Abstract
Background
Rett syndrome is a rare, severe neurodevelopmental disorder. Almost all cases occur in girls, in association with spontaneous (non-inherited) mutations involving the methyl-CpG-binding protein 2 gene located on the X chromosome. Diagnostic criteria for typical Rett syndrome require a period of regression, followed by recovery or stabilization, and fulfillment of all four main criteria (loss of purposeful hand skills, loss of spoken language, gait abnormalities, and stereotypic hand movements). Our objective was to estimate the prevalence of Rett syndrome in the general population, stratified by sex.
Methods
We conducted a search of PubMed, Embase, Web of Science, Cochrane Library, LILACS, and LIVIVO to retrieve studies published in English between Jan. 1, 2000, and June 30, 2021. Pooled prevalence with a 95% confidence interval (CI) was estimated using a random-effects meta-analysis based on a generalized linear mixed model with a logit link.
Results
Ten eligible studies were identified (all in females), with a combined sample size of 9.57 million women and 673 Rett syndrome cases. The pooled prevalence estimate (random effects) was 7.1 per 100,000 females (95% CI: 4.8, 10.5, heterogeneity p < 0.001). Despite greatly variable precision of estimation, all estimates were compatible with a prevalence range of approximately 5 to 10 cases per 100,000 females based on their respective 95% CIs.
Conclusion
These findings may facilitate planning of therapeutic trials in this indication in terms of target sample size and accrual times.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13643-023-02169-6.
Keywords: Epidemiology, Incidence, MECP2, Meta-analysis, Neurodevelopmental disorders, Prevalence, Rare diseases, Rett syndrome (RTT), Systematic literature review
Background
Rett syndrome (RTT) is a rare, severe neurodevelopmental disorder that affects almost exclusively females. The syndrome was first described in 1966 by Andreas Rett in the German medical literature [1]. However, RTT was not internationally recognized until 1983 when Hagberg et al. described the first cases in the English language literature [2]. In 1999, Amir et al. [3] identified mutations on the methyl-CpG binding protein 2 (MECP2) gene, which is located on the Xq28 chromosome band and encodes MECP2 in RTT patients. Mutations in MECP2 have been detected in approximately 95–97% of typical RTT cases and 85% of atypical RTT [1]. In addition to patients with RTT, mutations have been identified in individuals who do not have the clinical features of RTT. Because MECP2 mutations are neither necessary nor sufficient to make the diagnosis of RTT, the disorder remains a clinical diagnosis [4].
Development appears normal during the first 6 to 18 months of life but is followed by regression of motor and language skills. The clinical phenotype of RTT is broad and can be classified into two main categories: typical (classic) RTT and atypical (variant) RTT. Diagnostic criteria for typical RTT require a period of regression, followed by recovery or stabilization, and fulfillment of all four main criteria (loss of purposeful hand skills, loss of spoken language, gait abnormalities, and stereotypic hand movements) [4]. In some cases, deceleration of head growth can be one of the first signs of RTT. Further manifestations can include seizures, autistic features, intermittent breathing abnormalities, autonomic nervous system dysfunction, cardiac abnormalities, and sleep disturbances. Atypical RTT encompasses variants of RTT that have many but not all of the clinical features of typical RTT. The three defined atypical RTT variants are preserved speech, early onset seizure, and congenital variants [2].
Kirby et al. examined the longevity of patients with RTT in a cohort study conducted in the USA and Canada (N = 1928 subjects) [5] and found that most RTT-related deaths occurred before the age of 25 years. The researchers reported an overall survival of approximately 78% at age 25 years.
No cure nor effective disease-modifying therapy currently exists for RTT. Several pharmacologic treatments, including glatiramer acetate, dextromethorphan, and trofinetide, have been investigated in small clinical trials. Modest benefits were reported for endpoints such as gait velocity, respiratory function, seizures, and certain cognitive and behavioral parameters [6–8]. Gene therapy, which is in the drug development phase, demonstrates promise [9, 10].
One of the rate-limiting factors in the development of new pharmacologic therapies for RTT is the low prevalence of the disease, which makes conducting large clinical trials for this indication difficult. To date, no meta-analyses have reported on the prevalence of RTT. One meta-analysis that focused on the prevalence of autism spectrum disorders (ASDs) reported that 61% of children with RTT have ASD [11]. The aim of this systematic review and meta-analysis is to review the current literature pertaining to RTT and to estimate the prevalence of RTT in the general population, stratified by sex. These results may facilitate planning of future clinical trials for this indication in terms of target sample size and accrual times.
Methods
Search strategy
We performed a systematic search of electronic databases (PubMed, Embase, Web of Science, Cochrane Library, LILACS, and LIVIVO). Search strategies combined relevant terms for the disease (Rett, MECP2) with those for the occurrence (prevalence, incidence, epidemiology) (see Supplement 1: Database Search Strategies). The search was limited to records published from 1 January 2000 to 30 June 2021. We established the date limit of 2000 for study inclusion because the association of RTT with the MECP2 mutation was recognized in the year 1999 [3]. While the MECP2 mutation is neither necessary nor sufficient for the diagnosis of RTT, the current diagnostic criteria [4] acknowledge that identification of this mutation may result in a diagnosis of “possible RTT,” which can be further revised to a definite RTT diagnosis when the clinical criteria are fulfilled. The search was limited to publications in the English language and to human patients, and no geographic restriction was applied.
Study selection
Original, peer-reviewed articles reporting the prevalence and/or incidence of RTT (or sufficient data to calculate them) in the general population within a defined geographical area were eligible for inclusion. Review articles, conference abstracts, or unpublished manuscripts were excluded. If there were studies reporting duplicate data, the study with the most up-to-date and complete data was included. The full inclusion and exclusion criteria are listed in Table 1.
Table 1.
Study inclusion and exclusion criteria
| Category | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Study design/publication type |
-Observational studies (prospective, retrospective, cross-sectional, surveillance studies) -Meta-analysis or systematic reviews (to check the reference lists) -Human studies |
-Randomized controlled trials (RCTs) -Narrative review -Conference abstracts, unpublished manuscripts, expert opinions, editorials, comments, letters to the editor -Animal studies -Genetic or molecular laboratory studies |
| Country | All countries/worldwide | No countries excluded |
| Study topic/subject | Diagnosed RTT syndrome | Other or unspecified conditions |
| Study population | General population (also stratified by, e.g., gender, age, sex, geographical area, etc.) | Other populations |
| Outcomes of interest | Prevalence/incidence/proportion of diagnosed RTT | Other outcomes |
RCTs Randomized controlled trials, RTT Rett syndrome
Two reviewers (UP and DCD) examined the titles and abstracts of the retrieved publications in duplicate, and the full texts of selected articles were subsequently screened in duplicate. Reference lists of the included articles as well as of the review articles were manually screened to check for additional relevant articles. All records were transferred into the EndNote reference manager, where duplicates were automatically removed. In both screening steps, we achieved concordance of more than 98%. Disagreements on eligibility were resolved by discussion with a third reviewer (SLL). The Preferred Reporting Items for Systematic Reviews and Meta-Analysis standards 2021 guidelines were followed (Supplemental Table 2) [12]. The study protocol was not preregistered.
Data extraction and quality assessment
Relevant data were extracted using a standardized data collection form and included information on study design, study population, data collection period, location, diagnostic criteria/definitions for RTT, and sources of case ascertainment (Table 2). Prevalence estimates of RTT or raw numbers were recorded. The quality of eligible studies was assessed using the MetaXL User Guide Version 5.3 [23] predefined criteria list and included population representativeness, catchment area, disease assessment, and statistical methods. A quality score, which ranged from 0 to 11, was estimated, with a greater score indicating a better study quality (Table 3).
Table 2.
Characteristics of the included studies
| Authors, year | Country | Study period | Study design/population | Age of study population, years | Diagnosis criteria for Rett syndrome | Cases |
|---|---|---|---|---|---|---|
| Bienvenu et al. [13] (2006) | France | Born 1989–2000 | National population-based: Rett registry | 4–15 | Clinical and genetic | Rett |
| Magnússon et al. [14] (2001) | Iceland | Born 1974–1993 | National population-based: registers of tertiary hospitals | 5–24 | Clinical, pediatrician, psychiatrist (ICD-10) | ASD with Rett |
| Strømme et al. [15] (2000) | Norway, Akershus | Born 1980–1985 | Population-based: multiple search strategies | NR | Clinical (ICD-10 [code F84.2]) | MR with Rett |
| Isaksen et al. [16] (2012) | Norway, Oppland, and Hedmark | Born 1996–2002 | Population-based: multiple sources like registries, schools, hospitals, and public health services | 6–12 | Clinical, pediatrician, neurologists (ICD-10 [code F84.2]) | ASD with Rett |
| Sarajlija et al. [17] (2015) | Serbia | 1981–2001 | National population-based: registers of Mother and Child Health Institute of Serbia | <18 | Clinical and genetic | Rett |
| Aguilera et al. [18] (2007) | Spain, Seville | 2002–2003 | Population-based: all schools in the area | 3–21 | Clinical, confirmed by schools (DSM-IV, ICD-10) | ASD with Rett |
| Fombonne et al. [19] (2003) | UK, England, Wales, Scotland | 1999 | Population-based: Sample of Child Benefit Register | 5–15 | Clinical, psychiatrist (DSM-IV, ICD-10) | PDD with Rett |
| Chakrabarti et al. [20] (2005) | UK, Midlands | Born 1996–1998 | Population-based: Child Health Surveillance | 4–6 | Clinical, psychiatrist (DSM-IV) | PDD with Rett |
| Fehr et al. [21] (2011) | Australia | Born 1976–2006 | National population-based: multiple sources | 5–32 | Clinical and genetic | Rett |
| Wong et al. [22] (2007) | China, Hong Kong West | 2006 | Population-based: tertiary hospital | <35 | Clinical and genetic (DSM-IV) | Rett |
ASD Autism spectrum disorders, DSM-IV Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, ICD-10 International Classification of Diseases, Tenth Revision, MR Mental retardation, NR Not reported, PDD Pervasive developmental disorder, UK United Kingdom
Table 3.
MetaXL quality assessment score
| Study | 1. Population and observation period | 2. Diagnostic criteria | 3. Methods of case ascertainment | 4. Administration of measurement protocol | 5. Catchment area | 6. Prevalence measure | Total (max: 11) |
|---|---|---|---|---|---|---|---|
| Aguilera et al. [18] (2007) | 1 | 1 | 2 | 3 | 2 | 2 | 11 |
| Bienvenu et al. [13] (2006) | 1 | 1 | 2 | 1 | 2 | 2 | 9 |
| Isaksen et al. [22] (2012) | 1 | 1 | 2 | 3 | 2 | 2 | 11 |
| Chakrabarti et al. [20] (2005) | 1 | 1 | 2 | 2 | 2 | 1 | 9 |
| Fombonne et al. [19] (2003) | 1 | 1 | 1 | 3 | 2 | 2 | 10 |
| Magnússon et al. [14] (2001) | 1 | 1 | 1 | 1 | 2 | 2 | 8 |
| Strømme et al. [15] (2000) | 1 | 1 | 2 | 3 | 2 | 2 | 11 |
| Sarajlija et al. [17] (2015) | 1 | 1 | 1 | 2 | 2 | 2 | 9 |
| Fehr et al. [21] (2011) | 1 | 1 | 2 | 2 | 2 | 2 | 10 |
| Wong et al. [22] (2007) | 1 | 1 | 2 | 2 | 2 | 2 | 10 |
1) Yes = 1, no = 0
2) Diagnostic system reported = 1. Own system/symptoms described/no system/not specified = 0
3) Community survey/multiple institutions = 2. Inpatient/inpatients and outpatients/case registers = 1. Not specified = 0
4) Administered interview = 3. Systematic case note review = 2. Chart diagnosis/case records = 1. Not specified = 0
5) Broadly representative (national or multi-site survey) = 2. Small area/not representative (single community, single university) = 1. Convenience sampling/other (primary care sample/treatment group) = 0
6) Point prevalence (e.g., 1-month prevalence = 2; 12-month prevalence = 1; lifetime prevalence = 0)
Data synthesis and meta-analysis
All studies that were included used a cross-sectional design and estimated the prevalence, which was defined as the number of existing RTT cases expressed relative to the population size, in a well-defined population at one specific point in time. Studies were included in the meta-analysis if they reported the number of cases and the sample denominator or sufficient information to calculate the prevalence. The random-effects estimate of the pooled prevalence with a 95% confidence interval (CI) was calculated based on the generalized linear mixed model (GLMM) with a logit link function [24]. This approach results in valid inference with common or rare outcomes [24]. A heterogeneity test p-value for the null hypothesis of equal study-specific prevalence parameters was derived from the GLMM model [24]. The I2 statistic was calculated as I2 = (Q − df)/Q, where Q = CINV (1 − p-value, df), CINV is the chi-square inverse, and df = (number of studies − 1) [25]. For a visual examination of heterogeneity, individual study-specific prevalence estimates with 95% CIs were displayed together with the pooled prevalence estimate in a forest plot [25]. The 95% CIs for the study-specific prevalence parameters were calculated based on the exact binomial method [26]. The pooled prevalence estimate was based on the random-effects model due to evidence of heterogeneity, and “a priori” low plausibility of the homogeneity hypothesis, considering that the prevalence of most medical conditions is known to vary geographically and over time. A funnel plot of the estimated prevalence versus the margin of error (half-length of the 95% CI) was constructed to examine the variability of the study-specific estimates as a function of their estimated precision. In the absence of substantial heterogeneity, more precise estimates (i.e., those with the smaller margin of error) are expected to have relatively little spread in the plot, while outliers, if present, are expected to have large error margins. Outliers with small error margins are evidence of heterogeneity. Unlike studies of the treatment effects, however, prevalence studies are neither “positive” nor “negative.” Hence, the funnel plot does not provide information on publication bias in prevalence studies. Similarly, while power analysis is sometimes recommended for meta-analyses of treatment effects, where the absence of the treatment effect constitutes a natural null hypothesis [27, 28], this is usually not applicable to prevalence studies, where the focus is on point and interval estimation of the average prevalence parameter, as in the present work. Meta-analysis was performed based on all eligible studies combined and by subgroups defined by the use of genetic testing in the studies. All analyses were performed in SAS 9.4. The analysis code is available as Supplement 3.
Results
Study selection and characteristics
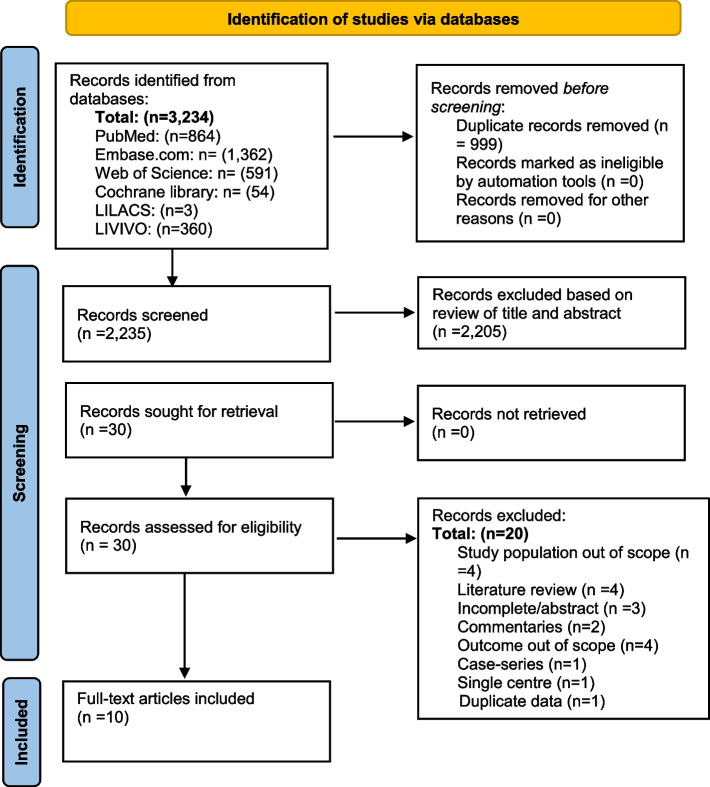
A total of 3234 articles were identified. After reviewing the titles and abstracts, 30 articles were selected for full-text evaluation. A review of the references of these studies identified one other article for inclusion. The review of the 30 full-text articles led to the selection of 10 studies that were considered relevant for the present review. These 10 studies were considered of sufficient quality, according to the MetaXL guidelines on assessing the study quality, and all 10 studies were included in the meta-analysis. A summary of the study quality assessment score is presented in Table 3. A summary of the article selection process is presented in Fig. 1.
Fig. 1.
PRISMA 2020 flow diagram for new systematic reviews. Searches of databases and registers only were included. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Four studies had an objective of estimating the prevalence of RTT: Bienvenu et al. from France, Sarajlija et al. from Serbia, Wong et al. from China, and Fehr et al. from Australia [13, 17, 21, 22]. Six studies had a broader diagnosis surveyed but presented stratifications of which RTT was a category [14–16, 18–20]. In our analyses, only the RTT information was included.
The sizes of the study populations ranged from 5227 to 4,337,627 and the majority of studies (five) included girls younger than age 18 years. Three of the studies included patients for which the age of the study population was 3 to 21 years [18], 5 to 24 years [14], or 5 to 32 years [21]. One other study observed patients younger than 35 years [22], while the age of the study population was not reported in one study [15]. All studies were population-based, and four were nationwide in their respective countries [13, 14, 17, 21]. Sampling methods differed between studies. For example, some studies reported multiple data sources (registers, schools, hospitals, and public health services) to ascertain RTT cases, whereas other studies used surveillance data or registers of tertiary hospitals. Table 2 provides characteristics of the included studies.
Meta-analysis
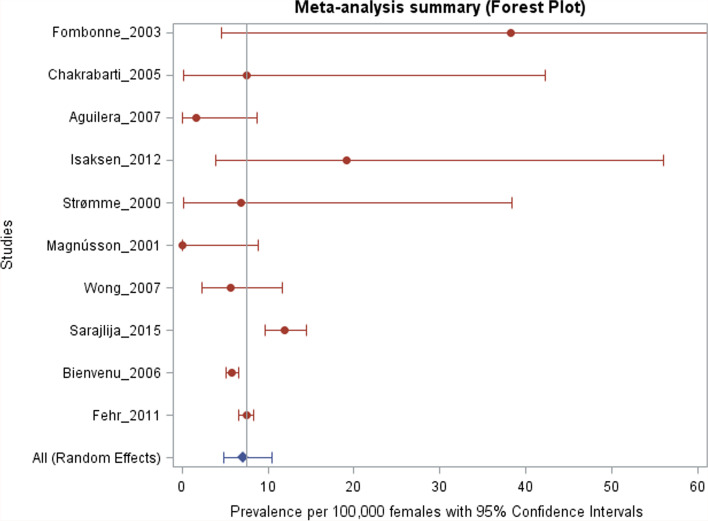
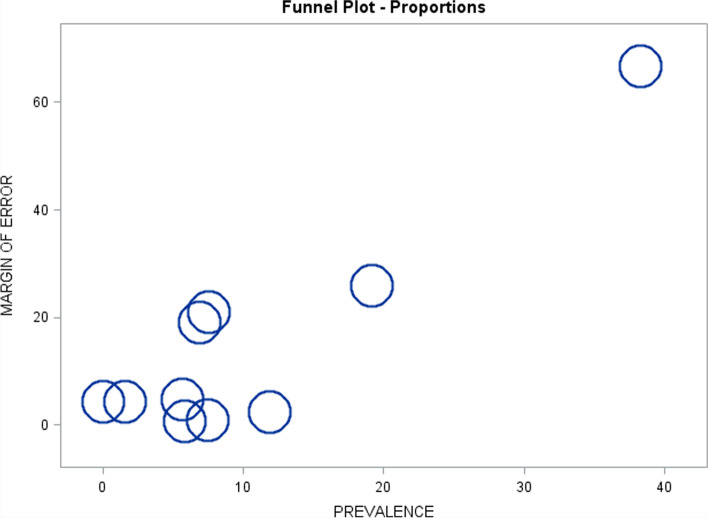
The meta-analysis is presented in Table 4, with forest and funnel plots presented in Figs. 2 and 3, respectively. Study-specific prevalence estimates per 100,000 females (95% CI) ranged from 0.0 (0.0, 8.8) to 38.3 (4.6, 138.0), with a highly significant heterogeneity test (p < 0.001, I2 = 0.831). However, much of this variability was due to a few imprecise estimates, such as Isaksen et al. [16], Fombonne et al. [19], Chakrabarti et al. [20], and Strømme et al. [15]. More precise prevalence estimates such as those reported by Fehr et al. [21], Bienvenu et al. [13], and Sarajlija et al. [17] were not highly variable (as observed in Figs. 2 and 3), and although some had non-overlapping 95% CIs (such that statistical evidence against the null hypothesis of equal prevalence parameters was rather strong), the magnitude of this variability was not great. The pooled prevalence estimate based on all eligible studies (random-effects model) was 7.1 cases per 100,000 females (95% CI: 4.8, 10.5). Pooled prevalence estimates within the two subgroups defined by the use of genetic testing in the studies were of similar magnitude and not significantly different from each other (p = 0.84), although the estimate in the first subgroup was much less precise than in the second due to the differences between the subgroups in the sample sizes and the case counts (Table 4). Interestingly, most estimates from the European region were of similar orders of magnitude as those from China and Australia. Despite greatly variable precision of estimation, all estimates in Table 4 are compatible with a prevalence range of approximately 5 to 10 cases per 100,000 females based on their respective 95% CIs. All studies had a quality score of eight points or greater.
Table 4.
Meta-analysis of Rett prevalence (per 100,000 females)
|
Case definition criteria Study |
Country |
Rett cases |
Female population |
Prevalence estimate |
95% LCL |
95% UCL |
|---|---|---|---|---|---|---|
| Clinical diagnosis onlya | ||||||
| Fombonne et al. (2003) [19] | UK | 2 | 5227 | 38.3 | 4.6 | 138.0 |
| Chakrabarti et al. (2005) [20] | UK | 1 | 13202 | 7.6 | 0.2 | 42.2 |
| Aguilera et al. (2007) [18] | Spain | 1 | 63675 | 1.6 | 0.0 | 8.7 |
| Isaksen et al. (2012) [16] | Norway | 3 | 15662 | 19.2 | 4.0 | 56.0 |
| Strømme et al. (2000) [15] | Norway | 1 | 14542 | 6.9 | 0.2 | 38.3 |
| Magnússon et al. (2001) [14] | Iceland | 0 | 41896 | 0.0 | 0.0 | 8.8 |
| Pooled prevalence | 8 | 154204 | 6.7 | 2.0 | 22.0 | |
| Clinical diagnosis + genetic testingb | ||||||
| Wong et al. (2007) [22] | China | 7 | 123968 | 5.6 | 2.3 | 11.6 |
| Sarajlija et al. (2015) [17] | Serbia | 102 | 857142 | 11.9 | 9.7 | 14.4 |
| Bienvenu et al. (2006) [13] | France | 251 | 4337627 | 5.8 | 5.1 | 6.5 |
| Fehr et al. (2011) [21] | Australia | 305 | 4094386 | 7.4 | 6.6 | 8.3 |
| Pooled prevalence | 665 | 9413123 | 7.6 | 5.4 | 10.8 | |
| All studiesc | 673 | 9567327 | 7.1 | 4.8 | 10.5 | |
| Pooled prevalence | ||||||
aGenetic testing not reported in the study publications (n = 6 studies, Q = 13.0; df = 5; heterogeneity p = 0.0231; I2 =0.616)
bGenetic testing reported in the study publications (n = 4 studies, Q = 36.6; df = 3; heterogeneity p<0.001; I2 = 0.918)
cAll eligible publications (n = 10 studies, Q = 53.3; df = 9; heterogeneity p<0.001; I2 = 0.831, subgroup difference p=0.84)
LCL Lower confidence limit, UCL Upper confidence limit
Fig. 2.
Forest plot demonstrating the study-specific prevalence of Rett syndrome estimates per 100,000 females (95% confidence interval)
Fig. 3.
Funnel plot demonstrating the estimated prevalence of Rett syndrome compared with margin of error (half-length of the 95% confidence interval) to examine the variability of the study-specific estimates as a function of their estimated precision
Discussion
This is the first systematic review and meta-analysis of RTT that reports pooled prevalence of RTT in the general female population. Our pooled prevalence estimate of 7.1 per 100,000 females (95% CI: 4.8, 10.5) is in line with the estimate reported on Orphanet (http://orpha.net; 10 per 100,000 live female births) [29], though the Orphanet estimate is limited by lack of a published description of its methods and data sources.
No studies that included patients with RTT older than 35 years were included. Studies report that, after reaching 25 years of age, adults with RTT have a mortality rate similar to the general population [5]. If that is indeed the case, the pooled prevalence estimates presented could be extrapolated to the general population. However, future studies should include patients of all ages to determine if the prevalence changes with age.
The strengths of this study were that the results encompassed the prevalence estimates from several nations and covered many different patient populations. Similar estimates were obtained for many different populations, and the true prevalence of RTT did not vary substantially from one region to another. To supplement the clinical criteria, some studies also used the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, International Classification of Disease, Tenth Revision, and genetic criteria.
The present meta-analysis has several limitations. The majority of the studies involved only females younger than age 24 years. No studies assessing the prevalence of all age groups have been completed. Subgroup information was not available from the original publications, so meta-analysis by subgroups could not be performed. Concerning the diagnostic criteria, the studies could have used different criteria given that these have changed in 1985, 2002, and 2010. Surprisingly, no studies from the USA were published during the review period (1 January 2000 to 30 June 2021). One study was identified, published in 1993 from a large population-based registry in Texas, in which the prevalence of classic RTT was estimated to be 4.4 per 100,000 females [30]. This is in line with what has been reported in the studies presented here (5 to 10 cases per 100,000 females). The protocol for this systematic review and meta-analysis was not preregistered on Prospective Register of Systematic Reviews (PROSPERO) or elsewhere, which is acknowledged as a limitation.
Conclusions
In summary, this is the first meta-analysis that estimates the prevalence of RTT. The results suggest that the prevalence remained stable for the last 20 years in the range of 5 to 10 cases per 100,000 females, without substantial regional variability. These findings may facilitate planning of therapeutic trials in this disease, especially for target sample size and accrual times.
Supplementary Information
Additional file 1: Supplement 1. Database search strategies.
Additional file 2: Supplemental Table 2. PRISMA checklist.
Acknowledgements
Editorial support was provided by Richard Barnett, of Kay Square Scientific, Newtown Square, PA. This support was funded by Novartis Gene Therapies, Inc.
Abbreviations
- ASD
Autism spectrum disorder
- CI
Confidence interval
- GLMM
Generalized linear mixed model
- MECP2
Methyl-CpG binding protein 2 gene
- PROSPERO
Prospective Register of Systematic Reviews
- RTT
Rett syndrome
- SAS
Statistical Analysis Systems
Authors’ contributions
Conceptualization of the work: SLL. Writing of the manuscript and literature review: UP, DCD, and SLL. Reviewing of the manuscript: UP, DCD, and SLL. Statistical analyses: SLL and ES. Designing and editing of the figures: ES. The authors read and approved the final manuscript.
Funding
Funding was provided by Novartis Gene Therapies, Inc.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
The manuscript does not contain clinical studies or patient data.
Consent for publication
Not applicable.
Competing interests
SLL and ES are employees of Novartis Pharmaceutical Company; the statements presented in the paper do not necessarily represent the position of the company. UP is an employee of Cognizant Technology Solutions working on behalf of Novartis; the statements presented in the paper do not necessarily represent the position of either company. DCD is a former employee of Novartis Pharmaceutical Company and a former employee of Rutgers University Ernest Mario School of Pharmacy. DCD is a current employee of Seqirus.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rett A. On an unusual brain atrophy syndrome in hyperammonemia in childhood. Wien Med Wochenschr. 1966;116:723–6. [PubMed] [Google Scholar]
- 2.Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: report of 35 cases. Ann Neurol. 1983;14:471–9. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- 3.Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–8. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 4.Neul JL, Kaufmann WE, Glaze DG, et al. Rett syndrome: revised diagnostic criteria and nomenclature. Ann Neurol. 2010;68:944–50. doi: 10.1002/ana.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirby RS, Lane JB, Childers J, et al. Longevity in Rett syndrome: analysis of the North American database. J Pediatr. 2010;156:135–8.e1. doi: 10.1016/j.jpeds.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Djukic A, Holtzer R, Shinnar S, et al. Pharmacologic treatment of Rett syndrome with glatiramer acetate. Pediatr Neurol. 2016;61:51–7. doi: 10.1016/j.pediatrneurol.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 7.Smith-Hicks CL, Gupta S, Ewen JB, et al. Randomized open-label trial of dextromethorphan in Rett syndrome. Neurology. 2017;89:1684–90. doi: 10.1212/WNL.0000000000004515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glaze DG, Neul JL, Kaufmann WE, et al. Rett 002 Study Group. Double-blind, randomized, placebo-controlled study of trofinetide in pediatric Rett syndrome. Neurology. 2019;92:e1912–25. doi: 10.1212/WNL.0000000000007316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bassuk AG. Gene therapy for Rett syndrome. Genes Brain Behav. 2022;21:e12754. doi: 10.1111/gbb.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collins BE, Merritt JK, Erickson KR, Neul JL. Safety and efficacy of genetic MECP2 supplementation in the R294X mouse model of Rett syndrome. Genes Brain Behav. 2022;21:e12739. doi: 10.1111/gbb.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richards C, Jones C, Groves L, Moss J, Oliver C. Prevalence of autism spectrum disorder phenomenology in genetic disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2015;2:909–16. doi: 10.1016/S2215-0366(15)00376-4. [DOI] [PubMed] [Google Scholar]
- 12.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18:e1003583. doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bienvenu T, Philippe C, De Roux N, et al. The incidence of Rett syndrome in France. Pediatr Neurol. 2006;34:372–5. doi: 10.1016/j.pediatrneurol.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Magnusson P, Saemundsen E. Prevalence of autism in Iceland. J Autism Dev Disord. 2001;31:153–63. doi: 10.1023/A:1010795014548. [DOI] [PubMed] [Google Scholar]
- 15.Strømme P, Diseth TH. Prevalence of psychiatric diagnoses in children with mental retardation: data from a population-based study. Dev Med Child Neurol. 2000;42:266–70. doi: 10.1017/S0012162200000451. [DOI] [PubMed] [Google Scholar]
- 16.Isaksen J, Diseth TH, Schjølberg S, Skjeldal OH. Observed prevalence of autism spectrum disorders in two Norwegian counties. Eur J Paediatr Neurol. 2012;16:592–8. doi: 10.1016/j.ejpn.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 17.Sarajlija A, Kisic-Tepavcevic D, Nikolic Z, et al. Epidemiology of Rett syndrome in Serbia: prevalence, incidence and survival. Neuroepidemiology. 2015;44:1–5. doi: 10.1159/000369494. [DOI] [PubMed] [Google Scholar]
- 18.Aguilera Jiménez A, Moreno Pérez FJ, Rodríguez Ortiz IR. Prevalence estimates of autism spectrum disorder in the school population of Seville. Spain. Br J Dev Disabil. 2007;53:97–109. doi: 10.1179/096979507799103405. [DOI] [Google Scholar]
- 19.Fombonne E, Simmons H, Ford T, Meltzer H, Goodman R. Prevalence of pervasive developmental disorders in the British nationwide survey of child mental health. Int Rev Psychiatry. 2003;15:158–65. doi: 10.1080/0954026021000046119. [DOI] [PubMed] [Google Scholar]
- 20.Chakrabarti S, Fombonne E. Pervasive developmental disorders in preschool children: confirmation of high prevalence. Am J Psychiatry. 2005;162:1133–41. doi: 10.1176/appi.ajp.162.6.1133. [DOI] [PubMed] [Google Scholar]
- 21.Fehr S, Bebbington A, Nassar N, et al. Trends in the diagnosis of Rett syndrome in Australia. Pediatr Res. 2011;70:313–9. doi: 10.1203/PDR.0b013e3182242461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong VC, Li SY. Rett syndrome: prevalence among Chinese and a comparison of MECP2 mutations of classic Rett syndrome with other neurodevelopmental disorders. J Child Neurol. 2007;22:1397–400. doi: 10.1177/0883073807307091. [DOI] [PubMed] [Google Scholar]
- 23.Barendregt JJ, Doi SA. MetaXL user guide version 5.3, 2016. http://www.epigear.com/index_files/. Accessed 21 Sept 2021.
- 24.Lin L, Chu H. Meta-analysis of proportions using generalized linear mixed models. Epidemiology. 2020;31:713–7. doi: 10.1097/EDE.0000000000001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 26.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–13. doi: 10.1093/biomet/26.4.404. [DOI] [Google Scholar]
- 27.Jackson D, Turner R. Power analysis for random-effects meta-analysis. Res Syn Meth. 2017;8:290–302. doi: 10.1002/jrsm.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valentine JC, Pigott TD, Rothstein HR. How many studies do you need? A primer on statistical power for meta-analysis. J Educ Behav Stat. 2010;35(2):215–247. doi: 10.3102/1076998609346961. [DOI] [Google Scholar]
- 29.Orphanet. Rett syndrome 2021. https://www.orpha.net/consor/cgi-bin/Disease_Search.php?lng=EN&data_id=91&Disease_Disease_Search_diseaseGroup=rett-syndrome&Disease_Disease_Search_diseaseType=Pat&Disease(s)/group%20of%20diseases=Rett-syndrome&title=Rett%20syndrome&search=Disease_Search_Simple. Accessed 21 Sept 2021.
- 30.Kozinetz CA, Skender ML, MacNaughton N, et al. Epidemiology of Rett syndrome: a population-based registry. Pediatrics. 1993;91:445. doi: 10.1542/peds.91.2.445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplement 1. Database search strategies.
Additional file 2: Supplemental Table 2. PRISMA checklist.
Data Availability Statement
Not applicable.