Abstract
As a bacterial product, Helicobacter pylori lipopolysaccharide (LPS) can originate in close proximity to parietal cells, but the role of this uniquely structured endotoxin on acid secretion has not been fully investigated and remains unclear. The purpose of this study was to test the direct effect of purified LPS (tested range, 0.1 to 100 μg/ml) from various strains of H. pylori and from one Helicobacter felis strain on histamine- and carbachol-stimulated acid secretion in vitro using mouse gastric glands and the accumulation of [14C]aminopyrine. In addition, we investigated whether H. pylori LPS can interfere with two native antisecretory substances, prostaglandin E2 (PGE2) and somatostatin, which may contribute to bacterial pathogenicity. Except for the LPS from H. pylori SS1 (Sydney strain), which gave a statistically significant increase in both histamine- and carbachol-stimulated acid output (38 and 24%, respectively; P < 0.05), no effect of the tested LPS was observed on acid secretion. H. pylori LPS purified from a patient isolate did not affect the potency or the efficacy of the inhibitory dose response curve to PGE2 or somatostatin. Bacterial interstrain variation in the direct stimulatory effect of Helicobacter-derived LPS on acid secretion was observed, which probably reflects the molecular structure of LPS and the potential to contribute to virulence. Importantly, the data showed that H. pylori LPS did not have any direct antisecretory properties. It can be speculated that the acid stimulatory properties of LPS from H. pylori SS1 may contribute to the gastric damage observed in the mouse model of H. pylori infection.
Helicobacter spp. are known to colonize the stomach of mice with the subsequent development of gastritis. This has led to the development and standardization of a mouse model of Helicobacter pylori-induced gastritis (15). Both bacterial factors and host resistance contribute to the severity of H. pylori-induced pathology (5). Gastric acid secretion can be beneficial while it acts as a part of the host defense mechanism preventing bacterial infection and hence pathogenicity. However, it also leads to erosive ulcers of the stomach and/or duodenum. Consequently, it also plays a key role in the colonization patterns of Helicobacter species, as increased acid secretion tends to keep bacterial colonization in the antral region of the stomach whereas decreased acid secretion permits bacterial colonization and spread throughout the corpus (14, 30). The presence of Helicobacter infection can modulate acid secretion by altering the physiology of G cells, D cells, and parietal cells (7). It can do this either by the direct presence of its metabolites or through induction of the inflammatory process as mediated by a wide range of cytokines.
Gram-negative bacterial lipopolysaccharide (LPS) from a number of bacterial species effectively inhibits gastric acid secretion in vivo (1, 6, 26, 28, 32). However, the mechanism of LPS action suggests the involvement of inflammatory products or mediators such as cytokines and prostaglandins (25, 27). Recently, H. pylori LPS was shown to inhibit acid secretion in vivo (22). H. pylori LPS has been implicated in the stimulation of pepsinogen and histamine secretion, inhibition of sulfated mucin synthesis, and the production of potentially destructive autoantibodies, which may all contribute to the loss of mucosal integrity (18). In addition, H. pylori LPS was shown to bind to the gastric mucosal somatostatin receptor (23). H. pylori LPS, as compared to Escherichia coli LPS, has a relatively low immunological activity (20) that potentially contributes to the persistence of infection.
The present study focuses on the potential effect of H. pylori LPS directly on acid secretion in order to elucidate possible mechanisms by which bacteria can affect parietal cells. H. pylori resides in the mucus layer close to the epithelium; therefore, its metabolites originate in relative and, in some circumstances, close proximity to acid-producing cells, but the role of this endotoxin on the secretory properties of parietal cells has not been fully investigated. As the mouse models of H. pylori infection have entered the mainstream of research (15), we have adapted and characterized the mouse gastric gland in vitro model of acid secretion. The purpose of this study was to test several purified Helicobacter-derived LPS preparations for a direct effect on carbachol- or histamine-stimulated acid secretion and its potential interference with the antisecretory actions of somatostatin and prostaglandin E2 (PGE2).
MATERIALS AND METHODS
Purification of LPS from H. pylori and Helicobacter felis.
Bacterial strains of H. pylori and H. felis were grown on blood agar to produce biomass as described previously (17). Bacteria were harvested in sterile distilled water, centrifuged at 5,000 × g (4°C, 30 min), and washed twice, and the bacterial pellets were freeze-dried. After pretreatment of the bacterial biomass with pronase E (8), LPS was extracted by the hot phenol-water technique (17). The LPS preparations were purified by treatment with DNase, RNase, and proteinase K and by ultracentrifugation as described previously (17). Five Helicobacter-derived LPS preparations were prepared and tested: water- and phenol-phase LPSs from the type strain H. pylori NCTC 11637 (purchased from the National Collection of Type Cultures, London, England), water-phase LPS from the Sydney strain H. pylori SS1 (obtained from A. Lee, Department of Microbiology and Immunology, University New South Wales, Sydney, Australia), water-phase LPS from an isolate from a duodenal ulcer patient (H. pylori patient isolate [PI]), and water-phase LPS from H. felis ATCC 49179 (purchased from the American Type Culture Collection, Rockville, Md.).
Animals.
Female BALB/c mice, 6 to 8 weeks old, were obtained from Charles River (St. Constant, PQ J5A 1Y2, Canada), kept under standard housing conditions at 21 to 23°C with a humidity of 40 to 50% and a 12/12 light/dark cycle, and fed Purina Lab Rodent Chow for up to 12 weeks. Ten mice for each acid assay were not fed for 24 h (water, ad libitum) prior to sacrifice by cervical dislocation. Subsequently, the stomachs were quickly removed, opened along the lesser curvature, and placed in oxygenated phosphate-buffered saline buffer, pH 7.3, at 37°C. Utilization of animals was approved by the Animal Research Ethics Board at McMaster University.
Preparation of gastric glands from mice.
Preparation of gastric glands was performed according to the method of Berglindh (4) with some modifications. Briefly, the gastric mucosa was scraped off the underlying muscle using a scalpel blade, pooled, and washed twice (approximately 200 g for 5 min) in phosphate-buffered saline. The scrapings were placed in an enzyme solution that contained (per ml) 2 mg of glucose, 1 mg of bovine serum albumin (Sigma A-7888), 0.25 mg of type II-s soybean trypsin inhibitor (Sigma T-9128), and 0.23 mg of type IV collagenase (Sigma C-5138). Since there were discrepancies in the acid-producing capacity of glands depending on the batch of collagenase used, different batches of collagenase were screened. Mouse gastric mucosa was enzymatically digested at 37°C for 45 min in a flat-bottom, covered, 150-ml Erlenmeyer flask and agitated by a magnetic stirrer (around 100 rpm). Subsequently, gastric glands were passed through nylon mesh (500-μm hole size) in order to separate debris and the undigested remains of the gastric mucosa. Then the preparation was washed three times (approximately 200 g for 5 min) in enzymatic buffer that did not contain collagenase or trypsin inhibitor. Finally, the preparation was resuspended in 50 ml of incubation medium containing 2 mM CaCl2, 1.2 mM MgSO4, 2 mg of bovine serum albumin/ml, and 2 mg of glucose/ml.
Measurement of acid secretion in mouse gastric glands.
Acid secretion was measured by the accumulation of a weak base, [14C]aminopyrine ([14C]AP), as described by Berglindh (4) with some modifications. Briefly, the experiment was carried out in closed 1.5-ml Eppendorf tubes containing 0.5 ml of resuspended gastric glands with added secretagogue (0.01 mM carbachol or 0.1 mM histamine), antisecretory compound (e.g., somatostatin or PGE2), and doses of test preparations of LPS (for control tubes that did not contain LPS, a corresponding volume of endotoxin-free distilled water was used). For testing basal acid secretion, tubes did not contain histamine or carbachol. Also, 20 μl (equal to 0.25 μCi) of [14C]AP was added to the tubes and incubated at 37°C for 60 min, with rotation. All tested reagents were coincubated with the gastric glands. The tubes were centrifuged at approximately 1,500 × g for 5 min, the supernatant was aspirated, and the pellet was washed three times in incubation buffer to minimize any nonspecific aminopyrine retention in the glands. The pellet was transferred to scintillation tubes and solubilized with 1 ml of tissue solubilizer (NCS-2; Amersham) overnight. Subsequently, 50 μl of glacial acetic acid was added to each tube containing the solubilized pellet in order to neutralize the highly basic tissue solubilizer. After the addition of 5 ml of ACS scintillation fluid (Amersham), radioactivity was determined in a Beckman scintillation counter (LS 5801). The radioactivity of the pellet correlated positively with the amount of acid secreted during 60 min of incubation. The radioactivity accumulated by glands with 0.1 mM dinitrophenol was subtracted from all data to compensate for any nonspecifically trapped [14C]AP, which accounted for less than 0.5% of the maximal histamine response. Each sample was tested in triplicate within each individual experiment, and each experiment was repeated with different gland preparations. This repetition is expressed by n (the number of individual experiments).
Chemicals.
E. coli LPS and all chemicals were of high purity and were purchased from Sigma unless otherwise stated.
Statistical analysis.
The data were calculated as the percentage of the maximal response in AP uptake to various stimulants; n represents the number of gland preparations for which each data point was tested in triplicate. The results are expressed as the mean ± the standard error of the mean of the preparation results. The significance of differences was tested by Student's t test, and differences were considered statistically significant if P was <0.05.
RESULTS
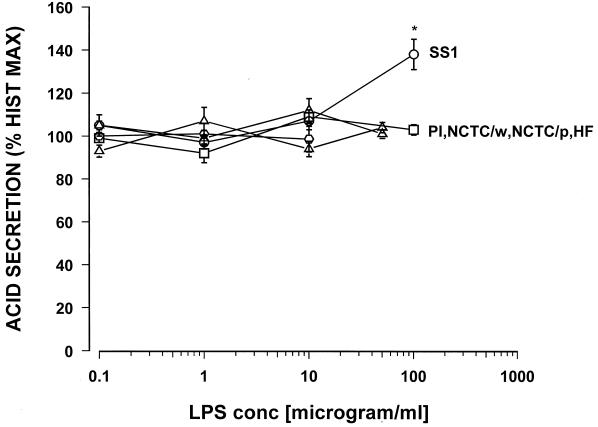
Concentration response curves were constructed for carbachol and histamine, with the maximal response being a 3- to 5-fold and 8- to 12-fold increase over basal acid secretion, respectively (data not shown). Maximum acid stimulation occurred at 10−5 M for carbachol and 10−4 M for histamine. Each LPS preparation was tested over the range from 0.1 to 100 μg/ml. For histamine-stimulated acid secretion, none of the tested H. pylori LPS showed a direct effect on acid secretion except H. pylori SS1 (Fig. 1).
FIG. 1.
Effect of various samples of Helicobacter LPS on histamine (10−4 M)-stimulated acid secretion in the mouse gastric glands as measured by [14C]AP accumulation. PI, H. pylori isolate from a duodenal ulcer patient; NCTC/p, H. pylori NCTC 11637 (phenol phase); NCTC/w, H. pylori NCTC 11637 (water phase); SS1, the Sydney strain of H. pylori; HF, H. felis; ∗, P < 0.05. n = 8 or 9.
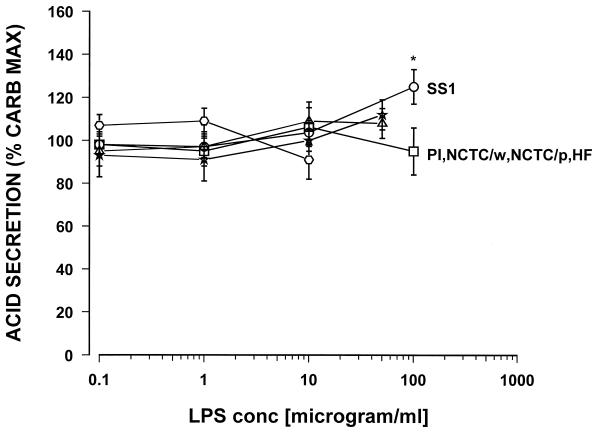
The highest concentration tested, 100 μg of the LPS of H. pylori SS1/ml, caused a significant 38% increase in histamine-stimulated acid secretion. Similarly, for carbachol-stimulated acid secretion, with the exception of the Sydney strain LPS, none of the tested H. pylori LPS preparations showed any direct effect on acid secretion (Fig. 2).
FIG. 2.
Effect of various samples of Helicobacter LPS on carbachol (10−5 M)-stimulated acid secretion in the mouse gastric glands as measured by [14C]AP accumulation. PI, H. pylori isolate from a duodenal ulcer patient; NCTC/p, H. pylori NCTC 11637 (phenol phase); NCTC/w, H. pylori NCTC 11637 (water phase); SS1, the Sydney strain of H. pylori; HF, H. felis; ∗, P < 0.05. n = 7 or 8.
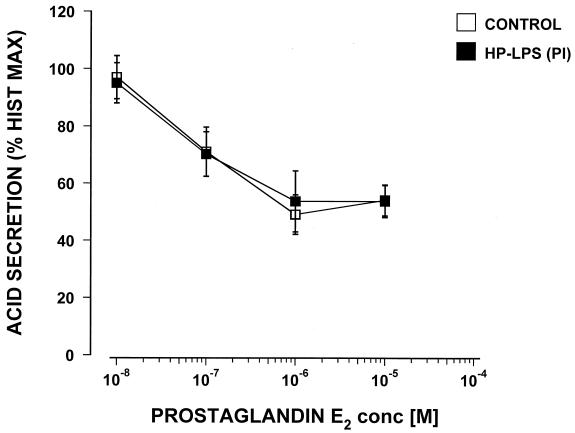
Again, only the highest concentration tested, 100 μg of the Sydney strain LPS/ml, caused a significant 23% increase in carbachol-stimulated acid secretion. No effect on acid secretion was seen with LPS from Helicobacter felis. Commercially available E. coli LPS did not show any effect on carbachol- or histamine-stimulated acid secretion in the mouse gland preparations when tested over the range from 1.0 pg to 100 μg/ml (data not shown). Dose response curves were constructed for PGE2 and somatostatin, and the possible effect of H. pylori LPS from a duodenal ulcer patient (PI strain) was tested. PGE2 caused a dose response-related inhibition of histamine-stimulated acid secretion with the maximum inhibition at 10−6 M and a 50% inhibitory concentration of 8 × 10−8 M (Fig. 3).
FIG. 3.
Effect of the LPS (25 μg/ml) of an H. pylori isolate from a duodenal ulcer patient (PI) on the inhibition by PGE2 of histamine-stimulated acid secretion in the mouse gastric glands as measured by [14C]AP accumulation (n = 3).
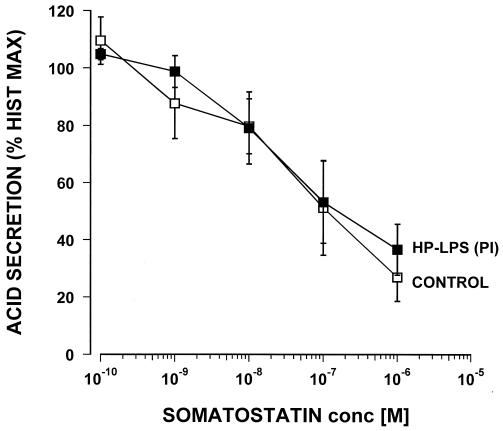
The LPS from H. pylori PI was coincubated for 60 min with 10−4 M of histamine and doses of PGE2 (from 10−8 to 10−5 M) and then compared with controls that did not contain any LPS. The LPS of H. pylori PI at a concentration of 25 μg/ml did not cause any significant changes in the efficacy or potency of the inhibitory effect of PGE2 (Fig. 3). Somatostatin inhibited histamine-stimulated acid secretion in a dose response manner with a 50% inhibitory concentration of around 10−8 M (Fig. 4). However, when tested with 25 μg of LPS/ml from H. pylori PI this failed to show any significant effect on the potency or efficacy of the somatostatin inhibition. Pretreatment of the glands for an additional 60 min with the tested LPS did not influence any of the reported results (data not shown).
FIG. 4.
Effect of the LPS (25 μg/ml) of an H. pylori isolate from a duodenal ulcer patient (PI) on the inhibition by somatostatin of histamine-stimulated acid secretion in the mouse gastric glands as measured by [14C]AP accumulation (n = 4).
DISCUSSION
The mouse gland preparation enables testing of the potential effect of Helicobacter-derived LPS in this in vitro model of acid secretion that lacks the potential involvement of immune inflammatory cells. The model allows testing of substances that act in a more direct fashion on the secretory function of mouse parietal cells. In the present study, only LPS of the Sydney strain of H. pylori caused direct stimulation of acid secretion, whereas the other tested preparations did not cause any significant change in acid secretion. A number of earlier reports have shown that LPS from gram-negative bacteria was able to inhibit acid secretion in vivo (1, 28, 32). In particular, these reports focused on the effect of E. coli-derived LPS, and its in vivo inhibitory effect has remained undisputed. Also, E. coli-derived LPS was shown to exert a lasting inhibitory effect on pepsinogen secretion (28). However, subsequent research revealed that the observed effect was reversible (29) and that the inhibitory effect of LPS from E. coli may have been caused by the involvement of inflammatory cytokines. In particular, interleukin-1 (IL-1) was suspected and this effect was blocked by indomethacin (25, 27). Indeed, administration of IL-1 in vivo (13, 24, 31) and, to some extent, in vitro (2) showed its potent antisecretory effects on acid secretion. Both a review of the articles presented in this discussion and studies that show the lack of direct antisecretory effect indicate that E. coli-derived LPS exerts strong antigenic and proinflammatory properties by which it inhibits acid and pepsin secretion. H. pylori LPS has, in general, much lower immunological activity than LPS purified from E. coli (18, 21). Thus, recently H. pylori LPS was shown to inhibit acid secretion in vivo at a dose over 10,000-fold higher than that of E. coli LPS (22), which correlates with its low immunological activity compared to that of E. coli LPS (18, 21). It was also suggested that these much lower acid inhibitory properties were the result of structural differences between H. pylori and E. coli LPS, again consistent with observed structure-bioactivity relationships for immunoactivity (21). However, one could also conclude that the observed antisecretory effect had a similar mechanism involving products of the host immune system, possibly IL-1 and prostaglandins. The antisecretory effect observed with H. pylori LPS could not be blocked by indomethacin. Nevertheless, it was also suggested that the inhibitory effect of IL-1 can be prostaglandin independent (31).
As the exact mechanism of the inhibitory action of LPS remains unclear, we tested the hypothesis that the observed effect of LPS could be of a more direct nature and be caused by inhibition of acid at the parietal cell level. However, no direct inhibitory effect by the tested LPS was observed in the present study, even with that derived from E. coli. Since these results were observed with an in vitro system, this suggests that the previously reported observed inhibitory effect of H. pylori- and E. coli-derived LPS on acid secretion stems, indeed, from its proinflammatory properties and is the consequence of the induction of inflammatory mediators. A number of reports have indicated that H. pylori sonicates inhibit secretion of parietal cells in vitro and that bacterial factors contributed up to 80% inhibition (3, 10, 11). However, based on the data from our present study, LPS as a possible mediator for the observed inhibition can be excluded. Also, it has been suggested that H. pylori LPS may bind to the gastric mucosal somatostatin receptor (23), but we did not find any potential interference with somatostatin receptors on parietal cells and subsequent inhibition of acid secretion. Similarly, H. pylori LPS failed to show any interaction with prostaglandin receptors on parietal cells and did not affect acid secretion in this fashion either. Supporting our findings, other studies have concluded that H. pylori LPS had no effect on acid secretion in an Ussing chamber study (20, 33). Therefore, it is unlikely that H. pylori LPS is directly responsible for the observed hyposecretion of acid in Helicobacter-related disease.
Whatever the mechanism of H. pylori-related inhibition of acid secretion, it is widely accepted that this effect promotes colonization of the gastric mucosa by H. pylori and may contribute to gastric ulcer disease, atrophy, and subsequent progress to cancer (9). In contrast to the described inhibitory properties of H. pylori LPS in vivo (22), we have shown that LPS from H. pylori SS1 can stimulate acid secretion, and since other LPS preparations did not, this is probably related to differences in the molecular structure of the tested LPS preparations. We have shown that H. felis LPS does not affect acid secretion, and although this bacterium colonizes the mouse stomach, development of active chronic gastritis is slow but is present 6 months postinfection (15). The Sydney strain of H. pylori was chosen to optimize the mouse model (15), and interestingly, H. pylori LPS from this particular strain in our studies had a stimulatory effect on acid secretion. Extensive work on the structure of the Sydney strain LPS (16) may help to explain why this strain is preferred in the mouse model of gastritis. Work on optimizing the mouse model of H. pylori-induced inflammation shows that this H. pylori strain can effectively mimic the antral gastritis observed in humans (15). The antral gastritis is generally linked to hypersecretion of gastric acid and tends to evolve into duodenal ulcer disease. It has been proposed that the local acid production will determine the extent of colonization by Helicobacter species in the stomach, with decreased acid production promoting the spread of colonization to the acid-producing body of the stomach as opposed to the increased acid production, which tends to restrict colonization predominantly to the gastric antrum (30). These results show for the first time that LPS from H. pylori can stimulate acid secretion, which possibly might contribute to mucosal damage of the stomach and duodenum. The second possible mechanism by which H. pylori LPS can stimulate acid secretion at the gland level derives from data showing that it can increase histamine release from rat ECL cells (12).
The clinical syndrome of septicemia caused by gram-negative organisms is mainly caused by an immune response to bacterial endotoxin, LPS. Nevertheless, there is no evidence that H. pylori infection and its endotoxin cause septicemia or septic shock, supporting the concept that it possesses low immunological activity. In our results the acid stimulatory effect was observed only at the highest dose tested of 100 μg/ml, and it is very unlikely that levels of this LPS in plasma can reach these concentrations or be of clinical significance. However, H. pylori bacteria can survive in close proximity to the surface of gastric mucosa, and in extreme situations inside gastric glands, local high concentrations of LPS cannot be excluded. The ability of LPS from H. pylori SS1 to increase acid secretion may prevent transgastric bacterial invasion and contribute to the predominantly antral colonization observed in BALB/c mice. We have also tested and previously reported the effect of H. pylori LPS, including the SS1 strain, on parietal cells obtained from C57BL/6 mice and found no differences, as obtained results were consistent and comparable in both strains of mice (I. T. Padol, A. P. Moran, S. O. Hynes, and R. H. Hunt, Abstr. Gastroenterology, vol. 118, no. 4, abstr. 3976, 2000). Therefore, the stimulatory effect of LPS from the SS1 strain can play only an accessory role in mouse models and the differences in the pattern of colonization are attributed mainly to another mechanism, possibly to the type of immune response evoked. Nevertheless, it seems that the inhibitory effect on acid secretion mediated by bacterum-induced inflammation can be counteracted by the ability of some strains of the bacterium to stimulate the parietal cell to secrete acid. Thus, it is possible that a dynamic balance exists between these processes, and depending on which one dominates, this may determine the pathogenic path for either duodenal ulcer or gastric ulcer.
Based on our data and the available literature, it can be concluded that the E. coli-derived LPS inhibits acid and pepsinogen secretion as a consequence of inflammation. In contrast, the LPS purified from the known gastric pathogen H. pylori has this antisecretory property greatly impaired and, depending on the strain of the bacterium, is able to stimulate directly both pepsinogen (19, 33) and acid secretion, potentially contributing to gastrointestinal pathology.
REFERENCES
- 1.Baume P E, Nicholls A, Baxter C H. Inhibition of gastric acid secretion by a purified bacterial lipopolysaccharide. Nature. 1967;215:59–60. doi: 10.1038/215059a0. [DOI] [PubMed] [Google Scholar]
- 2.Beales I L P, Calam J. Interleukin 1β and tumor necrosis factor α inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut. 1998;42:227–234. doi: 10.1136/gut.42.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beil W, Birkholz C, Wagner S, Sewing K-F. Interaction of Helicobacter pylori and its fatty acids with parietal cells and gastric H+/K+-ATPase. Gut. 1994;35:1176–1180. doi: 10.1136/gut.35.9.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berglindh T. Gastric glands and cells: preparation and in vitro methods. Methods Enzymol. 1990;192:93–107. doi: 10.1016/0076-6879(90)92064-k. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard T G, Czinn S J. Review article: immunological determinants that may affect the Helicobacter pylori cancer risk. Aliment Pharmacol Ther. 1998;12(Suppl. 1):83–90. doi: 10.1111/j.1365-2036.1998.00010.x. [DOI] [PubMed] [Google Scholar]
- 6.Blickenstaff D, Grossman M I. A quantitative study of the reduction of gastric acid secretion associated with pyrexia. Am J Physiol. 1950;160:567–571. doi: 10.1152/ajplegacy.1950.160.3.567. [DOI] [PubMed] [Google Scholar]
- 7.Calam J. Helicobacter pylori modulation of gastric acid secretion. Yale J Biol Med. 1999;72:195–202. [PMC free article] [PubMed] [Google Scholar]
- 8.Chester I R, Murray R G E. Analysis of the cell wall and lipopolysaccharide of Spirillum serpens. J Bacteriol. 1975;124:1168–1176. doi: 10.1128/jb.124.3.1168-1176.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman M. Gastrin secretion in health and disease. In: Sleisenger M H, Fortran J S, editors. Gastrointestinal disease. W. B. Philadelphia, Pa: Saunders; 1989. pp. 713–734. [Google Scholar]
- 10.Hoffman J S, King W W, Fox J G, Janik D, Cave D R. Rabbit and ferret parietal cell inhibition by Helicobacter species. Dig Dis Sci. 1995;40:147–152. doi: 10.1007/BF02063958. [DOI] [PubMed] [Google Scholar]
- 11.Jablonkowski H, Hengels K J, Kraemer N, Geis G, Opferkuch W, Strohmeyer G. Effects of Helicobacter pylori on histamine and carbachol stimulated acid secretion by human parietal cells. Gut. 1994;35:755–757. doi: 10.1136/gut.35.6.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidd M, Miu K, Tang L H, Perez-Perez G I, Blazer M J, Sandor A, Modlin I M. Helicobacter pylori lipopolysaccharide stimulates histamine release and DNA synthesis in rat enterochromaffin-like cells. Gastroenterology. 1997;113:1110–1117. doi: 10.1053/gast.1997.v113.pm9322505. [DOI] [PubMed] [Google Scholar]
- 13.Kondo S, Shinomura Y, Kanayama S, Kawabata S, Miyazaki Y, Imamura I, Fukui H, Matsuzawa Y. Interleukin-1 beta inhibits gastric histamine secretion and synthesis in the rat. Am J Physiol. 1994;267:G966–G971. doi: 10.1152/ajpgi.1994.267.6.G966. [DOI] [PubMed] [Google Scholar]
- 14.Lee A, Dixon M F, Danon S J, Kuipers E, Mégraud F, Larsson H, Mellgård B. Local acid production and Helicobacter pylori: a unifying hypothesis of gastroduodenal disease. Eur J Gastroenterol Hepatol. 1995;7:461–465. [PubMed] [Google Scholar]
- 15.Lee A, O'Rourke J, Corazon de Ungria M, Robertson B, Daskalopoulos G, Dixon M F. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 16.Monteiro M A, Appelmelk B J, Rasko D A, Moran A P, Hynes S O, MacLean L L, Chan K H, St. Michael F, Logan S M, O'Rourke J, Lee A, Taylor D E, Perry M B. Lipopolysaccharide structures of Helicobacter pylori genomic strains 26695 and J99, mouse model H. pylori Sydney strain, H. pylori P466 carrying sialyl Lewis X, and H. pylori UA915 expressing Lewis B. Classification of H. pylori lipopolysaccharides into glycotype families. Eur J Biochem. 2000;267:305–320. doi: 10.1046/j.1432-1327.2000.01007.x. [DOI] [PubMed] [Google Scholar]
- 17.Moran A P, Helander L M, Kosunen T U. Compositional analysis of Helicobacter pylori rough-form lipopolysaccharides. J Bacteriol. 1992;174:1370–1377. doi: 10.1128/jb.174.4.1370-1377.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moran A P. The role of lipopolysaccharide in Helicobacter pylori pathogenesis. Aliment Pharmacol Ther. 1996;10(Suppl. 1):39–50. doi: 10.1046/j.1365-2036.1996.22164004.x. [DOI] [PubMed] [Google Scholar]
- 19.Moran A P, Young G O, Lastovica A J. Pepsinogen induction by Helicobacter pylori lipopolysaccharides. Gut. 1998;43(Suppl. 2):A15. [Google Scholar]
- 20.Moran A P, Aspinall G O. Unique structural and biological features of Helicobacter pylori lipopolysaccharides. Proc Clin Biol Res. 1998;337:37–49. [PubMed] [Google Scholar]
- 21.Moran A P. Helicobacter pylori lipopolysaccharide-mediated gastric and extragastric pathology. J Physiol Pharmacol. 1999;50:787–805. [PubMed] [Google Scholar]
- 22.Ootsubo C, Okumura T, Takahashi N, Wakebe H, Imagawa K, Kikuchi M, Kohgo Y. Helicobacter pylori lipopolysaccharide inhibits acid secretion in pylorus-ligated conscious rats. Biochem Biophys Res Commun. 1997;236:532–537. doi: 10.1006/bbrc.1997.6999. [DOI] [PubMed] [Google Scholar]
- 23.Piotrowski J, Majka J, Slomiany A, Slomiany B L. Helicobacter pylori lipopolysaccharide inhibition of gastric mucosal somatostatin receptor. Biochem Mol Biol Int. 1995;36:491–498. [PubMed] [Google Scholar]
- 24.Taché Y, Saperas E. Potent inhibition of gastric acid secretion and ulcer formation by centrally and peripherally administered interleukin-1. Ann N Y Acad Sci. 1992;664:353–368. doi: 10.1111/j.1749-6632.1992.tb39774.x. [DOI] [PubMed] [Google Scholar]
- 25.Tsuji K, Uehara A, Okumura T, Taniguchi Y, Kitamori S, Takasugi Y, Namiki M. The gastric antisecretory action of lipopolysaccharide is blocked by indomethacin. Eur J Pharmacol. 1992;210:213–215. doi: 10.1016/0014-2999(92)90674-s. [DOI] [PubMed] [Google Scholar]
- 26.Tsuji T, Uehara A, Santos S B, Namiki M. Endotoxin protects the gastric mucosa against ulcerogenic stimuli. Biochem Biophys Res Commun. 1993;197:1326–1333. doi: 10.1006/bbrc.1993.2622. [DOI] [PubMed] [Google Scholar]
- 27.Uehara A, Okumura T, Sekiya C, Okamura K, Takasugi Y, Namiki M. Interleukin-1 inhibits the secretion of gastric acid in rats: possible involvement of prostaglandin. Biochem Biophys Res Commun. 1989;162:1578–1584. doi: 10.1016/0006-291x(89)90855-3. [DOI] [PubMed] [Google Scholar]
- 28.Uehara A, Okumura T, Okamura K, Takasugi Y, Namiki M. Lipopolysaccharide-induced inhibition of gastric acid and pepsin secretion. Eur J Pharmacol. 1990;181:141–145. doi: 10.1016/0014-2999(90)90256-6. [DOI] [PubMed] [Google Scholar]
- 29.Uehara A, Okumura T, Tsuji K, Taniguchi Y, Kitamori S, Takasugi Y, Namiki M. Evidence that gastric antisecretory action of lipopolysaccharide is not due to a toxic effect on gastric parietal cells. Dig Dis Sci. 1992;37:1039–1044. doi: 10.1007/BF01300284. [DOI] [PubMed] [Google Scholar]
- 30.Veldhuyzen Van Zanten S J O, Dixon M F, Lee A. The gastric transitional zones: neglected links between gastroduodenal pathology and Helicobacter ecology. Gastroenterology. 1999;116:1217–1229. doi: 10.1016/s0016-5085(99)70025-9. [DOI] [PubMed] [Google Scholar]
- 31.Wallace J L, Cucala M, Mugridge K, Parente L. Secretagogue-specific effects of interleukin-1 on gastric acid secretion. Am J Physiol. 1991;261:G559–G564. doi: 10.1152/ajpgi.1991.261.4.G559. [DOI] [PubMed] [Google Scholar]
- 32.Wyllie J H, Limbosch J M, Nyhus L M. Inhibition of gastric acid secretion by bacterial lipopolysaccharide. Nature. 1967;215:879. doi: 10.1038/215879a0. [DOI] [PubMed] [Google Scholar]
- 33.Young G O, Stemmet N, Lastovica A, van der Merwe E L, Louw J A, Modlin I M, Marks I N. Helicobacter pylori lipopolysaccharide stimulates gastric mucosal pepsinogen secretion. Aliment Pharmacol Ther. 1992;6:169–177. doi: 10.1111/j.1365-2036.1992.tb00260.x. [DOI] [PubMed] [Google Scholar]