast, activated platelets did not significantly inhibit proliferation of either PMPr strains ISP479r or 757-5 in simulated vegetations over the 8-h study period compared with the proliferation in the absence of activated platelets (Fig. 3A; P > 0.05 for each comparison). Additionally, the presence of activated platelets in the chamber fluid did not impair the overall growth kinetics of any of the three S. aureus strains studied in the fluid phase of the infection model, regardless of their intrinsic tPMP susceptibility phenotype (Fig. 3B).
FIG. 3.
S. aureus colonization and proliferation in the IE model in the presence or absence of thrombin-stimulated platelets. Bacterial inocula were introduced at time zero. Simulated vegetations and chamber fluid were quantitatively cultured at the subsequent time points indicated. (A) CFU/gram within the simulated vegetations over time. (B) CFU/milliliter within the chamber fluid over time.
(iii) Release of tPMPs in the IE model.
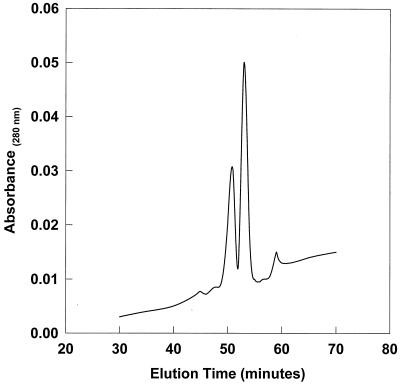
Supernatants from the thrombin-stimulated human platelets exhibited the anticipated microbicidal activities against the study tPMPs or tPMPr strains, B. subtilis and ISP479C or ISP479R and 757-5, respectively; these data were consistent with those previously determined for human tPMPs (data not shown) (9, 37). Furthermore, fluid-phase samples derived from IE models following platelet activation were analyzed for the presence of tPMPs using AU-PAGE and RP-HPLC. As illustrated in Fig. 4, two predominant protein peaks were isolated from these samples under chromatographic conditions previously used to isolate human tPMPs (Tang et al., abstr. 3626 and 2212). For example, the elution times for these peaks were 51.7 and 53.2 min, a result consistent with times determined in prior studies to be associated with two predominant human tPMPs (Tang et al., abstr. 3626). In addition, AU-PAGE analyses identified these peptides to have mass/charge ratios and migration characteristics consistent with known human tPMPs (data not shown). It is important to note that only very low concentrations of these proteins (<0.5 μg/ml) were detectable in model chamber fluid as determined by RP-HPLC.
FIG. 4.
Analytical RP-HPLC chromatogram of predominant protein peaks contained in the IE model fluid from the experiment depicted in Fig. 3. Purification using H2O-acetonitrile gradients containing 0.1% TFA at 1 ml/min (as previously described [33]). Note the presence of two predominant proteins consistent in elution time with known human tPMPs (Tang et al., abstr. 3626). Data from AU-PAGE analyses (not shown) corroborated these findings.
DISCUSSION
Several independent events have been proposed to occur in the establishment and evolution of IE (13). The formation of a nonbacterial thrombotic vegetation (NBTV) is perceived as one possible first step in this process. The NBTV usually forms on the surface of damaged endothelium, where platelets and fibrin comprise the matrix of this lesion and the evolving vegetation. To establish IE, organisms must access the bloodstream and reach the NBTV. Subsequently, the ability of the pathogen to colonize the NBTV is dependent on its adherence properties (11, 13). Alternatively, some bloodstream pathogens, such as S. aureus, may adhere directly to normal or abnormal vascular endothelial cells. Such adherence may elicit endothelial cells or local monocytes to elaborate tissue factor, prompting thrombin generation. In either pathway, the organisms, platelets, and/or endothelial cells initiate a procoagulant cascade, such that the organisms become entrapped in an evolving and complex biomatrix consisting predominantly of platelets and fibrin. Upon extensive intravegetation proliferation, virulence factor expression, and reseeding of the vascular compartment, hematogenous dissemination and metastatic complications may occur (13). Therefore, the ultimate fate of the organism in achieving IE through these pathogenic steps depends on it circumventing and/or surviving interactions with host defenses such as complement, antibody, leukocytes and platelets. The function of platelets in such a host defense role was the specific focus of the current study.
Prior investigations examining the role of tPMPs in limiting microbial pathogenesis of endovascular infections such as IE have been carried out in vitro and in experimental animal models (25, 26–37); Mercier et al., 37th ICAAC; Tang et al., abstr. 3626 and 2212). Our current project incorporated for the first time an in vitro model closely simulating IE to investigate the hypothesized antimicrobial host defense role(s) of platelets. There are limitations to this in vitro model, as there are for all in vitro models; for example, all aspects of antimicrobial host defense cannot be simultaneously represented. Nonetheless, in vitro models are important tools for dissecting the specific steps in pathogenesis (Mercier et al., 37th ICAAC). For example, our previous transmission electron microscopy studies in the current model (unpublished data) confirmed that staphylococci can be found within the platelet-fibrin matrix, extracellular to the platelets themselves. These results mirror those observed in human endocarditis (8, 9, 23).
Large numbers of platelets respond rapidly to local sites of endothelial infection within the vascular compartment. Given their multiple antimicrobial properties (e.g., tPMP release) (28) it is reasonable to hypothesize that platelets significantly contribute to host defense against induction and/or evolution of endovascular infection. Thus, platelets may potentially intercede in the development of IE by interfering with the colonization and/or proliferation of the pathogen at sites of injury or infection of the vascular endothelium. In the current studies, activated platelets had no detectable effect upon the colonization phase leading to establishment of S. aureus infection of the simulated IE vegetations, irrespective of the intrinsic tPMP susceptibility phenotype of the challenge strain. Therefore, it should be emphasized that activated platelets neither prevented nor promoted colonization of simulated IE by these S. aureus strains. This conclusion is based on the fact that the time to maximal bacterial binding to the vegetation occurs usually within 1 h of bacterial challenge in experimental IE in vivo, as opposed to the proliferation phase which normally proceeds over many hours later in the evolution of IE (11, 13). Our data indicate that the principal impact of activated platelets on the development of infection in the in vitro IE model was upon the later intravegetation proliferative phase of infection. Collectively, the results of our study suggest that activated platelets act principally to mitigate proliferation of S. aureus in the context of platelet-fibrin matrices, likely via the local release of tPMPs. It is important to note that the extent to which activated platelets mitigated this proliferative phase of IE was directly related to the intrinsic tPMP susceptibility phenotype of the infecting S. aureus strain.
Antibacterial activity due to tPMP release was associated with the chamber fluid over the 1- to 4-h time range following platelet activation, times corresponding with maximal observed platelet-mediated antibacterial effects within simulated IE vegetations. This antistaphylococcal activity was cationic and distinguishable from platelet lysozyme based on biochemical and microbiological findings, respectively. Furthermore, AU-PAGE and RP-HPLC revealed that the chamber fluid contained peptides consistent with human tPMPs (Tang et al., abstr. 3626). Moreover, in the in vitro IE model, the antistaphylococcal effects appeared to be predominant in the solid phase of the simulated vegetation and not in the fluid phase of the model compartment. However, in the fluid phase of the model, tPMPs were present, albeit in concentrations below their microbicidal thresholds for the S. aureus strains used in this study. These results suggest that tPMPs were responsible for the platelet-mediated antibacterial effects observed within simulated vegetations. These findings substantiate our hypothesis that tPMPs are released from stimulated platelets, achieve microbicidal concentrations in the immediate vicinity of endovascular infection (e.g., in the setting of infected endothelium), and may be further concentrated by accumulation upon target pathogens.
In conclusion, activated platelets appear to significantly limit the proliferation of tPMPs but not tPMPr S. aureus strains in this in vitro endovascular infection model. Furthermore, this antistaphylococcal effect is linked to release of PMPs from thrombin-stimulated platelets. This finding was observed despite the fact that the tPMPs and tPMPr strains were equivalent in their ability to initially colonize the simulated IE vegetations in the presence of thrombin-stimulated platelets. Furthermore, our data suggest that the anti-S. aureus activities of platelets in this model are predominant within the relative proximity of the simulated vegetations compared to the fluid phase of the chamber fluid. The precise mechanisms by which tPMPs exert these antibacterial effects are not entirely clear. However, recent studies have demonstrated that PMPs and tPMPs depolarize, de-energize, and permeabilize microbial cell membranes and may subsequently lead to the inhibition of macromolecular synthesis of target organisms (27, 28). Taken together, these findings support our hypothesis that tPMPs and PMPs released from activated platelets participate significantly in antimicrobial host defense.
ACKNOWLEDGMENTS
This work was supported by a grant to R.-C.M. from the Society of Infectious Diseases Pharmacists, in conjunction with Pfizer Pharmaceuticals, and NIH grants AI-39001 and AI39108 to M.R.Y. and A.S.B., respectively.
We thank Paul Sullam for helpful discussions related to these studies. We also thank Ambrose Cheung (Dartmouth University) for assistance in the construction of transductant strain, 757-5.
REFERENCES
- 1.Bayer A S, Cheng D, Yeaman M R, Corey G R, McClelland R S, Harrel L J, Fowler V G. In vitro resistance to thrombin-induced platelet microbicidal protein among clinical bacteremic isolates of Staphylococcus aureus correlates with an endovascular infectious source. Antimicrob Agents Chemother. 1998;42:3169–3172. doi: 10.1128/aac.42.12.3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayer A S, Ramos M D, Menzies B E, Yeaman M R, Shen A J, Cheung A L. Hyperproduction of alpha-toxin by Staphylococcus aureus results in paradoxically reduced virulence in experimental endocarditis: a host defense role for platelet microbicidal proteins. Infect Immun. 1997;65:4652–4660. doi: 10.1128/iai.65.11.4652-4660.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayer A S, Sullam P M, Ramos M, Li C, Cheung A L, Yeaman M R. Staphylococcus aureus induces platelet aggregation via a fibrinogen-dependent mechanism which is independent of principal platelet glycoprotein IIb/IIIa fibrinogen-binding domains. Infect Immun. 1995;63:3634–3641. doi: 10.1128/iai.63.9.3634-3641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergeron M G, Robert J, Beauchamp D. Pharmacodynamics of antibiotics in fibrin clots. J Antimicrob Chemother. 1993;31:113–136. doi: 10.1093/jac/31.suppl_d.113. [DOI] [PubMed] [Google Scholar]
- 5.Clawson C C. Platelet interaction with bacteria. III. Ultrastructure. Am J Clin Pathol. 1973;70:449–471. [PMC free article] [PubMed] [Google Scholar]
- 6.Cremieux A C, Maziere B, Vallois J M, Ottaviani M, Azancot A, Raffoul H, Bouvet A, Pocidalo J J, Carbon C. [3H]-spiramycin penetration into fibrin vegetations in an experimental model of streptococcal endocarditis. J Antimicrob Chemother. 1988;22:127–133. doi: 10.1093/jac/22.supplement_b.127. [DOI] [PubMed] [Google Scholar]
- 7.Dankert J, Van der Werff J, Zaat S A J, Joldersma W, Klein D, Hess J. Involvement of bactericidal factors from thrombin-stimulated platelets in clearance of adherent viridans streptococci in experimental infective endocarditis. Infect Immun. 1995;63:663–671. doi: 10.1128/iai.63.2.663-671.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhawan V K, Bayer A S, Yeaman M R. In vitro resistance to thrombin-induced platelet microbicidal protein is associated with enhanced progression and hematogenous dissemination in experimental Staphylococcus aureus infective endocarditis. Infect Immun. 1998;66:3476–3479. doi: 10.1128/iai.66.7.3476-3479.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhawan V K, Yeaman M R, Cheung A L, Kim E, Sullam P M, Bayer A S. Phenotypic resistance to thrombin-induced platelet microbicidal protein in vitro is correlated with enhanced virulence in experimental endocarditis due to Staphylococcus aureus. Infect Immun. 1997;65:3293–3299. doi: 10.1128/iai.65.8.3293-3299.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donaldson D M, Tew J G. Beta-lysin of platelet origin. Bacteriol Rev. 1977;41:501–513. doi: 10.1128/br.41.2.501-513.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durack D T, Besson P B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br J Exp Pathol. 1972;53:44–49. [PMC free article] [PubMed] [Google Scholar]
- 12.Durack D T, Besson P B, Petersdorf R G. Experimental bacterial endocarditis. III. Production and progression of the disease in rabbits. Br J Exp Pathol. 1973;54:142–151. [PMC free article] [PubMed] [Google Scholar]
- 13.Freedman L R. The pathogenesis of infective endocarditis. J Antimicrob Chemother. 1987;20(Suppl. A.):1–6. doi: 10.1093/jac/20.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- 14.Ganz T, Selsted M E, Lehrer R I. Defensins. Eur J Haematol. 1990;44:1–8. doi: 10.1111/j.1600-0609.1990.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 15.Houlihan H H, Mercier R C, Rybak M J. Pharmacodynamics of vancomycin alone and in combination with gentamicin at various dosing intervals against methicillin-resistant Staphylococcus aureus—infected fibrin platelet clots in an in vitro infection model. Antimicrob Agents Chemother. 1997;41:2497–2501. doi: 10.1128/aac.41.11.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaatz G W, Seo S M, Lamp K C, Bailey E M, Rybak M J. CI-960, a new fluoroquinolone, for therapy of experimental ciprofloxacin-susceptible and -resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1992;36:1192–1197. doi: 10.1128/aac.36.6.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang S L, Rybak M J. Pharmacodynamics of RP 59500 alone and in combination with vancomycin against Staphylococcus aureus in an in vitro-infected fibrin clot model. Antimicrob Agents Chemother. 1995;39:1505–1511. doi: 10.1128/aac.39.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keil S, Wiedemann B. Mathematical corrections for bacterial loss in pharmacodynamic in vitro dilution models. Antimicrob Agents Chemother. 1995;39:1054–1058. doi: 10.1128/aac.39.5.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koo S P, Yeaman M R, Bayer A S. Staphylocidal action of thrombin-induced platelet microbicidal protein is influenced by microenvironment and target cell growth phase. Infect Immun. 1996;64:3758–3764. doi: 10.1128/iai.64.9.3758-3764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGrath B J, Kang S L, Kaatz G W, Rybak M J. Bactericidal activities of teicoplanin, vancomycin and gentamicin alone and in combination against Staphylococcus aureus in an in vitro pharmacodynamic model of endocarditis. Antimicrob Agents Chemother. 1994;38:2034–2040. doi: 10.1128/aac.38.9.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicolau D P, Freeman C D, Nightingale C H, Coe C J, Quintiliani R. Minocycline versus vancomycin for treatment of experimental endocarditis caused by oxacillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1994;38:1515–1518. doi: 10.1128/aac.38.7.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rybak M J, Houlihan H H, Mercier R C, Kaatz G W. Pharmacodynamics of RP59500 (quinupristin/dalfopristin) administered by intermittent versus continuous infusion against Staphylococcus aureus-infected fibrin-platelet clots in an in vitro infection model. Antimicrob Agents Chemother. 1997;41:1359–1363. doi: 10.1128/aac.41.6.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scheld W M, Valone A, Sande M A. Bacterial adherence in the pathogenesis of endocarditis: interaction of bacterial dextran, platelets, and fibrin. J Clin Investig. 1978;61:1394–1404. doi: 10.1172/JCI109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sullam P M, Bayer A S, Foss W M, Cheung A L. Diminished platelet binding in vitro by Staphylococcus aureus is associated with reduced virulence in a rabbit endocarditis model. Infect Immun. 1996;64:4915–4921. doi: 10.1128/iai.64.12.4915-4921.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullam P M, Frank U, Yeaman M R, Tauber M G, Bayer A S, Chambers H F. Effect of thrombocytopenia on the early course of streptococcal endocarditis. J Infect Dis. 1993;168:910–914. doi: 10.1093/infdis/168.4.910. [DOI] [PubMed] [Google Scholar]
- 26.Wu T, Yeaman M R, Bayer A S. In vitro resistance to platelet microbicidal protein correlates with endocarditis source among bacteremic staphylococcal and streptococcal isolates. Antimicrob Agents Chemother. 1994;38:729–732. doi: 10.1128/aac.38.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiong Y Q, Yeaman M R, Bayer A S. In vitro antibacterial activities of platelet microbicidal protein and neutrophil defensin against Staphylococcus aureus are influenced by antibiotics differing in mechanism of action. Antimicrob Agents Chemother. 1999;43:1111–1117. doi: 10.1128/aac.43.5.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeaman M R. The role of platelets in antimicrobial host defense. Clin Infect Dis. 1997;25:951–970. doi: 10.1086/516120. [DOI] [PubMed] [Google Scholar]
- 29.Yeaman M R, Ibrahim A S, Edwards J E, Bayer A S, Ghannoum M A. Thrombin-induced rabbit platelet microbicidal protein is fungicidal in vitro. Antimicrob Agents Chemother. 1993;37:546–553. doi: 10.1128/aac.37.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeaman M R, Norman D C, Bayer A S. Platelet microbicidal protein enhances antibiotic-induced killing of and postantibiotic effect in Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:1665–1670. doi: 10.1128/aac.36.8.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeaman M R, Norman D C, Bayer A S. Staphylococcus aureus susceptibility to thrombin-induced platelet microbicidal protein is independent of platelet adherence and aggregation in vitro. Infect Immun. 1992;60:2368–2374. doi: 10.1128/iai.60.6.2368-2374.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeaman M R, Puentes S M, Norman D C, Bayer A S. Partial characterization and staphylocidal activity of thrombin-induced platelet microbicidal protein. Infect Immun. 1992;60:1202–1209. doi: 10.1128/iai.60.3.1202-1209.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeaman M R, Soldan S S, Ghannoum M A, Edwards J E, Filler S G, Bayer A S. Resistance to platelet microbicidal protein results in increased severity of experimental Candida albicans endocarditis. Infect Immun. 1996;64:1379–1384. doi: 10.1128/iai.64.4.1379-1384.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeaman M R, Sullam P M, Dazin P F, Bayer A S. Platelet microbicidal protein alone and in combination with antibiotics reduces Staphylococcus aureus adherence to platelets in vitro. Infect Immun. 1994;62:3416–3423. doi: 10.1128/iai.62.8.3416-3423.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeaman M R, Sullam P M, Dazin P F, Ghannoum M A, Edwards J E, Bayer A S. Fluconazole and platelet microbicidal protein inhibit Candida adherence to platelets in vitro. Antimicrob Agents Chemother. 1994;38:1460–1465. doi: 10.1128/aac.38.7.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yeaman M R, Sullam P M, Dazin P F, Norman D C, Bayer A S. Characterization of Staphylococcus aureus-platelet binding by quantitative flow cytometric analysis. J Infect Dis. 1992;166:65–73. doi: 10.1093/infdis/166.1.65. [DOI] [PubMed] [Google Scholar]
- 37.Yeaman M R, Tang Y Q, Shen A J, Bayer A S, Selsted M E. Purification and in vitro activities of rabbit platelet microbicidal proteins. Infect Immun. 1997;65:1023–1031. doi: 10.1128/iai.65.3.1023-1031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]