Abstract
Background:
Rotator cuff retears occur more often at the proximal region with the suture-bridge (SB) technique than at the typical footprint region with the single-row (SR) technique. Few longitudinal clinical trials have focused on the postoperative tendon quality of the repaired rotator cuff at different regions between the 2 techniques.
Purpose:
To compare tendon healing of the proximal and distal regions between the SB and SR techniques.
Study Design:
Cohort study; Level of evidence, 3.
Methods:
Included were consecutive patients who underwent arthroscopic rotator cuff repair and undertook clinical and magnetic resonance imaging (MRI) examinations at 3, 6, and 12 months postoperatively between 2016 and 2017. These patients were divided into the SB and SR groups according to the technique used. The repaired tendon was segmented into distal and proximal regions on ultrashort echo time–T2* mapping images. Clinical outcomes (Constant score, American Shoulder and Elbow Surgeons score, Fudan University Shoulder Score, and visual analog scale for pain) and MRI-based tendon healing (T2* values) of different regions were compared between the 2 groups. The differences in T2* values and clinical scores were determined by 1-way analysis of variance for repeated measurements.
Results:
A total of 31 patients (17 in SB group and 14 in SR group) were included. At 12-month follow-up, significant improvements from preoperatively were achieved for all patients in all clinical scores (P < .001 for all). No significant between-group differences were found in T2* values of the distal region at any time point; however, the mean T2* value of the proximal region at 3 months was significantly higher in the SB group compared with the SR group (P = .03). This difference became nonsignificant at subsequent follow-up time points.
Conclusion:
Significant clinical improvements over time can be expected in the first year after arthroscopic rotator cuff repair. In the early postoperative period, higher T2* values in the proximal region of the repaired tendon (representing inferior tendon quality) were seen with the SB technique compared with the SR technique; however, this phenomenon was resolved over time.
Keywords: rotator cuff, quantitative MRI, single row, suture bridge
Arthroscopic rotator cuff repair (ARCR) has gradually become the mainstream treatment option for rotator cuff tears, owing to its being less invasive. The single-row (SR), double-row (DR), and suture-bridge (SB) techniques are currently the most commonly used approaches. In recent years, the DR and SB techniques have attracted more attention than the SR technique because of their potential to increase the contact area, pressure, and force load. 8,25,26 Clinical studies have shown that the SB technique can achieve better biomechanical outcomes. 16,17,32 In addition, evidence suggests that the healing pattern of the proximal and distal regions of the repaired tendon with the SB and SR techniques is different; retears occur more often at the proximal area of the musculotendinous junction with the SB technique instead of at the distal area of the footprint, as typically found with the SR technique. 5,20 However, few longitudinal clinical trials have focused on the in vivo anatomic quality of the repaired rotator cuff at the musculotendinous junction and footprint regions between the 2 techniques.
Ultrashort echo time-T2* (UTE-T2*) mapping is a promising quantitative magnetic resonance imaging (MRI) method that is well suited for the evaluation of short T2 structures, such as tendons. 13 An earlier clinical study validated that UTE-T2* mapping of the shoulder is promising for the detection of biochemical healing conditions in the tendon, 37 where higher T2* values were associated with less organized collagen fibers, thus representing inferior tendon quality during the postoperative healing process. In addition, repeat imaging may be warranted for the surveillance of tendon healing progression in patients who seek operative treatment. 21,28
The purpose of this study was to serially evaluate the clinical scores and MRI-based tendon healing (T2* values) of the proximal and distal regions at 3, 6, and 12 months and to compare these outcomes between the 2 surgical techniques. We hypothesized that in the early follow-up period, higher T2* values in the proximal region and lower T2* values in the distal region would be seen with the SB technique compared with the SR technique.
Methods
Patient Population
This study was approved by the health science institutional review board of our hospital, and informed consent was obtained from all participants before enrollment. A consecutive series of patients who underwent unilateral ARCR at the Department of Sports Medicine of our hospital between 2016 and 2017 were invited to participate in this study. The inclusion criteria were as follows: small- to large-sized tear (1-5 cm) based on the criteria established by DeOrio and Cofield 10 and symptom duration before surgery <12 months. The exclusion criteria included grade 3 or 4 fatty infiltration in rotator cuff muscles on MRI according to the Goutallier classification, 12 acromiohumeral interval <7 mm, previous trauma or surgery, glenohumeral arthritis, and labral tears. Serial follow-up evaluations were carried out at 3, 6, and 12 months after surgery.
Surgical Technique and Postoperative Rehabilitation
Surgery was performed arthroscopically with the patient under general anesthesia in the lateral decubitus position. A single sports medicine fellowship-trained orthopaedic surgeon (with 26 years of experience in shoulder surgery) performed all the surgical procedures. The arthroscope was inserted through the posterior portal into the subacromial space. Acromioplasty was performed if an osteophyte was detected at the acromion or the acromion was revealed to be hooked or curved. If necessary, tenotomy or tenodesis was performed (for patients aged >65 years, only tenotomy). The rotator cuff tear size was measured in the anteroposterior dimension; retraction in the mediolateral dimension was assessed according to the Patte classification. 27 The SR or SB technique was determined by a consensus between the patient’s preference after an explanation of the repair techniques and the surgeon's expertise. Double- or triple-loaded medial-row anchors were placed at the edge of the articular cartilage (4.5 mm in the medial row [Helix; DePuy Mitek] and 4.5 mm in the lateral row [PushLock; Arthrex]). Lateral- or single-row anchors were inserted at the lateral edge of the footprint on the greater tuberosity, ensuring that the tendon edge was at the lateral edge of the footprint with low tension.
After surgery, all patients followed a standard rehabilitation program. Immobilization was maintained with an abduction brace immediately after surgery for 6 weeks. From the day of surgery, slight passive exercises were performed, including pendulum, forward flexion, abduction, and external rotation. Active-assisted exercises were started at 6 weeks postoperatively, and muscle-strengthening exercises were introduced gradually. Then, 6 months later, all sports activities and daily labor were permitted.
Clinical Assessments
The Constant score, 9 American Shoulder and Elbow Surgeons (ASES) score, 22 and Fudan University Shoulder Score (FUSS) 11 were used to evaluate the patient’s perception of functional recovery. The FUSS was not included in the preoperative assessments because it consists of an evaluation of satisfaction regarding the treatment. The visual analog scale (VAS), ranging from 0 (no pain) to 10 (extreme pain), was administered to record the subjective pain felt by the patients during ordinary activities over a 24-hour period. Complications including infections, synovitis, and suture anchor problems were recorded if they occurred.
Magnetic Resonance Imaging
MRI was performed with a 3.0-T system (Discovery MR750; GE Healthcare). All patients were placed in the supine position with a sandbag set in the supinated palm. An 8-channel phased array shoulder coil was used. Special attention was paid to ensure that the shoulder was equally parallel to the direction of the B 0 field to minimize potential magic angle effects. 38 The MRI sequences and parameters are presented in Table 1.
Table 1.
MRI Sequences and Parameters a
| Sequence | Coronal Oblique PDW | Sagittal Oblique T1W | Axial PDW | Coronal Oblique UTE |
|---|---|---|---|---|
| Repetition time, ms | 2682 | 553 | 2578 | 85.6 |
| Echo time, ms | 42 | 1 | 42 | 0.07, 3.1, 6.8, 10.2 |
| Field of view, mm | 18 × 18 | 20 × 20 | 17 × 17 | 14 × 14 |
| No. of excitations | 2.00 | 2.00 | 2.00 | 1.00 |
| Slice thickness, mm | 4 | 4 | 4 | 2.4 |
| No. of slices | 16 | 16 | 16 | 12 |
| Scan time, min:s | 1:31 | 1:57 | 1:59 | 5:24 |
| Bandwidth, Hz/pixel | 31.25 | 31.25 | 31.25 | 62.50 |
a MRI, magnetic resonance imaging; PDW, proton density–weighted; T1W, T1-weighted; UTE, ultrashort echo time.
A radiologist with 8 years of experience in musculoskeletal radiology (R1, Y.X.), who was blinded to the distribution of participants, reviewed proton density–weighted fat-saturated MRI scans and rated the repair status as 1 of 5 types according to the Sugaya classification 31 : type 1 (integrated tendon that had sufficient thickness with homogeneously low intensity), type 2 (sufficient thickness associated with a partial high-intensity area), type 3 (insufficient thickness with up to a 50% reduction without discontinuity), type 4 (minor discontinuity in >1 slice), or type 5 (major full-thickness discontinuity). Types 1, 2, and 3 represent attached repair sites, and types 4 and 5 represent retears. The T2* value of the attached tendons was calculated by fitting the acquired signal to a monoexponential decay model using the Advantage Workstation (Adw 4.6; GE Healthcare). The repaired tendon was manually segmented into 2 regions, the proximal region and distal region according to a method described previously, 1,28 on coronal oblique UTE-T2* images (Figure 1) by another musculoskeletal radiologist (R2, H.T., with 10 years of experience in musculoskeletal radiology), who was blinded to the distribution of participants. The repaired tendon between the greater tuberosity and the articular surface of the humerus was divided into 3 equal parts: lateral (distal region); middle (proximal region); and medial (muscle region), which was not studied in the present investigation. To assess the interobserver reliability of T2* values, segmentation was performed by R1 in 10 randomly selected patients and was then repeated by R2. R1 repeated these segmentation procedures after 1 month to evaluate intraobserver reproducibility.
Figure 1.
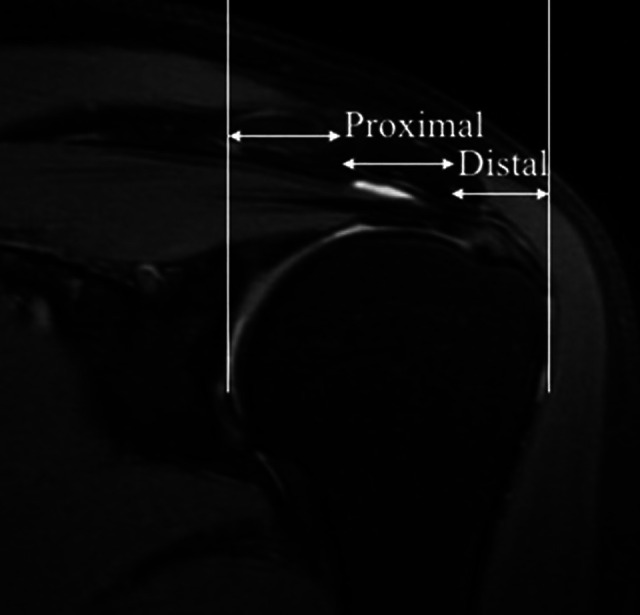
Representative coronal oblique proton density–weighted fat-saturated image of tendon segmentation. The white lines represent landmarks that were manually chosen at the greater tuberosity and the articular surface of the humerus to divide the tendon into 3 equal parts: lateral (distal region); middle (proximal region); and medial (muscle region), which was not studied in the present investigation.
Statistical Analysis
Data analysis was performed with R (Version 3.5.1; R Project for Statistical Computing), and data were presented as mean ± SD. The demographic data of the 2 groups were compared using the Student t test and the chi-square test. According to our prior calculated sample size (α = .05; power = 0.90), a minimum of 12 participants was required to detect a statistically significant difference in the T2* values. The differences in T2* values and clinical scores at the different time points (3, 6, and 12 months) were determined by 1-way analysis of variance for repeated measurements. The interobserver reliability and intraobserver reproducibility for T2* values were assessed with intraclass correlation coefficients (ICCs) using a 2-way random-effects model. 35 The ICCs were graded as follows: excellent reliability, 0.75 ≤ ICC ≤ 1.00; fair to good, 0.40 ≤ ICC < 0.75; and poor, 0.00 ≤ ICC < 0.40.
Results
Demographic and Surgical Characteristics
Patient demographics and surgical variables of the 2 groups are presented in Table 2. A total of 31 patients were included (17 in SB group and 14 in SR group). No one had complications throughout the follow-up period. Overall, no significant differences were observed regarding preoperative and surgical data between the groups (all P > .05) (Table 2). The total retear rate was 6.5% (2/31), with 1 in the SB group (Sugaya type 5) and 1 in the SR group (Sugaya type 4). Both retears were observed within 6 months postoperatively (one at 3 months and another at 6 months). Both patients refused to undergo revision surgery because the clinical outcomes were satisfactory. There were no significant differences regarding the healing pattern between the 2 groups according to the Sugaya classification (Table 2).
Table 2.
Patient and Surgical Characteristics a
| SB Group (n = 17) | SR Group (n = 14) | P Value | |
|---|---|---|---|
| Age at surgery, y | 55.1 ± 10.3 | 53.6 ± 11.1 | .58 |
| Body mass index | 22.2 ± 2.4 | 21.9 ± 3.2 | .34 |
| Sex, male/female | 5/12 | 6/8 | .68 |
| Smoking | 2 | 1 | >.99 |
| Fatty degeneration of supraspinatus, Goutallier grades 0/1/2 | 4/10/3 | 4/8/2 | .94 |
| Level of exercise, b high vs medium and low | 0/17 | 0/14 | >.99 |
| Surgery on dominant side, right/left | 10/7 | 9/5 | >.99 |
| Retraction, Patte stages 1/2/3 | 12/5/0 | 11/3/0 | >.99 |
| Previous treatment, medication/physical therapy/subacromial injection | 9/4/0 | 7/4/0 | .90 |
| Symptom duration before surgery, mo | 3.8 ± 2.4 | 4.1 ± 2.9 | .74 |
| Tear size in anteroposterior dimension, cm | 3.7 ± 1.9 | 3.2 ± 1.6 | .45 |
| Healing pattern at last follow-up, Sugaya types 1/2/3/4/5 | 4/5/7/0/1 | 2/7/4/1/0 | .47 |
a Data are presented as No. or mean ± SD. SB, suture-bridge; SR, single-row.
b Graded according to Chen et al. 4
Clinical Outcomes
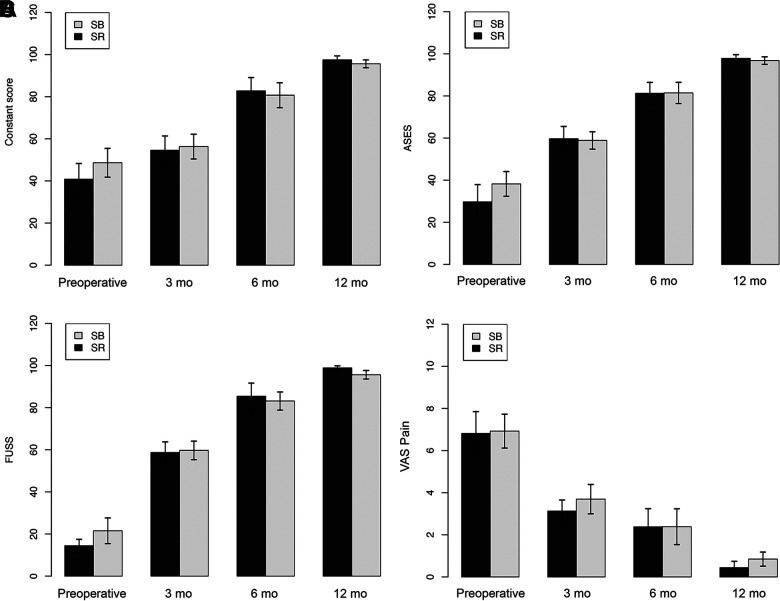
In both groups, there was a significant longitudinal improvement in functional scores from preoperatively (Constant, ASES, and FUSS) as well as VAS pain scores (all P < .001), with no significant differences between the groups at any follow-up time point (Figure 2). At the last follow-up, all patients (including 2 patients with retears) had excellent functional scores (Constant: 96.26 ± 4.77; ASES: 96.81 ± 4.47; FUSS: 96.74 ± 4.65) as well as a low VAS pain score (0.58 ± 0.67).
Figure 2.
Clinical outcomes over time between the suture-bridge (SB) and single-row (SR) groups. In both groups, the (A) Constant, (B) American Shoulder and Elbow Surgeons (ASES), (C) Fudan University Shoulder Score (FUSS), and (D) visual analog scale (VAS) pain scores markedly improved throughout the follow-up period (all P < .001), with no significant differences between the groups (all P > .05) at any time point.
T2* Values of Different Regions
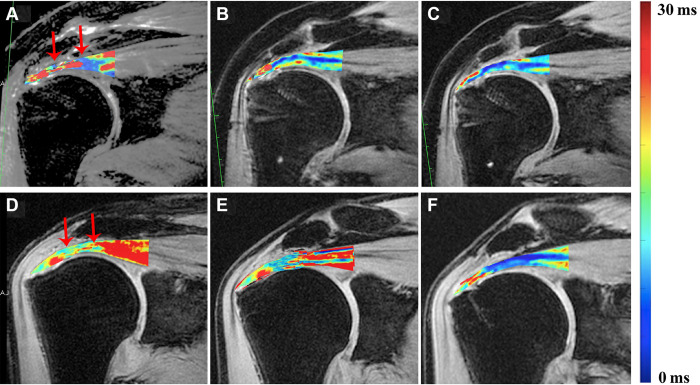
The ICC values of interobserver reliability and intraobserver reproducibility of T2* values were 0.90 and 0.82, respectively, indicating excellent reliability. Figure 3 displays longitudinal UTE-T2* maps of 2 representative patients at different time points from the SB and SR groups. The T2* values of the distal and proximal regions of attached tendons are compared between the 2 groups in Table 3 and Figure 4. In the SB group, the T2* values showed a tendency to decrease from 3 to 6 months (P = .38 for distal and P = .55 for proximal) and from 6 to 12 months (P = .04 for distal and P = .09 for proximal). Moreover, a significant decrease was observed between 3 and 12 months (P = .003 for distal and P = .02 for proximal). In the SR group, T2* values increased from 3 to 6 months (P = .13 for distal and P = .08 for proximal) and then decreased from 6 to 12 months (P = .006 for distal and P = .10 for proximal).
Figure 3.
Reformatted longitudinal ultrashort echo time–T2* images from (top row, A-C) a 52-year-old male patient from the suture-bridge group and (bottom row, D-F) a 49-year-old male patient from the single-row group at different follow-up time points: (A, D) 3 months, (B, E) 6 months, and (C, F) 12 months. The range of interest (ROI) of the distal region is directly over the medial-row anchor, and the ROI of the proximal region is directly over the humeral head. High T2* values are depicted as red and low T2* values as blue. The region between the red arrows mean the proximal region of repaired tendon.
Table 3.
T2* Values by Follow-up Time Point and Region a
| SB Group | SR Group | P Value | |
|---|---|---|---|
| 3 mo | |||
| Distal | 21.01 ± 4.66 | 18.06 ± 3.97 | .07 |
| Proximal | 17.79 ± 5.04 | 14.21 ± 3.19 | .03 |
| 6 mo | |||
| Distal | 19.53 ± 4.77 | 21.02 ± 5.38 | .44 |
| Proximal | 16.64 ± 5.75 | 17.48 ± 5.60 | .70 |
| 12 mo | |||
| Distal | 16.67 ± 2.78 | 15.71 ± 3.09 | .40 |
| Proximal | 13.60 ± 4.19 | 13.69 ± 5.72 | .97 |
a Data are presented as mean ± SD. Bolded P value indicates a statistically significant difference between groups (P < .05). SB, suture-bridge; SR, single-row.
Figure 4.
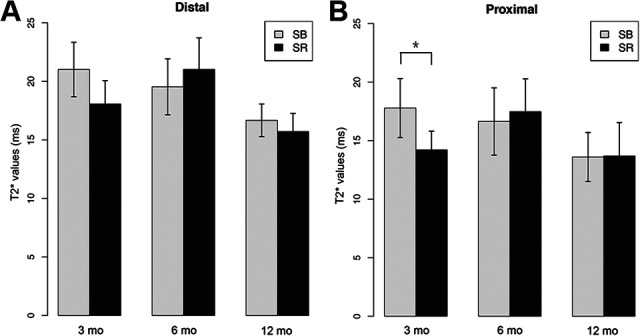
Comparison between the suture-bridge (SB) and single-row (SR) groups of mean longitudinal T2* values for the (A) distal and (B) proximal regions. Error bars represent SDs. *P < .05.
The SB group displayed significantly higher T2* values than the SR group (17.8 ± 5.0 vs 14.2 ± 3.2, respectively; P = .03) in the proximal region at 3 months (Figure 4). The differences became nonsignificant at subsequent time points for the proximal region. Moreover, no significant differences were detected for the distal region between the 2 groups throughout the follow-up period (Table 3 and Figure 4).
Discussion
The most important finding of this study is that in the early period (3 months) after surgery, higher T2* values at the proximal region of the repaired tendon were found in the SB group compared with the SR group (17.8 ± 5.0 vs 14.2 ± 3.2, respectively; P = .03), indicating inferior tendon quality at the musculotendinous junction with the SB technique.
In this study, the tendon was divided into distal and proximal regions as previously reported. 1,28 The distal region represents the healing site of the repaired tendon, whereas the proximal region indicates the tendon-muscle junction. Significant variations were observed in T2* values based on the anatomic region; lower values were obtained in the proximal region and higher values were registered in the distal region in the first follow-up year. It is well established that higher T2* values are associated with collagen fibers that are less organized and more water content based on studies using histological and biochemical reference standards and clinical results. 2,13,38 Therefore, the variations between anatomic regions were expected in the present study, as the distal region of the footprint underwent a healing process of inflammation and neovascularization, 18,30 consisting of more water content and less organized collagen fibers than that of the proximal region, thus resulting in higher T2* values.
An increased risk of retears at the proximal region instead of at the footprint has often been noticed and reported during the past decade with the SB technique. 6,7,14,15,19 This phenomenon reminded us of whether there were differences in the recovery pattern for the proximal region and distal region of the repaired tendon according to operative techniques and what the factors affecting the recovery pattern of arthroscopically repaired rotator cuff tears may be in the early follow-up period in our daily practice. In a study on medial rotator cuff failure after DR ARCR, Trantalis et al 33 considered that undue tension at the proximal region with the DR technique may have been the primary cause of the unusual failure pattern compared with the SR technique. They speculated that during DR surgery, surgeons generally aim for more medial suture tendon passage for a greater footprint coverage and a more robust biomechanical construct; this attempt leads to tendon compression at the proximal region instead of the distal region, which consequently weakens proximal tendon fibers more than the SR technique as reported before. 7,19 However, no evidence regarding in vivo anatomic-based tendon quality has been demonstrated on comparative analysis to date. In this regard, the higher T2* values at the proximal region that we observed in the SB group may provide direct evidence on differences regarding the retear pattern between the 2 techniques. Further research on the biomechanical characteristics of this anatomic-based pattern of tendon quality in rotator cuff healing with different repair techniques is necessary for detailed analysis; surgeons still need to be mindful of higher T2* values, which are associated with inferior tendon quality in the proximal region at early follow-up time points with the SB technique based on our pilot study. 7,19 A detailed understanding of the reasons that lead to differences in biochemical T2* values at the proximal region of the repaired rotator cuff may further contribute to improving the healing pattern.
At the subsequent follow-up time points of 6 and 12 months, no significant differences were observed for T2* values in both regions between the 2 techniques, indicating that tendon recovery tended to become similar after 6 months between the 2 techniques. This phenomenon was supported by the clinical outcomes, which were also similar between the 2 techniques as reported before. 37
In addition, UTE-T2* mapping was used to detect time-dependent changes in T2* values in order to monitor the healing process in the first follow-up year. With the SR technique, the T2* values increased from 3 to 6 months and decreased from 6 to 12 months in both regions. These results regarding T2* value–defined longitudinal tendon healing are consistent with those of a previous study. 37 After surgical repair of the rotator cuff, disorganized collagen scar tissue is produced in the early period, and then, the collagen fibers begin to rearrange over time. Similarly, the water content gradually decreases over time. Therefore, we can speculate that the repaired tendon did not gain recovery until 6 months, as shown by the increase in T2* values from 3 to 6 months, even if patients already experienced significant functional improvement and pain relief before 6-month follow-up with the SR technique. 5,21,36 On the other hand, with the SB technique, T2* values decreased over time in the first follow-up year. A laboratory study reported that the DR technique might promote short-term acute inflammation after surgery in contrast to the SR technique. 3 As previously mentioned, inflammation is a process associated with higher T2* values. 23,24,29 Therefore, we anticipated that the time-related decrease in T2* values revealed a recovery process of inflammation even from 3 months with the SB technique, indicating a faster healing response achieved by the SB technique than that by the SR technique as reported before. 26,34
Limitations
Certain limitations to this study need to be acknowledged. First, the sample size was relatively small. A larger sample size would strengthen our results about the clinical significance of our findings regarding higher T2* values at early follow-up in the SB group. Second, because satisfactory outcomes were obtained in most patients, they refused to return for clinical examinations, which prevented us from commenting on the longer term prognosis. Thus, the short duration of follow-up was another limitation. Third, we did not perform histological assessments and biomechanical measurements, which are the gold standards to document tendon healing after surgery. However, these are ethically problematic in human longitudinal clinical trials. Noninvasive imaging could provide a more global view of the structures involved and dynamic monitoring of the tendon-healing process. Finally, there may be selection bias because of patients’ decisions on repair techniques.
Conclusion
A significant improvement in clinical scores was seen in patients after ARCR. In the early postoperative period, higher T2* values in the proximal region of the repaired tendon (representing inferior tendon quality) were seen with the SB technique compared with the SR technique; however, this phenomenon was resolved over time.
Acknowledgment
The authors acknowledge Shiyi Chen and Jiwu Chen, from Huashan Hospital, Fudan University, for their help in interpreting the significance of the results in this study.
Footnotes
Final revision submitted August 19, 2022; accepted August 30, 2022.
One or more of the authors has declared the following potential conflict of interest or source of funding: This work was supported by the National Natural Science Foundation of China (grants 82102013 and 82171911). AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from Huashan Hospital (No. 2015M-010).
References
- 1. Anz AW, Lucas EP, Fitzcharles EK, et al. MRI T2 mapping of the asymptomatic supraspinatus tendon by age and imaging plane using clinically relevant subregions. Eur J Radiol. 2014;83(5):801–805. [DOI] [PubMed] [Google Scholar]
- 2. Ashir A, Ma Y, Jerban S, et al. Rotator cuff tendon assessment in symptomatic and control groups using quantitative MRI. J Magn Reson Imaging. 2020;52(3):864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baums MH, Schminke B, Posmyk A, et al. Effect of single- and double-row rotator cuff repair at the tendon-to-bone interface: preliminary results using an in vivo sheep model. Arch Orthop Trauma Surg. 2014;135(1):111–118. [DOI] [PubMed] [Google Scholar]
- 4. Chen Y, Jiang F, Li H, et al. Retears and concomitant functional impairments after rotator cuff repair: shoulder activity as a risk factor. Am J Sports Med. 2020;48(4):931–938. [DOI] [PubMed] [Google Scholar]
- 5. Chen Y, Li H, Qiao Y, et al. Double-row rotator cuff repairs lead to more intensive pain during the early postoperative period but have a lower risk of residual pain than single-row repairs. Knee Surg Sports Traumatol Arthrosc. 2019;27(10):3180–3187. [DOI] [PubMed] [Google Scholar]
- 6. Cho NS, Lee BG, Rhee YG. Arthroscopic rotator cuff repair using a suture bridge technique: is the repair integrity actually maintained? Am J Sports Med. 2011;39(10):2108–2116. [DOI] [PubMed] [Google Scholar]
- 7. Cho NS, Yi JW, Lee BG, Rhee YG. Retear patterns after arthroscopic rotator cuff repair: single-row versus suture bridge technique. Am J Sports Med. 2010;38(4):664–671. [DOI] [PubMed] [Google Scholar]
- 8. Cole BJ, ElAttrache NS, Anbari A. Arthroscopic rotator cuff repairs: an anatomic and biomechanical rationale for different suture-anchor repair configurations. Arthroscopy. 2007;23(6):662–669. [DOI] [PubMed] [Google Scholar]
- 9. Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clin Orthop Relat Res. 1987;214:160–164. [PubMed] [Google Scholar]
- 10. DeOrio JK, Cofield RH. Results of a second attempt at surgical repair of a failed initial rotator-cuff repair. J Bone Joint Surg Am. 1984;66(4):563–567. [PubMed] [Google Scholar]
- 11. Ge Y, Chen S, Chen J, Hua Y, Li Y. The development and evaluation of a new shoulder scoring system based on the view of patients and physicians: the Fudan University Shoulder Score. Arthroscopy. 2013;29(4):613–622. [DOI] [PubMed] [Google Scholar]
- 12. Goutallier D, Postel JM, Bernageau J, Lavau L, Voisin MC. Fatty muscle degeneration in cuff ruptures: pre- and postoperative evaluation by CT scan. Clin Orthop Relat Res. 1994;304:78–83. [PubMed] [Google Scholar]
- 13. Guo T, Ma YJ, High RA, et al. Assessment of an in vitro model of rotator cuff degeneration using quantitative magnetic resonance and ultrasound imaging with biochemical and histological correlation. Eur J Radiol. 2019;121:108706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hayashida K, Tanaka M, Koizumi K, Kakiuchi M. Characteristic retear patterns assessed by magnetic resonance imaging after arthroscopic double-row rotator cuff repair. Arthroscopy. 2012;28(4):458–464. [DOI] [PubMed] [Google Scholar]
- 15. Hein J, Reilly JM, Chae J, Maerz T, Anderson K. Retear rates after arthroscopic single-row, double-row, and suture bridge rotator cuff repair at a minimum of 1 year of imaging follow-up: a systematic review. Arthroscopy. 2015;31(11):2274–2281. [DOI] [PubMed] [Google Scholar]
- 16. Imam M, Sallam A, Ernstbrunner L, et al. Three-year functional outcome of transosseous-equivalent double-row vs. single-row repair of small and large rotator cuff tears: a double-blinded randomized controlled trial. J Shoulder Elbow Surg. 2020;29(10):2015–2026. [DOI] [PubMed] [Google Scholar]
- 17. Imam MA, Abdelkafy A. Outcomes following arthroscopic transosseous equivalent suture bridge double row rotator cuff repair: a prospective study and short-term results. SICOT J. 2016;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jensen PT, Lambertsen KL, Frich LH. Assembly, maturation, and degradation of the supraspinatus enthesis. J Shoulder Elbow Surg. 2018;27(4):739–750. [DOI] [PubMed] [Google Scholar]
- 19. Kim KC, Shin HD, Cha SM, Park JY. Comparisons of retear patterns for 3 arthroscopic rotator cuff repair methods. Am J Sports Med. 2014;42(3):558–565. [DOI] [PubMed] [Google Scholar]
- 20. Klouche S, Lefevre N, Herman S, Gerometta A, Bohu Y. Return to sport after rotator cuff tear repair: a systematic review and meta-analysis. Am J Sports Med. 2016;44(7):1877–1887. [DOI] [PubMed] [Google Scholar]
- 21. Liu S, Xie Y, Chen Q, et al. Tendon healing progression evaluated with magnetic resonance imaging signal intensity and its correlation with clinical outcomes within 1 year after rotator cuff repair with the suture-bridge technique. Am J Sports Med. 2020;48(3):697–705. [DOI] [PubMed] [Google Scholar]
- 22. Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons standardized shoulder assessment form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11(6):587–594. [DOI] [PubMed] [Google Scholar]
- 23. Moulin K, Viallon M, Romero W, et al. MRI of reperfused acute myocardial infarction edema: ADC quantification versus T1 and T2 mapping. Radiology. 2020;295(3):542–549. [DOI] [PubMed] [Google Scholar]
- 24. Nebelung S, Post M, Knobe M, et al. Detection of early-stage degeneration in human articular cartilage by multiparametric MR imaging mapping of tissue functionality. Sci Rep. 2019;9(1):5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Park MC, ElAttrache NS, Tibone JE, et al. Part I: footprint contact characteristics for a transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):461–468. [DOI] [PubMed] [Google Scholar]
- 26. Park MC, Tibone JE, ElAttrache NS, et al. Part II: biomechanical assessment for a footprint-restoring transosseous-equivalent rotator cuff repair technique compared with a double-row repair technique. J Shoulder Elbow Surg. 2007;16(4):469–476. [DOI] [PubMed] [Google Scholar]
- 27. Patte D. Classification of rotator cuff lesions. Clin Orthop Relat Res. 1990;254:81–86. [PubMed] [Google Scholar]
- 28. Pfalzer F, Huth J, Sturmer E, et al. Serial clinical and MRI examinations after arthroscopic rotator cuff reconstruction using double-row technique. Knee Surg Sports Traumatol Arthrosc. 2017;25(7):2174–2181. [DOI] [PubMed] [Google Scholar]
- 29. Qiao Y, Tao HY, Ma K, et al. UTE-T2* analysis of diseased and healthy Achilles tendons and correlation with clinical score: an in vivo preliminary study. Biomed Res Int. 2017;2017:2729807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shin MJ, Shim IK, Kim DM, et al. Engineered cell sheets for the effective delivery of adipose-derived stem cells for tendon-to-bone healing. Am J Sports Med. 2020;48(13):3347–3358. [DOI] [PubMed] [Google Scholar]
- 31. Sugaya H, Maeda K, Matsuki K, Moriishi J. Repair integrity and functional outcome after arthroscopic double-row rotator cuff repair: a prospective outcome study. J Bone Joint Surg Am. 2007;89(5):953–960. [DOI] [PubMed] [Google Scholar]
- 32. Tashjian RZ, Granger EK, Chalmers PN. Healing rates and functional outcomes after triple-loaded single-row versus transosseous-equivalent double-row rotator cuff tendon repair. Orthop J Sports Med. 2018;6(11):2325967118805365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Trantalis JN, Boorman RS, Pletsch K, Lo IK. Medial rotator cuff failure after arthroscopic double-row rotator cuff repair. Arthroscopy. 2008;24(6):727–731. [DOI] [PubMed] [Google Scholar]
- 34. Tuoheti Y, Itoi E, Yamamoto N, et al. Contact area, contact pressure, and pressure patterns of the tendon-bone interface after rotator cuff repair. Am J Sports Med. 2005;33(12):1869–1874. [DOI] [PubMed] [Google Scholar]
- 35. Weir JP. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J Strength Cond Res. 2005;19(1):231–240. [DOI] [PubMed] [Google Scholar]
- 36. Xie Y, Liu S, Qiao Y, et al. Quantitative T2 mapping-based tendon healing is related to the clinical outcomes during the first year after arthroscopic rotator cuff repair. Knee Surg Sports Traumatol Arthrosc. 2021;29(1):127–135. [DOI] [PubMed] [Google Scholar]
- 37. Xie Y, Liu S, Qu J, et al. Quantitative magnetic resonance imaging UTE-T2* mapping of tendon healing after arthroscopic rotator cuff repair: a longitudinal study. Am J Sports Med. 2020;48(11):2677–2685. [DOI] [PubMed] [Google Scholar]
- 38. Zhu Y, Cheng X, Ma Y, et al. Rotator cuff tendon assessment using magic-angle insensitive 3D ultrashort echo time cones magnetization transfer (UTE-Cones-MT) imaging and modeling with histological correlation. J Magn Reson Imaging. 2018;48(1):160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]