Summary
Aortic dissection (AD) caused by the tear in the aortic wall threatens aorta, causing severe chest pain, syncope and even death. Fortunately, development of genetic technology provides promising approaches for AD treatment. To analyze impacts of miR-15a-5p on modulating cell viability and migratory ability of vascular smooth muscle cells (VSMCs). Ang II (0, 0.05 and 0.1 μM) treatment were applied for inducing inflammatory reactions of VSMCs. RNA expressions of miR-15a-5p with Bcl-2 was examined using RT-qPCR. CCK-8 and transwell evaluated cell viability and migratory ability, respectively. The binding about miR-15a-5p with Bcl-2 were detected by luciferase reporter assay. Western blot detected protein expressions of Bcl-2, MCP-1 and MMP-9. Ang II treatment not only accelerated VSMCs viability and migratory abilities, but also upregulated MCP-1 and MMP-9 protein expressions. MiR-15a-5p was detected to be promoted by Ang II. However, miR-15a-5p inhibitor decreased VSMC cell viability and migratory ability and suppressed protein expressions of MCP-1 and MMP-9. Bcl-2 was targeted and downregulated by miR-15a-5p. Nevertheless, high VSMC cell viability and migration caused by miR-15a-5p overexpression were hindered with overexpressed Bcl-2. MiR-15a-5p mimics also elevated MCP-1 and MMP-9 protein expressions, which were inhibited by Bcl-2 upregulation.
Keywords: miR-15a-5p, Aortic dissection, Bcl-2, MCP-1, MMP-9
Introduction
Aortic dissection can be threatening because of a tear in the intima of aorta or bleeding in the wall of the aorta, leading to layer separation of the aorta wall [1]. It can be divided into acute aortic dissection (type A) and type B aortic dissection [2]. This disease usually occurs in 65–75 years of age and the morbidity is 35 cases/100000 people/year [3]. The mortality of acute aortic dissection in untreated patients is about 1–2 % per hour after onset of symptoms [4]. Hypertension has been verified to be a critical risk factor for aortic dissection, which could elevate aortic pressure, thereby increasing wall stress [5]. Atherosclerosis and Aortic aneurysm have also been detected to accelerate aortic dissection, in which aortic wall inflammation and calcification could lead to decreased aortic elasticity and elevated hemodynamic stress, thus causing aortic dissection [6]. Complications of aortic dissection contains lethal poor perfusion syndrome, heart failure, stroke and aortic insufficiency [7]. Pathological conditions of aortic dissection are mechanical wall stress and degradation of the medical wall of the aorta, in which VSMCs participates in this progression [8]. Abnormal phenotypic transformation, proliferation and migration of VSMCs are symbols of aortic dissection in thorax and increased apoptosis of VSMCs also associated aortic dissection [9,10]. Recently, noncoding RNAs have become promising biomarkers for various kinds of cardiovascular diseases, which also attracts attentions in progressions of aortic dissection [11].
In noncoding RNAs, microRNAs (miRNAs) are shorter than long noncoding RNAs with no protein-coding ability, which can inhibit the translation by targeting mRNAs [12]. MiRNAs have been verified to correlate closely with cardiovascular diseases, which has become a new way to diagnose, treat or predict progressions of this kind of disease [13]. Dysregulated miRNAs and related miRNA-mRNA networks have also been reported in aortic dissection. In the study of Su et al. [14] a total of 47 differently expressed miRNAs have been screened in aortic dissection, containing 12 upregulated ones and 35 suppressed ones [14]. MiR-15a-5p has been reported to facilitate apoptosis of pulmonary artery smooth muscle cells through upregulating vascular endothelial growth factor and suppressing p38 and matrix metalloproteinase-2 [15]. MiR-15a-5p has also been demonstrated to promote smooth muscle cell viabilities and inhibited cell apoptosis via targeting Cyclin Dependent Kinase Inhibitor 2B, thus accelerating abdominal aortic aneurysm development [16]. MiR-15a-5p were also detected to be highly expressed in patients with aortic dissection, implying a promoted role of miR-15a-5p in aortic dissection progression [17].
Members in the Bcl-2 family can promote or suppress cell apoptosis, in which B cell leukemia/lymphoma 2 (Bcl-2) plays an oncogenic role in progressions of malignancies [18]. Bcl-2 has been verified to increase cell proliferation while ablation of Bcl-2 accelerated apoptosis of VSMCs [19]. Based on TargetScan (https://www.targetscan.org), underlying binding spots between miR-15a-5p and Bcl-2 were displayed. Moreover, miR-15a-5p that was sponged by long noncoding RNA small nuclear RNA host gene 16 promoted human brain microvascular endothelial cells apoptosis via targeting Bcl-2, resulting in ischemia/reperfusion damage [20]. Therefore, miR-15a-5p might modulate progressions of aortic dissection through targeting Bcl-2.
Monocyte chemoattractant protein-1 (MCP-1), also named as Chemokine (CC-motif) ligand 2 (CCL2), has been demonstrated to elevate proinflammatory cytokines expressions in disorders [21]. MCP-1 was upregulated in VSMCs after high mobility group box 1 induction, resulting in accelerated vascular inflammation [22]. MCP-1 has also discovered to be elevated in Ang II-induced mouse aortic dissection model, resulting in accelerated inflammation [23]. Matrix metallopepti-dase 9 (MMP-9) has been found to be elevated in vascular inflammations and upregulate MCP-1 expressions [24]. Moreover, MMP-9 was upregulated by macrophage infiltration in aortic dissection and located in tearing area of aorta, thereby facilitating aortic dissection development [25]. Considering this, we hypothesized that miR-15a-5p might also modulate MCP-1 and MMP-9 expressions in aortic dissection.
Methods
Cell culture and transfection
Human vascular smooth muscle cells (VSMC, CRL-1999) were obtained from ATCC (USA). Cells were cultivated in T/G HA-VSMC cell culture medium (Procell, Wuhan, China) at 37 C, 5 % CO2 in a humid environment. After incubation, cells were treated by Ang II (0.01, 0.05 and 0.1 μM) for 12 h. HEK-293T cells purchased from ATCC were cultured in RPMI-1640 medium. As for transfection, VSMCs were incubated until the confluence reached 80 %. Thereafter, the inhibitor and the mimics of miR-15a-5p were synthesized by Sangon Biotech (Shanghai, China). Meanwhile, the overexpression of Bcl-2 was created by using the pcDNA3.1 vector and named oeBcl-2 while its negative control was called oeNC. Afterwards, using the Lipofectamine 3000 (Invitrogen, USA), NC/miR-15a-5p inhibitor, oeNC, oeBcl-2 and oeBcl-2 with miR-15a-5p mimics were transfected into VSMC cells followed by culturing for 24 h. Thereafter, Ang II (0.1 μM) was applied for treating transfected cells for 12 h. MiR-15a-5p and Bcl-2 expressions were evaluated by RT-qPCR.
RT-qPCR
Total RNA was segregated from VSMCs using the QIAzol Lysis Reagent (QIAGEN, Germany) after Ang II treatment and transfection. Next, the cDNA of Bcl-2 was compounded using the Transcriptor High Fidelity cDNA Synthesis Kit (Sigma-Aldrich, USA) and reverse transcription of miR-15a-5p was carried out by miRNA First-Strand cDNA Kit (TIANGEN, China). Thereafter, SYBR Green Realtime PCR Master Mix (TOYOBO, Japan) then was applied for detecting RNAs with QIAcuity Digital PCR System (QIAGEN). Primer sequences were shown in the Table 1. Conditions of RT-qPCR were also displayed as below, which were predenaturation, at 95 C, 4 min and 35 cycles of denaturation, at 95 C, 15 s; annealing, at 58 C, 15 s and extension, at 72 C, 1 min. Relative RNA expressions were examined by the 2−ΔΔCt method.
Table 1.
Primer sequences used for the RT-qPCR.
| Primer sequences (5′→3′) | |
|---|---|
| miR-15a-5p | Forward: GCCGAGTAGCAGCACACATAA Reverse: CAGTGCGTGTCGTGGAGT |
| Bcl-2 | Forward: CTTCGCCGAGATGTCCAG Reverse: GGCTCAGATAGGCACCCA |
| U6 | Forward: GCTTCGGCAGCACATATACTAAAAT Reverse: CGCTTCACGAATTTGCGTGTCAT |
| GAPDH | Forward: GCACCGTCAAGGCTGAGAAC Reverse: TGGTGAAGACGCCAGTGGA |
Cell viability
Parental or transfected VSMCs were seeded onto a 96-well plate with 5×103 cells/well, which were cultivated for 24 h and treated by Ang II (0.01, 0.05 and 0.1 μM) for 12 h. Next, 10 μl CCK-8 was added at specific time points (24 h, 48 h and 72 h) and then cultured with cells for another 1 h. Finally, optical density (OD) values of cells were examined using the SPECTROstar Nano (BMG LABTECH, Germany).
Transwell migration
Transwell assay was applied for detecting the migratory ability of parental VSMCs and transfected VSMCs. VSMCs 2×104 cells/well) were added in the upper compartment (Corning, USA) with serum-free medium while the lower component was filled with T/G HA-VSMC cell culture medium. Cells then were incubated for 8 h at 37 C, 5 % CO2. Thereafter, cells were treated by Ang II (0.1 μM) for 12 h. Next, 4 % paraformaldehyde (Sigma-Aldrich, USA) was applied for fixing cells and the average of cell numbers was counted using a microscope from 5 visual fields that were chosen randomly.
Luciferase report test
Using TargetScan (http://www.targetscan.org/vert_72/), binding spots of miR-15a-5p with Bcl-2 were offered. Afterward, wild and mutant type of Bcl-2 were inserted into psiCHECK-2 (Promega, USA) and named Bcl-2-wt/mut. Then, co-transfection of NC/miR-15a-5p mimics with Bcl-2-wt/mut into HEK-293T cells was carried out using the Lipofectamine 3000. After that, the fluorescence was evaluated by the Dual-Luciferase Reporter Assay System (Promega).
Western blot
MCP-1, MMP-9 and Bcl-2 protein expressions were examined using the western blot. First, total protein was isolated from cells using the RIPA buffer (Sigma-Aldrich, USA). Next, proteins were segregated by the SDS-PAGE and shifted onto PVDF membranes. Thereafter, Tris buffered saline (Sigma-Aldrich) was used for blocking membranes and then primary antibodies followed by cultivation with membranes overnight at 4 C. Primary antibodies were shown as below, which were anti-MCP-1 antibody (1:1000, ab9669, Abcam, UK), anti-MMP-9 antibody (1:1000, ab76003), and anti-GAPDH antibody (1:2000, ab9485). After incubation, membranes were cultivated with goat anti-rabbit IgG H&L (HRP) (1:800, ab7090) at 25 C for 1 h. ECL Substrate Kit (High Sensitivity) (ab133406) was applied for developing and gray values of proteins were analyzed using the Image J (NIH, USA).
Statistical analysis
All data were shown as mean ± SD and analyzed using GraphPad Prism 9 (GraphPad, USA). Differences of two groups were examined using the student’s t-test while one way ANOVA and two-way ANOVA were applied for measuring differences more than two groups. P<0.05 was meaningful statistically.
Ethical approval
All the studies were approved by People’s Hospital of Xingtai city, in line with the Declaration of Helsinki.
Results
Ang II induced high viability and high migratory ability of VSMCs
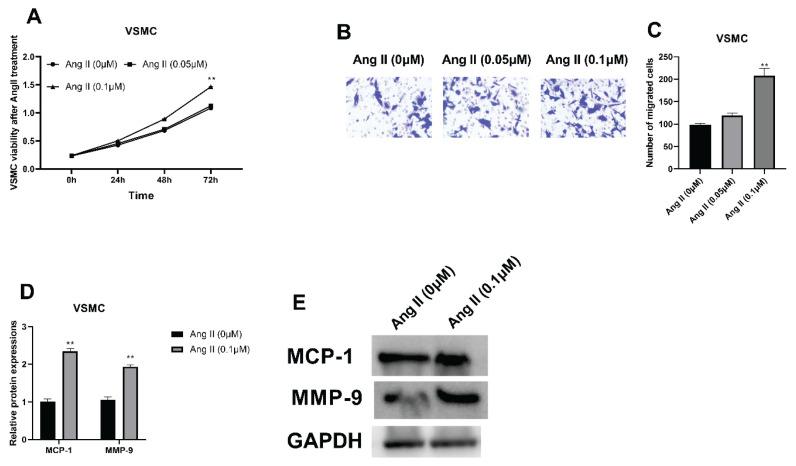
After the treatment of Ang II (0.01, 0.05 and 0.1 μM) for 12 h, the cell viability of VSMCs was promoted dose-dependently and the 0.1 μM of Ang II caused the highest cell viability (Fig. 1A, **** P<0.0001). The migratory ability of VSMCs were also detected, showing that the migration was accelerated dose dependently (Fig. 1B, C, **** P<0.0001). Thereafter, protein expressions of MCP-1 and MMP-9 were analyzed, revealing that both were upregulated after treated by Ang II and protein expressions reached the top in the 0.1 μM group (Fig. 1D, E, ** P=0.0013).
Fig. 1.
VSMC viabilities and migratory ability with Ang II treatment. (A) CCK-8 assay was applied for evaluating VSMC viabilities with Ang II treatment (0, 0.05 and 0.1 μM). (B, C) Transwell was used for detecting VSMC migration with Ang II treatment. (D, E) Western blot was applied to examine MCP-1 and MMP-9 protein expressions after Ang II treatment. ** P<0.05.
MiR-15a-5p was increased in VSMCs after Ang II induction
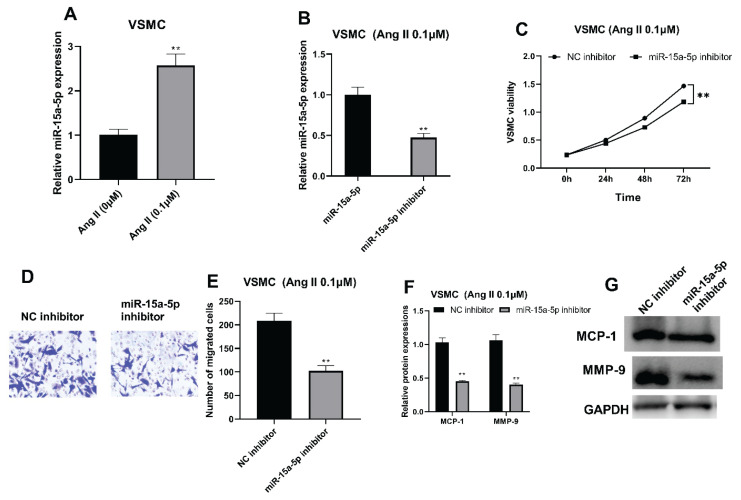
After biological functions of Ang II were explored, the underlying mechanism related to noncoding RNAs were analyzed. RT-qPCR results indicated that the miR-15a-5p was promoted by Ang II treatment (Fig. 2A, ** P<0.0039). Afterward, miR-15a-5p inhibitor transfection was performed in 0.1 μM Ang II-induced VSMCs, resulting in decreased RNA expression of miR-15a-5p in VSMCs (Fig. 2B, *** P<0.0009). Moreover, the cell viability was restrained with suppressed miR-15a-5p (Fig. 2C, **** P<0.0001). Meanwhile, the migration of VSMCs was facilitated after miR-15a-5p suppression (Fig. 2D, E, *** P<0.0007). Additionally, protein expressions of MCP-1 and MMP-9 were both downregulated in VSMCs after transfected by miR-15a-5p inhibition (Fig. 2F, G, **** P<0.0001).
Fig. 2.
MiR-15a-5p was promoted with Ang II treatment. (A) RT-qPCR was applied for validating miR-15a-5p expression in Ang II-induced VSMCs. (B) RT-qPCR was used for evaluating miR-15a-5p expression after miR-15a-5p suppression. (C) CCK-8 was applied to examine VSMC viabilities after miR-15a-5p inhibition. (D, E) Transwell was used to validate VSMC migratory capacities with suppressed miR-15a-5p. (F, G): Western blot was applied for analyzing MCP-1 and MMP-9 protein expressions with inhibited miR-15a-5p. ** P<0.05.
Bcl-2 was targeted by miR-15a-5p
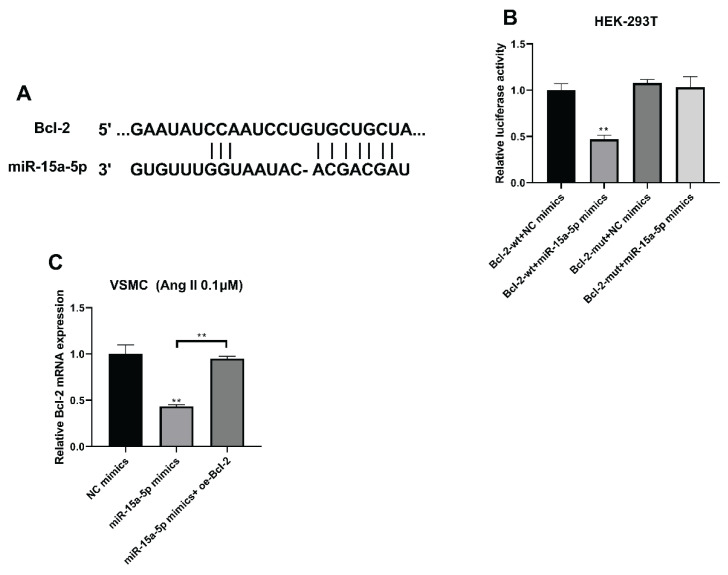
Using the TargetScan (http://www.targetscan.org/vert_72/), predicted binding spots of miR-15a-5p with Bcl-2 were offered (Fig. 3A). Furthermore, the fluorescence was the lowest in the wild type of Bcl-2 with overexpressed miR-15a-5p group, indicating that miR-15a-5p directly bound Bcl-2 (Fig. 3B, **** P<0.0001). Furthermore, Bcl-2 mRNA expression was suppressed with overexpressed miR-15a-5p, which were then prompted with Bcl-2 overexpression (Fig. 3C, **** P<0.0001).
Fig. 3.
Bcl-2 was bound by miR-15a-5p. (A) TargetScan displayed binding spots of miR-15a-5p with Bcl-2. (B) Fluorescence about miR-15a-5p with Bcl-2 was checked using luciferase reporter test. (C) Bcl-2 mRNA expressions were assessed using RT-qPCR with overexpressed miR-15a-5p and overexpressed Bcl-2. **** P<0.0001.
Bcl-2 regulated VSMCs viability and migration via binding miR-15a-5p
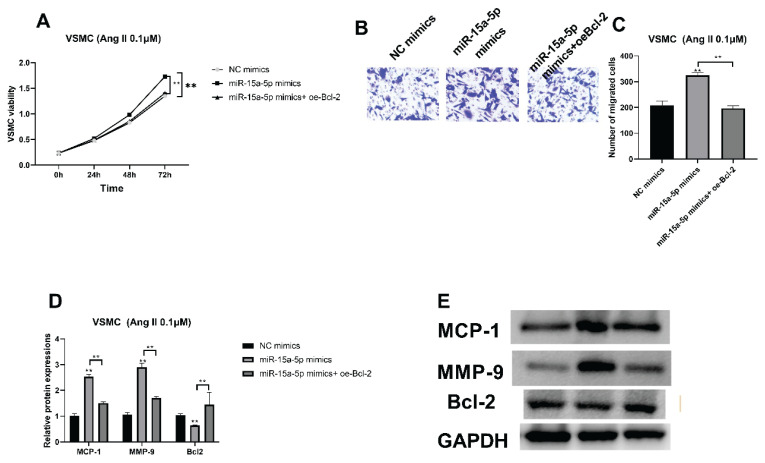
The interplay between miR-15a-5p and Bcl-2 were then analyzed. CCK-8 results showed that miR-15a-5p overexpression accelerated VSMC cell viability, which was then restrained by Bcl-2 upregulation (Fig. 4A, **** P<0.0001). Beyond that, upregulated VSMC migratory ability caused by miR-15a-5p mimics was also suppressed with Bcl-2 upregulation (Fig. 4B, C, **** P<0.0001). MCP-1 and MMP-9 protein expressions were prompted by miR-15a-5p overexpression, while overexpressed Bcl-2 reversed effects of miR-15a-5p by downregulating MCP-1 and MMP-9 protein expressions (Fig. 4D, E, **** P<0.0001). In contrast, decreased Bcl-2 protein caused by miR-15a-5p was prompted with Bcl-2 overexpression (Fig. 4D, E, **** P<0.0001).
Fig. 4.
Bcl-2 modulated VSMC viabilities and migratory capacities through binding miR-15a-5p. (A) CCK-8 was applied to validate VSMC viabilities with overexpressed miR-15a-5p and upregulated Bcl-2. (B, C) VSMC migratory capacities with miR-15a-5p overexpression and increased Bcl-2 were detected by transwell. (D, E) MCP-1, MMP-9 and Bcl-2 protein expressions with overexpressed miR-15a-5p and Bcl-2 overexpression were analyzed with western blot. **** P<0.0001.
Discussion
Present studies have indicated that miR-15a-5p was upregulated in Ang II-treated VSMCs while Bcl-2 was inhibited. Additionally, miR-15a-5p restrained cell viability and migratory ability and facilitated cell apoptosis through targeting Bcl-2. MAPK signaling pathway also participated in progressions of aortic dissection in VSMCs.
Angiotensin II has an effect on elevating blood pressure through inducing the shrink of myocardium and vascular smooth muscle [26]. According to previous studies, Ang II has been demonstrated to induce VSMCs proliferation, which was often used to stimulate aortic dissection in animal models [27]. Therefore, we also chose Ang II to simulate aortic dissection in VSMCs. After Ang II treatment, cell viability and migratory ability were facilitated. As a key chemokine of cell aggregation and migration, MCP-1 has been detected to be promoted by Ang II treatment in the study of Wu et al. [28]. Moreover, MMP-9 has also been verified to accelerate progressions of aortic dissection [25]. In this study, MCP-1 and MMP-9 protein expression were both upregulated by Ang II treatment. Hence, based on these experiments, we have got the same discovery that Ang II has the capacity to accelerate progressions of aortic dissection in VSMCs.
Previous evidence has elucidated essential impacts of miRNAs in many kinds of cardiovascular diseases including aortic dissection [29]. Moreover, miRNAs have also been selected as biomarkers for diagnosing this disease [30]. MiR-145 was largely expressed in normal human aortic walls, which restrained progression of aortic dissection of VSMCs through binding Krüppel-like factor 4 (KLF4) after mechanical stretching [31]. Additionally, miR-15a-5p was promoted by KLF4, resulting in decreased VSMC proliferation and angiogenesis [32]. In this study, miR-15a-5p was prompted in VSMCs after Ang II treatment. Furthermore, its suppression resulted in decreased cell viability and the migratory ability was also suppressed. Additionally, MCP-1 and MMP-9 protein expression were also downregulated by inhibited miR-15a-5p. According to these experiments, miR-15a-5p could enhance efficacy of Ang II in VSMCs through accelerating cell migration and viability.
Thereafter, we have examined targets of miR-15a-5p using bioinformatics tools, predicting that Bcl-2 was on target gene. Bcl-2 hampered Ang II-induced apoptosis of myocardial cells through targeting miR-30b [33]. Moreover, inhibitors of Bcl-2 could facilitate apoptosis of Human pulmonary artery smooth muscle cells [34]. In addition to that, miR-15a-5p targeted Bcl-2, decreasing hypoxia-induced invasiveness and migration of osteosarcoma cells [35]. Moreover, MiR-15a-5p accelerated apoptosis and hindered cell proliferation of human brain microvascular endothelial cells through targeting Bcl-2 [20]. In our study, Bcl-2 was bound directly by miR-15a-5p. As for their interaction, Bcl-2 expression was inhibited by overexpressed miR-15a-5p, which was increased after Bcl-2 overexpression. Additionally, high VSMC viabilities and migratory ability were facilitated with miR-15a-5p overexpression, which were hindered by Bcl-2 upregulation. Furthermore, upregulated MMP-9 and MCP-1 protein expressions caused by miR-15a-5p upregulation were also suppressed with Bcl-2 overexpression.
Conclusions
MiR-15a-5p was promoted in VSMCs after Ang II treatment, which resulted in promoted cell migration and viability via targeting Bcl-2. These results implied that miR-15a-5p and Bcl-2 might be novel biomarkers to diagnose and treat aortic dissection. Nevertheless, further studies in animal models and clinical stages are necessary to get more knowledge.
Acknowledgements
Not applicable.
Abbreviations
- Ang II
angiotensin II
- VSMC
vascular smooth muscle cell
- miRNA
microRNA
- MCP-1
monocyte chemoattractant protein-1
- MMP-9
matrix metallopeptidase 9
- Bcl-2
B cell leukemia/lymphoma 2
- PVDF
Polyvinylidene fluoride
- RIPA
Radio Immunoprecipitation Assay
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- KLF4
Krüppel-like factor 4
- ANOVA
one-way analysis of variance
- HEK
human embryonic kidney
- GAPDH
Glyceraldehyde 3-phosphate dehydrogenase
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1.Tchana-Sato V, Sakalihasan N, Defraigne JO. Aortic dissection. (Article in French) Rev Med Liege. 2018;73:290–295. [PubMed] [Google Scholar]
- 2.Zhu Y, Lingala B, Baiocchi B, Tao JJ, Arana VT, Khoo JW, Williams KM, et al. Type A Aortic Dissection-Experience Over 5 Decades: JACC Historical Breakthroughs in Perspective. J Am Coll Cardiol. 2020;76:1703–1713. doi: 10.1016/j.jacc.2020.07.061. [DOI] [PubMed] [Google Scholar]
- 3.Nienaber CA, Clough RE, Sakalihasan N, Suzuki T, Gibbs R, Mussa F, Jenkins MP, et al. Aortic dissection. Nat Rev Dis Primers. 2016;2:16053. doi: 10.1038/nrdp.2016.53. [DOI] [PubMed] [Google Scholar]
- 4.Gawinecka J, Schönrath F, von Eckardstein A. Acute aortic dissection: pathogenesis, risk factors and diagnosis. Swiss Med Wkly. 2017;147:w14489. doi: 10.4414/smw.2017.14489. [DOI] [PubMed] [Google Scholar]
- 5.Dong N, Piao H, Li B, Xu J, Wei S, Liu K. Poor management of hypertension is an important precipitating factor for the development of acute aortic dissection. J Clin Hypertens (Greenwich) 2019;21:804–812. doi: 10.1111/jch.13556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu D, Shen YH, Russell L, Coselli JS, LeMaire SA. Molecular mechanisms of thoracic aortic dissection. J Surg Res. 2013;184:907–924. doi: 10.1016/j.jss.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagan PG, Nienaber CA, Isselbacher EM, Bruckman D, Karavite DJ, Russman PL, Evangelista A, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA. 2000;283:897–903. doi: 10.1001/jama.283.7.897. [DOI] [PubMed] [Google Scholar]
- 8.Akutsu K. Etiology of aortic dissection. Gen Thorac Cardiovasc Surg. 2019;67:271–276. doi: 10.1007/s11748-019-01066-x. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Liu B, Wang C-S, Yang C-S. MST1 down-regulation in decreasing apoptosis of aortic dissection smooth muscle cell apoptosis. Eur Rev Med Pharmacol Sci. 2018;22:2044–2051. doi: 10.26355/eurrev_201804_14734. [DOI] [PubMed] [Google Scholar]
- 10.Riches K, Clark E, Helliwell RJ, Angelini TG, Hemmings KE, Bailey MA, Bridge I, Scott DJA, Porter KE. Progressive development of aberrant smooth muscle cell phenotype in abdominal aortic aneurysm disease. J Vasc Res. 2018;55:35–46. doi: 10.1159/000484088. [DOI] [PubMed] [Google Scholar]
- 11.Cheng M, Yang Y, Xin H, Li M, Zong T, He T, Yu T, Xin H. Non-coding RNAs in aortic dissection: From biomarkers to therapeutic targets. J Cell Mol Med. 2020;24:11622–11637. doi: 10.1111/jcmm.15802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sbarouni E, Georgiadou P. MicroRNAs in acute aortic dissection. J Thorac Dis. 2018;10:1256–1257. doi: 10.21037/jtd.2018.03.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barwari T, Joshi A, Mayr M. MicroRNAs in cardiovascular disease. J Am Coll Cardiol. 2016;68:2577–2584. doi: 10.1016/j.jacc.2016.09.945. [DOI] [PubMed] [Google Scholar]
- 14.Su Y, Li Q, Zheng Z, Wei X, Hou P. Integrative bioinformatics analysis of miRNA and mRNA expression profiles and identification of associated miRNA-mRNA network in aortic dissection. Medicine (Baltimore) 2019;98:e16013. doi: 10.1097/MD.0000000000016013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang W, Li Y, Xi X, Zhu G, Wang S, Liu S, Song M. MicroRNA-15a-5p induces pulmonary artery smooth muscle cell apoptosis in a pulmonary arterial hypertension model via the VEGF/p38/MMP-2 signaling pathway. Int J Mol Med. 2020;45:461–474. doi: 10.3892/ijmm.2019.4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao P, Si J, Yang B, Yu J. Upregulation of MicroRNA-15a contributes to pathogenesis of Abdominal Aortic Aneurysm (AAA) by modulating the expression of Cyclin-dependent kinase inhibitor 2B (CDKN2B) Med Sci Monit. 2017;23:881–888. doi: 10.12659/MSM.898233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong J, Bao J, Feng R, Zhao Z, Lu Q, Wang G, Li H, et al. Circulating microRNAs: a novel potential biomarker for diagnosing acute aortic dissection. Sci Rep. 2017;7:12784. doi: 10.1038/s41598-017-13104-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebrahim AS, Sabbagh H, Liddane A, Raufi A, Kandouz M, Al-Katib A. Hematologic malignancies: newer strategies to counter the BCL-2 protein. J Cancer Res Clin Oncol. 2016;142:2013–2022. doi: 10.1007/s00432-016-2144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perlman H, Sata M, Krasinski K, Dorai T, Buttyan R, Walsh K. Adenovirus-encoded hammerhead ribozyme to Bcl-2 inhibits neointimal hyperplasia and induces vascular smooth muscle cell apoptosis. Cardiovasc Res. 2000;45:570–578. doi: 10.1016/S0008-6363(99)00346-6. [DOI] [PubMed] [Google Scholar]
- 20.Teng H, Li M, Qian L, Yang H, Pang M. Long non-coding RNA SNHG16 inhibits the oxygen-glucose deprivation and reoxygenation-induced apoptosis in human brain microvascular endothelial cells by regulating miR-15a-5p/bcl-2. Mol Med Rep. 2020;22:2685–2694. doi: 10.3892/mmr.2020.11385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh S, Anshita D, Ravichandiran V. MCP-1: Function, regulation, and involvement in disease. Int Immunopharmacol. 2021;101:107598. doi: 10.1016/j.intimp.2021.107598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi JM, Baek SE, Kim JO, Jeon EY, Jang EJ, Kim CD. 5-LO-derived LTB4 plays a key role in MCP-1 expression in HMGB1-exposed VSMCs via a BLTR1 signaling axis. Sci Rep. 2021;11:11100. doi: 10.1038/s41598-021-90636-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito S, Hashimoto Y, Majima R, Nakao E, Aoki H, Nishihara M, Ohno-Urabe S, et al. MRTF-A promotes angiotensin II-induced inflammatory response and aortic dissection in mice. PLoS One. 2020;15:e0229888. doi: 10.1371/journal.pone.0229888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shih Y-C, Chen P-Y, Ko T-M, Huang P-H, Ma H, Tarng D-C. MMP-9 deletion attenuates arteriovenous fistula neointima through reduced perioperative vascular inflammation. Int J Mol Sci. 2021;22:5448. doi: 10.3390/ijms22115448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Liu D, Zhao L, Wang L, Li Y, Cho K, Tao C, Jiang B. Targeted depletion of monocyte/macrophage suppresses aortic dissection with the spatial regulation of MMP-9 in the aorta. Life Sci. 2020;254:116927. doi: 10.1016/j.lfs.2019.116927. [DOI] [PubMed] [Google Scholar]
- 26.Yamaki F, Obara K, Tanaka Y. Angiotensin II regulates excitability and contractile functions of myocardium and smooth muscles through autonomic nervous transmission. (Article in Japanese) Yakugaku Zasshi. 2019;139:793–805. doi: 10.1248/yakushi.19-00002. [DOI] [PubMed] [Google Scholar]
- 27.Huo YB, Gao X, Peng Q, Nie Q, Bi W. Dihydroartemisinin alleviates AngII-induced vascular smooth muscle cell proliferation and inflammatory response by blocking the FTO/NR4A3 axis. Inflamm Res. 2022;71:243–253. doi: 10.1007/s00011-021-01533-3. [DOI] [PubMed] [Google Scholar]
- 28.Wu Z, Dai F, Ren W, Liu H, Li B, Chang J. Angiotensin II induces apoptosis of human pulmonary microvascular endothelial cells in acute aortic dissection complicated with lung injury patients through modulating the expression of monocyte chemoattractant protein-1. Am J Transl Res. 2016;8:28–36. [PMC free article] [PubMed] [Google Scholar]
- 29.Vavuranakis M, Kariori M, Vrachatis D, Aznaouridis K, Siasos G, Kokkou E, Mazariset S, et al. MicroRNAs in aortic disease. Curr Top Med Chem. 2013;13:1559–1572. doi: 10.2174/15680266113139990105. [DOI] [PubMed] [Google Scholar]
- 30.Xu Z, Wang Q, Pan J, Sheng X, Hou D, Chong H, Wei Z, Zheng S, et al. Characterization of serum miRNAs as molecular biomarkers for acute Stanford type A aortic dissection diagnosis. Sci Rep. 2017;7:13659. doi: 10.1038/s41598-017-13696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qiu ZH, He J, Chai TC, Zhang Y-L, Zhou H, Zheng H, Chen X-S, Zhang L, Li Y-M, Chen L-W. miR-145 attenuates phenotypic transformation of aortic vascular smooth muscle cells to prevent aortic dissection. J Clin Lab Anal. 2021;35:e23773. doi: 10.1002/jcla.23773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng X, Li A, Zhao L, Zhou T, Shen Q, Cui Q, Qin Q. Key role of microRNA-15a in the KLF4 suppressions of proliferation and angiogenesis in endothelial and vascular smooth muscle cells. Biochem Biophys Res Commun. 2013;437:625–631. doi: 10.1016/j.bbrc.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Wei C, Li L, Gupta S. NF-κB-mediated miR-30b regulation in cardiomyocytes cell death by targeting Bcl-2. Mol Cell Biochem. 2014;387:135–141. doi: 10.1007/s11010-013-1878-1. [DOI] [PubMed] [Google Scholar]
- 34.Rybka V, Suzuki YJ, Shults NV. Effects of Bcl-2/Bcl-xL inhibitors on pulmonary artery smooth muscle cells. Antioxidants (Basel) 2018;7:150. doi: 10.3390/antiox7110150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leng J, Song Q, Zhao Y, Wang Z. miR-15a represses cancer cell migration and invasion under conditions of hypoxia by targeting and downregulating Bcl-2 expression in human osteosarcoma cells. Int J Oncol. 2018;52:1095–1104. doi: 10.3892/ijo.2018.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]