Abstract
The island nation of Madagascar is home to three endemic species of Old World fruit bat in the family Pteropodidae: Pteropus rufus, Eidolon dupreanum, and Rousettus madagascariensis, all three of which are IUCN Red Listed under some category of threat. Delineation of seasonal limits in the reproductive calendar for threatened mammals can inform conservation efforts by clarifying parameters used in population viability models, as well as elucidate understanding of the mechanisms underpinning pathogen persistence in host populations. Here, we define the seasonal limits of a staggered annual birth pulse across the three species of endemic Madagascar fruit bat, known reservoirs for viruses of high zoonotic potential. Our field studies indicate that this annual birth pulse takes place in September/October for P. rufus, November for E. dupreanum, and December for R. madagascariensis in central-eastern Madagascar where the bulk of our research was concentrated. Juvenile development periods vary across the three Malagasy pteropodids, resulting in near-synchronous weaning of pups for all species in late January–February at the height of the fruiting season for this region. We here document the size range in morphological traits for the three Malagasy fruit bat species, with P. rufus and E. dupreanum among the larger of pteropodids globally and R. madagascariensis among the smaller. All three species demonstrate subtle sexual dimorphism with males being larger than females. We explore seasonal variation in adult body condition by comparing observed body mass with body mass predicted by forearm length, demonstrating that pregnant females add weight during staggered gestation periods and males lose weight during the nutritionally deficit Malagasy winter. Finally, we quantify forearm, tibia, and ear length growth rates in juvenile bats, demonstrating both faster growth and more protracted development times for P. rufus as compared with E. dupreanum and R. madagascariensis. The longer development period for the already-threatened P. rufus further undermines the conservation status of this species as human hunting is particularly detrimental to population viability during reproductive periods. Our work highlights the importance of longitudinal field studies in collecting critical data for mammalian conservation efforts and human public health alike.
Keywords: Eidolon dupreanum, fruit bat, Madagascar, morphology, pteropodid, Pteropodidae, Pteropus rufus, Rousettus madagascariensis, seasonality
The Old World fruit bat family Pteropodidae, known colloquially as “flying foxes,” makes up one of the most endangered groups of mammals on Earth, with some 35% of species either extinct or threatened with extinction, a proportion almost three times higher than that reported (12%) for all other bat families combined (Species IUCN Red List Threat 2018). Fruit bats experience disproportionate rates of persecution, likely as a result of their propensity for small island endemism (Jones et al. 2009) and their large sizes—fruit bat wingspans can reach up to 2 m in the case of Pteropus vampyrus, the world’s largest bat (Corbet and Hill 1992), which make them targets for consumption by humans (Craig et al. 1994; Brooke 2002; Oleksy et al. 2003; Jenkins and Racey 2008; Kamins et al. 2011; Openshaw et al. 2016; Peel et al. 2017). Pteropodid bats offer critical services to surrounding ecosystems, playing important roles in the pollination and seed dispersal of numerous plant species across the Old World, particularly in island ecosystems often depauperate in other frugivores (McConkey and Drake 2006; Kunz et al. 2011).
Madagascar is one such island ecosystem recognized for its unusually depauperate frugivorous fauna (Goodman and Ganzhorn 1997; Dewar and Richard 2007; Federman et al. 2017). Primates (lemurs), rather than birds, are considered the primary seed dispersers on the island (Langrand 1990; Wright et al. 2011), in contrast to otherwise comparable tropical ecosystems in the New World (Terborgh 1983, 1986). In addition to lemurs, Madagascar is home to three endemic species of frugivorous bats from the family Pteropodidae—Pteropus rufus, Eidolon dupreanum, and Rousettus madagascariensis—all of which are known to pollinate flowers and disperse seeds from both native Malagasy and exotic plants (Bollen and Elsacker 2002; Andriafidison et al. 2006; Long and Racey 2007; Picot et al. 2007; Andrianaivoarivelo et al. 2011; Oleksy et al. 2015, 2017). Importantly, E. dupreanum may be the only extant pollinator of the endangered, endemic Malagasy baobab, Adansonia suarezensis (Andriafidison et al. 2006).
Despite their ecosystem value, Madagascar’s fruit bats are heavily persecuted. All three species are consumed across the island as a source of human food (Oleksy et al. 2003; Jenkins and Racey 2008; Cardiff et al. 2009; Randrianandrianina et al. 2010; Golden et al. 2014; Fernández-Llamazares et al. 2018; Brook et al. 2019b), and P. rufus, the largest and most heavily hunted, is sometimes targeted in response to its largely inaccurate characterization as a predator of human fruit crops (Raharimihaja et al. 2016). Respectively, P. rufus, E. dupreanum, and R. madagascariensis are currently IUCN Red Listed as “Vulnerable,” “Vulnerable,” and “Near-Threatened” species (Species IUCN Red List Threat 2018), though recent population viability analyses suggest that P. rufus, in particular, may be experiencing more severe population declines than have been previously reported (Brook et al. 2019b).
Bats are reservoir hosts for a majority of the world’s most virulent zoonotic viruses (Guth et al. 2019, 2022), as well as hosts for coronaviruses ancestral to the recently emerged SARS-CoV-2 (Zhou et al. 2020; Temmam et al. 2022). Globally, anti-bat sentiments have been on the rise as a result of the COVID-19 pandemic (Rocha et al. 2020); though no specific instances of COVID-related persecution have yet been documented for the Malagasy fruit bats, all three species are known to host potentially zoonotic pathogens (Iehlé et al. 2007; Razafindratsimandresy et al. 2009; Reynes et al. 2011; Wilkinson et al. 2012; Brook et al. 2015, 2019c; Razanajatovo et al. 2015; Ranaivoson et al. 2019), posing risks that negative public reactions may arise in the future.
Previous work suggests that roost population sizes and survival rates vary across the year for these three species (Brook et al. 2019b; Lalarivoniaina et al. 2019). Temporal fluctuations in nutritional status may alter bat immune responses, thus influencing pathogen dynamics (Brook et al. 2019c), as well as modulate the vulnerability of bats to seasonally variable hunting pressures (Brook et al. 2019b). All three Malagasy fruit bats are thought to reproduce seasonally in species-specific annual birth pulses (MacKinnon et al. 2003; Brook et al. 2019b). Previous work highlights how seasonal variation in hunting pressure for Malagasy lemurs poses elevated risks when directly overlapping their annual birth pulse (Brook et al. 2019a), a phenomenon that has been well-elucidated in systems outside of Madagascar as well (Kokko and Lindström 1998). As such, documentation of the timing of each annual birth pulse for Malagasy fruit bats is important for pinpointing periods of peak vulnerability to hunting.
Defining the temporal limits of the species-specific birth pulse for Malagasy fruit bats is also essential to understanding the mechanisms that underpin the maintenance and persistence of the numerous infectious agents that they host (Iehlé et al. 2007; Razafindratsimandresy et al. 2009; Reynes et al. 2011; Wilkinson et al. 2012; Brook et al. 2015, 2019c; Ranaivoson et al. 2019). Isolated E. helvum populations on islands off the west coast of Africa have been shown to support circulation of potentially zoonotic henipaviruses at population sizes well below the established critical community size for closely related paramyxoviruses in other systems (Bartlett 1957, 1960; Swinton et al. 1998; Peel et al. 2012). Some work has suggested that seasonally staggered births allowing for a protracted introduction of susceptible juveniles into the host population could play a role in pathogen persistence in these systems (Peel et al. 2013, 2014; Hayman 2015).
We sought to expand existing knowledge of seasonal variation in the reproductive calendar and nutritional status of all three Malagasy fruit bat species, to facilitate future conservation assessments and studies aimed at deciphering the dynamics of bat-hosted infections. In particular, for each of these species, we aimed to (i) quantify life history traits needed for population modeling, (ii) document seasonal variation in morphometrics and body conditions, and (iii) calculate juvenile growth rates throughout the postreproductive period. Our work emphasizes the importance of longitudinal field studies in accurately describing the ecology of frugivorous bats.
Materials and Methods
Study periods and sites
Field studies were carried out between 2013 and 2020 and combined with previously published work examining population viability and the dynamics of potentially zoonotic infections in Malagasy fruit bats (Brook et al. 2015, 2019b, 2019c; Ranaivoson et al. 2019). Bats were captured periodically throughout each year, with sampling spanning all months and all seasons (dry, wet, shoulder). Captures took place in several regions of Madagascar (Fig. 1): (i) Ankarana National Park in the northwest (−12.9S, 49.1E; 206 m); (ii) Makira Natural Park in the northeast (−15.1S, 49.6E; 536 m); (iii) Mahabo forest in the center-west (−20.5S, 44.7E; 66 m); and (iv) several sublocalities of the Manjakandriana and Moramanga Districts in the center-east, including the fragmented forests of Ambakoana (−18.5, 48.2; 902 m), Mangarivotra (−18.3S, 48.2E; 979 m), Marotsipohy (−18.4S, 48.1E; 969 m), Marovitsika (−18.8S, 48.1E; 936 m), Lakato (−19.2S, 48.4E; 616 m), and Mahialambo (−18.1S, 48.2E; 941 m); the special reserves of Angavokely (−18.9S, 47.8E; 1,490 m) and Angavobe (−18.9S, 47.9E; 1,438 m); and the new protected area of Maromizaha (−18.9S, 48.5E; 991 m). We focused our seasonal analyses on longitudinal data collected from repeatedly resampled roost sites in these central-eastern Manjakandriana/Moramanga Districts but used data from all sites to define the morphometric ranges for all three fruit bat species.
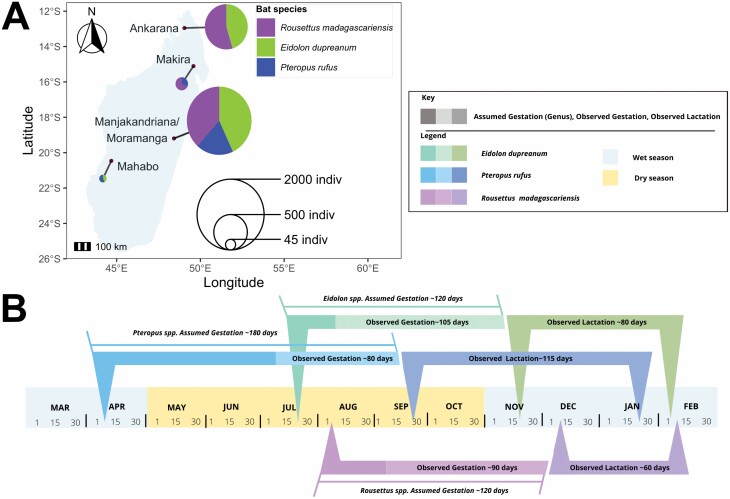
Fig. 1.
(A) Map of field sites and distribution of bat captures for Pteropus rufus, Eidolon dupreanum, and Rousettus madagascariensis in Madagascar. Pie size corresponds to total bats captured at each site: 1,700 in the Districts of Manjakandriana/Moramanga (P. rufus n = 317; E. dupreanum n = 732; R. madagascariensis n = 653), 380 in Ankarana National Park (E. dupreanum n = 172; R. madagascariensis n = 208), 47 in Makira Natural Park (P. rufus n = 15; R. madagascariensis n = 32), and 33 in Mahabo forest (P. rufus n = 19; E. dupreanum n = 14). (B) Gestation and lactation periods across the three Madagascar fruit bat species in the central-eastern Districts of Manjakandriana/Moramanga, calculated from the field data (observed) and reported in the literature (assumed). Respectively, for P. rufus, E. dupreanum, and R. madagascariensis, observed gestation begins on: 7 July, 3 August, and 11 September; birth occurs on: 29 September, 16 November, and 12 December; and lactation ceases on: 21 January, 2 February, and 19 February (Supplementary Data SD1.A).
Netting
Mist nets were deployed from 6 PM to midnight and from 3 AM to 8 AM around roosting or feeding sites of P. rufus, E. dupreanum, and R. madagascariensis and monitored continuously. Captured bats were placed in individual clean cloth bags while awaiting processing for infectious disease studies, as has been previously described (Brook et al. 2015, 2019c; Ranaivoson et al. 2019). For each sampling session, we conducted between 1 and 10 nights of netting, ending sessions early when 30 individuals of each species present at the site were captured. Upon capture, all bats were weighed (in grams) with a Pesola scale attached to the cloth bag and forearm, tibia, and ear were measured with a caliper or tape measure (in mm). Bats were classed by sex and age (juvenile vs. adult) and, for females, reproductive class (nonreproductive, pregnant, lactating). For females captured approximately within the period of possible gestation for each species, abdominal palpation was used to determine whether or not females were pregnant. All raw data used in this study are accessible in our open-access GitHub repository at: github.com/brooklabteam/Mada-Bat-Morphology.
This study was carried out in strict accordance with research permits obtained from the Madagascar Ministry of Forest and the Environment (permit numbers 251/13, 166/14, 075/15, 258/16, 170/18, 019/18, 170/18, 007/19, 14/20) and under guidelines posted by the American Veterinary Medical Association and the American Society of Mammalogists (Sikes et al. 2016). All field protocols employed were preapproved by the Princeton University and UC Berkeley Institutes for Animal Care and Use Committees (respectively, IACUC Protocol #1926 and ACUC Protocol # AUP-2017-10-10393), and every effort was made to minimize discomfort to animals.
Literature review
From the “Bat Species of the World” database (Simmons and Cirranello 2020), we compiled a list of 201 pteropodid species, then searched Google Scholar and Web of Science for any records documenting the mass, forearm, tibia, and ear length of each species. We only collected records that were sex-specific, and where possible, we documented the sample size from which those records were derived, if reported as an average. In cases where no sample size was reported, we assumed sample size to be one individual.
Statistical analysis
Data analysis was performed using R v.4.0.3. All raw data and corresponding code for these analyses can be accessed in our GitHub repository and in supplementary data files.
First, we aimed to define the seasonal limits of the reproductive calendar for each of the three Malagasy fruit bat species in the central-eastern Manjakandriana/Moramanga Districts where the bulk of our longitudinal data was collected. To this end, we queried the central-eastern subset of the data for the following metrics, unique for each species: (a) the earliest calendar day on which a pregnant female was observed, (b) the earliest calendar day on which a juvenile was observed, and (c) the latest calendar day on which a lactating female was observed. Metrics (a) and (b) corresponded to the date limits of gestation for each species, while metrics (b) and (c) corresponded to the date limits of lactation for each species. Because fruit bats of many species are known to delay embryonic implantation and fetal development for months after fertilization (Mutere 1967; Heideman 1988; Heideman and Powell 1998; Meenakumari and Krishna 2005), we assumed that abdominal palpation to determine reproductive status in the field would likely miss very early-stage pregnancies. Notably, delayed embryonic implantation and fetal development have not been described for Malagasy fruit bats. Nonetheless, to this end, we additionally searched the literature for records of gestation length in closely related pteropodids to compare against our records of observed gestation in Malagasy species.
We next sought to document morphological variation in adult P. rufus, E. dupreanum, and R. madagascariensis, as compared with other bats in family Pteropodidae. To this end, we calculated the sex-specific median and interquartile range of reported measurements of mean tibia and ear length (in mm) for adult pteropodids from the literature, as well as the range of values recorded for individuals within our field data set. For these analyses, we queried the entire pan-Madagascar field data set, rather than limiting ourselves to the seasonal central-eastern subset. To investigate any potential sexual dimorphism in our data set, we compared mean forearm, tibia, and ear length for male versus female distributions across data collected from the literature and from our Malagasy field data set using Welch’s 2-sample t-tests for independent distributions of unequal sample size.
We compared the relationship between sex-specific forearm length and mass for adult pteropodids surveyed in the literature against the ranges recorded in our own field data for the three Malagasy species. We first fitted a linear regression to log10-transformed values for both forearm length (predictor variable) and mass (response variable), separated by sex, both to species-level averages for pteropodids globally and to individual data points for adults of the three Malagasy species captured across all sampling sites in our data set.
Next, we explored seasonal variation in the relationship between adult body mass and forearm length within the central-eastern subset of our Malagasy field data. To facilitate this analysis, we refitted a composite linear regression using log10-transformed values for forearm length as predictors of log10-transformed values for mass. We included a fixed effect of bat species as an additional predictor to control for natural differences in fat content across the different species, then calculated the residual of each individual’s observed mass in the data against that predicted from the regression. This generated a body condition index metric for bats—individuals with positive mass:forearm residuals corresponded to those with higher masses than predicted by body size (broadly indicative of better nutritional condition), while individuals with negative mass:forearm residuals corresponded to those with lower masses than predicted by body size (broadly indicative of poorer nutritional condition). Because all individuals included in these analyses were adults, and previous work indicates that up to 96% of reproductively mature fruit bats give birth annually (Hayman et al. 2012; Brook et al. 2019b), we assumed all females observed during the defined gestation period for each species to be pregnant, regardless of reproductive class recorded from abdominal palpation. We based this assumption on previous findings from the literature for African E. helvum (Hayman et al. 2012); future work will need to validate reproductive rates for Malagasy fruit bats.
To assess seasonal variation in body condition, we fit a generalized additive model (GAM) in the mgcv package in R (Wood 2001) to the seasonal time series of mass:forearm residual across the longitudinally resampled central-eastern roost sites. We modeled the response variable of mass:forearm residual separately across each discrete species-sex subset of the data, as predicted by day of year as a cyclic cubic (“cc”) spline, with the number of smoothing knots (“k”) fixed at seven, as recommended by the package author (Wood 2001). Cyclic cubic splines can be used to capture annual seasonality, as the seasonal smoother on 1 January is modeled as a continuation from 31 December. Because some previous work has questioned the effectiveness with which body condition indices represent bat nutritional status (McGuire et al. 2018), we computed an additional set of supplementary GAMs that included the predictor variables of day of year (as a cyclic cubic smoothing spline) and forearm length (as a random effect) against the response variable of mass (in grams) for both sexes and all three species.
Finally, we explored juvenile growth rates for forearm, tibia, and ear across all three Malagasy fruit bat species in the central-eastern region, calculating the age in days since birth of each juvenile bat in our data set with “day 0” set equal to the first date of an observed juvenile in the data set for each species, as described above, up to 1 year of life (day 365). Using GAMs, we then modeled the response variables of forearm length, tibia length, and ear length against the smoothing predictor of age in days, using a thinplate smoothing spline (“tp”) with the number of smoothing knots fixed again at seven. After fitting each model, we then calculated the age-varying derivative of each fitted curve using the “gratia” package in R to facilitate comparison of growth rates across different species and morphological features.
Results
Field captures
In total, 2,160 fruit bats were captured and processed between August 2013 and March 2020 (Fig. 1A). The majority of bats (n = 1,700) were captured in roost sites located in the Districts of Manjakandriana/Moramanga in central-eastern Madagascar (P. rufus n = 316; E. dupreanum n = 732; R. madagascariensis n = 652), followed by Ankarana National Park in the northwest (n = 380; E. dupreanum n = 172; R. madagascariensis n = 208), Makira Natural Park in the northeast (n = 47; P. rufus n = 15; R. madagascariensis n = 32), and Mahabo forest in the center-west (n = 33; P. rufus n = 19; E. dupreanum n = 14; Supplementary Data SD1, Table S1).
Fruit bat reproductive calendars
Longitudinal data collected in the Districts of Manjakandriana/Moramanga allowed us to define the seasonal limits of a single annual reproduction event for all three fruit bat species in central-eastern Madagascar (Fig. 1B). We calculated the earliest calendar day on which a pregnant female was observed, respectively, for P. rufus, E. dupreanum, and R. madagascariensis, as 7 July, 3 August, and 11 September; the earliest calendar day on which a juvenile was observed as 29 September, 16 November, and 12 December; and the latest calendar day on which a lactating female was observed as 21 January, 2 February, and 19 February (Supplementary Data SD1). These dates allowed us to define the approximate duration of the observed gestation and lactation period for each species (observed gestation: P. rufus = ~80 days, E. dupreanum= ~105 days, and R. madagascariensis = ~90 days; observed lactation: P. rufus = ~115 days, E. dupreanum= ~80 days, and R. madagascariensis = ~60 days). Because gestation was documented via abdominal palpation in the field, we presumed that early-stage pregnancies for all three species might be missed. To account for this, we compared our observed gestation period for all three fruit bat species against that which has been previously described for closely related species: P. alecto, P. policephalus, and P. scapulatus (sister species to P. rufus) demonstrate a ~180-day gestation period on the Australian continent (McIlwee and Martin 2002); while E. helvum (sister species to E. dupreanum) and R. aegyptiacus (close relative to R. madagascariensis) both demonstrate gestation periods of ~120 days on the African continent (Odukoya et al. 2008; Barclay and Jacobs 2011). Extension of the gestation period for the three Malagasy species back in time from the birth pulse to match those recorded for related species elsewhere would place the mating period for P. rufus in the month of April, for E. dupreanum in the month of July, and for R. madagascariensis in the month of August. These estimates of mating period are consistent with previous reports for P. rufus (Long and Racey 2007) and R. madagascariensis (Lalarivoniaina et al. 2019); to our knowledge, no previous records of the reproductive calendar for E. dupreanum have been published.
In sum, we observed the longest gestation and lactation period for P. rufus, which births first, followed by E. dupreanum then R. madagascariensis, in order of decreasing body size. Despite differences in the timing and duration of gestation, lactating mothers for all three species weaned pups around the same time of the year (~late January to February), at the onset of peak fruit abundance in the hot-wet season in central-eastern Madagascar (Britt et al. 2002; Powzyk and Mowry 2002).
Morphological patterns
After searching the literature, we successfully compiled adult mass records from 103 pteropodid species for females and 106 species for males; adult forearm records from 146 species for females and 140 species for males; adult tibia records from 64 species for females and 64 species for males; and adult ear length records from 101 species for females and 99 species for males. We compared these records against morphological patterns witnessed in our own field data.
Morphometric data for ear length, tibia length, and forearm length from individual Malagasy fruit bats spanned the size range captured across mean values for all non-Malagasy pteropodid bats surveyed in the literature (Fig. 2), scaling downward from P. rufus to E. dupreanum to R. madagascariensis. For ear lengths, P. rufus and E. dupreanum distributions were largely overlapping, while R. madagascariensis were smaller; species-specific interquartile ranges for each morphological trait are summarized in Supplementary Data SD1, Table S2. Global data roughly approximated the range spanned from the R. madagascariensis minimum to the P. rufus maximum, with the median falling in between that of R. madagascariensis and E. dupreanum across all three metrics.
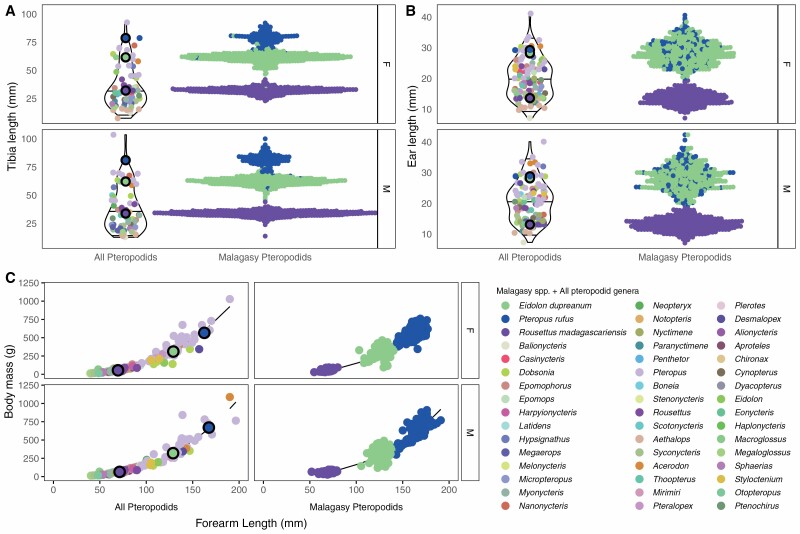
Fig. 2.
(A) Tibia, and (B) ear length across fruit bat species from the literature (left) and from our Madagascar field data (right), colored by genera according to legend; data are grouped by sex (upper = females, lower = males). Violin plots show range and 25%, 50%, and 75% quantiles for each distribution. (C) Linear regression of log10 body mass (in grams, y-axis) by log10 forearm length (in mm, x-axis) across pteropodids from the literature (left) and from all Madagascar field data (right), colored by genera according to legend; data are grouped by sex (upper = females, lower = males). Solid line corresponds to predictions from the fitted model (R2: all pteropodids, M = 0.96, F = 0.95; Malagasy pteropodids, M = 0.96, F = 0.97). Data are summarized in Supplementary Data SD1.B and SD1.C.
Welch’s 2-sample t-test comparisons indicated that length distributions for tibia and forearm length were significantly longer in adult males versus females for both P. rufus and R. madagascariensis (P < 0.001; Supplementary Data SD1 and SD2). For E. dupreanum, only tibia length was different between the sexes, with males again larger than females (P < 0.029). Ear lengths showed sexual dimorphism only in R. madagascariensis bats, for which observed female ear lengths were actually larger than those of males. Nonetheless, given that both tibia and forearm length were larger in R. mdagascariensis males versus females, we conclude that all three Malagasy fruit bat species demonstrated slight sexual dimorphism characterized by larger-bodied males and smaller females.
Linear regressions of log10 body mass as predicted by log10 forearm length for both all-pteropodid and Malagasy-specific data sets, separated by sex, demonstrated a good fit to the data with R2 values > 0.95. Roughly comparable slopes across all four models indicated 20- to 30-fold increases in bat mass (in grams) corresponding to every 10-fold increase in forearm length (in mm) across all species and sexes (Fig. 2C; Supplementary Data SD1).
Seasonality of mass:forearm relationships
Next, we refitted the regression of mass:forearm length across both sexes, incorporating bat species as a second fixed predictor of mass in our field data set (Supplementary Data SD1 and SD3), then computed mass:forearm residuals from the resulting regression models for each individual. We explored seasonal variation in these residuals from the central-eastern data subset, using GAMs. GAM results indicated significant seasonality in bat body condition for both male and female subsets of the P. rufus and E. dupreanum data and for the female subset of the R. madagascariensis data (P < 0.001; Supplementary Data SD1). Only male R. madagascariensis demonstrated no seasonal variation in mass:forearm residual. Finally, we plotted the GAM-predicted mass for each species and sex across, respectively, the reproductive and nutritional calendars for female and male fruit bats of the three Malagasy species in the central-eastern region (Fig. 3). As expected, we observed a seasonal peak in adult female mass:forearm which overlapped the staggered period of observed gestation for each species from Fig. 1, followed by a deficit overlapping the corresponding, species-specific lactation period. These results supported our assumption that the majority of female bats in our data set should be considered reproductive.
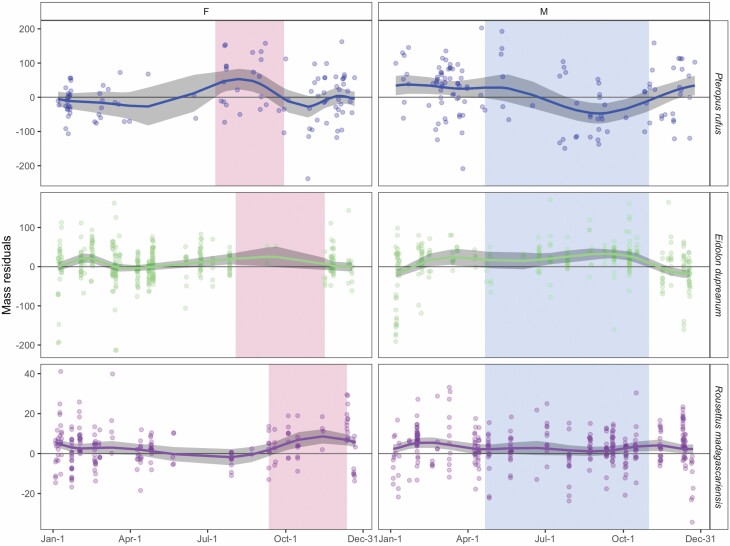
Fig. 3.
Seasonal variation in mass:forearm residual by sex (females = left, males = right) and species. Raw data from central-eastern Madagascar are shown as open circles with prediction from fitted generalized additive model (GAM) as solid line; 95% confidence intervals by standard error are shown by shading surrounding solid line (Supplementary Data SD1.E). For female plots, vertical shading corresponds to the species-specific gestation period; for male plots, vertical shading corresponds to the winter dry season.
We also observed a less extreme mass deficit that overlapped the resource-poor winter for male P. rufus and E. dupreanum but occurred earlier in the season for E. dupreanum than for P. rufus. Notably, GAMs for R. madagascariensis males, which showed no significant seasonality in body condition, predicted positive mass:forearm residuals across the entire calendar year, suggesting that bats in the central-eastern site had high mass:forearm ratios, as compared with those across the entire data set (sites outside of Manjakandriana/Moramanga Districts were not included in seasonal analyses). These results are logical, considering that, in our field study, outside of Moramanga, R. madagascariensis were predominantly captured in Ankarana National Park, an arid environment where bats are much more likely to experience food stress. Finally, supplementary GAMs used to model seasonal mass directly demonstrated comparable results to patterns for mass:forearm residuals across all three species and both sexes, with female masses peaking across gestation and male masses declining through the winter season (Supplementary Data SD4).
Juvenile growth rates
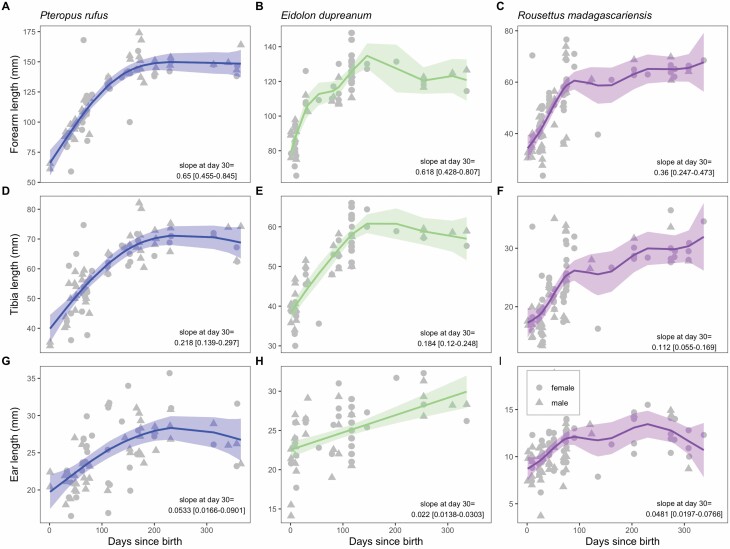
In our final analysis, we compared juvenile growth rates in forearm, tibia, and ear length across all three Malagasy fruit bat species in the first year of life. GAMs fitted to the response variable of each morphological trait demonstrated highly significant smoothing predictors of days since birth across all three metrics and all three species (Fig. 4; Supplementary Data SD1). Quantification of the derivative of each fitted GAM across the range of observed days since birth allowed us to compare growth rates across traits and species: in general, we observed the largest slopes, corresponding to the fastest growth rates for forearm lengths, then tibia lengths, and finally, ear lengths of all three species. Pteropus rufus grew at the fastest rate (largest slope in growth curve) for all three morphological traits, followed by E. dupreanum and R. madagascariensis. Despite faster growth rates, as the largest of the three species, P. rufus also demonstrated the most protracted development phase, approaching adult size (10-day average slope for forearm growth < 0.1) approximately 6 months after birth (180 days), as compared to 2 months (53 days) for E. dupreanum and 2.5 months (81 days) for R. madagascariensis. Species- and metric-specific growth rates from our fitted GAMs across the first year of life are summarized in Supplementary Data SD1.
Fig. 4.
Variation in juvenile forearm, tibia, and ear length with days since birth, corresponding to the date of first observed juvenile for each of three Madagascar species (29 September for Pteropus rufus, 16 November for Eidolon dupreanum, 12 December for Rousettus madagascariensis). Raw data are plotted as gray shapes (females = triangles, males = circles), with predictions from fitted generalized additive model (GAM) overlain as solid lines; 95% confidence intervals by standard error are shown by translucent shading (Supplementary Data SD1.F). The slope (derivative) of each fitted GAM at day 30 is identified in the bottom right; derivative results across the first year are summarized in Supplementary Data SD1.G.
Discussion
Here, we explore spatial–temporal and seasonal variation in morphological features for three endemic Malagasy bats in the Old World fruit bat family, Pteropodidae: P. rufus, E. dupreanum, and R. madagascariensis. Our work confirms that P. rufus, E. dupreanum, and R. madagascariensis birth in single annual pulses, temporally staggered across the three species, in Madagascar’s Manjakandriana/Moramanga Districts. In this central-eastern region, the P. rufus birth pulse occurred first in the months of September/October, followed by E. dupreanum in November, and R. madagascariensis in December. It is possible that the timing of this birth pulse may vary latitudinally based on climatic differences across the island (e.g. occurring earlier in warmer climates or later in cooler regions), though our birth pulse projections align well with previous records of the mating season for P. rufus in southeastern Madagascar (Long and Racey 2007) and R. madagascariensis in northwestern Madagascar (Lalarivoniaina et al. 2019); to our knowledge, no previous records defining the reproductive calendar for E. dupreanum have been published (Shi et al. 2014). Nonetheless, climate-related variation in birth pulse timing is well-described for populations of E. helvum, which range across the entirety of the African continent (Peel et al. 2013, 2017).
This birth timing of Malagasy fruit bats likely increases their vulnerability to seasonally varying population pressures. In particular, fruit bats are legally hunted during the Malagasy winter (1 May to 1 September), which overlaps the gestation period observed here for all three species, but most significantly for P. rufus, a species already known to be experiencing severe population declines due to anthropogenic threats (Golden et al. 2014; Brook et al. 2019b). Critically, the Malagasy fruit bat lactation periods are varied in duration such that, despite staggered birth pulses, juvenile weaning occurs largely coincidentally at the onset of the peak fruiting season in the hot-wet Malagasy summer, a pattern recapitulated across numerous species of frugivorous lemur (Britt et al. 2002; Powzyk and Mowry 2002; Wright et al. 2005). As a result, Malagasy fruit bat population viability will likely be sensitive to future shifts in fruiting phenology, which are predicted to accompany changing climates (Dunham et al. 2018). Importantly, our study quantifies life history traits needed to assess population viability for these species into the future (Dobson and Lyles 1989; Brook et al. 2019b).
In Madagascar, seasonally staggered birth pulses across the three fruit bat species could support the persistence of multispecies pathogens, such as bat-borne coronaviruses, which frequently transmit and recombine among different species of bats that co-roost in the same caves (Hu et al. 2017). Among Malagasy pteropodids, E. dupreanum and R. madagascariensis are known to share cave roosts, sometimes with insectivorous bats, while P. rufus inhabits single-species arboreal roosts (MacKinnon et al. 2003). Previous work suggests that sympatric cave-roosting likely plays a role in pathogen-sharing of diverse paramyxoviruses among Malagasy bats (Mélade et al. 2016), but considerable evidence also supports a largely single-host-species-to-single-pathogen relationship for many other bat-borne infections, including those described in Madagascar (Ng et al. 2015; Lagadec et al. 2016; Brook et al. 2019c; Joffrin et al. 2020). Notably, even bats co-roosting in the same cave can occupy disparate local environments within that cave, as is the case for E. dupreanum and R. madagascariensis in the Ankarana National Park cave system (Cardiff et al. 2009). In sum, it is likely that diverse inter- and intraspecies dynamics underpin the population-level persistence of different pathogen types in diverse bat ecosystems.
Because the dynamics of pathogen shedding and zoonotic spillover have been linked to reproductive and nutritional calendars across several bat-virus systems (Plowright et al. 2008; Amman et al. 2012; Schmidt et al. 2017; Brook et al. 2019c), documentation of seasonal variation in bat body condition and nutrition also has important implications for understanding immunity and pathogen maintenance. We here highlight significant seasonal changes observed in body condition for Malagasy fruit bats, apparently modulated by reproduction for females and corresponding more closely to the nutritional calendar for males. Further research confirming the reproductive status of adult female bats in this system—by either ultrasound in the field or assay of plasma progesterone from field-collected samples (Buchanan and Younglai 1986)—is needed to confirm this hypothesis of reproductive regulation of seasonal female bat masses. Additionally, future work elucidating seasonal and cross-species variation in fruit bat diet—and its impact on bat health—would do much to elucidate the observed discrepancy in the timing of the seasonal mass: forearm deficit for E. dupreanum (June–July) versus P. rufus (September) males. No seasonal pattern was found for male R. madagascariensis bats in our data set, which could result from a lack of statistical power to identify differences across a smaller body size range for this species, or which may signify perpetually abundant food resources for this species in the Moramanga District. Previous field studies in this system also found no evidence of seasonal variation in body mass for male R. madagascariensis (Andrianaivoarivelo et al. 2011).
Beyond the observed seasonality in body mass:forearm residual, which tracked reproduction for females and nutrition for males, we also documented sexual dimorphism (larger males vs. females) in tibia and forearm lengths for Malagasy fruit bats, a pattern that is common to pteropodids more generally (McNab and Armstrong 2001) and has been previously reported for R. madagascariensis (Goodman et al. 2017). Our study confirms that the size distribution of Malagasy pteropodids spans the range of that documented globally, with P. rufus and E. dupreanum among the larger 50% of previously described species and R. madagascariensis among the smaller. Mirroring adult size distributions, juvenile growth rates were highest and developmental periods longest in P. rufus, followed by E. dupreanum and R. madagascariensis. Critically, the longer development period for P. rufus further undermines the already-threatened conservation status of this species—which recent analysis suggests may be even more vulnerable than previously reported (Brook et al. 2019b). The rapid 2-month juvenile growth window witnessed for E. dupreanum in our data set suggests that this species may actually birth earlier than is recorded here; additional, intensive sampling throughout the reproduction period is needed to confirm the seasonal limits of each developmental stage for these three fruit bat species.
In conclusion, we quantify life history traits needed for population modeling, document seasonal variation in body condition, and elucidate the Malagasy fruit bat reproductive calendar, contributing important resources for future efforts to quantify both conservation trajectories and zoonotic pathogen transmission in this system. This work emphasizes the importance of longitudinal field studies in uncovering seasonal variability in ecological data, with critical implications for understanding of both population viability and infectious disease dynamics alike.
Supplementary Material
Acknowledgments
The authors thank Kimberly Rivera, Katie Fitzgerald, and Samantha Kreling for help in the field, the Virology Unit at the Institut Pasteur de Madagascar for logistical support, the Mention Zoologie et Biodiversité Animale at the Université d’Antananarivo for help in obtaining research permits, and the Brook lab at the University of Chicago for helpful contributions to the manuscript. We acknowledge funding from the National Institutes of Health (1R01AI129822-01 grant J-MH and CEB), DARPA (PREEMPT Program Cooperative Agreement no. D18AC00031 to CEB), the Adolph C. and Mary Sprague Miller Institute for Basic Research in Science (postdoctoral fellowship to CEB), the AAAS/Loréal-USA For Women in Science program (fellowship to CEB), and the Branco Weiss Society in Science (fellowship to CEB).
Contributor Information
Angelo Andrianiaina, Mention Zoologie et Biodiversité Animale, Université d’Antananarivo, Antananarivo 101, Madagascar.
Santino Andry, Mention Entomologie, Université d’Antananarivo, Antananarivo 101, Madagascar.
Anecia Gentles, Odum School of Ecology, University of Georgia, Athens 30609, Georgia, USA.
Sarah Guth, Department of Integrative Biology, University of California, Berkeley, Berkeley 94720, California, USA.
Jean-Michel Héraud, Virology Unit, Institut Pasteur de Madagascar, Antananarivo 101, Madagascar; Virology Department, Institut Pasteur de Dakar, Dakar 10200, Senegal; Ecole Doctorale Science de la Vie et de l’Environnement, Faculté des Sciences, Université d’Antananarivo, Antananarivo 101, Madagascar.
Hafaliana Christian Ranaivoson, Mention Zoologie et Biodiversité Animale, Université d’Antananarivo, Antananarivo 101, Madagascar; Virology Unit, Institut Pasteur de Madagascar, Antananarivo 101, Madagascar.
Ny Anjara Fifi Ravelomanantsoa, Mention Zoologie et Biodiversité Animale, Université d’Antananarivo, Antananarivo 101, Madagascar.
Timothy Treuer, Gund Institute for Environment, The University of Vermont, Burlington 05405, Vermont, USA.
Cara E Brook, Department of Integrative Biology, University of California, Berkeley, Berkeley 94720, California, USA; Department of Ecology and Evolution, University of Chicago, Chicago 60637, Illinois, USA.
Supplementary Data
Supplementary data are available at Journal of Mammalogy online.
Supplementary Data SD1.—Raw data and statistical output for all referenced analyses throughout manuscript. (A) Summary of bat captures by site and reproductive calendar by species from pan-Madagascar field sampling. (B) Interquartile ranges and Welch’s t-test results for male/female morphological traits across fruit bats (data are visualized in Supplementary Data SD2). (C) Regression statistics summary from bat mass:forearm ratios plotted in Fig. 2C, main text. (D) Summary output from multispecies mass:forearm linear regression. (E) Results summary from seasonal generalized additive model (GAM) of mass:forearm residual across Malagasy fruit bats. (F) Results summary from GAM of juvenile growth rates across all morphological traits for Malagasy fruit bats. (G) Time-varying GAM derivatives for juvenile growth rates across all morphological traits for Malagasy fruit bats.
Supplementary Data SD2.—Morphological trait distributions in forearm, tibia, and ear length for Pteropus rufus, Eidolon dupreanum, and Rousettus madagascariensis. Each panel compares distributions from females (F) versus males (M). Asterisks indicate significance in Welch’s 2-sample t-tests from data within each panel, based on significance codes: *** = 0.001; ** = 0.01; * = 0.05; . = 0.1. Data are numerically summarized in Supplementary Data SD1.B.
Supplementary Data SD3.—Forearm length (mm) versus mass (g) relationships for Malagasy fruit bats, separated by sex. Data are depicted as points colored by species. Solid black lines correspond to output from fitted linear regression model of log10 mass predicted by log10 forearm length, incorporating a fixed predictor of species. Residuals depicted in Fig. 3 (main text) were derived by subtracting predictions from data for each individual, as shown here. Model fits are summarized in Supplementary Data SD1.D.
Supplementary Data SD4.—Figure largely replicates Fig. 3 (main text) but here depicts the output of supplementary generalized additive models (GAMs) incorporating a direct response variable of bat mass (in g) predicted by day of year (as a cyclic cubic smoothing spline) with a random effect of forearm length. Random effects are silenced here for plotting purposes. As in Fig. 3, raw data are shown as open circles with prediction from fitted GAM model as solid line; 95% confidence intervals by standard error are shown by shading in gray. For female plots, pink shading corresponds to the species-specific gestation period; for male plots, blue shading corresponds to the winter dry season in Madagascar.
Literature Cited
- Amman B.R., Carroll S.A., Reed Z.D., Sealy T.K., Balinandi S., Swanepoel R., Kemp A., Erickson B.R., Comer J.A., Campbell S., et al. 2012. Seasonal pulses of Marburg virus circulation in juvenile Rousettus aegyptiacus bats coincide with periods of increased risk of human infection. PLoS Pathogens 8:e1002877. [This article has 26 authors]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriafidison D., Andrianaivoarivelo R.A., Ramilijaona O.R., Razanahoera M.R., Mackinnon J., Jenkins R.K.B., Racey P.A.. 2006. Nectarivory by endemic Malagasy fruit bats during the dry season. Biotropica 38:85–90. [Google Scholar]
- Andrianaivoarivelo R.A., Olga R., Racey P.A., Jenkins R.K.B.. 2011. Feeding ecology, habitat use and reproduction of Rousettus madagascariensis Grandidier, 1928 (Chiroptera: Pteropodidae) in eastern Madagascar. Mammalia 75:69–78. [Google Scholar]
- Barclay R.M.R., Jacobs D.S.. 2011. Differences in the foraging behaviour of male and female Egyptian fruit bats (Rousettus aegyptiacus). Canadian Journal of Zoology 89:466–473. [Google Scholar]
- Bartlett M.S. 1957. Measles periodicity and community size. Journal of the Royal Statistical Society, Series A 120:48–70. [Google Scholar]
- Bartlett M.S. 1960. The critical community size for measles in the United States. Journal of the Royal Statistical Society, Series A 123:37–44. [Google Scholar]
- Bollen A., Van E.L.. 2002. Feeding ecology of Pteropus rufus (Pteropodidae) in the littoral forest of Sainte Luce, SE Madagascar. Acta Chiropterologica 4:33–47. [Google Scholar]
- Britt A., Randriamandratonirina N.J., Glasscock K.D., Iambana B.R.. 2002. Diet and feeding behaviour of Indri indri in a low-altitude rain forest. Folia Primatologica 73:225–239. [DOI] [PubMed] [Google Scholar]
- Brooke A. 2002. Threats from overhunting to the flying fox, Pteropus tonganus, (Chiroptera: Pteropodidae) on Niue Island, South Pacific Ocean. Biological Conservation 103:343–348. [Google Scholar]
- Brook C.E., Bai Y., Dobson A.P., Osikowicz L.M., Ranaivoson H.C., Zhu Q., Kosoy M.Y.. 2015. Bartonella spp. in fruit bats and blood-feeding ectoparasites in Madagascar. PLoS Neglected Tropical Diseases 10:e0003532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook C.E., Herrera J.P., Borgerson C., Fuller E.C., Andriamahazoarivosoa P., Rasolofoniaina B.J.R., Randrianasolo J.L.R.R., Rakotondrafarasata Z.R.E., Randriamady H.J., Dobson A.P., et al. 2019a. Population viability and harvest sustainability for Madagascar lemurs. Conservation Biology 33:99–111. [This article has 11 authors]. [DOI] [PubMed] [Google Scholar]
- Brook C.E., Ranaivoson H.C., Andriafidison D., Ralisata M., Razafimanahaka J., Héraud J., Dobson A.P., Metcalf C.J.. 2019b. Population trends for two Malagasy fruit bats. Biological Conservation 234:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook C.E., Ranaivoson H.C., Broder C.C., Cunningham A.A., Héraud J.-M., Peel A.J., Gibson L., Wood J.L.N., Metcalf C.J., Dobson A.P.. 2019c. Disentangling serology to elucidate henipa- and filovirus transmission in Madagascar fruit bats. Journal of Animal Ecology 00:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan G.D., Younglai E.V.. 1986. Plasma progesterone levels during pregnancy in the little brown bat Myotis lucifugus (Vespertilionidae). Biology of Reproduction 34:878–884. [DOI] [PubMed] [Google Scholar]
- Cardiff S.G., Ratrimomanarivo F.H., Rembert G., Goodman S.M.. 2009. Hunting, disturbance and roost persistence of bats in caves at Ankarana, northern Madagascar. African Journal of Ecology 47:640–649. [Google Scholar]
- Corbet G.B., Hill J.E.. 1992. The mammals of the Indo-Malayan region. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- Craig P., Trail P., Morrell T.E.. 1994. The decline of fruit bats in American Samoa due to hurricanes and overhunting. Biological Conservation 69:261–266. [Google Scholar]
- Dewar R.E., Richard A.F.. 2007. Evolution in the hypervariable environment of Madagascar. Proceedings of the National Academy of Sciences of the United States of America 104:13723–13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson A.P., Lyles A.M.. 1989. The population dynamics and conservation of primate populations. Conservation Biology 3:362–380. [DOI] [PubMed] [Google Scholar]
- Dunham A.E., Razafindratsima O.H., Rakotonirina P., Wright P.C.. 2018. Fruiting phenology is linked to rainfall variability in a tropical rain forest. Biotropica 50:396–404. [Google Scholar]
- Federman S., Sinnott-Armstrong M., Baden A.L., Chapman A., Daly D.C., Richard A.R., Valenta K., Donoghue M.J.. 2017. The paucity of frugivores in Madagascar may not be due to unpredictable temperatures or fruit resources. PLoS One 12:e0168943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Llamazares Á., López-Baucells A., Rocha R., Andriamitandrina S.F.M., Andriatafika Z.E., Burgas D., Temba E.M., Torrent L., Cabeza M.. 2018. Are sacred caves still safe havens for the endemic bats of Madagascar? Oryx :1–5. [Google Scholar]
- Golden C.D., Bonds M.H., Brashares J.S., Rodolph Rasolofoniaina B.J., Kremen C.. 2014. Economic valuation of subsistence harvest of wildlife in Madagascar. Conservation Biology 28:234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S.M., Ganzhorn J.U.. 1997. Rarity of figs (Ficus) on Madagascar and its relationship to a depauperate frugivore community. Revue d’Ecologie 52:321–329. [Google Scholar]
- Goodman S.M., Rajemison F.I., Lalarivoniaina O.S.N.. 2017. Morphometric patterns of secondary sexual dimorphism and seasonal differences in Rousettus madagascariensis from northern Madagascar. Acta Chiropterologica 19:71–75. [Google Scholar]
- Guth S.E., Mollentze N., Renault K., Streicker D.G., Visher E., Boots M., Brook C.E.. 2022. Bats host the most virulent—but not the most dangerous—zoonotic viruses. Proceedings of the National Academy of Sciences of the United States of America 119:e2113628119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth S., Visher E., Boots M., Brook C.E.. 2019. Host phylogenetic distance drives trends in virus virulence and transmissibility across the animal–human interface. Philosophical Transactions of the Royal Society of London, B: Biological Sciences 374:20190296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman D.T.S. 2015. Biannual birth pulses allow filoviruses to persist in bat populations. Proceedings of the Royal Society of London, B: Biological Sciences 282:20142591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman D.T.S., McCrea R., Restif O., Suu-Ire R., Fooks A.R., Wood J.L.N., Cunningham A.A., Rowcliffe J.M.. 2012. Demography of straw-colored fruit bats in Ghana. Journal of Mammalogy 93:1393–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heideman P.D. 1988. The timing of reproduction in the fruit bat Haplonycteris fischeri (Pteropodidae): geographic variation and delayed development. Journal of Zoology 215:577–595. [Google Scholar]
- Heideman P.D., Powell K.S.. 1998. Age-specific reproductive strategies and delayed embryonic development in an Old World fruit bat, Ptenochirus jagori. Journal of Mammalogy 79:295–311. [Google Scholar]
- Hu B., et al. 2017. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathogens 13:1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iehlé C., Razafitrimo G., Razainirina J., Andriaholinirina N., Goodman S.M., Faure C.. 2007. Henipavirus and Tioman virus antibodies in pteropodid bats, Madagascar. Emerging Infectious Diseases 13:159–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R.K.B., Racey P.A.. 2008. Bats as bushmeat in Madagascar. Madagascar Conservation and Development 3:22–30. [Google Scholar]
- Joffrin L., et al. 2020. Bat coronavirus phylogeography in the western Indian Ocean. Scientific Reports 10:6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K.E., Mickleburgh S.P., Sechrest W., Walsh A.L.. 2009. Global overview of the conservation of island bats: importance, challenges and opportunities. In: Fleming T.H., Racey P.A., editors. Island bats: evolution, ecology & conservation. University of Chicago Press, Chicago; p. 496–531. [Google Scholar]
- Kamins A.O., Restif O., Ntiamoa-Baidu Y., Suu-Ire R., Hayman D.T.S., Cunningham A.A., Wood J.L.N., Rowcliffe J.M.. 2011. Uncovering the fruit bat bushmeat commodity chain and the true extent of fruit bat hunting in Ghana, West Africa. Biological Conservation 144:3000–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko H., Lindström J.. 1998. Seasonal density dependence, timing of mortality, and sustainable harvesting. Ecological Modelling 110:293–304. [Google Scholar]
- Kunz T.H., Braun de Torrez E., Bauer D., Lobova T., Fleming T.H.. 2011. Ecosystem services provided by bats. Annals of the New York Academy of Sciences 1223:1–38. [DOI] [PubMed] [Google Scholar]
- Lagadec E., Gomard Y., le Minter G., Cordonin C., Cardinale E., Ramasindrazana B., Dietrich M., Goodman S.M., Tortosa P., Dellagi K.. 2016. Identification of Tenrec ecaudatus, a wild mammal introduced to Mayotte Island, as a reservoir of the newly identified human pathogenic Leptospira mayottensis. PLoS Neglected Tropical Diseases 10:e0004933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalarivoniaina O.S.N., Rajemison F.I., Ramanantsalama R., Andrianarimisa A., Goodman S.M.. 2019. Population size and survival of the Malagasy fruit bat Rousettus madagascariensis (Pteropodidae) in Ankarana, Northern Madagascar. Acta Chiropterologica 21:103–113. [Google Scholar]
- Langrand O. 1990. Guide to the birds of Madagascar. Yale University Press, New Haven, Connecticut, USA. [Google Scholar]
- Long E., Racey P.A.. 2007. An exotic plantation crop as a keystone resource for an endemic megachiropteran, Pteropus rufus, in Madagascar. Journal of Tropical Ecology 23:397. [Google Scholar]
- MacKinnon J.L., Hawkins C.E., Racey P.A.. 2003. Pteropodidae, fruit bats, Fanihy, Angavo. In: Goodman S.M., Benstead J.P., editors. The natural history of Madagascar. The University of Chicago Press,Chicago; p. 1299–1302. [Google Scholar]
- McConkey K.R., Drake D.R.. 2006. Flying foxes cease to function as seed dispersers long before they become rare. Ecology 87:271–276. [DOI] [PubMed] [Google Scholar]
- McGuire L.P., et al. 2018. Common condition indices are no more effective than body mass for estimating fat stores in insectivorous bats. Journal of Mammalogy 99:1065–1071. [Google Scholar]
- McIlwee A.P., Martin L.. 2002. On the intrinsic capacity for increase of Australian flying-foxes (Pteropus spp., Megachiroptera). Australian Zoologist 32:76–100. [Google Scholar]
- McNab B.K., Armstrong M.I.. 2001. Sexual dimorphism and scaling of energetics in flying foxes of the genus Pteropus. Journal of Mammalogy 82:709–720. [Google Scholar]
- Meenakumari K.J., Krishna A.. 2005. Delayed embryonic development in the Indian short-nosed fruit bat, Cynopterus sphinx. Zoology 108:131–140. [DOI] [PubMed] [Google Scholar]
- Mélade J., McCulloch S., Ramasindrazana B., Lagadec E., Turpin M., Pascalis H., Goodman S.M., Markotter W., Dellagi K.. 2016. Serological evidence of Lyssaviruses among bats on Southwestern Indian Ocean Islands. PLoS One 11:e0160553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutere F.A. 1967. The breeding biology of equatorial vertebrates: reproduction in the fruit bat, Eidolon helvum, at latitude 0°20ʹN. Journal of Zoology 153:153–161. [Google Scholar]
- Ng M., et al. 2015. NPC1 contributes to species-specific patterns of Ebola virus infection in bats. eLife 4:e11785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odukoya S.A., Adeeyo O.A., Ofusori D.A., Caxton-Martins A.E., Ayoka O.A., Oyewo O.O., Babatunde L.S., Yusuf U.A., Adegoke A.A., Ishola O.O.. 2008. Histological investigation of the pregnant and non pregnant uterine limbs of the frugivorous bat (Eidolon helvum). International Journal of Integrative Biology 3:169–174. [Google Scholar]
- Oleksy R., Giuggioli L., McKetterick T.J., Racey P.A., Jones G.. 2017. Flying foxes create extensive seed shadows and enhance germination success of pioneer plant species in deforested Madagascan landscapes. PLoS One 12:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleksy R., Racey P.A., Jones G.. 2015. High-resolution GPS tracking reveals habitat selection and the potential for long-distance seed dispersal by Madagascan flying foxes Pteropus rufus. Global Ecology and Conservation 3:678–692. [Google Scholar]
- Oleksy R., Randrianandrianina F., Jenkins R.K.B.. 2003. Commercial hunting of foraging fruit bats in Western Madagascar. African Bat Conservation News 37:239–246. [Google Scholar]
- Openshaw J.J., Hegde S., Sazzad H.M.S., Khan S.U., Hossain M.J., Epstein J.H., Daszak P., Gurley E.S., Luby S.P.. 2016. Bat hunting and bat-human interactions in Bangladeshi villages: Implications for zoonotic disease transmission and bat conservation. Transboundary and Emerging Diseases 64:1287–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel A.J., Baker K.S., Crameri G., Barr A.J., Hayman D.T.S., Wright E., Broder C.C., Fernandez-Lorez A., Fooks A.R., Wang L.F., et al. 2012. Henipavirus neutralising antibodies in an isolated island population of African fruit bats. PLoS One 7:e30346. [This article has 12 authors]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel A.J., Pulliam J.R.C., Luis A.D., Plowright R.K., O'Shea T.J., Hayman D.T.S., Wood J.L.N., Webb C.T., Restif O., et al. 2014. The effect of seasonal birth pulses on pathogen persistence in wild mammal populations. Proceedings of the Royal Society of London, B: Biological Sciences 281:20132962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel A.J., Sargan A.R., Baker K.S., Hayman D.T.S., Barr J.A., Crameri G., Suu-Ire R., Broder C.C., Lembo T., Wang, et al. 2013. Continent-wide panmixia of an African fruit bat facilitates transmission of potentially zoonotic viruses. Nature Communications 4:2770. [This article has 14 authors]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel A.J., Wood J.L.N., Baker K.S., Breed K.C., de Carvalho A., Fernadez-Loras A., Gabrieli H.S., Gembu G.C., Kagengi, V.A., Kalebi P.M., Kityo, R.M., et al. 2017. How does Africa’s most hunted bat vary across the continent? Population traits of the straw-coloured fruit bat (Eidolon helvum) and its interactions with humans. Acta Chiropterologica 19:77–92. [This article has 18 authors]. [Google Scholar]
- Picot M., Jenkins R.K.B., Ramilijaona O., Racey P.A., Carrie S.M.. 2007. The feeding ecology of Eidolon dupreanum (Pteropodidae) in eastern Madagascar. African Journal of Ecology 45:645–650. [Google Scholar]
- Plowright R.K., Field H.E., Smith C., Divljan A., Palmer C., Tabor G., Daszak P., Foley J.E.. 2008. Reproduction and nutritional stress are risk factors for Hendra virus infection in little red flying foxes (Pteropus scapulatus). Proceedings of the Royal Society of London, B: Biological Sciences 275:861–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powzyk J.A., Mowry C.B.. 2002. Dietary and feeding differences between sympatric Propithecus diadema diadema and Indri indri. International Journal of Primatology 24:1143–1162. [Google Scholar]
- Raharimihaja T.E.A., Rakotoarison J.L.M., Racey P.A., Andrianaivoarivelo R.A.. 2016. A comparison of the effectiveness of methods of deterring pteropodid bats from feeding on commercial fruit in Madagascar. Journal of Threatened Taxa 8:9512–9524. [Google Scholar]
- Ranaivoson H.C., Héraud J.-M., Goethert H.K., TelfordS.R., III, Rabetafika L., Brook C.E.. 2019. Babesial infection in the Madagascan flying fox, Pteropus rufus É. Geoffroy, 1803. Parasites & Vectors 12:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randrianandrianina F.H., Racey P., Jenkins R.K.B.. 2010. Hunting and consumption of mammals and birds by people in urban areas of western Madagascar. Oryx 44:411–415. [Google Scholar]
- Razafindratsimandresy R., Jeanmaire E.M., Counor D., Vasconcelos P.F., Sall A.A., Reynes J.-M.. 2009. Partial molecular characterization of alphaherpesviruses isolated from tropical bats. Journal of General Virology 90:44–47. [DOI] [PubMed] [Google Scholar]
- Razanajatovo N.H., Nomenjanahary L.A., Wilkinson D.A., Razafimanahaka J.H., Goodman S.M., Jenkins R.K., Jones J.P., Heraud J.-M.. 2015. Detection of new genetic variants of Betacoronaviruses in endemic frugivorous bats of Madagascar. Virology Journal 12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynes J.-M., Andriamandimby S.F., Razafitrimo G.M., Razainirina J., Jeanmaire E.M., Bourhy H., Heraud J.-M.. 2011. Laboratory surveillance of rabies in humans, domestic animals, and bats in Madagascar from 2005 to 2010. Advances in Preventive Medicine 2011:727821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha R., et al. 2020. Bat conservation and zoonotic disease risk: a research agenda to prevent misguided persecution in the aftermath of COVID-19. Animal Conservation 24(3):303–307. [Google Scholar]
- Schmidt J.P., Park A.W., Kramer A.M., Han B.A., Alexander L.W., Drake J.M.. 2017. Spatiotemporal fluctuations and triggers of Ebola virus spillover. Emerging Infectious Diseases 23:415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J.J., Chan L.M., Peel A.J., Lai R., Yoder A.D., Goodman S.M.. 2014. A deep divergence time between sister species of Eidolon (Pteropodidae) with evidence for widespread panmixia. Acta Chiropterologica 16:279–292. [Google Scholar]
- Sikes R.S., and the Animal Care and Use Committee of the American Society of Mammalogists. 2016. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. Journal of Mammalogy 97:663–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons N., Cirranello A.. 2020. Bat species of the world: a taxonomic and geographic database.https://batnames.org/. Accessed 11 July 2020.
- Species IUCN Red List Threat. 2018. IUCN 2018. Version 2018-2. IUCN Red List. [Google Scholar]
- Swinton J., Harwood J., Grenfell B.T., Gilligan C.A.. 1998. Persistence thresholds for phocine distemper virus infection in harbour seal Phoca vitulina metapopulations. Journal of Animal Ecology 67:54–68. [Google Scholar]
- Temmam S., Vongphayloth K., Baquero E., Munier S., Bonomi M., Regnault B., Douangboubpha B., Karami Y., Chretien D., Sanamxay D., et al. 2022. Bat coronaviruses related to SARS-CoV-2 and infectious for human cells. Nature 604:330–336. doi: 10.1038/s41586-022-04532-4. [This article has 26 authors]. [DOI] [PubMed] [Google Scholar]
- Terborgh J. 1983. Five New World primates: a study in comparative ecology. Princeton University Press, Princeton, New Jersey, USA. [Google Scholar]
- Terborgh J. 1986. Keystone plant resources in the tropical forest. In: Soule M., editor. Conservation biology: the science of scarcity and diversity. Sunderland Sinauer Associates, Inc., Sunderland, Massachusetts; p. 330–344. [Google Scholar]
- Wilkinson D.A., Temmam S., Lebarbenchon C., Legadec E., Chotte J., Guillebaud J., Ramasindrazana B., Heraud J.M., de Lamballerie X., Goodman S.M.et al. 2012. Identification of novel paramyxoviruses in insectivorous bats of the Southwest Indian Ocean. Virus Research 170:159–163. [This article has 12 authors]. [DOI] [PubMed] [Google Scholar]
- Wood S.N. 2001. mgcv: GAMs and generalized ridge regression for R. R News 1(2):20–24. [Google Scholar]
- Wright P.C., Tecot S.R., Erhart E.M., Baden A.L., King S.J., Grassi C.. 2011. Frugivory in four sympatric lemurs: implications for the future of Madagascar’s forests. American Journal of Primatology 73:585–602. [DOI] [PubMed] [Google Scholar]
- Wright P.C., Vololontiana R., Pochron S.T.. 2005. The key to Madagascar frugivores. In: Dew J.L., Boubli J.P., editors. Tropical fruits and frugivores: the search for strong interactors. Springer, The Netherlands; p. 121–138. [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., et al. 2020. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579:270–273. [This article has 29 authors]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.