Abstract
A new approach named PEG-assist is introduced for the production of drug-loaded polymeric micelles. The method is based on the use of PEG as the non-selective solvent for PEG-b-PLA in the fabrication procedure. Both hydration temperature and PEG molecular weight are shown to have a significant effect on the encapsulation efficiency of PTX in PEG4kDa-b-PLA2kDa micelles. The optimal procedure for fabrication includes the use of PEG1kDa as the solvent at 60 °C, cooling the mixture to 40 °C, hydration at 40 °C, freezing at −80 °C and freeze-drying at −35 °C, 15 Pa. No significant difference (p>0.05) in PTX encapsulation, average particle size and polydispersity index is observed between the samples before freeze-drying and after reconstitution of the freeze-dried cake. The prepared PTX formulations are stable at room temperature for at least 8 hours. Scaling the batch size to 25X leads to no significant change (p>0.05) in PTX encapsulation, average particle size and polydispersity index. PEG-assist method is applicable to other drugs such as 17-AAG, and copolymers of varied molecular weights. The use of no organic solvent, simplicity, cost-effectiveness, and efficiency makes PEG-assist a very promising approach for large scale production of drug-loaded polymeric micelles.
Keywords: polyethylene glycol-block-polylactic acid (PEG-b-PLA), diblock copolymer, polymeric micelle, liquid-liquid phase separation, paclitaxel, scale up
1. Introduction
Despite the potential advantages, a relatively small number of nanomedicine formulations have been approved for clinical use [1]. Complex nature of nanomedicines compared with standard pharmaceuticals poses numerous challenges and obstacles in different stages of development. A reproducible scale-up and manufacturing process to obtain a consistent product with desired physicochemical and biological characteristics is an incontestable component of any formulation, especially in the later stages of development [2]. Switching from lab-scale to large-scale production may result in discrepancies in the product performance. The unsuccessful attempts in the marketplace to reproduce nab-paclitaxel are good examples of such discrepancies. High endotoxin and residual solvent beyond the safety limit, poor reproducibility in the manufacturing process and substantial inter-batch variations in particle size are some of the listed causes of failure. All appear to share one thing in common: fundamental differences with the genuine product resulting from differences in manufacturing [2]. Therefore, a potentially successful fabrication procedure needs to be scalable and comply with safety and regulatory challenges.
Polymeric micelles, first proposed as drug carriers by Bader et al. in 1984 [3], are an important subcategory of nanomedicine which form as a result of self-assembly of a diblock [4] or multiblock copolymer [5]. Solubilization of water-insoluble or poorly soluble drugs, controlled release, targeting and pharmacological activity of the carrier building blocks are the functions postulated for polymeric micelles in cancer treatment [6-10]. The latter i.e. the pharmacological activity of the carrier building blocks is more pronounced when the drug is chemically conjugated to the copolymer [6]. In contrast, the solubilization function can be well highlighted as it eliminates the need for using highly toxic organic solvents and/or low molecular weight surfactants such as ethanol/Cremophor EL conventionally used as drug solubilizers [11, 12]. In polymeric micelle formulations, polyethylene glycol (PEG) is often selected as the corona forming block due to its capability for reticuloendothelial system (RES) escape and prolonged circulation [13-15]. Furthermore, selectivity of water as a solvent for PEG prompts the formation of micelles with hydrophobic cores for drug encapsulation and consequent solubilization. PEG-b-PLA is a well-known example of micelle-forming block copolymer that does not disturb whole blood or its components and is compliant with the required standards for intravenous injection [16].
The existing methods of fabrication for drug-loaded polymeric micelles typically include the dissolution of both copolymer and drug in a non-selective organic solvent followed by addition of water as the selective solvent for one of the blocks to induce micellization. The latter step can be done either after complete removal of the organic solvent, such as in thin film hydration [17-22] and freeze-drying [23, 24] methods, or in the presence of organic solvent to replace, emulsify or change the selectivity, such as in dialysis [25-27], emulsification-solvent evaporation [28-31] or cosolvent azeotrope evaporation [32] techniques, respectively. In the latter group of methods, the micelles form in the presence of organic solvent when a critical water concentration is reached [33, 34] and the final product can be obtained by the complete removal of the organic solvent thereafter. The use of organic solvent, complex processes and specialty equipment in all the above-mentioned fabrication routes leads to serious complications in bench-to-bedside translation. It includes but is not limited to technical, safety, environmental and economic challenges during the production as well as regulatory concerns about the final product due to residual solvent and reproducibility of nanoscale properties in large-scale batches [2, 35-38]. Therefore, alternative procedures for the fabrication of drug loaded polymeric micelles are critically needed.
The aim of this study was to develop a safe and potentially scalable method for the fabrication of drug-loaded polymeric micelles. The method is called “PEG-assist” and offers the use of PEG instead of organic solvents due to the capability of PEG to dissolve hydrophobic drugs at elevated temperatures [39, 40]. The pragmatism of using PEG in pharmaceutical formulations [41] eliminates the safety and regulatory concerns of removing it and simplifies the fabrication process for scale-up. The PEG, diblock copolymer and drug mixture turns into drug-loaded polymeric micelles upon hydration. Since the solvation power of PEG is molecular weight- and temperature-dependent, the mixture and consequently the preparation outcome is affected by both. Therefore, the effect of temperature and PEG molecular weight on particle size, encapsulation efficiency and critical water concentration is presented for PEG4kDa-b-PLA2.2kDa as the micelle building block and paclitaxel (PTX) as the model drug. The applicability of the method to other drugs and copolymers, scalability, freeze-drying and the stability of the prepared formulations are also studied.
2. Experimental details
Materials
Polyethylene glycol-b-poly(D, L-lactide) (PEG-b-PLA, PEG block Mw: 4 kDa, PDLLA block Mw: 2.2 kDa, PDI=1.08) diblock copolymer was purchased from JenKem Technology (Plano, TX). PEG-b-PLA copolymer with different molecular weights were purchased from Advanced Polymer Materials Inc. (Quebec, Canada). PEGs of different molecular weights (i.e. 200, 400, 600 and 1000 Da) were purchased from Sigma-Aldrich (St. Louis, MO). Paclitaxel (PTX) was obtained from LC Laboratories (Woburn, MA). All other chemicals were of reagent grade.
Crystallization – Differential Scanning Calorimetry (DSC)
PEG4kDa-b-PLA2.2kDa diblock copolymer granules (5.0 wt.%) were added to liquid PEG (Mw 200-600 Da), heated to 60 °C and vortexed to obtain a transparent mixture. After 30 min incubation, one drop of each solution (ca. 20 mg) was transferred to aluminum crucibles and allowed to cool down to room temperature prior to DSC analysis (DSC 404 F1 Pegasus, NETZSCH, Germany). The samples were heated to 80 °C and kept at this temperature for 15 min in order to erase thermal history in the first scan. The samples were then cooled down to different temperatures ranging from 25 to 40 °C for various periods ranging from 0.5 to 48 hours to induce isothermal crystallization. Heating the samples to 80 °C in the second scan yielded the melting onset temperature and enthalpy. A heating/cooling rate of 5 °C/min was used for all the scans. The maximum crystallizable content (100%) was obtained by incubating samples in the corresponding temperature for 10 days followed by DSC scan using the same parameters.
Micelle preparation protocol
Polymeric micelles were obtained by hydration of the PEG-b-PLA diblock copolymer/PEG mixture at different temperatures. Briefly, a transparent mixture (5.0 wt.% PEG-b-PLA in PEGs of different Mw) was obtained by heating the mixture to 60 °C in borosilicate glass vials, vortexing and incubation at the same temperature for 30 min. The transparent mixture was kept at 60 °C or cooled down to 40 or 25 °C, incubated for 2 h and hydrated by addition of DI water with the same temperature.
In order to prepare drug-loaded micelles, PTX (at a weight ratio of 1:2 w/w PTX: PEG4kDa-b-PLA2.2kDa corresponding to target loading of ca. 33 wt.%) was added to the mixture prior to heating to 60 °C. The amount of water when PTX was present in the formulation was adjusted to aim for 4 mg/mL PTX level. Such a high target loading and PTX level was used for optimization of preparation parameters only. The hydration process was performed under different conditions including rapid hydration (vortexing after addition of water) or slow hydration (a two-step hydration protocol consisting of addition of half of the target water volume followed by mixing and then addition of the rest followed by gentle mixing at 50 rpm in a rotary mixer). Micelles with the target loadings of 10 and 20 wt.% at 1 mg/mL PTX target level were prepared after optimizing the process parameters and used for other experiments such as stability measurement, scalability and freeze-drying. To prepare formulations with 1 mg/mL PTX target level at 10 or 20 wt.% target loading, 1 mg PTX together with 9 or 4 mg copolymer were mixed with 170 or 75 mg PEG, respectively, and heated to 60 °C to obtain a transparent mixture. The added amounts of PEG keep the block copolymer concentration at 5.0 wt.% in the mixture. The mixture was cooled down to 40 °C, incubated for 2 h followed by hydration with 1 mL DI water at 40 °C to obtain drug-loaded polymeric micelles. The samples were cooled down to room temperature and analyzed.
Micelle formation and particle size analysis
Dynamic light scattering (DLS, nano ZS, Malvern) was used to measure particle size distribution and to investigate micelle formation. For particle size analysis, the measurements were carried out at 25 °C using water as dispersant.
For micelle formation study, DLS was carried out for all the samples at two different temperatures (60 and 40°C). The intensity of scattered light detected at 173° was recorded for different concentrations of PEG-b-PLA in PEG1000 ranging from 0.0 to 5.0 wt.% in the absence or presence of water (water/PEG weight ratio ranging from 0 to 3). The measured value for “count rate”, which represents the intensity of scattered light, is independent of the input values for viscosity, RI and dielectric constant of the solvent. The mentioned values for PEG1000 are listed in Table S1. Micelle formation results in a considerable increase in the intensity of scattered light and enhanced signal to noise ratio in autocorrelation function. According to Topel et al. study [42], DLS is as sensitive as fluorescence spectroscopy using pyrene in determining the critical micelle concentration (CMC) of a copolymer. We defined a parameter named “Relative Scattered Light (RSL)” as the ratio of “scattered light intensity by sample” to “scattered light intensity by dispersant” in order to minimize the effect of dispersion medium (water/PEG mixture):
| eq. 1 |
The onset of RSL increase can be considered as the onset of micelle formation which in turn gives CMC and critical water concentration (CWC). DLS correlograms (correlation coefficient vs. time) were used to study micelle formation near the critical concentrations. Correlation coefficient indicates how fast a scattered light signal originated from certain entities in the sample decay by correlating the signal intensity with itself at very short intervals. Perfect and no correlation between the two signals are indicated by unity and zero, respectively. The signal decay (i.e. reduction of correlation coefficient from 1 to 0) is due to Brownian motion of particles and is correlated with both particle size and medium viscosity.
Encapsulation efficiency
To measure the encapsulation efficiency throughout the study, unencapsulated drug was first removed by centrifugation at 10000 g for 10 min. Following centrifugation, 50 μl supernatant of each sample was mixed with 50 μl DI water and 400 μl acetonitrile (ACN). A high-performance liquid chromatography system (HPLC, Prominence, Shimadzu, Japan) equipped with an autosampler, a C18 column and a UV-Vis detector was employed to measure PTX content. PTX was detected at 228 nm absorption wavelength by injecting 10 μl sample through an 80/20 ACN/water v/v mixture as the mobile phase under the flow rate of 0.5 mL/min. Encapsulation efficiency was defined as the weight ratio of encapsulated drug to the initial drug used. The target drug loading was defined as:
| eq. 2 |
Freeze-drying
For freeze-drying, first, the melting onset of the water/PEG eutectic mixture was determined using DSC. The PEG4kDa-b-PLA2kDa /PEG1000/water mixture was cooled down to −80 °C at 10 °C/min. The frozen mixture was then heated to room temperature at 10 °C/min to detect the melting transitions of the eutectic mixture and the ice crystals. The sample composition for freeze drying was 1 mg PTX, 4 mg copolymer, 75 mg PEG1000 and 1 mL water in a 3.7 mL standard glass vial. The freezing step was performed at −80 °C overnight. The freeze-dryer shelf temperature, based on the collected data, was set at −35 °C and freeze-drying was performed in a VirTis Advantage Pro shelf freeze-dryer at 15 Pa for 72 h.
Statistical analysis
All the measurements are reported as Mean ± SEM. One-way or two-way ANOVA were used to analyze statistical differences between different groups followed by Tukey’s HSD or Sidak’s test as multiple comparison tests. The differences were considered significant if p<0.05.
3. Results and Discussion
Hydration temperature and PEG molecular weight influence the physicochemical properties of drug-loaded polymeric micelles
Diblock copolymers self-assemble into polymeric micelles in a selective environment for one of the blocks. Formation of PEG-b-PLA micelles in water, for example, is due to its selectivity for the PEG block. Therefore, dissolving the copolymer in a non-selective solvent (e.g. acetonitrile) followed by replacement with or addition of a selective solvent (e.g. water) constructs the basis of most fabrication methods for polymeric micelles [18-30, 32]. Addition of drugs to the initial solution results in drug encapsulation during self-assembly. We aimed to use PEG instead of organic solvents in the preparation procedure. To begin with, we studied the solubility of PEG-b-PLA in liquid PEG.
At elevated temperatures, mixing PEG4kDa-b-PLA2.2kDa with PEG of different molecular weights at 5.0 wt.% yields a transparent solution. Cooling this mixture to room temperature causes turbidity over time and results in a waxy solid. We assumed that this phenomenon is a result of crystallization of the PEG block of the copolymer at reduced temperatures. Therefore, PEG-b-PLA solubility in PEG is temperature dependent. In this case, the mixture is undersaturated at 60 °C and supersaturated at 25 °C (see Figure S1 in supporting info). We assessed the approximate saturation temperature for the mentioned composition by monitoring crystal formation at different temperatures over time using DSC. No crystals could be detected after 48 hours of isothermal hold at 40 °C and this temperature was supposed as approximate saturation temperature (Figure S1). We concluded that 5.0 wt.% PEG4kDa-b-PLA2.2kDa in liquid PEG is undersaturated at 60 °C, saturated at 40 °C and supersaturated at 25 °C. The supersaturated solution at 25 °C, if incubated long enough, will result in formation of copolymer crystals in equilibrium with a saturated solution.
Since the solubility of PEG-b-PLA in liquid PEG is considerably temperature-dependent, we examined the effect of temperature on encapsulation efficiency and particle size of PTX-loaded micelles obtained using PEG4kDa-b-PLA2.2kDa/PTX/liquid PEG. We selected 25, 40 or 60 °C to characterize different states of the solution. Addition of water to PEG-b-PLA/PTX/liquid PEG is expected to result in the formation of polymeric micelles by increasing the selectivity of the environment for the PEG block of the copolymer. In addition to temperature, we investigated the effect of PEG molecular weight as it may influence the solubilization power of the environment throughout the self-assembly process.
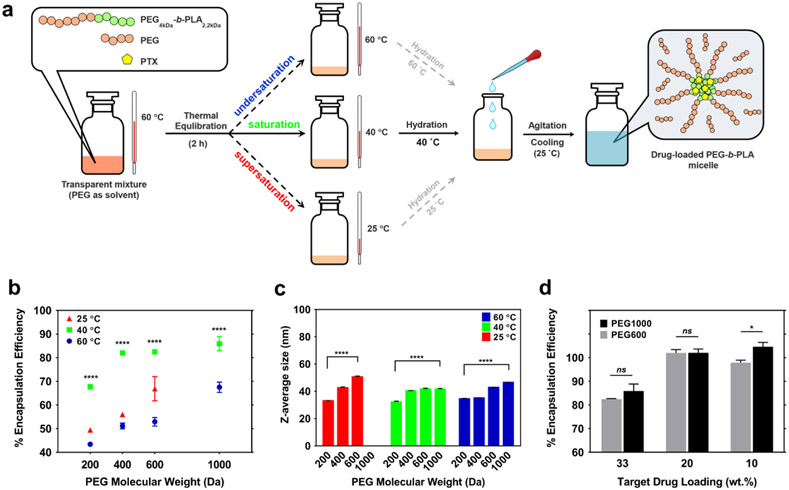
We incubated the copolymer/PTX/PEG (5.0 : 2.5 : 92.5 wt.%) for 2 h at 25, 40 or 60 °C. The micelles were obtained by rapid hydration of the mixture at the same temperature (Figure 1a). In other words, water was added to the mixture, then the sample was vortexed, cooled down to room temperature and analyzed. As shown in Figure 1b, both PEG molecular weight (200-600 Da) and the incubation temperature (25-60 °C) influence the encapsulation efficiency. The direct relationship between the molecular weight of PEG and encapsulation efficiency encouraged us to examine the formulation prepared by PEG1000 at 40 and 60 °C as well. It should be noted that PEG1000 is the highest possible molecular weight to obtain a liquid mixture at 40 °C for comparison. Higher molecular weights of PEG require higher processing temperatures. Two-way ANOVA suggests an extremely significant interaction between the two variables i.e. PEG molecular weight and temperature (5.2% of the total variance, p<0.0001). The effect of both variables on encapsulation efficiency is extremely significant (p<0.0001). Tukey’s multiple comparisons test implies a significantly higher encapsulation efficiency when the mixture is hydrated at 40 °C (p<0.0001) compared to both 25 and 60 °C for all molecular weights of PEG. Hydration at 25 °C leads to a significantly higher encapsulation efficiency compared to 60 °C only for PEG600 (p<0.0001). PEG4kDa-b-PLA2.2kDa used in this study is expected to produce 25-50 nm PTX encapsulated micelles depending on the preparation process [43, 44]. PEG molecular weight and hydration temperature both significantly (p<0.0001) influence the average particle size as well (Figure 1c). According to two-way ANOVA, the effect of PEG molecular weight is more pronounced – accounting for 68.9% of the total variance (vs. 16.6% for temperature). As seen in Figure 1c, the average particle size increases with increasing the PEG molecular weight.
Figure 1.
a) Schematic representation of micelle preparation protocol (PEG-assist). The effects of PEG molecular weight and hydration temperature on b) encapsulation efficiency (33 wt.% target loading) and c) average particle size of the micelles. d) Encapsulation efficiency as a function of target drug loading when the micelles prepared using optimal parameters. Statistical analysis: n=6, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
According to the obtained results, for all the examined molecular weights, liquid PEG containing PEG-b-PLA in saturated state offers the best outcome. The maximum encapsulation efficiency for PEG4kDa-b-PLA2.2kDa is achieved using PEG1000, with addition of water performed at 40 °C. It should be noted that the saturation temperature and consequently the optimal temperature for fabrication may vary if one wishes to use higher molecular weights of the copolymer and/or homo PEG. Although the optimization was performed using 5.0 wt.% PEG4kDa-b-PLA2.2kDa and rapid hydration (vortexing), the observed trend was found to be reproducible for other concentrations and hydration criteria. We experimented i) rapid hydration of 10.0 wt.% PEG4kDa-b-PLA2.2kDa with the same target loading (i.e. 33%) and ii) a slow hydration process consisting of rapid prehydration at the same temperature followed by complete hydration in a rotary mixer at 50 rpm for both 5.0 and 10.0 wt.% PEG4kDa-b-PLA2.2kDa. The results are displayed in Figure S2. The same conclusion can be made for the supplementary experiments: using higher molecular weight of PEG and hydration at 40 °C gives the highest encapsulation efficiency. Lastly, we studied the effect of reducing PTX target loading and PTX final concentration in water on the encapsulation efficiency by rapid hydration of 5.0 wt.% PEG4kDa-b-PLA2.2kDa in PEG600-1000 at 40 °C. As illustrated in Figure 1d, the PEG-assist method with the optimal parameters can produce PTX-loaded PEG4kDa-b-PLA2.2kDa with almost 100% encapsulation efficiency for 10 and 20 wt.% target loading at 1 mg/mL target drug level. PEG1000 still slightly excels PEG 600. Given the collected data, we selected to prepare the micelles at 20 wt.% target loading using 5.0 wt.% PEG-b-PLA, PEG1000 and rapid hydration at 40 °C as the optimal preparation parameters for the remainder of the experiments.
A biphasic mixture forms prior to the formation of micelles in PEG-b-PLA/PEG/water
In order to gain a better understanding of how the PEG-assist method efficiently produces drug-loaded polymeric micelles, five important questions were enquired: i) does homo PEG play a solubilization role in the system, or is drug mainly encapsulated in the micelles’ core? ii) how does hydration of copolymer/PEG/drug mixture lead to formation of drug-loaded micelles? iii) does the application of a small molecule organic solvent under the same condition offer comparable outcome? If not, why? iv) how does increasing the homo PEG molecular weight improve encapsulation efficiency? v) how does saturation temperature (e.g. 40 °C) outperform undersaturation (e.g. 60 °C) and supersaturation (e.g. 25 °C)?
To begin with, we measured the solubility of PTX in mixtures of water and PEG of different molecular weights as a function of water content. Hydrophobic drugs such as PTX are highly soluble in liquid PEG. Addition of water, however, if surpasses a critical point, will result in precipitation of PTX (see Figure S3 in supporting info). This critical point is positioned near 50.0 wt.% water content (or water:PEG 1:1 w/w ratio) for all the examined molecular weights. In other words, presence of equal or larger amount of water in a PEG/water mixture prohibits PTX solubility. Considering the PEG-assist preparation procedure, the water content always well exceeds the critical value in the final product (ca. 85.0, 92.0 or 96.0 wt.% water content for 1 mg/mL PTX level at 10, 20 or 33 wt.% target loading, respectively). Therefore, PEG/water mixture cannot play any solubilization role for PTX in the proposed system, and the drug is expected to mainly reside in the PEG-b-PLA micelles’ core.
Next, we used DLS to study micelle formation near the CMCs at specific water:PEG ratios and CWCs at specific PEG-b-PLA concentrations in PEG. Those critical values can be estimated by recording the intensity of scattered light by each sample [42, 45, 46]. To eliminate the variations caused by ratio change in our bicomponent dispersant i.e. PEG/water for different samples, we defined the parameter “RSL” which is obtained by normalizing the intensity of scattered light by the sample to its dispersant. When the micelles form, a sharp increase in RSL is observed as shown in Figure S4. The onset of RSL increase was determined for different concentrations of PEG4kDa-b-PLA2.2kDa in PEG1000 in various water:PEG ratios at 40 °C to generate micelle formation diagram as illustrated in Figure 2a. In the red region of the diagram, the PEG-b-PLA and water concentrations are adequate for micelle formation. For instance, the CMC in 3:1 (w/w) water:PEG mixture is reached when the initial PEG-b-PLA concentration in PEG1000 is ca. 0.4 wt.% or higher (red dashed box in Figure 2a). For initial concentration of 5.0 wt.% PEG-b-PLA in PEG1000, the CWC is between 10-20% water:PEG weight ratio (red dotted box in Figure 2a).
Figure 2.
a) Micelle formation for PEG4kDa-b-PLA2.2kDa/PEG1000/water mixture at 40 °C; the compositions within the red region produce polymeric micelles; the boundary line between red and blue regions gives approximate CMC for a specific water:PEG ratio or CWC for a specific initial copolymer concentration in PEG1000; the dashed and dotted boxes illustrate CMC at 3:1 water:PEG w/w ratio and CWC at 5.0 wt.% initial copolymer concentration, respectively. DLS correlograms for compositions near b) CMC at 3:1 water:PEG1000 w/w ratio, c) CWC at 5.0 wt.% initial copolymer concentration in PEG1000 (“cp” stands for “copolymer”), c’) Phase microscope images of PEG4kDa-b-PLA2.2kDa/PEG1000/water mixture for 5.0 wt.% initial copolymer concentration and 15.0% (w/w) water:PEG1000 incubated for 1 or 48 h at 40 °C. d) CWC at 5.0 wt.% initial copolymer concentration in PEG1000 and 33 wt.% PTX target loading (“cp” stands for “copolymer”). e) Schematic representation of the proposed mechanism of PEG-b-PLA micelle formation in PEG-assist approach. f) Encapsulation efficiency of PTX when loaded in polymeric micelles by rapid mixing using acetonitrile (ACN) or PEG1000 as the solvent. Statistical analysis: n=6, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
We then analyzed the correlation functions in the vicinity of critical concentrations, CMC and CWC, at 40 °C. Figure 2b displays the correlation coefficient for varied concentrations of PEG-b-PLA in 3:1 (w/w) water:PEG. The presence of large entities with long decay times is evident for low initial PEG-b-PLA concentration (0.1 wt.%). When the initial PEG-b-PLA concentration is increased to 0.2 wt.%, the signal for those entities deteriorates. The large entities vanish when the concentration is further increased to 0.4 wt.% which is the onset of RSL jump as well (Figure 2a, dashed box). For higher concentrations, the correlation coefficient represents a uniform dispersion of PEG-b-PLA micelles with short decay times (Figure 2b). Similarly, large entities with long decay times can be detected in 5.0 wt.% copolymer mixed with water when the water concentration is below CWC (Figure 2c). In this case, the signal for large entities intensifies by addition of water up to 15.0% (w/w) water:PEG. The signal partially vanishes when the water concentration is increased to 17.5% and completely disappears for 20.0% water or higher. The onset of large entities evanescence (i.e 17.5% water) accords the onset of RSL jump (Figure 2a, dotted box). For higher water concentrations, the correlation coefficient represents a uniform dispersion of PEG-b-PLA micelles with short decay times (Figure 2c). We also collected phase microscope images of the sample containing 15.0% water incubated at 40 °C over time (Figure 2c’). The images confirm the presence of large entities in the form of droplets at such a water concentration. Interestingly, the size of those droplets increases tremendously by prolonging the incubation time which shows the dynamic nature of those entities. Very similar trend can be observed even if a high content of PTX is added to the mixture (Figure 2d). It should be noted that shorter decay times of PEG-b-PLA micelles in Figure 2b compared to Figure 2c is due to the lower viscosity of the dispersant (containing 300.0% vs. 25.0% water). The mixture behaves very similarly in the vicinity of corresponding CWC at 60 °C (see Figure S5 in supporting info). Interestingly, at 60 °C, the large entities to micelle transformation occurs at slightly higher water concentrations (22.5% for 60 °C vs. 17.5% for 40 °C).
It is evident that the formation of these large entities precedes PEG-b-PLA micelle formation at both temperatures. We think the large entities can be a result of liquid-liquid phase separation prior to micelle formation as suggested by Sato and Takahashi [47]. Liquid-liquid phase separation during amphiphilic self-assembly has been recently probed by Laniro et al. [48] through liquid-phase electron microscopy. Their results suggest the formation of polymer-rich liquid droplets as a precursor for polymeric micelles and vesicles when the amphiphilicity is increased by changing the solvency of the copolymer blocks. The presence of large entities with long decay times prior to micelle formation and their evanescence by further hydration (Figure 2b-c) is in line with such a hypothesis. Higher water content needed for such transition at 60 °C compared to 40 °C can be attributed to improved solubility of the copolymer in PEG at 60 °C which necessitates the addition of more water to induce phase separation, alter the selectivity of the environment and prompt micelle formation. In other words, lower temperatures may facilitate liquid-liquid phase separation. We didn’t examine the onset of liquid-liquid phase separation in the current study. It is possible that phase separation occurs even in the absence of water at certain temperatures and is further encouraged by addition of water.
Based on the collected data, we propose a mechanism for PEG-assist process wherein liquid-liquid phase separation precedes micelle formation as illustrated in Figure 2e. Hydration of a PEG-b-PLA/PEG/drug mixture at adequately low temperatures leads to formation of a biphasic mixture consisting of a copolymer-rich and a copolymer-poor phase prior to the formation of drug-loaded polymeric micelles. The copolymer-rich phase efficiently transforms to drug-loaded polymeric micelles and gives high encapsulation efficiencies.
If the same protocol is followed but using a common organic solvent such as acetonitrile (ACN) instead of PEG, the outcome is very disappointing. Figure 2f compares the outcome of the same protocol for PEG1000 and ACN used to load 20 wt.% PTX at 1 mg/mL into PEG4kDa-b-PLA2.2kDa micelles. As seen, ACN fails to yield drug-loaded micelles when it’s rapidly replaced by water. To further explore the possible causes of low or no encapsulation when ACN is used as the solvent, we measured the onset of micelle formation or CWC for 5.0 wt.% PEG4kDa-b-PLA2.2kDa in ACN as well as the solubility of PTX in water/ACN mixture as a function of water content (Figure S6). The onset of RSL jump, indicating CWC as previously elucidated, was spotted at ca. 58.5% (w/w) water:ACN (Figure S6a) which is equal to 36.9 wt.% water content. The correlograms (Figure S6b) confirms the formation of micelles at higher water contents than the obtained CWC. The CWC for 5.0 wt.% PEG4kDa-b-PLA2.2kDa when PEG1000 is used as the solvent is 17.5% (w/w) water:PEG1000 (Figure 2a) which is equal to 14.8 wt.% water content. It is obvious that higher water content is needed for micelle formation when ACN is used as the solvent, simply because ACN is a better solvent for both blocks of the copolymer and the entropy of mixing is increased when ACN is used as the solvent. On the other hand, solubility data suggests that water/PEG mixture can barely maintain PTX at 1.0 mg/mL beyond 40.0 wt.% water content (Figure S3) while PTX is fully soluble in water/ACN mixture at 1.0 mg/mL when the water content is as high as 60.0 wt.% (Figure S6c). Micelle formation at lower water content and lower solubility of PTX in external water/PEG mixture combined with precedence of liquid-liquid phase separation may work together to encourage partitioning of the drug into the core of the micelles when PEG is used as the solvent. When ACN is used, micelle formation is shifted towards higher water contents, where the drug is still highly soluble in the external water/ACN mixture. This may prohibit efficient partitioning of the drug into the micelles’ core and cause drug precipitation by further dilution. Gradual solvent replacement is needed, like in dialysis method, to allow gradual partitioning and achieve encapsulation by common organic solvents such as ACN. In contrast, the application of PEG as the solvent can grant full encapsulation even through relatively rapid mixing. Therefore, PEG may outperform common organic solvents such as ACN when used as the non-selective solvent in the fabrication procedure under the mentioned circumstances.
The results obtained for dependency of encapsulation efficiency on PEG molecular weight and temperature (40 vs. 60 °C) can be explained using the proposed mechanism in Figure 2e. Increasing PEG molecular weight decreases PTX solubility in external PEG-rich phase (or water/PEG mixture) and favors its partitioning into the copolymer-rich droplets and consequently PLA micelle core. As a result, higher PEG molecular weight is expected to give a higher encapsulation efficiency which is compatible with the trend observed in the presented data. Lowering the temperature from 60 to 40 °C is expected to have a similar effect. In other words, the PTX solubility in PEG-rich phase is lower at 40 °C, favoring drug partitioning into the copolymer-rich phase and the derived micelles. Furthermore, temperature can affect the composition of the separated phases. In a mixture with upper critical solution temperature (UCST), for example, reducing temperature can further enrich the copolymer-rich phase with the copolymer and deplete the copolymer from the copolymer-poor phase. This in turn may influence drug solubility, drug distribution between the phases and eventually encapsulation. While being valid for 60 vs. 40 °C, the hypothesis is not applicable to 25 °C. Surprisingly, further decrease in temperature not only poses no positive effect on encapsulation but also ruins it. We conducted a set of experiments to explain the cause for higher encapsulation at 40 °C compared to 25 °C which we thought is the phase transition at latter temperature.
We assumed that the unexpected reduction in encapsulation efficiency at 25 °C is linked to supersaturation and the resulted isothermal crystallization (Figure S1c). To examine this hypothesis, we prepared a PTX-free sample by mixing PEG4kDa-b-PLA2.2kDa and liquid PEG at the same ratio (5.0 wt.% copolymer in PEG) and hydrated it after 2 h incubation at different temperatures. Particle size analysis revealed the formation of uniform polymeric micelles when hydration was performed at 60 or 40 °C (Figure 3a). In contrast, hydration of the obtained waxy sample at 25 °C, depending on the PEG used, led to a unimodal distribution of large particles or a bimodal distribution composed of both polymeric micelles and larger particles as illustrated in Figure 3a. It was known from the DSC data that crystal formation at 25 °C is a time-dependent process. To confirm that crystallization in the mixture leads to formation of large particles when hydrated, we examined the effect of incubation time at 25 °C on the particle size distribution (Figure 3b). According to the obtained results, hydration of the mixture before the onset of crystallization leads to formation of polymeric micelles whereas large particles start to appear and grow in population as crystallization proceeds over time (Figure 3b). The onset of crystallization occurs at shorter times for lower molecular weights of PEG (Figure S1c). The mixture made with PEG200 has the shortest onset (< 0.5 h) followed by PEG400 and PEG600, respectively (both > 0.5 h). As seen in Figure 3b, hydration of PEG200 mixture after 0.5 h incubation at room temperature led to a bimodal particle size distribution composed of both sub-100 nm polymeric micelles and large particles. By increasing the crystal content through prolonging incubation times, the large particles dominated the polymeric micelles in the distribution profile. Incubation for 0.5 h which is shorter than the onset of crystallization for PEG400 and 600 made mixtures, led to formation of unimodal polymeric micelles while prolonging the incubation time produced a bimodal distribution containing large particles (Figure 3b). The collected data convinced us that crystallization in the mixture leads to formation of large particles through hydration. The cause of lower than expected encapsulation at 25 °C might be the exclusion of those large particles by centrifugation during preparation. Hydration of the mixture at higher temperatures (e.g. 40 °C) can prevent the formation of those large particles. We also investigated if altering the cooling rate or prehydration at 40 °C prior to cooling to 25 °C can have a positive effect on PTX encapsulation. According to Sidak’s multiple comparison test, neither the cooling rate (Figure 3c) nor prehydration (Figure 3d) would lead to significantly different encapsulation (see supporting info section 7 for details) – particularly for high molecular weight PEGs (p>0.05). Therefore, complete hydration at 40 °C remains the optimal protocol for micelle preparation.
Figure 3.
a) The effect of incubation temperature on the particle size distribution for a PTX-free sample. b) Particle size distribution for micelles obtained by hydration of the copolymer/PEG200-600 mixture incubated at 25 °C for different periods. c) The effect of cooling rate to 25 °C on encapsulation efficiency. d) The effect of prehydration at 40 °C followed by hydration at 25 °C on encapsulation efficiency. Statistical analysis: n=6, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
In addition to solvency, PEG can play the lyoprotectant role to produce stable, scalable and freeze-dried micellar formulations
We considered stability, applicability to other drugs and copolymers, scalability and long-term preservation as four more criteria to evaluate the PEG-assist method. The stability of PTX-loaded PEG-b-PLA micelles prepared at 20 wt.% target loading with 1 mg/mL target PTX level is shown in Figure 4a. All the samples were prepared at saturation temperature i.e. 40 °C. The effect of molecular weight of the homo PEG used (600 vs. 1000 Da) is also demonstrated. As seen, the formulation is stable at room temperature for at least 8 hours after hydration. Two-way ANOVA suggests that within the examined time range, PEG molecular weight and incubation time have no significant effect on the PTX content (p>0.05). In other words, PTX remains soluble after 8 hours incubation at room temperature without any significant loss of drug for any of the PEG molecular weights (p>0.05). Particle size analysis also reveals no significant change in average size over the studied time course (see Figure S7a). We observed that all the samples were completely transparent without any visual precipitation within the 8 h time frame. It should be noted that PTX, when loaded physically, may prove instable beyond this time scale. The inherent incompatibility of PTX with the micelle core can be alleviated using acyl and ester prodrug strategies for physical loading purposes [22, 44, 49-51].
Figure 4.
a) The stability of PTX-loaded micelles at room temperature. b) Encapsulation efficiency and stability of 17-AAG-loaded polymeric micelles at room temperature. c) The effect of molecular weight of PEG and PLA blocks of PEG-b-PLA copolymer on the characteristics of the corresponding PTX-loaded micelles. d) The effect of 25X scale-up on the characteristics of PTX-loaded micelles made by PEG-assist using PEG4kDa-b-PLA2kDa. e) DSC thermogram of PEG4kDa-b-PLA2kDa/PEG1000/water mixture; heated at 10 °C/min after getting frozen at the same rate to detect the eutectic melting transition. e') The characteristics of PTX-loaded micelles before and after freeze-drying at −35 °C and 15 Pa for 72 h. Statistical analysis: n=6, * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
As the proof of concept for applicability to other drugs, we used PEG-assist (PEG1000, 40 °C) to encapsulate 17-AAG at a target loading and target drug level of 20 wt.% and 1 mg/mL, respectively. 17-AAG can be encapsulated at 80.2 ± 2.1% efficiency and prove stable for at least 24 h (Figure 4b) with no visual precipitation and no significant drop in encapsulated drug content as confirmed by one-way ANOVA (p>0.05). Particle size analysis further supports the stability within the examined time frame as no significant change in average size is observed (Figure S7b).
We applied the PEG-assist method using PEG1000 at 40 °C to PEG-b-PLA block copolymers with different molecular weights of the two blocks. First, the effect of changing the molecular weight of the PLA block while keeping the PEG block molecular weight at 4 kDa was investigated. As seen in Figure 4c, increasing the PLA block molecular weight reduces the PTX encapsulation and increases the particle size. Overall, the encapsulation efficiency remains high (>95.0%) when the PLA block molecular weight is smaller than the PEG block. For equal molecular weights (i.e. PEG4kDa-b-PLA4kDa), the encapsulation efficiency dropped down to 56.7 ± 1.8%. We then studied the effect of PEG block molecular weight variation while keeping the PLA block molecular weight at 2 kDa. According to our results (Figure 2c), increasing the PEG block molecular weight from 2 to 4 or 7 kDa increases the encapsulation efficiency but reduces the particle size. It should be noted that a very probable cause for such an observation is that the parameters used are the optimal ones for PEG4kDa block. Using the same parameters for PEG2kDa or PEG7kDa blocks may result in under- or supersaturation, respectively. Therefore, it is expected that optimizing the PEG-assist method parameters for a particular copolymer gives a higher encapsulation efficiency. Overall, obtaining encapsulation efficiencies over 95.0% for most of the formulations proves the applicability of PEG-assist to different molecular weights of the PEG-b-PLA block copolymer.
Next, we evaluated the feasibility of scaling the batch size to 25X. Again, PTX target loading and final level of 20 wt.% and 1 mg/mL was used, respectively. In other words, the batch contained 25 mg PTX, 100 mg copolymer, 1875 mg PEG1000 and was hydrated with 25 mL water. As seen in Figure 4d, 25X scale-up using PEG1000 as the solvent and 40 °C as the processing temperature led to no significant change in encapsulation efficiency, average particle size and polydispersity (p>0.05).
Lastly, we probed the feasibility of freeze-drying for long-term storage and reconstitution of PTX formulation for injection. PEGs of high molecular weight have been previously used as lyoprotectants [52, 53]. For freeze-drying, samples were fabricated at 40 °C using PEG1000. It is well-known that PEG and water form a eutectic when mixed [54]. We first identified the eutectic melting temperature for PEG4kDa-b-PLA2kDa/PEG1000/water mixture. The ratios in the mixture were adjusted as required to obtain 20 wt.% target loading and 1 mg/mL target drug level for any drug of interest. The mixture was cooled down to −80 °C at 10 °C/min to freeze, followed by heating at the same rate to detect the eutectic melting onset. As seen in Figure 4e, such a mixture produces a eutectic system with a melting onset of ca. −23.4 °C.
Freeze-drying of PTX formulation with 20 wt.% loading and 1 mg/mL PTX level was performed at −35 °C shelf temperature to ensure the sample temperature remains well below the eutectic melting onset [55]. Such a protocol yields a high-quality cake that when hydrated, produces drug-loaded micelles that are statistically equivalent (p>0.05) in terms of encapsulation efficiency, average particle size and polydispersity index to the initial sample before freeze-drying (Figure 4e’). According to our DSC data (not shown), PEG1000 in the freeze-died cake is crystalline with a melting onset of 35.6 °C, melting peak of 38.1 °C and enthalpy of fusion equal to 103.2 J/g. It should be noted that lower molecular weights of PEG such as PEG600 are impractical for freeze-drying due to very low temperatures required for solidification which in turn prohibits adequate drying on account of low ice vapor pressure at such temperatures. Practically, PEG1000 is the lowest molecular weight that can be used to freeze-dry the samples.
The optimal PEG-assist procedure to obtain a freeze-dried cake that can produce drug-loaded PEG4kDa-b-PLA2kDa micelles containing PTX upon reconstitution has been schematically illustrated in Figure 5. One of the important advantages of using high molecular weight PEGs at elevated temperatures in PEG-assist over using low molecular weight liquid PEGs [39] is the abolition of the need to remove PEG before freeze-drying. Furthermore, no other excipient or lyoprotectant is needed for successful freeze-drying. In other words, high molecular weight PEG can play a dual role, both as the solvent and the lyoprotectant, in PEG-assist method. However, elevated temperatures needed for higher molecular weights can be detrimental to thermolabile payloads. The main drawback of PEG-assist method is therefore the application of heat which may cause thermal degradation of labile molecules such as proteins, nucleosides and nucleotides [56].
Figure 5.
Schematic illustration of PEG-assist method optimized for PEG4kDa-b-PLA2kDa to obtain freeze-dried samples.
We would like to highlight the fact that PEG-assist can be optimized for different molecular weights of homo PEG and PEG-b-PLA block copolymer. For a different combination, hydration should be performed near the solution saturation temperature and freeze-drying done at a temperature below the eutectic point.
Overall, PEG-assist is a very promising approach for large scale production of drug-loaded polymeric micelles as i) no organic solvent is needed for the fabrication step (not including the polymer synthesis); ii) the procedure is very simple, rapid and cost-effective; iii) the method yields high encapsulation efficiencies and uniform particle size distribution; iv) micelle characteristics can be controlled by altering the biphasic mixture through temperature and composition change; v) not only is PEG unnecessary to be removed, but it can also be employed as the lyoprotectant for freeze-drying.
PEG-assist commences a new approach for large scale production of polymeric micelles and nanoparticles. Due to the differences in manufacturing, the obtained formulations by PEG-assist differ from the currently developed formulations such as Genexol-PM® and BIND-014 in terms of physiochemical properties and formulation excipients. Genexol-PM® is a polymeric micellar formulation of paclitaxel approved in South Korea for the treatment of breast and non-small cell lung cancers [11, 57]. Genexol-PM® formulation is different from PEG-assist formulations in terms of molecular weight of the diblock copolymer used, particle size and the lyoprotectant. Genexol-PM® is composed of a low molecular weight block copolymer (PEG2kDa-b-PLA2kDa) which gives a smaller particle size (ca. 24 nm [58]). The applicability of PEG-assist to a wide range of molecular weights makes it a feasible method for fabrication of a wide range of formulations. Genexol-PM® is made at elevated temperatures using ethanol as the solvent followed by freeze-drying using lactose as the lyoprotectant [59]. BIND-014, on the other hand, is a high molecular weight PEGylated PLA nanoparticular formulation of docetaxel developed for the treatment of prostate cancer. BIND-014 is made using emulsion-based processes and has a particle size of ca. 100 nm [60]. In contrast to PEG-assist, the production of both Genexol-PM® and BIND-014 relies on the use organic solvent during formulation. It should be noted that despite avoiding the use of organic solvent during formulation by PEG-assist, organic solvents are still needed for synthesis of the formulation excipients such as PEG and the copolymer. To assess whether PEG-assist is more environmentally friendly compared to the existing methods of fabrication requires a detailed tecno-economic analysis which is beyond the scope of current study.
4. Conclusion
PEG-assist method was developed as a potentially scalable approach for the production of drug-loaded polymeric micelles. PEG was used as the solvent in the proposed fabrication method for PTX-loaded PEG4kDa-b-PLA2kDa micelles. The encapsulation efficiency was found to be directly correlated with the molecular weight of the PEG. Hydration at 40 °C as the approximate saturation temperature of 5.0 wt.% PEG4kDa-b-PLA2kDa in liquid PEG was realized to yield the highest encapsulation efficiency for all molecular weights of PEG. The optimal procedure was, therefore, established as the hydration of PEG1kDa/PTX/ PEG4kDa-b-PLA2kDa at 40 °C. Scaling the batch size to 25X using the optimal procedure had no significant effect (p>0.05) on encapsulation and particle size distribution. The formulation was freeze-dried successfully without any significant change (p>0.05) in encapsulation and particle size distribution. PEG1kDa played a dual role in the formulation, both as the solvent for drug and copolymer, and as the lyoprotectant for freeze-drying. PEG-assist needs no organic solvent, is rapid, simple and cost-effective, and gives high encapsulation efficiency for different drugs and copolymers.
Supplementary Material
Highlights.
PEG-assist method for fabrication of drug-loaded polymeric micelles is introduced.
The procedure for optimizing the fabrication temperature and PEG molecular weight is presented.
The approach is suggested as being proficient for large-scale production and freeze-drying without removing the PEG or adding any other excipient.
Acknowledgements
This research was supported by National Cancer Institute of the NIH under the award number R01-CA257837.
Footnotes
Declaration of competing interest
The authors disclose no conflict of interest.
References
- 1.Pillai G, Nanomedicines for cancer therapy: an update of fda approved and those under various stages of development. SOJ Pharm Pharm Sci 1 (2): 13. Nanomedicines for Cancer Therapy: An Update of FDA Approved and Those under Various Stages of Development, 2014. [Google Scholar]
- 2.Desai N, Challenges in development of nanoparticle-based therapeutics. The AAPS journal, 2012. 14(2): p. 282–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bader H, Ringsdorf H, and Schmidt B, Watersoluble polymers in medicine. Die Angewandte Makromolekulare Chemie: Applied Macromolecular Chemistry and Physics, 1984. 123(1): p. 457–485. [Google Scholar]
- 4.Jones M-C and Leroux J-C, Polymeric micelles–a new generation of colloidal drug carriers. European journal of pharmaceutics and biopharmaceutics, 1999. 48(2): p. 101–111. [DOI] [PubMed] [Google Scholar]
- 5.Zhu C, et al. , Co-delivery of siRNA and paclitaxel into cancer cells by biodegradable cationic micelles based on PDMAEMA–PCL–PDMAEMA triblock copolymers. Biomaterials, 2010. 31(8): p. 2408–2416. [DOI] [PubMed] [Google Scholar]
- 6.Yokoyama M, Polymeric micelles as a new drug carrier system and their required considerations for clinical trials. Expert opinion on drug delivery, 2010. 7(2): p. 145–158. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto T, et al. , What are determining factors for stable drug incorporation into polymeric micelle carriers? Consideration on physical and chemical characters of the micelle inner core. Journal of controlled release, 2007. 123(1): p. 11–18. [DOI] [PubMed] [Google Scholar]
- 8.Kabanov AV, Batrakova EV, and Alakhov VY, Pluronic® block copolymers for overcoming drug resistance in cancer. Advanced drug delivery reviews, 2002. 54(5): p. 759–779. [DOI] [PubMed] [Google Scholar]
- 9.Kabanov AV, Batrakova EV, and Alakhov VY, An essential relationship between ATP depletion and chemosensitizing activity of Pluronic® block copolymers. Journal of controlled release, 2003. 91(1–2): p. 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bae Y, et al. , Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: Polymeric micelles that are responsive to intracellular pH change. Angewandte Chemie, 2003. 115(38): p. 4788–4791. [DOI] [PubMed] [Google Scholar]
- 11.Lee KS, et al. , Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast cancer research and treatment, 2008. 108(2): p. 241–250. [DOI] [PubMed] [Google Scholar]
- 12.Kim T-Y, et al. , Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clinical cancer research, 2004. 10(11): p. 3708–3716. [DOI] [PubMed] [Google Scholar]
- 13.Wang Q, et al. , Targeted delivery of low-dose dexamethasone using PCL–PEG micelles for effective treatment of rheumatoid arthritis. Journal of Controlled Release, 2016. 230: p. 64–72. [DOI] [PubMed] [Google Scholar]
- 14.Xu M, et al. , PEG-detachable polymeric micelles self-assembled from amphiphilic copolymers for tumor-acidity-triggered drug delivery and controlled release. ACS applied materials & interfaces, 2019. 11(6): p. 5701–5713. [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, et al. , Rod-shaped micelles based on PHF-g-(PCL-PEG) with pH-triggered doxorubicin release and enhanced cellular uptake. Biomacromolecules, 2019. 20(3): p. 1167–1177. [DOI] [PubMed] [Google Scholar]
- 16.Maghak L and Larsen J, Intact or in Pieces? A Look at How Clinically Approved, Biodegradable Block Co-Polymers Affect Blood Components. ACS Biomaterials Science & Engineering, 2020. [DOI] [PubMed] [Google Scholar]
- 17.Lavasanifar A, Samuel J, and Kwon GS, Micelles self-assembled from poly (ethylene oxide)-block-poly (N-hexyl stearate L-aspartamide) by a solvent evaporation method: effect on the solubilization and haemolytic activity of amphotericin B. Journal of controlled release, 2001. 77(1–2): p. 155–160. [DOI] [PubMed] [Google Scholar]
- 18.Ma X, et al. , Esterase-activatable β-lapachone prodrug micelles for NQO1-targeted lung cancer therapy. Journal of Controlled Release, 2015. 200: p. 201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang L, et al. , Paclitaxel-loaded polymeric micelles based on poly (ε-caprolactone)-poly (ethylene glycol)-poly (ε-caprolactone) triblock copolymers: in vitro and in vivo evaluation. Nanomedicine: Nanotechnology, Biology and Medicine, 2012. 8(6): p. 925–934. [DOI] [PubMed] [Google Scholar]
- 20.Chaibundit C, et al. , Micellization and gelation of mixed copolymers P123 and F127 in aqueous solution. Langmuir, 2007. 23(18): p. 9229–9236. [DOI] [PubMed] [Google Scholar]
- 21.Tam YT, et al. , Stereocomplex prodrugs of oligo (lactic acid) n-gemcitabine in poly (ethylene glycol)-block-poly (D, L-lactic acid) micelles for improved physical stability and enhanced antitumor efficacy. ACS nano, 2018. 12(7): p. 7406–7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tam YT, Gao J, and Kwon GS, Oligo (lactic acid) n-paclitaxel prodrugs for poly (ethylene glycol)-block-poly (lactic acid) micelles: loading, release, and backbiting conversion for anticancer activity. Journal of the American Chemical Society, 2016. 138(28): p. 8674–8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fournier E, et al. , A novel one-step drug-loading procedure for water-soluble amphiphilic nanocarriers. Pharmaceutical research, 2004. 21(6): p. 962–968. [DOI] [PubMed] [Google Scholar]
- 24.Gaucher G, et al. , Block copolymer micelles: preparation, characterization and application in drug delivery. Journal of controlled release, 2005. 109(1–3): p. 169–188. [DOI] [PubMed] [Google Scholar]
- 25.Kohori F, et al. , Process design for efficient and controlled drug incorporation into polymeric micelle carrier systems. Journal of controlled release, 2002. 78(1–3): p. 155–163. [DOI] [PubMed] [Google Scholar]
- 26.Feng YH, et al. , How is a micelle formed from amphiphilic polymers in a dialysis process: Insight from mesoscopic studies. Chemical Physics Letters, 2020: p. 137711. [Google Scholar]
- 27.Lin S, et al. , Overcoming the anatomical and physiological barriers in topical eye surface medication using a peptide-decorated polymeric micelle. ACS applied materials & interfaces, 2019. 11(43): p. 39603–39612. [DOI] [PubMed] [Google Scholar]
- 28.Chu H, et al. , Morphology and in vitro release kinetics of drug-loaded micelles based on well-defined PMPC–b–PBMA copolymer. International journal of pharmaceutics, 2009. 371(1–2): p. 190–196. [DOI] [PubMed] [Google Scholar]
- 29.Sant VP, Smith D, and Leroux J-C, Enhancement of oral bioavailability of poorly water-soluble drugs by poly (ethylene glycol)-block-poly (alkyl acrylate-co-methacrylic acid) self-assemblies. Journal of Controlled Release, 2005. 104(2): p. 289–300. [DOI] [PubMed] [Google Scholar]
- 30.Kataoka K, et al. , Doxorubicin-loaded poly (ethylene glycol)–poly (β-benzyl-l-aspartate) copolymer micelles: their pharmaceutical characteristics and biological significance. Journal of Controlled Release, 2000. 64(1-3): p. 143–153. [DOI] [PubMed] [Google Scholar]
- 31.Kwon G, et al. , Block copolymer micelles for drug delivery: loading and release of doxorubicin. Journal of Controlled Release, 1997. 48(2–3): p. 195–201. [Google Scholar]
- 32.Jette KK, et al. , Preparation and drug loading of poly (ethylene glycol)-block-poly (ε-caprolactone) micelles through the evaporation of a cosolvent azeotrope. Pharmaceutical research, 2004. 21(7): p. 1184–1191. [DOI] [PubMed] [Google Scholar]
- 33.Lim Soo P and Eisenberg A, Preparation of block copolymer vesicles in solution. Journal of Polymer Science Part B: Polymer Physics, 2004. 42(6): p. 923–938. [Google Scholar]
- 34.Wang W, Zhang K, and Chen D, From Tunable DNA/Polymer Self-Assembly to Tailorable and Morphologically Pure Core–Shell Nanofibers. Langmuir, 2018. 34(50): p. 15350–15359. [DOI] [PubMed] [Google Scholar]
- 35.Payyappilly SS, et al. , Organic solvent-free low temperature method of preparation for self assembled amphiphilic poly (∈-caprolactone)–poly (ethylene glycol) block copolymer based nanocarriers for protein delivery. Colloids and Surfaces B: Biointerfaces, 2015. 135: p. 510–517. [DOI] [PubMed] [Google Scholar]
- 36.Grodowska K and Parczewski A, Organic solvents in the pharmaceutical industry. Acta Pol Pharm, 2010. 67(1): p. 3–12. [PubMed] [Google Scholar]
- 37.Guideline IHT, Impurities: Guideline for residual solvents Q3C (R5). Current Step, 2005. 4: p. 1–25. [Google Scholar]
- 38.Feng J, et al. , Translational formulation of nanoparticle therapeutics from laboratory discovery to clinical scale. Journal of translational medicine, 2019. 17(1): p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seo M-H, Yi Y-W, and Yu J-W, Method for the preparation of polymeric micelle via phase separation of block copolymer. 2009, Google Patents. [Google Scholar]
- 40.Oh KS, et al. , Paclitaxel-loaded Pluronic nanoparticles formed by a temperature-induced phase transition for cancer therapy. Journal of controlled release, 2010. 148(3): p. 344–350. [DOI] [PubMed] [Google Scholar]
- 41.D’souza AA and Shegokar R, Polyethylene glycol (PEG): a versatile polymer for pharmaceutical applications. Expert opinion on drug delivery, 2016. 13(9): p. 1257–1275. [DOI] [PubMed] [Google Scholar]
- 42.Topel Ö, et al. , Determination of critical micelle concentration of polybutadiene-block-poly (ethyleneoxide) diblock copolymer by fluorescence spectroscopy and dynamic light scattering. Journal of Molecular Liquids, 2013. 177: p. 40–43. [Google Scholar]
- 43.Tomoda K, et al. , Triolimus: A multi-drug loaded polymeric micelle containing paclitaxel, 17-AAG, and rapamycin as a novel radiosensitizer. Macromolecular bioscience, 2017. 17(1): p. 1600194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tam YT, et al. , Poly (ethylene glycol)-block-poly (d, l-lactic acid) micelles containing oligo (lactic acid) 8-paclitaxel prodrug: In Vivo conversion and antitumor efficacy. Journal of Controlled Release, 2019. 298: p. 186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Note MA, Surfactant Micelle Characterization using Dynamic Light Scattering. The Journal of Physical Chemistry B, 2006: p. 1–5. [Google Scholar]
- 46.Croy SR and Kwon GS, The effects of Pluronic block copolymers on the aggregation state of nystatin. Journal of controlled release, 2004. 95(2): p. 161–171. [DOI] [PubMed] [Google Scholar]
- 47.Sato T and Takahashi R, Competition between the micellization and the liquid–liquid phase separation in amphiphilic block copolymer solutions. Polymer Journal, 2017. 49(2): p. 273–277. [Google Scholar]
- 48.Ianiro A, et al. , Liquid–liquid phase separation during amphiphilic self-assembly. Nature chemistry, 2019. 11(4): p. 320–328. [DOI] [PubMed] [Google Scholar]
- 49.Repp L, et al. , Acyl and oligo(lactic acid) prodrugs for PEG-b-PLA and PEG-b-PCL nano-assemblies for injection. J Control Release, 2021. 330: p. 1004–1015. [DOI] [PubMed] [Google Scholar]
- 50.Forrest ML, et al. , Paclitaxel prodrugs with sustained release and high solubility in poly (ethylene glycol)-b-poly (ε-caprolactone) micelle nanocarriers: pharmacokinetic disposition, tolerability, and cytotoxicity. Pharmaceutical research, 2008. 25(1): p. 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ansell SM, et al. , Modulating the therapeutic activity of nanoparticle delivered paclitaxel by manipulating the hydrophobicity of prodrug conjugates. Journal of medicinal chemistry, 2008. 51(11): p. 3288–3296. [DOI] [PubMed] [Google Scholar]
- 52.Yang ZL, et al. , Amphotericin B-loaded poly (ethylene glycol)–poly (lactide) micelles: Preparation, freeze-drying, and in vitro release. Journal of Biomedical Materials Research Part A: An Official Journal of The Society for Biomaterials, The Japanese Society for Biomaterials, and The Australian Society for Biomaterials and the Korean Society for Biomaterials, 2008. 85(2): p. 539–546. [DOI] [PubMed] [Google Scholar]
- 53.Abdelwahed W, et al. , Freeze-drying of nanoparticles: formulation, process and storage considerations. Advanced drug delivery reviews, 2006. 58(15): p. 1688–1713. [DOI] [PubMed] [Google Scholar]
- 54.Kuttich B, et al. , X-ray scattering study on the crystalline and semi-crystalline structure of water/PEG mixtures in their eutectic phase diagram. Soft Matter, 2020. 16(45): p. 10260–10267. [DOI] [PubMed] [Google Scholar]
- 55.Kasper JC and Friess W, The freezing step in lyophilization: physico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopharmaceuticals. European journal of pharmaceutics and biopharmaceutics, 2011. 78(2): p. 248–263. [DOI] [PubMed] [Google Scholar]
- 56.Fang M, et al. , Thermal degradation of small molecules: a global metabolomic investigation. Analytical chemistry, 2015. 87(21): p. 10935–10941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim D-W, et al. , Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Annals of oncology, 2007. 18(12): p. 2009–2014. [DOI] [PubMed] [Google Scholar]
- 58.Werner ME, et al. , Preclinical evaluation of Genexol-PM, a nanoparticle formulation of paclitaxel, as a novel radiosensitizer for the treatment of non-small cell lung cancer. International Journal of Radiation Oncology* Biology* Physics, 2013. 86(3): p. 463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trieu V and Motamed K, Nanoparticle Formulations, Google Patents No. 2014, WO2014165829A2.
- 60.Sanna V, Pala N, and Sechi M, Targeted therapy using nanotechnology: focus on cancer. International journal of nanomedicine, 2014. 9: p. 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.