Abstract
Background
Neurological symptoms, in particular cognitive deficits, are common in post-COVID-19 syndrome (PCS). There is no approved therapy available, and the underlying disease mechanisms are largely unknown. Besides others, autoimmune processes may play a key role.
Design
We here present data of a prospective study conducted between September 2020 and December 2021 and performed at two German University hospitals with specialized Neurology outpatient clinics. Fifty patients with self-reported cognitive deficits as main complaint of PCS and available serum and CSF samples were included. Cell-based assays and indirect immunofluorescence on murine brain sections were used to detect autoantibodies against intracellular and surface antigens in serum and CSF and analyzed for associations with cognitive screening assessment.
Results
Clearly abnormal cognitive status (MoCA ≤ 25/30 points) was only seen in 18/50 patients with self-reported cognitive deficits. Most patients (46/50) had normal routine CSF parameters. anti-neuronal autoantibodies were found in 52 % of all patients: n = 9 in serum only, n = 3 in CSF only and n = 14 in both, including those against myelin, Yo, Ma2/Ta, GAD65 and NMDA receptor, but also a variety of undetermined epitopes on brain sections. These included cerebral vessel endothelium, Purkinje neurons, granule cells, axon initial segments, astrocytic proteins and neuropil of basal ganglia or hippocampus as well as a formerly unknown perinuclear rim pattern. Pathological MoCA results were associated with the presence of anti-neuronal antibodies in CSF (p = 0.0004).
Conclusions
Autoantibodies targeting brain epitopes are common in PCS patients and strongly associate with pathological cognitive screening tests, in particular when found in CSF. Several underlying autoantigens still await experimental identification. Further research is needed to inform on the clinical relevance of these autoantibodies, including controlled studies that explore the potential efficacy of antibody-depleting immunotherapy in PCS.
Keywords: Post-COVID-19, CSF, Autoantibody, Neurology, Neurocognitive disorder
1. Introduction
1.1. Neurological symptoms in post-COVID-19 syndrome
A variety of symptoms has been observed in patients with post-COVID-19 syndrome (PCS). Cognitive deficits are frequently reported (Huang et al., 2021) as one of the major symptoms of PCS. PCS is defined by new or ongoing symptoms three months after the onset of acute COVID-19 that last for at least 2 months, fluctuate in appearance, and are not explained by another diagnosis (Soriano et al., 2022). To date pathophysiological mechanisms of PCS are scarcely understood. The lack of predictive biomarkers impedes the objectivation of patients’ complaints and development of therapeutic options. It is debated whether (direct) viral infection of nervous tissue can cause PCS. In most published studies, SARS-CoV-2 RNA was not detected in cerebrospinal fluid (CSF), and a study on intrathecally produced SARS-CoV-2 directed IgG antibodies was not suggestive of chronic viral infection as the cause of PCS (Schweitzer et al., 2022). In contrast, the finding of autoantibodies in patients during acute COVID-19 suggested that autoimmunity might contribute to PCS (Wang et al., 2021, Woodruff et al., 2022). Such mechanisms are well-known from other viral infections, such as post-viral NMDA receptor encephalitis after herpes simplex virus encephalitis (HSE) (Prüss, 2021, Prüss et al., 2012). We therefore examined the presence of anti-neuronal and anti-glial autoantibodies in serum and CSF of PCS patients with self-reported cognitive deficits.
2. Methods
Between September 2020 and December 2021, patients fulfilling the criteria of PCS were screened at two tertiary care centers (Charité – Universitätsmedizin Berlin, and Universitätsklinikum Köln). Patients presenting with self-reported cognitive deficits as main symptom underwent clinical and laboratory work up including cranial MR imaging, cognitive screening using the Montreal Cognitive Assessment (MoCA) and blood examination. A fraction of patients underwent a lumbar puncture, at the discretion of the treating physician and as part of the clinical routine. Written informed consent for research and publication was obtained (ethics committee approval, Berlin: EA2/066/20, Cologne: 20-1501). Autoantibodies against intracellular and surface antigens relevant for central nervous system diseases were measured by line blots, ELISA and cell-based assays (Labor Berlin, Germany) and included antibodies against amphiphysin, CV2 (CRMP5), GAD65, Hu, Ri, Yo, Ma2/Ta, Tr (DNER), GAD65, glutamate receptor (AMPAR1/2, NMDAR), DPPX, GABAAR, GABABR, mGluR5, LGI1, myelin, Caspr2, dopamine-2 receptor, aquaporin-4, skeletal muscle and phospholipids (cardiolipin, beta2-glycoprotein, annexin). In addition, indirect immunofluorescence on unfixed murine brain sections was performed to search for novel autoantibodies not included in the clinical routine assays, according to established protocols (Prüss et al., 2012, Kreye et al., 2020, Franke et al., 2021). One-way ANOVA was used to analyze MoCA results for patients with autoantibodies detected in CSF (AbCSF)), detected only in serum (Abserum) or without detection of autoantibodies (Ababsent).
3. Results
3.1. Patient characteristics
A total of 360 patients with positive SARS-CoV-2 PCR testing during acute COVID-19 and residual neurological symptoms were screened for this study at the Neurology outpatient clinics in Berlin (260 patients) and Cologne (100 patients) after a median of 250 days [100–597 days] since the acute infection. None of the patients had been vaccinated against SARS-CoV-2 at the time of infection. Self-reported cognitive deficits were indicated as the main symptom in 232 patients; 128 patients reported other predominant neurological complaints. Patients with other causes likely responsible for their cognitive deficits (e.g. metabolic disease, preexisting neurological disease including sleep apnea syndrome and/or preexisting psychiatric disease including depression) were excluded. They did not receive MoCA testing and were referred for further diagnostic and therapy. A lumbar puncture was recommended in all other unexplained cases, and performed in 50 patients (median age 47 [22–78 years], 17 male).
3.2. Cognitive screening, laboratory findings and imaging in patients with self-reported cognitive deficits
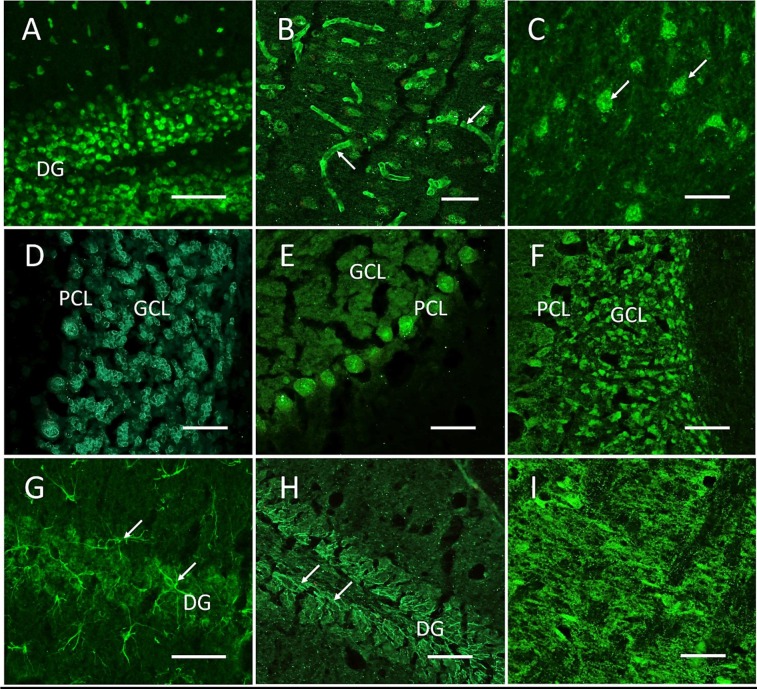
Of the 50 patients receiving a lumbar puncture, 18 presented with pathological MoCA score results (MoCA ≤ 25 points (median 23, [13–25 points]). Thirty-two patients presented with MoCA > 25 (median 27, [26–29 points]) and were considered as subjective cognitive decline (SCD). Mild pleocytosis was found in 2/50 patients. CSF protein was elevated in 2/50 and CSF-specific oligoclonal bands (OCB) present in 4/50 patients. Using routine diagnostics, serum antibodies were identified against myelin (n = 12), Yo (n = 2), Ma2/Ta (n = 1), and GAD65 (n = 1). One patient showed NMDA receptor (NMDAR) antibodies (IgG) in serum (titer 1:10) and CSF (titer 1:1) in cell-based assays indicating autoimmune activation, however, lack of staining with indirect immunofluorescence on mouse brain sections excluded NMDAR encephalitis. CSF analysis for the presence of anti-neuronal autoantibodies in a complementary assay using indirect immunofluorescence on unfixed mouse brain sections reproducibly showed strong autoreactivity in 16/50 patients, following nine distinct patterns (Table 1 , Fig. 1 ). IgG staining patterns included vessel endothelium, Purkinje neurons, granule cells, axon initial segments, astrocytic proteins and neuropil of basal ganglia or hippocampus as well as a formerly unknown perinuclear rim pattern (Fig. 1). Cranial MR imaging did not reveal pathological findings correlating with cognitive impairment including atrophy.
Table 1.
Patient characteristics and laboratory findings.
| Patient N° | Sex | MoCA | CSF |
OCB |
Autoantibody panel(a) |
Indirect immunofluorescence (IgG)(b) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell count [<5/µl] | Glucose [mg/dl] | Lactate[<22 mg/dl] | Protein [<450 mg/l] | Serum | CSF | Serum | CSF | Serum | CSF | ||||
| #1 | 44 | m | 25 | 1 | 56 | 12,4 | 398 | neg | neg | Myelin 1:100 | neg. | ANA | ANA |
| #2 | 27 | f | 25 | 5 | 60 | 12,2 | 357 | neg. | pos. | neg. | neg. | LN | LN |
| #3 | 51 | m | 20 | 0 | 116 | 20,4 | 393 | neg | neg | Myelin 1:100 & GAD |
neg. | ANA; VS;GC | ANA; VS; GC |
| #4 | 51 | m | 24 | 1 | 72 | 15,3 | 252 | neg. | neg | neg. | neg. | neg | VS |
| #5 | 43 | f | 23 | 2 | 59 | 16,8 | 255 | pos. | pos. | neg. | neg. | GC; NP | GC; NP |
| #6 | 58 | m | 25 | 3 | 67 | 15,9 | 275 | neg. | pos. | Myelin 1:100 | Yo | neg | ANA |
| #7 | 57 | f | 24 | 1 | 66 | 13,8 | 268 | neg. | neg. | neg. | neg. | neg | Astro |
| #8 | 66 | m | 25 | 0 | 66 | 15,1 | 406 | neg. | neg. | neg. | neg. | ANA | ANA |
| #9 | 45 | f | 21 | 3 | 77 | 18,1 | 336 | neg. | neg. | Myelin 1:100 | neg. | neg | PRP |
| #10 | 52 | f | 27 | 3 | 78 | 17,6 | 596 | neg. | neg. | Myelin 1:100 | neg. | neg | AIS |
| #11 | 43 | f | 25 | 3 | 66 | 15 | 236 | neg. | neg. | neg. | neg. | neg | PN |
| #12 | 78 | m | 21 | 1 | 91 | 20 | 418 | pos. | pos. | neg. | neg. | ANA; PN | ANA; PN |
| #13 | 60 | m | 28 | 1 | 63 | 12 | 377 | neg. | neg. | Myelin 1:100 | neg. | PRP | PRP |
| #14 | 70 | f | 25 | 2 | 59 | 12,9 | 357 | neg. | neg. | neg. | neg. | ANA; GC | ANA; GC |
| #15 | 49 | f | 25 | 2 | 59 | 13,2 | 281 | neg. | neg. | neg. | neg. | ANA | ANA |
| #16 | 53 | f | 25 | 1 | 54 | 14 | 333 | neg. | neg. | neg. | neg. | ANA | ANA |
| #17 | 36 | f | 26 | 1 | 60 | 17,7 | 257 | neg. | neg. | Ma2/Ta | neg. | neg. | neg. |
| #18 | 39 | f | 28 | 2 | 63 | 13,9 | 235,8 | neg. | neg. | neg. | neg. | neg. | neg. |
| #19 | 22 | f | 26 | 3 | 63 | 14 | 191 | neg. | neg. | GAD | neg. | neg. | neg. |
| #20 | 51 | m | 26 | 1 | 69 | 17,2 | 250 | neg. | neg. | Myelin 1:100 | neg. | neg. | neg. |
| #21 | 28 | f | 26 | 1 | 60 | 11,5 | 158 | neg. | neg. | neg. | neg. | neg. | neg. |
| #22 | 67 | f | 20 | 3 | 69 | 14,6 | 275 | neg. | neg. | Myelin 1:1000, NMDAR-IgG 1:10 | NMDAR-IgG 1:1 | neg. | neg. |
| #23 | 43 | f | 24 | 1 | 64 | 12 | 209 | neg. | neg. | Myelin 1:100 | neg. | neg. | neg. |
| #24 | 49 | f | 26 | 2 | 70 | 17 | 294 | neg. | neg. | Yo | neg. | neg. | neg. |
| #25 | 51 | f | 28 | 2 | 60 | 15,6 | 267 | pos. | pos. | Yo | neg. | neg. | neg. |
| #26 | 46 | m | 27 | 4 | 55 | 12,2 | 334 | neg. | neg. | Myelin 1:100 | neg. | neg. | neg. |
| #27 | 49 | f | 28 | 1 | 53 | 11,8 | 205 | neg. | pos. | Myelin 1:100 | neg. | neg. | neg. |
| #28 | 53 | f | 29 | 7 | 58 | 12 | 360 | neg. | neg. | neg. | neg. | neg. | neg. |
| #29 | 44 | f | 29 | 1 | 63 | 14,5 | 206 | neg. | neg. | neg. | neg. | neg. | neg. |
| #30 | 57 | f | 28 | 8 | 60 | 15,5 | 414 | neg. | neg. | neg. | neg. | neg. | neg. |
| #31 | 39 | f | 26 | 1 | 61 | 13,8 | 238 | pos. | pos. | neg. | neg. | neg. | neg. |
| #32 | 38 | f | 29 | 1 | 57 | 14,3 | 191 | neg. | neg. | neg. | neg. | neg. | neg. |
| #33 | 63 | m | 24 | 4 | 62 | 16,5 | 548 | neg. | neg. | neg. | neg. | neg. | neg. |
| #34 | 31 | f | 28 | 2 | 60 | 16,4 | 287 | neg. | neg. | neg. | neg. | neg. | neg. |
| #35 | 40 | f | 30 | 3 | 66 | 13,8 | 302 | neg. | neg. | neg. | neg. | neg. | neg. |
| #36 | 32 | f | 30 | 2 | 57 | 13,3 | 235 | neg. | neg. | neg. | neg. | neg. | neg. |
| #37 | 24 | f | 26 | 1 | 55 | 13,4 | 221 | neg. | neg. | neg. | neg. | neg. | neg. |
| #38 | 56 | m | 26 | 2 | 68 | 17,5 | 404 | neg. | neg. | neg. | neg. | neg. | neg. |
| #39 | 36 | m | 28 | 4 | 65 | 13,9 | 342 | neg. | neg. | neg. | neg. | neg. | neg. |
| #40 | 39 | f | 27 | 3 | 53 | 16,4 | 185 | neg. | neg. | neg. | neg. | neg. | neg. |
| #41 | 45 | f | 29 | 2 | 59 | 13,7 | 276 | neg. | neg. | neg. | neg. | neg. | neg. |
| #42 | 40 | m | 24 | 6 | 70 | 12,6 | 390 | neg. | neg. | neg. | neg. | neg. | neg. |
| #43 | 48 | f | 26 | 0 | 54 | 13,5 | 300 | neg. | neg. | neg. | neg. | neg. | neg. |
| #44 | 34 | m | 29 | 0 | 55 | 14,5 | 420 | neg. | neg. | neg. | neg. | neg. | neg. |
| #45 | 57 | m | 26 | 2 | 71 | 19,1 | 345 | neg. | neg. | neg. | neg. | neg. | neg. |
| #46 | 51 | f | 28 | 2 | 70 | 15,3 | 376 | neg. | neg. | neg. | neg. | neg. | neg. |
| #47 | 49 | m | 29 | 3 | 61 | 13,9 | 412 | neg. | pos. | neg. | neg. | neg. | neg. |
| #48 | 55 | f | 26 | 3 | 76 | 15,8 | 358 | neg. | neg. | Myelin 1:100 | neg. | neg. | neg. |
| #49 | 51 | m | 27 | 3 | 63 | 13,1 | 362 | neg. | neg. | neg. | neg. | neg. | neg. |
| #50 | 36 | f | 27 | 1 | 61 | 13,7 | 306 | neg. | neg. | neg. | neg. | neg. | neg. |
Abbreviations: ANOVA: one-way analysis of variance; Cohen’s d: effect size; AbCSF: autoantibodies in cerebrospinal fluid (CSF), n = 17 patients (with or without autoantibodies in serum); Abserum: autoantibodies in serum only, n = 9 patients; Ababsent: no detection of autoantibodies n = 24 patients.
(a) includes antibodies against amphiphysin, CV2 (CRMP5), GAD65, Hu, Ri, Yo, Ma2/Ta, Tr (DNER), GAD65, glutamate receptor (AMPAR1/2, NMDAR), DPPX, GABAAR, GABABR, mGluR5, LGI1, myelin, Caspr2, dopamine-2 receptor, aquaporin-4, skeletal muscle and phospholipids (cardiolipin, beta2-glycoprotein, annexin).
(b) Autoantibody detection by indirect immuno-fluorescence (IgG) on unfixed murine brain sections using serum and CSF are grouped into the following nine patterns (corresponding to Fig. 1): ANA, anti-nuclear antibodies; vS vessel; LN, large neurons; PRP, perinuclear rim pattern; PN, Purkinje neurons; GC, granule cells; Astro, astrocytes; AIS, axon initial segment; NP, neuropil.
Fig. 1.
CSF of patients with neurocognitive disorders in post-COVID-19 syndrome frequently shows autoreactivity on unfixed mouse brain sections. Representative images of indirect immunofluorescence using undiluted CSF with incubation overnight at 4 °C demonstrate autoantibody binding following nine major patterns including anti-nuclear antibodies (A, from patient #8), vessel endothelium (B), large neurons (C, from patient #2), perinuclear rim pattern (D, from patient #13), Purkinje neurons (E, from patient #12), cerebellar granule cells (F, from patient #5), astrocytic proteins (G, from patient #7), axon initial segments (H, from patient #10) and neuropil of basal ganglia (I, from patient #5) or hippocampus.
3.3. MoCA results in relation to anti-neuronal antibodies
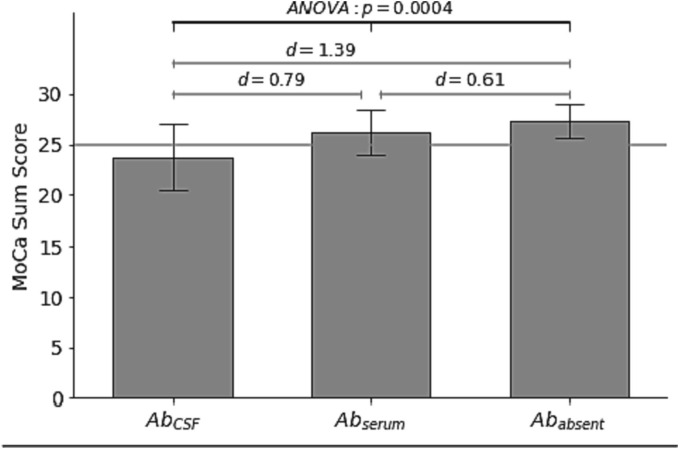
In 52 % (n = 26) of all patients of our cohort autoantibodies were detected. While most patients with CSF autoantibodies also had autoantibodies in serum (n = 14), three patients had autoantibodies selectively in CSF and nine patients selectively in serum. The patients with autoantibodies in CSF (with or without autoantibodies in serum) showed significantly lower MoCA scores compared to the PCS patients who also reported on predominant cognitive deficits but without detection of autoantibodies (Fig. 2 ). One-way ANOVA revealed a difference of MoCA results between the three patient groups (F(2) = 9.43, p = 0.0004), and post-hoc analysis showed intermediate to large effects (AbCSF-Abserum: T = 2.19, p = 0.019, d = 0.79; Abserum-Ababsent: T = 1.54, p = 0.071, d = 0.61; AbCSF-Abserum: T = 3.85, p = 0.0005, d = 1.39), indicating clinically important differences for all comparisons (Fig. 2).
Fig. 2.
Results of the cognitive screening for PCS patient groups with any anti-neuronal autoantibodies found in CSF (left) or in serum only (middle) or antibody-negative patients (right).
4. Discussion
We report on the high frequency of anti-neuronal autoantibodies in patients with predominant cognitive deficits in PCS and no other reason for cognitive dysfunction. Interestingly, correlation of cognitive deficits measured by MoCA with autoantibodies were particularly strong when antibodies were present in CSF. The findings support the concept that humoral autoimmunity may contribute to the development of cognitive impairment in some PCS patients. The patterns of anti-neuronal and anti-glial autoantibodies in serum and CSF overlap with those seen in acute COVID-19. For example, NMDA receptor, GAD and myelin antibodies were also detected in patients with neurological manifestations during the acute infection9, 10. The brain immunofluorescence ‘perinuclear rim pattern’ (Fig. 1, D) has been detected exclusively in patients with acute or post-COVID-19, indicating a potentially disease-specific epitope binding. The routine parameters of CSF (such as protein concentration, lactate or white blood cell counts) and structural MR imaging of the brain were normal in most patients, thus not well reflecting the patient’s presentation, partially with severe clinical impairment, and therefore seem less helpful in identification of patients with suggestive autoimmune pathomechanisms. This is different from CSF findings during acute COVID-19 where disruption of the blood-CSF barrier was consistently found in the absence of intrathecal inflammation (Franke et al., 2021, Jarius et al., 2022).
Besides autoimmunity, various alternative mechanisms in PCS pathogenesis are currently under investigation, such as those centering on viral persistence (Song et al., 2021), secondary unspecific inflammation (Meinhardt et al., 2021), endothelial dysregulation (Varga et al., 2020) and humoral targets (Murphy and Longo, 2022, Bertin et al., 2021). Given the strong medical need to care for PCS patients and the absence of systematic clinical immunotherapy trials in this population, implementation of CSF analyses in clinical routine diagnostics of PCS may help guiding individual treatment attempts in the future.
5. Strengths and limitations
A strength of our study is the prospective design and the blinded assessment of clinical and laboratory findings. All participants were clinically examined including the MoCA by the same investigator at each site, eliminating inter-observer bias. Laboratory findings including CSF analysis for the presence of anti-neuronal autoantibodies were performed with commercial routine assays, and a team unaware of the clinical status of the patients performed indirect immunofluorescence. Our finding of several groups of anti-neuronal autoantibodies in PCS patients warrants experimental identification of the target proteins as those may not only help to better understand post-viral disease mechanisms but also to develop improved diagnostic assays.
One of the obvious limitations of our study is the lack of CSF from fully healthy volunteers, related to the invasive nature of the lumbar puncture and the strict ethical regulation regarding voluntary CSF sampling in Germany. The here observed frequency of CSF autoantibodies exceeds the number in other disease cohorts, in routine samples of our diagnostic laboratory and in published controls analyzed with the identical methodology (Schumacher et al., 2019, Franke et al., 2021, Lutt et al., 2018, Doss et al., 2014). In-depth investigation and larger cohorts are needed to analyze whether the described autoantibodies may serve as biomarkers, as well as their (clinical) relevance, if present in serum and/or CSF. Further autoantibodies will likely be found in the future, such as those against G protein-coupled receptors seen in serum and CSF in one study assessing patients with Long-COVID (Wallukat et al., 2021), even though correlation with clinical symptoms is pending. This is particularly important as PCS-like symptoms were equally frequent in patients with and without SARS-CoV-2 infection in some studies (Matta et al., 2022), supporting the strong medical need for appropriate biomarkers.
Screening assessment of cognitive function using MoCA is commonly used in neurological outpatient clinics although MoCA might have a low sensitivity for minor cognitive deficits in non-neurodegenerative conditions (Nersesjan et al., 2022). Perhaps due to its practicability and especially in the context of cognitive impairment in PCS the MoCA is commonly used, leading to robust comparability of the various studies within the same context (Crivelli et al., 2022). Besides the necessity of further and in-depth diagnostic testing to fully assess neurological and neuropsychiatric impairment, appropriate and thorough neuropsychological test batteries should be exerted additionally to screening instruments.
6. Conclusions
The high frequency of autoantibodies in CSF more than in serum only in patients with PCS and their correlation with pathological MoCA results suggests a potentially causal association with cognitive deficits, frequently reported by PCS patients. While several target autoantigens still await identification in future studies, presence of autoantibodies may explain some aspects of neurological manifestations in PCS and support investigation of immunotherapies.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
Data will be made available on request.
References
- Bertin D., Kaphan E., Weber S., et al. Persistent IgG anticardiolipin autoantibodies are associated with post-COVID syndrome. Int. J. Infect. Dis. 2021;113:23–25. doi: 10.1016/j.ijid.2021.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crivelli L., Palmer K., Calandri I., et al. Changes in cognitive functioning after COVID-19: A systematic review and meta-analysis. Alzheimers Dement. 2022;18:1047–1066. doi: 10.1002/alz.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doss S., Wandinger K.P., Hyman B.T., et al. High prevalence of NMDA receptor IgA/IgM antibodies in different dementia types. Ann. Clin. Transl. Neurol. 2014;1:822–832. doi: 10.1002/acn3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke C., Ferse C., Kreye J., et al. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav. Immun. 2021;93:415–419. doi: 10.1016/j.bbi.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Huang L., Wang Y., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarius S., Pache F., Kortvelyessy P., et al. Cerebrospinal fluid findings in COVID-19: a multicenter study of 150 lumbar punctures in 127 patients. J. Neuroinflammation. 2022;19:19. doi: 10.1186/s12974-021-02339-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreye J., Reincke S.M., Kornau H.C., et al. A Therapeutic Non-self-reactive SARS-CoV-2 Antibody Protects from Lung Pathology in a COVID-19 Hamster Model. Cell. 2020;183(1058–1069):e1019. doi: 10.1016/j.cell.2020.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutt A., Michel K., Kruger D., et al. High prevalence and functional effects of serum antineuronal antibodies in patients with gastrointestinal disorders. Neurogastroenterol. Motil. 2018;30:e13292. doi: 10.1111/nmo.13292. [DOI] [PubMed] [Google Scholar]
- Matta J., Wiernik E., Robineau O., et al. Association of Self-reported COVID-19 Infection and SARS-CoV-2 Serology Test Results With Persistent Physical Symptoms Among French Adults During the COVID-19 Pandemic. JAMA Intern. Med. 2022;182:19–25. doi: 10.1001/jamainternmed.2021.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt J., Radke J., Dittmayer C., et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- Murphy W.J., Longo D.L. A Possible Role for Anti-idiotype Antibodies in SARS-CoV-2 Infection and Vaccination. N. Engl. J. Med. 2022;386:394–396. doi: 10.1056/NEJMcibr2113694. [DOI] [PubMed] [Google Scholar]
- Nersesjan V., Fonsmark L., Christensen R.H.B., et al. Neuropsychiatric and Cognitive Outcomes in Patients 6 Months After COVID-19 Requiring Hospitalization Compared With Matched Control Patients Hospitalized for Non-COVID-19 Illness. JAMA Psychiat. 2022;79:486–497. doi: 10.1001/jamapsychiatry.2022.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prüss H. Autoantibodies in neurological disease. Nat. Rev. Immunol. 2021;21:798–813. doi: 10.1038/s41577-021-00543-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prüss H., Finke C., Holtje M., et al. N-methyl-D-aspartate receptor antibodies in herpes simplex encephalitis. Ann. Neurol. 2012;72:902–911. doi: 10.1002/ana.23689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher H., Wenke N.K., Kreye J., et al. IgA autoantibodies against native myelin basic protein in a patient with MS. Neurol. Neuroimmunol. Neuroinflamm. 2019;6:e569. doi: 10.1212/NXI.0000000000000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer F., Goereci Y., Franke C., et al. Cerebrospinal Fluid Analysis Post-COVID-19 Is Not Suggestive of Persistent Central Nervous System Infection. Ann. Neurol. 2022;91:150–157. doi: 10.1002/ana.26262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song E., Zhang C., Israelow B., et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021;218 doi: 10.1084/jem.20202135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano J.B., Murthy S., Marshall J.C., Relan P., Diaz J.V. Condition WHOCCDWGoP-C-. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022;22:e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallukat G., Hohberger B., Wenzel K., et al. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J. Transl. Autoimmun. 2021;4 doi: 10.1016/j.jtauto.2021.100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E.Y., Mao T., Klein J., et al. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021;595:283–288. doi: 10.1038/s41586-021-03631-y. [DOI] [PubMed] [Google Scholar]
- Woodruff M.C., Ramonell R.P., Haddad N.S., et al. Dysregulated naive B cells and de novo autoreactivity in severe COVID-19. Nature. 2022 doi: 10.1038/s41586-022-05273-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.