Abstract
Primary cutaneous mucinous carcinoma (PCMC) is a rare malignant skin adnexal tumor. Recurrences are most often localized, and long-term follow-up after complete surgery consists essentially of self-examination of skin. We report one case of metastatic PCMC with elevated levels of serum CEA and CA15.3. Because of the difficulty to differentiate PCMC and metastasis of mucinous breast cancer, the hypothesis of a metastasized breast cancer was ruled out. These tumor markers contributed to the monitoring of the metastatic disease. Since metastatic disease was diagnosed after several years of seeming complete remission, CEA and CA15.3 would likely have allowed the clinicians to detect the relapse earlier. Although the use of tumor biomarkers in PCMC is not rooted in clinical practice and not mentioned in guidelines, we suggest that CEA and CA15.3 could be of particular interest to monitor and detect early metastatic PCMC.
Keywords: Mucinous skin cancer, Monitoring, Serum tumor marker, CA15.3, CEA
Introduction
Primary cutaneous mucinous carcinoma (PCMC) is a rare and slow-growing low-grade malignant skin adnexal tumor [1]. Most cases arise on the face, especially on the eyelid and scalp, but presentation in the axilla and trunk has been reported [1]. Clinically, the lesion presents as an erythematous or gray-blue painless nodule (0.5–7 cm in diameter) which may be indurated or ulcerated [1, 2]. Prognosis is good when diagnosed and treated promptly by surgical resection. However, in rare cases, metastatic dissemination can occur and is associated with a poor outcome [1]. Indeed, low effectiveness of chemotherapy and radiotherapy on metastatic disease has been shown [3]. It is noteworthy that no evidence-based guidelines are available for early detection and monitoring of the disseminated disease. In addition, to the best of our knowledge, there is no indication in the literature that using serum tumor markers could help detect and monitor the disseminated disease. We report a case of metastatic PCMC in which tumor markers CEA and CA15.3 were elevated and contributed to the follow-up of the metastatic disease.
Case Report
A 58-year-old woman presented in 2009 with an occipital sub-cutaneous lesion of the scalp (6 cm along its longer axis) suspected to be a lipoma. Complete surgical resection showed a polylobed nodule located in the dermis and hypodermis with a 1-mm histological safety margin in depth. Immunohistochemistry showed the following staining pattern: CK7+, CK20−, CEA+ (heterogenous), p53−, Mib-1/Ki67+ (<5% of tumor cells), and α-smooth muscle actin−. Histopathological analysis concluded a sweat gland tumor with myxoid stroma and the final diagnosis was a low-grade primary mucinous cutaneous carcinoma or a cutaneous mixed tumor.
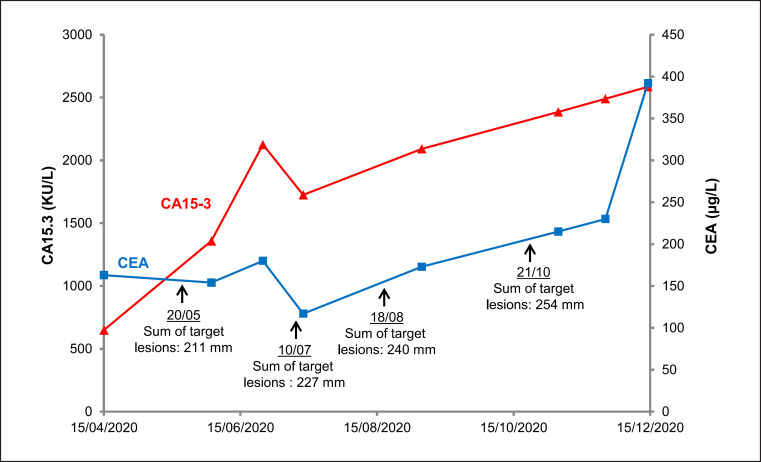
In 2010, at distance from the initial lesion, recurrence occurred with two skin lesions localized in temporal and parietal areas. Corrective surgery allowed a complete resection of the tumor, and histology was similar to that of the initial tumor. Immunohistochemistry showed ER+ and PR+ and tamoxifen was initiated. Given that clinical examination and brain CT scan showed no recurrence after a 5-year course of treatment, tamoxifen was discontinued. Quarterly then annual monitoring with physical examination and brain CT scan showed no recurrence in the following 5 years. However, 5 years after discontinuation of tamoxifen, the patient presented in April 2020 with chest pain. CT TAP scan revealed a mass in the right lung base, hepatic pedicle and mediastinal adenopathies, and a secondary liver cancer. PET scan confirmed a disseminated tumoral disease in the lung, mediastinum, liver, peritoneum, bone, and lymph node. Liver biopsies were taken; histological and immunohistochemistry analysis confirmed metastasis of a primary mucinous cutaneous carcinoma with GATA3+, ER+, PR−, CDX2−, TTF1− (shown in Fig. 1). It is noteworthy that bilateral ACR3 mammographic lesions and lymphadenopathies were evidenced, but biopsies showed only benign mammary lesion and reactive lymphoid hyperplasia. Serum tumor markers CEA and CA15.3, commonly used to monitor breast cancer, were assayed for the first time and interestingly were elevated with concentration of 163 μg/L (N <4.7) and 649 kU/L (N < 26.4), respectively. Because of the lack of systemic therapy guidelines and based on similarities between this PCMC and breast cancer, in particular, estrogen receptor expression, a treatment associating fulvestrant and palbociclib was initiated in June 2020. CEA level was stable (154 μg/L), but CA15.3 increased to 1,358 kU/L at the initiation of therapy. The course of tumor markers together with target lesion size is shown in Figure 2. After 2 months, because CT-scan and serum tumor markers evidenced disease progression (including tumor progression in liver), fulvestrant and palbociclib were stopped and second-line chemotherapy with carboplatin-taxol was started in September 2020. Despite changing chemotherapy, the disease progressed, as evidenced by CT scan (shown in Fig. 3) and tumor markers (shown in Fig. 2), and management was limited to palliative care.
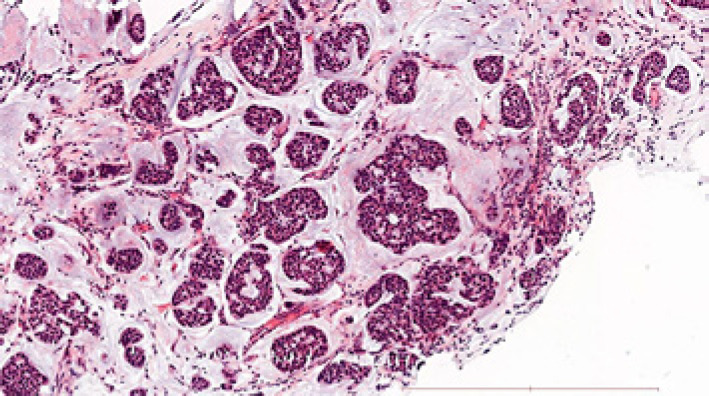
Fig. 1.
Liver metastasis core needle biopsy sample histology. Well-differentiated mucinous adenocarcinoma characterized by abundant pools of mucin and epithelial islands of atypical cells with ductal differentiation (HE staining, ×100 magnification, scale bar, 500 μm). The immunophenotype of tumor cells was GATA3+, TTF1−, CDX2−, ER+, PR−.
Fig. 2.
Serum tumor markers CEA and CA15.3 levels with respect to the sum of target lesions size during metastatic PCMC monitoring. CEA is expressed in µg/L (right axis) and CA15-3 in kU/L (left axis). Courses of tumor markers CEA and CA15.3 are presented by blue and red line, respectively. References values are <4.7 μg/L and <26.4 kU/L for CEA and CA15.3, respectively. Target lesions sizes were measured with CT scan.
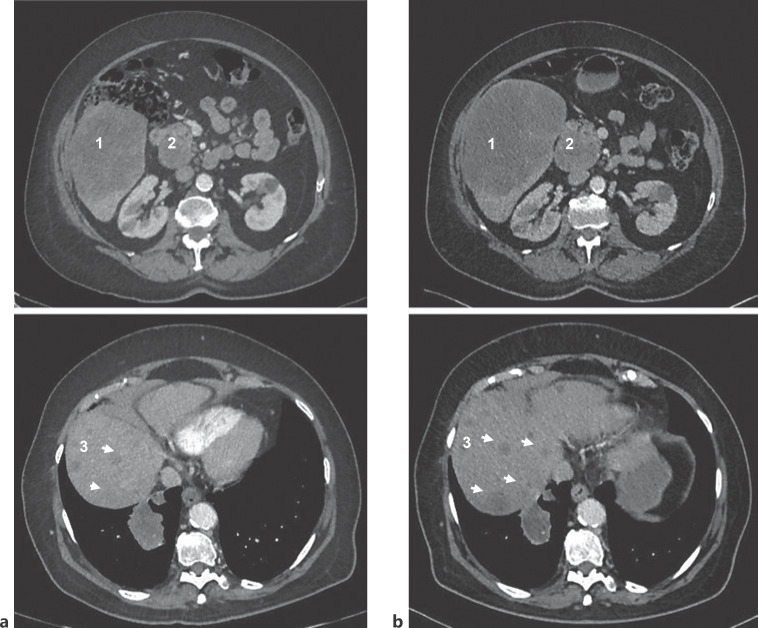
Fig. 3.
Two CT scans, repeated at an interval of about 3 months, show disease progression. a CT scan performed in July 2020. b CT scan performed in October 2020. CT scan slices enable to visualize hepatic lesion (identified by «1»), hilar lymphadenopathy (identified by «2»), and multiple hepatic lesions (identified by «3»). Comparison shows an increase of about 33% for hepatic lesion and 8% for hilar lymphadenopathy as well as enlargement of multiple hepatic lesions.
Discussion
We report a case of metastatic PCMC with elevated levels of serum CEA and CA15.3, tumor markers commonly used for breast cancer monitoring. At first sight, elevated tumor markers of breast cancer associated with PCMC could lead to suspect a misdiagnosis. Indeed, because of homologies between PCMC and mucinous lesions of breast cancer [4], distinction between PCMC and metastasis of breast cancer is particularly difficult. For the patient, breast imaging was carried out and allowed to formally exclude breast cancer. Interestingly, CA15.3 levels are well correlated with disease progression assessed by CT scan. CEA was also closely linked to disease worsening (shown in Fig. 1). Note that CA125 and CA19.9, other mucins used as tumor markers, were normal. Moreover increased CA15.3 and CEA were also evidenced in another of our patients with primary cutaneous endocrine mucin-producing sweat gland carcinoma, a cutaneous adnexal carcinoma subtype close to PCMC [5]. Immunohistochemistry staining pattern was CK7+, CK8+, CK20−, ER+, PR+, synaptophysin+, CGA+, TTF1−, PS100−, p63−, GCDFP15−, Ki67 ≈ 15%.
In addition to their role in monitoring noninvasively metastatic PCMC, CEA and CA15.3 could probably help detect early recurrence or disseminated disease. The case presented here shows that metastatic relapse can occur after several years of seeming complete remission. Indeed, despite complete resection and regular monitoring with physical examination and brain CT scan, metastatic stage was diagnosed late when symptoms developed. Because of high levels of CEA and CA15.3 evidenced when metastatic disease was detected, there is much to suggest that including serum tumor markers for monitoring would have permitted earlier detection of the relapse.
Conclusion
To the best of our knowledge, this report is the first to highlight elevated serum CEA and CA15.3 in metastatic PCMC. CEA and CA15.3 are well correlated with disease progression and therefore can be considered as tumor markers of PCMC. As evidenced here and in other reports, late recurrences and metastases can occur in PCMC [1], and so, if other studies confirm their role, CEA and CA15.3 could improve disease monitoring and early recurrence detection.
Statement of Ethics
This study was approved by the Ethical Review Committee for publication of the Cochin university Hospital (CLEP) (No.: AAA-2022-08003). Written informed consent was obtained from the next-of-kin for publication of the details of their medical case and any accompanying images.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
No funding was received.
Author Contributions
Antonin Ginguay suggested to report this case, performed serum tumor markers measurement, and wrote the manuscript. Solène Lecolant helped collect clinical and biological data and design the figure. Nora Kramkimel, Jennifer Arrondeau, and François Goldwasser managed the patient and contributed with their clinical expertise. Maxime Battistella contributed with their expertise in anatomopathology. Nora Kramkimel, Solène Lecolant, Maxime Battistella, François Goldwasser, and Jennifer Arrondeau helped draft the manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Acknowledgements
We are grateful to the patient's next-of-kin for allowing us to publish this case report. We also thank Pr Dominique Wendum (Saint-Antoine Hospital, AP-HP, Paris, France) for providing histology slide of the liver metastasis core needle biopsy.
Funding Statement
No funding was received.
References
- 1.Kamalpour L, Brindise RT, Nodzenski M, Bach DQ, Veledar E, Alam M. Primary cutaneous mucinous carcinoma a systematic review and meta-analysis of outcomes after surgery. JAMA Dermatol. 2014;150((4)):380–384. doi: 10.1001/jamadermatol.2013.6006. [DOI] [PubMed] [Google Scholar]
- 2.Javaid H, Raza N, Ejaz U, Sarfraz T. Unusual skin mass (primary cutaneous mucinous carcinoma) BMJ Case Rep. 2018;2018:bcr2017222546. doi: 10.1136/bcr-2017-222546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breiting L, Christensen L, Dahlstrøm K, Breiting V, Winther JF. Primary mucinous carcinoma of the skin a population-based study. Int J Dermatol. 2008;47((3)):242–245. doi: 10.1111/j.1365-4632.2008.03558.x. [DOI] [PubMed] [Google Scholar]
- 4.Kazakov DV, Suster S, LeBoit PE, Calonje E, Bisceglia M, Kutzner H, et al. Mucinous carcinoma of the skin and secondary a clinicopathologic study of 63 cases with emphasis on the morphologic spectrum of primary cutaneous forms: homologies with mucinous lesions in the breast. Am J Surg Pathol. 2005;29((6)):764–782. doi: 10.1097/01.pas.0000159104.02985.6b. [DOI] [PubMed] [Google Scholar]
- 5.Agni M, Raven ML, Bowen RC, Laver NV, Chevez-Barrios P, Milman T, et al. An update on endocrine mucin-producing sweat gland carcinoma clinicopathologic study of 63 cases and comparative analysis. Am J Surg Pathol. 2020;44((8)):1005–1016. doi: 10.1097/PAS.0000000000001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further inquiries can be directed to the corresponding author.