Abstract
Summary
Diabetes is closely connected with skeletal muscle dysfunction. Ellagic acid (EA) possesses a variety of bio-effects and is applied to the improvement of diabetes. The purpose of this study was to explore the potential improvement effect and mechanisms of EA in streptozotocin (STZ)-induced diabetic muscle atrophy. The model of diabetic mice was established by intra-peritoneal STZ to evaluate treatment effect of EA (100 mg/kg/d for 8 weeks) on muscle atrophy. Our data exhibited that EA enhanced fiber size and weight of gastrocnemius, and promoted grip strength to relieve STZ-induced muscle lesions. In serum, the levels of Creatine kinase (CK), lactate dehydrogenase (LDH), total cholesterol (TC), triglyceride (TG), low-density lipoprotein cholesterol (LDL) were inhibited, while high-density lipoprotein cholesterol (HDL) level was enhanced by EA treatment in diabetic mice. In gastrocnemius, EA decreased Atrogin-1 and MuRF-1 expressions to relieve STZ-induced muscle atrophy. Moreover, EA increased NRF-1 and PGC-1α expressions to alleviate mitochondrial disorder. Meanwhile, EA suppressed CHOP and GRP-87 levels to relieve ER stress. Lastly, EA inhibited BAX expressions and enhanced Bcl-2 expressions to mitigate apoptosis. In conclusion, EA is preventing the event of STZ-induced gastrocnemia by amelioration of mitochondrial dysfunction, ER stress and apoptosis, and could be used in the protection and therapeutic of muscle atrophy in diabetes.
Keywords: Ellagic acid, Muscle atrophy, Mitochondrion, Endoplasmic reticulum stress, Apoptosis
Introduction
The leading cause of diabetes is the loss of functional beta-cell mass in the pancreas, which threatens insensitivity and deficiency of insulin. Insulin, as a vital regulator of glucose homeostasis, is closely relevant to the regulation of hyperglycemia and incidence of diabetes [1,2]. In other words, the primary clinical manifestation of diabetes is uncontrollable high glucose. A large number of previous studies have shown that altered glucose homeostasis causes multiple tissues and organs injury to evoke diabetic complications [3]. Nowadays, diabetic complication has become a crucial research topic owing to its growing number of patients [4]. Many drugs used in the treatment of diabetic complications are becoming more and more widespread, but its side effects are also increasing correspondingly. Hence, the case for developing an effective agent with low side effects is imperative.
Skeletal muscle is an essential component of our body and is necessary for physical capacity. In addition, skeletal muscle is involved in glucose uptake [5]. However, diabetes has been proved to affect skeletal muscle health via reducing muscle mass, cross-sectional area and strength [6]. The main molecular mechanism for development of diabetic muscle atrophy is connected with ubiquitin-proteasome system UPS dysfunction as represented by accumulating ubiquitinated proteins and increasing proteasomal activity [7]. It also disturbs other biological functions, such as endoplasmic reticulum stress, mitochondrial disorder and apoptosis, which are closely involved in muscle wasting [8–10]. Therefore, improvement of ubiquitin-proteasome system is considered as a critical way to prevent diabetic muscle atrophy.
Ellagic acid is a natural polyphenol widespread in fruits and vegetables. EA has a variety of therapeutic effects both on diabetes and muscle injury due to its multiple biological and pharmacological properties [11,12]. Previous studies showed that EA could stimulate insulin production and reduce glucose to improve diabetic complications [13]. In diabetes-induced testicular injury, EA was proved to inhibit apoptosis [14]. In addition, EA decreased MDA level and increased SOD activity in ischemia/reperfusion-induced skeletal muscle damage [15]. In cuprizone-induced muscular dysfunction, EA improved motor coordination and enhanced ATP production via regulation of mitochondrial respiratory chain activity [16]. However, there is no report on the efficacy of EA on STZ-induced muscle injury, and its molecular pathogenesis is not illuminated. We hypothesized that the improvement of EA on diabetic muscle atrophy was attributed to its regulations on ER stress, mitochondrial function, and apoptosis. In this study, we aimed to address the protective functions of EA on diabetic muscle atrophy.
Methods
Animals
Male ICR mice (n=60, 20±2 g) were acquired from Hunan SJA Laboratory animal (Changsha, China). The age of mice was about 8 weeks old. Mice were housed in normal light and dark (12:12 h) cycles with applicable humidity and temperature. During the experimental period, mice were ad libitum fed standard food and water. In this study, animal experiments were inspected by the Ethics Committee of Hunan University of Arts and Science (No. HUAS-2021-TY-158).
Chemicals and reagents
Ellagic acid (purity: ≥98 %) and streptozotocin were obtained from Sangon Biotech (Shanghai, China). The antibodies of BAX, Bcl-2 and CHOP were obtained from Proteintech (Wuhan, China). The antibodies of Atrogin-1, GRP-78, MuRF-1, NRF-1 and PGC-1α were obtained from Sangon Biotech (Shanghai, China). The assay kits of HDL, LDL, TC and TG were purchased from Nanjing Jiancheng Biotechnology Institute (Nanjing, China).
Experimental design
Experimental mice were assigned into control group (CON group, n=20), diabetes mellitus group (DM group, n=20), and ellagic acid treatment group (DM + EA group, n=20). STZ was dissolved in citrate buffer (0.1 M, pH 4.5). The model of diabetic mice was established by intra-peritoneal STZ. To confirm experimental diabetes, blood was taken from caudal vein to test glucose level. Mice with the glucose level of 16.7 mmol/l and above were deemed as a suitable diabetic model and chosen for further experiment [17]. Clinical manifestations of diabetic mice were observed and recorded every day. In diabetic model, mice were intragastrically fed with EA (100 mg/kg/day for 8 weeks), which was considered as DM + EA group. The control (CON) group was accordingly treated with an equivalent amount of saline.
Grip strength
Muscle force was detected by a dynamometer (YLS13, Anhui Zhenghua Bioinstrumentation). During force test, experimental mouse steadily gripped stick and pulled backward [18]. The peak of force was observed and recorded. The grip strength was measured three times to calculate an average value.
Sample preparation
After muscle force test, mice were sacrificed by anesthesia with the injection of pentobarbital. Blood was taken from eyeball for biochemical assessment. Gastrocnemia was excised and weighed. A part of gastrocnemia was preserved at −80°C for the measurement of protein expression. The remaining tissue was fixed in 4 % paraformaldehyde for histopathological analysis.
Biochemical assessment
CK and LDH were examined to estimate muscle injury. TC, TG, LDL and HDL were examined to estimate lipid metabolism in blood. CK, LDH, TC, TG, LDL and HDL levels were tested by spectrophotometer.
Histological analysis
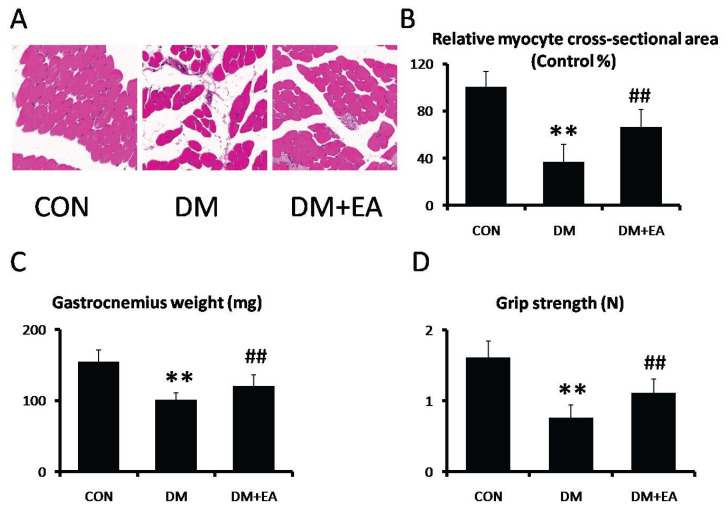
Haematoxylin and Eosin (HE) staining was utilized to detect the morphological characteristics of gastrocnemia [19]. After fixation, gastrocnemia was dehydrated with alcohol, cleared with xylene, and embedded in paraffin. Then, paraffin block was cut into 5 μm by a rotary microtome. For histological evaluation, sections were stained with haematoxylin and eosin. The staining was observed under a light microscope. Muscle fiber size was statistically assessed and measured by Image J. The results showed the relative myocyte cross-sectional area of gastrocnemia from CON group, DM group, and DM + EA group.
Western blot
Gastrocnemia was homogenized at low temperatures by a homogenizer. Lysis buffer and proteinase inhibitors were added into homogenates, which were centrifuged to obtain supernatants for protein expression analysis [20]. SDS-PAGE was utilized to separate lysate proteins. Wet electroblotting was utilized to transfer individual proteins onto PVDF membrane. After blocking with 5 % milk, PVDF membrane was incubated with primary antibodies. PVDF membrane was washed with TBS for 3 times. The corresponding HRP-conjugated antibodies were added onto PVDF membrane. After washing with TBS, the signals were detected by ECL chemiluminescence. The protein bands were observed under imaging system and its density was recorded. β-actin was considered as loading control. The results were expressed as protein band density relative to β-actin.
Statistics
All data were showed as mean ± SD. Statistical difference was demonstrated by ANOVA test with Tukey’s post hoc test. p<0.05 was deemed statistically significant.
Results
Effects of EA on STZ-induced skeletal muscle atrophy
HE was used to appraise the improvement of EA against STZ-induced morphological characteristics in gastrocnemius. The muscle fiber size was markedly reduced in DM group, while representative myocyte cross-sections were dramatically enhanced by EA treatment (Fig. 1A, B). Moreover, gastrocnemia weight and grip strength were markedly reduced in DM group, while these changes were dramatically reversed by EA treatment (Fig. 1C–D).
Fig. 1.
Effects of EA on STZ-induced skeletal muscle atrophy. (A, B) morphological characteristics were analyzed by HE staining in gastrocnemia (200×). (C) Gastrocnemia weight. (D) Grip strength. ** p<0.01 vs. CON; ## p<0.01 vs. DM.
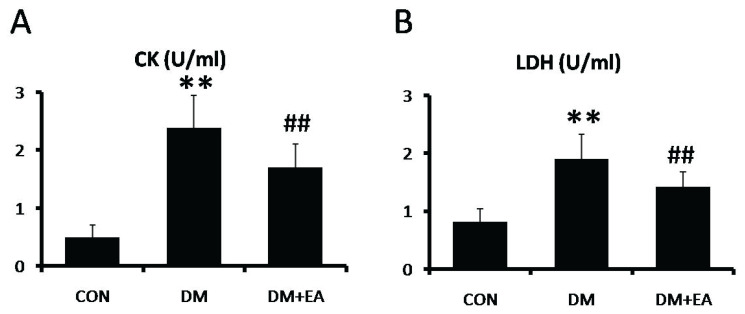
Effects of EA on CK and LDH
To appraise the modulation of EA on STZ-induced muscle damage, CK and LDH activities were detected in serum. The activities of CK and LDH were markedly enhanced in DM group, while CK and LDH activities were dramatically reduced by EA treatment (Fig. 2A, B).
Fig. 2.
Effects of EA on CK and LDH in serum. (A) CK and (B) LDH levels were detected by spectrophotometer. ** p<0.01 vs. CON; ## p<0.01 vs. DM.
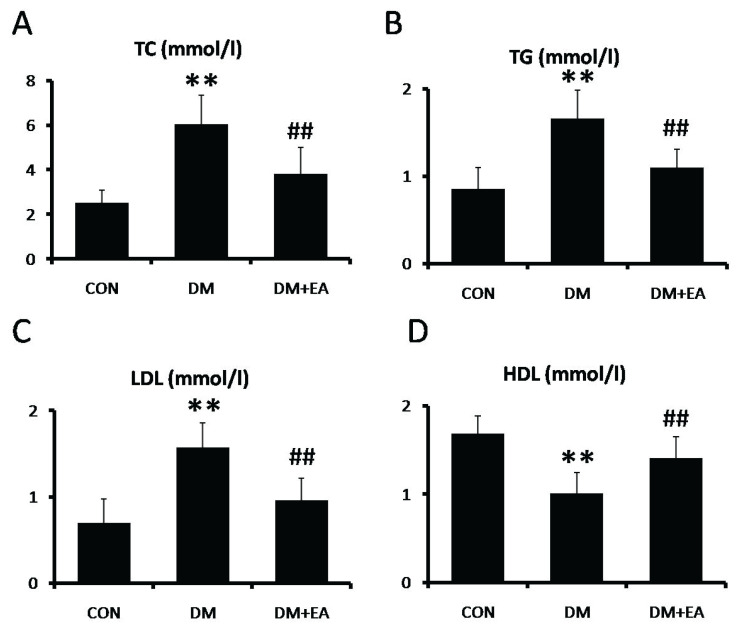
Effects of EA on TC, TG, LDL and HDL
To appraise the modulation of EA on STZ-induced lipid metabolic abnormality, TC, TG, LDL and HDL levels were detected in serum. In DM group, the levels of TC, TG and LDL were markedly enhanced (Fig. 3A–C), while HDL level was markedly reduced (Fig. 3D). In contrast, these lipid profile alterations were dramatically improved by EA treatment (Fig. 3).
Fig. 3.
Effects of EA on lipid metabolic in serum. (A) TC, (B) TG, (C) LDL and (D) HDL levels were detected by spectrophotometer. ** p<0.01 vs. CON; ## p<0.01 vs. DM.
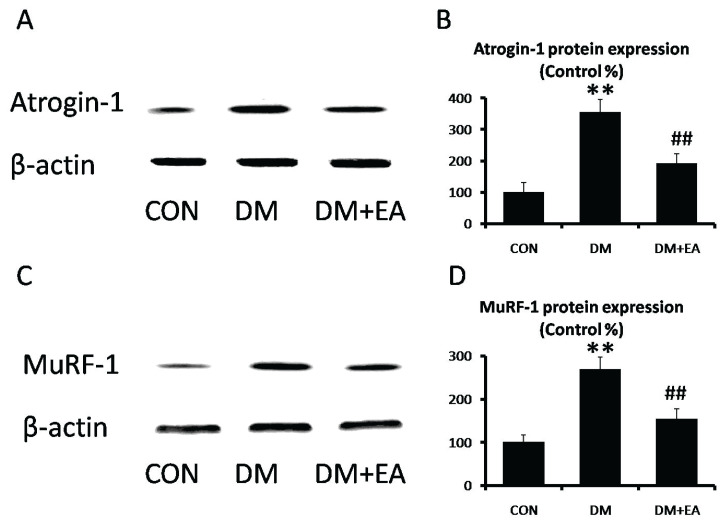
Effects of EA on protein degradation
To appraise the regulation of EA on STZ-induced UPS dysfunction, the expressions of Atrogin-1 and MuRF-1 were detected in gastrocnemius. Atrogin-1 and MuRF-1 expressions were markedly enhanced in DM group, while EA dramatically reduced these muscle-specific E3 ubiquitin ligases to improve ubiquitin proteasome pathway (Fig. 4).
Fig. 4.
Effects of EA on protein degradation in gastrocnemius. (A) Atrogin-1 and (B) MuRF-1 levels were detected by Western blot. (C) Atrogin-1 and (D) MuRF-1 relative expressions were quantized. ** p<0.01 vs. CON; ## p<0.01 vs. DM.
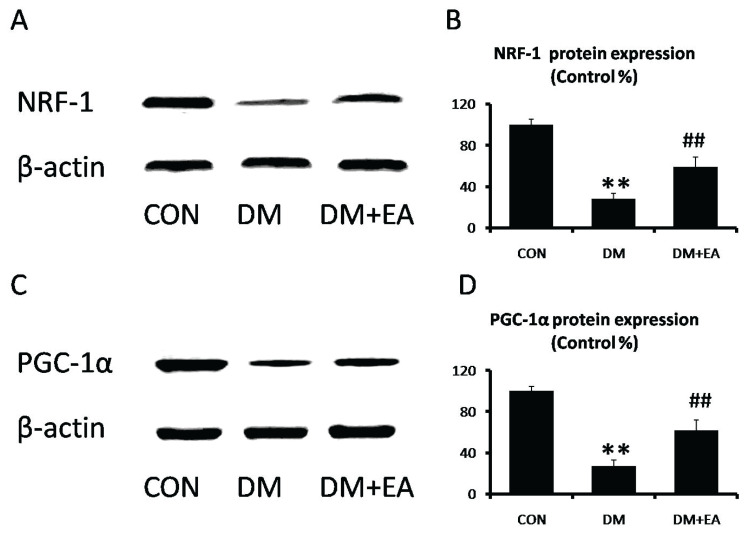
Effects of EA on mitochondrial disorder
NRF-1 and PGC-1α are vital and reliable markers of mitochondrial function [21,22]. NRF-1 and PGC-1α expressions were markedly reduced in DM group, while EA dramatically enhanced these expressions to moderate mitochondrial disorder in gastrocnemia (Fig. 5).
Fig. 5.
Effects of EA on mitochondrial function in gastrocnemius. (A) NRF-1 and (B) PGC-1α levels were detected by Western blot. (C) NRF-1 and (D) PGC-1α relative expressions were quantized. ** p<0.01 vs. CON; ## p<0.01 vs. DM.
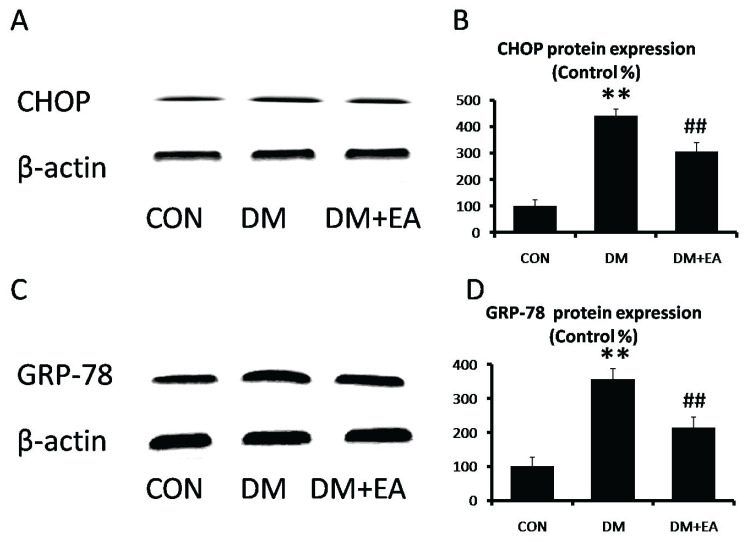
Effects of EA on ER stress
CHOP and GRP-78 are involved in regulation of ER stress [23]. CHOP and GRP-78 expressions were markedly enhanced in DM group, while EA dramatically reversed STZ-induced increase of ER stress markers in gastrocnemia (Fig. 6).
Fig. 6.
Effects of EA on ER stress in gastrocnemius. (A) CHOP and (B) GRP-78 levels were detected by Western blot. (C) CHOP and (D) GRP-78 relative expressions were quantized. ** p<0.01 vs. CON; ## p<0.01 vs. DM.
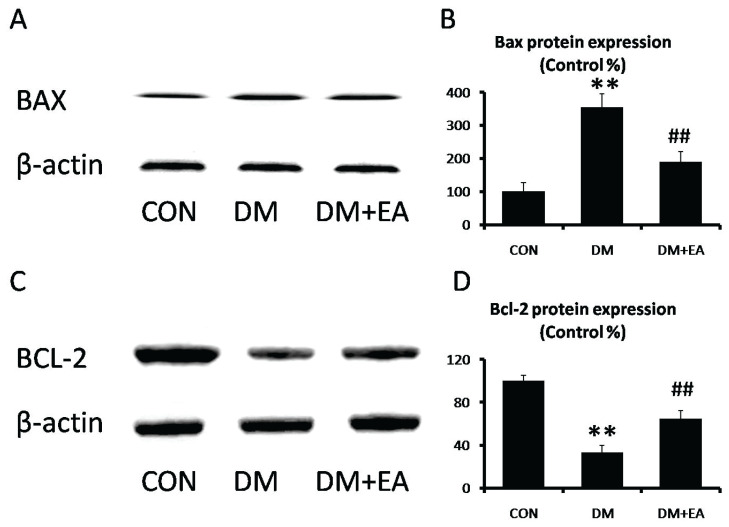
Effects of EA on apoptosis
BAX and Bcl-2 are well-known indicators of apoptotic, which was associated with cellular damage [24]. BAX level was markedly enhanced in DM group, while EA observably suppressed STZ-induced BAX expression in gastrocnemia (Fig. 7A, B). However, Bcl-2 level was markedly inhibited in DM group, while EA observably enhanced Bcl-2 expression in gastrocnemia (Fig. 7C, D).
Fig. 7.
Effects of EA on apoptotic in gastrocnemius. (A) BAX and (B) Bcl-2 levels were detected by Western blot. (C) Bax and (D) Bcl-2 relative expressions were quantized. ** p<0.01 vs. CON; ## p<0.01 vs. DM.
Discussion
Skeletal muscle is a locomotive organ in human body. Damage to skeletal muscle can disrupt motor activity and strength. In diabetes, skeletal muscle, as an endocrine organ, plays a vital role in glucose uptake [25]. Previous Studies showed EA might enhance GLUT4 expression in skeletal muscle of diabetic rats to modulate blood glucose levels [26]. Emerging research has demonstrated hyperglycemia is a high-risk factor for muscle injury [27]. In this study, our results showed diabetes led to weakening muscle mass, fiber size and force generation in skeletal muscle, which are the main characteristics of muscular atrophy. However, EA reversed these changes in STZ-induced diabetic mice. Clinically, CK and LDH are common biochemical indexes, excessive levels of which are used to demonstrate muscle injury. In this study, CK and LDH levels were increased in serum of diabetic mice, while EA relieved CK and LDH levels. Therefore, EA was proved to improve muscle morphology and blood biochemical parameters in diabetic mice.
UPS, as a primary component of the proteolytic system, is responsible for protein anabolic and catabolic processes. In diabetes, UPS is elementary to maintaining muscle weight and its disorder results in protein degradation to cause muscle atrophy [28]. Atrogin-1 and MuRF1 are protein ubiquitination-related proteins and involved in regulating protein degradation, excess expressions of which lead to UPS disorder [28]. Hyperglycemia increased Atrogin-1 and MuRF-1 expressions to degrade intracellular proteins in skeletal muscle [30]. In this study, our results showed EA maintained UPS by inhibiting Atrogin-1 and MuRF-1 expressions in gastrocnemius, suggesting EA improved protein anabolism and catabolism to relieve diabetic muscle atrophy.
Mitochondrial biogenesis is involved in energy generation for skeletal muscle to adaptation exercise. In STZ-induced diabetes, mitochondrial biology, such as mitochondrial fusion, fission and autophagy, was disturbed [31]. NRF-1 and PGC-1α play an important role in the maintenance of mitochondrial homeostasis. PGC-1α, as a transcriptional regulator, is also responsible for mitochondrial content and ATP production by modulating NRF-1 [32–34]. Moreover, diabetes inhibits NRF-1 and PGC-1α expressions to evoke mitochondrial malfunction in various organs [35]. Previous studies showed that EA was involved in the beige remodeling of white adipose tissue by promoting the expression of NRF-1 and PGC-1α [36]. In this study, our results showed EA enhanced mitochondrial biogenesis in gastrocnemius, as represented by upregulating NRF-1 and PGC-1α expression, to relieve diabetic muscle atrophy.
ER was responsible for protein modification by folding and processing polypeptide chains into functional proteins [37]. An accumulation of misfolded or unfolded proteins participates in the induction of participating in the induction of apoptosis [38]. The activated ER stress is proven to be linked to diabetic muscle atrophy [39]. As a critical modulator of ER stress, CHOP and GRP-87 are up-regulated in diabetic complications. Previous studies showed that hyperglycemia increased the production of CHOP and GRP-87 to cause muscle atrophy [27]. In addition, EA could inhibit UVA-induced ER stress in human keratinocyte cells [40]. In this study, our results showed EA relieved ER stress by reducing CHOP and GRP-87 expressions in gastrocnemius, suggesting EA was involved in protein modification to alleviate diabetic muscle atrophy.
Apoptosis is a controllable mechanism for orderly death of cells to maintain a stable internal environment. A disruption of apoptotic signal induces muscle damage in diabetes [41]. BAX and Bcl-2 have been suggested to be central to the apoptotic signals. Previous studies showed that hyperglycemia increased BAX expression and decreased Bcl-2 expression in diabetic complications [42]. Moreover, hyperglycemia-induced mitochondrial malfunction and excess ER stress activate apoptosis. Previous studies showed that EA possessed anti-apoptotic effects in diabetic cardiomyopathy [43]. In muscle, EA reduced CCl4-induced muscle tissue injury via regulation of apoptosis pathway [44]. In this study, our results showed EA possessed anti-apoptotic effects by down-regulating BAX expression and up-regulating Bcl-2 expression in gastrocnemius, suggesting EA was useful for an orderly process of cell death in the treatment of diabetic muscle atrophy.
Conclusions
Our research revealed that EA attenuated diabetic muscle atrophy which was linked to its accommodation of ER stress, mitochondrial function and apoptosis. These results demonstrated EA could be developed as a novel and natural medicine to alleviate muscle atrophy in diabetes.
Acknowledgements
This study was supported by Hunan University of Arts and Science Doctor Foundation (Grant No.18BSQD26). We would like to thank all the people who participated in the study and the researchers.
Footnotes
Conflict of Interest
There is no conflict of interest.
References
- 1.Holeček M. The role of skeletal muscle in the pathogenesis of altered concentrations of branched-chain amino acids (valine, leucine, and isoleucine) in liver cirrhosis, diabetes, and other diseases. Physiol Res. 2021;70:293–305. doi: 10.33549/physiolres.934648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yaribeygi H, Farrokhi FR, Butler AE, Sahebkar A. Insulin resistance: Review of the underlying molecular mechanisms. J Cell Physiol. 2019;234:8152–8161. doi: 10.1002/jcp.27603. [DOI] [PubMed] [Google Scholar]
- 3.Huang DD, Shi G, Jiang Y, Yao C, Zhu C. A review on the potential of Resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed Pharmacother. 2020;125:109767. doi: 10.1016/j.biopha.2019.109767. [DOI] [PubMed] [Google Scholar]
- 4.Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62:3–16. doi: 10.1007/s00125-018-4711-2. [DOI] [PubMed] [Google Scholar]
- 5.Chadt A, Al-Hasani H. Glucose transporters in adipose tissue, liver, and skeletal muscle in metabolic health and disease. Pflugers Arch. 2020;472:1273–1298. doi: 10.1007/s00424-020-02417-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo S, Chen Q, Sun Y, Chen J. Nicotinamide protects against skeletal muscle atrophy in streptozotocin-induced diabetic mice. Arch Physiol Biochem. 2019;125:470–477. doi: 10.1080/13813455.2019.1638414. [DOI] [PubMed] [Google Scholar]
- 7.Ishida T, Iizuka M, Ou Y, Morisawa S, Hirata A, Yagi Y, Jobu K, Morita Y, Miyamura M. Juzentaihoto Suppresses Muscle Atrophy in Streptozotocin-Induced Diabetic Mice. Biol Pharm Bull. 2019;42:1128–1133. doi: 10.1248/bpb.b18-00983. [DOI] [PubMed] [Google Scholar]
- 8.Zhang IX, Raghavan M, Satin LS. The endoplasmic reticulum and calcium homeostasis in pancreatic beta cells. Endocrinology. 2020;161:bqz028. doi: 10.1210/endocr/bqz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romanello V, Sandri M. The connection between the dynamic remodeling of the mitochondrial network and the regulation of muscle mass. Cell Mol Life Sci. 2021;78:1305–1328. doi: 10.1007/s00018-020-03662-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Sun H, Zhang J, Xia Z, Chen W. Streptozotocin-induced diabetic cardiomyopathy in rats: ameliorative effect of PIPERINE via Bcl2, Bax/Bcl2, and caspase-3 pathways. Biosci Biotechnol Biochem. 2020;84:2533–2544. doi: 10.1080/09168451.2020.1815170. [DOI] [PubMed] [Google Scholar]
- 11.Amor AJ, Gómez-Guerrero C, Ortega E, Sala-Vila A, Lázaro I. Ellagic acid as a tool to limit the diabetes burden: Updated evidence. Antioxidants (Basel) 2020;9:1226. doi: 10.3390/antiox9121226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khodaei F, Rashedinia M, Heidari R, Rezaei M, Khoshnoud MJ. Ellagic acid improves muscle dysfunction in cuprizone-induced demyelinated mice via mitochondrial Sirt3 regulation. Life Sci. 2019;237:116954. doi: 10.1016/j.lfs.2019.116954. [DOI] [PubMed] [Google Scholar]
- 13.Harakeh S, Almuhayawi M, Jaouni SA, Almasaudi S, Hassan S, Amri TA, Azhar N, Abd-Allah E, Ali S, El-Shitany N, Mousa SA. Antidiabetic effects of novel ellagic acid nanoformulation: Insulin-secreting and anti-apoptosis effects. Saudi J Biol Sci. 2020;27:3474–3480. doi: 10.1016/j.sjbs.2020.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akarca Dizakar SÖ, Saribas GS, Tekcan A. Effects of ellagic acid in the testes of streptozotocin induced diabetic rats. Drug Chem Toxicol. 2021:1–8. doi: 10.1080/01480545.2021.1908714. [DOI] [PubMed] [Google Scholar]
- 15.Ekinci Akdemir FN, Gülçin İ, Karagöz B, Soslu R, Alwasel SH. A comparative study on the antioxidant effects of hesperidin and ellagic acid against skeletal muscle ischemia/reperfusion injury. J Enzyme Inhib Med Chem. 2016;31(Sup4):114–118. doi: 10.1080/14756366.2016.1220378. [DOI] [PubMed] [Google Scholar]
- 16.Khodaei F, Rashedinia M, Heidari R, Rezaei M, Khoshnoud MJ. Ellagic acid improves muscle dysfunction in cuprizone-induced demyelinated mice via mitochondrial Sirt3 regulation. Life Sci. 2019;237:116954. doi: 10.1016/j.lfs.2019.116954. [DOI] [PubMed] [Google Scholar]
- 17.Chen H, Jin G. Downregulation of salusin-β protects renal tubular epithelial cells against high glucose-induced inflammation, oxidative stress, apoptosis and lipid accumulation via suppressing miR-155-5p. Bioengineered. 2021;12:6155–6165. doi: 10.1080/21655979.2021.1972900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, Sun L, Fan X, Yin B, Kang Y, An S, Tang L. Pulsed electromagnetic fields alleviate streptozotocin induced diabetic muscle atrophy. Mol Med Rep. 2018;18:1127–1133. doi: 10.3892/mmr.2018.9067. [DOI] [PubMed] [Google Scholar]
- 19.Sun W, Li T, Yao H, Kang L, Dong F. Effects of concentrated growth factor and nanofat on aging skin of nude mice induced by D-galactose. Physiol Res. 2021;70:425–435. doi: 10.33549/physiolres.934640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang W, Chen Y, Huang C, Wang W, Huang C, Li Y. MiR-18a Inhibits PI3K/AKT signaling pathway to regulate PDGF BB-induced airway smooth muscle cell proliferation and phenotypic transformation. Physiol Res. 2021;70:883–892. doi: 10.33549/physiolres.934753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klinge CM. Estrogenic control of mitochondrial function. Redox Biol. 2020;31:101435. doi: 10.1016/j.redox.2020.101435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakamoto I, Ishihara A. Effects of voluntary running exercise on skeletal muscle properties in nonobese rats with type 2 diabetes. Physiol Res. 2020;69:73–84. doi: 10.33549/physiolres.934178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rana SVS. Endoplasmic reticulum stress induced by toxic elements-a review of recent developments. Biol Trace Elem Res. 2020;196:10–19. doi: 10.1007/s12011-019-01903-3. [DOI] [PubMed] [Google Scholar]
- 24.Geng H, Chen L, Su Y, Xu Q, Fan M, Huang R, Li X, Lu X, Pan M. miR-431-5p regulates apoptosis of cardiomyocytes after acute myocardial infarction via targeting selenoprotein T. Physiol Res. 2022;71:55–62. doi: 10.33549/physiolres.934683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merz KE, Thurmond DC. Role of skeletal muscle in insulin resistance and glucose uptake. Compr Physiol. 2020;10:785–809. doi: 10.1002/cphy.c190029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nankar RP, Doble M. Hybrid drug combination: anti-diabetic treatment of type 2 diabetic Wistar rats with combination of ellagic acid and pioglitazone. Phytomedicine. 2017;37:4–9. doi: 10.1016/j.phymed.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Tseng YT, Chang WH, Lin CC, Chang FR, Wu PC, Lo YC. Protective effects of Liuwei dihuang water extracts on diabetic muscle atrophy. Phytomedicine. 2019;53:96–106. doi: 10.1016/j.phymed.2018.09.032. [DOI] [PubMed] [Google Scholar]
- 28.Reddy SS, Shruthi K, Joy D, Reddy GB. 4-PBA prevents diabetic muscle atrophy in rats by modulating ER stress response and ubiquitin-proteasome system. Chem Biol Interact. 2019;306:70–77. doi: 10.1016/j.cbi.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Cheng TL, Lin ZY, Liao KY, Huang WC, Jhuo CF, Pan PH, Chen CJ, Kuan YH, Chen WY. Magnesium lithospermate B attenuates high-fat diet-induced muscle atrophy in C57BL/6J mice. Nutrients. 2021;14:104. doi: 10.3390/nu14010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yin L, Chen X, Li N, Jia W, Wang N, Hou B, Yang H, Zhang L, Qiang G, Yang X, Du G. Puerarin ameliorates skeletal muscle wasting and fiber type transformation in STZ-induced type 1 diabetic rats. Biomed Pharmacother. 2021;133:110977. doi: 10.1016/j.biopha.2020.110977. [DOI] [PubMed] [Google Scholar]
- 31.Wang D, Sun H, Song G, Yang Y, Zou X, Han P, Li S. Resveratrol improves muscle atrophy by modulating mitochondrial quality control in STZ-induced diabetic mice. Mol Nutr Food Res. 2018;62:e1700941. doi: 10.1002/mnfr.201700941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joseph AM, Pilegaard H, Litvintsev A, Leick L, Hood DA. Control of gene expression and mitochondrial biogenesis in the muscular adaptation to endurance exercise. Essays Biochem. 2006;42:13–29. doi: 10.1042/bse0420013. [DOI] [PubMed] [Google Scholar]
- 33.Lira VA, Benton CR, Yan Z, Bonen A. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;299:E145–E161. doi: 10.1152/ajpendo.00755.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vainshtein A, Tryon LD, Pauly M, Hood DA. Role of PGC-1α during acute exercise-induced autophagy and mitophagy in skeletal muscle. Am J Physiol Cell Physiol. 2015;308:C710–C719. doi: 10.1152/ajpcell.00380.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akhtar S, Siragy HM. Pro-renin receptor suppresses mitochondrial biogenesis and function via AMPK/SIRT-1/PGC-1α pathway in diabetic kidney. PLoS One. 2019;14:e0225728. doi: 10.1371/journal.pone.0225728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Park WY, Park J, Ahn KS, Kwak HJ, Um JY. Ellagic acid induces beige remodeling of white adipose tissue by controlling mitochondrial dynamics and SIRT3. FASEB J. 2021;35:e21548. doi: 10.1096/fj.202002491R. [DOI] [PubMed] [Google Scholar]
- 37.Oakes SA, Papa FR. The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol. 2015;10:173–194. doi: 10.1146/annurev-pathol-012513-104649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Delbrel E, Soumare A, Naguez A, Label R, Bernard O, Bruhat A, Fafournoux P, Tremblais G, Marchant D, Gille T, Bernaudin JF, Callard P, Kambouchner M, Martinod E, Valeyre D, Uzunhan Y, Planès C, Boncoeur E. HIF-1α triggers ER stress and CHOP-mediated apoptosis in alveolar epithelial cells, a key event in pulmonary fibrosis. Sci Rep. 2018;8:17939. doi: 10.1038/s41598-018-36063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reddy SS, Shruthi K, Prabhakar YK, Sailaja G, Reddy GB. Implication of altered ubiquitin-proteasome system and ER stress in the muscle atrophy of diabetic rats. Arch Biochem Biophys. 2018;639:16–25. doi: 10.1016/j.abb.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 40.Hseu YC, Chou CW, Senthil Kumar KJ, Fu KT, Wang HM, Hsu LS, Kuo YH, Wu CR, Chen SC, Yang HL. Ellagic acid protects human keratinocyte (HaCaT) cells against UVA-induced oxidative stress and apoptosis through the upregulation of the HO-1 and Nrf-2 antioxidant genes. Food Chem Toxicol. 2012;50:1245–1255. doi: 10.1016/j.fct.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 41.Ono T, Takada S, Kinugawa S, Tsutsui H. Curcumin ameliorates skeletal muscle atrophy in type 1 diabetic mice by inhibiting protein ubiquitination. Exp Physiol. 2015;100:1052–1063. doi: 10.1113/EP085049. [DOI] [PubMed] [Google Scholar]
- 42.Bagheri F, Amri J, Salehi M, Karami H, Alimoradian A, Latifi SA. Effect of Artemisia absinthium ethanolic extract on oxidative stress markers and the TLR4, S100A4, Bax and Bcl-2 genes expression in the kidney of STZ-induced diabetic rats. Horm Mol Biol Clin Investig. 2020;41:10. doi: 10.1515/hmbci-2020-0028. [DOI] [PubMed] [Google Scholar]
- 43.Altamimi JZ, Alfaris NA, Alshammari GM, Alagal RI, Aljabryn DH, Aldera H, Alkhateeb MA, Yahya MA. Ellagic acid protects against diabetic cardiomyopathy in rats by stimulating cardiac silent information regulator 1 signaling. J Physiol Pharmacol. 2020;71:10. doi: 10.26402/jpp.2020.6.12. [DOI] [PubMed] [Google Scholar]
- 44.Aslan A, Beyaz S, Gok O, Erman O. The effect of ellagic acid on caspase-3/Bcl-2/Nrf-2/NF-kB/TNF-α/COX-2 gene expression product apoptosis pathway: a new approach for muscle damage therapy. Mol Biol Rep. 2020;47:2573–2582. doi: 10.1007/s11033-020-05340-7. [DOI] [PubMed] [Google Scholar]