Summary
It has been 30 years since the first member of the protease-activated receptor (PAR) family was discovered. This was followed by the discovery of three other receptors, including PAR2. PAR2 is a G protein-coupled receptor activated by trypsin site-specific proteolysis. The process starts with serine proteases acting between arginine and serine, creating an N-terminus that functions as a tethered ligand that binds, after a conformational change, to the second extracellular loop of the receptor, leading to activation of G-proteins. The physiological and pathological functions of this ubiquitous receptor are still elusive. This review focuses on PAR2 activation and its distribution under physiological and pathological conditions, with a particular focus on the pancreas, a significant producer of trypsin, which is the prototype activator of the receptor. The role in acute or chronic pancreatitis, pancreatic cancer, and diabetes mellitus will be highlighted.
Keywords: PAR2, PRSS1, Trypsin, Pancreas, Pancreatitis, Pancreatic ductal adenocarcinoma
Introduction
Thrombin receptor activated by a novel mechanism, i.e. site-specific proteolysis, was discovered 30 years ago [1]. Since then, three other receptors with similar activation mechanisms have been discovered and are known as protease-activated receptors (PARs). Proteolysis cleaves off the N-terminal segment of the receptor, making a new N-terminal end that acts as a tethered ligand. After the subsequent conformational change, the tethered ligand interacts with the second extracellular loop (ECL-2) of the receptor and activates a site-specific G-protein on the third intracellular loop (ICL-3). The activated G-protein leads to intracellular consequences, including respective second-messengers (inositol triphosphate (IP3), calcium mobilization, mitogen-activated protein kinases (MAPK), protein kinase C (PKC), etc.) [2–4]. The tethered ligand can also activate neighboring receptors of the same PAR-type as well as other PAR-types [5]. Additionally, synthetic analogs of the respective tethered ligand can activate PARs, generally recognized as PAR-activating peptides [6]. Among the four identified types of PARs, types 1, 3, and 4 (PAR1, PAR3, PAR4) [1,7,8] are predominantly activated by thrombin and similar enzymes. The type 2 receptor (PAR2) is activated by site-specific proteolysis caused by trypsin or tryptase (produced mainly by mast cells) [9–11]. Trypsin is predominantly produced in the exocrine pancreas as an inactive form called cationic trypsinogen, which is a proteinaceous product of the PRSS1 gene [12]. Our brief review is intended to bring attention to the relationship between trypsin, either normal or mutated, and PAR2 under physiological and pathological conditions, with a special focus on the pancreas.
Regional distribution and function of PAR2
PAR2 is a ubiquitous membrane receptor found in all tissues, including vessels, immune-competent cells, epithelial cell layers, and all organs, including the heart and brain. Its distribution is not stable and varies between physiological and pathological conditions, as well as throughout embryonic development. Minimal expression is observed at the genesis of organs; expression then rises and culminates at specific times for each organ. This is followed by a decrease to levels usually seen in adult tissues. It is suggested that these changes in PAR2 expression correspond to the level of differentiation [13].
In the central nervous system, neurons and astrocytes normally exhibit weak positivity of PAR2 [14]. However, increased levels of PAR2 can be observed under pathological conditions, including HIV infection, Alzheimer’s disease (AD) [15], multiple sclerosis [16], and Parkinson’s disease [17]. Radiation injury in the murine model an increase in PAR2 positivity in cortical neurons was described. Authors suggest blood-brain barrier damage and reaction to the leak of free blood proteinases, including trypsin, as an explanation [18]. In a spinal cord injury model, activation of PAR2 via c-Jun N-terminal kinases (JNK) promotes glial scar formation. The inhibition by either PAR2 or JNK prevents scar formation and could prolong axonal regeneration times [19].
PAR2 is an integral part of the cardiovascular system, including lymphatic vessels. PAR2 is abundantly present on the endothelial cells and smooth muscle cells of arteries [20]. PAR2 stimulation on endothelium results in vasodilation and hypotension [21]. Atherosclerosis, a common arterial disease, is also related to PAR2 stimulation because of the high concentration of PAR2 inside atherosclerotic plaques [22]. Furthermore, atherosclerosis was less developed in murine PAR2 gene knock-out models (PAR2-KO mice) than in the control group. Bone marrow transplantation reversed the PAR2 gene knock-out effect, showing the involvement of blood cells in the process [22].
Inside the mesenteric lymphatic vessels of guinea pigs, PAR2 is expressed on the endothelium. Stimulation of the receptor and subsequent production of NO via endothelial cyclooxygenase resulted in a decrease in spontaneous transient depolarizations and frequency of lymphatic vessel contractions. The author of this experiment concluded that this response plays a role in inflammation [23].
In the heart, PAR1 and PAR2 are expressed on cardiomyocytes, the stimulation of which promotes increased spontaneous automaticity (via elevated Ca2+ levels), survival of cardiomyocytes (activation of c-Jun, AKT cascades), and induces hypertrophy [24]. In zones surrounding myocardial infarctions, inflammatory stimuli via PAR2 can induce arrhythmias leading to poor outcomes [24]. In patients suffering from diastolic heart failure with preserved ejection fraction, one of the key factors is advanced myocardial fibrosis [25], which is linked to stimulation of PAR1 and TGF-β receptors resulting in increased collagen I production by heart fibroblasts [25].
In the respiratory tract, bronchial and alveolar epithelium, as well as the smooth muscle cells and endothelium, exhibit strong positivity for PAR2 [14]. In a murine asthma model, inhibition of PAR2 alleviated eosinophilic inflammation and allergen-induced airway hyper-responsivity [26]. PAR2 is strongly involved in developing idiopathic pulmonary fibrosis (IPF), a scarring disease of the lungs refractive to treatment; PAR2 stimulation by mast cell tryptase promotes proliferation of fibroblasts and extracellular matrix production [27]. Furthermore, treatment with PAR2 inhibitor diminishes bleomycin-induced pulmonary fibrosis in murine models [28].
PAR2 is strongly expressed in the gastrointestinal tract on epithelium and goblet cells [29]. Basal stimulation regulates mucus and electrolyte (HCO3− and Cl−) secretion, while apical stimulation increases paracellular permeability. Proteases released from the mucosa can stimulate the PAR2 present on vegetative nerves resulting in hyperalgesia. Furthermore, stimulation of PAR1, PAR2, and PAR4 modulates smooth muscle contractility in the intestinal wall [30]. In the kidneys, PAR2 is expressed in epithelial, mesangial, inflammatory cells, fibroblasts, and blood vessels. The suggested role is ion transport, tissue protection, inflammation, repair, and fibrosis [31]. PAR2 is also involved in the immune system. PAR2 is expressed on various leukocytes (T-cells, NK-cells, monocytes, neutrophils, eosinophils, and mastocytes) [32], and combined PAR2-mediated activation of leukocytes and endothelial cells increases capillary permeability and diapedesis [33].
PAR2 localization and role in healthy pancreas
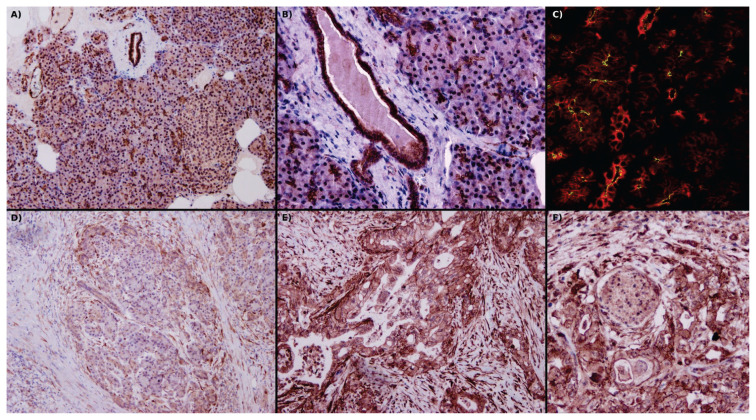
Kawabata et al. immunolocalized PAR2 to the apical part of pancreatic acini, and stimulation facilitated amylase secretion [10]. Using a canine pancreatic model, Nguyen et al. observed a granular pattern of PAR2 expression inside pancreatic ductal epithelial cells (PDEC) of intralobular and interlobular ducts [34]. In another study with bovine pancreatic explants, immunolocalization showed a strong presence of PAR2 in the apical membrane of PDEC; the authors concluded that different expression patterns resulted from better tissue preservation [35]. Olejar et al. observed PAR2 expression on the endothelium of venules and arterioles; discrete positivity in Langerhans islets was also present [36]. In addition to the exocrine and endocrine compartments and interstitium the pancreas contains dispersed quiescent pancreatic stellate cells (PSC) [37]. Masamune et al. observed expression of PAR2 in freshly isolated rat PSC [38]. Figure 1A–C demonstrates localization of PAR2 receptor in formalin-fixed paraffin-embedded tissue block of archival human pancreas.
Fig. 1.
(A) Image of a physiological pancreas, with strong expression of PAR2 in the apical part of the acini and intralobular ducts, diffuse in interlobular ducts and the endothelium of vessels, and weak diffuse positivity in Langerhans islets. Fibroblasts are negative. (B) Detail view of PAR2 positivity in interlobular ducts, acini, and venules. (C) Apical positivity of PAR2 (green) on acini and interlobular duct cells, where cytokeratins 8/18 diffusely fills cytoplasm (red). (D) In pancreatitis, acini and intralobular duct cell PAR2 positivity decreases and takes on a diffuse cytoplasmic pattern. At the periphery, there is enhanced PAR2 positivity in fibroblasts and immune cells. (E) In pancreatic ductal adenocarcinoma, there is strong membranous positivity of PAR2 as well as greatly enhanced expression of PAR2 in fibroblasts. (F) Proximity of ductal adenocarcinoma to nerves results in enhanced neural PAR2 positivity. In A, B, D, E & F use immunohistochemical localization of PAR2, and in C, localization of PAR2 is by immunofluorescence in a confocal microscope. Original magnification 40× in A and D; 100× in B, E & F; 630× in C. P. Suhaj provided these archival pictures.
In pancreatic acini, direct activation of PAR2 increases the secretion of zymogen granules from acinar cells [39]. Furthermore, PAR2 activation increases mucus exocytosis [40] as well as increases HCO3− secretion from PDEC [41]. These mechanisms modulate the luminal environment and influence trypsinogen activation and trypsin activity. PAR2 acts as a protective mechanism against the development and progression of pancreatitis and is discussed further below [39–41].
PAR2 in acute pancreatitis
Pancreatitis is an inflammatory disease of the pancreas classified as either acute or chronic according to the clinical course; the acute form can be further classified as mild or severe. Mild pancreatitis tends to be a self-limited disease dominated by the loss of tight junctions and focal apoptosis [42]. Severe pancreatitis is a destructive disease associated with local complications (abscess, pseudocyst, necrosis with loss of exocrine and endocrine functions) [43,44], as well as systemic complications (massive amounts of pancreatic enzymes in the systemic circulation followed by multiorgan failure and death) [44].
PAR2 plays several roles in protecting from acute pancreatitis. The common molecular basis behind the protective mechanism is PAR2-induced dephosphorylation of MAP/ERK followed by downregulation of MAP kinase [39]. Direct activation of PAR2 increases the secretion of zymogen granules from acinar cells, protecting them from intracellular activation and digestion [39]. Another protective mechanism mediated by PAR2 (via Ca2+ and PKC) is increased mucus exocytosis by PDEC [40], which most likely creates a thin mucus layer that protects PDEC from enzymatic damage. Activated PAR2 increases HCO3− secretion via increased expression of Cl−/HCO3− exchangers on PDEC. The following increase in pH protects the pancreas from spontaneous trypsinogen activation and consequential damage [41]. On the other hand, PAR2 can initiate and cause the progression of pancreatitis indirectly by vasoconstriction (via PAR2 on smooth muscle cells) followed by vasodilation (PAR2 on endothelia). This sequence of events damages epithelial structures via the ischemia-reperfusion mechanism [45]. Systemic complications of severe pancreatitis were simulated when rat models were treated with trypsin or PAR2-AP. In the lungs, this resulted in activation of mast cells and macrophages, increasing activity of tryptase and myeloperoxidase, respectively. This results in PAR2-induced lung injury, which can be partially decreased by a PAR2 inhibitor [46]. In summary, in edematous pancreatitis, PAR2-induced local protective effects prevail, and the disease tends to be limited to the pancreas. Taken together, the supposed failure of local protective mechanisms in severe pancreatitis results in massive trypsin activity in the systemic circulation, followed by PAR2-induced life-threatening organ complications [47].
PAR2 in chronic pancreatitis
Chronic pancreatitis is a progressive fibroinflammatory disease of the pancreas in which healthy tissue is replaced with fibrous tissue produced by PSC [48,49]. This disease is associated with alcohol, smoking, and specific genetic mutations (e.g. PRSS1, cystic fibrosis conductance regulators (CFTR), serine protease inhibitor Kazal type 1 (SPINK1)). Complications include maldigestion (due to a lack of enzymes and repeated steatorrhea), pancreatic diabetes mellitus combined with sudden changes in glucose levels, bouts of chronic neuropathic pain due to the affection of nearby nerves, and carcinogenesis [49]. These complications decrease the patient’s quality of life and life expectancy [49].
Restricting known modifiable risk factors can limit exacerbations and slow disease progression [49]. A well-known but unmodifiable risk factor is a genetic mutation in the PRSS1 gene, which results in a hereditary form of chronic pancreatitis. A missense mutation leads to increased transcription of PRSS1, autoactivation of trypsinogen, and decreased trypsin degradation [50]. The disease itself results in increased trypsin activity (and activation of PAR2) [51], tissue damage followed by exocrine and endocrine insufficiency, and an increased risk of cancer development [50]. For other causes of chronic pancreatitis, the involvement of PAR2 is only partially explained. In chronic pancreatitis, PAR2 downregulates apical anion exchangers and CFTR on PDEC [51]. This set of events decreases the secretion of bicarbonate, as well as lowers the pH, and autoactivates trypsinogen [44,51]. PDEC can respond by downregulating PAR2 on the transcriptional level and translocating PAR2 into the cytoplasm [51].
Unlike the healthy pancreas, activated PSC acquires a myofibroblast-like phenotype and overproduces collagen and metalloproteinases inhibitors, resulting in overproduction of collagen and replacement of original tissues [37]. Freshly isolated rat PSC express PAR2, and activation by PAR2-AP (SLIGRL-NH2) pushes PSC to increased proliferation activity and collagen production (via c-JUN and p38-MAP) [38]. Abdominal neuropathic pain in chronic pancreatitis, as well as pancreatic adenocarcinoma, are associated with pancreatic neuritis. Inflammatory infiltrates show specific enrichment of mast cells, producing tryptase capable of PAR2 stimulation of nerves [52,53].
PAR2 in Langerhans islet pathologies
In 1970 Bertaccini et al. described significant increases in blood insulin concentrations after administration of caerulein to anesthetized dogs [54]. In taurocholate-induced pancreatitis, the level of PAR2 expression in Langerhans islets dramatically increases [36]. These findings suggest that severe pancreatitis could directly modulate insulin secretion (apparently by PAR2 activation).
Diabetes mellitus (DM) is a group of metabolic diseases resulting in hyperglycemia, non-enzymatic glycation of proteins and organ complications. Most DM cases can be classified as type I or type II. Type I is an autoimmune disease associated with complete ablation of β-cells and a complete absence of insulin production at an early age [55]. Recently a new, phenomena were described, putatively associated with PAR2. In murine model of hereditary pancreatitis Langerhans islet hyperplasia was observed [56]. On the other hand, stimulation of PAR2 in islets devoid of β-cells resulted in transdifferentiation of α-cells into β-cells [57]. After further elucidation, targeting of PAR2 could serve as long term treatment of the type I DM.
Type II usually appears later in life and is connected with a relative lack of insulin due to increased insulin resistance [55]. Insulin resistance is caused by adipose tissue and immune system dysfunction and is usually associated with modern diets (high in lipids and carbohydrates). In human and rat adipose tissue, an increase in PAR2 expression correlates with an increased BMI, potentiated by diets high in palmitic, stearic, and myristic acids, with the greatest impact linked to palmitic acid. Three-quarters of this PAR2 increase was associated with the non-adipose stromal vascular cells fraction; PAR2 was more common compared to other PARs. Administration of the PAR2 antagonist, GB88, reduced body weight, visceral fat deposition (central obesity), and fatty tissue inflammation (involving macrophages and mast cells), eliminated liver and pancreatic dysfunction by normalized expression of glucose transporter 2 (GLUT2), normalized glucose-stimulated insulin secretion, decreased insulin resistance, and reduced complications of metabolic syndrome [58].
PAR2 in non-pancreatic cancers
Associated tumors are heterogeneous groups of autonomous proliferative diseases in which trypsin-PAR2 mediates various mechanisms that support tumor progression. Colon cancer is a typical example of a neoplasia producing trypsin and stimulating local PAR2 in an autocrine manner [59]. Co-stimulation of PAR2 and tissue factor (TF) in colon cancer cell lines results in a complex intracellular cascade consisting of protein kinase C (PKC) followed by an extracellular signal-regulated kinase (ERK 1/2). This results in increased mitotic activity (phosphorylation and upregulation of c-Jun/AP1 proto-oncogene) [60], as well as facilitated migration (upregulated production of matrix metalloproteinase (MMP), which digests the surrounding extracellular matrix [61]. Another example is a cholangiocarcinoma cell experiment, in which PAR2 activating peptide (PAR2-AP) increased migration through collagen membranes by the formation of filopodia spikes (mediated by an intracellular cascade consisting of a tyrosine-kinase met (MET) receptor, a mitogen-activated protein kinase (MAPK) and a signal transducer and activator of transcription 3 (STAT3)) [62]. In hepatocellular carcinoma, activation of PAR2-ERK leads to an epithelial-mesenchymal transition that is associated with enhanced migration and invasion capability. Furthermore, increased PAR2 expression corresponds to larger tumors, increased microvascular invasion, and advanced tumor stage [63]. In such cases, the level of PAR2 expression could be a valuable prognostic factor [63].
Besides the direct influence on tumors, PAR2 influences the tumor microenvironments. PAR2 induces a desmoplastic reaction (i.e. increased collagen production) which decreases the permeability of solutes and drugs (one cause of tumor drug resistance). At the same time, PAR2 suppresses lymphatic vessel formation and lymphatic propagation of neoplasias [64]. This dual role of PAR2, which has been described in other neoplasias as well [65], poses an obstacle in the pharmacological treatment of cancers [66].
PAR2 in pancreatic tumors and their precursors
Benign pancreatic lesions with an increased risk of transforming into pancreatic ductal adenocarcinoma (PDAC) are Pancreatic intraepithelial neoplasia (PanIN), Intraductal papillary mucinous neoplasia (IPMN), and Mucinous cystic neoplasia (MCN) [67]. PanIN involves microscopic lesions that can form flat lesions, tufts, or small papillae affecting the pancreatic ducts and mucin-producing columnar epithelium [68]. In experiments using the PRSS1-R116C mutation in transgenic mice (a model for hereditary pancreatitis), histopathological findings consist of pancreatic duct inflammation and epithelial dysplasia (i.e. defective structure and cell arrangement, disorganization, epithelial mucilage) that corresponds to PanIN [56]. Human patients with severe hereditary pancreatitis frequently develop early PanIN. Fifty percent of these patients have PanIN with high-grade dysplasia and a higher risk of PDAC [69]. Involvement of mutated pancreatic trypsin and subsequently PAR2 activation is indicated. Yada et al. reported that the majority of IPMNs strongly express PAR2 in the cytoplasm, just like PDAC. According to this finding author suggests IPMN-PDAC sequence [70].
PDAC, according to 5-year survival rates, is one of the deadliest malignant tumors. Annually PDAC causes 331,000 deaths, with the highest incidence in developed countries [71]. PDAC is a neoplasm resembling the pancreatic duct system, typically forming tubular structures [72]. PDAC usually harbors the p53 mutation, which may be the driver for malignant transformation since p53 is absent in PanIN and IPMN [73]. In experimental models, increased expression and activation of PAR2 were associated with increased pancreatic cancer cell proliferation via the MAP-kinase pathway. Co-expression of trypsinogen inside these malignant cells suggests autocrine stimulation of this receptor and results in greater autonomy and progression of the malignancy [74]. In experiments with the PANC-1 cell line harboring the PRSS1-R116C mutation or overexpressing the PRSS1 gene, excessive stimulation of PAR2 was described [56]. PAR2 activation starts the phosphorylation cascade PKC-JAK1-STAT5 and results in upregulated expression of trypsin (autocrine stimulation of PAR2), increased proliferation (enhanced transition of G1/S phase), and invasion (MMPs) [75]. Increased production and release of MMPs by these neoplasias results in connective tissue degradation that facilitates infiltrative growth [75–77]. Another PAR pathway in PDAC initiates the production of IL-8, causing stimulation of PSC and intercellular crosstalk. This chain reaction results in progressive desmoplasia in the vicinity of malignant cells, allowing evasion of therapeutic drugs (drug resistance) and disease progression [78,79]. Activated PAR2 can stimulate PDAC indirectly by increased expression of VEGF-α via the MEK pathway, which induces neo-angiogenesis and tumor-related thrombosis [80]. The pain of neuropathic origin is a frequent complication of patients with PDAC [61]. Colocalization of neuronal markers with PAR2 in the tumor’s proximity suggests the involvement of trypsin in the development of neuropathic pain [53]. A series of invasive and non-invasive methods can significantly reduce pain and improve the patient’s quality of life [81]. According to murine experiments, administration of a PAR2 antagonist after intraperitoneal administration of tumorous cells led to pain relief. The development of specific anti-PAR2 agents could have great potential in this field [81].
Another highly malignant pancreatic tumor is acinar cell carcinoma, which resembles acinar structures and usually produces exocrine granules. In experiments with the TGP49 cell line (analog of pancreatic acinar cell carcinoma), PAR2-AP supports cellular growth and eventually overcomes harmful cell mutations. The use of a PAR2 antagonist results in decreased proliferation. The experiment showed that cell growth is dependent on autocrine stimulation of PAR2 [82].
PAR2 in experimental models of pancreatic diseases
Two experimental murine models of acute pancreatitis are frequently in use to represent the toxonutritive and cholestatic etiology of the disease. The first one is pancreatitis induced by caerulein, a cholecystokinin analog, which represents early intracellular trypsinogen activation followed by acinar injury [83]. The other is pancreatitis induced by taurocholate, a component of bile salts, representing injury caused by detergent action on cell membranes [84]. Supraphysiological doses of caerulein and taurocholate led directly to an interruption of apical exocrine secretion, intracellular activation, and basolateral secretion resulting in autodigestion and direct initiation of pancreatitis [83,84]. This is consistent with findings from Maeda et al. describing a change in the PAR2 staining pattern (antibodies focused against the cleavage site) in the apical membrane of acini and lobular ducts to the basolateral part of these cells [85].
Furthermore, in taurocholate model shows increased expression of PAR2 in acinar cells, ductal cells, Langerhans islets, and the smooth muscle layer of vessels. This possibly enhances the effect of both protective and harmful mechanisms [37]. However, these models are not interchangeable: since deletion of PAR2 decreases acinar cell injury and intracellular signaling in taurocholate-induced pancreatitis, but in caerulein-induced pancreatitis, it has the opposite effect [86].
A transgenic murine model with PRSS1-R116C represents a hereditary form of chronic pancreatitis. One observed phenomenon is Langerhans islet hyperplasia [56]. In this model, histopathological changes of the exocrine pancreas consist of dysplastic changes resembling PanIN [56]. Further research with this model is needed for elucidation of chronic activation of PAR2, the effect on Langerhans islets, PanIN development, and PanIN-PDAC sequence.
In islets void of β-cells, Piran et al. observed PAR2-induced trans-differentiation of α-cells into β-cells [57]. Consecutive shrinkage of the α-cell population and consequently lower blood glucagon levels resulted, followed by the genesis of new α-cells. Together, both processes could help ameliorate DM type I and, after comprehensive elucidation, may represent a treatment [87,88].
PAR2 as a target of treatment
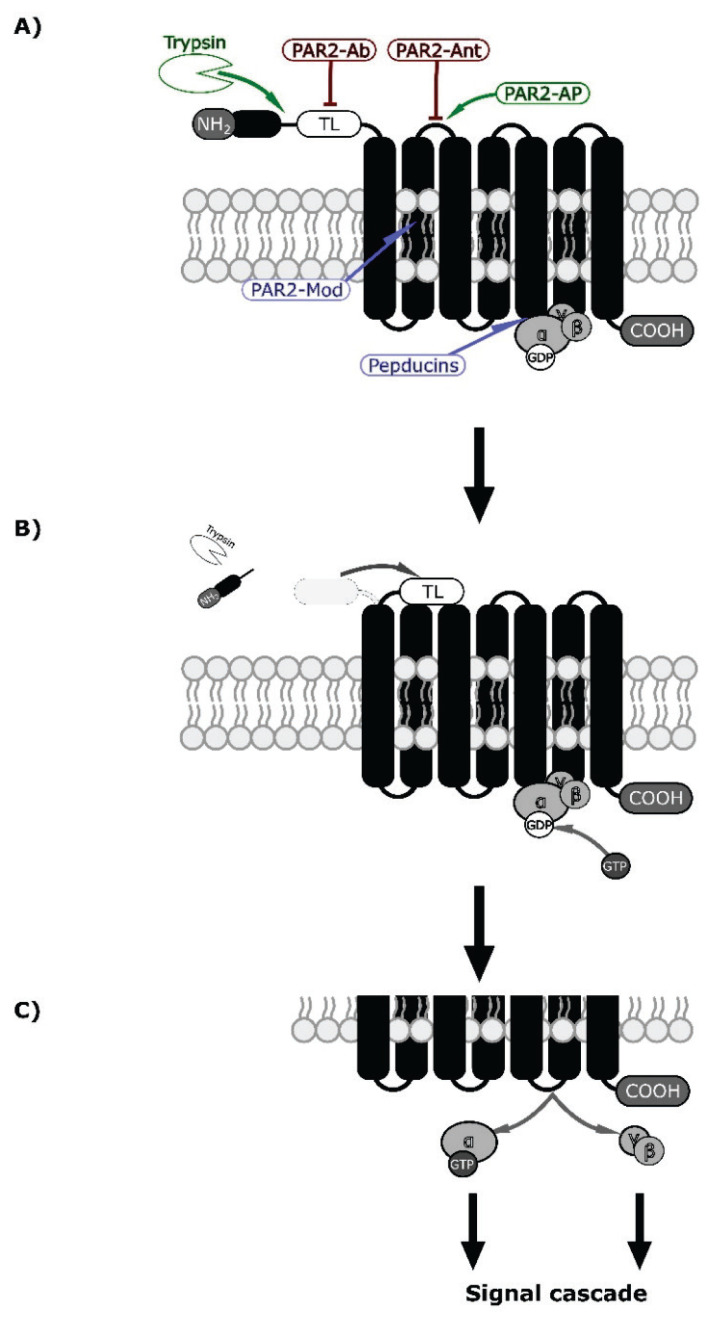
There are several sites where the function of PAR2 can be influenced; as such, they are potential treatment targets. PAR2-AP (e.g. SLIGKV-NH2 or 2-furoyl-LIGRL-NH2) resemble TL, and their binding to orthosteric pockets on ECL-2 results in non-enzymatic activation [89]. On the other hand, activation of PAR2 could be blocked by monoclonal antibody (PAR2-Ab) binding to TL or PAR2 antagonists (PAR2-Ant) binding to orthosteric pockets. PAR2-Ant consist of peptides (FSLLRY-NH2), non-peptide small molecules (e.g. GB88), and antigen-binding fragments (Fab) [89]. PAR2 modulators (PAR2-Mod, e.g. AZ3451 and AZ8838) bind to allosteric pockets formed by hydrophobic parts of transmembrane domains (TMDs) or TMDs and ECL2; the subsequent conformational change affects transduction [90]. Lastly, pepducins are a group of short lipidated peptides (e.g. P2pal-18S) that penetrate plasma membranes and bind with ICL-3, where G-protein pockets are present [89]. Figure 2A demonstrates binding sites of these drugs on PAR2.
Fig. 2.
PAR2 mechanism of activation by specific proteolysis [9] and putative binding sites of drugs (agonists are green, antagonists red and modulators blue) [89,90]. (A) PAR2 receptor consists of N-terminal end (NH2 and peptide) followed by tethered ligand (TL), 7 transmembrane domains (black columns bound together by 6 loops), and C-terminal (COOH). This receptor is coupled to the inactive G-protein (a group composed of α, β, and γ domains, with attached GDP to the α domain) in a nucleotide-binding pocket on the third intracellular loop [9,97]. The enzyme (white indented ellipsis) cuts between the N-terminal and tethered ligands. At the same level, PAR2-AP binds to the first extracellular loop and causes non-enzymatic activation (TL works similarly on a different PAR [9]. (B) N-terminal end is cleaved off, and the tethered ligand is uncovered. This results in a conformational change and receptor activation by the tethered ligand. In both mechanisms, the activation of the receptor is then transferred to the G-protein, resulting in the release of GDP and replacement by GTP [98]. (C) The α-subunit with the GTP is separated from βγ -subunits, and both can induce intracellular signal cascades [97,98].
Currently, there are no PAR2-targeting drugs in clinical use. However, Vorapaxar (PAR1 competitive antagonist) has already been approved for preventing cardiovascular events (myocardial infarction and peripheral arterial diseases) [91]. One of the promising PAR2-targeting drugs is MEDI0618 (PAR2 monoclonal antibody), which is in phase I trials and is intended to treat chronic pain from osteoarthritis [89].
Other drugs offer a novel approach to inflammatory diseases. FSLLRY-NH2 significantly decreases the responsiveness of respiratory epithelium and bronchial smooth muscle cells. This could be a potential adjuvant agent to asthma therapy [92]. I-287 (PAR2 negative allosteric modulator) selectively inhibits the Gq and G12/13 pathways (PLC-IP3/DAG-PKC; SRF-RE; FAK; ERK1/2; IL-8) without altering the Gi/o pathway (β-arrestin). As such, it could effectively inhibit pro-inflammatory responses [93]. In the pancreas, P2pal-18S diminishes injury caused by bile salts in murine models and may prove helpful in pancreatitis induced by Endoscopic-retrograde cholangiopancrea-tography (ERCP) [94].
PAR2 drugs could find a place in the treatment of neoplasias as well. For example, FSLLRY-NH2 in HeLa cell cultures significantly inhibits proliferation and increases apoptosis, and in in vivo experiments, it suppresses cancer growth via the STAT3 pathway [95]. P2pal-18S in NSCLC attenuates ERK phosphorylation that forces epithelial-mesenchymal transition, which induces EGFR transactivation and PD-L1 expression and results in Osimertinib resistance (third-generation EGFR tyrosine kinase inhibitor). The combined use of P2pal-18S and Osimertinib further block cell viability, migration, 3D sphere formation, and in vivo tumor growth [96].
Conclusions
PAR2, the “trypsin” receptor, is involved in physiological and pathological processes in all tissues. Especially in the pancreas, where large amounts of the PAR2 enzyme are produced, activation participates in the development or progression of acute and chronic pancreatitis, insulin resistance in type 2 diabetes mellitus, and pancreatic ductal adenocarcinoma. Logically, targeting the PAR2 receptor seems like a rational treatment modality, but currently, there are no PAR2-targeting drugs available. There are several promising candidates representing activators, antagonists, and modulators, which have the potential to thwart the course of these, often fatal, pancreatic conditions.
Acknowledgements
This work was supported by MH CZ – DRO (Thomayer University Hospital – TUH, 00064190). Authors thanks Thomas Ownsby Secrest M.Sc. for the English correction.
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1.Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-V. [DOI] [PubMed] [Google Scholar]
- 2.Faraut B, Barbier J, Ravel-Chapuis A, Doyennette MA, Jandrot-Perrus M, Verdiere-Sahuque M, Schaeffer L, Koenig J, Hantai D. Thrombin downregulates muscle acetylcholine receptors via an IP3 signaling pathway by activating its G-protein-coupled protease-activated receptor-1. J Cell Physiol. 2003;196:105–112. doi: 10.1002/jcp.10280. [DOI] [PubMed] [Google Scholar]
- 3.van der Merwe JQ, Moreau F, MacNaughton WK. Protease-activated receptor-2 stimulates intestinal epithelial chloride transport through activation of PLC and selective PKC isoforms. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1258–G1266. doi: 10.1152/ajpgi.90425.2008. [DOI] [PubMed] [Google Scholar]
- 4.Yan S, Ding H, Peng J, Wang X, Pang C, Wei J, Wei J, Chen H. Down-regulation of protease-activated receptor 2 ameliorated osteoarthritis in rats through regulation of MAPK/NF-KB signaling pathway in vivo and in vitro. Biosci Rep. 2020;40:BSR20192620. doi: 10.1042/BSR20192620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scarborough RM, Naughton MA, Teng W, Hung DT, Rose J, Vu TK, Wheaton VI, Turck CW, Coughlin SR. Tethered ligand agonist peptides. Structural requirements for thrombin receptor activation reveal mechanism of proteolytic unmasking of agonist function. J Biol Chem. 1992;267:13146–13149. doi: 10.1016/S0021-9258(18)42184-9. [DOI] [PubMed] [Google Scholar]
- 6.Seiler SM, Goldenberg HJ, Michel IM, Hunt JT, Zavoico GB. Multiple pathways of thrombin-induced platelet activation differentiated by desensitization and a thrombin exosite inhibitor. Biochem Biophys Res Commun. 1991;181:636–643. doi: 10.1016/0006-291X(91)91238-8. [DOI] [PubMed] [Google Scholar]
- 7.Ishihara H, Connolly A, Zeng D. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature. 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- 8.Xu WF, Andersen H, Whitmore TE, Presnell SR, Yee DP, Ching A, Gilbert T, Davie EW, Foster DC. Cloning and characterization of human protease-activated receptor 4. Proc Natl Acad Sci U S A. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohm SK, Kong W, Bromme D, Smeekens SP, Anderson DC, Connolly A, Kahn M, Nelken NA, Coughlin SR, Payan DG, Bunnett NW. Molecular cloning, expression and potential functions of the human proteinase-activated receptor-2. Biochem J. 1996;314:1009–1016. doi: 10.1042/bj3141009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawabata A, Kuroda R, Nishida M, Nagata N, Sakaguchi Y, Kawao N, Nishikawa H, Arizono N, Kawai K. Protease-activated receptor-2 (PAR-2) in the pancreas and parotid gland: Immunolocalization and involvement of nitric oxide in the evoked amylase secretion. Life Sci. 2002;71:2435–2446. doi: 10.1016/S0024-3205(02)02044-1. [DOI] [PubMed] [Google Scholar]
- 11.Wronkowitz N, Gorgens SW, Romacho T, Villalobos LA, Sanchez-Ferrer CF, Peiro C, Sell H, Eckel J. Soluble DPP4 induces inflammation and proliferation of human smooth muscle cells via protease-activated receptor 2. Biochim Biophys Acta. 2014;1842:1613–1621. doi: 10.1016/j.bbadis.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Chen JM, Ferec C. Gene conversion-like missense mutations in the human cationic trypsinogen gene and insights into the molecular evolution of the human trypsinogen family. Mol Genet Metab. 2000;71:463–469. doi: 10.1006/mgme.2000.3086. [DOI] [PubMed] [Google Scholar]
- 13.Jenkins AL, Chinni C, De Niese MR, Blackhart B, Mackie EJ. Expression of protease-activated receptor-2 during embryonic development. Dev Dyn. 2000;218:465–471. doi: 10.1002/1097-0177(200007)218:3<465::AID-DVDY1013>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 14.D'Andrea MR, Derian CK, Leturcq D, Baker SM, Brunmark A, Ling P, Darrow AL, Santulli RJ, Brass LF, Andrade-Gordon P. Characterization of protease-activated receptor-2 immunoreactivity in normal human tissues. J Histochem Cytochem. 1998;46:157–164. doi: 10.1177/002215549804600204. [DOI] [PubMed] [Google Scholar]
- 15.Afkhami-Goli A, Noorbakhsh F, Keller AJ, Vergnolle N, Westaway D, Jhamandas JH, Andrade-Gordon P, Hollenberg MD, Arab H, Dyck RH, Power C. Proteinase-activated receptor-2 exerts protective and pathogenic cell type-specific effects in Alzheimer's disease. J Immunol. 2007;179:5493–5503. doi: 10.4049/jimmunol.179.8.5493. [DOI] [PubMed] [Google Scholar]
- 16.Noorbakhsh F, Tsutsui S, Vergnolle N, Boven LA, Shariat N, Vodjgani M, Warren KG, Andrade-Gordon P, Hollenberg MD, Power C. Proteinase-activated receptor 2 modulates neuroinflammation in experimental autoimmune encephalomyelitis and multiple sclerosis. J Exp Med. 2006;203:425–435. doi: 10.1084/jem.20052148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu P, Sun L, Zhao XL, Zhang P, Zhao XM, Zhang J. PAR2-mediated epigenetic upregulation of a-synuclein contributes to the pathogenesis of Parkinson's disease. Brain Res. 2014;1565:82–89. doi: 10.1016/j.brainres.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Olejar T, Matej R, Zadinova M, Pouckova P. Proteinase-activated receptor-2 expression on cerebral neurones after radiation damage: immunohistochemical observation in Wistar rats. Int J Tissue React. 2002;24:81–88. [PubMed] [Google Scholar]
- 19.Li TZ, Deng H, Liu Q, Xia YZ, Darwazeh R, Yan Y. Protease-activated receptor-2 regulates glial scar formation via JNK signaling. Physiol Res. 2019;68:305–316. doi: 10.33549/physiolres.933908. [DOI] [PubMed] [Google Scholar]
- 20.Molino M, Raghunath PN, Kuo A, Ahuja M, Hoxie JA, Brass LF, Barnathan ES. Differential expression of functional protease-activated receptor-2 (PAR-2) in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1998;18:825–832. doi: 10.1161/01.ATV.18.5.825. [DOI] [PubMed] [Google Scholar]
- 21.McGuire JJ, Hollenberg MD, Andrade-Gordon P, Triggle CR. Multiple mechanisms of vascular smooth muscle relaxation by the activation of proteinase-activated receptor 2 in mouse mesenteric arterioles. Br J Pharmacol. 2002;135:155–169. doi: 10.1038/sj.bjp.0704469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones SM, Mann A, Conrad K, Saum K, Hall DE, McKinney LM, Robbins N, et al. PAR2 (Protease-Activated Receptor 2) deficiency attenuates atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2018;38:1271–1282. doi: 10.1161/ATVBAHA.117.310082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan AK, Vergnolle N, Hollenberg MD, von der Weid PY. Proteinase-activated receptor 2 activation modulates guinea-pig mesenteric lymphatic vessel pacemaker potential and contractile activity. J Physiol. 2004;560:563–576. doi: 10.1113/jphysiol.2004.071399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabri A, Muske G, Zhang H, Pak E, Darrow A, Andrade-Gordon P, Steinberg SF. Signaling properties and functions of two distinct cardiomyocyte protease-activated receptors. Circ Res. 2000;86:1054–1061. doi: 10.1161/01.RES.86.10.1054. [DOI] [PubMed] [Google Scholar]
- 25.Friebel J, Weithauser A, Witkowski M, Rauch BH, Savvatis K, Dorner A, Tabaraie T, et al. Protease-activated receptor 2 deficiency mediates cardiac fibrosis and diastolic dysfunction. Eur Heart J. 2019;40:3318–3332. doi: 10.1093/eurheartj/ehz117. [DOI] [PubMed] [Google Scholar]
- 26.Asaduzzaman M, Nadeem A, Arizmendi N, Davidson C, Nichols HL, Abel M, Ionescu LI, et al. Functional inhibition of PAR2 alleviates allergen-induced airway hyperresponsiveness and inflammation. Clin Exp Allergy. 2015;45:1844–1855. doi: 10.1111/cea.12628. [DOI] [PubMed] [Google Scholar]
- 27.Wygrecka M, Dahal BK, Kosanovic D, Petersen F, Taborski B, von Gerlach S, Didiasova M, et al. Mast cells and fibroblasts work in concert to aggravate pulmonary fibrosis: role of transmembrane SCF and the PAR-2/PKC-a/Raf-1/p44/42 signaling pathway. Am J Pathol. 2013;182:2094–2108. doi: 10.1016/j.ajpath.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 28.Lin C, von der Thusen J, Daalhuisen J, ten Brink M, Crestani B, van der Poll T, Borensztajn K, Spek CA. Pharmacological targeting of protease-activated receptor 2 affords protection from bleomycin-induced pulmonary fibrosis. Mol Med. 2015;21:576–583. doi: 10.2119/molmed.2015.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jarry A, Dorso L, Gratio V, Forgue-Lafitte ME, Laburthe M, Laboisse CL, Darmoul D. PAR-2 activation increases human intestinal mucin secretion through EGFR transactivation. Biochem Biophys Res Commun. 2007;364:689–694. doi: 10.1016/j.bbrc.2007.10.073. [DOI] [PubMed] [Google Scholar]
- 30.Kawabata A, Matsunami M, Sekiguchi F. Gastrointestinal roles for proteinase-activated receptors in health and disease. Br J Pharmacol. 2008;153(Suppl 1):S230–S240. doi: 10.1038/sj.bjp.0707491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vesey DA, Hooper JD, Gobe GC, Johnson DW. Potential physiological and pathophysiological roles for protease-activated receptor-2 in the kidney. Nephrology (Carlton) 2007;12:36–43. doi: 10.1111/j.1440-1797.2006.00746.x. [DOI] [PubMed] [Google Scholar]
- 32.Lopez ML, Soriano-Sarabia N, Bruges G, Marquez ME, Preissner KT, Schmitz ML, Hackstein H. Expression pattern of protease activated receptors in lymphoid cells. Cell Immunol. 2014;288:47–52. doi: 10.1016/j.cellimm.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Steinhoff M, Buddenkotte J, Shpacovitch V, Rattenholl A, Moormann C, Vergnolle N, Luger TA, Hollenberg MD. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev. 2005;26:1–43. doi: 10.1210/er.2003-0025. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen TD, Moody MW, Steinhoff M, Okolo C, Koh DS, Bunnett NW. Trypsin activates pancreatic duct epithelial cell ion channels through proteinase-activated receptor-2. J Clin Invest. 1999;103:261–269. doi: 10.1172/JCI2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvarez C, Regan JP, Merianos D, Bass BL. Protease-activated receptor-2 regulates bicarbonate secretion by pancreatic duct cells in vitro. Surgery. 2004;136:669–676. doi: 10.1016/j.surg.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 36.Olejar T, Matej R, Zadinova M, Pouckova P. Expression of proteinase-activated receptor 2 during taurocholate-induced acute pancreatic lesion development in Wistar rats. Int J Gastrointest Cancer. 2001;30:113–121. doi: 10.1385/IJGC:30:3:113. [DOI] [PubMed] [Google Scholar]
- 37.Hrabák P, Kalousová M, Krechler T, Zima T. Pancreatic stellate cells - rising stars in pancreatic pathologies. Physiol Res. 2021;70(Suppl 4):S597–S616. doi: 10.33549//physiolres.934783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masamune A, Kikuta K, Satoh M, Suzuki N, Shimosegawa T. Protease-activated receptor-2-mediated proliferation and collagen production of rat pancreatic stellate cells. J Pharmacol Exp Ther. 2005;312:651–658. doi: 10.1124/jpet.104.076232. [DOI] [PubMed] [Google Scholar]
- 39.Namkung W, Yoon JS, Kim KH, Lee MG. PAR2 exerts local protection against acute pancreatitis via modulation of MAP kinase and MAP kinase phosphatase signaling. Am J Physiol Gastrointest Liver Physiol. 2008;295:G886–G894. doi: 10.1152/ajpgi.00053.2008. [DOI] [PubMed] [Google Scholar]
- 40.Singh VP, Bhagat L, Navina S, Sharif R, Dawra RK, Saluja AK. Protease-activated receptor-2 protects against pancreatitis by stimulating exocrine secretion. Gut. 2007;56:958–964. doi: 10.1136/gut.2006.094268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim MH, Choi BH, Jung SR, Sernka TJ, Kim S, Kim KT, Hille B, Nguyen TD, Koh DS. Protease-activated receptor-2 increases exocytosis via multiple signal transduction pathways in pancreatic duct epithelial cells. J Biol Chem. 2008;283:18711–18720. doi: 10.1074/jbc.M801655200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Merilainen S, Makela J, Anttila V, Koivukangas V, Kaakinen H, Niemela E, Ohtonen P, Risteli J, Karttunen T, Soini Y, Juvonen T. Acute edematous and necrotic pancreatitis in a porcine model. Scand J Gastroenterol. 2008;43:1259–1268. doi: 10.1080/00365520802158580. [DOI] [PubMed] [Google Scholar]
- 43.Bradley EL., 3rd A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13,1992. Arch Surg. 1993;128:586–590. doi: 10.1001/archsurg.1993.01420170122019. [DOI] [PubMed] [Google Scholar]
- 44.Namkung W, Han W, Luo X, Muallem S, Cho KH, Kim KH, Lee MG. Protease-activated receptor 2 exerts local protection and mediates some systemic complications in acute pancreatitis. Gastroenterology. 2004;126:1844–1859. doi: 10.1053/j.gastro.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 45.Toyama MT, Lewis MP, Kusske AM, Reber PU, Ashley SW, Reber HA. Ischaemia-reperfusion mechanisms in acute pancreatitis. Scand J Gastroenterol. 1996;219(Suppl):20–23. doi: 10.3109/00365529609104994. [DOI] [PubMed] [Google Scholar]
- 46.De-Madaria E, del Mar Frances M, Gea-Sorli S, Gutierrez LM, Viniegra S, Perez-Mateo M, Closa D, Lopez-Font I. Role of protease-activated receptor 2 in lung injury development during acute pancreatitis in rats. Pancreas. 2014;43:895–902. doi: 10.1097/MPA.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 47.Matej R, Housa D, Olejar T. Acute pancreatitis: proteinase-activated receptor-2 as Dr. Jekyll and Mr Hyde. Physiol Res. 2006;55:467–474. doi: 10.33549/physiolres.930798. [DOI] [PubMed] [Google Scholar]
- 48.Beyer G, Habtezion A, Werner J, Lerch MM, Mayerle J. Chronic pancreatitis. Lancet. 2020;396:499–512. doi: 10.1016/S0140-6736(20)31318-0. [DOI] [PubMed] [Google Scholar]
- 49.Vege SS, Chari ST. Chronic Pancreatitis. N Engl J Med. 2022;386:869–878. doi: 10.1056/NEJMcp1809396. [DOI] [PubMed] [Google Scholar]
- 50.Howes N, Greenhalf W, Stocken DD, Neoptolemos JP. Cationic trypsinogen mutations and pancreatitis. Gastroenterol Clin North Am. 2004;33:767–787. doi: 10.1016/j.gtc.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 51.Pallagi P, Venglovecz V, Rakonczay Z, Jr, Borka K, Korompay A, Ozsvari B, Judak L, et al. Trypsin reduces pancreatic ductal bicarbonate secretion by inhibiting CFTR Cl− channels and luminal anion exchangers. Gastroenterology. 2011;141:2228–2239. doi: 10.1053/j.gastro.2011.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demir IE, Schorn S, Schremmer-Danninger E, Wang K, Kehl T, Giese NA, Algul H, Friess H, Ceyhan GO. Perineural mast cells are specifically enriched in pancreatic neuritis and neuropathic pain in pancreatic cancer and chronic pancreatitis. PLoS One. 2013;8:e60529. doi: 10.1371/journal.pone.0060529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu J, Miao XR, Tao KM, Zhu H, Liu ZY, Yu DW, Chen QB, Qiu HB, Lu ZJ. Trypsin-protease activated receptor-2 signaling contributes to pancreatic cancer pain. Oncotarget. 2017;8:61810–61823. doi: 10.18632/oncotarget.18696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bertaccini G, De Caro G, Melchiorri P. The effects of caerulein on insulin secretion in anaesthetized dogs. Br J Pharmacol. 1970;40:78–85. doi: 10.1111/j.1476-5381.1970.tb10612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(Suppl 1):S62–S69. doi: 10.2337/dc11-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu Q. PRSS1 mutation: a possible pathomechanism of pancreatic carcinogenesis and pancreatic cancer. Mol Med. 2019;2:44. doi: 10.1186/s10020-019-0111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Piran R, Lee SH, Kuss P, Hao E, Newlin R, Millan JL, Levine F. PAR2 regulates regeneration, transdifferentiation, and death. Cell Death Dis. 2016;7:e2452. doi: 10.1038/cddis.2016.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lim J, Iyer A, Liu L, Suen JY, Lohman RJ, Seow V, Yau MK, Brown L, Fairlie DP. Diet-induced obesity, adipose inflammation, and metabolic dysfunction correlating with PAR2 expression are attenuated by PAR2 antagonism. FASEB J. 2013;27:4757–4767. doi: 10.1096/fj.13-232702. [DOI] [PubMed] [Google Scholar]
- 59.Ducroc R, Bontemps C, Marazova K, Devaud H, Darmoul D, Laburthe M. Trypsin is produced by and activates protease-activated receptor-2 in human cancer colon cells: evidence for new autocrine loop. Life Sci. 2002;70:1359–1367. doi: 10.1016/S0024-3205(01)01519-3. [DOI] [PubMed] [Google Scholar]
- 60.Vogt PK. Fortuitous convergences: the beginnings of JUN. Nat Rev Cancer. 2002;2:465–469. doi: 10.1038/nrc818. [DOI] [PubMed] [Google Scholar]
- 61.Hu L, Xia L, Zhou H, Wu B, Mu Y, Wu Y, Yan J. TF/FVIIa/PAR2 promotes cell proliferation and migration via PKCa and ERK-dependent c-Jun/AP-1 pathway in colon cancer cell line SW620. Tumour Biol. 2013;34:2573–2581. doi: 10.1007/s13277-013-0803-2. [DOI] [PubMed] [Google Scholar]
- 62.Kaufmann R, Hascher A, Mussbach F, Henklein P, Katenkamp K, Westermann M, Settmacher U. Proteinase-activated receptor 2 (PAR(2)) in cholangiocarcinoma (CCA) cells: effects on signaling and cellular level. Histochem Cell Biol. 2012;138:913–924. doi: 10.1007/s00418-012-1006-4. [DOI] [PubMed] [Google Scholar]
- 63.Sun L, Li PB, Yao YF, Xiu AY, Peng Z, Bai YH, Gao YJ. Proteinase-activated receptor 2 promotes tumor cell proliferation and metastasis by inducing epithelial-mesenchymal transition and predicts poor prognosis in hepatocellular carcinoma. World J Gastroenterol. 2018;24:1120–1133. doi: 10.3748/wjg.v24.i10.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi K, Queiroz KC, Roelofs JJ, van Noesel CJ, Richel DJ, Spek CA. Protease-activated receptor 2 suppresses lymphangiogenesis and subsequent lymph node metastasis in a murine pancreatic cancer model. J Pathol. 2014;234:398–409. doi: 10.1002/path.4411. [DOI] [PubMed] [Google Scholar]
- 65.Olejar T, Vetvicka D, Zadinova M, Pouckova P, Kukal J, Jezek P, Matej R. Dual role of host Par2 in a murine model of spontaneous metastatic B16 melanoma. Anticancer Res. 2014;34:3511–3515. [PubMed] [Google Scholar]
- 66.Sareide K, Roalsa M, Aunan JR. Is there a Trojan horse to aggressive pancreatic cancer biology? A review of the trypsin-PAR2 axis to proliferation, early invasion, and metastasis. J Pancreat Cancer. 2020;6:12–20. doi: 10.1089/pancan.2019.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Basturk O, Hong SM, Wood LD, Adsay NV, Albores-Saavedra J, Biankin AV, Brosens LA, et al. A revised classification system and recommendations from the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am J Surg Pathol. 2015;39:1730–1741. doi: 10.1097/PAS.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim JY, Hong SM. Precursor lesions of pancreatic cancer. Oncol Res Treat. 2018;41:603–610. doi: 10.1159/000493554. [DOI] [PubMed] [Google Scholar]
- 69.Rebours V, Levy P, Mosnier JF, Scoazec JY, Soubeyrand MS, Flejou JF, Turlin B, Hammel P, Ruszniewski P, Bedossa P, Couvelard A. Pathology analysis reveals that dysplastic pancreatic ductal lesions are frequent in patients with hereditary pancreatitis. Clin Gastroenterol Hepatol. 2010;8:206–212. doi: 10.1016/j.cgh.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 70.Yada K, Shibata K, Matsumoto T, Ohta M, Yokoyama S, Kitano S. Protease-activated receptor-2 regulates cell proliferation and enhances cyclooxygenase-2 mRNA expression in human pancreatic cancer cells. J Surg Oncol. 2005;89:79–85. doi: 10.1002/jso.20197. [DOI] [PubMed] [Google Scholar]
- 71.Luchini C, Capelli P, Scarpa A. Pancreatic ductal adenocarcinoma and its variants. Surg Pathol Clin. 2016;9:547–560. doi: 10.1016/j.path.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 72.Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22:9694–9705. doi: 10.3748/wjg.v22.i44.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wada K. p16 and p53 gene alterations and accumulations in the malignant evolution of intraductal papillary-mucinous tumors of the pancreas. J Hepatobiliary Pancreat Surg. 2002;9:76–85. doi: 10.1007/s005340200007. [DOI] [PubMed] [Google Scholar]
- 74.Shimamoto R, Sawada T, Uchima Y, Inoue M, Kimura K, Yamashita Y, Yamada N, Nishihara T, Ohira M, Hirakawa K. A role for protease-activated receptor-2 in pancreatic cancer cell proliferation. Int J Oncol. 2004;24:1401–1406. [PubMed] [Google Scholar]
- 75.Ikeda O, Egami H, Ishiko T, Ishikawa S, Kamohara H, Hidaka H, Mita S, Ogawa M. Expression of proteinase-activated receptor-2 in human pancreatic cancer: a possible relation to cancer invasion and induction of fibrosis. Int J Oncol. 2003;22:295–300. doi: 10.3892/ijo.22.2.295. [DOI] [PubMed] [Google Scholar]
- 76.Shi K, Queiroz KC, Stap J, Richel DJ, Spek CA. Protease-activated receptor-2 induces migration of pancreatic cancer cells in an extracellular ATP-dependent manner. J Thromb Haemost. 2013;11:1892–1902. doi: 10.1111/jth.12361. [DOI] [PubMed] [Google Scholar]
- 77.Xie L, Duan Z, Liu C, Zheng Y, Zhou J. Protease-activated receptor 2 agonist increases cell proliferation and invasion of human pancreatic cancer cells. Exp Ther Med. 2015;9:239–244. doi: 10.3892/etm.2014.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ikeda O, Egami H, Ishiko T, Ishikawa S, Kamohara H, Hidaka H, Takahashi M, Ogawa M. Signal of proteinase-activated receptor-2 contributes to highly malignant potential of human pancreatic cancer by up-regulation of interleukin-8 release. Int J Oncol. 2006;28:939–946. doi: 10.3892/ijo.28.4.939. [DOI] [PubMed] [Google Scholar]
- 79.Bynigeri RR, Jakkampudi A, Jangala R, Subramanyam C, Sasikala M, Rao GV, Reddy DN, Talukdar R. Pancreatic stellate cell: Pandora's box for pancreatic disease biology. World J Gastroenterol. 2017;23:382–405. doi: 10.3748/wjg.v23.i3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chang LH, Pan SL, Lai CY, Tsai AC, Teng CM. Activated PAR-2 regulates pancreatic cancer progression through ILK/HIF-a-induced TGF-a expression and MEK/VEGF-A-mediated angiogenesis. Am J Pathol. 2013;183:566–575. doi: 10.1016/j.ajpath.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 81.Dobosz E, Kaczor M, Stefaniak TJ. Pain in pancreatic cancer: review of medical and surgical remedies. ANZ J Surg. 2016;86:756–761. doi: 10.1111/ans.13609. [DOI] [PubMed] [Google Scholar]
- 82.Li W, Nakagawa T, Koyama N, Wang X, Jin J, Mizuno-Horikawa Y, Gu J, Miyoshi E, Kato I, Honke K, Taniguchi N, Kondo A. Down-regulation of trypsinogen expression is associated with growth retardation in alpha1,6-fucosyltransferase-deficient mice: attenuation of proteinase-activated receptor 2 activity. Glycobiology. 2006;16:1007–1019. doi: 10.1093/glycob/cwl023. [DOI] [PubMed] [Google Scholar]
- 83.Halangk W, Sturzebecher J, Matthias R, Schulz HU, Lippert H. Trypsinogen activation in rat pancreatic acinar cells hyperstimulated by caerulein. Biochim Biophys Acta. 1997;1362:243–251. doi: 10.1016/S0925-4439(97)00082-3. [DOI] [PubMed] [Google Scholar]
- 84.Aho HJ, Koskensalo SM, Nevalainen TJ. Experimental pancreatitis in the rat. Sodium taurocholate-induced acute haemorrhagic pancreatitis. Scand J Gastroenterol. 1980;15:411–416. doi: 10.3109/00365528009181493. [DOI] [PubMed] [Google Scholar]
- 85.Maeda K, Hirota M, Kimura Y, Ichihara A, Ohmuraya M, Sugita H, Ogawa M. Proinflammatory role of trypsin and protease-activated receptor-2 in a rat model of acute pancreatitis. Pancreas. 2005;31:54–62. doi: 10.1097/01.mpa.0000163178.37050.0d. [DOI] [PubMed] [Google Scholar]
- 86.Laukkarinen JM, Weiss ER, van Acker GJ, Steer ML, Perides G. Protease-activated receptor-2 exerts contrasting model-specific effects on acute experimental pancreatitis. J Biol Chem. 2008;283:20703–20712. doi: 10.1074/jbc.M801779200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee SH, Hao E, Scharp D, Levine F. Insulin acts as a repressive factor to inhibit the ability of PAR2 to induce islet cell transdifferentiation. Islets. 2018;10:1–12. doi: 10.1080/19382014.2018.1472839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell. 2009;138:449–462. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McIntosh KA, Cunningham MR, Bushell T, Plevin R. The development of proteinase-activated receptor-2 modulators and the challenges involved. Biochem Soc Trans. 2020;48:2525–2537. doi: 10.1042/BST20200191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheng RKY, Fiez-Vandal C, Schlenker O, Edman K, Aggeler B, Brown DG, Brown GA, et al. Structural insight into allosteric modulation of protease-activated receptor 2. Nature. 2017;545:112–115. doi: 10.1038/nature22309. [DOI] [PubMed] [Google Scholar]
- 91.Baker NC, Lipinski MJ, Lhermusier T, Waksman R. Overview of the 2014 Food and Drug Administration Cardiovascular and Renal Drugs Advisory Committee meeting about vorapaxar. Circulation. 2014;130:1287–1294. doi: 10.1161/CIRCULATIONAHA.114.011471. [DOI] [PubMed] [Google Scholar]
- 92.Ocasio-Rivera M, Marin-Maldonado F, Trossi-Torres G, Ortiz-Rosado A, Rodriguez-Irizarry V, Rodriguez-Lopez E, Martinez S, Almodovar S, Suarez-Martinez E. Targeting of protease activator receptor-2 (PAR-2) antagonist FSLLRY-NH2 as an asthma adjuvant therapy. Medicine (Baltimore) 2020;99:e22351. doi: 10.1097/MD.0000000000022351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Avet C, Sturino C, Grastilleur S, Gouill CL, Semache M, Gross F, Gendron L, Bennani Y, Mancini JA, Sayegh CE, Bouvier M. The PAR2 inhibitor I-287 selectively targets Gaq and Ga12/13 signaling and has anti-inflammatory effects. Commun Biol. 2020;3:719. doi: 10.1038/s42003-020-01453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Michael ES, Kuliopulos A, Covic L, Steer ML, Perides G. Pharmacological inhibition of PAR2 with the pepducin P2pal-18S protects mice against acute experimental biliary pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2013;304:G516–G526. doi: 10.1152/ajpgi.00296.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shanshan H, Lan X, Xia L, Huang W, Meifang Z, Ling Y. Inhibition of protease-activated receptor-2 induces apoptosis in cervical cancer by inhibiting signal transducer and activator of transcription-3 signaling. J Int Med Res. 2019;47:1330–1338. doi: 10.1177/0300060518820440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jiang Y, Zhuo X, Wu Y, Fu X, Mao C. PAR2 blockade reverses osimertinib resistance in non-small-cell lung cancer cells via attenuating ERK-mediated EMT and PD-L1 expression. Biochim Biophys Acta Mol Cell Res. 2022;1869:119144. doi: 10.1016/j.bbamcr.2021.119144. [DOI] [PubMed] [Google Scholar]
- 97.Duc NM, Kim HR, Chung KY. Structural mechanism of G protein activation by G protein-coupled receptor. Eur J Pharmacol. 2015;763:214–222. doi: 10.1016/j.ejphar.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 98.Pfeil EM, Brands J, Merten N, Vogtle T, Vescovo M, Rick U, Albrecht IM, et al. Heterotrimeric G protein subunit Gaq is a master switch for Gβy-mediated calcium mobilization by Gi-coupled GPCRs. Mol Cell. 2020;80:940–954. doi: 10.1016/j.molcel.2020.10.027. [DOI] [PubMed] [Google Scholar]