Abstract
Anti-metabotropic glutamate receptor 1 (mGluR1) encephalitis is a rare autoimmune disorder manifesting with cerebellar syndrome. Patients with mGluR1 encephalitis have been treated with immunomodulatory therapies; however, little is known about the efficacy of this therapy. A 58-year-old Japanese woman presented with dizziness when walking and standing up. Symptoms persisted and the patient gradually deteriorated. The neurological examination revealed a broad-based gait, horizontal and slightly gaze-evoked nystagmus, noticeable head titubation, and truncal ataxia without limb ataxia. Magnetic resonance imaging was normal. The <sup>123</sup>I-isopropyl-iodoamphetamine single-photon emission-computed tomography scans showed normal cerebellar perfusion. Based on a positive antibody test for anti-mGluR1, the patient was diagnosed with anti-mGluR1 encephalitis. She was treated with intravenous methylprednisolone and intravenous immunoglobulin (IVIg). Symptoms gradually improved over 1 month and almost disappeared after additional IVIg therapy. Anti-mGluR1 encephalitis is a rare disease, and effective treatment is unclear. In this case, a favorable outcome was obtained with immunomodulatory therapy, even though the neurological disability of the disease course is worse. We emphasize the importance of early diagnosis and therapeutic intervention, suspecting the disease on the basis of its characteristic symptoms.
Keywords: Anti-mGluR1 encephalitis, Cerebellar ataxia, Head titubation, Intravenous immunoglobulin therapy, Immunomodulatory therapy
Introduction
Metabotropic glutamate receptor 1 (mGluR1) is enriched in the olfactory bulb, hippocampus, globus pallidus, thalamus, substantia nigra colliculus, and cerebellum in the rat brain [1]. Mice deficient in mGluR1 have severe motor coordination and spatial learning deficits. The absence of mGluR1 induces a severe motor deficit due to cerebellar dysfunction [2]. Sillevis Smitt et al. [3] described 2 patients with cerebellar ataxia whose serum and cerebrospinal fluid IgG was bound to mGluR1 receptors in the brain and showed that anti-mGluR1 antibodies caused ataxia in mice. That was the first report of patients with paraneoplastic cerebellar ataxia due to anti-mGluR1 antibodies. Subsequently, several reports have described non-paraneoplastic cerebellar ataxia caused by anti-mGluR1 autoantibodies that was treated with immunomodulatory therapies [4, 5, 6, 7, 8, 9, 10, 11, 12].
Anti-mGluR1 encephalitis is a rare autoimmune disorder, which manifests mainly as cerebellar syndrome in middle-aged adults [4]. However, the effective therapy for anti-mGluR1 encephalitis is unclear. Herein, we present a case of anti-mGluR1 encephalitis successfully treated with intravenous methylprednisolone (IV MTP) and intravenous immunoglobulin (IVIg). In addition, we review the literature regarding the treatment of anti-mGluR1 encephalitis.
Case Presentation
A 58-year-old Japanese woman was admitted to our hospital because of dizziness when walking and standing up. The patient had been in her usual state of health until 5 months before her first visit to our hospital. At that time (July 2020), she suddenly experienced dizziness when she got up from the chair, but she was able to walk unaided. She owned a bar, but she quit her job because of the dizziness. She visited an orthopedist and an otolaryngologist, but the cause of the dizziness was not identified. During the next 4 months, the dizziness worsened.
In late December 2020, she visited our hospital for the first time and without support. However, 11 days after the first visit, she was admitted to our hospital because of worsening symptoms. On admission, the neurological examination revealed a broad-based gait, horizontal and slightly gaze-evoked nystagmus, head titubation, and truncal ataxia without limb ataxia but with orthostatic dysautonomia. Her speech, cognitive function, and mental state were normal. Insertion of an intrauterine device was the only significant past medical history. She smoked one pack of cigarettes per day for 40 years. She reported drinking socially but had previously drunk a lot. There was no family history of genetic or neurologic disorders. A biochemical examination, including vitamin E, soluble interleukin-2 receptor (sIL-2R), and thyroid function, was normal. Serum antinuclear antibody was weakly positive (1:40; normal: <1:40); however, the patient was negative for other antibodies associated with systemic and neurological autoimmune diseases, including anti-Sm, anti-Jo-1, anti-Ro/SS-A, anti-La/SS-B, anti-U1 RNP, neutrophil proteins leukocyte proteinase 3 antineutrophil cytoplasmic, myeloperoxidase antineutrophil cytoplasmic, and antigliadin antibodies; antithyroglobulin; antithyroid peroxidase; and anti-voltage-gated calcium channel. Paraneoplastic antibodies (anti-AMPH, CV2, PNMA2, Ri, Yo, Hu, recoverin, SOX1, titin, zic4, GAD65, and Tr) were also negative other than SOX1(±). A cerebrospinal fluid examination revealed no pleocytosis (1 leukocyte/µL) with normal glucose and protein levels and no oligoclonal IgG bands. Magnetic resonance imaging was normal. 123I-isopropyl-iodoamphetamine single-photon emission-computed tomography scans showed normal cerebellar perfusion. Whole-body computed tomography, esophagogastroduodenoscopy, and colonoscopy did not reveal any cancer. Positron emission tomography with 18F-fluoro-2-deoxy-D-glucose showed abnormal uptake in the uterus endometrium. A gynecologist performed a histological examination of the uterus endometrium. No malignancy was found, but bacterial infection of the intrauterine device was detected, and the device was removed. The cerebellar ataxia gradually worsened. She was unable to perform activities of daily living because of truncal ataxia. She did not receive any treatments during the first hospital stay.
In mid-January 2021, she visited our hospital in a wheelchair and was admitted (additional file 1: online suppl. video 1; for all online suppl. material, see www.karger.com/doi/10.1159/000526632). Cerebellar ataxia was assessed using the scale for the assessment, and the extent of ataxia (scale for the assessment and rating of ataxia [SARA]) [13] and activities of daily living were assessed using the modified Rankin Scale (mRS). The SARA score was 19 points, and the mRS score was 4 before therapy. Truncal ataxia was severe (gait, stance, and sitting), while extremity ataxia (finger chase, nose-finger test, heel-knee test, and fast alternating hand movement) was mild. Head titubation and associated involuntary movements of the trunk disappeared when she put the back of her head on the wall or laid in bed in the supine position. Slight lateral gaze-evoked nystagmus was present. Speech was normal. Additional blood examinations were performed. Antigliadin antibodies and paraneoplastic antibodies were negative, including SOX1, but the anti-mGluR1 antibody was detected in serum with a cell-based assay using COS7 cells expressing mGluR1.
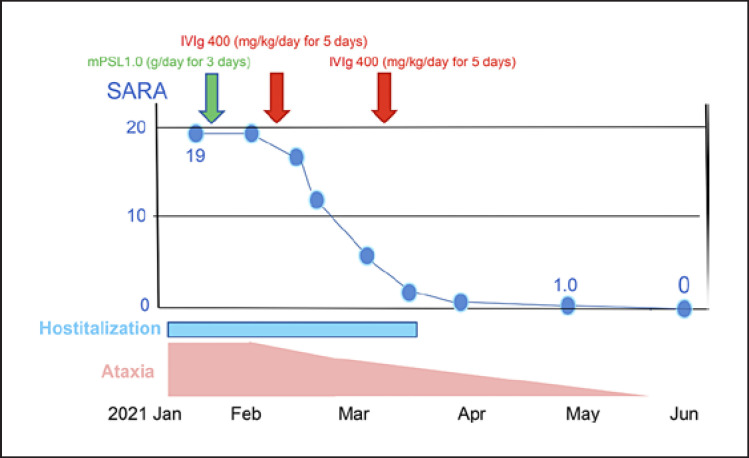
In late January, IV MTP therapy was initiated at a dose of 1,000 mg/day for 3 days. However, the therapy was ineffective. In early February, IVIg (IVIg: 400 mg/kg/day) was administered for 5 days. Truncal ataxia improved slightly 4 days after the IVIg therapy and continued to improve gradually over 1 month. After 1 month, the SARA score improved to 7 points. Additional IVIg (IVIg: 400 mg/kg/day) was administered for 5 days in early March, and the SARA score improved to 3.5 points at the end of the therapy (additional file 2: online suppl. Video 2). Her walk normalized, and only slight truncal ataxia remained. She was discharged to her home without support. At the last follow-up, 2 months after the discharge, her ataxia further improved (SARA score decreased to 1 point, and mRS score was 1), and 3 months after the discharge, her ataxia has disappeared (SARA score was 0 points, and mRS score was 0) (Fig. 1). In January 2022, approximately 1 year from the initiation of therapy, no relapse occurred, and no additional medication was required.
Fig. 1.
Timeline for the clinical course of the case. The ataxia and SARA score almost normalized by 4 months after the first immunomodulatory therapy. IVIg, intravenous immunoglobulin; PSL, prednisolone; SARA, scale for the assessment and rating of ataxia.
Discussion
Autoantibodies against mGluR1 affect the excitability, plasticity, and survival of Purkinje cells, leading to deficits in both cerebellar motor performance and motor learning [14]. Anti-mGluR1 encephalitis occurs mainly in middle-aged adults, although it occurs rarely in children. Patients present with non-isolated cerebellar syndrome accompanied by other neurologic symptoms, including behavioral changes and cognitive impairment [4]. Our patient manifested only cerebellar ataxia, which was mainly truncal ataxia.
Anti-mGluR1 encephalitis is described in only a few reviews [4, 5]. Hence, therapies for anti-mGluR1 encephalopathy are unclear. Spatola et al. [4] reviewed all reported cases with mGluR1 antibodies before this review was published; 17 patients (89%; 17/19) received first-line immunomodulatory therapies (oral prednisolone, IV MTP, IVIg, and plasma exchange), and 12 patients (63%; 12/19) received second-line immunomodulatory therapies (azathioprine, cyclophosphamide, hydroxychloroquine, mycophenolate mofetil, and rituximab) (Table 1). Some patients improved with the administration of only the first-line immunomodulatory therapies, whereas others improved with the administration of second-line immunomodulatory therapies. Therefore, the available data may not allow a conclusion to be drawn on patients who may need the first-line or second-line immunomodulatory therapies.
Table 1.
Previously reported cases of mGluR1 encephalitis from nine studies
| Case | Reference | Age/sex | Therapy |
|---|---|---|---|
| 1 | Spatola et al., (2020) [4] | 29/M | IV MTP, IVIg, CYC, RTX |
| 2 | Spatola et al., (2020) [4] | 22/F | IVIg, RTX, CYC |
| 3 | Spatola et al., (2020) [4] | 45/F | IV MTP, IVIg, RTX, CYC, AZA |
| 4 | Spatola et al., (2020) [4] | 54/M | Oral PSL, IV MTP, HQQ, MMF |
| 5 | Spatola et al., (2020) [4] | 56/M | Oral PSL, IV MTP, MMF |
| 6 | Spatola et al., (2020) [4] | 38/F | IV MTP, IVIg, RTX, CYC |
| 7 | Spatola et al., (2020) [4] | 49/F | IVIg, AZA |
| 8 | Spatola et al., (2020) [4] | 6/M | IV MTP, IVIg |
| 9 | Spatola et al., (2020) [4] | 62/M | Oral PSL, IV MTP |
| 10 | Lopez-Chiriboga et al., (2016) [5] | 54/M | Steroid, IVIg |
| 11 | Lopez-Chiriboga et al., (2016) [5] | 51/M | Steroid, IVIg, PE |
| 12 | Lopez-Chiriboga et al., (2016) [5] | 33/F | Steroid |
| 13 | Lopez-Chiriboga et al., (2016) [5] | 64/M | Steroid, RTX |
| 14 | Lopez-Chiriboga et al., (2016) [5] | 81/M | IVIg |
| 15 | Lopez-Chiriboga et al., (2016) [5] | 77/M | Steroid, IVIg |
| 16 | Lopez-Chiriboga et al., (2016) [5] | 60/F | PSL |
| 17 | Marignier et al., (2010) [6] | 50/F | Oral PSL, IVIg, MMF |
| 18 | Iorio et al., (2013) [7] | 65/M | Oral PSL, IVIg |
| 19 | Yoshikura et al., (2018) [8] | 51/F | Oral PSL, IV MTP, IVIg, PE, RTX, TCR, AZA |
| 20 | Christ et al., (2019) [9] | 45/M | Oral PSL, IV MTP, IVIg, RTX |
| 21 | Bien et al., (2020) [10] | 3/M | IV MTP, oral PSL |
| 22 | Chaumont et al., (2019) [11] | 22/F | IVIg, RTX, CYC |
| 23 | Golion C et al., (2018) [12] | 64/M | Steroid, IVIg |
IV MTP, intravenous methylprednisolone; IVIg, intravenous immunoglobulin; CYC, cyclophosphamide; RTX, rituximab; AZA, azathioprine; HQQ, hydroxychloroquine; MMF, mychophenolate mofetil; PSL, prednisolone; PE, plasma exchange; TCR, tacrolimus.
In their study, comparing patients with good and bad outcomes following anti-mGluR1 encephalitis based on an mRS score of 2 as the cutoff, Spatola et al. [4] reported that the patients with bad outcomes exhibited worse neurological disability at the peak of the disease course and were more likely to require walking assistance compared to those with good outcomes. However, the authors did not find significant differences in age, sex, clinical presentation, tumor association, or brain abnormalities at initial magnetic resonance imaging, immunotherapy, or the length of delay from onset to treatment between the two groups. In the present case, the SARA score was 19 and the mRS score was 4 at the peak of the disease course. However, the symptoms resolved with first-line therapy, suggesting that improvement might be possible even in patients with severe clinical presentation. The reason for the better outcome observed in the present case remains unclear, although there is a possibility that immunotherapy was initiated before irreversible cellular damage in the cerebellum. It is challenging to evaluate cerebellar damage; however, the magnetic resonance imaging was normal, and the 123I-isopropyl-iodoamphetamine single-photon emission-computed tomography scans showed normal cerebellar perfusion in the present case, suggesting limited morphological and functional damage. It is probable that the initiation of first-line therapy before cerebellar damage might be associated with the better outcome observed in this case.
The neurological examination of the current patient revealed noticeable head titubation, truncal ataxia without limb ataxia, and gait and trunk instability (Video 1). Head/trunk tremor or titubation and gait/trunk instability are observed in most patients with anti-mGluR1 encephalitis [4]. Pedroso et al. [15] indicated that subacute head titubation should raise suspicion for mGluR1-associated autoimmune cerebellitis. As observed in the current case, subacute progressive head/trunk tremors or titubation and gait/trunk instability may be key features for the differential diagnosis of anti-mGluR1 encephalitis and may lead to early diagnosis, which can improve outcomes by allowing the early initiation of therapeutic intervention before cerebellar damage.
We emphasize that suspecting the disease on the basis of characteristic symptoms leads to early diagnosis and enables early therapeutic intervention. This can result in a better outcome even though the neurological disability of the disease course is worse.
Statement of Ethics
This study has been carried out as per the Declaration of Helsinki. This study protocol was reviewed and approved by the Ethics Committee of the National Hospital Organization Hyogo-Chuo National Hospital (Approval No. 21-03). Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This work was supported by grants-in-aid from the Research Committee of Ataxia, Health Labor Sciences Research Grant, the Ministry of Health Labor and Welfare, Japan (JPMH20FC1041).
Author Contributions
Kento Sakashita, Katsuya Nishida, and Naonobu Futamura carried out the clinical examination of the patient and contributed to the clinical description. Kento Sakashita gathered all clinical data, carried out literature review, and wrote the manuscript. Kento Sakashita, Katsuya Nishida, Yu Takenaka, Ichiro Yokota, Hiroshi Yamasaki, Keisuke Nishimoto, Kunihiko Kawamoto, Maki Mitani, Itaru Funakawa, Nobuaki Yoshikura, Akio Kimura, Takayoshi Shimohata, and Naonobu Futamura have read and approved the final manuscript.
Data Availability Statement
All data and material (additional files 1 and 2) supporting our findings have been provided. The data that support the findings of this study are openly available in “figshare” at http://doi.org/[doi], reference number (video 1 “Video presentation of the patient with ataxia before therapy”, video 2 “Video presentation of the patient with ataxia after therapy”)
Supplementary Material
Supplemental Video
Supplemental Video
Acknowledgments
We thank Dr. Masakatsu Motomura and Dr. Shunsuke Yoshimura for the voltage-gated calcium channel antibody testing. We are thankful to the patient and her family for their valuable cooperation.
Funding Statement
This work was supported by grants-in-aid from the Research Committee of Ataxia, Health Labor Sciences Research Grant, the Ministry of Health Labor and Welfare, Japan (JPMH20FC1041).
References
- 1.Martin LJ, Blackstone CD, Huganir RL, Price DL. Cellular localization of a metabotropic glutamate receptor in rat brain. Neuron. 1992 Aug;9((2)):259–270. doi: 10.1016/0896-6273(92)90165-a. [DOI] [PubMed] [Google Scholar]
- 2.Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, et al. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994 Nov;372((6503)):237–243. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- 3.Sillevis Smitt P, Kinoshita A, De Leeuw B, Moll W, Coesmans M, Jaarsma D, et al. Paraneoplastic cerebellar ataxia due to autoantibodies against a glutamate receptor. N Engl J Med. 2000 Jan;342((1)):21–27. doi: 10.1056/NEJM200001063420104. [DOI] [PubMed] [Google Scholar]
- 4.Spatola M, Petit Pedrol M, Maudes E, Simabukuro M, Muñiz-Castrillo S, Pinto AL, et al. Clinical features prognostic factors and antibody effects in anti-mGluR1 encephalitis. Neurology. 2020 Sep;95((22)):e3012–e3025. doi: 10.1212/WNL.0000000000010854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Chiriboga AS, Komorowski L, Kümpfel T, Probst C, Hinson SR, Pittock SJ, et al. Metabotropic glutamate receptor type 1 autoimmunity clinical features and treatment outcomes. Neurology. 2016 Mar;86((11)):1009–1013. doi: 10.1212/WNL.0000000000002476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marignier R, Chenevier F, Rogemond V, Sillevis Smitt P, Renoux C, Cavillon G, et al. Metabotropic glutamate receptor type1 autoantibody-associated cerebellitis a primary autoimmune disease? Arch Neurol. 2010 May;67((5)):627–630. doi: 10.1001/archneurol.2010.51. [DOI] [PubMed] [Google Scholar]
- 7.Iorio R, Damato V, Mirabella M, Vita MG, Hulsenboom E, Plantone D, et al. Cerebellar degeneration associated with mGluR1 autoantibodies as a paraneoplastic manifestation of prostate adenocarcinoma. J Neuroimmunol. 2013 Oct;263((1–2)):155–158. doi: 10.1016/j.jneuroim.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 8.Yoshikura N, Kimura A, Fukata M, Fukata Y, Yokoi N, Harada N, et al. Long-term clinical follow-up of a patient with non-paraneoplastic cerebellar ataxia associated with anti-mGluR1 autoantibodies. J Neuroimmunol. 2018 Jun;319:63–67. doi: 10.1016/j.jneuroim.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Christ M, Müller T, Bien C, Hagen T, Naumann M, Bayas A. Autoimmune encephalitis associated with antibodies against the metabotropic glutamate receptor type 1 case report and review of the literature. Ther Adv Neurol Disord. 2019 May;12:1756286419847418. doi: 10.1177/1756286419847418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bien CG, Braig S, Bien CI. Antibodies against metabotropic glutamate receptor type 1 in a toddler with acute cerebellitis. J Neuroimmunol. 2020 Nov;348:577366. doi: 10.1016/j.jneuroim.2020.577366. [DOI] [PubMed] [Google Scholar]
- 11.Chaumont H, Petit A, Mameri T, Schollhammer R, Honnorat J, Lannuzel A. Successful management of anti-mGluR1 encephalitis with immunosuppressive treatment dengue virus as a trigger? Mov Disord Clin Pract. 2019 Oct;6((8)):727–728. doi: 10.1002/mdc3.12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gollion C, Dupouy J, Roberts M, Simonetta-Moreau M, Brefel Courbon C, Rascol O, et al. Reversible myoclonus-ataxia encephalitis related to anti-mGluR1 autoantibodies. Mov Disord. 2019 Mar;34((3)):438–439. doi: 10.1002/mds.27634. [DOI] [PubMed] [Google Scholar]
- 13.Schmitz-Hübsch T, du Montcel ST, Baliko L, Berciano J, Boesch S, Depondt C, et al. Scale for the assessment and rating of ataxia development of a new clinical scale. Neurology. 2006 Jun;66((11)):1717–1720. doi: 10.1212/01.wnl.0000219042.60538.92. [DOI] [PubMed] [Google Scholar]
- 14.Benarroch EE. Metabotropic glutamate receptors synaptic modulators and therapeutic targets for neurologic disease. Neurology. 2008 Mar;70((12)):964–968. doi: 10.1212/01.wnl.0000306315.03021.2a. [DOI] [PubMed] [Google Scholar]
- 15.Pedroso JL, Dutra LA, Espay AJ, H¨oftberger R, Barsottini OGP. Video NeuroImages head titubation in anti-mGluR1 autoantibody-associated cerebellitis. Neurology. 2018 April;90((16)):746–747. doi: 10.1212/WNL.0000000000005338. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video
Supplemental Video
Data Availability Statement
All data and material (additional files 1 and 2) supporting our findings have been provided. The data that support the findings of this study are openly available in “figshare” at http://doi.org/[doi], reference number (video 1 “Video presentation of the patient with ataxia before therapy”, video 2 “Video presentation of the patient with ataxia after therapy”)