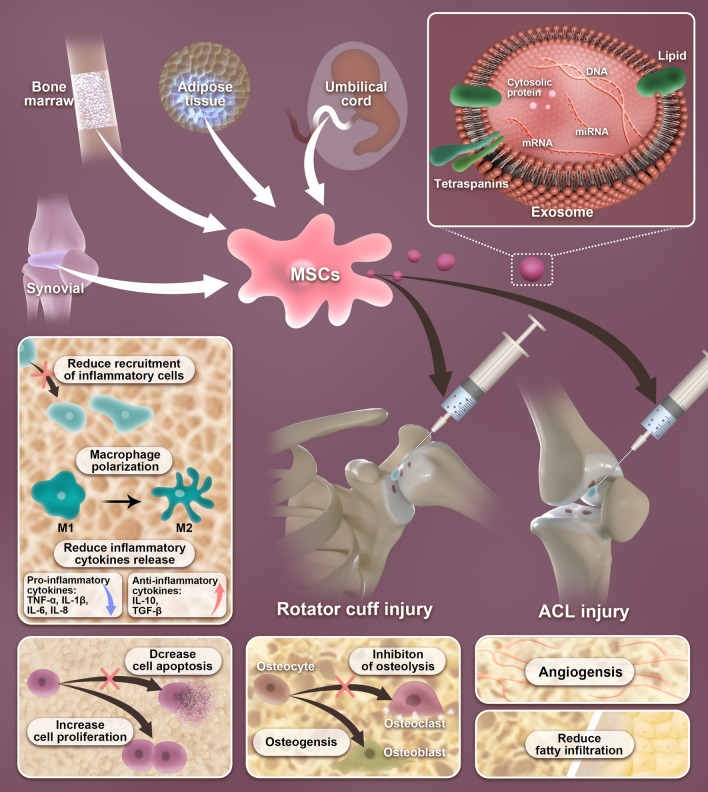
Abstract
Tendon–bone insertion (TBI) injuries, such as anterior cruciate ligament injury and rotator cuff injury, are the most common soft tissue injuries. In most situations, surgical tendon/ligament reconstruction is necessary for treating such injuries. However, a significant number of cases failed because healing of the enthesis occurs through scar tissue formation rather than the regeneration of transitional tissue. In recent years, the therapeutic potential of mesenchymal stem cells (MSCs) has been well documented in animal and clinical studies, such as chronic paraplegia, non-ischemic heart failure, and osteoarthritis of the knee. MSCs are multipotent stem cells, which have self-renewability and the ability to differentiate into a wide variety of cells such as chondrocytes, osteoblasts, and adipocytes. Numerous studies have suggested that MSCs could promote angiogenesis and cell proliferation, reduce inflammation, and produce a large number of bioactive molecules involved in the repair. These effects are likely mediated by the paracrine mechanisms of MSCs, particularly through the release of exosomes. Exosomes, nano-sized extracellular vesicles (EVs) with a lipid bilayer and a membrane structure, are naturally released by various cell types. They play an essential role in intercellular communication by transferring bioactive lipids, proteins, and nucleic acids, such as mRNAs and miRNAs, between cells to influence the physiological and pathological processes of recipient cells. Exosomes have been shown to facilitate tissue repair and regeneration. Herein, we discuss the prospective applications of MSC-derived exosomes in TBI injuries. We also review the roles of MSC–EVs and the underlying mechanisms of their effects on promoting tendon–bone healing. At last, we discuss the present challenges and future research directions.
Graphical Abstract
Keywords: Tendon–bone healing, Mesenchymal stem cells, Exosomes, Drug delivery, Nanocarriers, Biomaterials, Nanomedicine
Introduction
Tendon injuries are common musculoskeletal system disorders in orthopedic clinical practices that can cause excruciating pain and disability. Such injuries mostly occur during sports and routine daily activities [1]. The anterior cruciate ligament (ACL) and rotator cuff (RC) are two of the most commonly injured soft tissue structures. The interface where tendons and ligaments attach to the skeleton is referred to as the enthesis, which is the site of stress concentration in the region. Therefore, ACL and RC are prone to overuse injuries [2]. The incidence rate of RC injuries is very high in musculoskeletal diseases. Statistical data show that over 270,000 surgeries are performed annually in the United States to repair torn RC tendons [3]. In addition, approximately 200,000 patients per annum suffer from ACL injuries [4].
Clinically, surgical tendon/ligament reconstruction is required for treating RC and ACL enthesis injuries in most situations, which is accomplished by a tendon graft inserted into the bone tunnel to establish tendon-to-bone healing. However, the recurrent tear rate after the surgical intervention is high; for example, the re-tear rate for repaired RC was estimated to be as high as 94%, while the average ACL reconstruction failure rate was 11.7% [5, 6]. Sufficient tendon graft–bone healing depends on the degree of the bone ingrowth into the graft, and the regeneration of fibrocartilage zones is critical for satisfactory clinical outcomes of these injuries [7, 8]. The tendon–bone insertion (TBI), the transition between the soft tendinous tissue and the hard bony tissue, heals slowly as a result of hypovascularity, especially in the fibrocartilage zone. Thus, fibrocartilage healing occurs through scar tissue rich in type I collagen formation after an injury [5, 9, 10]. The fibrovascular scar with low biomechanical properties results in an inferior interface and increases the risk of injuries again because scar tissue lacks the native tendon–bone interface gradient structure and the alignment of collagen fiber [11]. To reduce the re-tear rate and achieve satisfactory surgical results, various approaches, such as using different suturing ways, interference screws, tissue engineering, and growth factors, have been applied to promote tendon–bone healing in animal models and patients. However, their efficacy is limited due to hypocellularity and poor vascularization of the tendon [12]. It is especially difficult for TBI regeneration when compared to bone-to-bone healing [13]. Consequently, fibrocartilage transition regeneration is currently limited and imposes a formidable challenge in TBI repair.
Recently, increasing studies have focused on mesenchymal stem cells (MSCs), which are multipotent stem cells with self-renewal and multilineage differentiation potential. Their use has been reported to be a promising therapeutic strategy for tissue regeneration in regenerative medicine [14]. Stem cells are found in nearly all adult tissues. MSCs with different origins have been reported to be used for promoting tendon-to-bone junction repair [15–21]. However, the transplantation of MSCs also has risks that may cause immune rejection, bear the potential of tumorigenicity, and result in cancer drug resistance [22]. Therefore, the further development of stem cell therapy encounters great difficulties. Recently, a large body of evidence has shown that the positive effects of stem cell therapy are likely mediated by the paracrine mechanisms of MSCs, particularly by secreting exosomes [9, 23]. Mesenchymal stem cell-derived exosomes (MSC-exos), nano-sized extracellular vesicles, have been identified as emerging nano-scale acellular therapeutic agents for tissue regeneration. They have been employed in cutaneous wound healing, ischemic brain injury, renal injuries, osteoarthritis, fracture healing, and degenerative bone disease in recent years [24–28]. Thus, cell-free therapy is anticipated to provide a new perspective for researchers in promoting tendon–bone healing.
Clinically, traditional conservative management or operative treatment is difficult to reconstruct anatomic continuity after TBI injuries. Hence, a better understanding of the mechanisms of exosomes has important implications for the design of novel therapeutic scenarios for TBI injuries. In this paper, we review the potential role of MSC-exos and the mechanisms underlying these actions in the improvement of tendon–bone healing. In addition, we discuss the possible application of MSC-exos for promoting of tendon–bone healing. Finally, the current challenges and future research directions of MSC-exos-based strategies for improving tendon–bone healing are proposed.
Tendon–bone healing
Physical structure of a tendon–bone insertion
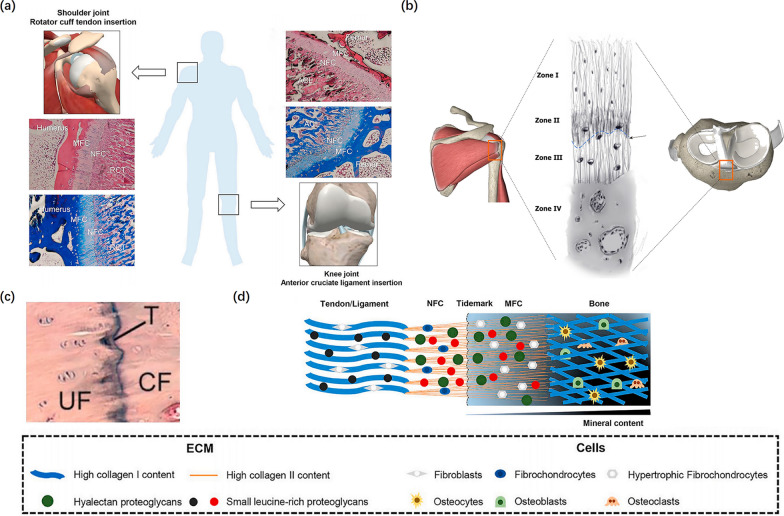
The musculoskeletal system functions through the coordination of multiple types of connective tissues. Among them, a highly specialized and organized tissue, called tendon–bone insertion, incorporates tendon/ligament to bone [29]. This complex transition site from hard bone to soft tendon/ligament provides a strong supporting strength to promote joint movement [30]. The intact healthy enthesis effectively facilitates the progressive transfer of complex mechanical loads through its gradient structure between the two distinct, inhomogeneous tissues: tendon/ligament and bone [31]. TBIs can be divided into two types, namely, indirect and direct insertions. For example, the medial collateral ligament runs parallel to the bone and inserts into the tibial through indirect insertions. Indirect insertion sites attach to the metaphysis and diaphysis of bone by connecting the superficial layers of the tendon/ligament to the periosteum, supported by specialized collagen fibers, called fibers, which provide anchorage between the bone and tendon/ligament. In contrast, direct insertions, such as the insertion of the ACL and RC, insert onto a bone by a gradient structure consisting of four distinct yet continuous layers in the sequence from soft to hard tissues, i.e., tendon, unmineralized fibrocartilage, mineralized fibrocartilage, and bone (Fig. 1) [5, 31–33]. The increase of tissue stiffness along the insertion site from tendon/ligament to bone may be regulated by the alignment of collagen fiber and a progressive increase in mineralization [34]. The fibrocartilage zone plays a key role in absorbing stress concentration between the tendon and bone. The demineralized region withstands excessive pressure or strain within the distal tendon, while the mineralized region protects the bone from excessive shear stress [31].
Fig. 1.
Physical Structure of tendon-bone insertion. a The histological staining of tendon/ligament-to-bone insertion (H&E, and Masson staining). Reproduced with permission [46]. b Zone I consists of the ligament. Zone II comprises nonmineralized fibrocartilage. Zone III is composed of mineralized cartilage. Zone IV consists of bone. Tidemark between Zone II and Zone III (black arrow) is shown. Reproduced with permission [7]. c The tidemark stained with H&E. Reproduced with permission [46]. d The schematic of tendon/ligament-to-bone insertion. RCT indicates rotator cuff tendon; ACL indicates anterior cruciate ligament; NFC indicates non-mineralized fibrocartilage; MFC indicates mineralized fibrocartilage; UF indicates uncalcified fibrocartilage; CF indicates calcified fibrocartilage; T indicates tidemark; ECM indicates extracellular matrix. Reproduced with permission [46]
Healing processes of tendon–bone insertion
Histologically, the native insertion site is a complex layered structure, which ensures its unique function, but once the tendon–bone junctions are injured, restoration of the anatomic structures is difficult. Based on current experimentation on animals, scholars have elucidated the chronological and morphological changes in TBI [35–37]. During surgical reconstruction, because the graft is placed and pulled through bone tunnels, the transitional tissue of the native direct insertion is not regenerated. Instead, the graft heals with the formation of fibrovascular scar tissue at the graft–tunnel interface [38]. The fibrovascular tissue is disorganized at the beginning. Subsequently, the formation of perpendicular fibers resembling the fibers of an indirect insertion begins 3 to 4 weeks after graft placement [39]. In the end, gradual bone ingrowth into this interface tissue 1 year after the operation promotes the graft attachment strength and integrates the graft into the surrounding bone [38]. Studies have demonstrated that the scar tissue exhibits weaker mechanical strength owing to the disorganized collagen fibers and the lack of the gradient of mineral distribution, leading to the increase in re-tear rate [40, 41]. Currently, there is no strategy to enhance the anatomic regeneration of the enthesis. Therefore, enhancing the functional and biological recovery of TBI is significant and urgent in regenerative medicine.
The healing process of TBI is divided into four different phases: inflammation phase, proliferation phase, remodeling phase, and maturation phase [42]. For the inflammatory phase, macrophages and neutrophils were among the first to be recruited in the healing of TBI and clearing tissue debris, which was eventually followed by fibroblast infiltration and the deposition of collagen type III, leading to the formation of the fibrovascular scar tissue at the interface between the graft and the bone tunnel [43]. During the proliferation phase, the cytokines and growth factors released by interface cells stimulate the migration, proliferation, and differentiation of stem cells, contributing to revascularization [44]. Next, during the remodeling phase, these healing cells synthesize and deposit a new extracellular matrix with bone ingrowth into the graft. Gradually, the new bone, fibrocartilage, and Sharpey's fibers are formed, which establish continuity of collagen fibers between the tendon graft and the bone [45]. Finally, cellularity and vascularity at the interface gradually decrease, and the collagen fibers are mostly parallel to each other in the maturation phase, indicating gradual restoration of the biomechanical strength of the interface.
Current biological treatments for promoting tendon–bone healing
Most cases of tendon/ligament rupture and enthesis injuries require surgical interventions. However, insufficient osteointegration after surgical reconstruction is one of the leading causes resulting in unsatisfactory clinical outcomes [47]. In 1993, Rodeo et al. reported that the mechanical strength of the TBI was positively associated with the degree of bone ingrowth into the graft, mineralization, and tissue maturation at the interface [36]. Over the past decade, researchers have studied biological treatments meticulously for accelerating and promoting tendon–bone healing in basic science studies of orthopedics. In recent years, using growth factors/cytokines [48], platelet-rich plasma [49], physical therapies [50], cell-based therapies [51], tissue engineering [52], and a range of delivery and inducement approaches have been applied and developed boomingly.
Currently, a variety of growth factors/cytokines, including transforming growth factor-β (TGF-β), bone morphogenetic protein (BMP), and granulocyte colony-stimulating factor (G-CSF), have been applied to promote tendon graft healing within a bone tunnel in various animal model studies. For instance, improving the integration of the transplanted tendon grafts in the bone tunnel with bone morphogenetic protein-7 (BMP-7) treatment after reconstruction of the ACL has been reported in sheep models [48]. However, there are still a few limitations to the clinical application of these methods. Most importantly, these biological factors are very difficult to retain in the tendon/ligament injury site and would flow away or undergo rapid clearance [6].
Cell-based therapies using stem cells exhibit the potential to become an effective treatment alternative in tissue engineering of TBI. MSCs are a stem cell population, which have the ability to self-renew and differentiate into a wide variety of cell types, including adipocytes, osteoblasts, and chondrocytes, while showing low immunogenicity upon transplantation. In addition, MSCs are capable of secreting a large number of bioactive molecules to enhance soft and hard tissue repair and regeneration [53]. In the field of tendon–bone healing, the most commonly used stem cells have been isolated from bone marrow (BMSCs) or adipose tissue (ASCs). However, several recent studies have shown that MSCs derived from the periosteum, synovium, and tendon have been widely used for improving enthesis regeneration at the tendon–bone healing site [54–56]. Despite the promising outcomes of using cellular therapies, they have a few limitations. For instance, there is no uniform standard for the dosage and frequency of stem cell therapy to accomplish the optimal effect, which is a certain obstacle to the clinical application of stem cells [7]. Besides, the potential risks of causing tumor formation and other adverse effects may occur after the administration of stem cells [57].
In brief, these limitations have necessitated the exploration of a novel therapeutic method to meet clinical requirements and enhance tendon–bone healing.
Therapeutic potential of MSCs’ secretory products in tendon–bone healing
In recent years, a series of studies have shown that the positive effects of stem cell therapy may not be restricted to cellular regeneration alone, but they are also mediated by a large number of bioactive molecules secreted by the paracrine action of MSCs. Stem cells can secrete growth factors, cytokines, and extracellular vesicles that can modulate the molecular component of the local environment to elicit responses from resident cells, presenting antiapoptotic, angiogenic, anti-scarring, immunomodulatory, and chemoattractant functions [58]. In regenerative medicine, secretory products of stem cells, such as secretomes and exosomes, have been tested for tendon–bone healing. Recent research has demonstrated that the efficacy of MSC-derived exosome therapies is comparable to those of stem-cell-based therapies without the potentially harmful effects of entire cell transplantations [59].
Secretome/conditioned medium (CM)
Secretome or CM, a medium where stem cells are cultured, contains various bioactive molecules secreted by living cells [60]. When the local microenvironment is altered, the MSCs released secretome or CM into the culture media [61]. The MSCs’ secretome or CM is made up of soluble proteins, lipids, nucleic acid, extracellular vesicles, and abundant cytokines/growth factors, such as vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), TGF, and epidermal growth factor (EGF) [62, 63]. Secretome or CM plays a pivotal role in tissue regeneration, promoting cell proliferation, migration, and differentiation [64]. Recently, several studies have suggested the beneficial effects of secretome or CM on tendon–bone healing. Sevivas et al. demonstrated that in vitro tendon cell viability and density and in vivo biochemical and histological properties of TBI were increased significantly in the CM-treated group compared with the control group [65]. Sun et al. demonstrated that intra-articular injection of a CM of human bone marrow-derived stem cells (hBMSC-CM) could promote tendon graft–bone healing in a rat model of ACL reconstruction [66]. Chen et al. found that hBMSC-CM could enhance tendon–bone healing by regulating macrophage polarization in a rat model of RC repair [32]. In light of this, we believe that MSCs’ secretome/CM is a promising candidate clinically for tendon-bone healing. However, the exact mechanism that which MSCs’ secretome/CM has the potential to be used as a therapeutic tool has not been elucidated.
Exosomes
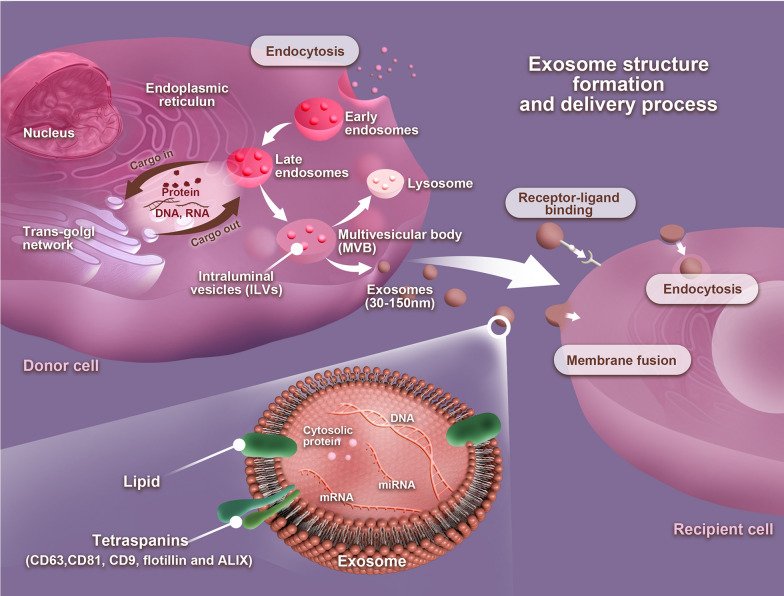
Exosomes are a class of extracellular vesicles with a diameter ranging around 30–150 nm [67]. Exosomes are nano-sized particles with a lipid bilayer and a membrane structure that are naturally released by various cell types under both physiological and pathological conditions by cytosolic exocytosis [68]. The formation of exosomes can be divided into three different stages. Stage 1 is when the cell’s limiting membrane invaginates to form early endosomes and then gradually forms late endosomes. Stage 2 involves the inward budding of late endosomal membranes that form a multivesicular body (MVB). Stage 3 involves the fusion of an MVB with the plasma membrane that contributes to the release of late endosomal contents, i.e., intraluminal vesicles (ILVs) with a variety of information into the extracellular space to form exosomes [69, 70]. In the extracellular space, exosomes can be taken up by recipient cells via three patterns: receptor–ligand binding, endocytosis, or membrane fusion (Fig. 2) [71]. Exosomes have been widely found in cell cultures and various body fluids, and several studies have proved that exosomes play an important role in intercellular communication by transferring bioactive lipids, proteins, and nucleic acids, such as DNAs, mRNAs, miRNAs, and lncRNAs between cells to influence physiological and pathological processes of recipient cells, for instance, improving ischemic brain injury, accelerated cutaneous wound healing by enhancing angiogenesis, and therapeutic potential in osteoarthritis [26, 72, 73]. As a key secretion product of MSCs, MSC-exos are shown to have similar regenerative properties to parental MSCs. Consequently, MSC-exos have a very high potential for diagnostic or prognostic biomarkers, drug delivery systems, as well as vehicles for gene therapy in clinical applications.
Fig. 2.
Exosome structure, formation, and delivery process. The plasma membrane invaginates to form early endosomes and then gradually forms late endosomes. Multivesicular bodies (MVBs) form by inward budding of the late endosomal limiting membrane. The MVB can either fuse with lysosomes or autophagosomes to be degraded or fuse with the plasma membrane to the release of late endosomal contents, i.e., intraluminal vesicles (ILVs) with a variety of information into the extracellular space to form exosomes. In the extracellular space, exosomes can be taken up by recipient cells via three patterns: receptor–ligand binding, endocytosis, or membrane fusion
Current progress in the application of exosomes to TBI injuries
Exosome isolation and identification
In studies of exosomes and their practical usage, the extraction and identification methods of exosomes are the research bases. First, they must be separated and purified from both biological fluids and in vitro cell cultures. The choice of exosome isolation techniques noticeably affects the exosome yield. Selection and modification of isolation techniques should vary according to the size, shape, density, and surface proteins of exosomes isolated from the biological fluid or cell culture. In this part, we briefly discuss the principles and methods of several commonly used exosome separation approaches and then summarize their respective advantages and disadvantages (Table 1) [74–79]. Additionally, the tissue source for parental MSCs for exosome studies has been isolated from a variety of tissues including bone marrow, umbilical cord, adipose tissue, embryonic stem cells (ESCs), or induced pluripotent stem cells (iPSCs). To date, the most common isolation strategy for purity exosomes from conditioned media has been ultracentrifugation and precipitation methods [80]. Unfortunately, these approaches suffer from many disadvantages, such as potential damage to exosomes and the co-precipitation of other non-exosomal contaminants like proteins [77]. Due to the limitations of current conventional methods, it is challenging to obtain the high yield, high purity, and better function of exosomes, especially MSC-exos, as the number of exosomes produced by MSCs is relatively small compared with other types of cells or body fluids. Moreover, there are homogeneity issues. Therefore, further research and development on the biological properties and extraction technologies of exosomes are urgently needed to improve and optimize the large-scale production of exosomal formulations that could meet their clinical application.
Table 1.
Comparison of different exosome isolation methods
| Exosome-based features | Isolation techniques | Equipment requirements | Time | Specificity | Pros and cons | Refs. |
|---|---|---|---|---|---|---|
| Density | Ultracentrifugation | Normal | Long | Yes/no | Standard gold method, low cost but the potential damage to exosomes | [76, 79, 83] |
| Size | Size-exclusion chromatography | High | Short | No | High concentration but low sample utilization | [77, 83] |
| Surface proteins | Polymer precipitation | Low | Short | No | High concentration but low purity and low sample utilization | [76, 78] |
| Immunoaffinity capture | Low | Short | Yes | High concentration but low purity | [75, 79, 83] | |
| Microfluidics isolation | High | Short | Yes | High purity but expensive | [74, 83] |
Next, it is also essential to identify the extracted exosomes for their biomedical application. According to morphological characteristics, size, and marker proteins, the identification approaches of exosomes currently primarily include nanoparticle tracking analysis, scanning electron microscopy or transmission electron microscopy observation, and marker protein expression detection as western blot analysis and flow cytometry [81, 82].
Exosomes as nanocarriers for drug delivery system
Exosomes can be secreted by a variety of cell types, travel throughout the body by the circulatory system, and naturally cross the biological barriers and other tissues, which play an important role in intercellular communication by delivering miRNAs, mRNAs, proteins, and other bioactive substances into recipient cells [84]. At present, the native cargo delivery of exosomes has been developed as various drug delivery carriers for disease treatment, which has the advantages of no cytotoxicity, low immunogenicity, high stability in circulation, and good biocompatibility compared with cell-based therapies [85]. The cell-targeting functions of exosomes potentiate their role as drug delivery nanoplatforms. Recently, numerous studies on exosomes that act as nanocarriers have focused on miRNA molecules (Table 2). miRNAs are a class of small non-coding RNA molecules. Exosomes contain various miRNAs, which have been increasingly recognized as one of the most important regulators in disease (such as inflammation and fibrosis) and the biological processes of tissue repair and regeneration, involving angiogenesis, cellular proliferation, and differentiation by regulating the gene expression of recipient cells through translational or post-transcriptional repression [86, 87]. The key role of miRNAs in mediating exosomes to facilitate tendon–bone healing has been proven. In a seminal study, Cui et al. reported that macrophage-derived miR-21-5p-containing exosomes induced peritendinous fibrosis after tendon injury by directly targeting Smad7 in a rat model [86]. Interestingly, it has recently been found that exosomal miR-21-5p could promote fibrogenesis and enhance tendon-bone integration by reducing SMAD7 expression levels in a rat model of ACLR [88]. Furthermore, Feng et al. discovered that exosomes derived from genetically modified Scleraxis-overexpressing PDGFRα(+) BMMSCs delivered miR-6924–5p to prevent osteoclast formation during tendon–bone healing and enhance the biomechanical strength of the TBI [89]. A recent study conducted by Wu et al. found that exosomes derived from low-intensity pulsed ultrasound stimulation (LIPUS)-preconditioned bone marrow mesenchymal stem cells (LIPUS-BMSC-Exos) could improve fibrocartilage regeneration at the tendon–bone interface and decrease supraspinatus fatty infiltration in a murine RC repair model by promoting BMSC chondrogenesis and anti-adipogenesis, which was primarily by delivering miR-140 [90]. Similarly, Z. Li et al. demonstrated that BMSC-derived exosomes (BMSC-Exos) overexpressing miR-23a-3p could promote M1 macrophage to M2 macrophage polarization, reduce the early inflammatory reaction at the tendon–bone interface, and promote early healing after ACL reconstruction [91]. However, currently, most data on miRNA-mediated mechanisms of tendon–bone healing are based on the use of unpackaged miRNAs, while little is known about the effects of miRNAs existing as cargo of MSC-exos, which hinders the development of exos-derived miRNAs into clinical practices. Thus, further exploration of the mechanisms of targeted delivery of exosomal miRNAs is required, which could offer a therapeutic strategy for tendon–bone healing. Additionally, which miRNA can perform the function of exosomes should be identified. However, a recent study has shown that MSC-derived exosomes may play a functional role due to their proteins rather than miRNAs. The study demonstrated that BMSC-Exos of polyaspartic acid polylactic acid-glycolic acid (PASP-PLGA) microcapsules could facilitate tendon–bone healing by delivering bone morphogenetic protein-2 (BMP-2), which belongs to the family of transforming growth factors, and polylactic acid (PLA) [92]. This is a new point of view, like miRNAs in MSC-derived exosomes, the MSC-derived exosomes proteome plays a pivotal role in many biological processes and has the potential to modulate various biological functions related to disease pathogenesis, tissue repair, and regeneration, which may provide a new direction for researchers in enhancing tendon–bone healing.
Table 2.
Summary of exosomal miRNAs involved in tendon-bone healing
| miRNAs | Stem cell source | Models | Biological functions in tendon–bone healing | Refs. |
|---|---|---|---|---|
| miR-21-5p | Magnetically actuated human BMSCs | A rat model of ACLR | Significantly prevented peri-tunnel bone loss, promoted more osseous ingrowth into the tendon graft, increased fibrocartilage formation at the tendon-bone tunnel interface, promoted ECM production and tissue fibrosis in tendon-bone healing | [88] |
| miR-23a-3p | Genetically modified Scleraxis-overexpressing PDGFRα(+) BMSCs | A mouse model of the anterior cruciate ligament tendon-bone healing | Dramatically reduced osteoclast formation and improved tendon-bone healing strength | [89] |
| miR-140 | Low-intensity pulsed ultrasound stimulation-preconditioned mice BMSCs | A murine rotator cuff repair model | Promoted tendon-bone interface fibrocartilage regeneration and ameliorated supraspinatus fatty infiltration | [90] |
| miR-23a-3p | Rat BMSCs | A rat model of ACLR | Promoted M1 macrophage to M2 macrophage polarization, reduced the early inflammatory reaction at the tendon-bone interface and promoted early healing after ACLR | [91] |
Furthermore, the crux to using exosomes as drug delivery carriers for enhancing tendon-bone healing is the effective loading of the small therapeutic molecules (siRNAs, miRNAs, mRNA, and even proteins) into exosomes. Currently, there are several methods to load drug moieties into exosomes, including sonication, incubation, transfection, electroporation, extrusion, freeze–thaw cycles, surface treatments, hypotonic dialysis, and the pH gradient method [93]. The electroporation method is the gold standard, however, it influences exosome membrane stability and integrity, which reduces drug loading efficiency [94]. Another commonly used method is to incubate exosomes with the cargo for a period of time at a specific temperature. The method is simple to operate, inexpensive, and has been widely applied. But the packaging rate is relatively low [95]. These methods have their advantages and drawbacks. Thus, in-depth research on drug loading approaches will significantly promote the therapeutic effect on TBI injuries.
Exosomes combined with biomaterials as therapeutics
MSC-exos can be used directly, alone, or in combination with biomaterials to facilitate targeting and therapeutic efficacy. Typically, the application of exosomes involves the direct injection of exosome aqueous solution into the circulation system or body cavities to enhance tissue repair [12]. However, it may not be feasible to directly inject exosomes into the injury region as the free exosomes in an aqueous solution are difficult to retain in the targeted area, resulting in flowing away or undergoing rapid clearance, which contributes to exosomes being unable to exert their full biological function [96]. Therefore, a vehicle of exosomes is needed for injury repair, and it should have the properties of the controlled release of exosomes in a dose- and time-dependent manner, no adverse effects for internalization of exosomes, and a suitable degradation rate [97]. Research has evidenced that exosomes and biomaterials can be combined to promote efficacy. Currently, the most commonly used biomaterials are the following: degradable implants, tissue-derived materials, and growth factors. Furthermore, varieties of biomaterial scaffolds have been explored to deliver bioactive factors and cellular products in a controlled manner. Ideal scaffold biomaterials should have the following characteristics in interface tissue engineering to be able to promote the regeneration of the TBI: (1) the ability to reproduce the specific structure of the TBI as much as possible, including matrix composition, microstructure, and mechanical properties; (2) the scaffold can support the adhesion, proliferation, and differentiation of specific phenotypes of different types of stem or progenitor cells; and (3) the scaffold should be degradable, and the degradation rate should be comparable to the rate of tissue regeneration, which can continue to maintain the physiological load [10]. Recent studies have suggested that biomaterial scaffolds have been effectively used in tendon tissue engineering to serve as delivery vehicles for cells and growth factors, offer proper structural support, and even biochemical cues to enhance the healing and mechanical properties of the repaired tendons, indicating the possibilities of synchronous exosome delivery and tendon tissue engineering [98, 99]. In addition, hydrogels are polymer networks with high water content, which is a biologically derived and FDA-approved product with similar properties to the extracellular matrix, including excellent biocompatibility, biodegradability, and beneficial characteristics for cell infiltration and adhesion. It has been widely used in various scientific studies and clinical practices [100]. There is research that shows bioactive molecules combined with hydrogels are able to retain their structure and function for a longer period, compared to their hydrogel-free administration [101]. Recently, hydrogels have been effectively used in TBI injuries as a delivery carrier of exosomes (Fig. 3). Interestingly, it not only offers a good microenvironment for exosome storage and their gradual absorption but also delivers a great number of exosomes to the target site [97, 101]. Huang et al. found that BMSC-Exos combined with hydrogels to sustain the release of exosomes could facilitate the formation of fibrocartilage in a mouse tendon–bone construction model [102]. Similarly, in a rat RC model, Fu et al. reported that ASC-Exo loaded with hydrogels markedly enhanced osteogenic tenogenic and chondrogenic differentiation of tendon stem cells [103]. Consistent with these findings, a recent study has indicated that infrapatellar fat pad (IPFP) MSC–derived exosomes loaded in a sodium alginate hydrogel promoted tendon–bone healing and intra-articular graft remodeling in a rat ACL construction model [104]. This may provide a new therapeutic option for the application of exosomes.
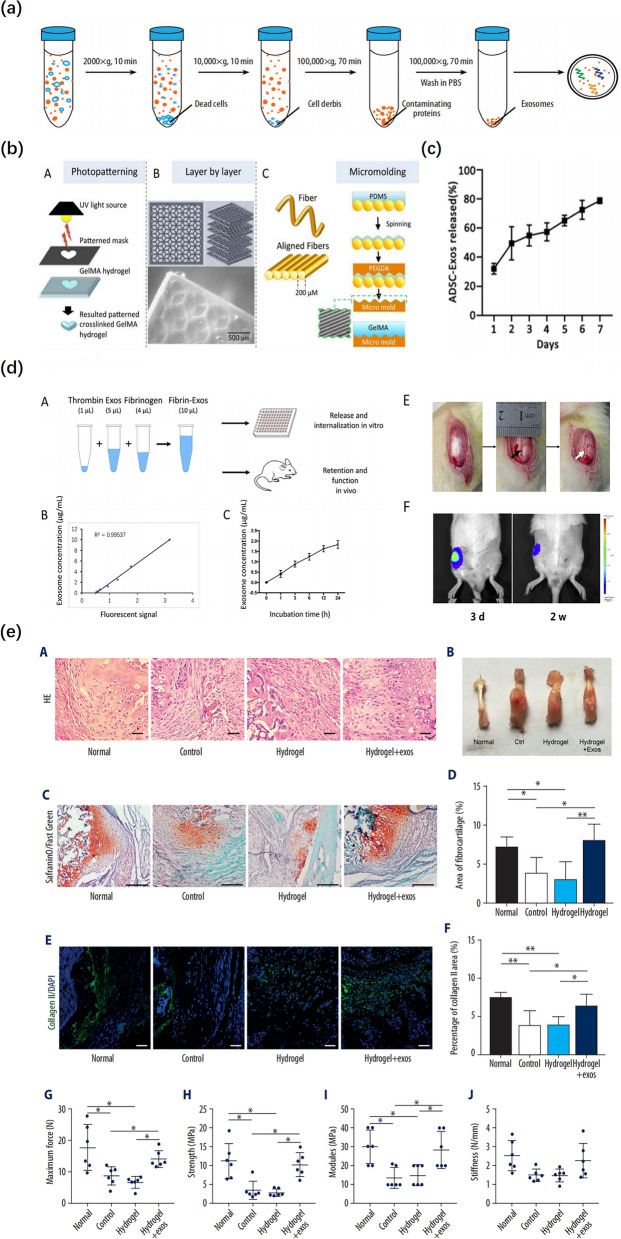
Fig. 3.
Exosomes combined with hydrogel promoted tendon-bone healing. a Schematic diagram of the isolation of exosomes. Reproduced with permission [105]. b Schematic representation of photopatterning of GelMA using a pre-patterned photomask. Stacked layers of patterned GelMA hydrogels fabricated using a micro-mirror projection stereolithography system. Schematic representation of a fiber-assisted micromolding technique for the production of parallel microgrooved surfaces that serve as a template for micropatterning GelMA. Reproduced with permission [106]. c Profile of ADSC-Exos released from the GelMA. Reproduced with permission [12]. d Release in vitro and retention in vivo of the BMSCs-exos embedded in fibrin-exo. The black arrow indicates the patellar window defect; The white arrow indicates the implanted fibrin-exo/fibrin-vesicle. Reproduced with permission [97]. e BMSC-Exos combined with hydrogel promoted fibrocartilage regeneration and enhanced the biomechanical properties in tendon-bone healing (H&E, safranin O-fast green, DAPI, and collagen type II staining). Reproduced with permission [105]
Tendon–bone healing is a highly complex process. Thus, further exploration is required into the application of exosomes in tendon–bone healing to acquire a better understanding of the possible implications of exosomes for future therapies. In addition, whether exosomes need to be used for a fixed period remains to be further studied.
Mechanisms of MSC-exos on tendon–bone healing
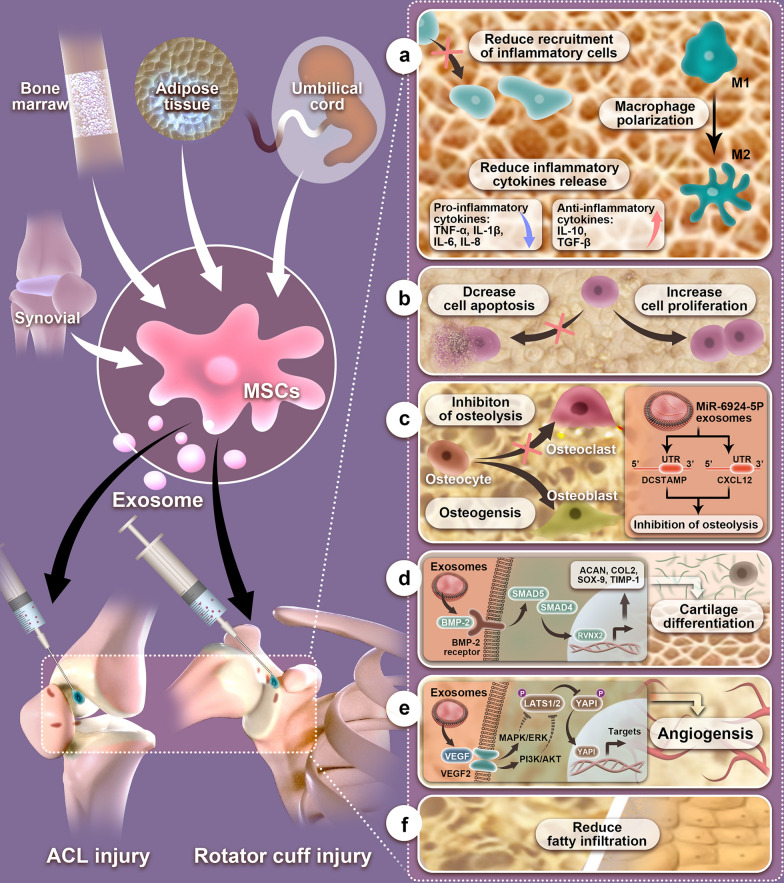
Tendon–bone healing is a complex repair and healing process due to various reasons. First, the fibrocartilage zone has relative avascularity at the injury site, leading to slow or nonunion of the TBI; Second, once this structure is damaged, as the injury heals, fibrovascular scar tissue forms in the TBI in general, rather than through the regeneration of the fibrocartilage junction, which results in poor biomechanical properties. Third, osteointegration typically recovers slowly, leading to impairing the pullout strength and stiffness of the TBI [107–109]. Recent research has revealed the potential therapeutic benefits of using MSC-exos for TBI injuries. A better understanding of the mechanisms underlying the actions of MSC-exos on the promotion of tendon–bone healing would facilitate the development of MSC-exos as a new therapeutic strategy for the treatment of TBI injuries. The mechanisms of MSC-exos for improving tendon–bone healing are primarily ascribed to three aspects: (1) the anti-inflammatory effects of MSC-exos can reduce the infiltration of inflammatory cells into the TBI, such as macrophages, and induce macrophage polarization from the M1 phenotype to the M2 phenotype, which can reduce the pro-inflammatory cytokines, decrease cell apoptosis, increase cell proliferation, decrease the formation of fibrovascular scar tissue, and enhance the regeneration of fibrocartilaginous enthesis; (2) the angiogenesis effects of MSC-exos can provide sufficient vascular invasion around the TBI, which is essential for promoting tendon–bone healing; and (3) the osteogenesis and inhibitory osteolysis effects of MSC-exos can promote the osteointegration of the tendon graft into the bone tunnel surface, which will obtain a promising tendon–bone healing outcome in the long run. The major mechanisms that explain the rationale behind using exosomes for TBI injuries are depicted in Fig. 4.
Fig. 4.
Mechanisms of exosomes on TBI injuries. a Exosomes could attenuate early inflammatory response via reducing inflammatory, polarizing macrophage from a pro-inflammatory M1 phenotype to a pro-regenerative M2 phenotype, and reducing inflammatory cytokines release. b Exosomes could enhance cell proliferation and reduce apoptosis. c Exosomes rich in miR-6924-5p could directly inhibit osteoclast formation by binding to the 3′-untranslated regions (3′ UTRs) of OCSTAMP and CXCL12. d Exosome-delivered BMP-2 promotes cartilage differentiation via Smad/RUNX2 signaling pathway. Activation of BMP-2/Smad signaling induces Runx2 expression through the activation of Smad4 and Smad5, after which Runx2 induces the expression of cartilage differentiation-related proteins, Aggrecan, Collagen II, SOX-9, and TIMP-1. e Exosomes promote angiogenesis by activating the VEGF and Hippo signaling pathways. When VEGF binds to the vascular endothelial growth factor receptor (VEGFR), the VEGF‐VEGFR interaction inhibits LATS1/2 and YAP1 phosphorylation by activating the PI3K and MAPK signaling pathways. The leads to increased expression of YAP1 in the nucleus inducing angiogenesis. f Exosomes could reduce fatty infiltration
Table 3 summarizes the major mechanisms studying the therapeutic effects of MSC-exos on tendon–bone healing.
Table 3.
MSC-exos mediated mechanisms that enhance tendon-bone healing
| Animal model | Exosomes (source/dosage/frequency) | Time point | Results | The underlying mechanisms | Refs. |
|---|---|---|---|---|---|
| Mice model of the anterior cruciate ligament tendon-bone healing | Genetically modified Scleraxis-overexpressing PDGFRα( +) BMSC-derived exosomes/ particles/once | 1 week, 2, and 3 weeks after surgery | Local injection of -exos or miR-6924–5p dramatically reduced osteoclast formation and improved tendon-bone healing strength; inhibition of miR-6924–5p expression reversed the prevention of osteoclastogenic differentiation by -exos; delivery of miR-6924–5p efficiently inhibited the osteoclastogenesis of human monocytes | Exosomes rich in miR-6924–5p could directly inhibit osteoclast formation by binding to the 3′-untranslated regions (3′ UTRs) of OCSTAMP and CXCL12 | [89] |
| Rat model of rotator cuff reconstruction | Rat BMSC-derived exosomes/200 μg/once | 4, and 8 weeks after surgery | BMSC-Exos increased the breaking load and stiffness of the rotator cuff after reconstruction in rats, induced angiogenesis around the rotator cuff endpoint, and promoted the growth of the tendon-bone interface | Promotion of angiogenesis through the VEGF and Hippo signaling pathways; inhibition of inflammation by inhibiting M1 macrophage polarization and M1 macrophage secretion of pro-inflammatory factors | [102] |
| Rat model of rotator cuff injury | Human ADSC-derived exosomes/25 μl of ADSC-Exos (0.3 mg/ml) mixed with 75 μl hydrogel/once | 4, and 8 weeks after surgery | The hydrogel with ADSC-exos significantly improved the osteogenic and adipogenesis differentiation and improved the biomechanical RCT healing | Upregulation of osteogenic markers (RUNX2), cartilage markers (Sox-9), and tenogenesis genes (TNC, TNMD, and Scx) | [103] |
| Rat model of the anterior cruciate ligament construction | Infrapatellar fat pad (IPFP) MSC–derived exosomes/0.1 ml of IPFP MSC–derived exosomes ( particles/mL) mixed with sodium alginate hydrogel (SAH)/once | 2, 4, and 8 weeks after surgery | IPFP MSC–derived exosomes group showed significantly higher biomechanical properties, a thinner graft-to-bone healing interface with more fibrocartilage, greater new bone ingrowth, and significantly fewer proinflammatory M1 macrophages and larger numbers of reparative M2 macrophages than in the other groups | Decreased M1 expression and increased M2 expression by the immunomodulation of macrophage polarization | [104] |
| Mice model of Achilles tendon-bone reconstruction | Mice BMSC-derived exosomes/dosage not reported/once | 7 days, 14 days, and 1 month after surgery | At 1 month after surgery, there was more fibrocartilage in the hydrogel + BMSC-Exos group than in the other groups. The biomechanical properties of the tendon-bone junction were significantly promoted in the hydrogel + BMSC-Exos group | Decreased the M1 macrophages, the proinflammatory factors (IL-1b and IL-6) in local tissues and cell apoptosis, increased cell proliferation, reduced ECM deposition, and suppressed excessive scar formation by regulation of the transition of macrophages from M1 to M2 | [105] |
| Rabbit model of chronic rotator cuff tears | Human ASC-derived exosomes/dosage not reported/once | 6 and 18 weeks after injury | At the end of week 18, ASC-Exos group showed significantly lower fatty infiltration, a higher histological score with more newly regenerated fibrocartilage, and greater biomechanical properties than the saline group | May reduce the infiltration of inflammatory cells (such as macrophages) into the tendon-bone interface, which can decrease the formation of fibrous scar tissue and enhance the regeneration of the normal tendon-bone insertion site | [119] |
Regulation of macrophage polarization
When the graft is transplanted into bone tunnels, the earliest cellular response is the accumulation of various inflammatory cells [110]. In particular, macrophages are recruited at the graft–tunnel interface (Fig. 5a) [43]. An increasing number of studies have demonstrated that macrophages play a key role in the onset and progression of TBI injuries (Fig. 5b). Chamberlain et al. indicated that nonspecific inhibition of macrophages was detrimental to early matrix formation and ligament strength [111]. Likewise, Hays et al. reported that macrophage depletion with clodronate liposomes following ACL reconstruction resulted in significantly improved morphological and biomechanical properties at the healing tendon–bone interface, perhaps due to decreased expression of macrophage-induced TGF-β [112]. Excessive numbers of macrophages or M1 polarization can enhance cell apoptosis, reduce cell proliferation, and induce fibroblasts to secrete excessive extracellular matrix resulting in peritendinous fibrosis and the formation of scar tissue [105, 113]. Persistent inflammation leads to the release of various inflammatory mediators that impede the regeneration of fibrocartilaginous enthesis and the intra-articular graft remodeling process [114, 115].
Fig. 5.
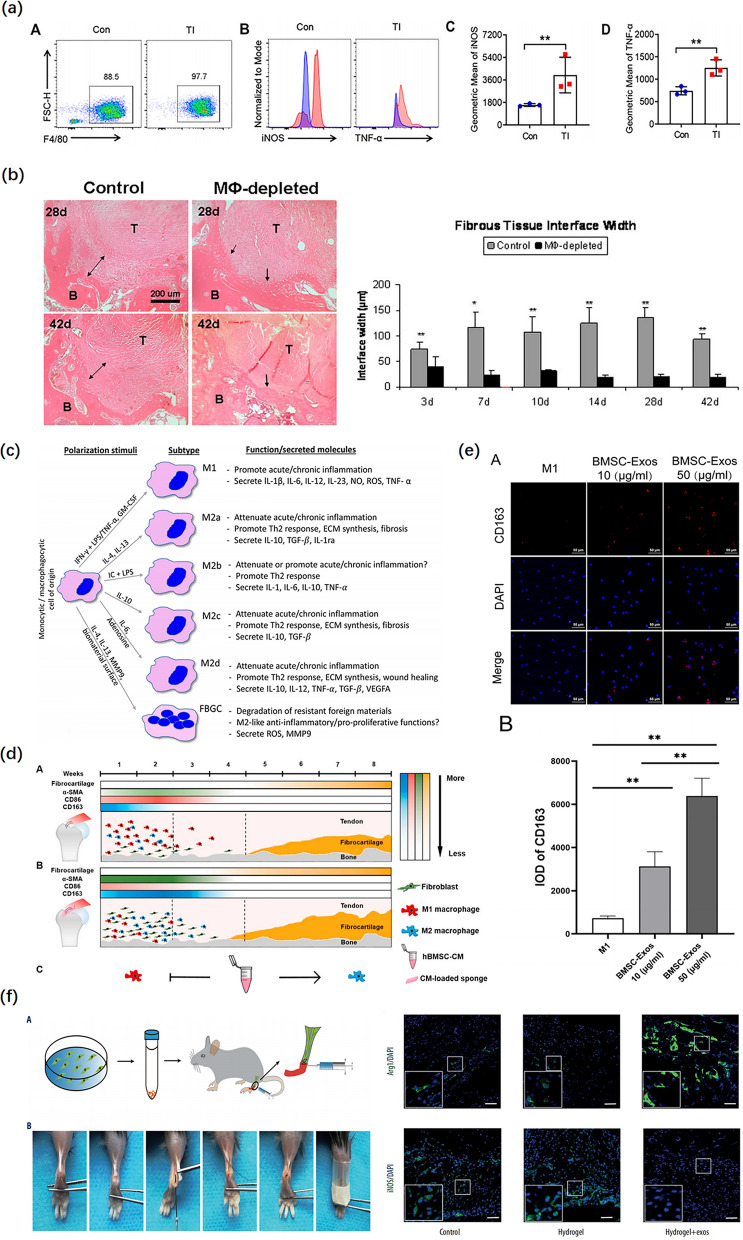
The effect of exosomes on the regulation of the transition of macrophages from M1 to M2 during tendon-bone healing. a An Increase in the pro-inflammatory phenotype of macrophages after a mouse tendon-bone injury (Flow cytometric analysis). Reproduced with permission [122]. b At all time points, the width of the fibrous tissue interface was significantly reduced following macrophage depletion (H&E staining). Reproduced with permission [112]. c Classification of macrophages by their inducing stimulus. Reproduced with permission [118]. d Brief summary of tendon-bone healing after rotator cuff repair in rats. CM indicates conditioned medium. Reproduced with permission [32]. e The BMSC-Exos polarized macrophages from the M1 phenotype into M2 (DAPI and CD163 staining). Reproduced with permission [91]. f The mouse tendon-bone reconstruction model and surgical procedure. BMSC-Exos induce M2 macrophage polarization during tendon-bone healing (DAPI, Arg1, and iNOS staining). Reproduced with permission [105]
Macrophages in areas of inflammatory tissue are derived from monocytes in the bloodstream, which are a heterogeneous group of cells having different phenotypes and functions. Activated macrophages can be categorized into two major phenotypes: first, the M1 phenotype, classically activated macrophages phenotype, participates in cellular debris clearance and host defense and accelerates the development of inflammation by secreting a large number of pro-inflammatory cytokines; second, the M2 phenotype, alternatively activated macrophage phenotype, is antiparasitic and can exert anti-inflammatory functions by releasing anti-inflammatory cytokines (such as IL-10 and TGF-β) and promote tissue regeneration and repair (Fig. 5c) [116, 117]. In the tendon–bone healing process, extensive M1 macrophage accumulation is an early event at the reconstructed place [43]. In addition, M1 macrophages can effectively polarize into M2 macrophages during the successful integration of tissue and biomaterial [118]. Consequently, regulation of the transition of macrophages from M1 to M2 attenuates the macrophage inflammatory reaction, which plays a key role in improving tendon–bone healing.
Numerous studies have shown that MSC-exos can promote tendon–bone healing by polarizing macrophages from a pro-inflammatory M1 phenotype to an anti-inflammatory M2 phenotype to reduce the inflammatory response after TBI injuries (Fig. 5d). In this regard, Wang et al. found that local injection of adipose stem cell-derived exosomes (ASC-Exos) could reduce fatty infiltration, enhance tendon–bone healing, and promote biomechanical properties in a rabbit model of a chronic RC tear, possibly due to the anti-inflammatory effects of ASC-Exos resulting in decreasing the infiltration of inflammatory cells (such as macrophages) into TBI [119]. To elucidate mechanisms underlying exosomes, Shi et al. demonstrated that the local administration of BMSC-Exos can induce M2 macrophages polarization to reduce the expression of pro-inflammatory cytokines, such as IL-1β and IL-6, as well as stimulate the expression of anti-inflammatory cytokines, such as IL-10, TGF-β, and IGF, which contributed to promoting fibrocartilage regeneration at the tendon–bone interface and improving biomechanical properties (Fig. 5f) [105]. Similarly, Huang et al. discovered that BMSC-Exos promoted tendon–bone healing after RC reconstruction in rats by inhibiting M1 macrophage polarization and M1 macrophage secretion of pro-inflammatory cytokines to suppress inflammation [102]. Consistent with these findings, a recent study has shown that IPFP MSC–derived exosomes accelerated tendon–bone healing and intra-articular graft remodeling in a rat model of ACL construction, which may have resulted from the immunomodulation of macrophage polarization, leading to decreasing M1 infiltration while promoting M2 infiltration [104]. In general, such features of exosomes make them promising tools for attenuating early inflammatory response, which is the prior condition for successful tissue healing processes [120]. However, the precise mechanism by which MSC-exos can regulate M2 macrophage polarization and function in TBI injuries has not been fully elucidated and requires further investigation.
In recent years, a growing number of studies have found that exosomes can change the polarization phenotype of macrophages by stimulating the nuclear factor-κB (NF-κB) signaling pathway. NF-κB is a key transcription factor in macrophages that regulates macrophage polarization. In a seminal study, Shen et al. demonstrated that ASC-Exos loaded onto a collagen sheet attenuated the early tendon inflammatory response, which directly targeted macrophages and subsequently reduced NF‐κB activity and downstream Il1b expression in injured tendons [121]. More interestingly, a recent study has confirmed that miR-23a-3p in BMSC-Exos enhanced the polarization of M2 macrophages and reduced the inflammatory response at the tendon–bone interface by targeting the inhibition of the IRF1 and NF-κB pathway in macrophages to promote tendon–bone healing after ACL reconstruction (Fig. 5e) [91]. Moreover, Zhou et al. reported that disulfiram (DSF), an aldehyde dehydrogenase inhibitor, significantly facilitated the transition of macrophages from M1 to M2 phenotype and decreased macrophage pro-inflammatory phenotype by targeting gasdermin D (GSDMD) to attenuate macrophage cell pyroptosis, interleukin-1β (IL-1β), and high-mobility group box 1 protein (HMGB1) release. Deficiency or inhibition of GSDMD significantly suppressed peritendinous fibrosis formation around the injured tendon and was accompanied by increased regenerated bone and fibrocartilage in tendon–bone injury. The authors observed that DSF significantly reduced the proliferation and migration of fibroblasts after BMDM exosome treatment [122]. In recent years, GSDMD with high levels of expression has been reported in the macrophages, which is involved in a variety of diseases. Heilig et al. found that GSDMD pore formation resulted in very rapid cell lysis and stimulated IL-1β secretion [123]. Therefore, NF‐κB and GSDMD may represent promising therapeutic targets for enhancing tendon–bone healing, which must be further explored.
Regulation of angiogenesis
Tendons generally have limited vascularization when compared to muscles, especially at the TBI. After a TBI injury, poor vascularization at the tendon–bone junction decreases the availability of oxygen, growth factors, and other nutrients essential for tendon–bone healing, leading to poor biomechanical properties, eventually affecting tendon–bone healing [124]. An increasing number of studies have demonstrated that neovascularization is crucial for enhancing tendon–bone healing. Interestingly, Demirag et al. indicated that increased vascularization at the healing interface led to the more mature interface tissue that contained numerous perpendicular collagen bundles called Sharpey’s fibers [125]. Therefore, the exploration of the underlying mechanisms of angiogenesis via special pathways is critical for promoting tendon–bone healing.
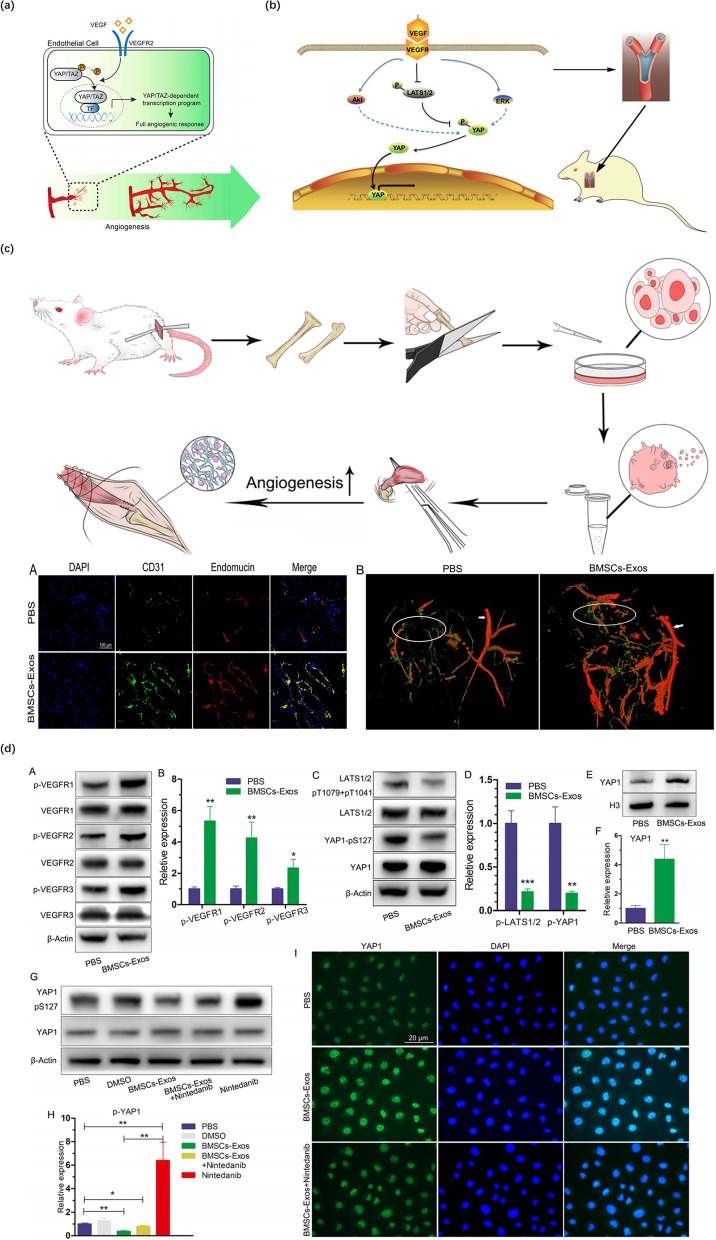
In recent years, MSC-exos have been reported to enhance tendon–bone healing, but only a few studies have investigated the effects of exosomes on tendon–bone healing associated with improving angiogenesis. In a notable study, BMSC-Exos were injected into the tail vein of rats after RC reconstruction, which improved tendon–bone healing by promoting angiogenesis through the VEGF and Hippo signaling pathways (Fig. 6c and d) [102]. Besides, Wang et al. found that YAP/TAZ, as pivotal mediators of VEGF signaling, were essential for the formation of blood vessels, and their activity was mainly regulated via the Hippo pathway [126] (Fig. 6a). VEGF is a key regulator of physiological angiogenesis that takes part in the activation, proliferation, and migration of endothelial cells in a variety of pathological processes, which can enhance blood supply in the grafted tendon in surgical reconstruction and accordingly promote tendon–bone healing (Fig. 6b) [127, 128]. Similarly, Takayama et al. discovered that suppressing VEGF expression decreased revascularization and inhibited grafted tendon maturation and biomechanical strength following ACL reconstruction surgery. Meanwhile, they also discovered that over-expression of VEGF hampered enhancement in tendon graft biomechanical strength [129]. Consequently, further studies on the mechanisms by which exosomes promote angiogenesis via the VEGF signaling pathway may provide avenues for enhancing tendon–bone healing.
Fig. 6.
The effect of exosomes on enhancing angiogenesis. a YAP/TAZ orchestrate VEGF signaling during developmental angiogenesis. Reproduced with permission [126]. b VEGF‐induced angiogenesis via the VEGF–VEGFR–Hippo signaling pathway axis and promoted rotator tendon‐bone healing in rats. Reproduced with permission [128]. c BMSC-Exos promoted angiogenesis around the tendon-bone interface of the rotator cuff in rats (DAPI, CD31, endomucin staining, and angiography). Reproduced with permission [102]. d Functional effects of BMSC-Exos on angiogenesis-related signaling pathways in HUVECs (Western blot analysis, DAPI, and YAP1 staining). Reproduced with permission [102]
Regulation of bone metabolism
Bone metabolism is tightly regulated by a balance of bone formation mediated by osteoblasts and bone resorption mediated by osteoclasts. Increased osteoclastic activity and reduced osteogenic activity play an important role in TBI injuries, resulting in tendon–bone healing failure after surgery. On the one hand, Rodeo et al. discovered that the tendon graft–bone interface strength positively correlated with the degree of osseous ingrowth [36]. Bone formation is a highly regulated process that involves the direct differentiation of BMSC to osteoblasts in bone remodeling in the living organism. Runt-related transcription factor-2 (RUNX2) and Osterix (Osx or Sp7), osteoblast-specific transcription factors, are essential for osteoblast differentiation and regulate many bone-related gene expressions, so-called markers of osteogenic differentiation [130, 131]. Exosomes in the TBI injury can influence osteoblast proliferation, migration, and differentiation by increasing the expression of the transcription factors RUNX2 and Osx. In this regard, Fu et al. noted that local injection of ADSC-exos-hydrogel complex in the shoulder enhanced RC repair and tendon–bone healing in a rat RC injury model, which could be because ADSC-exos induced adipogenesis and osteogenesis of tendon-derived stem cells, leading to the significant upregulation of osteogenic marker RUNX2, chondrogenic marker SOX-9, and tenogenesis genes TNC, TNMD, and Scx (Fig. 7b) [103]. On the other hand, bone resorption, i.e., osteolysis significantly impedes bony growth into the grafted tendon and decreases the pullout strength and stiffness of the TBI, resulting in delayed union and surgical failure in the early stage [132]. Tunnel osteolysis or widening after ACL reconstruction is a common complication due to increased osteoclast activity, which can occur in post-operation patients who undergo ACL reconstruction [133]. Osteoclasts are the only cell type involved in the destruction and resorption of bone tissue in vivo, which are differentiated from hematopoietic stem cell-derived monocytes and macrophage lineage progenitors (progenitors of osteoclasts). Thus, suppressing osteolysis and osteogenesis are the two central factors influencing the osteointegration of the tendon graft into the bone tunnel surface.
Fig. 7.
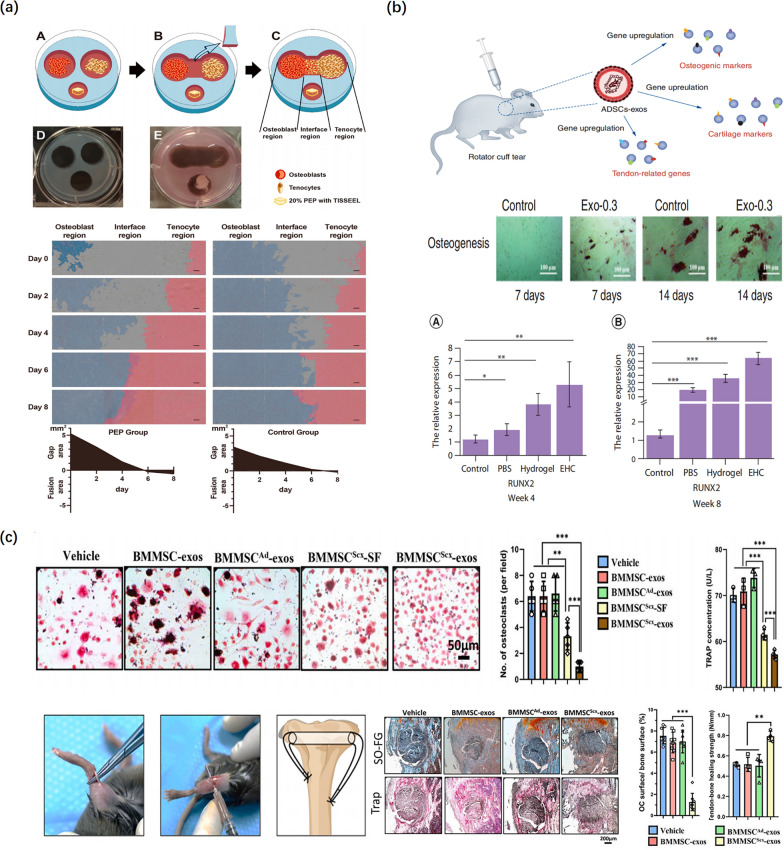
The effect of exosomes on the regulation of bone metabolism. a Schematic representation of the co-culture model. Cell growth and migration were significantly increased in all regions following exposure to PEP. PEP indicates purified exosome product. Reproduced with permission [73]. b The tendon-derived stem cell differentiation is enhanced by adipose-derived stem cell exosomes (Alizarin Red S staining). Real-time PCR analysis showed the EHC significantly induced upregulation of osteogenic marker RUNX2. EHC: Adipose-derived stem cell exosome–hydrogel complex. Reproduced with permission [103]. c Schematic diagram of the tendon-bone healing model. -exos inhibit osteoclastogenesis and improve tendon-bone healing. (TRAP, and safranin O-fast green staining). indicates Scx-overexpressing PDGFRα (+) BMMSCs; indicates the control group; SF indicates soluble factors. Reproduced with permission [89]
Numerous preclinical and clinical studies have shown the potential of using exosomes for the enhancement of tendon–bone healing via the promotion of osteogenesis and inhibition of osteolysis. In a seminal study, Feng et al. reported that MiR-6924-5p-rich exosomes derived from genetically modified Scleraxis-overexpressing PDGFRα(+) BMMSCs could efficiently suppress tunnel osteolysis and promote the biomechanical strength of tendon–bone healing via targeting two osteoclastogenic regulators OCSTAMP and CXCL12 (Fig. 7c) [89]. In another study, Ren et al. found that purified exosome products and circulating exosomes enhanced the proliferation, migration, and confluence of osteoblast cells and tenocytes in an in vitro co-culture model, especially during direct cell–cell contact, and facilitated tendon–bone healing in a rat RC tear model (Fig. 7a) [73]. Similarly, a recent study has reported that the BMSC-Exos of PASP-PLGA microcapsules via delivering bone morphogenetic protein-2 (BMP-2) and PLA facilitated the formation of bone tissue and fibrocartilage tissue as well as the stiffness and the ultimate load strength of the tendon interface by Smad/RUNX2 pathway, leading to enhancing tendon–bone healing in a rabbit model of acute RC rupture [92]. Consequently, the inhibition of osteoclast activity or osteoclastogenesis and promotion of osteogenesis provide potential targets for the enhancement of osteointegration at the TBI to facilitate tendon–bone healing.
Challenges and future perspectives
Clinically, most patients with TBI injuries require surgical repair and reconstruction rather than nonoperative management. During the past decades, various biological strategies, such as biomaterials, stem cells, and growth factors, have been used to facilitate tendon–bone healing. However, restoring the structure of the physiological transition between tendon/ligament and bone remains a huge challenge. Poor tendon–bone healing may contribute to the re-tear of the TBI, which can dramatically reduce patients’ quality of life. In recent years, numerous studies have reported the promotional effects of MSC-exos, but these results need to be further validated in clinical research. At the same time, the clinical translation related to MSC-exos therapies still has great challenges. First, it is the absence of a gold standard protocol for the isolation of exosomes with high yield and pure product. Second, the ability, which distinguishes exosomes from other EVs, especially functional microvesicles, is scarce. Third, the optimal frequency of exosome injection is uncertain, and whether more injections could produce better results than a single injection is unclear. Extensive research must be conducted to confirm the optimal dosage and injection frequency. Fourth, exosomes can be secreted by various cell types, each with different functions; thus, further studies are required to compare exosomes derived from various cell types in clinical settings to provide the optimal choice for tendon–bone healing. Fifth, most exosome therapy trials are focused on small animal models, which have a faster rate of the tendon–bone healing process than human patients. More investigations to test the efficacy and safety of this application in large animals are necessary to prove its clinical viability. Sixth, due to the diversity and complexity of the components in exosomes, further exploration is required to determine the specific substances improving tendon–bone healing in exosomes. Lastly, further research is needed to optimize the targeting of exosomes as drug delivery vehicles, which will also resolve problems encountered by stem-cell-based therapies, and may provide a novel “all-in-one” cell-free therapeutic strategy method.
The MSC-exos research has recently become the active hotspot in the field of TBI injuries. Advancement of technologies and related research may provide more valuable information for revealing the content of MSC-exos and the underlying mechanism of MSC-exos functions. The use of exosomes as nanocarriers has a very high potential in TBI injuries. In addition, reprogrammed or redesigned exosomes for improving tendon–bone healing are promising in the future. Further studies on the mechanisms of targeted delivery of MSC-exos would clarify the engineering of MSC-exos as therapeutic carriers for drug and gene delivery, which is of great significance for the future clinical application of MSC-exos.
Conclusion
In regenerative medicine, stem cell therapy is an effective strategy for tissue repair and regeneration. In recent years, an increasing number of studies have shown that the beneficial effects of stem cell therapy are mediated by exosomes secreted by the paracrine action of MSCs, which provide a new potential cell-free therapy for enhancing tendon–bone healing. With further research, we have found that MSC-exos may regulate macrophage polarization, promote angiogenesis, enhance osteogenesis, and suppress tunnel osteolysis. Furthermore, MSC-exos are rich in a variety of nucleic acids, multiple proteins, and bioactive lipids, which primarily function as intercellular communication carriers to deliver these between cells to trigger biological responses in recipient cells. Recent studies have shown that exosomes can combine biomaterials to facilitate tendon–bone healing. These findings may provide emerging research directions for the treatment of TBI injuries. In summary, although these studies did not establish a precise healing mechanism, they identified the great potential of exosomes in the treatment of TBI injuries.
Acknowledgements
Not applicable.
Abbreviations
- TBI
Tendon–bone insertion
- MSCs
Mesenchymal stem cells
- EVs
Extracellular vesicles
- ACL
Anterior cruciate ligament
- RC
Rotator cuff
- MSCs-exos
Mesenchymal stem cells-derived exosomes
- RCT
Rotator cuff tendon
- NFC
Non-mineralized fibrocartilage
- MFC
Mineralized fibrocartilage
- UF
Uncalcified fibrocartilage
- CF
Calcified fibrocartilage
- T
Tidemark
- ECM
Extracellular matrix
- TGF-β
Transforming growth factor-β
- BMP
Bone morphogenetic protein
- G-CSF
Granulocyte colony-stimulating factor
- BMSCs
Bone marrow
- ASCs
Adipose tissue
- CM
Conditioned medium
- VEGF
Vascular endothelial growth factor
- PDGF
Platelet-derived growth factor
- EGF
Epidermal growth factor
- hBMSC-CM
Human bone marrow-derived stem cells
- MVB
Multivesicular body
- ILVs
Intraluminal vesicles
- ESCs
Embryonic stem cells
- iPSCs
Induced pluripotent stem cells
- LIPUS
Low-intensity pulsed ultrasound stimulation
- BMSC-Exos
BMSC-derived exosomes
- PASP-PLGA
Polyaspartic acid polylactic acid-glycolic acid
- PLA
Polyaspartic acid
- IPFP
Infrapatellar fat pad
- 3′ UTRs
3′-Untranslated regions
- VEGFR
Vascular endothelial growth factor receptor
- ASC-Exos
Adipose stem cell-derived exosomes
- NF-κB
Nuclear factor-κB
- DSF
Disulfiram
- GSDMD
Gasdermin D
- IL-1β
Interleukin-1β
- HMGB1
High-mobility group box 1 protein
- RUNX2
Runt-related
- PEP
Purified exosome product
Scx-overexpressing PDGFRα (+) BMMSCs
- SF
Soluble factors
Author contributions
JXZ and WNY defined the focus of the review. JXZ and WNY summarized studies. JXZ drafted the manuscript. WSC and CSL participated in some parts of the final manuscript. HBW, AL, and JC revised the manuscript. All authors reviewed the final version of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81972077) and the Young Scientist Fund of the National Natural Science Foundation of China (Grant No. 82001458).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiaxuan Zou and Weinan Yang contributed equally to this work
Contributor Information
Jing Chen, Email: phdchenj@gmail.com.
An Liu, Email: la@zju.edu.cn.
Haobo Wu, Email: 2505014@zju.edu.cn.
References
- 1.Wang Y, He G, Guo Y, Tang H, Shi Y, Bian X, et al. Exosomes from tendon stem cells promote injury tendon healing through balancing synthesis and degradation of the tendon extracellular matrix. J Cell Mol Med. 2019;23:5475–5485. doi: 10.1111/jcmm.14430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benjamin M, Toumi H, Ralphs JR, Bydder G, Best TM, Milz S. Where tendons and ligaments meet bone: attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J Anat. 2006;208:471–490. doi: 10.1111/j.1469-7580.2006.00540.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saveh-Shemshaki N, Nair LS, Laurencin CT. Nanofiber-based matrices for rotator cuff regenerative engineering. Acta Biomater. 2019;94:64–81. doi: 10.1016/j.actbio.2019.05.041. [DOI] [PubMed] [Google Scholar]
- 4.Silvers-Granelli HJ, Bizzini M, Arundale A, Mandelbaum BR, Snyder-Mackler L. Does the FIFA 11+ injury prevention program reduce the incidence of ACL injury in male soccer players? Clin Orthop. 2017;475:2447–2455. doi: 10.1007/s11999-017-5342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H, Li S, Xiao H, Wu B, Zhou L, Hu J, et al. Effect of exercise intensity on the healing of the bone-tendon interface: a mouse rotator cuff injury model study. Am J Sports Med. 2021;49:2064–2073. doi: 10.1177/03635465211011751. [DOI] [PubMed] [Google Scholar]
- 6.Wang J, Xu J, Wang X, Sheng L, Zheng L, Song B, et al. Magnesium-pretreated periosteum for promoting bone–tendon healing after anterior cruciate ligament reconstruction. Biomaterials. 2021;268:120576. doi: 10.1016/j.biomaterials.2020.120576. [DOI] [PubMed] [Google Scholar]
- 7.Xu Y, Zhang W-X, Wang L-N, Ming Y-Q, Li Y-L, Ni G-X. Stem cell therapies in tendon–bone healing. World J Stem Cells. 2021;13:753–775. doi: 10.4252/wjsc.v13.i7.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shengnan Q, Bennett S, Wen W, Aiguo L, Jiake X. The role of tendon derived stem/progenitor cells and extracellular matrix components in the bone tendon junction repair. Bone. 2021;153:116172. doi: 10.1016/j.bone.2021.116172. [DOI] [PubMed] [Google Scholar]
- 9.Lui PPY. Mesenchymal stem cell-derived extracellular vesicles for the promotion of tendon repair—an update of literature. Stem Cell Rev Rep. 2021;17:379–389. doi: 10.1007/s12015-020-10023-8. [DOI] [PubMed] [Google Scholar]
- 10.Patel S, Caldwell J, Doty SB, Levine WN, Rodeo S, Soslowsky LJ, et al. Integrating soft and hard tissues via interface tissue engineering. J Orthop Res. 2018;36:1069–1077. doi: 10.1002/jor.23810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connor DE, Paulus JA, Dabestani PJ, Thankam FK, Dilisio MF, Gross RM, et al. Therapeutic potential of exosomes in rotator cuff tendon healing. J Bone Miner Metab. 2019;37:759–767. doi: 10.1007/s00774-019-01013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu H, Zhang M, Shi M, Zhang T, Lu W, Yang S, et al. Adipose-derived mesenchymal stromal cell-derived exosomes promote tendon healing by activating both SMAD1/5/9 and SMAD2/3. Stem Cell Res Ther. 2021;12:338. doi: 10.1186/s13287-021-02410-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gulotta LV, Kovacevic D, Ying L, Ehteshami JR, Montgomery S, Rodeo SA. Augmentation of tendon-to-bone healing with a magnesium-based bone adhesive. Am J Sports Med. 2008;36:1290–1297. doi: 10.1177/0363546508314396. [DOI] [PubMed] [Google Scholar]
- 14.Lim WL, Liau LL, Ng MH, Chowdhury SR, Law JX. Current progress in tendon and ligament tissue engineering. Tissue Eng Regen Med. 2019;16:549–571. doi: 10.1007/s13770-019-00196-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harada Y, Mifune Y, Inui A, Sakata R, Muto T, Takase F, et al. Rotator cuff repair using cell sheets derived from human rotator cuff in a rat model. J Orthop Res Off Publ Orthop Res Soc. 2017;35:289–296. doi: 10.1002/jor.23289. [DOI] [PubMed] [Google Scholar]
- 16.Kaizawa Y, Franklin A, Leyden J, Behn AW, Tulu US, Sotelo Leon D, et al. Augmentation of chronic rotator cuff healing using adipose-derived stem cell-seeded human tendon-derived hydrogel. J Orthop Res. 2019;37:877–886. doi: 10.1002/jor.24250. [DOI] [PubMed] [Google Scholar]
- 17.Lu H, Liu F, Chen H, Chen C, Qu J, Xu D, et al. The effect of low-intensity pulsed ultrasound on bone-tendon junction healing: initiating after inflammation stage. J Orthop Res Off Publ Orthop Res Soc. 2016;34:1697–1706. doi: 10.1002/jor.23180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Q, Yu Y, Reisdorf RL, Qi J, Lu CK, Berglund LJ, et al. Engineered tendon-fibrocartilage-bone composite and bone marrow-derived mesenchymal stem cell sheet augmentation promotes rotator cuff healing in a non-weight-bearing canine model. Biomaterials. 2019;192:189–198. doi: 10.1016/j.biomaterials.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 19.Rothrauff BB, Smith CA, Ferrer GA, Novaretti JV, Pauyo T, Chao T, et al. The effect of adipose-derived stem cells on enthesis healing after repair of acute and chronic massive rotator cuff tears in rats. J Shoulder Elbow Surg. 2019;28:654–664. doi: 10.1016/j.jse.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki A, Mizuno M, Ozeki N, Katano H, Otabe K, Tsuji K, et al. Canine mesenchymal stem cells from synovium have a higher chondrogenic potential than those from infrapatellar fat pad, adipose tissue, and bone marrow. PLoS ONE. 2018;13:e0202922. doi: 10.1371/journal.pone.0202922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Utsunomiya H, Sekiya I, Uchida S. Editorial commentary: are we ready to apply stem cell therapy in rotator cuff tear surgery? Arthrosc J Arthrosc Relat Surg. 2020;36:86–87. doi: 10.1016/j.arthro.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Lu V, Tennyson M, Zhang J, Khan W. Mesenchymal stem cell-derived extracellular vesicles in tendon and ligament repair—a systematic review of in vivo studies. Cells. 2021;10:2553. doi: 10.3390/cells10102553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun H, Pratt RE, Hodgkinson CP, Dzau VJ. Sequential paracrine mechanisms are necessary for the therapeutic benefits of stem cell therapy. Am J Physiol Cell Physiol. 2020;319:C1141–C1150. doi: 10.1152/ajpcell.00516.2019. [DOI] [PubMed] [Google Scholar]
- 24.Bruno S, Tapparo M, Collino F, Chiabotto G, Deregibus MC, Soares Lindoso R, et al. Renal regenerative potential of different extracellular vesicle populations derived from bone marrow mesenchymal stromal cells. Tissue Eng Part A. 2017;23:1262–1273. doi: 10.1089/ten.tea.2017.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pan Q, Kuang X, Cai S, Wang X, Du D, Wang J, et al. miR-132-3p priming enhances the effects of mesenchymal stromal cell-derived exosomes on ameliorating brain ischemic injury. Stem Cell Res Ther. 2020;11:260. doi: 10.1186/s13287-020-01761-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qiu X, Liu J, Zheng C, Su Y, Bao L, Zhu B, et al. Exosomes released from educated mesenchymal stem cells accelerate cutaneous wound healing via promoting angiogenesis. Cell Prolif. 2020;53:e12830. doi: 10.1111/cpr.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ni Z, Zhou S, Li S, Kuang L, Chen H, Luo X, et al. Exosomes: roles and therapeutic potential in osteoarthritis. Bone Res. 2020;8:25. doi: 10.1038/s41413-020-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Hao Z, Wang P, Xia Y, Wu J, Xia D, et al. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019;52:e12570. doi: 10.1111/cpr.12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li M, Tang Y, Chen C, Zhou J, Zheng C, Chen H, et al. Comparison of bone surface and trough fixation on bone–tendon healing in a rabbit patella–patellar tendon injury model. J Orthop Transl. 2020;21:49–56. doi: 10.1016/j.jot.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao X, Zhou Y, Li J, Zhang C, Wang J. Opportunities and challenges of hydrogel microspheres for tendon–bone healing after anterior cruciate ligament reconstruction. J Biomed Mater Res B Appl Biomater. 2022;110:289–301. doi: 10.1002/jbm.b.34925. [DOI] [PubMed] [Google Scholar]
- 31.Thangarajah T, Pendegrass CJ, Shahbazi S, Lambert S, Alexander S, Blunn GW. Augmentation of rotator cuff repair with soft tissue scaffolds. Orthop J Sports Med. 2015;3:232596711558749. doi: 10.1177/2325967115587495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen W, Sun Y, Gu X, Cai J, Liu X, Zhang X, et al. Conditioned medium of human bone marrow-derived stem cells promotes tendon-bone healing of the rotator cuff in a rat model. Biomaterials. 2021;271:120714. doi: 10.1016/j.biomaterials.2021.120714. [DOI] [PubMed] [Google Scholar]
- 33.Cheng P, Han P, Zhao C, Zhang S, Wu H, Ni J, et al. High-purity magnesium interference screws promote fibrocartilaginous entheses regeneration in the anterior cruciate ligament reconstruction rabbit model via accumulation of BMP-2 and VEGF. Biomaterials. 2016;81:14–26. doi: 10.1016/j.biomaterials.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Genin GM, Kent A, Birman V, Wopenka B, Pasteris JD, Marquez PJ, et al. Functional grading of mineral and collagen in the attachment of tendon to bone. Biophys J. 2009;97:976–985. doi: 10.1016/j.bpj.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinczewski LA, Clingeleffer AJ, Otto DD, Bonar SF, Corry IS. Integration of hamstring tendon graft with bone in reconstruction of the anterior cruciate ligament. Arthrosc J Arthrosc Relat Surg. 1997;13:641–643. doi: 10.1016/S0749-8063(97)90194-8. [DOI] [PubMed] [Google Scholar]
- 36.Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75:1795–803. doi: 10.2106/00004623-199312000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Shino K, Kawasaki T, Hirose H, Gotoh I, Inoue M, Ono K. Replacement of the anterior cruciate ligament by an allogeneic tendon graft. An experimental study in the dog. J Bone Joint Surg Br. 1984;66:672–81. doi: 10.1302/0301-620X.66B5.6501359. [DOI] [PubMed] [Google Scholar]
- 38.Hao Z-C, Wang S-Z, Zhang X-J, Lu J. Stem cell therapy: a promising biological strategy for tendon-bone healing after anterior cruciate ligament reconstruction. Cell Prolif. 2016;49:154–162. doi: 10.1111/cpr.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grana WA, Egle DM, Mahnken R, Goodhart CW. An analysis of autograft fixation after anterior cruciate ligament reconstruction in a rabbit model. Am J Sports Med. 1994;22:344–351. doi: 10.1177/036354659402200309. [DOI] [PubMed] [Google Scholar]
- 40.Lu HH, Thomopoulos S. Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu Rev Biomed Eng. 2013;15:201–226. doi: 10.1146/annurev-bioeng-071910-124656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakagawa H, Morihara T, Fujiwara H, Kabuto Y, Sukenari T, Kida Y, et al. Effect of footprint preparation on tendon-to-bone healing: a histologic and biomechanical study in a rat rotator cuff repair model. Arthrosc J Arthrosc Relat Surg. 2017;33:1482–1492. doi: 10.1016/j.arthro.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 42.Kovacevic D, Rodeo SA. Biological augmentation of rotator cuff tendon repair. Clin Orthop. 2008;466:622–633. doi: 10.1007/s11999-007-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kawamura S, Ying L, Kim H-J, Dynybil C, Rodeo SA. Macrophages accumulate in the early phase of tendon-bone healing. J Orthop Res. 2005;23:1425–1432. doi: 10.1016/j.orthres.2005.01.014.1100230627. [DOI] [PubMed] [Google Scholar]
- 44.Haus J, Refior HJ. A study of the synovial and ligamentous structure of the anterior cruciate ligament. Int Orthop. 1987;11:117–124. doi: 10.1007/BF00266696. [DOI] [PubMed] [Google Scholar]
- 45.Deehan DJ, Cawston TE. The biology of integration of the anterior cruciate ligament. J Bone Joint Surg Br. 2005;87:889–895. doi: 10.1302/0301-620X.87B7.16038. [DOI] [PubMed] [Google Scholar]
- 46.Lei T, Zhang T, Ju W, Chen X, Heng BC, Shen W, et al. Biomimetic strategies for tendon/ligament-to-bone interface regeneration. Bioact Mater. 2021;6:2491–2510. doi: 10.1016/j.bioactmat.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ménétrey J, Duthon VB, Laumonier T, Fritschy D. “Biological failure” of the anterior cruciate ligament graft. Knee Surg Sports Traumatol Arthrosc. 2008;16:224–231. doi: 10.1007/s00167-007-0474-x. [DOI] [PubMed] [Google Scholar]
- 48.Mihelic R, Pecina M, Jelic M, Zoricic S, Kusec V, Simic P, et al. Bone morphogenetic protein-7 (osteogenic protein-1) promotes tendon graft integration in anterior cruciate ligament reconstruction in sheep. Am J Sports Med. 2004;32:1619–1625. doi: 10.1177/0363546504263703. [DOI] [PubMed] [Google Scholar]
- 49.Cervellin M, de Girolamo L, Bait C, Denti M, Volpi P. Autologous platelet-rich plasma gel to reduce donor-site morbidity after patellar tendon graft harvesting for anterior cruciate ligament reconstruction: a randomized, controlled clinical study. Knee Surg Sports Traumatol Arthrosc. 2012;20:114–120. doi: 10.1007/s00167-011-1570-5. [DOI] [PubMed] [Google Scholar]
- 50.Lu H, Liu F, Chen C, Wang Z, Chen H, Qu J, et al. Low-intensity pulsed ultrasound stimulation for tendon-bone healing: a dose-dependent study. Am J Phys Med Rehabil. 2018;97:270–277. doi: 10.1097/PHM.0000000000000844. [DOI] [PubMed] [Google Scholar]
- 51.Mifune Y, Matsumoto T, Ota S, Nishimori M, Usas A, Kopf S, et al. Therapeutic potential of anterior cruciate ligament-derived stem cells for anterior cruciate ligament reconstruction. Cell Transplant. 2012;21:1651–1665. doi: 10.3727/096368912X647234. [DOI] [PubMed] [Google Scholar]
- 52.Murray MM, Spindler KP, Ballard P, Welch TP, Zurakowski D, Nanney LB. Enhanced histologic repair in a central wound in the anterior cruciate ligament with a collagen-platelet-rich plasma scaffold. J Orthop Res. 2007;25:1007–1017. doi: 10.1002/jor.20367. [DOI] [PubMed] [Google Scholar]
- 53.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 54.De Bari C, Dell’Accio F, Luyten FP. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001;44:85–95. doi: 10.1002/1529-0131(200101)44:1<85::AID-ANR12>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 55.Ju Y-J, Muneta T, Yoshimura H, Koga H, Sekiya I. Synovial mesenchymal stem cells accelerate early remodeling of tendon-bone healing. Cell Tissue Res. 2008;332:469–478. doi: 10.1007/s00441-008-0610-z. [DOI] [PubMed] [Google Scholar]
- 56.Lui PPY, Wong OT, Lee YW. Application of tendon-derived stem cell sheet for the promotion of graft healing in anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42:681–689. doi: 10.1177/0363546513517539. [DOI] [PubMed] [Google Scholar]
- 57.Røsland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, et al. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 2009;69:5331–5339. doi: 10.1158/0008-5472.CAN-08-4630. [DOI] [PubMed] [Google Scholar]
- 58.da Meirelles L, Nardi NB. Methodology, biology and clinical applications of mesenchymal stem cells. Front Biosci. 2009;14:4281–98. doi: 10.2741/3528. [DOI] [PubMed] [Google Scholar]
- 59.Phinney DG, Pittenger MF. Concise review: MSC-derived exosomes for cell-free therapy. Stem Cells Dayt Ohio. 2017;35:851–858. doi: 10.1002/stem.2575. [DOI] [PubMed] [Google Scholar]
- 60.Beer L, Mildner M, Ankersmit HJ. Cell secretome based drug substances in regenerative medicine: when regulatory affairs meet basic science. Ann Transl Med. 2017;5:170. doi: 10.21037/atm.2017.03.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Phelps J, Sanati-Nezhad A, Ungrin M, Duncan NA, Sen A. Bioprocessing of mesenchymal stem cells and their derivatives: toward cell-free therapeutics. Stem Cells Int. 2018;2018:9415367. doi: 10.1155/2018/9415367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Driscoll J, Patel T. The mesenchymal stem cell secretome as an acellular regenerative therapy for liver disease. J Gastroenterol. 2019;54:763–773. doi: 10.1007/s00535-019-01599-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pawitan JA. Prospect of stem cell conditioned medium in regenerative medicine. BioMed Res Int. 2014;2014:965849. doi: 10.1155/2014/965849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.El Moshy S, Radwan IA, Rady D, Abbass MMS, El-Rashidy AA, Sadek KM, et al. Dental stem cell-derived secretome/conditioned medium: the future for regenerative therapeutic applications. Stem Cells Int. 2020;2020:1–29. doi: 10.1155/2020/7593402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sevivas N, Teixeira FG, Portugal R, Direito-Santos B, Espregueira-Mendes J, Oliveira FJ, et al. Mesenchymal stem cell secretome improves tendon cell viability in vitro and tendon-bone healing in vivo when a tissue engineering strategy is used in a rat model of chronic massive rotator cuff tear. Am J Sports Med. 2018;46:449–459. doi: 10.1177/0363546517735850. [DOI] [PubMed] [Google Scholar]
- 66.Sun Y, Chen W, Hao Y, Gu X, Liu X, Cai J, et al. Stem cell-conditioned medium promotes graft remodeling of midsubstance and intratunnel incorporation after anterior cruciate ligament reconstruction in a rat model. Am J Sports Med. 2019;47:2327–2337. doi: 10.1177/0363546519859324. [DOI] [PubMed] [Google Scholar]
- 67.Abels ER, Breakefield XO. Introduction to extracellular vesicles: biogenesis, RNA cargo selection, content, release, and uptake. Cell Mol Neurobiol. 2016;36:301–312. doi: 10.1007/s10571-016-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nooshabadi VT, Mardpour S, Yousefi-Ahmadipour A, Allahverdi A, Izadpanah M, Daneshimehr F, et al. The extracellular vesicles-derived from mesenchymal stromal cells: a new therapeutic option in regenerative medicine. J Cell Biochem. 2018;119:8048–8073. doi: 10.1002/jcb.26726. [DOI] [PubMed] [Google Scholar]
- 69.Latifkar A, Hur YH, Sanchez JC, Cerione RA, Antonyak MA. New insights into extracellular vesicle biogenesis and function. J Cell Sci. 2019;132:jcs222406. doi: 10.1242/jcs.222406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zakeri Z, Salmaninejad A, Hosseini N, Shahbakhsh Y, Fadaee E, Shahrzad MK, et al. MicroRNA and exosome: key players in rheumatoid arthritis. J Cell Biochem. 2019 doi: 10.1002/jcb.28499. [DOI] [PubMed] [Google Scholar]
- 71.Li Z, Wang Y, Xiao K, Xiang S, Li Z, Weng X. Emerging role of exosomes in the joint diseases. Cell Physiol Biochem. 2018;47:2008–2017. doi: 10.1159/000491469. [DOI] [PubMed] [Google Scholar]
- 72.Keshtkar S, Azarpira N, Ghahremani MH. Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Res Ther. 2018;9:63. doi: 10.1186/s13287-018-0791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ren Y, Zhang S, Wang Y, Jacobson DS, Reisdorf RL, Kuroiwa T, et al. Effects of purified exosome product on rotator cuff tendon–bone healing in vitro and in vivo. Biomaterials. 2021;276:121019. doi: 10.1016/j.biomaterials.2021.121019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alzhrani GN, Alanazi ST, Alsharif SY, Albalawi AM, Alsharif AA, Abdel-Maksoud MS, et al. Exosomes: isolation, characterization, and biomedical applications. Cell Biol Int. 2021;45:1807–1831. doi: 10.1002/cbin.11620. [DOI] [PubMed] [Google Scholar]
- 75.An Y, Zhao J, Nie F, Qin Z, Xue H, Wang G, et al. Exosomes from adipose-derived stem cells (ADSCs) overexpressing miR-21 promote vascularization of endothelial cells. Sci Rep. 2019;9:12861. doi: 10.1038/s41598-019-49339-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bu H, He D, He X, Wang K. Exosomes: isolation, analysis, and applications in cancer detection and therapy. Chembiochem. 2019;20:451–461. doi: 10.1002/cbic.201800470. [DOI] [PubMed] [Google Scholar]
- 77.Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7:789–804. doi: 10.7150/thno.18133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ryu KJ, Lee JY, Park C, Cho D, Kim SJ. Isolation of small extracellular vesicles from human serum using a combination of ultracentrifugation with polymer-based precipitation. Ann Lab Med. 2020;40:253–258. doi: 10.3343/alm.2020.40.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New technologies for analysis of extracellular vesicles. Chem Rev. 2018;118:1917–1950. doi: 10.1021/acs.chemrev.7b00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elahi FM, Farwell DG, Nolta JA, Anderson JD. Preclinical translation of exosomes derived from mesenchymal stem/stromal cells. Stem Cells. 2020;38:15–21. doi: 10.1002/stem.3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lässer C, Eldh M, Lötvall J. Isolation and characterization of RNA-containing exosomes. J Vis Exp. 2012 doi: 10.3791/3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao R, Zhao T, He Z, Cai R, Pang W. Composition, isolation, identification and function of adipose tissue-derived exosomes. Adipocyte. 2021;10:587–604. doi: 10.1080/21623945.2021.1983242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramirez MI, Amorim MG, Gadelha C, Milic I, Welsh JA, Freitas VM, et al. Technical challenges of working with extracellular vesicles. Nanoscale. 2018;10:881–906. doi: 10.1039/C7NR08360B. [DOI] [PubMed] [Google Scholar]
- 84.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu M, Yang Q, Sun X, Wang Y. Recent advancements in the loading and modification of therapeutic exosomes. Front Bioeng Biotechnol. 2020;8:586130. doi: 10.3389/fbioe.2020.586130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cui H, He Y, Chen S, Zhang D, Yu Y, Fan C. Macrophage-derived miRNA-containing exosomes induce peritendinous fibrosis after tendon injury through the miR-21-5p/Smad7 pathway. Mol Ther Nucleic Acids. 2019;14:114–130. doi: 10.1016/j.omtn.2018.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu X, Odenthal M, Fries JWU. Exosomes as miRNA carriers: formation-function-future. Int J Mol Sci. 2016;17:2028. doi: 10.3390/ijms17122028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu X-D, Kang L, Tian J, Wu Y, Huang Y, Liu J, et al. Exosomes derived from magnetically actuated bone mesenchymal stem cells promote tendon-bone healing through the miR-21-5p/SMAD7 pathway. Mater Today Bio. 2022;15:100319. doi: 10.1016/j.mtbio.2022.100319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Feng W, Jin Q, Ming-yu Y, Yang H, Xu T, You-xing S, et al. MiR-6924-5p-rich exosomes derived from genetically modified scleraxis-overexpressing PDGFRα(+) BMMSCs as novel nanotherapeutics for treating osteolysis during tendon-bone healing and improving healing strength. Biomaterials. 2021;279:121242. doi: 10.1016/j.biomaterials.2021.121242. [DOI] [PubMed] [Google Scholar]
- 90.Wu B, Chen H, Shi X, Wang L, Zhang T, Guan C, et al. Exosomes derived from bone marrow mesenchymal stem cell preconditioned by low-intensity pulsed ultrasound stimulation promote bone-tendon interface fibrocartilage regeneration and ameliorate rotator cuff fatty infiltration. Med Sci Monit. 2021 doi: 10.21203/rs.3.rs-809653/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li Z, Li Q, Tong K, Zhu J, Wang H, Chen B, et al. BMSC-derived exosomes promote tendon-bone healing after anterior cruciate ligament reconstruction by regulating M1/M2 macrophage polarization in rats. Stem Cell Res Ther. 2022;13:295. doi: 10.1186/s13287-022-02975-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Han L, Liu H, Fu H, Hu Y, Fang W, Liu J. Exosome-delivered BMP-2 and polyaspartic acid promotes tendon bone healing in rotator cuff tear via Smad/RUNX2 signaling pathway. Bioengineered. 2022;13:1459–1475. doi: 10.1080/21655979.2021.2019871. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 93.Kimiz-Gebologlu I, Oncel SS. Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release. 2022;347:533–543. doi: 10.1016/j.jconrel.2022.05.027. [DOI] [PubMed] [Google Scholar]
- 94.Fu S, Wang Y, Xia X, Zheng JC. Exosome engineering: current progress in cargo loading and targeted delivery. NanoImpact. 2020;20:100261. doi: 10.1016/j.impact.2020.100261. [DOI] [Google Scholar]
- 95.Herrmann IK, Wood MJA, Fuhrmann G. Extracellular vesicles as a next-generation drug delivery platform. Nat Nanotechnol. 2021;16:748–759. doi: 10.1038/s41565-021-00931-2. [DOI] [PubMed] [Google Scholar]
- 96.Imai T, Takahashi Y, Nishikawa M, Kato K, Morishita M, Yamashita T, et al. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J Extracell Vesicles. 2015;4:26238. doi: 10.3402/jev.v4.26238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu H, Cheng J, Shi W, Ren B, Zhao F, Shi Y, et al. Bone marrow mesenchymal stem cell-derived exosomes promote tendon regeneration by facilitating the proliferation and migration of endogenous tendon stem/progenitor cells. Acta Biomater. 2020;106:328–341. doi: 10.1016/j.actbio.2020.01.051. [DOI] [PubMed] [Google Scholar]
- 98.Chen Y, Lee K, Kawazoe N, Yang Y, Chen G. ECM scaffolds mimicking extracellular matrices of endochondral ossification for the regulation of mesenchymal stem cell differentiation. Acta Biomater. 2020;114:158–169. doi: 10.1016/j.actbio.2020.07.049. [DOI] [PubMed] [Google Scholar]
- 99.Sakiyama-Elbert Combining stem cells and biomaterial scaffolds for constructing tissues and cell delivery. StemBook. 2008 doi: 10.3824/stembook.1.1.1. [DOI] [PubMed] [Google Scholar]
- 100.Xiao S, Zhao T, Wang J, Wang C, Du J, Ying L, et al. Gelatin methacrylate (GelMA)-based hydrogels for cell transplantation: an effective strategy for tissue engineering. Stem Cell Rev Rep. 2019;15:664–679. doi: 10.1007/s12015-019-09893-4. [DOI] [PubMed] [Google Scholar]
- 101.Akbari A, Jabbari N, Sharifi R, Ahmadi M, Vahhabi A, Seyedzadeh SJ, et al. Free and hydrogel encapsulated exosome-based therapies in regenerative medicine. Life Sci. 2020;249:117447. doi: 10.1016/j.lfs.2020.117447. [DOI] [PubMed] [Google Scholar]
- 102.Huang Y, He B, Wang L, Yuan B, Shu H, Zhang F, et al. Bone marrow mesenchymal stem cell-derived exosomes promote rotator cuff tendon-bone healing by promoting angiogenesis and regulating M1 macrophages in rats. Stem Cell Res Ther. 2020;11:496. doi: 10.1186/s13287-020-02005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fu G, Lu L, Pan Z, Fan A, Yin F. Adipose-derived stem cell exosomes facilitate rotator cuff repair by mediating tendon-derived stem cells. Regen Med. 2021;16:359–372. doi: 10.2217/rme-2021-0004. [DOI] [PubMed] [Google Scholar]
- 104.Xu J, Ye Z, Han K, Zheng T, Zhang T, Dong S, et al. Infrapatellar fat pad mesenchymal stromal cell-derived exosomes accelerate tendon-bone healing and intra-articular graft remodeling after anterior cruciate ligament reconstruction. Am J Sports Med. 2022;50:662–673. doi: 10.1177/03635465211072227. [DOI] [PubMed] [Google Scholar]
- 105.Shi Y, Kang X, Wang Y, Bian X, He G, Zhou M, et al. Exosomes derived from bone marrow stromal cells (BMSCs) enhance tendon-bone healing by regulating macrophage polarization. Med Sci Monit. 2020;26:e923328. doi: 10.12659/MSM.923328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254–271. doi: 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lu H, Qin L, Cheung W, Lee K, Wong W, Leung K. Low-intensity pulsed ultrasound accelerated bone-tendon junction healing through regulation of vascular endothelial growth factor expression and cartilage formation. Ultrasound Med Biol. 2008;34:1248–1260. doi: 10.1016/j.ultrasmedbio.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 108.Nebelung W, Becker R, Urbach D, Röpke M, Roessner A. Histological findings of tendon-bone healing following anterior cruciate ligament reconstruction with hamstring grafts. Arch Orthop Trauma Surg. 2003;123:158–163. doi: 10.1007/s00402-002-0463-y. [DOI] [PubMed] [Google Scholar]
- 109.Wong MWN, Qin L, Tai JKO, Lee SKM, Leung KS, Chan KM. Engineered allogeneic chondrocyte pellet for reconstruction of fibrocartilage zone at bone-tendon junction–a preliminary histological observation. J Biomed Mater Res B Appl Biomater. 2004;70:362–367. doi: 10.1002/jbm.b.30049. [DOI] [PubMed] [Google Scholar]
- 110.Gulotta LV, Rodeo SA. Biology of autograft and allograft healing in anterior cruciate ligament reconstruction. Clin Sports Med. 2007;26:509–524. doi: 10.1016/j.csm.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 111.Chamberlain CS, Leiferman EM, Frisch KE, Wang S, Yang X, van Rooijen N, et al. The influence of macrophage depletion on ligament healing. Connect Tissue Res. 2011;52:203–211. doi: 10.3109/03008207.2010.511355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hays PL, Kawamura S, Deng X-H, Dagher E, Mithoefer K, Ying L, et al. The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am. 2008;90:565–579. doi: 10.2106/JBJS.F.00531. [DOI] [PubMed] [Google Scholar]
- 113.Manning CN, Havlioglu N, Knutsen E, Sakiyama-Elbert SE, Silva MJ, Thomopoulos S, et al. The early inflammatory response after flexor tendon healing: a gene expression and histological analysis. J Orthop Res. 2014;32:645–652. doi: 10.1002/jor.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Janssen RPA, Scheffler SU. Intra-articular remodelling of hamstring tendon grafts after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2014;22:2102–2108. doi: 10.1007/s00167-013-2634-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li S, Xu Z, Wang Z, Xiang J, Zhang T, Lu H. Acceleration of bone-tendon interface healing by low-intensity pulsed ultrasound is mediated by macrophages. Phys Ther. 2021;101:pzab055. doi: 10.1093/ptj/pzab055. [DOI] [PubMed] [Google Scholar]
- 116.Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Klopfleisch R. Macrophage reaction against biomaterials in the mouse model—phenotypes, functions and markers. Acta Biomater. 2016;43:3–13. doi: 10.1016/j.actbio.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 119.Wang C, Hu Q, Song W, Yu W, He Y. Adipose stem cell-derived exosomes decrease fatty infiltration and enhance rotator cuff healing in a rabbit model of chronic tears. Am J Sports Med. 2020;48:1456–1464. doi: 10.1177/0363546520908847. [DOI] [PubMed] [Google Scholar]
- 120.Fatima F, Ekstrom K, Nazarenko I, Maugeri M, Valadi H, Hill AF, et al. Non-coding RNAs in mesenchymal stem cell-derived extracellular vesicles: deciphering regulatory roles in stem cell potency, inflammatory resolve, and tissue regeneration. Front Genet. 2017;8:161. doi: 10.3389/fgene.2017.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shen H, Yoneda S, Abu-Amer Y, Guilak F, Gelberman RH. Stem cell-derived extracellular vesicles attenuate the early inflammatory response after tendon injury and repair. J Orthop Res. 2020;38:117–127. doi: 10.1002/jor.24406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou Q, Wang W, Yang F, Wang H, Zhao X, Zhou Y, et al. Disulfiram suppressed peritendinous fibrosis through inhibiting macrophage accumulation and its pro-inflammatory properties in tendon bone healing. Front Bioeng Biotechnol. 2022;10:823933. doi: 10.3389/fbioe.2022.823933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Heilig R, Dick MS, Sborgi L, Meunier E, Hiller S, Broz P. The gasdermin-D pore acts as a conduit for IL-1β secretion in mice. Eur J Immunol. 2018;48:584–592. doi: 10.1002/eji.201747404. [DOI] [PubMed] [Google Scholar]
- 124.Randelli P, Menon A, Ragone V, Creo P, Bergante S, Randelli F, et al. Lipogems product treatment increases the proliferation rate of human tendon stem cells without affecting their stemness and differentiation capability. Stem Cells Int. 2016;2016:4373410. doi: 10.1155/2016/4373410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Demirag B, Sarisozen B, Ozer O, Kaplan T, Ozturk C. Enhancement of tendon-bone healing of anterior cruciate ligament grafts by blockage of matrix metalloproteinases. J Bone Joint Surg Am. 2005;87:2401–2410. doi: 10.2106/JBJS.D.01952. [DOI] [PubMed] [Google Scholar]
- 126.Wang X, Freire Valls A, Schermann G, Shen Y, Moya IM, Castro L, et al. YAP/TAZ orchestrate VEGF signaling during developmental angiogenesis. Dev Cell. 2017;42:462–478.e7. doi: 10.1016/j.devcel.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 127.Yoshikawa T, Tohyama H, Katsura T, Kondo E, Kotani Y, Matsumoto H, et al. Effects of local administration of vascular endothelial growth factor on mechanical characteristics of the semitendinosus tendon graft after anterior cruciate ligament reconstruction in sheep. Am J Sports Med. 2006;34:1918–1925. doi: 10.1177/0363546506294469. [DOI] [PubMed] [Google Scholar]
- 128.Huang Y, Pan M, Shu H, He B, Zhang F, Sun L. Vascular endothelial growth factor enhances tendon-bone healing by activating Yes-associated protein for angiogenesis induction and rotator cuff reconstruction in rats. J Cell Biochem. 2020;121:2343–2353. doi: 10.1002/jcb.29457. [DOI] [PubMed] [Google Scholar]
- 129.Takayama K, Kawakami Y, Mifune Y, Matsumoto T, Tang Y, Cummins JH, et al. The effect of blocking angiogenesis on anterior cruciate ligament healing following stem cell transplantation. Biomaterials. 2015;60:9–19. doi: 10.1016/j.biomaterials.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 130.Lian JB, Stein GS, Javed A, van Wijnen AJ, Stein JL, Montecino M, et al. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:1–16. doi: 10.1007/s11154-006-9001-5. [DOI] [PubMed] [Google Scholar]
- 131.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/S0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 132.Hjorthaug GA, Søreide E, Nordsletten L, Madsen JE, Reinholt FP, Niratisairak S, et al. Negative effect of zoledronic acid on tendon-to-bone healing. Acta Orthop. 2018;89:360–366. doi: 10.1080/17453674.2018.1440189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bhullar R, Habib A, Zhang K, de Sa D, Horner NS, Duong A, et al. Tunnel osteolysis post-ACL reconstruction: a systematic review examining select diagnostic modalities, treatment options and rehabilitation protocols. Knee Surg Sports Traumatol Arthrosc. 2019;27:524–533. doi: 10.1007/s00167-018-5142-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.