To the Editor
Vaccination is a key prevention method against COVID-19, but emerging SARS-CoV-2 variants of concern (VOC), especially the Omicron VOC, have impaired the effectiveness of the original, wild-type SARS-CoV-2–based COVID-19 vaccines [1,2]. Consequently, bivalent COVID-19 vaccines combining the wild-type spike mRNA with Omicron VOC BA.1 or BA.4-5 spike mRNA became available. For the bivalent mRNA-1273.214 vaccine (Wuhan-Hu-1/BA.1), slightly higher rates of the predominant adverse reactions have been reported [3]. Because of approval without an additional clinical study, to date, no evidence is available on adverse reactions and inability to work following a BA.4-5 adapted, bivalent COVID-19 vaccination.
This nonrandomized controlled study examined adverse reactions, as-needed medication intake, and inability to work after a fourth vaccination (i.e. second booster) among healthcare workers of the prospective CoVacSer study including follow-up participation until 17 November 2022. All enrolled individuals previously had been administered three COVID-19 vaccine doses. The second booster was performed with the monovalent BNT162b2 mRNA vaccine or the bivalent BNT162b2 mRNA original/Omicron BA.4-5 vaccine. Study participants administered with a different COVID-19 vaccine as second booster were excluded. As coadministration of COVID-19 and influenza vaccines might influence immunogenicity and side effects [4], individuals who received a simultaneous influenza vaccine were also excluded.
The study protocol was approved by the ethics committee of the University of Wuerzburg (file no. 79/21). Data were collected through a questionnaire using Research Electronic Data Capture (projectredcap.org). Data analysis was performed using GraphPad Prism 9.4.1 (GraphPad Software). Null-hypothesis testing was performed using Fisher's exact test (for gender, smoking, SARS-CoV-2 convalescence, side effects, as-needed drug intake, and percentage inability to work) and Mann-Whitney U test (for body mass index [BMI], age, and time intervals). The two-tailed significance level α was set to 0.05.
One hundred four healthcare workers received a fourth dose of COVID-19 vaccine between 13 August 2021 and 28 October 2022 with either the original, monovalent BNT162b2 mRNA (38.5%, 40/104) or bivalent BNT162b2 mRNA original/Omicron BA.4-5 vaccines (61.5%, 64/104). Individuals who received the bivalent vaccine showed no statistically significant differences to those who received the monovalent vaccine regarding gender (82.8% vs. 77.5% women), median age (51 [interquartile range: 40–66] vs. 47 [34–58] years), median BMI (24.1 [21.6–29.1] vs. 24.5 [22.1–29.7] kg/m2), smoking (15.6% vs. 15.0%), COVID-19 convalescence rate (31.3% vs. 17.5%), and time between infection and fourth dose (210 [198–235] vs. 156 [120–242] days). All infections except one occurred after the third vaccine dose. No participant reported having been infected more than once. The median interval between first and second COVID-19 booster vaccinations was significantly longer among bivalent-vaccinated participants compared with monovalent-vaccinated participants (329 [320–335] vs. 199 [118–265] days, p < 0.0001), the median interval between fourth vaccination dose and filling out the questionnaire shorter among bivalent-vaccinated participants (18 [15–22] vs. 22 [15–50] days, p = 0.02).
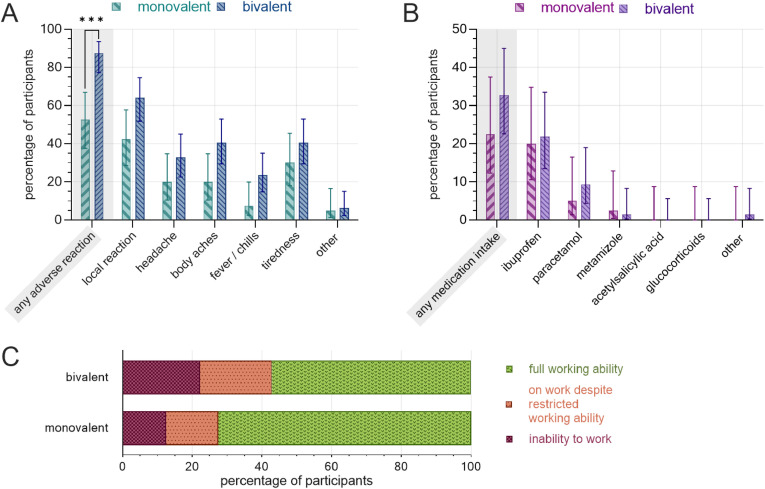
The rate of adverse reactions for the second booster dose was significantly higher among participants receiving the bivalent vaccine (87.5% [95% CI, 77.2%–93.5%; 56/64]) compared with those receiving the monovalent vaccine (52.5% [95% CI, 37.5%–67.1%; 21/40]) vaccine (p = 0.0002). Bivalent-vaccinated participants further reported higher rates of adverse reactions in all subcategories (Fig. 1 (a)). Also, there were more frequent intake of as-needed medication (Fig. 1(b)), numerically higher rates of workability restrictions (Fig. 1(c)), and longer mean duration of the inability to work (2.1 ± 3.5 vs. 1.2 ± 0.4 days) in the bivalent-vaccinated group.
Fig. 1.
Postvaccination adverse reactions, as-needed medication, and inability to work following the second COVID-19 booster administration, separated by vaccine. (a) Rate of adverse reactions by subcategory. (b) Rate of as-needed medication. (c) Workability restrictions. Monovalent: BNT162b2 mRNA (n = 40), bivalent: BNT162b2 mRNA original/Omicron BA.4-5 (n = 64). Error bars indicate 95% CI. ∗∗∗: p < 0.001.
In a multiple logistic regression including vaccine type, convalescence, gender, age, smoking, BMI, and interval between third and fourth vaccination and between fourth vaccination and filling out the questionnaire, significant effects of the bivalent vaccine (p < 0.0001) and a shorter interval between third and fourth vaccine dose (p = 0.03) on a higher rate of adverse reactions could be observed along with nonsignificant effects of the bivalent vaccine on more frequent as-needed medication intake (p = 0.27) and workability restrictions (p = 0.15). SARS-CoV-2 convalescence showed a significant effect on more frequent workability restrictions (p = 0.01) and nonsignificant effects on a higher rate of adverse reactions (p = 0.23) and as-needed medication intake (p = 0.32) after the fourth vaccine dose.
Individuals receiving a second COVID-19 booster vaccination with the bivalent BNT162b2 mRNA original/Omicron BA.4-5 vaccine reported adverse reactions more frequently compared with those receiving the monovalent vaccine. There was a trend towards an increased rate of inability to work and intake of as-needed medication following bivalent vaccination. Limitations of this study are the retrospective questionnaire–based assessment, the lack of randomization and blinding, and the difference in the interval between both booster vaccinations in the two groups. Owing to underdetection of asymptomatic SARS-CoV-2 infections, the convalescent rate might be underestimated. Our study focused on a direct comparison between the monovalent BNT162b2 mRNA and the corresponding bivalent vaccine. In the light of preprints reporting inconclusive results in neutralising antibody levels between the compared vaccines [[5], [6], [7]], our results and further studies on safety and reactogenicity of bivalent COVID-19 booster vaccines are very important to aid clinical decision-making in the choice between bivalent and monovalent vaccinations.
Author contributions
All authors had unlimited access to all data. I.W. and M.K. take responsibility for data integrity and accuracy of data analysis. Conception and design: O.K., N.P., M.K. Trial management: I.W., J.R., N.P., M.K. Statistical analysis: I.W., J.R., A.G., L.K., M.K. Obtained funding: O.K. First draft of the manuscript: I.W., L.K., M.K. The manuscript was reviewed and approved by all authors.
Transparency declaration
M.K. receives honoraria from GlaxoSmithKline and Pfizer outside the submitted work. All other authors declare no potential conflicts of interest.
This study was funded by the Federal Ministry of Education and Science, Germany (BMBF) through a grant provided to the University Hospital of Wuerzburg by the Network University Medicine on COVID-19 (B-FAST, grant-No 01KX2021) as well as by the Free State of Bavaria with COVID-research funds provided to the University of Wuerzburg, Germany. N.P. is supported by the German Research Foundation (DFG) funded scholarship UNION CVD.
This study was initiated by the investigators. The sponsoring institutions had no function in study design, data collection, and analysis and interpretation of data as well as in the writing of the manuscript. All authors had unlimited access to all data. I.W., J.R., A.G., N.P., and M.K. had the final responsibility for the decision to submit for publication.
Data sharing statement
Additional data that underlie the results reported in this article, after deidentification (text, tables, figures, and appendices) as well as the study protocol, statistical analysis plan, and analytic code is made available to researchers who provide a methodologically sound proposal addressed to the corresponding author.
Previous presentation of the information reported in the manuscript
The preliminary analysis of the study has been published as a preprint on medRxiv (https://www.medrxiv.org/content/10.1101/2022.11.07.22281982v1).
Acknowledgements
We explicitly thank Professor Ulrich Vogel, Infection Control and Antimicrobial Stewardship Unit, University Hospital Würzburg, Germany, for conception and design as well as funding support. He played a major role in the CoVacSer study but could not approve the final manuscript version as he died on 4 October 2022. We miss him as an enthusiastic colleague and friend who showed great dedication to his work, family, and friends.
Handling Editor: L. Leibovici
References
- 1.Andrews N., Stowe J., Kirsebom F., Toffa S., Rickeard T., Gallagher E., et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchan S.A., Chung H., Brown K.A., Austin P.C., Fell D.B., Gubbay J.B., et al. Estimated effectiveness of COVID-19 vaccines against Omicron or Delta symptomatic infection and severe outcomes. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.32760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalkias S., Harper C., Vrbicky K., Walsh S.R., Essink B., Brosz A., et al. A bivalent Omicron-containing booster vaccine against Covid-19. N Engl J Med. 2022;387:1279–1291. doi: 10.1056/NEJMoa2208343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagenhäuser I., Reusch J., Gabel A., Höhn A., Lâm T.-T., Almanzar G., et al. Immunogenicity and safety of coadministration of COVID-19 and influenza vaccination. Eur Respir J. 2023;61 doi: 10.1183/13993003.01390-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Q., Bowen A., Valdez R., Gherasim C., Gordon A., Liu L., et al. Antibody responses to Omicron BA.4/BA.5 bivalent mRNA vaccine booster shot. bioRxiv. 2022:2022. doi: 10.1101/2022.10.22.513349. preprint. [DOI] [Google Scholar]
- 6.Collier A-ry., Miller J., Hachmann N.P., McMahan K., Liu J., Bondzie E.A., et al. Immunogenicity of the BA.5 bivalent mRNA vaccine boosters. bioRxiv. preprint 2022 doi: 10.1101/2022.10.24.513619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis-Gardner M.E., Lai L., Wali B., Samaha H., Solis D., Lee M., et al. Neutralization against BA.2.75.2, BQ.1.1, and XBB from mRNA bivalent booster. N Engl J Med. 2022;388:183–185. doi: 10.1056/NEJMc2214293. [DOI] [PMC free article] [PubMed] [Google Scholar]