Abstract
Background:
The interrelationships between gait, cerebral small vessel disease (CSVD), and cognitive impairments in aging are not well-understood—despite their common co-occurrence.
Objective:
To systematically review studies of gait impairment in CSVD, pre-dementia, and dementia, and to identify key gaps for future research and novel pathways toward intervention.
Methods:
A Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)-guided search strategy was implemented in PubMed to identify relevant studies. Potential articles (n = 263) published prior to 1 December 2021 were screened by two reviewers. Studies with sample sizes >20 and including some adults over > 65 years (n = 202) were included.
Results:
The key findings were that (1) adverse gait and cognitive outcomes were associated with several (rather than select) CSVD pathologies distributed across the brain, and (2) poor gait and CSVD pathologies were more strongly associated with dementia with a vascular, rather than an Alzheimer’s disease-related, cause.
Discussion:
A better understanding of the interrelationships between gait performance in CSVD, pre-dementia, and dementia requires studies examining (1) comprehensive patterns in the clinical manifestations of CSVD, (2) racially/ethnically diverse samples, (3) samples followed for extended periods of time or across the adult life span, (4) non-traditional CSVD neuroimaging markers (e.g. resting-state functional magnetic resonance imaging (fMRI)), and (5) continuous (e.g. wearable sensors) and complex (e.g. dual-task) walking performance.
Keywords: Cerebral small vessel disease, gait, cognition, aging, cognitive impairment, dementia, gait abnormalities
Introduction
Poor gait performance is observed in older adults with cerebral small vessel disease (CSVD), pre-dementia, and dementia. Traditional neuroimaging markers of CSVD, such as subcortical infarcts, lacunes, and white matter hyperintensities (WMHs), for instance, are associated with gait impairments. These gait impairments are also associated with future onset of pre-dementias and dementias, including those that could be the downstream results of CSVD (e.g. vascular dementia). Note that pre-dementia syndromes, such as mild cognitive impairment (MCI), are marked by poor cognitive performance, but are not accompanied with the daily functional limitations observed in individuals with dementia. The objective of this systematic review was to generate a better understanding of the interrelationships between qualitative (e.g. apraxia) and quantitative (e.g. gait speed) gait performance in older adults with CSVD, pre-dementia, and dementia—and to identify important areas of research, and novel paths toward intervention. The focus and scope of this review is further illustrated in Figure 1.
Figure 1.
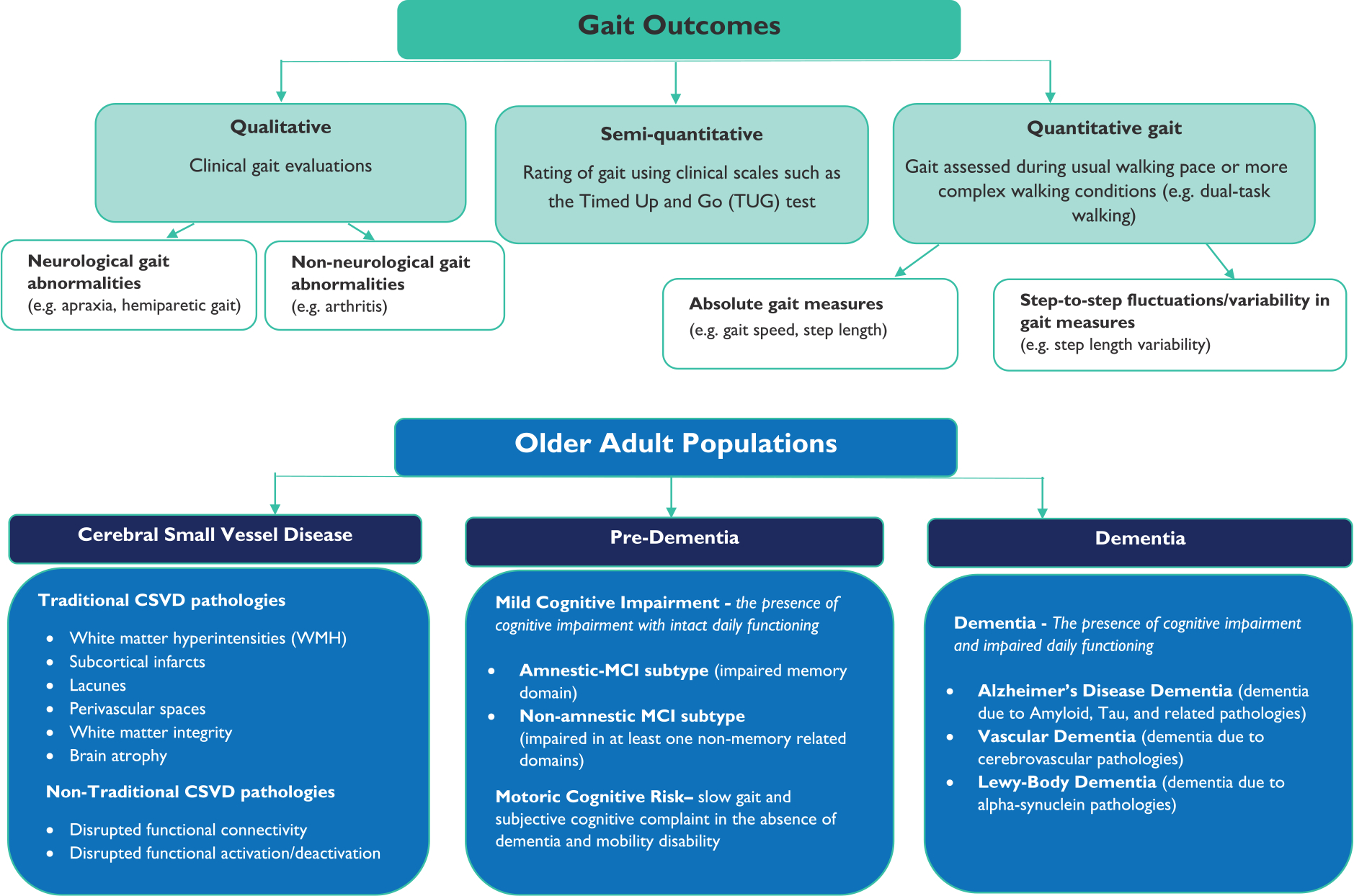
Gait outcomes and older adult populations examined in this review.
Methods
Search strategy and selection criteria
The search strategy was guided by Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and is summarized in Figure 2(a).1 PubMed was systematically screened to identify studies of qualitative and quantitative gait in CSVD, pre-dementia, and dementia populations. The specific search terms are provided in Figure 2(b). The identified studies were first independently and then collaboratively screened by H.M.B and O.J to ensure they met inclusion/exclusion criteria. Key findings and demographic characteristics were then tabulated (Supplementary Tables 2–4) and categorized (Supplementary Figures 1–2). To reduce bias, both positive and negative findings were considered. Clinical gait abnormalities and quantitative gait outcomes in CSVD were reviewed in the “Clinical gait abnormalities and CSVD,” “Quantitative gait and CSVD,” “Gait decline and CSVD,” and “Gait and CSVD in pre-dementia and dementia” sections Clinical gait abnormalities and quantitative gait outcomes associated with cognitive decline, MCI, and dementia were reviewed in the “Clinical gait abnormalities in pre-dementia and dementia,” “Quantitative gait measures as markers for MCI and dementia,” and “Gait as a predictor of cognitive decline, MCI, and dementia” sections.
Figure 2.
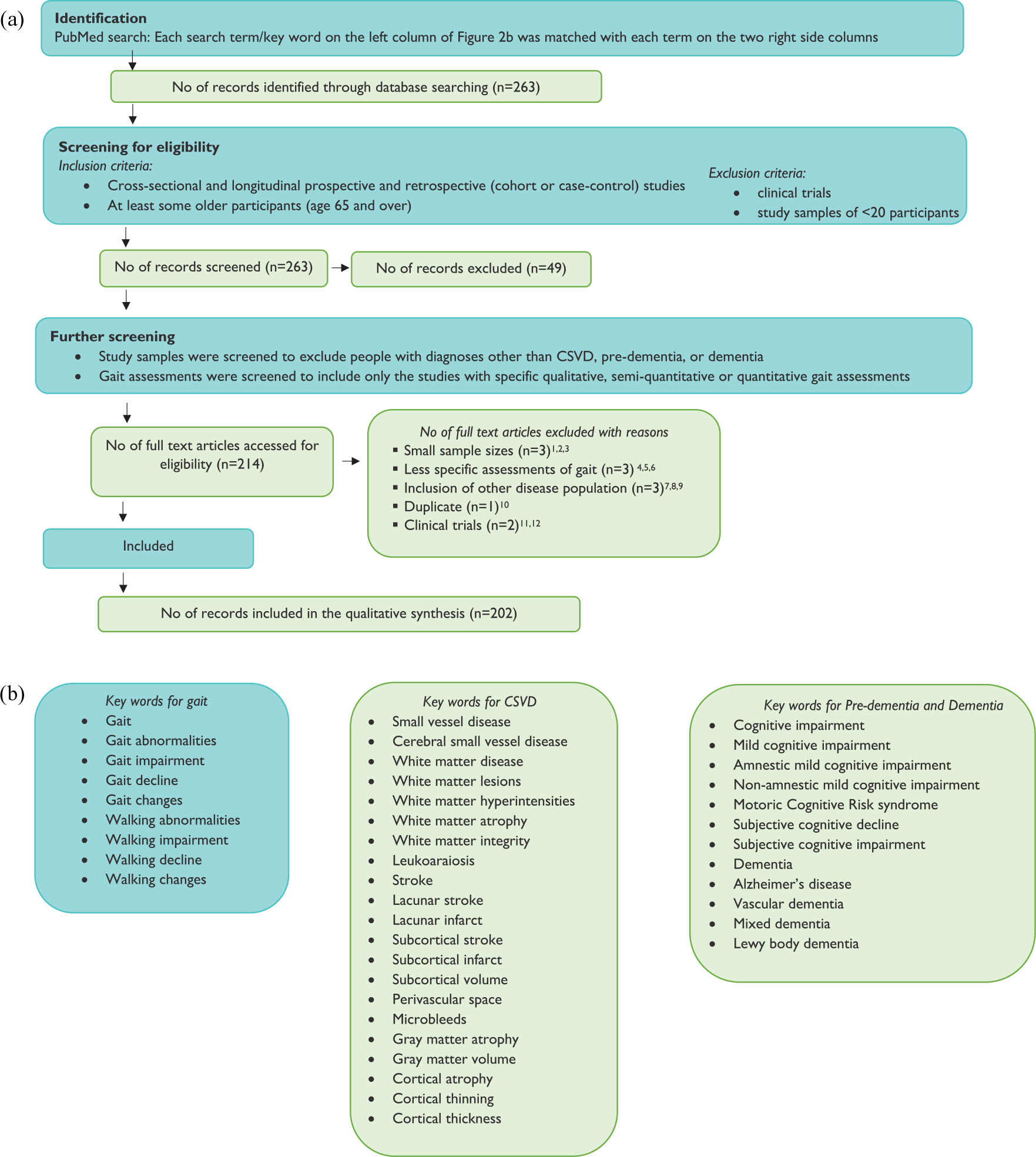
(a) PRISMA flow diagram of study selection and (b) key words used in systematic PubMed search.
Note: The key words for gait were sequentially paired with each CSVD, pre-dementia, and dementia keyword during PubMed search, using an AND statement.
1Bugalho P, Guimarães J. Gait disturbance in normal pressure hydrocephalus: a clinical study. Parkinsonism & related disorders. 2007 Oct 1;13(7):434–7.
2Rutherford BR, Choi J, Slifstein M, O’Boyle K. Abi-Dargham A, Brown PJ, Wall MW, Vanegas-Arroyave N, Sakhardande J, Stern Y, Roose SP. Neuroanatomical predictors of L-DOPA response in older adults with psychomotor slowing and depression: A pilot study. Journal of affective disorders. 2020 Mar 15;265:439–44.
3Wolfson L, Wei X, Hall CB, et al. Accrual of MRI white matter abnormalities in elderly with normal and impaired mobility. 2005; 232: 23–27.
4Abbott RD, White LR, Ross GW, et al. Walking and dementia in physically capable elderly men. Jama 2004; 292: 1447–1453.
5Aggarwal NT, Wilson RS, Beck TL, Bienias JL, Bennett DA. Motor dysfunction in mild cognitive impairment and the risk of incident Alzheimer disease. Archives of Neurology. 2006;63(12):1763–1769
6Waite L, Grayson D, Piguet O, Creasey H, Bennett H, Broe G. Gait slowing as a predictor of incident dementia: 6-year longitudinal data from the Sydney Older Persons Study. Journal of the neurological sciences. 2005;229:89–93
7Katsumata Y, Todoriki H, Yasura S, Dodge HH. Timed up and go test predicts cognitive decline in healthy adults aged 80 and older in Okinawa: Keys to Optimal Cognitive Aging (KOCOA) Project. Journal of the American Geriatrics Society. 2011;59(11):2188
8Alves G, Larsen JP, Emre M, Wentzel-Larsen T, Aarsland D. Changes in motor subtype and risk for incident dementia in Parkinson’s disease. Movement disorders: official journal of the Movement Disorder Society. 2006;21(8):1123–1130
9Senanarong V, Harnphadungkit K, Poungvarin N, Vannasaeng S, Chongwisal S, Chakorn T, Jamjumrus P, Raksthaput A, Chaichanettee S, Aoonkaew N, Udompunthurak S. The dementia and disability project in Thai elderly: rational, design, methodology and early results. BMC neurology. 2013 Dec; 13(1):1–1.
10Staekenborg SS, Van der Flier WM, Van Straaten EC, Lane R, Barkhof F, Scheltens P. Neurological signs in relation to type of cerebrovascular disease in vascular dementia. Stroke. 2008 Feb 1;39(2):317–22.
11Nadkarni NK, Perera S, Studenski SA, Rosano C, Aizenstein HJ, VanSwearingen JM. Callosal hyperintensities and gait speed gain from two types of mobility interventions in older adults. Archives of physical medicine and rehabilitation. 2015 Jun 1;96(6):1154–7.
12Voss MW, Heo S, Prakash RS, Erickson KI, Alves H, Chaddock L, Szabo AN, Mailey EL. Wo jcicki TR, White SM, Gothe N, McAuley E, Sutton BP, Kramer AF. 2013; The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: results of a one-year exercise intervention. Human Brain Mapping.;34:2972–85.
Results
A total of 263 studies were screened, 202 studies were reviewed (Supplementary Tables 1–5 and Supplementary Figures 1–2), and 72 of these studies were qualitatively interpreted below.
Clinical gait abnormalities and CSVD
Studies of clinical gait abnormalities and CSVD pathologies were primarily cross-sectional. Gait abnormalities or gait disturbance severity were associated with several CSVD pathologies and brain regions—including subcortical infarcts, overall, subcortical, periventricular, and frontal WMH, white matter integrity in frontal, parietal, and corpus callosum regions, and thickness of the corpus callosum.2–6 Apraxia (frontal gait) was associated with WMH in frontal and basal ganglia regions, white matter integrity in the genu of corpus callosum, and subcortical and lacunar strokes.4,7,8
The distributed CSVD pathologies associated with clinical gait abnormalities suggest that examining patterns in the pathological and clinical manifestations of CSVD could improve our understanding of gait abnormalities and CSVD. A recent example of this approach employed cluster analyses to identify different neuroimaging profiles in individuals with severe CSVD.9 One cluster displayed widespread periventricular WMH, multiple lacunes and microbleeds, atrophy, severe cognitive and gait abnormalities, and down-regulation of vascular endothelial growth factor-A (indicative of widespread vascular wall damage). The other cluster displayed deep WMH, white matter lacunes, and enlarged perivascular spaces, but relatively less microbleeds and atrophy, less severe cognitive and gait abnormalities, and upregulation of tumor necrosis factor alpha (indicative of systemic inflammation). This study underscored the heterogeneity of the neuroimaging and clinical manifestations of severe CSVD and identified two distinct pathways that could guide future intervention design.
Quantitative gait and CSVD
Cross-sectional studies of quantitative gait and CSVD in aging can be categorized into (1) those contrasting CSVD in mobility-impaired versus mobility-intact individuals—determined by gait speed cuts (using a stopwatch), clinical evaluation, and/or semi-quantitative gait measures (e.g. Timed Up and Go (TUG) test);10 (2) those examining associations between CSVD and quantitative gait with instrumented walkways.
Mobility impairment and CSVD.
Mobility impairments were associated with CSVD pathologies in distributed brain regions, evident as greater overall WMH burden, more periventricular and deep WMH, more WMH in posterior, occipito-parietal, frontal and corpus callosum regions, worse white matter integrity in the thalamic radiation and corpus callosum, and a smaller caudate nucleus.11–17 Non-traditional CSVD neuroimaging markers shed light onto how the brain adapted to (or compensated for) CSVD. One study of cerebral perfusion revealed that mobility-impaired (compared to mobility-intact) individuals with CSVD showed relative under-activation of supplementary motor, thalamus, and basal ganglia regions and over-activation of premotor regions.18 Another study of resting-state functional magnetic resonance imaging (fMRI) revealed that mobility-impaired (compared to mobility-intact) individuals displayed reduced functional connectivity in the supplementary motor and superior parietal components of sensorimotor and fronto-parietal resting-state networks—and between supplementary motor and temporal lobe regions.19
Gait speed and CSVD.
Slow gait speed during usual pace (single-task) walking was associated with widepread-WMH,20–27 frontal and thalamic lacunes, microbleeds, widespread cortical and hippocampal atrophy, and reduced white matter integrity in specific tracts.21,23,24,28–32 A better understanding of the temporal interrelationship between adverse gait outcomes and CSVD requires studying these associations across the adult life span.
Dual-task walking and CSVD.
Associations between dual-task walking (DTW; walking while reciting alternate letters of the alphabet) and CSVD were inconsistent.33 One study suggested that DTW speed was not associated with WMH, whereas in another study increased dual-task costs (the percent difference between single-task and DTW-speed) was associated with less gray matter in medial prefrontal, cingulate, and thalamic regions.17,32 Furthermore, functional connectivity in sensorimotor, visual, vestibular, and left frontal-parietal resting-state networks were associated with both single-task and DTW-speeds—yet the supplementary and prefrontal components of sensorimotor and fronto-parietal networks were more strongly associated with DTW than single-task walking.34 Thus, current evidence suggests that DTW is associated with some but not all CSVD pathologies.
Additional gait measures and CSVD.
Absolute measures and step-to-step variability in stride length (distance between initial contacts of the same foot) and double support time (time when both feet are on the ground) were associated with different CSVD pathologies and brain regions. Shorter stride/step length was associated with overall, periventricular, and frontal WMH,21,24 a composite measure of CSVD (lacunes, WMH, microbleeds, and periventricular space),35 and lower overall, sensorimotor, and fronto-parietal volumes.36,37 Greater step length variability was associated with WMH and basal ganglia infarcts,38 and a widespread gray matter pattern that included frontal, temporal, insular, occipital, and cerebellar regions.39 Longer double support time was associated with WMH and less volume in sensorimotor and fronto-parietal regions.21,37 Finally, greater double support time variability was associated with gray matter volume in frontal, medial, temporal, anterior cingulate, insular, cerebellar and striatal regions, and cortical thickness in frontal and temporal regions.39,40 These studies suggest that distributed CSVD pathologies are associated with additional gait measures.
Gait decline and CSVD
The current literature on gait decline and CSVD is limited and mixed. Some studies of older adults without dementia suggested that baseline and the progression of overall and periventricular WMH, hippocampal atrophy, and the integrity of the corona radiata were associated with gait speed and step length decline.41–45
In contrast, in relatively young older adults (50–85 years) baseline lacunes, microbleeds, white matter integrity, and brain volume were not associated with gait speed decline after 5 years.46
Gait and CSVD in pre-dementia and dementia
In individuals with MCI, more subcortical WMH, less gray matter (in motor, frontal, middle temporal, cuneus, precuneus, and striatal regions) and greater lateral ventricular volume were associated with slower gait and greater stride time variability during single-task walking and DTW.47–49 The motoric cognitive risk syndrome (MCR syndrome; slow gait speed and subjective cognitive complaint) was associated with widespread cortical thinning and gray matter atrophy.50,51 In older Indian adults without dementia, cortical microbleeds were not associated with MCI or MCR, and frontal lacunes were associated only with MCR.52 In individuals with vascular dementia (VaD), WMH, cerebral infarcts, and thalamic lesions were associated with clinical gait abnormalities (hemiplegic or Parkinsonian gait).53 Interestingly, the relationship between dual-task gait and CSVD differed by MCI subtype.49,54 Less superior and middle frontal, temporal, striatal, and cerebellar volumes were associated with slower DTW-speed in non-amnestic MCI, while less occipital, parahippocampal, cuneus, and inferior frontal volumes were associated with DTW speed in amnestic MCI.49,54
Clinical gait abnormalities in pre-dementia and dementia
Neurological gait abnormalities (e.g. Parkinsonian gait) were more common in MCI than in cognitively healthy individuals,55 but results were inconclusive regarding whether they were different between amnestic and non-amnestic MCI.55,56 Individuals with MCR had a higher prevalence of non-neurological gait abnormalities (i.e. arthritis) than those without MCR.57 Neurological gait abnormalities were common in dementia than in cognitively healthy individuals—and in those with non-Alzheimer dementias (e.g. VaD or Lewy body dementia (LBD)) than those with AD.58
Quantitative gait measures as markers for MCI and dementia
Gait speed, usual pace walking and DTW.
Usual pace walking in individuals with dementia was slower than in MCI, and in those with MCI than cognitively healthy individuals.59 In subtypes, individuals with non-amnestic MCI had slower gait speed and faster gait speed decline than amnestic MCI.59,60 Interestingly, most studies found slower gait speed in those with non-AD dementia, including VaD and LBD, compared to those with AD.61,62 Finally, in individuals with MCI and dementia, DTW-speed was slower than usual pace gait speed, and compared to cognitively healthy individuals.63,64
Additional gait measures, MCI, and dementia.
Individuals with MCI had shorter stride length,65 smaller cadence,56,63 greater step, stance, and double support time during usual pace and DTW,66 compared to cognitively healthy individuals. Worse performance was observed among those with non-amnestic MCI than amnestic MCI.56,59 In one study, variability in stride length and stride width during usual pace walking were greater in non-amnestic MCI compared to amnestic MCI.59 In other studies, stride length variability and gait speed variability were greater in amnestic than non-amnestic MCI, and cognitively healthy older adults.56,67 Individuals with dementia showed poorer performance than MCI and cognitively healthy individuals on most gait measures59,68 and people with VaD had shorter stride length than those with AD.61
Gait as a predictor of cognitive decline, MCI, and dementia
Slow usual pace gait speed predicted decline in global cognition,69 memory,69–71 and non-memory related (executive function, visuospatial function, processing speed, and language) functions.69,70,72 Greater variability in space-related (i.e. stride length) and time-related (i.e. double support time, stance time) gait measures were also associated with decline in memory, executive function, and language.70,72 During DTW, slow speed and greater stride time variability were associated with decline in global cognition, but there was limited evidence on decline in specific cognitive functions.73,74 Additional studies showed that slow gait speed during usual and DTW conditions predicted cognitive impairment and MCI.73,75,76 Interestingly, those who converted to MCI had faster decline in usual pace gait speed than non-converters—which started to accelerate approximately 12 years before MCI diagnosis in men, and 6 years before MCI diagnosis in women.76
Neurological gait patterns (e.g. apraxia) were associated with increased risk of dementia and VaD.77 Slow gait speed and accelerated gait speed decline predicted risk of incident dementia and AD in cognitively healthy older adults, and in those with subjective cognitive impairment or MCI.78 Although there were some inconsistencies regarding whether gait is a stronger predictor of AD,79,80 than non-AD dementias a large-scale study (n = 3663; follow-up: 9 years) found that slow gait was associated with a two-fold increased risk for AD, and a 12-fold increased risk for VaD.78 DTW was a stronger predictor of dementia than usual pace gait speed in cognitively intact and MCI individuals.81,82 Finally, slower DTW speed, greater dual-task cost and greater swing time variability were associated with increased risk of dementia and VaD.73,82
Discussion
Most clinical and quantitative gait measures in aging are associated with several CSVD pathologies (see Figure 3)—and these pathologies were widely distributed across the brain. A better understanding of the interrelationship and shared pathophysiology of CSVD and adverse gait outcomes in aging will come from (1) examining comprehensive patterns in gait and neuroimaging manifestations of CSVD, (2) identifying the earliest signs of gait decline and CSVD from studies across the adult life span and longitudinal studies with extensive follow-up, and (3) using non-traditional CSVD markers (e.g. resting-state functional connectivity). Gait impairments in pre-dementia and dementia also differed as a function of whether they have a vascular- or AD-related cause. Yet, more longitudinal studies, and direct comparisons of gait, CSVD, and different forms of dementia are needed to speak the temporal interrelationships between them, and to guide future disease monitoring and development of interventions.
Figure 3.
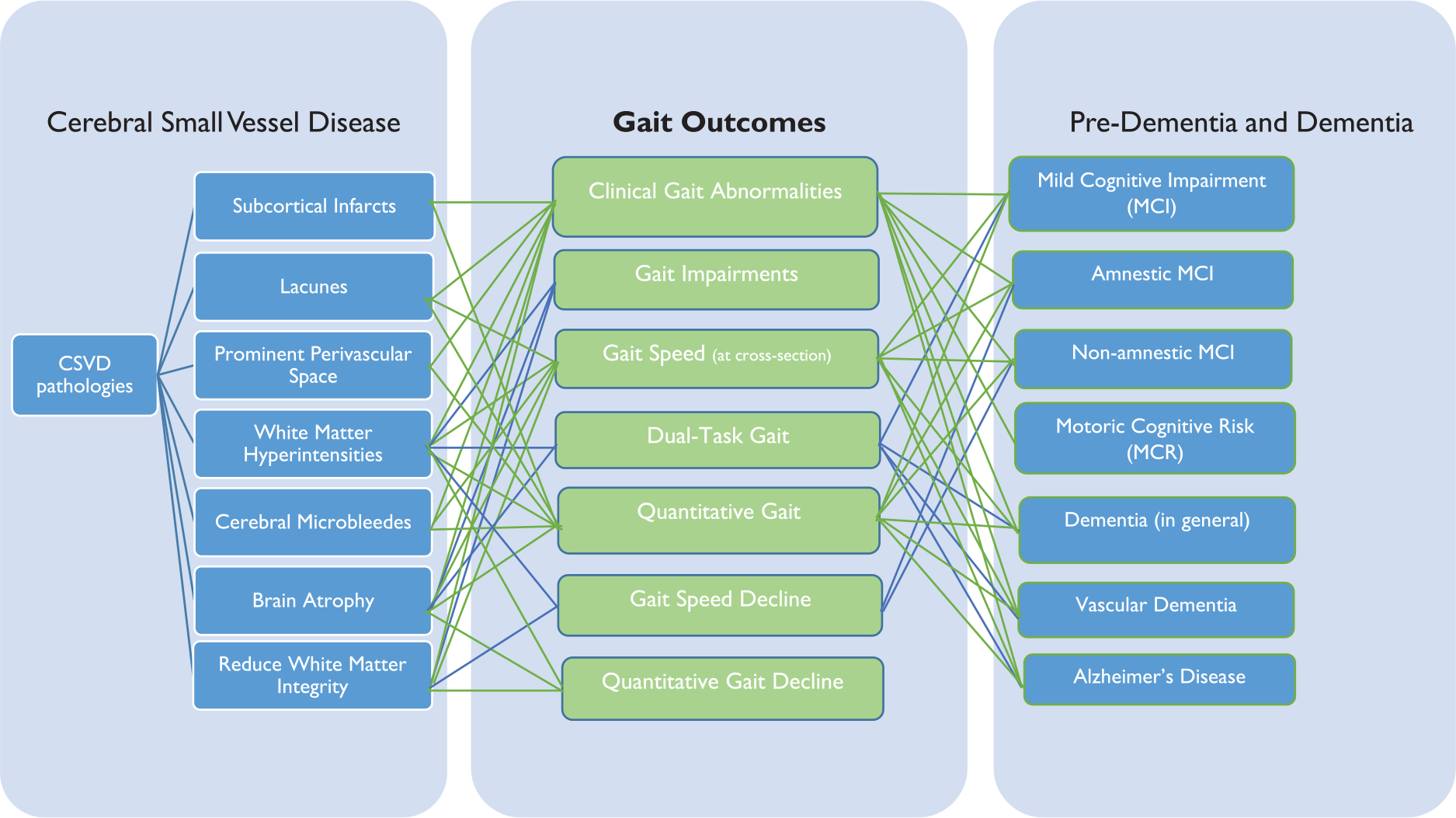
The interrelationship between gait outcomes and cerebral small vessels disease, pre-dementia, and dementia discussed in this review. A connecting line between gait outcomes and cerebral small vessel disease and pre-dementia and dementia indicates that at least one article discussed observed a significant association.
Strong evidence also suggest that poor qualitative and quantitative gait performance are important markers for distinguishing between individuals with and without cognitive impairment, and for early identification of those at increased risk for cognitive decline, MCI, and dementia. Yet, additional studies need to determine the potential of gait in predicting different subtypes of MCI, and to validate gait measures for differential diagnosis (e.g. compared to neuroimaging and other biomarkers). Additional studies also need to determine if more regular monitoring of gait (e.g. wearable or in-home sensors versus clinical gait assessments) or more complex gait performance (e.g. DTW) can assist in identifying the earliest signs of gait, cognitive, and CSVD changes in aging. Taken together, this systematic review strongly confirms that gait impairment, CSVD, pre-dementia, and dementia often co-occur in aging—and recommends early, simultaneous, regular and longitudinal tracking of gait, CSVD and cognitive measures to support the development of novel, and potentially more successful pathways toward intervention.
It is important to recognize that this review (like other reviews) was subject to different biases with unknown effects, including publication biases. Most of the studies reviewed herein sampled (primarily white) Western older adults, and evidence suggests that the relationship between CSVD and cognitive impairment may be different in Eastern populations.52 The anatomical distribution of CSVD, for example, has been shown to be different in Eastern and Western populations—indicating pathophysiological differences with potential implications for the diagnosis and treatment of CSVD.83 Thus, future studies are needed to examine the interrelationship between gait, CSVD, pre-dementia and dementia in different racial/ethnic groups.
Supplementary Material
Acknowledgements
The authors thank Daniel Schlehofer, Dachel Sanchez-Castellanos, and Bennett Kautz for their assistance in identifying articles.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: National Institute on Aging Grants (1R01AG062659-01A1; R01AG057548-01A1) played no role in data collection or interpretation.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Supplemental material
Supplemental material for this article is available online.
References
- 1.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev 2021; 10: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baloh RW, Yue Q, Socotch TM and Jacobson KM. White matter lesions and disequilibrium in older people: I. Case-control comparison. Arch Neurol 1995; 52: 970–974. [DOI] [PubMed] [Google Scholar]
- 3.Briley DP, Wasay M, Sergent S and Thomas S. Cerebral white matter changes (leukoaraiosis), stroke, and gait disturbance. J Am Geriatr Soc 1997; 45: 1434–1438. [DOI] [PubMed] [Google Scholar]
- 4.Pohjasvaara T, Mäntylä R, Ylikoski R, Kaste M and Erkinjuntti T. Clinical features of MRI-defined subcortical vascular disease. Alzheimer Dis Assoc Disord 2003; 17: 236–242. [DOI] [PubMed] [Google Scholar]
- 5.Moretti M, Carlucci G, Di Carlo A, et al. Corpus callosum atrophy is associated with gait disorders in patients with leukoaraiosis. Neurol Sci 2005; 26: 61–66. [DOI] [PubMed] [Google Scholar]
- 6.Kim YJ, Kwon HK, Lee JM, et al. Gray and white matter changes linking cerebral small vessel disease to gait disturbances. Neurology 2016; 86: 1199–1207. [DOI] [PubMed] [Google Scholar]
- 7.Okroglic S, Widmann CN, Urbach H, Scheltens P and Heneka MT. Clinical symptoms and risk factors in cerebral microangiopathy patients. PLoS ONE 2013; 8: e53455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fling BW, Dale ML, Curtze C, Smulders K, Nutt JG and Horak FB. Associations between mobility, cognition and callosal integrity in people with parkinsonism. NeuroImage Clin 2016; 11: 415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobrynina LA, Zabitova MR, Shabalina AA, et al. MRI types of cerebral small vessel disease and circulating markers of vascular wall damage. Diagnostics 2020; 10: 354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Podsiadlo D and Richardson S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991; 39: 142–148. [DOI] [PubMed] [Google Scholar]
- 11.Guttmann CR, Benson R, Warfield S, et al. White matter abnormalities in mobility-impaired older persons. Neurology 2000; 54: 1277–1283. [DOI] [PubMed] [Google Scholar]
- 12.Benson RR, Guttmann C, Wei X, et al. Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology 2002; 58: 48–55. [DOI] [PubMed] [Google Scholar]
- 13.Brodoefel H, Ramachandran R, Pantol G, et al. Association between linear measurements of corpus callosum and gait in the elderly. Eur Radiol 2013; 23: 2252–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhadelia RA, Price LL, Tedesco KL, et al. Diffusion tensor imaging, white matter lesions, the corpus callosum, and gait in the elderly. Stroke 2009; 40: 3816–3820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumurgier J, Crivello F, Mazoyer B, et al. MRI atrophy of the caudate nucleus and slower walking speed in the elderly. NeuroImage 2012; 60: 871–878. [DOI] [PubMed] [Google Scholar]
- 16.Bolandzadeh N, Liu-Ambrose T, Aizenstein H, et al. Pathways linking regional hyperintensities in the brain and slower gait. NeuroImage 2014; 99: 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashimoto M, Takashima Y, Uchino A, Yuzuriha T and Yao H. Dual task walking reveals cognitive dysfunction in community-dwelling elderly subjects: the Sefuri brain MRI study. J Stroke Cerebrovasc Dis 2014; 23: 1770–1775. [DOI] [PubMed] [Google Scholar]
- 18.Iseki K, Hanakawa T, Hashikawa K, et al. Gait disturbance associated with white matter changes: a gait analysis and blood flow study. NeuroImage 2010; 49: 1659–1666. [DOI] [PubMed] [Google Scholar]
- 19.Zhou X, Zhang C, Li L, et al. Altered brain function in cerebral small vessel disease patients with gait disorders: a resting-state functional MRI study. Front Aging Neurosci 2020; 12: 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Starr JM, Leaper S, Murray AD, et al. Brain white matter lesions detected by magnetic resonance imaging are associated with balance and gait speed. J Neurol Neurosurg Psychiatry 2003; 74: 94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosano C, Brach J, Longstreth WT Jr and Newman AB. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology 2006; 26: 52–60. [DOI] [PubMed] [Google Scholar]
- 22.Rosano C, Brach J, Studenski S, Longstreth WT Jr and Newman AB. Gait variability is associated with subclinical brain vascular abnormalities in high-functioning older adults. Neuroepidemiology 2007; 29: 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srikanth V, Phan TG, Chen J, Beare R, Stapleton JM and Reutens DC. The location of white matter lesions and gait—a voxel-based study. Ann Neurol 2010; 67: 265–269. [DOI] [PubMed] [Google Scholar]
- 24.De Laat KF, Van Norden AG, Gons RA, et al. Gait in elderly with cerebral small vessel disease. Stroke 2010; 41: 1652–1658. [DOI] [PubMed] [Google Scholar]
- 25.De Laat KF, Tuladhar AM, Van Norden AG, Norris DG, Zwiers MP and de Leeuw F-E. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain 2010; 134: 73–83. [DOI] [PubMed] [Google Scholar]
- 26.Murray ME, Senjem ML, Petersen RC, et al. Functional impact of white matter hyperintensities in cognitively normal elderly subjects. Arch Neurol 2010; 67: 1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su N, Zhai FF, Zhou LX, et al. Cerebral small vessel disease burden is associated with motor performance of lower and upper extremities in community-dwelling populations. Front Aging Neurosci 2017; 9: 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stijntjes M, de Craen AJ, van der Grond J, Meskers CG, Slagboom PE and Maier AB. Cerebral microbleeds and lacunar infarcts are associated with walking speed independent of cognitive performance in middle-aged to older adults. Gerontology 2016; 62: 500–507. [DOI] [PubMed] [Google Scholar]
- 29.Callisaya ML, Srikanth VK, Lord SR, et al. Sub-cortical infarcts and the risk of falls in older people: combined results of TASCOG and Sydney MAS studies. Int J Stroke 2014; 9: 55–60. [DOI] [PubMed] [Google Scholar]
- 30.Ezzati A, Katz MJ, Lipton ML, Lipton RB and Verghese J. The association of brain structure with gait velocity in older adults: a quantitative volumetric analysis of brain MRI. Neuroradiology 2015; 57: 851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blumen HM, Brown LL, Habeck C, et al. Gray matter volume covariance patterns associated with gait speed in older adults: a multi-cohort MRI study. Brain Imaging Behav 2019; 13: 446–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tripathi S, Verghese J and Blumen HM. Gray matter volume covariance networks associated with dual-task cost during walking-while-talking. Hum Brain Mapp 2019; 40: 2229–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verghese J, Holtzer R, Lipton RB and Wang C. Mobility stress test approach to predicting frailty, disability, and mortality in high-functioning older adults. J Am Geriatr Soc 2012; 60: 1901–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan J, Blumen HM, Verghese J and Holtzer R. Functional connectivity associated with gait velocity during walking and walking-while-talking in aging: a resting-state fMRI study. Hum Brain Mapp 2015; 36: 1484–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou Y, Li Y, Yang S, Qin W, Yang L and Hu W. Gait impairment and upper extremity disturbance are associated with total magnetic resonance imaging cerebral small vessel disease burden. Front Aging Neurosci 2021; 13: 640844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Callisaya ML, Beare R, Phan TG, Chen J and Srikanth VK. Global and regional associations of smaller cerebral gray and white matter volumes with gait in older people. PLoS ONE 2014; 9: e84909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosano C, Aizenstein H, Brach J, Longenberger A, Studenski S and Newman AB. Gait measures indicate underlying focal gray matter atrophy in the brain of older adults. J Gerontol A Biol Sci Med Sci 2008; 63: 1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosano C, Aizenstein HJ, Studenski S and Newman AB. A regions-of-interest volumetric analysis of mobility limitations in community-dwelling older adults. J Gerontol A Biol Sci Med Sci 2007; 62: 1048–1055. [DOI] [PubMed] [Google Scholar]
- 39.Jayakody O, Breslin M, Beare R, Srikanth VK, Blumen HM and Callisaya ML. The associations between grey matter volume covariance patterns and gait variability—the Tasmanian Study of Cognition and Gait. Brain Topogr 2021; 34: 478–488. [DOI] [PubMed] [Google Scholar]
- 40.Jayakody O, Breslin M, Beare R, Blumen HM, Srikanth VK and Callisaya ML. Regional associations of cortical thickness with gait variability —the Tasmanian Study of Cognition and Gait. J Gerontol B Biol Sci 2020; 75: 1537–1544. [DOI] [PubMed] [Google Scholar]
- 41.Silbert L, Nelson C, Howieson D, Moore MM and Kaye JA. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology 2008; 71: 108–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soumaré A, Elbaz A, Zhu Y, et al. White matter lesions volume and motor performances in the elderly. Ann Neurol 2009; 65: 706–715. [DOI] [PubMed] [Google Scholar]
- 43.Callisaya ML, Beare R, Phan TG, et al. Brain structural change and gait decline: a longitudinal population-based study. J Am Geriatr Soc 2013; 61: 1074–1079. [DOI] [PubMed] [Google Scholar]
- 44.Willey JZ, Scarmeas N, Provenzano FA, Luchsinger JA, Mayeux R and Brickman AM. White matter hyperintensity volume and impaired mobility among older adults. J Neurol 2013; 260: 884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van der Holst HM, Tuladhar AM, Zerbi V, et al. White matter changes and gait decline in cerebral small vessel disease. NeuroImage Clin 2018; 17: 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Der Holst HM, Van Uden IW, De Laat KF, et al. Baseline cerebral small vessel disease is not associated with gait decline after five years. Mov Disord Clin Pract 2017; 4: 374–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Annweiler C, Beauchet O, Bartha R, et al. Motor cortex and gait in mild cognitive impairment: a magnetic resonance spectroscopy and volumetric imaging study. Brain 2013; 136: 859–871. [DOI] [PubMed] [Google Scholar]
- 48.Annweiler C, Beauchet O, Bartha R, Montero-Odasso M and WALK Team-Working Group Angers-London for Knowledge. Slow gait in MCI is associated with ventricular enlargement: results from the Gait and Brain Study. J Neural Transm 2013; 120: 1083–1092. [DOI] [PubMed] [Google Scholar]
- 49.Doi T, Blumen HM, Verghese J, et al. Gray matter volume and dual-task gait performance in mild cognitive impairment. Brain Imaging Behav 2017; 11: 887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blumen HM, Schwartz E, Allali G, et al. Cortical thickness, volume, and surface area in the motoric cognitive risk syndrome. J Alzheimers Dis 2021; 81: 651–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Blumen HM, Allali G, Beauchet O, Lipton RB and Verghese J. A gray matter volume covariance network associated with the motoric cognitive risk syndrome: a multicohort MRI study. J Gerontol A Biol Sci 2019; 74: 884–889. [DOI] [PubMed] [Google Scholar]
- 52.Wang N, Allali G, Kesavadas C, et al. Cerebral small vessel disease and motoric cognitive risk syndrome: results from the Kerala-Einstein study. J Alzheimers Dis 2016; 50: 699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Staekenborg SS, van der Flier WM, van Straaten EC, Lane R, Barkhof F and Scheltens P. Neurological signs in relation to type of cerebrovascular disease in vascular dementia. Stroke 2008; 39: 317–322. [DOI] [PubMed] [Google Scholar]
- 54.Allali G, Montembeault M, Saj A, et al. Structural brain volume covariance associated with gait speed in patients with amnestic and non-amnestic mild cognitive impairment: a double dissociation. J Alzheimers Dis 2019; 71: S29–S39. [DOI] [PubMed] [Google Scholar]
- 55.Boyle P, Wilson R, Aggarwal N, et al. Parkinsonian signs in subjects with mild cognitive impairment. Neurology 2005; 65: 1901–1906. [DOI] [PubMed] [Google Scholar]
- 56.Verghese J, Robbins M, Holtzer R, et al. Gait dysfunction in mild cognitive impairment syndromes. J Am Geriatr Soc 2008; 56: 1244–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ayers E and Verghese J. Gait dysfunction in motoric cognitive risk syndrome. J Alzheimers Dis 2019; 71: S95–S103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allan LM, Ballard CG, Burn DJ and Kenny RA. Prevalence and severity of gait disorders in Alzheimer’s and non-Alzheimer’s dementias. J Am Geriatr Soc 2005; 53: 1681–1687. [DOI] [PubMed] [Google Scholar]
- 59.Allali G, Annweiler C, Blumen HM, et al. Gait phenotype from mild cognitive impairment to moderate dementia: results from the GOOD initiative. Eur J Neurol 2016; 23: 527–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dodge H, Mattek N, Austin D, Hayes TL and Kaye JA. In-home walking speeds and variability trajectories associated with mild cognitive impairment. Neurology 2012; 78: 1946–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tanaka A, Okuzumi H, Kobayashi I, Murai N, Meguro K and Nakamura T. Gait disturbance of patients with vascular and Alzheimer-type dementias. Percept Mot Skills 1995; 80: 735–738. [DOI] [PubMed] [Google Scholar]
- 62.Fritz NE, Kegelmeyer DA, Kloos AD, et al. Motor performance differentiates individuals with Lewy body dementia, Parkinson’s and Alzheimer’s disease. Gait Posture 2016; 50: 1–7. [DOI] [PubMed] [Google Scholar]
- 63.Gillain S, Warzee E, Lekeu F, et al. The value of instrumental gait analysis in elderly healthy, MCI or Alzheimer’s disease subjects and a comparison with other clinical tests used in single and dual-task conditions. Ann Phys Rehabil Med 2009; 52: 453–474. [DOI] [PubMed] [Google Scholar]
- 64.MacAulay RK, Wagner MT, Szeles D and Milano NJ. Improving sensitivity to detect mild cognitive impairment: cognitive load dual-task gait speed assessment. J Int Neuropsychol Soc 2017; 23: 493–501. [DOI] [PubMed] [Google Scholar]
- 65.Xie H, Wang Y, Tao S, Huang S, Zhang C and Lv Z. Wearable sensor-based daily life walking assessment of gait for distinguishing individuals with amnestic mild cognitive impairment. Front Aging Neurosci 2019; 11: 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghoraani B, Boettcher LN, Hssayeni MD, Rosenfeld A, Tolea MI and Galvin JE. Detection of mild cognitive impairment and Alzheimer’s disease using dual-task gait assessments and machine learning. Biomed Signal Process Control 2021; 64: 102249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beauchet O, Allali G, Thiery S, Gautier J, Fantino B and Annweiler C. Association between high variability of gait speed and mild cognitive impairment: a cross-sectional pilot study. J Am Geriatr Soc 2011; 59: 1973–1974. [DOI] [PubMed] [Google Scholar]
- 68.Nadkarni NK, Mawji E, McIlroy WE and Black SE. Spatial and temporal gait parameters in Alzheimer’s disease and aging. Gait Posture 2009; 30: 452–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mielke MM, Roberts RO, Savica R, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci 2012; 68: 929–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Savica R, Wennberg AM, Hagen C, et al. Comparison of gait parameters for predicting cognitive decline: the Mayo Clinic Study of Aging. J Alzheimers Dis 2017; 55: 559–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gale CR, Allerhand M, Sayer AA, Cooper C and Deary IJ. The dynamic relationship between cognitive function and walking speed: the English Longitudinal Study of Ageing. Age 2014; 36: 9682–9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jayakody O, Breslin M, Srikanth VK and Callisaya ML. Gait characteristics and cognitive decline: a longitudinal population-based study. J Alzheimers Dis 2019; 71: S5–S14. [DOI] [PubMed] [Google Scholar]
- 73.Rosso AL, Metti AL, Faulkner K, et al. Complex walking tasks and risk for cognitive decline in high functioning older adults. J Alzheimers Dis 2019; 71: S65–S73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beauchet O, Launay CP, Sekhon H, et al. Association of increased gait variability while dual tasking and cognitive decline: results from a prospective longitudinal cohort pilot study. Geroscience 2017; 39: 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marquis S, Moore MM, Howieson DB, et al. Independent predictors of cognitive decline in healthy elderly persons. Arch Neurol 2002; 59: 601–606. [DOI] [PubMed] [Google Scholar]
- 76.Buracchio T, Dodge HH, Howieson D, Wasserman D and Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol 2010; 67: 980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ and Buschke H. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N Engl J Med 2002; 347: 1761–1768. [DOI] [PubMed] [Google Scholar]
- 78.Dumurgier J, Artaud F, Touraine C, et al. Gait speed and decline in gait speed as predictors of incident dementia. J Gerontol A Biol Sci 2017; 72: 655–661. [DOI] [PubMed] [Google Scholar]
- 79.Abellan van Kan G, Rolland Y, Gillette-Guyonnet S, et al. Gait speed, body composition, and dementia. The EPIDOS-Toulouse cohort. J Gerontol A Biol Sci Med Sci 2012; 67: 425–432. [DOI] [PubMed] [Google Scholar]
- 80.Verghese J, Wang C, Lipton RB, Holtzer R and Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry 2007; 78: 929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ceïde ME, Ayers EI, Lipton R and Verghese J. Walking while talking and risk of incident dementia. Am J Geriatr Psychiatry 2018; 26: 580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Montero-Odasso M, Sarquis-Adamson Y, Speechley M, et al. Association of dual-task gait with incident dementia in mild cognitive impairment: results from the gait and brain study. JAMA Neurol 2017; 74: 857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yakushiji Y, Wilson D, Ambler G, et al. Distribution of cerebral microbleeds in the East and West: individual participant meta-analysis. Neurology 2019; 92: e1086–e1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


