Abstract
Background and Objectives
There are disparities in the prevalence of obesity by race, and the relationship between obesity and cognitive decline is unclear. The objective of this study was to determine whether obesity is independently associated with cognitive decline and whether the association between obesity and cognitive decline differs in Black and White adults. We hypothesized that obesity is associated with greater cognitive decline compared with normal weight and that the effect of obesity on cognitive decline is more pronounced in Black adults compared with their White counterparts.
Methods
We pooled data from 28,867 participants free of stroke and dementia (mean, SD: age 61 [10.7] years at the first cognitive assessment, 55% female, 24% Black, and 29% obese) from 6 cohorts. The primary outcome was the annual change in global cognition. We performed linear mixed-effects models with and without time-varying cumulative mean systolic blood pressure (SBP) and fasting plasma glucose (FPG). Global cognition was set to a t-score metric (mean 50, SD 10) at a participant's first cognitive assessment; a 1-point difference represents a 0.1 SD difference in global cognition across the 6 cohorts. The median follow-up was 6.5 years (25th percentile, 75th percentile: 5.03, 20.15).
Results
Obese participants had lower baseline global cognition than normal-weight participants (difference in intercepts, −0.36 [95% CI, −0.46 to −0.17]; p < 0.001). This difference in baseline global cognition was attenuated but was borderline significant after accounting for SBP and FPG (adjusted differences in intercepts, −0.19 [95% CI, −0.39 to 0.002]; p = 0.05). There was no difference in the rate of decline in global cognition between obese and normal-weight participants (difference in slope, 0.009 points/year [95% CI, −0.009 to 0.03]; p = 0.32). After accounting for SBP and FPG, obese participants had a slower decline in global cognition (adjusted difference in slope, 0.03 points/year slower [95% CI, 0.01 to 0.05]; p < 0.001). There was no evidence that race modified the association between body mass index and global cognitive decline (p = 0.34).
Discussion
These results suggest that obesity is associated with lower initial cognitive scores and may potentially attenuate declines in cognition after accounting for BP and FPG.
Obesity prevalence has risen considerably over the past few decades. Cross-sectional data from the National Health and Nutrition Examination Survey indicate that 42% of adults were obese in 2018, up from 31% in 2000.1 Obesity is associated with an increased risk of mortality, cardiovascular disease, stroke, diabetes, and cancer.2 Race, a proxy for socioeconomic status and allostatic load, may contribute to differences in obesity prevalence.3,4 National studies show that the prevalence of obesity in non-Hispanic Black adults is 50%, compared with 42% in non-Hispanic White adults.1 Disparities in obesity prevalence and its associated cardiometabolic outcomes have been linked to lower levels of education, literacy, and financial adequacy—risk factors for cognitive impairment and dementia (CID).5 Older Black adults have a 2-fold higher risk of CID than their White counterparts.6
The relationship between obesity and CID is unclear. Some studies show no association or a mildly increased risk between midlife obesity and CID and no or decreased risk of late‐life obesity with CID, likely due to the prodromal weight loss phenomenon that occurs up to 10 years before dementia diagnosis.7-10 Less is known about differences in the relationship between obesity and CID in Black and White adults, and longitudinal studies with racial diversity are particularly sparse. Studies have found that the effect of vascular risk factors on the brain might differ between Black and White adults.11 In particular, increases in body mass index (BMI) over time were associated with lower total brain volumes in Black adults, but no evidence of an association in White adults.12 Furthermore, whether racial differences in obesity prevalence explain disparities in CID is uncertain, and whether the association of obesity and cognitive decline is independent of obesity's effect on blood pressure (BP) and glucose levels has not been well elucidated.
This analysis leverages 6 diverse, well-characterized, population-based cohort studies with repeated objective measures of cognition to examine whether obesity independently confers an increased risk of cognitive decline and whether race alters the association of obesity with cognitive trajectories. We hypothesized that obesity is associated with greater cognitive decline compared with normal weight and that the effect of obesity on cognitive decline is more pronounced in Black adults compared with their White counterparts.
Methods
Study Design
The report follows the STROBE reporting guidelines for cohort studies.13 This meta-analysis examined individual participant data from well-characterized American prospective cohort studies from 1971 to 2017: Atherosclerosis Risk in Communities (ARIC) study,14 Coronary Artery Risk Development in Young Adults (CARDIA) study,15 Cardiovascular Health Study (CHS),16 Framingham Offspring Study (FOS),17 Multi-Ethnic Study of Atherosclerosis (MESA),18 and Northern Manhattan Study (NOMAS)19 (eMethods, links.lww.com/WNL/C411).
Standard Protocol Approvals, Registrations, and Patient Consents
The University of Michigan Institutional Review Board approved this study (Blood Pressure-Cognition ). Participating institutions approved the cohort studies. Participants provided written informed consent.
Study Population
We required ≥1 measurements of cognition and ≥1 measurements of BMI before the first measurement of cognition. We excluded participants reporting a baseline history of stroke and those with incident stroke (because stroke can alter cognitive trajectories)20 or cohort-defined incident dementia at or before the first cognitive assessment.21
Measurement of BMI
BMI was measured closest to, but not after, the first cognitive assessment using participants weight in kilograms divided by height in square meters.
Measurement of Race/Ethnicity
We excluded participants who reported race other than Black or White (n = 3,385) because there were few other races, which precluded examining the association between other race and the dependent variable. We excluded participants reporting Hispanic ethnicity from the MESA and NOMAS because other cohorts did not collect information on Hispanic ethnicity or had few participants reporting Hispanic ethnicity, making it difficult to separate the effect of the MESA and NOMAS cohorts from the effect of Hispanic ethnicity.21
Cognitive Function Assessments
Trained cohort staff administered cognitive function tests longitudinally in person to participants using tests validated in Black and White adults22,23 and consistent with the Vascular Cognitive Impairment Harmonization Standards.24 In 3 cohorts (ARIC study, NOMAS, and CHS), trained staff administered global cognitive function tests (but not memory or executive function tests) by telephone for participants unable to attend some of the examination visits in person. Cognitive tests can be measured reliably and precisely over the telephone in adults with comparable results.25,26
To make inferences about cognitive domains instead of individual cognitive test items, and to resolve the challenge of different cognitive tests administered across cohorts, we cocalibrated available cognitive test items into factors representing global cognition (global cognitive performance), learning and memory (learning and delayed recall/recognition), and executive function (complex and/or speed cognitive functions) using item response theory methods that leverage all available cognitive information across cohorts and test items unique to particular cohorts.27,28 Cognitive factor scores, estimated using the regression-based method in Mplus,29,30 were set to a t-score metric (mean 50, SD 10 at a participant's first cognitive assessment); a 1-point difference represents a 0.1 SD difference in the distribution of cognition across the 6 cohorts. Higher cognitive scores indicate better performance (eMethods, links.lww.com/WNL/C411). The primary outcome was change in global cognition. Secondary outcomes were change in executive function and memory.26
Covariates
We used covariates measured closest to, but not after, the first cognitive assessment. Demographics included age, sex, education, and cohort study. Vascular risk factors included current cigarette smoking, physical activity, low-density lipoprotein (LDL) cholesterol, and history of atrial fibrillation. Cohorts measured current hypertension medication use by evidence of medication bottles and self-report. Each cohort study measured BP and fasting plasma glucose (FPG) at in-person visits using standard protocols and equipment.21
Statistical Analysis
Following a prespecified analysis plan, we compared participant characteristics by standard World Health Organization (WHO) BMI categories (normal weight: 18.5 to <25 kg/m2, overweight: ≥25 to <30 kg/m2, and obese: ≥30 kg/m2)31 using analysis of variance, Kruskal-Wallis test, or χ2 test as appropriate. Underweight participants (BMI <18.5 kg/m2) were excluded from this analysis to minimize reverse causation from smoking or preexisting illness. Linear mixed-effects models estimated changes in each continuous cognitive outcome over time by BMI category and other covariates. Time was treated as a continuous covariate and defined as years since the first measurement of each cognitive outcome. Because the pooled data involved a small number of cohorts (n = 6), we associated a fixed effect with cohorts.21 To estimate differences in cognitive decline by BMI category, models included a BMI factor × follow-up time interaction term. The models included covariates in Table 1, interaction terms for age at the time of the first cognitive assessment × follow-up time, sex × follow-up time, race × follow-up time, and subject-specific random effects for intercepts and slopes. All continuous covariates were centered at the overall median, except cumulative mean systolic BP (SBP), which was centered at 120 mm Hg. FPG, LDL cholesterol, and SBP values were divided by 10 so that parameter estimates reflect a 10-unit change in the variables. For each outcome, all available cognitive observations were used in the primary analysis except those after adjudicated incident stroke during follow-up because incident stroke alters the cognitive trajectory.20,26 Previous studies found no evidence of nonlinear effects of covariates on cognitive trajectories.
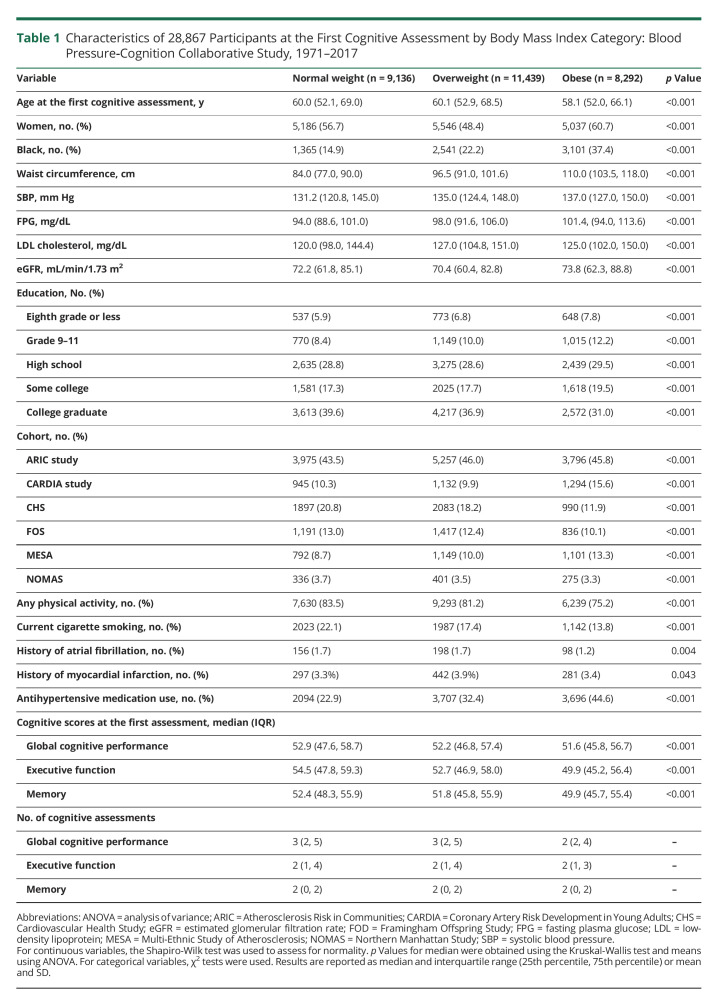
Table 1.
Characteristics of 28,867 Participants at the First Cognitive Assessment by Body Mass Index Category: Blood Pressure‐Cognition Collaborative Study, 1971–2017
Cumulative mean SBP and FPG are likely confounders of the association between obesity and cognitive function. Therefore, to examine whether obesity is a risk factor for cognitive decline independent of BP and FPG, model A included covariates and aforementioned interaction terms without time-varying cumulative mean SBP and FPG, whereas model B included time-varying cumulative mean SBP, cumulative mean SBP × follow-up time, time-varying cumulative mean FPG, and cumulative mean FPG × follow-up time.
To address whether race modifies the association of BMI and cognitive decline, a race × BMI × follow-up time interaction term was added to model B. We examined the effect modification of the BMI-cognitive decline association by age because studies have suggested a positive association of midlife obesity and dementia, but opposite associations in late life.8 We found no evidence of a significant age × BMI × follow-up time interaction on global cognition trajectories. We found evidence of a significant age × BMI (intercept) interaction (p < 0.001) and improved model fit, so the interaction term was included in the final models (eTable 1, links.lww.com/WNL/C411). Statistical significance for all analyses was set as p < 0.05 (2 sided). Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and Stata, IC version 16.0 (StataCorp LP, College Station, TX).
Sensitivity Analyses
We repeated analyses including participants' cognitive observations after the time of incident stroke and after adding kidney function (estimated glomerular filtration rate [eGFR]32) and history of myocardial infarction because they may be on the causal pathway.26 We also examined the interaction of BMI and APOE ε4 genotype on cognitive decline.33
Although the use of BMI for the measurement of overweight and obesity is advantageous because of its widespread use in research and clinical practice and its association with many cardiovascular risk factors, it has limitations. BMI is not a perfect indicator of visceral adiposity and does not consider differences in body composition with aging. We repeated analyses using WC categories and added race × WC × follow-up time interaction terms to models. We also analyzed BMI and WC as continuous measures, and both exposures were included in models. To minimize the effect of reverse causality, we repeated analyses excluding participants aged ≥65 years. We examined the unadjusted association between global cognition and BMI category and whether baseline BMI or cognition was associated with fewer cognitive assessments.
Data Availability
Anonymized data from the cohort studies used in this analysis were available from each study's respective coordinating centers. Specific policies governing each study's data and the process to access data can be found online (ARIC study: sites.cscc.unc.edu/aric/; CARDIA study: cardia.dopm.uab.edu/; MESA: mesa-nhlbi.org/; CHS: chs-nhlbi.org/; FOS: framinghamheartstudy.org/; and NOMAS: columbianomas.org/study.html).
Results
The study sample included 28,867 participants. The mean age of study participants at the first cognitive assessment was 61 years; 55% were women, 24% were Black, and 29% were obese. Figure 1 shows the derivation of the meta-cohort. Table 1 presents characteristics by BMI category measured at or before the first cognitive assessment. During a median follow-up of 6.5 years (25th, 75th percentile, 5.0–20.1), the median number of global cognition assessments was 3 (25th, 75th percentile, 2–5) for normal-weight and overweight adults and 2 for obese adults (25th, 75th percentile, 2–4). The median number of executive function assessments was 2 (25th, 75th percentile, 1–4) for normal-weight, overweight, and obese adults. The median number of memory assessments was 2 (25th, 75th percentile, 0–2) for all BMI categories. Compared with their normal-weight counterparts, obese participants were more likely to be female, Black, and have a higher SBP and FPG. Obese participants were less likely to smoke or engage in physical activity and had lower mean baseline global cognition, executive function, and memory scores compared with normal-weight participants.
Figure 1. Derivation of the Cohort.
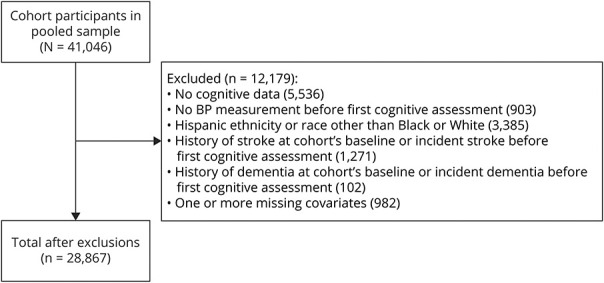
Categories for missing data on covariates are not mutually exclusive. Missing data include body mass index (n = 40), glucose (n = 351), waist circumference (n = 118), smoking (n = 8), physical activity (n = 50), LDL cholesterol (n = 350), education (n = 189), antihypertensive medication (n = 26), history of atrial fibrillation (n = 1), and history of myocardial infarction (n = 1). BP = blood pressure; LDL = low-density lipoprotein.
Differences in Baseline and Slope of Global Cognition
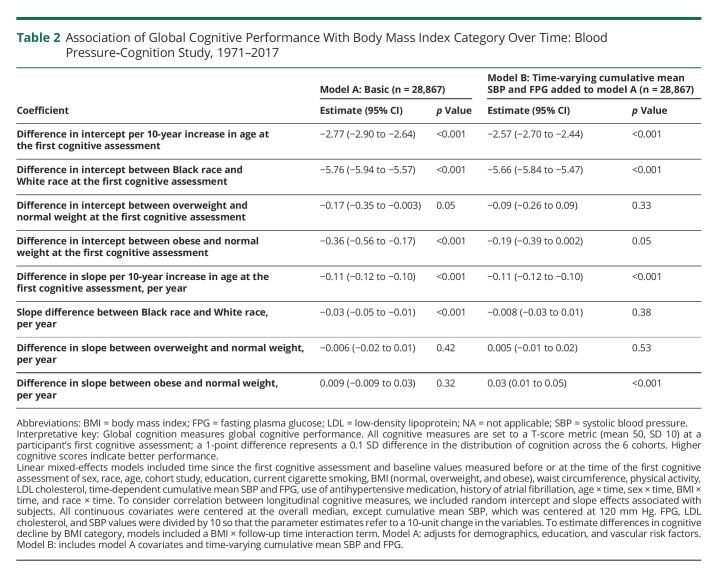
Table 2 and Figure 2 describe the adjusted association of global cognition with BMI category. Overweight (difference in intercepts, −0.17 [95% CI, −0.35 to −0.003]; p = 0.05; model A) and obese (difference in intercepts, −0.39 [95% CI, −0.56 to −0.17]; p < 0.001; model A) participants had lower baseline global cognition compared with normal-weight participants. This difference in baseline global cognition in overweight (adjusted differences in intercepts, −0.09 [95% CI, −0.26 to 0.09; p = 0.3]; model B) and obese (adjusted differences in intercepts, −0.19 [95% CI, −0.39 to 0.002]; p = 0.05; model B) participants was attenuated after adjusting for time-varying cumulative mean SBP and FPG. Before adjusting for time-varying cumulative mean SBP and FPG, there was no difference in the slopes of global cognition in overweight (difference in slope, −0.006 points/year; [95% CI, −0.02 to 0.01; p = 0.42]; model A) and obese (difference in slope, 0.009 points/year [95% CI, −0.009 to 0.03]; p = 0.3; model A) participants compared with their normal-weight counterparts. However, after adjustment for time-varying cumulative mean SBP and FPG, obese participants had slower decline in global cognition (adjusted differences in slope, 0.03 points/year slower [95% CI, 0.01 to 0.05]; p < 0.001; model B). There was no difference in the slope of global cognition in overweight participants compared with normal-weight participants after adjusting for SBP and FPG. Black participants had faster decline in global cognition compared with their White counterparts (adjusted differences in slope, 0.03 points/year faster [95% CI, −0.05 to −0.01]; p < 0.001 model A]). Race did not modify the association of BMI on the intercept (p = 0.33) and slope (p = 0.34) of global cognition.
Table 2.
Association of Global Cognitive Performance With Body Mass Index Category Over Time: Blood Pressure‐Cognition Study, 1971–2017
Figure 2. Predicted Global Cognitive Trajectory by BMI Category.
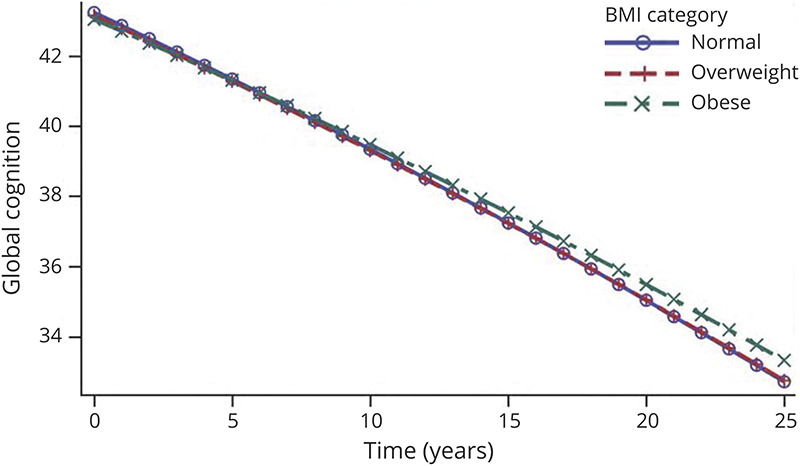
Participant-specific (conditional) predicted values of cognition were calculated for a 60-year-old Black participant (female vs male) with the following values of all covariates at or before the first cognitive assessment: NOMAS cohort, eighth grade or lower education, nonsmoking, LDL cholesterol (123.8 mg/dL) and glucose (95.7 mg/dL) that increases by 0.1 mg/dL each year, no history of atrial fibrillation, no hypertension treatment, and a baseline SBP of 150 mm Hg that increases by 1 mm each year. Random effects were set to zero. Linear mixed-effects models included time since the first cognitive assessment and baseline values measured before or at the time of the first cognitive assessment of body mass index (BMI; normal, overweight, and obese), age, race, sex, cohort study, education, current cigarette smoking, waist circumference (WC), physical activity, LDL cholesterol, time-dependent cumulative mean systolic blood pressure (SBP) and fasting plasma glucose (FPG), use of antihypertensive medication, history of atrial fibrillation, age × time, sex × time, BMI × time, and race × time. LDL = low-density lipoprotein.
Differences in Baseline and Slope of Executive Function
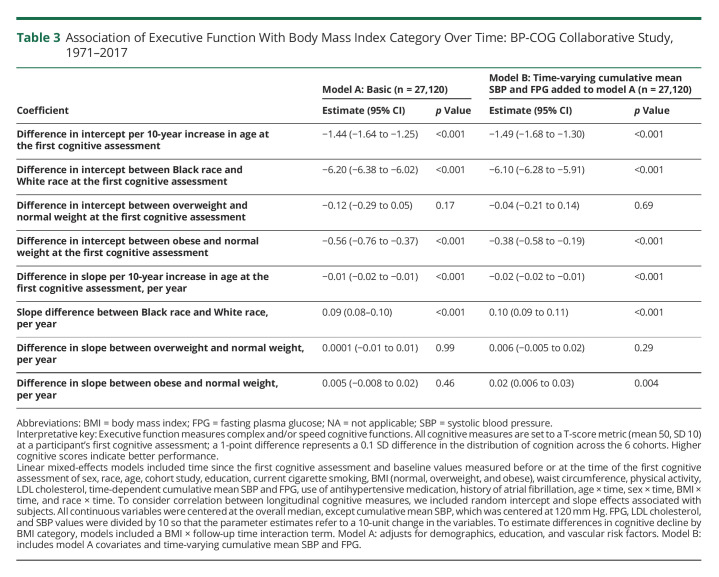
Table 3 describes the association of executive function with BMI category. There was no significant difference in baseline executive function in overweight participants (adjusted differences in intercepts, −0.12 [95% CI, −0.29 to 0.05]; p = 0.17; model A) compared with normal-weight participants. Obese participants had lower baseline executive function compared with normal-weight participants (adjusted differences in intercepts, −0.56 [95% CI, −0.76 to −0.37]; p < 0.001; model A). This difference in baseline executive function in obese participants compared with their normal-weight counterparts was slightly attenuated after adjusting for cumulative mean SBP and FPG (adjusted differences in intercepts, −0.38 [95% CI, −0.58 to −0.19]; p < 0.001; model B). There were no differences in changes in executive function over time between obese and normal-weight participants (differences in slope, 0.005 points/year [95% CI, −0.008 to 0.02]; p = 0.46; model A). After adjustment for cumulative mean SBP and FPG, obese participants had slower cognitive decline in executive function (adjusted differences in slope, 0.02 points/year slower [95% CI, 0.006 to 0.03]; p = 0.004; model B). There was no difference in the slope of executive function in overweight participants compared with normal-weight participants. Black participants had slower decline in executive function compared with their White counterparts (adjusted differences in slope, 0.10 points/year slower [95% CI, 0.09 to 0.11]; p < 0.001 model A). Race modified the association of BMI on the intercept (p = 0.02), but not slope (p = 0.76) of executive function.
Table 3.
Association of Executive Function With Body Mass Index Category Over Time: BP-COG Collaborative Study, 1971–2017
Differences in Baseline and Slope of Memory
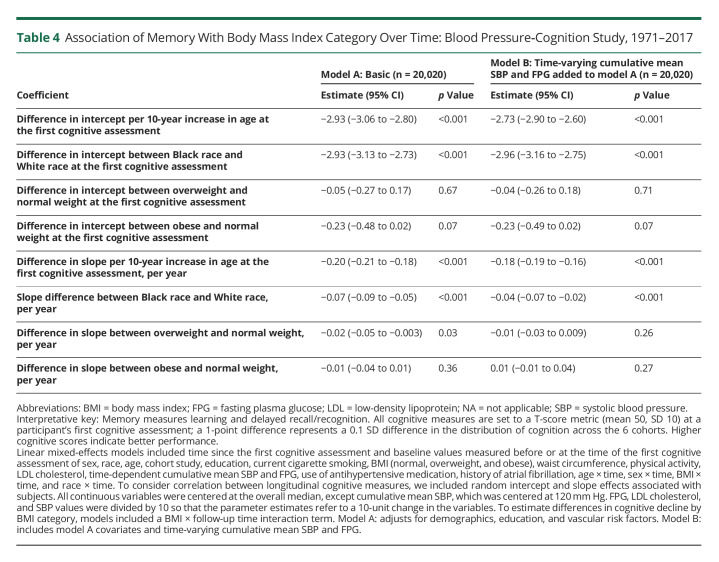
Table 4 describes the association of memory with BMI category. Overweight (difference in intercepts, −0.05 [95% CI, −0.27 to 0.17]; p = 0.67; model A) and obese (difference in intercepts, −0.23 [95% CI, −0.48 to 0.02]; p = 0.07; model A) participants did not have lower baseline memory scores compared with normal-weight participants. This difference in baseline memory scores in overweight (adjusted differences in intercepts, −0.04 [95% CI, −0.26 to −0.18]; p = 0.7; model B) and obese (adjusted differences in intercepts, −0.23 [95% CI, −0.49 to 0.02]; p = 0.07; model B) participants compared with their normal-weight counterparts was not attenuated after adjusting for cumulative mean SBP and FPG. Compared with normal-weight participants, overweight participants had faster decline in memory (difference in slope, −0.03 points/year faster [95% CI, −0.05 to −0.003]; p = 0.03; model A), but this difference in memory decline was attenuated after adjusting for SBP and FPG (adjusted differences in slope, −0.03 to 0.009; p = 0.26; model B). There was no statistically significant difference in memory decline in obese participants before (differences in slope, −0.01 points/year [95% CI, −0.04 to 0.01]; p = 0.36; model A) and after (adjusted differences in slope, −0.01 to 0.04; p = 0.27; model B) adjustment for SBP and FPG. Black participants had faster decline in memory compared with their White counterparts (adjusted differences in slope, 0.07 points/year faster [95% CI, −0.09 to −0.05]; p < 0.001 model A). Race did not modify the association of BMI on the intercept (p = 0.11) and slope (p = 0.46) of memory scores.
Table 4.
Association of Memory With Body Mass Index Category Over Time: Blood Pressure‐Cognition Study, 1971–2017
Sensitivity Analyses
Results were similar in sensitivity analyses including participants' cognitive observations after the time of incident stroke and adding eGFR and history of myocardial infarction as covariates (eTables 1–3, links.lww.com/WNL/C411). Independent of waist circumference (WC), higher BMI was associated with slower declines in global cognition (eTables 4–5). Replacing BMI with WC as a continuous or categorical variable yielded similar results (eTables 6–7), but there was evidence of a significant race × WC (continuous) × time interaction on the slope (p = 0.009), but not intercept (p = 0.5) of global cognition (eTable 6). Black participants with elevated WC (≥80 cm in women and ≥94 cm in men) had higher baseline and slower decline in global cognition than Black participants with normal WC (<80 cm in women and <94 cm in men). In contrast, there was no difference in global cognition slopes in White participants with normal WC compared with White participants with elevated WC (eFigure 1). Participants with 1 or 2 APOE ε4 alleles (moderate to high risk34) had lower baseline global cognition scores and faster cognitive decline compared with participants without an APOE ε4 allele (low risk). There was no evidence of a significant APOE ε4 × BMI (categorial) × follow-up time interaction (eTable 8). Excluding participants aged ≥65 years did not change results (eTable 9). Being overweight was associated with fewer cognitive assessments in global cognition and executive function but not memory after accounting for demographic, education, and vascular risk factors. Obesity was associated with fewer cognitive assessments in memory (eTable 10–14). Obesity was associated with lower baseline cognitive performance compared with normal weight (difference in intercepts, −1.7 [95% CI, −1.9 to −1.5]; p < 0.001), but not faster cognitive decline (differences in slope, 0.01 points/year [95% CI, −0.006 to 0.03]; p = 0.2) in unadjusted models (eFigure 2).
Discussion
In this biracial meta-analysis of 28,867 middle-aged and older adults from 6 community-based cohort studies across the United States, we found that obesity, compared with normal weight, was associated with lower baseline performance in global cognition and executive function but not memory. Contrary to our hypothesis, obese adults, compared with normal-weight adults, had slower declines in global cognition and executive function but not memory. The obesity-related differences in cognitive decline were only supported after inclusion of the time-varying cumulative SBP, FPG, and related time interaction terms.
There is conflicting evidence regarding the association of midlife and late-life obesity and cognitive decline. Studies have shown that being obese in midlife is associated with an increased risk of decline in executive function and dementia compared with being normal weight8,35; however, other studies have shown improved cognition and decreased dementia risk associated with late-life obesity.8,36 Several hypotheses have been posited regarding these findings seen in observational studies. First, previous studies indicate that dementia-related weight loss begins several years before diagnosis,10 and therefore, underestimation of cognitive decline associated with obesity may be due to attrition of participants with dementia-associated weight loss over the study duration. Second, weight loss in older age is associated with worsening health status and increased mortality.37 Together, these hypotheses may suggest an epidemiologic distortion rather than a true biological effect of obesity on cognitive function. Our results provide additional evidence that midlife and late-life obesity potentially attenuated declines in cognition given that our results persisted after excluding underweight participants and participants aged ≥65 years (eTable 15, links.lww.com/WNL/C411). This finding is consistent with the studies suggesting that maintenance of lean mass via higher BMI is associated with a lower risk of cognitive impairment.38
The results of this study provide evidence suggesting that higher BP and FPG typically seen in obesity-associated metabolic syndrome might contribute to the differences in initial cognitive scores between obese and normal-weight participants. One study found that higher cumulative mean SBP is associated with faster decline in global cognition, executive function, and memory.26 Another study showed that elevated FPG, even among adults without diabetes, was associated with higher dementia risk.39 Despite a body of evidence linking the cardiovascular and metabolic complications of obesity to CID, biological mechanisms of obesity and cognitive decline are not fully understood. There is growing evidence of increased levels of proinflammatory cytokines (interleukin‐1β)40 in the brain that are associated with obesity, which may explain the persistence of a statistically significant association between obesity and initial executive function scores and a borderline association between obesity and initial global cognition scores after adjusting for SBP and FPG. Furthermore, one study showed that obesity in early old age was associated with smaller cortical thickness.41 Altogether, these studies suggest that obesity and its cardiometabolic sequelae are linked to increased microvascular brain injury and neuroinflammatory events that can lead to cognitive dysfunction.
Vascular risk factors typically affect executive function,42 whereas memory deficits are considered signs of Alzheimer disease.43 Our study examined multiple domains of cognition to understand obesity's potential differential effect. Our results are consistent with one study that showed that obesity-associated metabolic syndrome is linked to lower initial executive function scores but not memory.44 Another explanation for these findings is that memory indicators may be less sensitive than global cognition and executive function to detect differences in baseline cognitive scores by BMI category. We were surprised by slower declines in global cognition and executive function but not memory after adjustment for cumulative mean SBP and FPG. However, fewer cognitive assessments of memory (Table 1) and the variable quality of memory assessments across cohorts may likely have reduced the precision of memory estimates.
Studies examining how the association between adiposity and CID varies by race often lack a sufficient sample of Black adults to detect significant effect modification. Race is a proxy for differences in socioeconomic status and allostatic load that vary across populations and are associated with obesity.3,4 Black adults have lower initial cognitive scores and faster declines in global cognition, executive function, and memory compared with their White counterparts after adjusting for age, sex, education, and vascular risk factors.26 Our study, which comprised a large sample of Black adults, did not find evidence that the association between BMI and slopes of global cognition, memory, and executive function varied by race. Surprisingly, we found that elevated WC was associated with slower decline in global cognition and executive function, but not memory, in Black adults but not in White adults (eFigure 1, links.lww.com/WNL/C411). This finding is not consistent with one study showing lower brain volumes in Black adults compared with White adults with increasing adiposity.12 There is insufficient evidence in our study and the literature to explain the Black-White differences in the association of WC, a marker of visceral adiposity, and cognitive decline. We recommend a cautious interpretation of this finding without additional confirmation and more methodological approaches to address the limitations in the current study.
In our study, we found that obesity was associated with lower initial cognitive scores. If the observed differences between obesity and normal weight in declines in global cognition and executive function are causal, they would be clinically significant, equivalent to 1–2 years of potentially slower declines in cognitive aging. The slower declines in mean cognitive scores associated with obesity can approximate equivalent changes in years of brain or cognitive aging by calculating the ratio of slope coefficients for obesity and baseline age on cognition. Experts have defined clinically meaningful cognitive decline as a decline in cognitive function of ≥0.5 SDs from baseline cognitive scores.45 Obese adults will reach the threshold of a 0.5-SD decrease from the baseline score more slowly than normal-weight adults: 0.74 years slower for global cognition, 1.89 years slower for memory, and 1.05 years slower for executive function. (eTable 10, links.lww.com/WNL/C411). Our results suggest that any reductions in the rate of obesity-associated cognitive decline are offset by markedly lower baseline cognitive scores that are likely due to obesity-associated cardiometabolic complications (e.g., inflammation, hypertension, and diabetes).
Our study has clinical implications. BMI as a risk factor is often associated with paradoxical associations with different health outcomes, particularly when comparing midlife with late life. Although we found that obesity potentially attenuated declines in cognition, our results also show that obese adults have lower initial cognitive scores, likely due to the deleterious effects of obesity and its cardiometabolic sequelae. Increased lean mass through high-intensity resistance exercise has been shown to promote better cognition in adults with mild cognitive impairment while also protecting Alzheimer disease–vulnerable hippocampal subfields from degeneration—reinforcing the health benefits of continued physical activity in older adults.38,46 Ultimately, however, further prospective studies, and where possible randomized trials, will be needed to determine definitively the benefits of exercise and weight modification on cognitive outcomes.
There are several strengths of this study. First, this is a longitudinal study of well-characterized middle-aged and older adults from 6 geographically diverse communities in the United States. Second, this study had repeated harmonized measures of global cognition, memory, and executive function and assessed clinically meaningful differences in cognitive decline by BMI category. Third, this study had many Black participants, which aided in the assessment of racial differences in the association of BMI and cognitive decline. Fourth, we had measured and not self-reported anthropometric measurements, which reduces the likelihood of systematic bias. Finally, this study had 21 years of follow-up.
This study has several limitations. The standard WHO BMI categories commonly used in clinical practice reflect the mass of all tissues and not solely adiposity. In addition, measures of adiposity and their relationship with cognition may differ by adiposity depot.9 However, the assessment of the association between WC categories and cognitive function showed that high WC potentially attenuates declines in cognition, similar to our results using BMI. Although we excluded underweight participants and participants aged 65 years and older in sensitivity analyses, we did not examine whether weight loss was associated with attrition from the study and thus cannot completely rule out reverse causation or attrition bias as partly contributing to the paradoxical association of BMI and cognitive decline. Like other longitudinal studies, the estimates of the effect of BMI and cognitive decline may be distorted by survivor bias. Inclusion of smokers in this study may result in residual confounding.
Although we adjusted for years of education, we were unable to adjust for literacy, educational quality, or other socioeconomic factors (income) due to the heterogeneity of data collection among studies. However, studies have shown that socioeconomic factors tend to influence baseline cognition (intercept) rather than the change in cognitive scores over time (slope).47 Most participants in this study are older. Therefore, survivor bias may contribute to the discrepant findings between the baseline and slope measures in the association between obesity and cognitive decline. Furthermore, educational attainment may not reflect educational quality equally across race and geographic location.48 Therefore, residual confounding cannot be excluded and may bias effect estimates. In the ARIC study, race and study center are confounded, and Black adults in the CHS may not be representative of all Black participants as they have higher socioeconomic status and are healthier.
Race was self-reported in this study. Black adults were more likely to be excluded than White adults because of stroke or dementia before the first cognitive assessment. This suggests that our estimates of the Black-White differences in cognitive decline seen in this study may be conservative. In addition, although we had many Black adults in this study, the smaller sample size and fewer cognitive assessments may reduce the reliability of estimates of cognitive decline in Black adults. This study did not examine incident dementia because some cohort studies lacked these data. We also did not adjust for the initial cognitive scores at the first cognitive assessment49 because some cohorts only had 2 cognitive assessments over time. Having fewer cognitive tests per cognitive domain in some cohorts might affect the statistical validity of the summary estimate of the effect of obesity on cognitive decline in the pooled cohort. Informative missingness and death of cognitively impaired participants may underestimate the rate of cognitive decline in this study.50
This biracial pooled longitudinal study of middle-aged and older adults demonstrates that obesity is associated with lower baseline cognitive scores after accounting for BP and FPG levels. Additional studies are needed to confirm whether obesity potentially attenuates declines in cognition.
Glossary
- ARIC
Atherosclerosis Risk in Communities
- BMI
body mass index
- BP‐COG
Blood Pressure-Cognition
- CARDIA
Coronary Artery Risk Development in Young Adults
- CHS
Cardiovascular Health Study
- CID
cognitive impairment and dementia
- eGFR
estimated glomerular filtration rate
- FPG
fasting plasma glucose
- LDL
low-density lipoprotein
- SBP
systolic blood pressure
- WC
waist circumference
- WHO
World Health Organization
Appendix. Authors
Study Funding
This research project is supported by a grant R01 NS102715 from the National Institute of Neurological Disorders and Stroke (NINDS), NIH, and Department of Health and Human Service. The NINDS was not involved in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication except one representative (author CBW) of the funding agency reviewed the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the NIH. Additional funding was provided by National Institute of Aging (NIA) grant R01 AG051827 (Levine), NIA Claude Pepper Center grant P30 AG024824 (Galecki), NIA grants K01 AG050699 (Gross) and K01 AG050723 (Tom), and NIA Michigan Alzheimer's Disease Research Center grant P30 AG053760 (Giordani). Cohort funding/support: The ARIC study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, NIH, and Department of Health and Human Services, under contract nos. HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I. Neurocognitive data are collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917, and R01 AG040282 from the NIH (NHLBI, NINDS, NIA, and NIDCD). The authors thank the staff and participants of the ARIC study for their important contributions. The Coronary Artery Risk Development in Young Adults (CARDIA) study is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201800005I and HHSN268201800007I), Northwestern University (HHSN268201800003I), University of Minnesota (HHSN268201800006I), and Kaiser Foundation Research Institute (HHSN268201800004I). This manuscript has been reviewed by CARDIA for scientific content. This research was supported by contracts HHSN268201200036C, HHSN268200800007C, HHSN268201800001C, N01HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, N01HC85086, and 75N92021D00006 and grants U01HL080295 and U01HL130114 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by R01AG023629 from the National Institute on Aging (NIA). A full list of principal CHS investigators and institutions can be found at CHS-NHLBI.org. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The Framingham Heart Study is a project of the National Heart Lung and Blood Institute of the NIH and Boston University School of Medicine. This project has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, NIH, and Department of Health and Human Services, under contract no. HHSN268201500001. The Northern Manhattan Stroke study has been funded at least in part with federal funds from the NIH and National Institute of Neurological Disorders and Stroke by R01 NS29993. This research was supported by contracts 75N92020D00001, HHSN268201500003I, N01-HC-95159, 75N92020D00005, N01-HC-95160, 75N92020D00002, N01-HC-95161, 75N92020D00003, N01-HC-95162, 75N92020D00006, N01-HC-95163, 75N92020D00004, N01-HC-95164, 75N92020D00007, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, and N01-HC-95169 from the National Heart, Lung, and Blood Institute and by grants UL1-TR-000040, UL1-TR-001079, and UL1-TR-001420 from the National Center for Advancing Translational Sciences (NCATS). Cognitive testing at Exam 6 in the MESA has been funded by grants R01HL127659 from the National Heart, Lung, and Blood Institute (NHLBI) and R01AG054069 and R01AG054474 from the NIH. The authors thank the other investigators, the staff, and the participants of the MESA for their valuable contributions. A full list of participating MESA investigators and institutions can be found at mesa-nhlbi.org.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017-2018. NCHS Data Brief; 2020:1-8. [PubMed] [Google Scholar]
- 2.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298(17):2028-2037. [DOI] [PubMed] [Google Scholar]
- 3.Black JL, Macinko J. Neighborhoods and obesity. Nutr Rev. 2008;66(1):2-20. [DOI] [PubMed] [Google Scholar]
- 4.Scott KA, Melhorn SJ, Sakai RR. Effects of chronic social stress on obesity. Curr Obes Rep. 2012;1:16-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yaffe K, Falvey C, Harris TB, et al. ; Health ABC Study. Effect of socioeconomic disparities on incidence of dementia among biracial older adults: prospective study. BMJ. 2013;347:f7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang MX, Cross P, Andrews H, et al. Incidence of AD in African-Americans, Caribbean Hispanics, and Caucasians in northern Manhattan. Neurology. 2001;56(1):49-56. [DOI] [PubMed] [Google Scholar]
- 7.Pedditzi E, Peters R, Beckett N. The risk of overweight/obesity in mid-life and late life for the development of dementia: a systematic review and meta-analysis of longitudinal studies. Age Ageing. 2016;45(1):14-21. [DOI] [PubMed] [Google Scholar]
- 8.Fitzpatrick AL, Kuller LH, Lopez OL, et al. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch Neurol. 2009;66(3):336-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parker KG, Lirette ST, Deardorff DS, et al. Relationships of clinical and computed tomography-imaged adiposity with cognition in middle-aged and older African Americans. J Gerontol A Biol Sci Med Sci. 2018;73(4):492-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart R, Masaki K, Xue QL, et al. A 32-year prospective study of change in body weight and incident dementia: the Honolulu-Asia Aging Study. Arch Neurol. 2005;62(1):55-60. [DOI] [PubMed] [Google Scholar]
- 11.Howard G, Lackland DT, Kleindorfer DO, et al. Racial differences in the impact of elevated systolic blood pressure on stroke risk. JAMA Intern Med. 2013;173(1):46-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Windham BG, Lirette ST, Fornage M, et al. Associations of brain structure with adiposity and changes in adiposity in a middle-aged and older biracial population. J Gerontol A Biol Sci Med Sci. 2017;72(6):825-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344-349. [DOI] [PubMed] [Google Scholar]
- 14.The Atherosclerosis Risk in Communities (ARIC) study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129:687-702. [PubMed] [Google Scholar]
- 15.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41(11):1105-1116. [DOI] [PubMed] [Google Scholar]
- 16.Fried LP, Borhani NO, Enright P, et al. The cardiovascular health study: design and rationale. Ann Epidemiol. 1991;1(3):263-276. [DOI] [PubMed] [Google Scholar]
- 17.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham offspring study. Design and preliminary data. Prev Med. 1975;4:518-525. [DOI] [PubMed] [Google Scholar]
- 18.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871-881. [DOI] [PubMed] [Google Scholar]
- 19.Sacco RL, Boden-Albala B, Gan R, et al. Stroke incidence among white, black, and Hispanic residents of an urban community: the Northern Manhattan Stroke Study. Am J Epidemiol. 1998;147(3):259-268. [DOI] [PubMed] [Google Scholar]
- 20.Levine DA, Galecki AT, Langa KM, et al. Trajectory of cognitive decline after incident stroke. JAMA. 2015;314(1):41-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine DA, Gross AL, Briceno EM, et al. Sex differences in cognitive decline among US adults. JAMA Netw Open. 2021;4(2):e210169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lucas JA, Ivnik RJ, Willis FB, et al. Mayo's Older African Americans Normative Studies: normative data for commonly used clinical neuropsychological measures. Clin Neuropsychol. 2005;19(2):162-183. [DOI] [PubMed] [Google Scholar]
- 23.Ferraro FR. Minority and Cross-Cultural Aspects of Neuropsychological Assessment. Lisse: Swets & Zeitlinger; 2002. [Google Scholar]
- 24.Hachinski V, Iadecola C, Petersen RC, et al. National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke. 2006;37(9):2220-2241. [DOI] [PubMed] [Google Scholar]
- 25.Manly JJ, Schupf N, Stern Y, Brickman AM, Tang MX, Mayeux R. Telephone-based identification of mild cognitive impairment and dementia in a multicultural cohort. Arch Neurol. 2011;68(5):607-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine DA, Gross AL, Briceno EM, et al. Association between blood pressure and later-life cognition among black and white individuals. JAMA Neurol. 2020;77(7):810-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Griffith L, van den Heuvel E, Fortier I, et al. Harmonization of Cognitive Measures in Individual Participant Data and Aggregate Data Meta-Analysis. ; 2013. [PubMed] [Google Scholar]
- 28.Samejima F. Estimation of Latent Ability Using a Response Pattern of Graded Scores: Psychometric Society; 1969. [Google Scholar]
- 29.Muthén LK, Muthén B. Mplus User's Guide: Statistical Analysis with Latent Variables, User's Guide. Muthén & Muthén; 2017. [Google Scholar]
- 30.Asparouhov T, Muthén B. Plausible Values for Latent Variables Using Mplus; 2010. [Google Scholar]
- 31.Joint FAO/WHO Expert Committee on Food Additives. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;896(i-xii):1-128. [PubMed] [Google Scholar]
- 32.Levey AS, Stevens LA, Schmid CH, et al. ; CKD-EPI Chronic Kidney Disease Epidemiology Collaboration. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajan KB, Skarupski KA, Rasmussen HE, Evans DA. Gene-environment interaction of body mass index and apolipoprotein E ε4 allele on cognitive decline. Alzheimer Dis Assoc Disord. 2014;28(2):134-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker KA, Sharrett AR, Wu A, et al. Association of midlife to late-life blood pressure patterns with incident dementia. JAMA. 2019;322(6):535-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77(5):461-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun Z, Wang ZT, Sun FR, et al. ; Alzheimer’s Disease Neuroimaging Initiative. Late-life obesity is a protective factor for prodromal Alzheimer's disease: a longitudinal study. Aging (Albany NY). 2020;12(2):2005-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alley DE, Metter EJ, Griswold ME, et al. Changes in weight at the end of life: characterizing weight loss by time to death in a cohort study of older men. Am J Epidemiol. 2010;172(5):558-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noh HM, Oh S, Song HJ, et al. Relationships between cognitive function and body composition among community-dwelling older adults: a cross-sectional study. BMC Geriatr. 2017;17(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crane PK, Walker R, Larson EB. Glucose levels and risk of dementia. N Engl J Med. 2013;369(19):1863-1864. [DOI] [PubMed] [Google Scholar]
- 40.Guo DH, Yamamoto M, Hernandez CM, Khodadadi H, Baban B, Stranahan AM. Visceral adipose NLRP3 impairs cognition in obesity via IL-1R1 on CX3CR1+ cells. J Clin Invest. 2020;130(4):1961-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caunca MR, Gardener H, Simonetto M, et al. Measures of obesity are associated with MRI markers of brain aging: the Northern Manhattan Study. Neurology. 2019;93(8):e791–e803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levine DA, Galecki AT, Langa KM, et al. Blood pressure and cognitive decline over 8 Years in middle-aged and older black and white Americans. Hypertension. 2019;73(2):310-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jahn H. Memory loss in Alzheimer's disease. Dialogues Clin Neurosci. 2013;15(4):445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuo H-K, Jones RN, Milberg WP, et al. Effect of blood pressure and diabetes mellitus on cognitive and physical functions in older adults: a longitudinal analysis of the advanced cognitive training for independent and vital elderly cohort. J Am Geriatr Soc. 2005;53(7):1154-1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolinsky FD, Unverzagt FW, Smith DM, Jones R, Stoddard A, Tennstedt SL. The ACTIVE cognitive training trial and health-related quality of life: protection that lasts for 5 years. J Gerontol A Biol Sci Med Sci. 2006;61(12):1324-1329. [DOI] [PubMed] [Google Scholar]
- 46.Broadhouse KM, Singh MF, Suo C, et al. Hippocampal plasticity underpins long-term cognitive gains from resistance exercise in MCI. NeuroImage. 2020;25:102182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zahodne LB, Glymour MM, Sparks C, et al. Education does not slow cognitive decline with aging: 12-year evidence from the victoria longitudinal study. J Int Neuropsychol Soc. 2011;17(6):1039-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev. 2008;18(3):223-254. [DOI] [PubMed] [Google Scholar]
- 49.Glymour MM, Weuve J, Berkman LF, Kawachi I, Robins JM. When is baseline adjustment useful in analyses of change? An example with education and cognitive change. Am J Epidemiol. 2005;162(3):267-278. [DOI] [PubMed] [Google Scholar]
- 50.Salthouse TA. Selectivity of attrition in longitudinal studies of cognitive functioning. J Gerontol B Psychol Sci Soc Sci. 2014;69(4):567-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data from the cohort studies used in this analysis were available from each study's respective coordinating centers. Specific policies governing each study's data and the process to access data can be found online (ARIC study: sites.cscc.unc.edu/aric/; CARDIA study: cardia.dopm.uab.edu/; MESA: mesa-nhlbi.org/; CHS: chs-nhlbi.org/; FOS: framinghamheartstudy.org/; and NOMAS: columbianomas.org/study.html).