Abstract
The related peptides pituitary adenylate cyclase-activating polypeptide (PACAP) and vasoactive intestinal peptide (VIP) have diverse biological functions in peripheral tissues and the central nervous system. Therefore, these peptides and their three receptors represent potential drug targets for several conditions, including neurological and pain-related disorders. However, very little is known about how these peptides regulate their receptors through processes such as internalization. Therefore, we developed tools to study receptor regulation through the synthesis of fluorescently labeled analogues of PACAP-38, PACAP-27, and VIP using copper-mediated 1,3-dipolar cycloaddition of the Cy5 fluorophore. The functionality of Cy5-labeled peptides at their receptors was confirmed in cAMP accumulation assays. Internalization of the Cy5-labeled peptides was then examined and quantified at two distinct PAC1 receptor splice variants, VPAC1 and VPAC2 receptors in transfected cells. All labeled peptides were functional, exhibiting comparable cAMP pharmacology to their unlabeled counterparts and underwent internalization in a time-dependent manner. Temporal differences in the internalization profiles were observed between Cy5-labeled peptides at the PAC1n, PAC1s, VPAC1, and VPAC2 receptors. Interestingly, the pattern of Cy5-labeled peptide activity differed for cAMP accumulation and internalization, indicating that these peptides differentially stimulate cAMP accumulation and internalization and therefore display biased agonism. This novel insight into PACAP-responsive receptor signaling and internalization may provide a unique avenue for future therapeutic development. The fluorescently labeled PACAP and VIP peptides described herein, which we validated as tools to study receptor internalization, will have utility across a broad range of applications and provide greater insight into this receptor family.
Keywords: PACAP, VIP, Cy5, internalization, bias
The pituitary adenylate cyclase-activating polypeptide (PACAP) peptide family includes two biologically active forms of PACAP: the full-length 38 amino acid peptide (PACAP-38) and shorter 27 amino acid peptide (PACAP-27) in addition to the related 28 amino acid vasoactive intestinal peptide (VIP).1 PACAP and VIP are linked to diverse biological functions in both peripheral tissues and the central nervous system.2−4 Not surprisingly, these peptides are implicated in numerous disorders, including neurodegenerative conditions and migraine.1,2,5
The important role PACAP plays in migraine has led to the development of clinical drug candidates targeting the PACAP peptide and receptor family.4,6,7 These drug candidates act to block the activity of PACAP and prevent downstream signaling events following receptor activation. An anti-PACAP antibody has been developed and is currently in clinical trials.4,6 However, an anti-PAC1 receptor antibody, which blocks receptor activation, did not exhibit clinical efficacy against migraine.7 Efficacy in other neurological disorders or diseases has yet to be examined. The reason for this lack of efficacy is unclear but emphasizes the need to better understand how these peptides activate and regulate their receptors to develop effective therapeutics.
The PACAP family of peptides produces their effects through three different PACAP-responsive G protein-coupled receptors; PAC1, VPAC1, and VPAC2. Characteristically, both PACAP-38 and PACAP-27 exhibit higher activity than VIP at the PAC1 receptor, whereas PACAP and VIP activate the VPAC1 and VPAC2 receptors equivalently.1 However, the PAC1 receptor can undergo alternative splicing to generate variants that may differ in their pharmacology and signaling behaviors.8−10 PAC1 receptor splice variants are broadly categorized into two groups based on the location and type of splicing event: either N-terminal deletions or intracellular loop 3 (ICL3) insertions. The receptor with a complete N-terminus and no ICL3 insertion is considered the PAC1n variant. N-terminal variants are known as PAC1s and PAC1vs and ICL3 variants as PAC1Hop and PAC1Hip.11 Although PACAP is generally reported to have a higher potency than VIP at PAC1 receptors, this is not the case at the PAC1s receptor splice variant, where PACAP and VIP display similar potency.8,10,12
The activation of a PACAP-responsive receptor initiates a series of intracellular signaling events, which canonically involve the accumulation of cAMP.10 Interestingly, biased agonism, which refers to the activation of different signaling pathways by different ligands, has been reported for the PAC1 receptor.13−15 Thus, different peptides could activate the same PACAP-responsive receptor but stimulate different signaling events, yielding diverse pharmacological outcomes.
Receptor internalization is an important regulator of intracellular signaling responses by limiting the number of receptors at the cell surface and preventing further cell surface receptor activation. This occurs through the translocation of activated receptors from the cell surface into endosomal vesicles, which can then be targeted for degradation or recycling.16,17 However, evidence now suggests that internalized receptors can continue to signal from endosomes, where they can activate similar signaling pathways to those at the cell surface or specific endosomal signaling pathways.16,18,19 Interestingly, endosomal signaling through the PAC1 receptor has been linked to chronic pain and anxiety-like responses.20,21 This is consistent with other reports where endosomal signaling contributes to sustained neural excitation, which results in enhanced pain behaviors.16,19,22
Investigation of PACAP-responsive receptor internalization has been undertaken.23−27 Studies using tagged receptors or fluorescently labeled peptides suggest that these receptors can undergo internalization in a time-dependent manner with most studies focusing on PAC1.25−29 However, typically only a single peptide, receptor subtype, or splice variant have been examined in any individual study. Consequently, there is currently an incomplete picture of PACAP-responsive receptor internalization, and potential peptide or receptor-dependent differences may have been missed. Such differences could have significant implications for understanding the role of the PACAP peptide family in physiological functions. Thorough comparisons using multiple peptide ligands at multiple receptor subtypes are required to elucidate these behaviors.
Traditionally, radiolabeled peptide probes have been used to investigate receptor binding and peptide-stimulated receptor internalization.30,31 More recently, fluorescent imaging techniques have matured and are increasingly used to investigate these aspects of receptor biology.32,33 These methods involve using either fluorescently labeled peptides or antibodies that target the receptor to allow visualization and quantification of internalization. Characterized fluorescently labeled probes are invaluable pharmacological tools that aid the understanding of agonist-mediated internalization for a wide range of receptors.32−35 Fluorescently labeled PACAP or VIP has previously been generated and used to investigate receptor internalization or tissue distribution.23,24,36−38 However, several of these tools were not site-specifically labeled, as the synthesis approach used could generate a mixture of peptides labeled at different sites, or were not robustly pharmacologically characterized.
This study aimed to develop and validate a series of fluorescently labeled PACAP and VIP peptides that could be used as tools to study cellular aspects of PACAP-responsive receptor behavior, including receptor internalization. We synthesized Cy5-conjugated PACAP-38, PACAP-27, and VIP peptides by click chemistry and pharmacologically characterized their ability to stimulate PAC1n, PAC1s, VPAC1, and VPAC2 receptor-mediated cAMP accumulation in transfected cells. These were then used to investigate internalization by fluorescent imaging at all three receptor subtypes, including the previously unexplored PAC1s receptor splice variant.
Results and Discussion
Synthesis of Cy5-Labeled PACAP and VIP Peptides
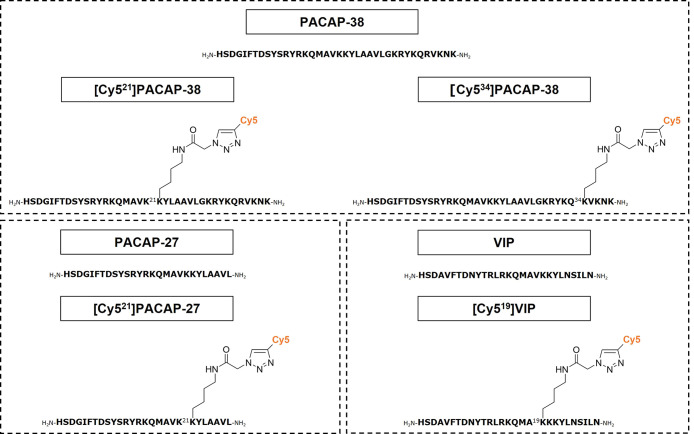
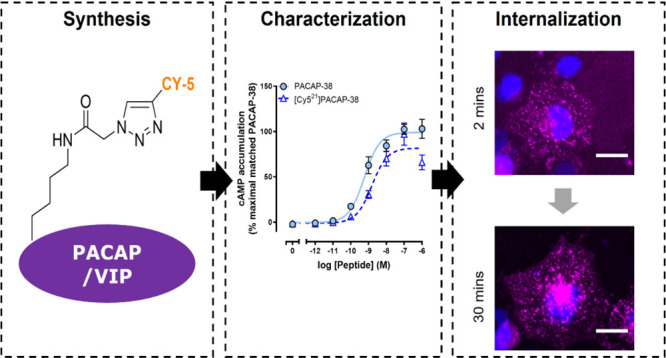
Our selection of labeling sites was guided by published targeted mutagenesis studies as at the time of initiating these studies, only limited structural data were available.39−41 These suggested that several positions within PACAP and VIP may tolerate the incorporation of an azido-lysine and the subsequent conjugation of a Cy5 alkyne fluorophore. Position 21 of both PACAP-27 and PACAP-38 was selected as mutagenesis to glutamic acid had a minimal effect on peptide binding at the three receptor subtypes.39 In addition, position 34 of PACAP-38 was selected to synthesize a second Cy5-labeled PACAP-38 analogue as mutagenesis to lysine did not significantly affect peptide binding.40 Alanine scanning and screening of VIP binding at the VPAC1 receptor suggested that modification at position 19 would be well tolerated.41 Therefore, 1,3-dipolar cycloaddition of an in-house Cy5 alkyne fluorophore was employed to synthesize the following site-specifically labeled fluorescent peptides: [Cy521]PACAP-38, [Cy534]PACAP-38, [Cy521]PACAP-27, and [Cy519]VIP, as depicted in Figure 1.
Figure 1.
Chemical structures of fluorescently labeled peptides illustrating the position of Cy5 attachment to PACAP-38, PACAP-27, and VIP.
Incorporation of Cy5 into PACAP and VIP Was Tolerated Pharmacologically
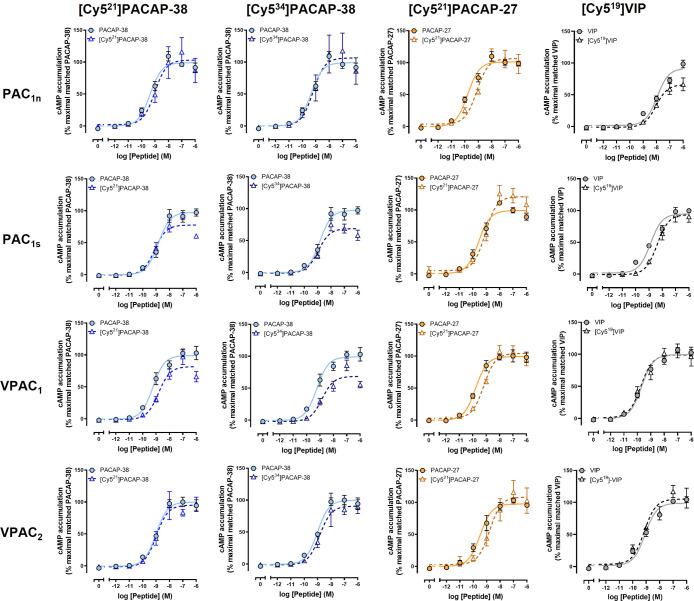
Following synthesis, it was essential to characterize the pharmacological activity of the Cy5-labeled peptides at their receptors to determine if the activity had been affected. To achieve this, peptide-induced cAMP accumulation was measured in receptor-transfected Cos7 cells. Cos7 cells were selected as they do not respond to PACAP endogenously.10,42,43 cAMP accumulation was selected as this pathway was robustly activated in the Cos7 cell model system and displayed similar relative pharmacological profiles to other signaling pathways.10 Furthermore, cAMP accumulation is a well-described measure of receptor activation, which is relative to receptor binding for the PACAP-responsive receptor family.8,12,43 We therefore opted to use cAMP accumulation as a measure of receptor activation and an indirect indicator of receptor binding. Multiple peptide concentrations were used to generate concentration–response curves from which the peptide potency (pEC50) and maximal response (Emax) at each receptor were calculated and compared to the corresponding unlabeled peptide. [Cy521]PACAP-38 and [Cy534]PACAP-38 were equipotent to unlabeled PACAP-38 at stimulating cAMP accumulation at the PAC1n, PAC1s, VPAC1, and VPAC2 receptors (Figure 2, Table S1). However, a significantly lower Emax was observed for both labeled peptides at the PAC1s and VPAC1 receptors. [Cy521]PACAP-27 was significantly less potent compared to unlabeled PACAP-27 at all receptors; however, this difference was relatively small at less than 5-fold (Figure 2, Table S2). The retention of potency for both Cy5-labeled PACAP-38 and PACAP-27 is not surprising based on cryo-EM structures of the peptide-bound PAC1 and VPAC2 receptors that are now available.44−46 These studies identified that position 21 of PACAP did not make crucial receptor interactions, while the C-terminus of PACAP-38 could not be resolved and is likely a highly flexible region that forms weak receptor interactions. [Cy519]VIP stimulated cAMP accumulation at all receptors equipotently to its unlabeled peptide but exhibited a significantly lower Emax at the PAC1n receptor (Figure 2, Table S3). No VIP-bound receptor structure has been solved, but the findings of the current study suggest the valine at position 19 is not required for high-affinity binding. Overall, Cy5 labeling of all peptides was well tolerated at the PAC1n, PAC1s, VPAC1, and VPAC2 receptors. Peptide potencies were consistent or within a ∼5-fold range to those reported in the literature for unlabeled PACAP and VIP.8,10,43 The fluorescent peptides were therefore further characterized for their ability to undergo receptor-mediated internalization.
Figure 2.
Stimulation of cAMP accumulation by unlabeled and Cy5-labeled PACAP-38, PACAP-27, and VIP in Cos7 cells transfected with the PAC1n, PAC1s, VPAC1, or VPAC2 receptor. Data were normalized to the maximal responses produced by the corresponding unlabeled peptide and expressed as a percentage. Data points are the mean ± SEM of the combined data from 5 independent experiments.
Internalization of Cy5-Labeled PACAP and VIP Peptides
To confirm that PACAP-responsive receptors were capable of internalization in receptor-transfected Cos7 cells, ligand-stimulated internalization of HA-tagged (HA) PAC1 receptors was measured using cell surface ELISA. PACAP-38 stimulated a time-dependent reduction in HA-PAC1n and HA-PAC1s receptor cell surface expression, indicative of internalization (Figure S1). In contrast, PACAP-27 appeared to stimulate a reduction in HA-PAC1s but not HA-PAC1n receptor surface expression; however, this difference did not reach significance (Figure S1). This suggests that PAC1 receptors can internalize and that the transfected Cos7 cell model was suitable for investigation of Cy5-labeled peptide-stimulated internalization. For analysis of internalization, a Cy5-labeled peptide concentration of 10 nM was used because this produced robust stimulation of cAMP production at each receptor (Figure 2). Diffuse fluorescent staining would be observed in the absence of internalization, whereas distinct fluorescent spots within the cell cytoplasm are indicative of peptide-bound receptor internalization into endosomes. To differentiate between spots on the cell surface and within the cytoplasm, the cytoplasmic stain CellMask Green was used (Figures S2–S5). The time points were chosen to reflect the rapid nature of receptor internalization which can begin within 5 min of peptide addition.
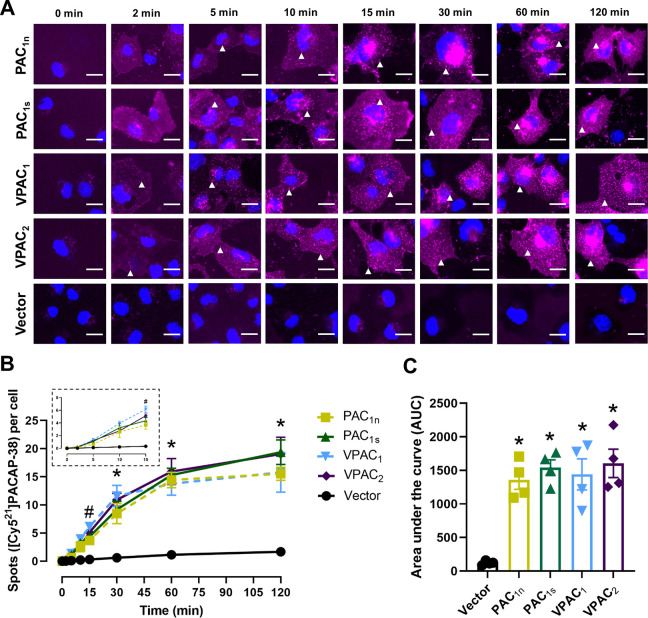
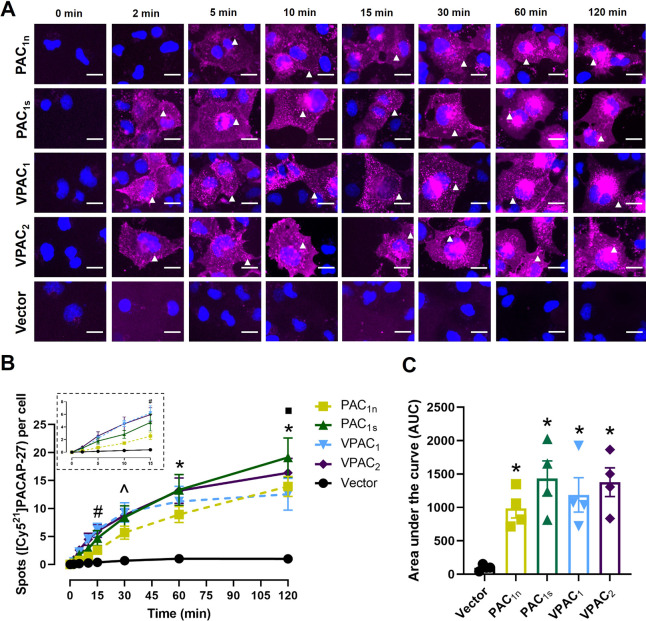
A time-dependent increase in the number of [Cy521]PACAP-38 spots was observed in cells transfected with the PAC1n, PAC1s, VPAC1, and VPAC2 receptors but not the vector control (Figure 3A and 3B). Interestingly, the VPAC1 and VPAC2 receptors exhibited a slightly different temporal profile from the PAC1 receptors, where distinct fluorescent spots were evident at 2 min for VPAC1 and VPAC2 but were comparatively delayed at the PAC1 receptors (10 min). The temporal difference was reflected in quantification, whereby the number of spots reached statistical significance for the VPAC receptors by 15 min, compared to 30 min for the PAC1 receptors (Figure 3B). All receptors reached a similar maximum at ∼60 min, and the degree of [Cy521]PACAP-38-stimulated internalization (area under the curve) was comparable between all receptors examined (Figure 3C, Table S4).
Figure 3.
Internalization of [Cy521]PACAP-38 (10 nM) over time in Cos7 cells transfected with the PAC1n, PAC1s, VPAC1, and VPAC2 receptors. (A) [Cy521]PACAP-38 fluorescence shown in magenta and nuclear 4′,6-diamindino-2-phenylindole (DAPI) in blue; white arrowheads indicate examples of spots. Images are representative of one field of view from 4 independent experiments. Scale bar, 20 μm. (B) Quantification of the number of [Cy521]PACAP-38 spots per cell at each time point. (Inset) Magnified view of the spot number from 0 to 15 min. Statistical significance was determined by repeated measures two-way ANOVA with post hoc Tukey’s test comparing all receptors and vector-transfected cells to each other at each time point. * p < 0.05 for all receptors compared to vector-transfected cells, and # p < 0.05 for VPAC1 and VPAC2 compared to vector-transfected cells. (C) Area under the spot counting curve from B. Statistical significance was determined by one-way ANOVA with post hoc Tukey’s test. * p < 0.05 compared to vector-transfected cells. Data in B and C are plotted as the mean ± SEM combined from 4 independent experiments.
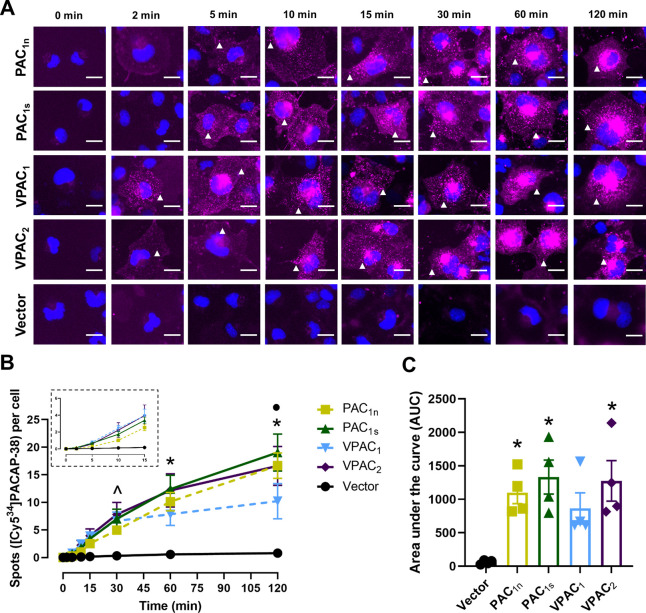
[Cy534]PACAP-38 produced a time-dependent increase in the number of spots at all receptors, though the temporal profile between receptors was slightly different (Figure 4A and 4B). The PAC1s, VPAC1, and VPAC2 receptors, but not the PAC1n receptor, exhibited significantly more fluorescent spots compared to vector at 30 min (Figure 4B). However, the PAC1n receptor had reached significance from 60 min. The number of spots per cell increased gradually until 120 min for all receptors except VPAC1, which appeared to plateau after 30 min (Figure 4B). Overall, [Cy534]PACAP-38 stimulated a comparable degree of internalization at the PAC1 and VPAC2 receptors, whereas internalization at the VPAC1 receptor was lower and not significantly different from the vector (Figure 4C, Table S4). [Cy534]PACAP-38 appeared to be less effective at stimulating internalization than [Cy521]PACAP-38; however, this was not significantly different (Figure S6).
Figure 4.
Internalization of [Cy534]PACAP-38 (10 nM) over time in Cos7 cells transfected with the PAC1n, PAC1s, VPAC1, and VPAC2 receptors. (A) [Cy534]PACAP-38 fluorescence shown in magenta and nuclear DAPI in blue; white arrowheads indicate examples of spots. Images are representative of one field of view from 4 independent experiments. Scale bar, 20 μm. (B) Quantification of the number of [Cy534]PACAP-38 spots per cell at each time point. (Inset) Magnified view of the spot number from 0 to 15 min. Statistical significance was determined by repeated measures two-way ANOVA with post hoc Tukey’s test comparing all receptors and vector-transfected cells to each other at each time point. * p < 0.05 for all receptors compared to vector-transfected cells, ● p < 0.05 for VPAC1 compared to all receptors. and ^ p < 0.05 for PAC1s, VPAC1, and VPAC2 compared to vector-transfected cells. (C) Area under the spot counting curve from B. Statistical significance was determined by one-way ANOVA with post hoc Tukey’s test. * p < 0.05 compared to vector-transfected cells. Data in B and C are plotted as the mean ± SEM combined from 4 independent experiments.
A time-dependent increase in the number of [Cy521]PACAP-27 spots was observed at all receptors except the PAC1n receptor, which appeared to have a delayed response (Figure 5A and 5B). Significant differences from the vector were detected from 15 min for the VPAC1 and VPAC2 receptors, 30 min for the PAC1s receptor, and 60 min for the PAC1n receptor (Figure 5B). Although the number of spots increased gradually over time, VPAC1 receptor internalization appeared to plateau from 60 min and was significantly lower than the PAC1s receptor at 120 min (Figure 5B). Overall, [Cy521]PACAP-27 stimulated a comparable degree of internalization at the PAC1s and VPAC2 receptors, whereas responses at PAC1n and VPAC1 appeared to be more limited; however, these were not significantly different (Figure 5C, Table S4). Furthermore, the degree of [Cy521]PACAP-27-stimulated internalization was comparable to both Cy5-labeled PACAP-38 peptides (Figure S6).
Figure 5.
Internalization of [Cy521]PACAP-27 (10 nM) over time in Cos7 cells transfected with the PAC1n, PAC1s, VPAC1, and VPAC2 receptors. (A) [Cy521]PACAP-27 fluorescence shown in magenta and nuclear DAPI in blue; white arrowheads indicate examples of spots. Images are representative of one field of view from 4 independent experiments. Scale bar, 20 μm. (B) Quantification of the number of [Cy521]PACAP-27 spots per cell at each time point. (Inset) Magnified view of the spot number from 0 to 15 min. Statistical significance was determined by repeated measures two-way ANOVA with post hoc Tukey’s test comparing all receptors and vector-transfected cells to each other at each time point. * p < 0.05 for all receptors compared to vector-transfected cells, ■ p < 0.05 for VPAC1 compared to PAC1s, ^ p < 0.05 for PAC1s, VPAC1, and VPAC2 compared to vector-transfected cells, and # p < 0.05 for VPAC1 and VPAC2 compared to vector-transfected cells. (C) Area under the spot counting curve from B. Statistical significance was determined by one-way ANOVA with post hoc Tukey’s test. * p < 0.05 compared to vector-transfected cells. Data in B and C are plotted as the mean ± SEM combined from 4 independent experiments.
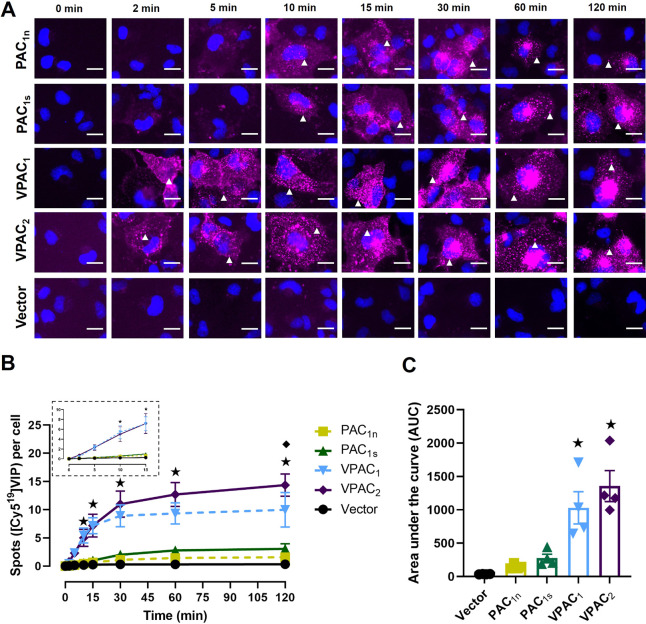
[Cy519]VIP stimulated a time-dependent increase in fluorescent spots in cells transfected with the PAC1n, PAC1s, VPAC1, and VPAC2 receptors but not the vector control (Figure 6A and 6B). Fluorescent spots were initially observed at 2 min at the VPAC1 and VPAC2 receptors followed by the PAC1n and PAC1s receptors from 10 min, and the VPAC receptors exhibited significantly more fluorescent spots at each time point compared to the PAC1 receptors (Figure 6B). Interestingly, the number of observed spots at the VPAC1 and VPAC2 receptors appeared to plateau at 30 min with VPAC2 reaching a significantly higher maximum compared to VPAC1 at 120 min (Figure 6B). Overall, [Cy519]VIP stimulated a comparable degree of internalization at the VPAC1 and VPAC2 receptors with very low levels of internalization observed at the PAC1n and PAC1s receptors (Figure 6C, Table S4).
Figure 6.
Internalization of [Cy519]VIP (10 nM) over time in Cos7 cells transfected with the PAC1n, PAC1s, VPAC1, and VPAC2 receptors. (A) [Cy519]VIP fluorescence shown in magenta and nuclear DAPI in blue; white arrowheads indicate examples of spots. Images are representative of one field of view from 4 independent experiments. Scale bar, 20 μm. (B) Quantification of the number of [Cy519]VIP spots per cell at each time point. (Inset) Magnified view of the spot number from 0 to 15 min. Statistical significance was determined by repeated measures two-way ANOVA with post hoc Tukey’s test comparing all receptors and vector-transfected cells to each other at each time point. ★ p < 0.05 for VPAC1 and VPAC2 compared to PAC1n, PAC1s, and vector-transfected cells, and ◆ p < 0.05 for VPAC1 compared to VPAC2. (C) Area under the spot counting curve from B. Statistical significance was determined by one-way ANOVA with post hoc Tukey’s test. ★ p < 0.05 compared to PAC1n, PAC1s, and vector-transfected cells. Data in B and C are plotted as the mean ± SEM combined from 4 independent experiments.
Internalization of PACAP or PACAP-mediated receptor internalization has been investigated, but very few studies characterize the tools used or examined responses at multiple receptor subtypes in the same study. Site-specific FITC-labeled PACAP-38 and PACAP-27 peptides have been reported and were used to show time-dependent internalization of both PACAP-38 and PACAP-27.36 In this study, the internalization response plateaued within 30 min, whereas only VPAC1 receptor internalization with Cy5-PACAP plateaued in the current study (Figures 4B and 5B). Furthermore, the FITC-PACAP internalization was reported to be receptor independent, which contrasts with our results as internalization was not observed with the Cy5-labeled PACAP-38 or PACAP-27 in vector-transfected cells. This difference in results could reflect distinct properties of the labeled peptide or the cells used. Alternatively, many cells contain endogenous PACAP-responsive receptors.
Another study generated and investigated the internalization of an AlexaFluor488-labeled PACAP-27.23 Time-dependent internalization of PACAP-27 was reported in cells expressing the PAC1Hop1 receptor; however, in contrast to the current study with [Cy521]PACAP-27, a more pronounced plateau was observed from 30 min. PACAP-38-mediated receptor internalization has been observed using the PAC1Hop1 receptor tagged with green fluorescent protein (GFP).25 Following receptor activation, GFP was observed to accumulate in endosome-like structures after 3 min, which was comparable to that observed in the current study. PACAP-27-stimulated internalization has also been investigated using a GFP-tagged PAC1Hop1 receptor.27,47 Consistent with the internalization profile of [Cy521]PACAP-27 in the current study, they reported a disappearance of cell surface fluorescence within 10–20 min following PACAP-27 stimulation and an increase in fluorescence within the cytoplasm (Figure 5A).27,47 A similar pattern of internalization has been reported for the PAC1n receptor.30,48,49
The current study represents the first to explicitly look at the internalization of the PAC1s receptor. The combination of our data using the PAC1n and PAC1s receptors with prior studies suggests that the internalization profiles are similar for different PAC1 receptor splice variants. However, PACAP-27 appeared to stimulate internalization more rapidly and robustly at the untagged and HA-tagged PAC1s receptor compared to the PAC1n receptor, which may indicate unique temporal differences in responses between variants. Despite PACAP-38 and PACAP-27 being potent agonists of VPAC receptors, no previous studies have investigated PACAP-38-mediated internalization of either the VPAC1 or the VPAC2 receptor. The current study now shows that both forms of PACAP can promote receptor internalization across the PACAP-responsive receptor subtypes.
Compared to the PACAP peptides, internalization of VIP or VIP-stimulated receptor internalization has been more thoroughly investigated. Three previous studies have investigated the internalization of fluorescently labeled VIP peptides.24,37,38 The internalization and localization of Cy3-VIP within the cytoplasm of Panc1 cells have been reported after 15 min.38 However, this internalization could not be attributed to a specific receptor subtype as Panc1 cells endogenously express PAC1, VPAC1, and VPAC2 receptors. Consistent with the profile observed by [Cy519]VIP, a second study using FITC-VIP reported internalization of the peptide from 5 min in cells expressing the VPAC1 receptor.24 Using antibodies against the VPAC1 and VPAC2 receptors, VIP (100 nM or 1 μM) was reported to stimulate a rapid decrease of cell surface fluorescence and increase in cytosolic fluorescence within 30 min, indicative of receptor internalization.26,28,29,49 The observation of internalization occurred more rapidly than that seen by [Cy519]VIP at the VPAC1 or VPAC2 receptor in the current study; this could be due to the much lower concentration of VIP used.50
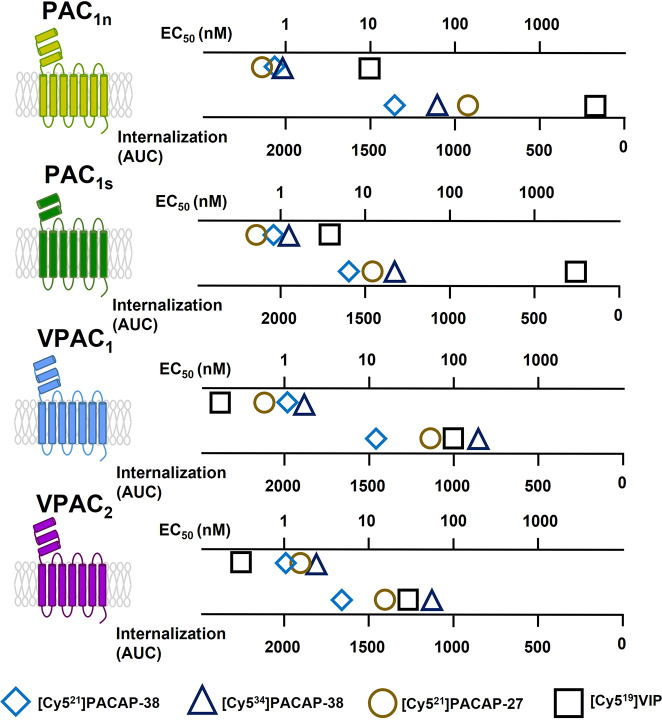
Receptor activation and intracellular signaling are intimately connected to receptor internalization, whereby the presence of different agonists or intracellular signaling proteins can have dramatic effects on receptor internalization.15−18 The experimental design of the current study involved testing multiple peptide agonists at multiple PACAP-responsive receptor subtypes over several time points. This allowed peptide-mediated internalization to be quantified and directly compared across different receptor subtypes for the first time. Furthermore, this approach allowed a comparison between peptide-mediated cAMP accumulation and internalization to be performed (Figure 7). Differences between the relative pattern of cAMP signaling and internalization may indicate the presence of biased signaling where specific peptides may display preferential activation of cAMP signaling over internalization at individual PACAP-responsive receptors. However, the relative potency of PACAP-responsive receptor internalization appears to be consistently lower than cAMP accumulation, which may indicate that differential coupling between these processes may be a confounding factor. It is therefore possible that differences in receptor coupling, downstream signaling events, or sensitivity between our ability to detect cAMP and receptor internalization may contribute to the overall pattern. We previously observed greater stimulation of Gs (cAMP accumulation) compared to Gq (IP1 accumulation), ERK1/2 phosphorylation, and Akt phosphorylation at the PACAP-responsive receptors.10 The data suggested that these receptors may couple more effectively to Gs than Gq and other downstream signaling events. PACAP-responsive receptor internalization may therefore correlate more closely to other pathways than cAMP accumulation. This possibility is particularly intriguing as PACAP-mediated phosphorylation of ERK1/2 and Akt is reported to require internalized PAC1 in the endosomal compartment.27,48 Further exploration of Gq and other downstream signaling events may therefore prove valuable in understanding receptor internalization.
Figure 7.
Summary of Cy5-labeled peptide cAMP accumulation potency and internalization capacity. Internalization was measured with 10 nM peptide. AUC = area under the curve.
Consistent with the cAMP signaling profile of VIP at the PAC1n receptor, the degree of [Cy519]VIP internalization was significantly less when compared to the Cy5-labeled PACAP-38 and PACAP-27 (Figure 7, Figure S6). Interestingly, although [Cy519]VIP produced a potent cAMP response at the PAC1s receptor, it exhibited less internalization than expected, which could indicate potential biased signaling toward cAMP accumulation over internalization (Figure 7, Figure S6). Alternatively, the relatively low VIP concentration used (10 nM) may have influenced the amount of detectable signal as this concentration does not produce a maximal cAMP response at the PAC1s receptor, and [Cy519]VIP is slightly less potent than unlabeled VIP (Figure 2). However, a study investigating VIP-stimulated internalization of the PAC1 receptor reported that PAC1 remained at the cell surface, even when higher concentrations of VIP were used.49 This along with the lack of observed [Cy519]VIP internalization in the current study at the PAC1 receptors may suggest VIP is not as readily internalized at this receptor subtype compared to the PACAP peptides. Furthermore, the lack of potent VIP internalization at the PAC1s receptor indicates this receptor variant may exhibit unique agonist profiles for previously unexplored signaling pathways. The relative difference between cAMP and internalization at the PAC1s receptor we observed was greater for VIP than either PACAP-38 or PACAP-27; this suggests that functional selectivity or biased signaling may be involved where VIP is biased away from internalization relative to PACAP-38. However, in a prior study using the Cos7 cell model, although VIP-stimulated IP1 accumulation displayed a trend toward bias, significant biased signaling was not observed relative to PACAP-38 at the PAC1s receptor.10 Interestingly, relative to PACAP-38, the VIP-mediated IP1 response at the PAC1s receptor displayed the most divergence from reported binding affinities and cAMP accumulation responses.8,10 Formal quantification of this potential bias requires further investigation, including direct comparisons between receptor internalization and additional signaling molecules. Similarly, at the VPAC1 and VPAC2 receptors, [Cy519]VIP yielded the most potent stimulator of cAMP accumulation. However, when internalization was measured, [Cy521]PACAP-38 appeared to exhibit the greatest response (Figure 7, Figure S6). This difference could represent biased signaling of [Cy519]VIP for cAMP accumulation over internalization when compared to [Cy521]PACAP-38. However, formal investigation and quantification of biased signaling is required to confirm this observation.
Conclusions
To effectively target the PACAP peptide system in neurological conditions, including neurodegenerative and pain-related disorders, a deeper understanding of how these receptors behave is required. Internalized peptides, or their receptors, could present a novel target for therapeutic development, but we currently lack sufficient, well-characterized tools to explore this process. This study reports the synthesis of novel, functional, and characterized Cy5-labeled PACAP and VIP peptide analogues that can be used as tools to examine internalization. The ability of PACAP-38, PACAP-27, and VIP to internalize demonstrates they may all play an important role in mediating the endosomal signaling of PACAP-responsive receptors, including those involved in pain behaviors. Furthermore, this is the first study to consider internalization at the PAC1s receptor splice variant and identify that biased agonism across the PACAP peptide family may involve internalization. This could have significant implications for understanding the biology of these receptors and drug development. The Cy5-labeled analogues developed herein provide insight into the role internalization plays across the PACAP peptide family. These novel tools represent a new resource that could be used to further study internalization and investigate peptide binding sites in tissues important in pain and other physiological functions.
Methods
General Peptide Synthesis
Native PACAP-38, PACAP-27, and VIP were synthesized in house by a Nα-9-fluorenylmethoxycarbonyl (Fmoc) solid-phase synthesis strategy as previously described (Supporting Information).10 Two analogues of Cy5-labeled PACAP-38 were generated: one labeled at amino acid 21 and the second at amino acid 34. PACAP-27 was labeled at amino acid 21 and VIP at amino acid 19. To facilitate Cy5 labeling, an azido-lysine was substituted into the sequence at these positions in place of the native amino acid. 1,3-Dipolar cycloaddition of an in-house Cy5 alkyne fluorophore was then performed and purified, and fractions were collected and analyzed by ESI-MS and RP-RPLC. Fractions with correct m/z were combined and lyophilized to afford the desired product with a purity of >98%. Full details of the Cy5-labeled peptide synthesis can be found in the Supporting Information. All peptides were assumed to have 80% peptide content and were made up as 1 mM stock solutions in sterile water under reduced light and stored at −30 °C.
Cell Culture and Transfection
Cos7 cells were selected for this study as they do not respond endogenously to PACAP and were cultured as previously described.51 Cos7 cells were plated at 20 000 cells per well into 96-well SpectraPlates (PerkinElmer, MA, USA) for measurement of cAMP and ELISA and into 96-well CellCarrier Ultra plates (PerkinElmer) at a density of 15 000 cells per well for internalization studies. Cos7 cells were transiently transfected using polyethylenimine with 0.25 μg per well of either vector plasmid (pcDNA3.1) or receptor containing plasmid as previously described.51 Experiments were performed 48 h after transfection. The human PAC1n, VPAC1, and VPAC2 receptors in the pcDNA3.1 vector were purchased from the cDNA Resource Centre (Bloomsburg University, PA, USA). The human PAC1s receptor was originally obtained in the pCMV6-XL6 vector (Origene, MD, USA) and subsequently cloned into pcDNA3.1 as previously described.10 Empty pcDNA3.1 was used as the vector control.
cAMP Measurement
Peptide-stimulated cAMP accumulation was measured as previously described.52 Briefly, Cy5-labeled and unlabeled peptides were serially diluted in cAMP assay media (DMEM containing 0.1% (w/v) BSA and 1 mM 3-isobutyl-1-methylxanthine) to give a final concentration range from 1 pM to 1 μM. Cos7 cells transiently transfected with receptor were incubated with peptide, media alone, or the positive control forskolin for 15 min at 37 °C. The reaction was stopped by aspiration of the media and addition of 50 μL of ice-cold absolute ethanol. The ethanol was then evaporated from the wells and cAMP reconstituted in 50 μL of cAMP detection buffer (PerkinElmer). cAMP was then quantified using the LANCE cAMP detection kit (PerkinElmer) as per the manufacturer’s instructions.
Fluorescent Imaging
Cells were serum starved in DMEM containing 0.1% (w/v) BSA for 30 min at 37 °C. The cells were then incubated at 37 °C with 10 nM fluorescent peptide for 0–120 min. Following this, the media was aspirated, and cells were fixed with 8% paraformaldehyde (PFA). Cells were then washed in PBS and incubated with Cell Mask Green (1:1000; Life Technologies, CA, USA) and DAPI for 30 min at 37 °C. Cells were washed a further two times with PBS and imaged on an Operetta High-content Imager (PerkinElmer) using a 20× nonconfocal 0.75 high NA lens.
Measurement of HA-PAC1 Receptor Cell Surface Expression by ELISA
Cos7 cells were transfected with HA-tagged hPAC1n and hPAC1s receptors and assayed for receptor cell surface expression as described previously with minor modifications.51,53 Briefly, transfected cells were stimulated with 100 nM unlabeled PACAP-38 or PACAP-27 for 0–120 min and fixed using 4% PFA. Cells were washed with PBS and incubated with 0.6% hydrogen peroxide for 20 min. Cells were blocked with 10% goat serum/PBS for 1 h, followed by a 30 min incubation with anti-HA primary antibody (1:1000, Biolegend, CA, USA). Cells were washed in PBS, incubated with antimouse horseradish peroxidase (HRP) secondary antibody (1:500, GE Healthcare, IL, USA) for 1 h, and washed again in PBS. Sigma FAST OPD was added to each well and incubated in the dark for 15 min. Sulfuric acid was added to stop the reaction, and the absorbance was measured.
Image Analysis
Spot counting was performed using the Columbus software package (Figure S7). Cells were identified by nuclear staining of DAPI and CellMask Green staining of the cell cytoplasm. The cytoplasm region was set at 90% of the cell volume to ensure that fluorescent peptide at the cell surface was not counted.34 Fluorescent peptide spots were quantified within the cytoplasm region of the cells in each field of view. Each condition was performed in duplicate. Data are the average of 8 fields of view for each well outputted as the number of spots per cell. The area under the curve was calculated and plotted as a bar graph.
For presentation, the raw TIFF files were acquired using the Harmony software on the Operetta and merged, pseudocolored, and adjusted for brightness and contrast, and scale bars were added in ImageJ. Image adjustments were applied uniformly for all time points related to a peptide at a particular receptor. The brightness and contrast of vector-transfected cells were matched to each time point from [Cy521]PACAP-38, [Cy534]PACAP-38, and [Cy521]PACAP-27 at the PAC1n receptor or [Cy519]VIP at the VPAC1 receptor.
Data and Statistical Analysis
All graphing, curve fitting, and statistical analysis were performed using GraphPad Prism 7.0. Statistical significance was defined as p < 0.05.
cAMP Measurement
Data points are the mean ± SEM from 5 independent experiments performed with 2–3 technical replicates (wells). Data from individual experiments were initially fitted with a four-parameter logistic equation. F tests were then used to determine if the Hill slope was significantly different from 1. In the majority of experiments, the Hill slope was not significantly different from 1, and the curves were then refitted to a three-parameter logistic equation to obtain the peptides pEC50. The mean pEC50 from independent experiments was calculated for each peptide, and significant differences were determined using an unpaired two-tailed t-test (PACAP-27 and VIP) or one-way ANOVA with Dunnett’s test (PACAP-38). Significant differences in peptide Emax were determined from the non-normalized peptide values by a ratio-paired two-tailed t-test (PACAP-27 and VIP) or log transformed followed by a repeated measures one-way ANOVA with Dunnett’s test (PACAP-38). For presentation purposes, the concentration–response data from individual experiments were normalized to the curve-fitted maximum (Emax) and minimum (Emin) cAMP response produced by the matched unlabeled peptide and expressed as a percentage.
ELISA Assays
Data points are the mean ± SEM from 4 or 5 (PACAP-27 at HA-PAC1n) independent experiments performed with 2 technical replicates (wells). In each independent experiment, values were normalized to vector-transfected cells (0%) and the receptor expression in unstimulated cells at 0 min (100%).
The mean normalized receptor surface expression was then combined from individual experiments. Significant differences to vehicle-treated wells at each time point were determined by repeated measures two-way ANOVA with post hoc Bonferroni’s test. The area under the curve of PACAP-stimulated receptors was calculated and plotted as a bar graph. Significant differences to vehicle-treated wells were determined using a one-way ANOVA with post hoc Dunnett’s test.
Internalization Spot Count Analysis
For spot counting, significant differences for each peptide were determined using a two-way ANOVA with post hoc Tukey’s test comparing all receptors and vector-transfected cells to each other at each time point. Significant differences in the spot count area under the curve were determined by one-way ANOVA with post hoc Tukey’s test.
Acknowledgments
This work was supported by a Marsden Fast-start grant from the Royal Society of New Zealand to C.S.W. C.S.W. acknowledges receipt of a Sir Charles Hercus Health Research Fellowship from the Health Research Council, New Zealand.
Glossary
Abbreviations
- PACAP
pituitary adenylate cyclase-activating polypeptide
- VIP
vasoactive intestinal peptide
- cAMP
cyclic adenosine monophosphate.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.2c00124.
Additional biology methodology (spot count analysis), colocalization of Cy5 peptides with a cytoplasm marker (CellMask green), ELISA results with PACAP-stimulated HA-PAC1 receptors, table of peptide potencies and degree of internalization, and detailed chemistry methods describing the synthesis of fluorescent peptides (PDF)
Author Contributions
∥ T.I.A. and Z.T. contributed equally to this work.
Author Contributions
This study was conceived and designed by D.L.H. and C.S.W. A.S., M.A.B., and P.W.R.H. performed peptide synthesis. T.I.A., Z.T., and T.A.R. performed peptide characterization and internalization experiments. The manuscript was written through contributions from T.I.A., Z.T., A.S., M.A.B., P.W.R.H., D.L.H., and C.S.W.
The authors declare the following competing financial interest(s): D.L.H. is or has been a consultant or speaker for Lilly, Amgen, Teva, Intarcia, and Merck Sharp and Dohme and has received research funding from Living Cell Technologies and AbbVie in the past 3 years. C.S.W. has received research support from Living Cell Technologies and AbbVie.
Special Issue
Published as part of the ACS Pharmacology & Translational Science virtual special issue “GPCR Signaling”.
Supplementary Material
References
- Harmar A. J.; Fahrenkrug J.; Gozes I.; Laburthe M.; May V.; Pisegna J. R.; Vaudry D.; Vaudry H.; Waschek J. A.; Said S. I. Pharmacology and functions of receptors for vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide: IUPHAR review 1. Br. J. Pharmacol. 2012, 166 (1), 4–17. 10.1111/j.1476-5381.2012.01871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody T. W.; Ito T.; Osefo N.; Jensen R. T. VIP and PACAP. Recent insights into their functions/roles in physiology and disease from molecular and genetic studies. Current opinion in endocrinology, diabetes, and obesity 2011, 18 (1), 61–67. 10.1097/MED.0b013e328342568a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson T.; Mitchell R.; Robberecht P.; Fleetwood-Walker S. M. The role of VIP/PACAP receptor subtypes in spinal somatosensory processing in rats with an experimental peripheral mononeuropathy. Neuropharmacology 1999, 38 (1), 167–180. 10.1016/S0028-3908(98)00171-3. [DOI] [PubMed] [Google Scholar]
- Rustichelli C.; Lo Castro F.; Baraldi C.; Ferrari A. Targeting pituitary adenylate cyclase-activating polypeptide (PACAP) with monoclonal antibodies in migraine prevention: a brief review. Expert Opin Investig Drugs 2020, 29 (11), 1269–1275. 10.1080/13543784.2020.1811966. [DOI] [PubMed] [Google Scholar]
- Sundrum T.; Walker C. S. Pituitary adenylate cyclase-activating polypeptide receptors in the trigeminovascular system: implications for migraine. British journal of pharmacology 2018, 175 (21), 4109–4120. 10.1111/bph.14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan Loomis C.; Dutzar B.; Ojala E. W.; Hendrix L.; Karasek C.; Scalley-Kim M.; Mulligan J.; Fan P.; Billgren J.; Rubin V.; Boshaw H.; Kwon G.; Marzolf S.; Stewart E.; Jurchen D.; Pederson S. M.; Perrino McCulloch L.; Baker B.; Cady R. K.; Latham J. A.; Allison D.; Garcia-Martinez L. F. Pharmacologic Characterization of ALD1910, a Potent Humanized Monoclonal Antibody against the Pituitary Adenylate Cyclase-Activating Peptide. Journal of Pharmacology and Experimental Therapeutics 2019, 369 (1), 26. 10.1124/jpet.118.253443. [DOI] [PubMed] [Google Scholar]
- Ashina M.; Dolezil D.; Bonner J. H.; Zhou L.; Klatt J.; Picard H.; Mikol D. D. A phase 2, randomized, double-blind, placebo-controlled trial of AMG 301, a pituitary adenylate cyclase-activating polypeptide PAC1 receptor monoclonal antibody for migraine prevention. Cephalalgia 2021, 41 (1), 33–44. 10.1177/0333102420970889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautzenberg F. M.; Mevenkamp G.; Wille S.; Hauger R. L. N-terminal splice variants of the type I PACAP receptor: isolation, characterization and ligand binding/selectivity determinants. J. Neuroendocrinol 1999, 11 (12), 941–9. 10.1046/j.1365-2826.1999.00411.x. [DOI] [PubMed] [Google Scholar]
- Pisegna J. R.; Wank S. A. Cloning and characterization of the signal transduction of four splice variants of the human pituitary adenylate cyclase activating polypeptide receptor. Evidence for dual coupling to adenylate cyclase and phospholipase C. J. Biol. Chem. 1996, 271 (29), 17267–17274. 10.1074/jbc.271.29.17267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasma Z.; Siow A.; Harris P. W. R.; Brimble M. A.; Hay D. L.; Walker C. S. Characterisation of agonist signalling profiles and agonist-dependent antagonism at PACAP-responsive receptors: Implications for drug discovery. Br. J. Pharmacol. 2022, 179 (3), 435–453. 10.1111/bph.15700. [DOI] [PubMed] [Google Scholar]
- Blechman J.; Levkowitz G. Alternative Splicing of the Pituitary Adenylate Cyclase-Activating Polypeptide Receptor PAC1: Mechanisms of Fine Tuning of Brain Activity. Frontiers in Endocrinology 2013, 4, 55. 10.3389/fendo.2013.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz E. M.; Ronaldson E.; Shaw P.; Johnson M. S.; Holland P. J.; Mitchell R. Characterization of novel splice variants of the PAC1 receptor in human neuroblastoma cells: consequences for signaling by VIP and PACAP. Molecular and Cellular Neurosciences 2006, 31 (2), 193–209. 10.1016/j.mcn.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Spongier D.; Waeber C.; Pantaloni C.; Holsboer F.; Bockaert J.; Seeburgt P. H.; Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature 1993, 365 (6442), 170–175. 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Walker C. S.; Sundrum T.; Hay D. L. PACAP receptor pharmacology and agonist bias: analysis in primary neurons and glia from the trigeminal ganglia and transfected cells. British journal of pharmacology 2014, 171 (6), 1521–1533. 10.1111/bph.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb P.; Kenakin T.; Alexander S. P. H.; Bermudez M.; Bohn L.M.; Breinholt C.S.; Bouvier M.; Hill S.J.; Kostenis E.; Martemyanov K.A.; Neubig R.R.; Onaran H.O.; Rajagopal S.; Roth B.L.; Selent J.; Shukla A.K.; Sommer M.E.; Gloriam D.E. Community guidelines for GPCR ligand bias: IUPHAR review 32. Br. J. Pharmacol. 2022, 179 (4), 3651. 10.1111/bph.15811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calebiro D.; Godbole A. Internalization of G-protein-coupled receptors: Implication in receptor function, physiology and diseases. Best Pract Res. Clin Endocrinol Metab 2018, 32 (2), 83–91. 10.1016/j.beem.2018.01.004. [DOI] [PubMed] [Google Scholar]
- McMahon H. T.; Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2011, 12 (8), 517–33. 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- Thomsen A. R. B.; Plouffe B.; Cahill T. J. 3rd; Shukla A. K.; Tarrasch J. T.; Dosey A. M.; Kahsai A. W.; Strachan R. T.; Pani B.; Mahoney J. P.; Huang L.; Breton B.; Heydenreich F. M.; Sunahara R. K.; Skiniotis G.; Bouvier M.; Lefkowitz R. J. GPCR-G Protein-beta-Arrestin Super-Complex Mediates Sustained G Protein Signaling. Cell 2016, 166 (4), 907–919. 10.1016/j.cell.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarwood R. E.; Imlach W. L.; Lieu T.; Veldhuis N. A.; Jensen D. D.; Klein Herenbrink C.; Aurelio L.; Cai Z.; Christie M. J.; Poole D. P.; Porter C. J. H.; McLean P.; Hicks G. A.; Geppetti P.; Halls M. L.; Canals M.; Bunnett N. W. Endosomal signaling of the receptor for calcitonin gene-related peptide mediates pain transmission. Proc. Natl. Acad. Sci. U. S. A. 2017, 114 (46), 12309–12314. 10.1073/pnas.1706656114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May V.; Parsons R. L. G Protein-Coupled Receptor Endosomal Signaling and Regulation of Neuronal Excitability and Stress Responses: Signaling Options and Lessons From the PAC1 Receptor. Journal of Cellular Physiology 2017, 232 (4), 698–706. 10.1002/jcp.25615. [DOI] [PubMed] [Google Scholar]
- Missig G.; Mei L.; Vizzard M. A.; Braas K. M.; Waschek J. A.; Ressler K. J.; Hammack S. E.; May V. Parabrachial Pituitary Adenylate Cyclase-Activating Polypeptide Activation of Amygdala Endosomal Extracellular Signal-Regulated Kinase Signaling Regulates the Emotional Component of Pain. Biol. Psychiatry 2017, 81 (8), 671–682. 10.1016/j.biopsych.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal J. S.; Ramirez-Garcia P. D.; Shenoy P. A.; Poole D. P.; Veldhuis N. A. Internalized GPCRs as Potential Therapeutic Targets for the Management of Pain. Front Mol. Neurosci 2019, 12, 273. 10.3389/fnmol.2019.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germano P. M.; Stalter J.; Le S. V.; Wu M.; Yamaguchi D. J.; Scott D.; Pisegna J. R. Characterization of the pharmacology, signal transduction and internalization of the fluorescent PACAP ligand, fluor-PACAP, on NIH/3T3 cells expressing PAC1. Peptides 2001, 22 (6), 861–6. 10.1016/S0196-9781(01)00410-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie J.-C.; Rouyer-Fessard C.; Couvineau A.; Nicole P.; Devaud H.; Benna J.; Laburthe M. Serine 447 in the Carboxyl Tail of Human VPAC1 Receptor Is Crucial for Agonist-Induced Desensitization but Not Internalization of the Receptor. Molecular pharmacology 2003, 64, 1565–74. 10.1124/mol.64.6.1565. [DOI] [PubMed] [Google Scholar]
- Shintani Y.; Hayata-Takano A.; Moriguchi K.; Nakazawa T.; Ago Y.; Kasai A.; Seiriki K.; Shintani N.; Hashimoto H. beta-Arrestin1 and 2 differentially regulate PACAP-induced PAC1 receptor signaling and trafficking. PLoS One 2018, 13 (5), e0196946 10.1371/journal.pone.0196946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachtergael I.; Gaspard N.; Langlet C.; Robberecht P.; Langer I. Asn229 in the third helix of VPAC1 receptor is essential for receptor activation but not for receptor phosphorylation and internalization: Comparison with Asn216 in VPAC2 receptor. Cellular Signalling 2006, 18 (12), 2121–2130. 10.1016/j.cellsig.2006.03.006. [DOI] [PubMed] [Google Scholar]
- May V.; Buttolph T. R.; Girard B. M.; Clason T. A.; Parsons R. L. PACAP-induced ERK activation in HEK cells expressing PAC1 receptors involves both receptor internalization and PKC signaling. American journal of physiology. Cell physiology 2014, 306 (11), C1068–C1079. 10.1152/ajpcell.00001.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlet C.; Langer I.; Vertongen P.; Gaspard N.; Vanderwinden J. M.; Robberecht P. Contribution of the carboxyl terminus of the VPAC1 receptor to agonist-induced receptor phosphorylation, internalization, and recycling. J. Biol. Chem. 2005, 280 (30), 28034–43. 10.1074/jbc.M500449200. [DOI] [PubMed] [Google Scholar]
- McDonald T. P.; Dinnis D. M.; Morrison C. F.; Harmar A. J. Desensitization of the human vasoactive intestinal peptide receptor (hVIP2/PACAP R): evidence for agonist-induced receptor phosphorylation and internalization. Ann. N.Y. Acad. Sci. 1998, 865, 64–72. 10.1111/j.1749-6632.1998.tb11164.x. [DOI] [PubMed] [Google Scholar]
- Pisegna J. R.; Lyu R. M.; Germano P. M. Essential structural motif in the C-terminus of the PACAP type I receptor for signal transduction and internalization. Ann. N.Y. Acad. Sci. 2000, 921, 195–201. 10.1111/j.1749-6632.2000.tb06966.x. [DOI] [PubMed] [Google Scholar]
- Lyu R. M.; Germano P. M.; Choi J. K.; Le S. V.; Pisegna J. R. Identification of an essential amino acid motif within the C terminus of the pituitary adenylate cyclase-activating polypeptide type I receptor that is critical for signal transduction but not for receptor internalization. J. Biol. Chem. 2000, 275 (46), 36134–42. 10.1074/jbc.M004612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comeo E.; Trinh P.; Nguyen A. T.; Nowell C. J.; Kindon N. D.; Soave M.; Stoddart L. A.; White J. M.; Hill S. J.; Kellam B.; Halls M. L.; May L. T.; Scammells P. J. Development and Application of Subtype-Selective Fluorescent Antagonists for the Study of the Human Adenosine A1 Receptor in Living Cells. J. Med. Chem. 2021, 64 (10), 6670–6695. 10.1021/acs.jmedchem.0c02067. [DOI] [PubMed] [Google Scholar]
- Allikalt A.; Purkayastha N.; Flad K.; Schmidt M. F.; Tabor A.; Gmeiner P.; Hubner H.; Weikert D. Fluorescent ligands for dopamine D2/D3 receptors. Sci. Rep 2020, 10 (1), 21842. 10.1038/s41598-020-78827-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingell J. J.; Rees T. A.; Hendrikse E. R.; Siow A.; Rennison D.; Scotter J.; Harris P. W. R.; Brimble M. A.; Walker C. S.; Hay D. L. Distinct Patterns of Internalization of Different Calcitonin Gene-Related Peptide Receptors. ACS Pharmacol Transl Sci. 2020, 3 (2), 296–304. 10.1021/acsptsci.9b00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yule L. R.; Garelja M.L.; Hendrikse E.R.; Gingell J.J.; Poyner D.R.; Harris P. W. H.; Brimble M.A.; Hay D.L. A potent fluorescent calcitonin gene-related peptide analogue enables visualization of receptor internalization. Peptide Sci. 2019, 111 (6), e24126. 10.1002/pep2.24126. [DOI] [Google Scholar]
- Doan N. D.; Chatenet D.; Letourneau M.; Vaudry H.; Vaudry D.; Fournier A. Receptor-independent cellular uptake of pituitary adenylate cyclase-activating polypeptide. Biochim. Biophys. Acta 2012, 1823 (4), 940–9. 10.1016/j.bbamcr.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Becker A.; Hessenius C.; Bhargava S.; GrÖTzinger C.; Licha K. A. I.; Schneider-Mergener J.; Wiedenmann B.; Semmler W. Cyanine Dye Labeled Vasoactive Intestinal Peptide and Somatostatin Analog for Optical Detection of Gastroenteropancreatic Tumors. Ann. N.Y. Acad. Sci. 2000, 921 (1), 275–278. 10.1111/j.1749-6632.2000.tb06976.x. [DOI] [PubMed] [Google Scholar]
- Ortner A.; Wernig K.; Kaisler R.; Edetsberger M.; Hajos F.; Kohler G.; Mosgoeller W.; Zimmer A. VPAC receptor mediated tumor cell targeting by protamine based nanoparticles. J. Drug Target 2010, 18 (6), 457–67. 10.3109/10611860903508796. [DOI] [PubMed] [Google Scholar]
- Yung S. L.; Dela Cruz F.; Hamren S.; Zhu J.; Tsutsumi M.; Bloom J. W.; Caudle M.; Roczniak S.; Todd T.; Lemoine L.; MacDougall M.; Shanafelt A. B.; Pan C. Q. Generation of highly selective VPAC2 receptor agonists by high throughput mutagenesis of vasoactive intestinal peptide and pituitary adenylate cyclase-activating peptide. J. Biol. Chem. 2003, 278 (12), 10273–81. 10.1074/jbc.M211945200. [DOI] [PubMed] [Google Scholar]
- Ramos-Álvarez I.; Mantey S. A.; Nakamura T.; Nuche-Berenguer B.; Moreno P.; Moody T. W.; Maderdrut J. L.; Coy D. H.; Jensen R. T. A structure-function study of PACAP using conformationally restricted analogs: Identification of PAC1 receptor-selective PACAP agonists. Peptides 2015, 66, 26–42. 10.1016/j.peptides.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicole P.; Lins L.; Rouyer-Fessard C.; Drouot C.; Fulcrand P.; Thomas A.; Couvineau A.; Martinez J.; Brasseur R.; Laburthe M. Identification of key residues for interaction of vasoactive intestinal peptide with human VPAC1 and VPAC2 receptors and development of a highly selective VPAC1 receptor agonist. Alanine scanning and molecular modeling of the peptide. J. Biol. Chem. 2000, 275 (31), 24003–12. 10.1074/jbc.M002325200. [DOI] [PubMed] [Google Scholar]
- Dickinson T.; Fleetwood-Walker S. M.; Mitchell R.; Lutz E. M. Evidence for roles of vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) receptors in modulating the responses of rat dorsal horn neurons to sensory inputs. Neuropeptides 1997, 31 (2), 175–185. 10.1016/S0143-4179(97)90087-1. [DOI] [PubMed] [Google Scholar]
- Dickson L.; Aramori I.; Sharkey J.; Finlayson K. VIP and PACAP Receptor Pharmacology: A Comparison of Intracellular Signaling Pathways. Ann. N.Y. Acad. Sci. 2006, 1070 (1), 239–242. 10.1196/annals.1317.021. [DOI] [PubMed] [Google Scholar]
- Liang Y. L.; Belousoff M. J.; Zhao P.; Koole C.; Fletcher M. M.; Truong T. T.; Julita V.; Christopoulos G.; Xu H. E.; Zhang Y.; Khoshouei M.; Christopoulos A.; Danev R.; Sexton P. M.; Wootten D. Toward a Structural Understanding of Class B GPCR Peptide Binding and Activation. Mol. Cell 2020, 77 (3), 656–668. 10.1016/j.molcel.2020.01.012. [DOI] [PubMed] [Google Scholar]
- Wang J.; Song X.; Zhang D.; Chen X.; Li X.; Sun Y.; Li C.; Song Y.; Ding Y.; Ren R.; Harrington E. H.; Hu L. A.; Zhong W.; Xu C.; Huang X.; Wang H. W.; Ma Y. Cryo-EM structures of PAC1 receptor reveal ligand binding mechanism. Cell Res. 2020, 30 (5), 436–445. 10.1038/s41422-020-0280-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y.; Feng W.; Zhou Q.; Liang A.; Li J.; Dai A.; Zhao F.; Yan J.; Chen C.-W.; Li H.; Zhao L.-H.; Xia T.; Jiang Y.; Xu H. E.; Yang D.; Wang M.-W. A distinctive ligand recognition mechanism by the human vasoactive intestinal polypeptide receptor 2. Nat. Commun. 2022, 13 (1), 2272–2272. 10.1038/s41467-022-30041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriam L. A.; Baran C. N.; Girard B. M.; Hardwick J. C.; May V.; Parsons R. L. Pituitary adenylate cyclase 1 receptor internalization and endosomal signaling mediate the pituitary adenylate cyclase activating polypeptide-induced increase in guinea pig cardiac neuron excitability. Journal of neuroscience: the official journal of the Society for Neuroscience 2013, 33 (10), 4614–4622. 10.1523/JNEUROSCI.4999-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May V.; Lutz E.; MacKenzie C.; Schutz K. C.; Dozark K.; Braas K. M. Pituitary adenylate cyclase-activating polypeptide (PACAP)/PAC1HOP1 receptor activation coordinates multiple neurotrophic signaling pathways: Akt activation through phosphatidylinositol 3-kinase gamma and vesicle endocytosis for neuronal survival. J. Biol. Chem. 2010, 285 (13), 9749–9761. 10.1074/jbc.M109.043117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz S.; Rocken C.; Mawrin C.; Weise W.; Hollt V.; Schulz S. Immunocytochemical identification of VPAC1, VPAC2, and PAC1 receptors in normal and neoplastic human tissues with subtype-specific antibodies. Clin. Cancer Res. 2004, 10 (24), 8235–42. 10.1158/1078-0432.CCR-04-0939. [DOI] [PubMed] [Google Scholar]
- Shetzline M. A.; Walker J. K.; Valenzano K. J.; Premont R. T. Vasoactive intestinal polypeptide type-1 receptor regulation. Desensitization, phosphorylation, and sequestration. J. Biol. Chem. 2002, 277 (28), 25519–26. 10.1074/jbc.M201815200. [DOI] [PubMed] [Google Scholar]
- Tasma Z.; Wills P.; Hay D. L.; Walker C. S. Agonist bias and agonist-dependent antagonism at corticotrophin releasing factor receptors. Pharmacol Res. Perspect 2020, 8 (3), e00595 10.1002/prp2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley M. J.; Reynolds C. A.; Simms J.; Walker C. S.; Mobarec J. C.; Garelja M. L.; Conner A. C.; Poyner D. R.; Hay D. L. Receptor activity-modifying protein dependent and independent activation mechanisms in the coupling of calcitonin gene-related peptide and adrenomedullin receptors to Gs. Biochem. Pharmacol. 2017, 142, 96–110. 10.1016/j.bcp.2017.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins H. A.; Walker C. S.; Ly K. N.; Bailey R. J.; Barwell J.; Poyner D. R.; Hay D. L. Receptor activity-modifying protein-dependent effects of mutations in the calcitonin receptor-like receptor: implications for adrenomedullin and calcitonin gene-related peptide pharmacology. Br. J. Pharmacol. 2014, 171 (3), 772–88. 10.1111/bph.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.