Abstract
Vascular endothelial growth factor (VEGF) is a pro-angiogenic factor that mediates the differentiation and function of vascular endothelial cells. VEGF has been implicated in modulating various pains. However, the effects of VEGF in Parkinson’s disease (PD)-related pain have not been studied. The goal of this study was to understand the effects of VEGF-expressing mesenchymal stem cells (MSCs) on PD-related pain and the involved mechanisms. We used two types of MSCs: hAMSC-Vector-GFP and hAMSC-VEGF189-GFP in PD mice. Then, the expression of VEGF and the viability have been compared between two types of MSCs. To demonstrate the therapeutic effect of hAMSC-VEGF189-GFP, we transplanted each cell line in a PD mouse model. Head mechanical withdrawal thresholds were examined. hAMSC-VEGF189-GFP was associated with significantly increased VEGF expression and slightly increased viability, compared with hAMSC-Vector-GFP. The transplanted hAMSC-VEGF189-GFP significantly improved mechanical allodynia and inhibited transient receptor potential vanilloid 1 (TRPV1) expression in site. And such pain relief effects could be partially blocked by TRPV1 agonist. However, we did not observe tumor generation or neuron degeneration in hAMSC-VEGF189-GFP-transplanted animals. Taken together, our data suggest that hAMSC-VEGF189-GFP is safely therapeutically appropriate for treating PD-related pain. VEGF inhibits TRPV1 expression, which may contribute to its analgesic properties.
Keywords: vascular endothelial growth factor, mesenchymal stem cells, transient receptor potential vanilloid 1, pain, Parkinson’s disease
Introduction
Pain is the most bothersome nonmotor symptom of Parkinson’s disease (PD), which is ranked after three motor symptoms1. It is estimated that around 40%–85% of PD patients suffer from pain, and the prevalence increases with disease progression2,3. Pain in PD is often associated with disease severity4, disease duration5, depression6, anxiety7, and suicide8. For these reasons, various therapies have been investigated, including analgesics, neurosurgical interventions, and stem cell therapy. However, pharmacotherapy and neurosurgical lesioning have disappointing efficacy and undesirable risk-benefit ratio for long-term therapy.
Recently, several studies have examined the therapeutic effect of stem cells on pain. Mesenchymal stem cells (MSCs) are suggested to be a promising candidate for biological delivery vehicles for the following theories: (1) MSCs can be transplanted safely and effectively; (2) MSCs can differentiate into multiple cell lineages; and (3) MSCs have a trophic factor releasing paracrine effects9. Some research works revealed that MSCs significantly improved angiogenesis in a variety of diseases in a paracrine manner10,11. Then, Bahlakeh et al.12 demonstrated that adipose-derived MSCs can be employed as neurotrophic release machines to restore neurogenesis in Alzheimer’s disease. We have used human adipose-derived MSCs (hAMSCs) as a delivery vehicle for the treatment of PD and glioma in our previous studies13,14. However, there are some limitations in such therapy, including a low survival rate of transplanted cells and difficulty in finding an efficient trophic factor.
Vascular endothelial growth factor A (VEGF-A) is a crucial regulator in angiogenesis that exerts a wide effect on the nerve system15–17. It has been shown to improve cell survival18, increase peripheral nerve density19, and ameliorate pain20. Targeting VEGF can decrease pain behaviors in a variety of animal models21,22. Meanwhile, some studies have reported on the therapeutic possibility of using VEGF-expressing stem cell for treatment of pain23,24. Thus, we investigated whether VEGF-expressing hAMSCs (hAMSC-VEGF) are effective for the treatment of hyperalgesia and a higher survival rate of MSCs in the PD mice model. We aimed to search for an effective strategy and gain further understanding of the pathological mechanics of pain in PD.
Materials and Methods
Cell-Based Delivery System
Following approval by the Huazhong Science and Technology University Institutional Review Board, early passaged primary hAMSCs (hAMSC 173) were obtained from patients undergoing neurosurgical procedures as described in our previous studies14,25. The primary hAMSCs were isolated using the collagenase digestion method (collagenase-A; Thermo Fisher, Carlsbad, CA, USA) as described before13. The cells were cultured in MSC complete media [MesenPRO RS basal media with one vial of MesenPRO RS growth supplement (Gibco), 1% Glutamax (Gibco), and 1% penicillin/streptomycin (Gibco)] and incubated at 37°C in a humidified atmosphere containing 5% CO2. Lentiviral vector–driven expression of VEGF (LV-VEGF-GFP) (Viraltherapy Technologies) was used to transduce the hAMSCs. VEGF expression was assessed by Western blot. All lentiviral (LV) constructs were packaged as LV vector in HEK 293 cells. After collection, the hAMSCs (hAMSC-Vector-GFP, hAMSC-Vector-GFP/Fluc, hAMSC-VEGF189-GFP, hAMSC-VEGF189-GFP/Fluc) were sorted by a Moflo cytometer (Beckman Coulter, Indianapolis, IN, USA).
MTT Assay
For the measurement of viability, passages 4–6 of 4 × 105 cells were seeded on 24-well plates. After 48 h, 20 μl of DMEM/F12 containing 10% 3-(4,5-dimenthylthiazol-2-yl)-2,5-diphenylterazolium bromide solution (5 mg/ml; Sigma) was added into each well for 5 h. After incubation, media was removed and formazan crystals were dissolved in dimethyl sulfoxide (Sigma). The absorbance of dissolved samples was measured at 570 nm.
Western Immunoblotting
The Western blot analysis was performed as described recently26. The hAMSCs were lysed in a radioimmunoprecipitation assay buffer (Sigma) with phenylmethylsulfonyl fluoride (PMSF) and the trigeminal subnucleus caudalis (Vc) area was lysed in a solubilization buffer [50 mM Tris HCl, pH 8.0, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, 1% NP-40, 0.5% deoxycholic acid, 0.1% sodium dodecyl sulfate (SDS), 1 mM Na3VO4, 1 U/ml aprotinin, 20 μg/ml leupetin, 20 μg/ml pepstatin A]. Protein samples were resolved by SDS-PAGE (polyacrylamide gel electrophoresis) and transferred to a nitrocellulose membrane. The following primary antibodies and dilutions were used: rabbit anti-VEGF (1:200, Abcam, ab46154), guinea pig anti-transient receptor potential vanilloid 1 (TRPV1) (1:1,000, Alomone labs, AGP-118), and mouse monoclonal anti-beta-actin (1:5,000, Abcam). The following secondary antibodies and dilutions were used: goat anti-rabbit horseradish peroxidase (HRP) [1:5,000, Thermo Fisher Scientific (TFS), 62-6120], goat anti-guinea pig HRP (1:10,000, TFS, A18769), and anti-mouse HRP (1:5,000, TFS, 62-6520). Densitometric analysis was performed using Image J software (National Institute of Health, USA).
Immunostaining
After treatment, the mice were anesthetized with 4% chloral hydrate and perfused with saline and 4% paraformaldehyde. Brains including Vc and trigeminal ganglia (TG) were removed and post-fixed overnight in 4% paraformaldehyde at 4°C. Transverse sections (20 μm) cut with a cryostat were incubated in 3% normal goat serum, followed by incubation with relevant primary antibodies: striatum (STR) and substantia nigra (SN) with rabbit anti-TH (tyrosine hydroxylase) antibody (1:1,000, abcam, ab112), Vc with guinea pig anti-TRPV1 (1:500, Alomone labs, AGP-118), mouse monoclonal anti-VEGF (1:50, Santa Cruz Biotechnology, SC-7269), rabbit anti-Vimentin (1:200, Sigma, SAB1305096), and mouse anti-Sm actin (1:100, Abcam, ab5694) overnight at 4°C. The sections were then incubated with species-specific secondary antibodies: goat anti-rabbit Alexa488 (1:1,000, Abcam, ab150077), goat anti-mouse Alexa488 (1:1,000, Abcam, ab150113), and goat anti–guinea pig Alexa594 secondary antibody (1:1,000, Abcam, ab150188) with 4′,6-diamidino-2-phenylindole (DAPI) nuclear counterstain (Vector Laboratories, China). Stained slides were examined with a bright-field microscope (Olympus). For TH+ neuron analysis, three sections randomly chosen from four mice per group were analyzed (12 pictures/group). The survival graft volume was measured with GFP/Fluc-stained sections, and the extent was noted by tracing the outlines of each section throughout the anteroposterior axis of the graft on those stained sections. The volume was calculated according to the Cavalieri principle using the following formula: volume = the sum of areas × the inverse of the sampling fraction × the section thickness. Macros were custom-developed in ImageJ to quantify dopaminergic neuron and graft volume.
In Vivo Bioluminescence Imaging of Transfected hAMSCs
To identify the safety of hAMSC-VEGF189 in the normal brain, 2 × 105 hAMSC-VEGF189-GFP/Fluc were stereotactically injected into the right Vc. Following injection, these animals were imaged using an in vivo imaging system (IVIS) for small animal (Perkin Elmer) at different time periods (1, 2, 3, 4, 5, and 6 weeks after injection). Then the mice brains were perfused and fully cryo-sectioned at a 20-μm thickness. The hAMSC-GFP/DAPI/α-smooth muscle actin (Sm-actin) and hAMSC-GFP/DAPI/Vimentin were used to stain and measure the effect in normal brain.
Animals
Male C57BL/6J mice (8–9 weeks of age) were used in accordance with the ethical guidelines set by Huazhong Science and Technology University Institutional Animal Care and Use Committees. Mice were grouped and kept under conditions of a 12-h light/dark cycle at an ambient temperature of 22°C. Food and water were available ad libitum.
Stereotaxic Surgery
Stereotaxic surgery was performed under anesthesia with 5% chloral hydrate (350 mg/kg, intraperitoneally) as described previously27. Using coordinates relative to the Bregma, stereotaxic injection of 6-hydroxidopamine (6-OHDA) [left STR; 1 μl at A/P +0.3 mm, M/L +2.2 mm, and D/V −3.0 mm and 1 μl at A/P +1.1 mm, M/L +1.7 mm, and D/V −2.9 mm; 3 μg/μl in saline containing 0.02% ascorbic acid; Sigma], hAMSC-VEGF (right Vc; A/P −7.8 mm, M/R +1.6 mm, D/V −4.5 mm; 2 × 105 in phosphate-buffered saline; 0.5 μl), and respective control was done according to the atlas of Paxinos and Watson, the TRPV1 agonist capsaicin (right Vc; A/P −7.8 mm, M/R +1.6 mm, D/V −4.5 mm; 1 μM; 0.5 μl).
Behavior Test
Our previous study demonstrated that 6-OHDA-induced semi PD mice model displayed thermal and mechanical pain hypersensitivity but not spontaneous pain28. So, we chose mechanical allodynia to demonstrate the effects of hAMSC-VEGF. Mice were allowed to acclimate for approximately 30 min before all behavior tests. d-Amphetamine (5 mg/kg, intraperitoneally) was used to monitor ipsilateral rotations which were counted for 1 h at 1 and 2 weeks post 6-OHDA, and 2 or 4 weeks post hAMSC-VEGF. The mechanical hyperalgesia was measured with a series of calibrated von Frey filaments. The von Frey filaments were applied to the skin near the center of the vibrissa pad. The mechanical pain threshold was quantified as EF50, the von Frey filament force (g) that produced a 50% frequency of the withdrawal responses of the head.
Statistical Analysis
All data are expressed as mean ± standard error of the mean. Data within the two groups were assessed by unpaired Student’s t test using the Instat 3.05 software package (GraphPad Software, San Diego, CA, USA). Animal behavior data were analyzed with two-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test. Statistical significance was defined as P < 0.05.
Results
VEGF Expression and Cell Viability
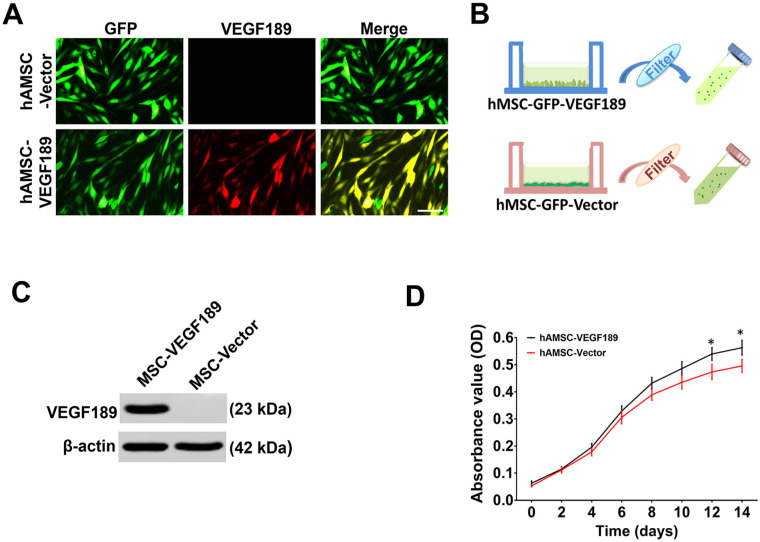
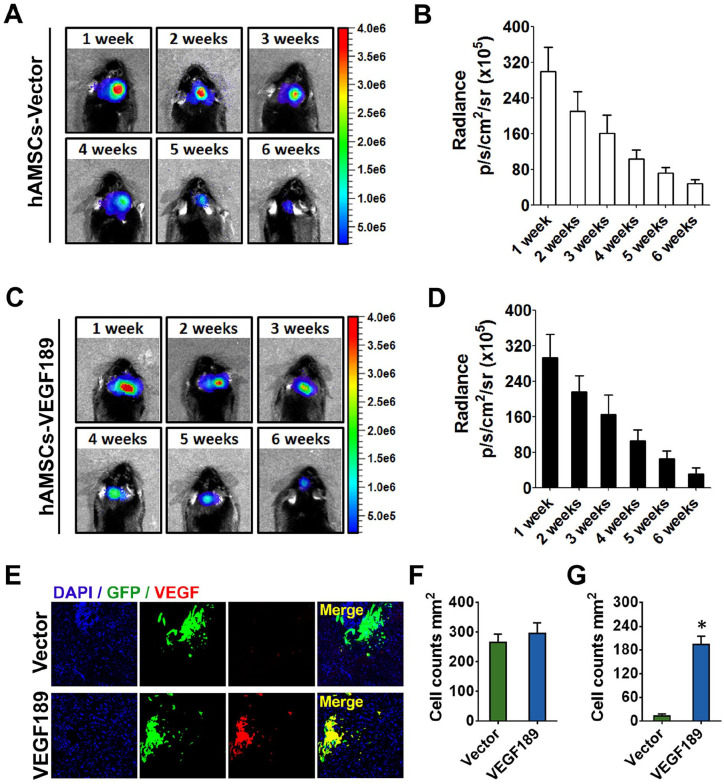
We measured VEGF expression level of hAMSC-Vector-GFP and hAMSC-VEGF189-GFP using immunocytochemical staining (Fig. 1A) and Western blotting (Fig. 1B, C). The expression of intracellular and extracellular VEGF levels was significantly elevated in hAMSC-VEGF189-GFP compared with hAMSC-Vector-GFP. To examine the viability of each cell line, we performed the MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. The viability of hAMSC-VEGF189-GFP was increased in comparison with hAMSC-Vector-GFP (Fig. 1D). It has been reported before that VEGF improves the cell proliferation and migration effects in vitro24. To investigate the effects in vivo, IVIS was carried out after hAMSC transplantation from 1 week to 6 weeks. The survival of transplanted hAMSCs remained at 6 weeks post-transplantation assessed by IVIS. There was no significant difference in grafted cell survival between hAMSC-VEGF189-GFP and hAMSC-Vector-GFP (Fig. 2A–D). Moreover, the expression of VEGF in hAMSC-VEGF189-GFP-transplanted mice is robustly increased when compared with that in hAMSC-Vector-GFP-transplanted mice (Fig. 2E–G).
Figure 1.
VEGF189 expression and cell viability of engineered hAMSCs. (A) Immunofluorescence staining was used to determine the VEGF expression in hAMSC-VEGF189 and hAMSC-Vector. Scale bar, 100 μm. (B) Schema showing the collection of the MSC-conditioned media (MSC-CM). (C) Western blots were performed to test the VEGF expression of hAMSC-VEGF189 and hAMSC-Vector. Cell lysate from the hAMSC-Vector served as a negative control. VEGF level in hAMSC-VEGF189 is significantly higher than in hAMSC-Vector. (D) MTT was performed to test the viability of hAMSC-VEGF189 and hAMSC-Vector. hAMSCs: human adipose-derived mesenchymal stem cells; VEGF: vascular endothelial growth factor; MSC: mesenchymal stem cell; MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide. *P < 0.05, significantly different from hAMSC-Vector. Unpaired t test analysis.
Figure 2.
The survival of engineered hAMSCs after transplantation. (A) Bioluminescence for the hAMSC-Vector-bearing mice was checked on weeks 1, 2, 3, 4, 5, and 6. (B) Bioluminescence radiance was maintained between week 1 and week 6 for hAMSC-Vector, whereas there was an obvious decrease in week 6. (C) Bioluminescence for the hAMSC-VEGF189-bearing mice was checked on weeks 1, 2, 3, 4, 5, and 6. (D) Bioluminescence radiance was maintained between week 1 and week 6 for hAMSC-VEGF189, whereas there was an obvious decrease in week 6. (E) Immunofluorescence staining was used to determine the VEGF expression after transplantation. Scale bar, 100 μm. (F) Number of hAMSC-VEGF189 and hAMSC-Vector in Vc at 2 weeks after transplantation. (G) Number of VEGF+ MSCs in Vc at 2 weeks after transplantation. n = 6 in each group. Data are presented as mean ± SEM. Unpaired t test and two-way analysis of variance with Tukey’s multiple comparisons test. hAMSCs: human adipose-derived mesenchymal stem cells; VEGF: vascular endothelial growth factor; MSCs: mesenchymal stem cells; SEM: standard error of the mean; DAPI: 4′,6-diamidino-2-phenylindole; Vc: subnucleus caudalis. *P < 0.05, significantly different from hAMSC-Vector.
Improvement of Mechanical Allodynia by VEGF
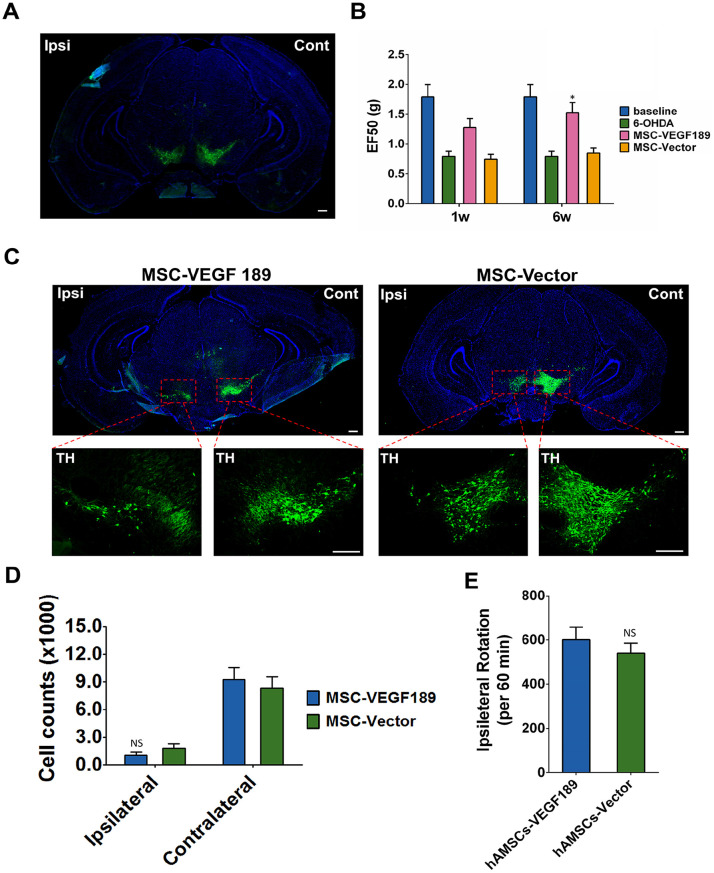
In this study, a unilateral 6-OHDA administration protocol was applied to generate semi PD mice model. We confirmed this PD model by degeneration of dopaminergic neuron seen in left SN (Fig. 3A). To confirm mechanical allodynia, we carried out the Von Frey test and measured the threshold force in unilateral 6-OHDA-lesioned mice (Fig. 3B). Consistent with our previous report29, unilateral 6-OHDA-lesioned mice display contralateral mechanical pain hypersensitivity. In the hAMSC-VEGF189-GFP transplant group, the threshold force was 1.791 ± 0.506 g before 6-OHDA injection, 0.792 ± 0.225 g after injection, 1.278 ± 0.371 g 1 week after hAMSC-VEGF189-GFP transplantation, and 1.523 ± 0.424 g 6 weeks after transplantation. This group showed a fast improvement in mechanical allodynia and returned to an almost normal threshold at 6 weeks post-transplant. However, in the hAMSC-Vector-GFP transplant group, the EF50s of the mice were 0.746 ± 0.200 g one week after transplantation and 0.847 ± 0.217 g 6 weeks after transplantation. Mechanical allodynia of this group slowly improved as 6 weeks post-transplant and the difference was not significant.
Figure 3.
hAMSC-VEGF189 alleviates hyperalgesia in 6-OHDA-lesioned mice without affecting dopaminergic neuron. (A) Visualization of loss of dopaminergic neuron in ipsilateral SN stained with TH after treatment with 6-OHDA. Scale bar, 200 μm. (B) The effects of hAMSC-VEGF189 on mechanical hyperalgesia induced by 6-OHDA at the orofacial region. *P < 0.05, significantly different from 6-OHDA without MSC. (C) Visualization of loss of dopaminergic neuron in ipsilateral SN stained with TH of 6-OHDA-lesioned mice after hAMSC-VEGF189 transplantation. Scale bar, 200 μm. (D) Number of TH+ neurons in the SN pars compacta. NS = P > 0.05, compared with hAMSC-Vector. (E) Ipsilateral rotations compared between hAMSC-VEGF189 and hAMSC-Vector groups. NS = P > 0.05, compared with hAMSC-Vector. n = 6 in each group. B, n = 6 in each group. Data are presented as mean ± standard error of the mean. Unpaired t test and two-way analysis of variance with Tukey’s multiple comparisons test. hAMSCs: human adipose-derived mesenchymal stem cells; SN: substantia nigra; TH: tyrosine hydroxylase; MSC: mesenchymal stem cell; hMSC: human mesenchymal stem cell; NS: not significant; 6-OHDA: 6-hydroxidopamine.
Effects of hAMSC-VEGF on Dopamine Neurons
To evaluate the effects of hAMSC-VEGF189-GFP on dopamine neurons in 6-OHDA-lesioned animals, we imaged SN sections of PD mice with treatment of hAMSC-VEGF189-GFP at 6 weeks after transplantation. Both in hAMSC-VEGF189-GFP and in hAMSC-Vector-GFP transplant groups, very few dopamine neurons exhibited TH expression in impaired lateral compared with unimpaired lateral (Fig. 3C). Meanwhile, there was no significant difference in the expression of TH in dopamine neurons between hAMSC-VEGF189-GFP and hAMSC-Vector-GFP transplant groups (Fig. 3D). Ipsilateral rotation was not relieved by either hAMSC-VEGF189-GFP or hAMSC-Vector-GFP transplantation (Fig. 3E).
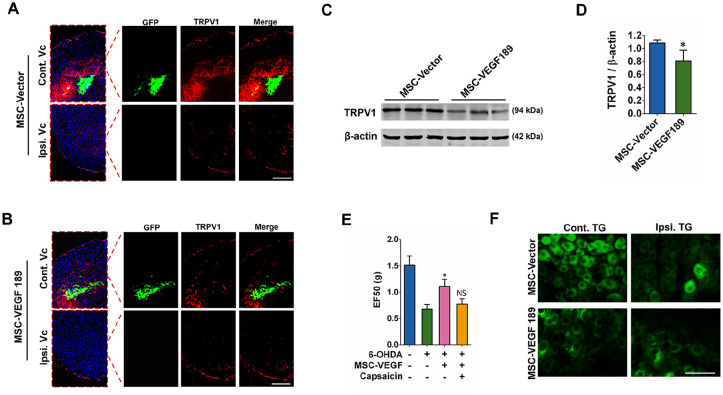
Effects of hAMSC-VEGF on TRPV1
Modulation of TRPV1 function and/or expression is fundamental to the development of hyperalgesia30,31. Central terminal sensitization influencing pain was evaluated by staining of TRPV1 at Vc (Fig. 4A, B). Strikingly, in the hAMSC-VEGF189-GFP transplant group, enhanced expression of TRPV1 in Vc was slightly reduced when compared with the hAMSC-Vector-GFP injected group (Fig. 4A, B). This effect was also confirmed by Western blotting (Fig. 4C, D). To determine the effect of TRPV1 in hAMSC-VEGF189-GFP-mediated pain relief, we microinject the TRPV1 agonist (capsaicin) with transplanted cells in Vc and perform Von Frey test 1 week after transplantation. Administration of locally applied hAMSC-VEGF189-GFP to Vc attenuated mechanical allodynia, which was partially blocked by coadministration of capsaicin (Fig. 4E). We then determined whether TRPV1 expression in sensory neurons was affected. Treatment with hAMSC-VEGF189-GFP in Vc also slightly reduced TRPV1 expression in TG neurons (Fig. 4F). These results indicate that hAMSC-VEGF189-GFP can reduce TRPV1 sensitization in central terminal and peripheral sensory neurons. Pharmacological activation of TRPV1 eliminated hAMSC-VEGF189-GFP-mediated pain relief, indicating that the mechanism of action of hAMSC-VEGF189-GFP involves, at least in part, downregulation of TRPV1.
Figure 4.
hAMSC-VEGF189 alleviates hyperalgesia in 6-OHDA-lesioned mice. (A) Vc slices from mice 6 weeks after MSC-Vector microinjection were stained with anti-TRPV1 (red) antibodies. Scale bar, 500 μm. (B) Visualization of downregulation of TRPV1 in Vc of 6-OHDA-lesioned mice after hMSC-VEGF189 microinjection in Vc. Scale bar, 500 μm. (C and D) A representative Western blot image using anti-TRPV1 antibody. *P < 0.05, significantly different from 6-OHDA with MSC-Vector. (E) The effects of capsaicin on mechanical hyperalgesia induced by 6-OHDA at the orofacial region. *P < 0.05, significantly different from 6-OHDA without MSC and capsaicin. NS = P > 0.05, compared with 6-OHDA with MSC, but without capsaicin. (F) TG slices from mice 6 weeks after MSC-Vector or hMSC-VEGF189 microinjection were stained with anti-TRPV1 (green) antibodies. Scale bar, 50 μm. D, n = 3 in each group. E, n = 6 in each group. Data are presented as mean ± standard error of the mean. Unpaired t test and two-way analysis of variance with Tukey’s multiple comparisons test. hAMSCs: human adipose-derived mesenchymal stem cells; MSC: mesenchymal stem cell; TRPV1: transient receptor potential vanilloid 1; NS: not significant; TG: trigeminal ganglia; VEGF: vascular endothelial growth factor; Vc: subnucleus caudalis; hMSC: human mesenchymal stem cell.
Tumor Formation by hAMSC-VEGF Transplantation
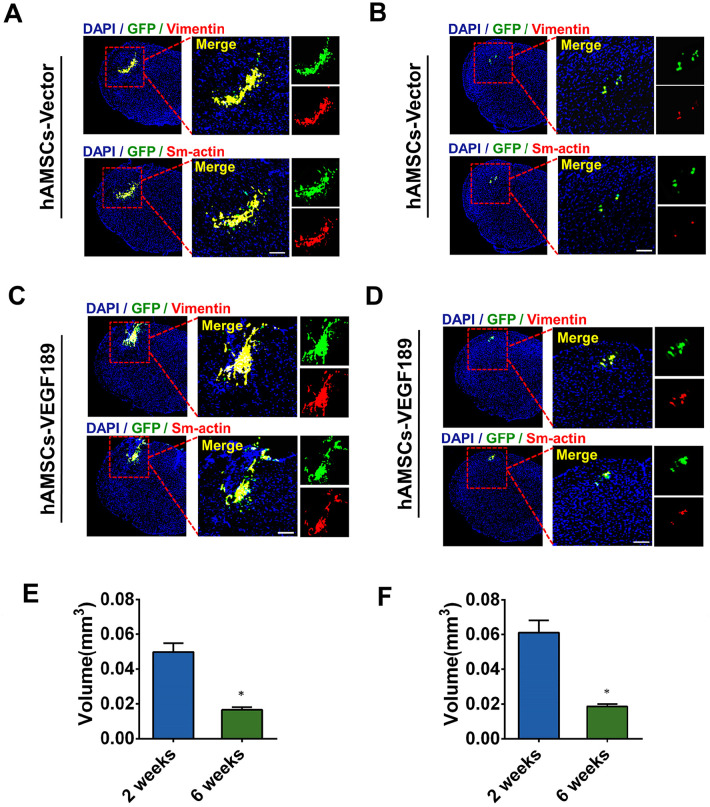
The tumor generation effect of VEGF32 or MSCs33 has been studied in some research works. To demonstrate the therapeutic effect of hAMSC-VEGF189-GFP on PD-related pain, we used transplanted cells in this study. So the tumor generation effect of hAMSC-VEGF189-GFP has to be investigated in this study. The immunofluorescence staining of vimentin and Sm-actin significantly decreased at 6 weeks after transplantation when compared with that at 2 weeks after transplantation, in both hAMSC-Vector-GFP (Fig. 5A, B) and hAMSC-VEGF189-GFP groups (Fig. 5C, D). The vimentin and Sm-actin always appear in fibroblastic cells, which have been used by a developing cancer34. The decrease in vimentin and Sm-actin is inconsistent with the character of developing cancer. Graft survival in both groups at 2 and 6 weeks after transplantation was calculated. Stereological measurement showed an average survival volume of 0.050 ± 0.005 mm3 at 2 weeks and 0.017 ± 0.001 mm3 at 6 weeks after transplantation in the hAMSC-Vector-GFP group (Fig. 5E). Compared with the hAMSC-Vector-GFP group, hAMSC-VEGF189-GFP had a slightly larger volume of surviving grafts, but the difference is not significant (0.061 ± 0.007 mm3 vs 0.050 ± 0.005 mm3 at 2 weeks; 0.019 ± 0.001 mm3 vs 0.017 ± 0.001 mm3 at 6 weeks; P > 0.05) (Fig. 5F).
Figure 5.
hAMSC-VEGF does not generate tumor in PD mice. (A and B) Visualization of Vimentin and Sm-actin expression at 2 weeks and 6 weeks after hAMSC-Vector microinjection in 6-OHDA-lesioned mice. Vimentin and Sm-actin expression significantly decreased at 6 weeks, compared with that at 2 weeks post-transplantation. Scale bar, 50 μm. (C and D) Visualization of Vimentin and Sm-actin expression at 2 and 6 weeks after hAMSC-VEGF189 microinjection in 6-OHDA-lesioned mice. Vimentin and Sm-actin expression significantly decreased at 6 weeks, compared with that at 2 weeks post-transplantation. Scale bar, 50 μm. (E) The graft volume was analyzed in the hAMSC-Vector microinjection group. *P < 0.05, significantly different from 2 weeks. (F) The graft volume was analyzed in the hAMSC-VEGF189 microinjection group. *P < 0.05, significantly different from 2 weeks. Data are presented as mean ± standard error of the mean. Unpaired t test analysis. hAMSCs: human adipose-derived mesenchymal stem cells; PD: Parkinson’s disease; DAPI: 4′, 6-diamidino-2-phenylindole; 6-OHDA: 6-hydroxidopamine.
Discussion
Several recent studies have shown that VEGF A plays an important role in pain modulation. Some of them revealed that VEGF A participates in peripheral sensitization via activation of TRPV1 in the dorsal root ganglion28. However, there are no relevant reports concerning whether VEGF A modulates PD-related pain and the central terminal sensitization of TRPV1 in the dorsal horn. In this study, we demonstrated that intra-Vc injection of VEGF189-expressing MSCs (hAMSC-VEGF189) could significantly relieve mechanical allodynia in PD mice (P = 0.012) at 6 weeks after transplantation, and the mechanism involves downregulation of TRPV1 in the dorsal horn. Notably, our current study excluded the possibility that MSC itself may also block TRPV1, thereby partially contributing to the alleviation of PD-related pain.
In this study, we used two types of stable human MSCs: hAMSC-VEGF189-GFP and hAMSC-Vector-GFP. We compared and analyzed intracellular and extracellular expression of VEGF189, cell proliferation, and cell migration. In the hAMSC-VEGF189-GFP group, extracellular VEGF189 expression and proliferation were higher than in the hAMSC-Vector-GFP group. The migration of hAMSC-VEGF189-GFP was not significantly different compared with the other ones. Such properties were similar with the VEGF-expressing neuron stem cells as reported24. The long-term fate of MSCs in the Vc of PD mice was investigated after intracellular injection of Fluc-labeled MSCs, which is a live imaging method that does not affect the characteristics or viability of the MSCs35. The fluorescent signals from naïve MSCs were observed for 6 weeks. Our retention period was longer than that reported in the study after intra-arterial injection (2 weeks)36 and was shorter than that reported in the study after intra-articular injection (10 weeks)35. The fluorescent signals of the hAMSC-VEGF189-GFP were observed until 6 weeks after injection. This result is consistent with previous studies using engineered MSC implantation for therapy37.
When we transplanted these stable MSC lines in a mouse model, hAMSC-VEGF189-GFP was shown to have the most impressive therapeutic effect, yielding improvement in pain reduction. Mechanical allodynia improvement was consistent at 6 weeks post-transplantation. Some researchers found that VEGF gene therapy has therapeutic possibility on neuropathic pain38–40. Others also demonstrated that transplantation with VEGF-expressing neural stem cells aids pain reduction in a sciatic nerve injury model23,24 and a spinal cord injury model41. Despite already knowing that MSCs have therapeutic effect in various types of pain, we could not confirm the therapeutic effect of MSCs in PD-related pain in this study42.
Although the mechanism of PD-related pain remains unclear, several studies have examined TRPV1 activation in a neuropathic pain model31,43,44 and even in a PD-related pain model29. The essential role of TRPV1 in the development of mechanical hyperalgesia is medicated by a direct sensitization of peripheral terminals and central terminals31. The control of TRPV1 may be crucial to VEGF-mediated pain relief. It is reported that VEGF189 can bind the receptor tyrosine kinases VEGFR1 and VEGFR2, and noncatalytic receptor neuropilin 1 (NRP1)45. Then the colocalization of VEGFR2 and TRPV1 has been found in DRG neurons20. Meanwhile, another study demonstrated that VEGF can block increases in TRPV1-evoked calcium responses in DRG neurons28. Our study also reports TRPV1 suppression by VEGF expression in Vc. All these findings agree on a crosstalk between VEGF and TRPV1 throughout the central terminal. Therefore, VEGF-expressing system prevents the development of mechanical hyperalgesia.
We did not induce dopaminergic neuron regeneration in this study, which is inconsistent with some research works. Some researchers reported that VEGF signaling may result in neuroprotective effects to enhance dopaminergic neuron survival directly in the striatum46–48. However, the effects of VEGF in Vc are not studied in PD models recently. Moreover, the studies investigate that the effects of MSCs in various disorders did not find migration of MSC in vivo after local injection by IVIS and immunostaining24,37. So the transplantation of hAMSC-VEGF189-GFP in Vc may not affect dopaminergic neuron in PD models.
Previously, several researchers attempted to use stem cell therapy to alleviate various pains. However, stem cell therapies often come with limitations, particularly low efficiency. In this research, we improved the efficiency of transplanted stem cells via induction of VEGF. We indeed demonstrated the therapeutic effect of VEGF-expressing MSCs in PD-related pain. And we found that downregulation of TRPV1 may participate in the VEGF-expressing MSC-mediated pain relief. However, our research has several limitations. First, the neuronal sensitization and microglial activation in the spinal dorsal horn have not been detected. Second, although we did not find that a signal dose of hAMSC-VEGF189 increased the risk of tumor formation at the site of gene delivery in PD mice, we did not confirm the presence or absence of tumor formation longer than 6 weeks.
Conclusion
MSCs cannot be used to treat PD-related pain solely. However, VEGF189 gene transfection may potentiate the therapeutic efficacy of MSCs for PD-related pain. In PD mice, hAMSC-VEGF189 showed better pain relief and TRPV1 suppression in Vc than conventional naïve MSCs. We conclude that VEGF189 gene transfection potentiates the therapeutic efficacy of MSCs for PD-related pain. Downregulation of TRPV1 in Vc may be involved in the mechanisms.
Footnotes
Author Contributions: Man Li: Helped in experimental design, experimental execution, and manuscript preparation. Ji Li: Helped in experimental execution. Hong Chen: Helped in experimental execution. Mingxin Zhu: Helped in experimental design, data analysis, and manuscript preparation.
Ethical Approval: This study was approved by the Ethics Committee of Huazhong University of Science and Technology (IRB ID: TJ20170201).
Statement of Human and Animal Rights: The experimental procedure was performed according to the animal care guidelines of the Institutional Animal Care and Use Committees (IRB ID: TJ20170201).
Statement of Informed Consent: Not applicable.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by National Natural Science Foundation of China (No. 82001193).
ORCID iD: Mingxin Zhu  https://orcid.org/0000-0002-0985-3857
https://orcid.org/0000-0002-0985-3857
References
- 1. Ha AD, Jankovic J. Pain in Parkinson’s disease. Mov Disord. 2012;27(4):485–91. [DOI] [PubMed] [Google Scholar]
- 2. Mostofi A, Morgante F, Edwards MJ, Brown P, Pereira EAC. Pain in Parkinson’s disease and the role of the subthalamic nucleus. Brain. 2021;144(5):1342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silverdale MA, Kobylecki C, Kass-Iliyya L, Martinez-Martin P, Lawton M, Cotterill S, Chaudhuri KR, Morris H, Baig F, Williams N, Hubbard L, et al. A detailed clinical study of pain in 1957 participants with early/moderate Parkinson’s disease. Parkinsonism Relat Disord. 2018;56:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zambito Marsala S, Tinazzi M, Vitaliani R, Recchia S, Fabris F, Marchini C, Fiaschi A, Moretto G, Giometto B, Macerollo A, Defazio G. Spontaneous pain, pain threshold, and pain tolerance in Parkinson’s disease. J Neurol. 2011;258(4):627–33. [DOI] [PubMed] [Google Scholar]
- 5. Lee MA, Walker RW, Hildreth TJ, Prentice WM. A survey of pain in idiopathic Parkinson’s disease. J Pain Symptom Manage. 2006;32(5):462–69. [DOI] [PubMed] [Google Scholar]
- 6. Ehrt U, Larsen JP, Aarsland D. Pain and its relationship to depression in Parkinson disease. Am J Geriatr Psychiatry. 2009;17(4):269–75. [DOI] [PubMed] [Google Scholar]
- 7. Hanagasi HA, Akat S, Gurvit H, Yazici J, Emre M. Pain is common in Parkinson’s disease. Clin Neurol Neurosurg. 2011;113(1):11–13. [DOI] [PubMed] [Google Scholar]
- 8. Shepard MD, Perepezko K, Broen MPG, Hinkle JT, Butala A, Mills KA, Nanavati J, Fischer NM, Nestadt P, Pontone G. Suicide in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2019;90:822–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gimble JM. Adipose tissue-derived therapeutics. Expert Opin Biol Ther. 2003;3(5):705–13. [DOI] [PubMed] [Google Scholar]
- 10. Mohammadi E, Nassiri SM, Rahbarghazi R, Siavashi V, Araghi A. Endothelial juxtaposition of distinct adult stem cells activates angiogenesis signaling molecules in endothelial cells. Cell Tissue Res. 2015;362(3):597–609. [DOI] [PubMed] [Google Scholar]
- 11. Rezaie J, Mehranjani MS, Rahbarghazi R, Shariatzadeh MA. Angiogenic and restorative abilities of human mesenchymal stem cells were reduced following treatment with serum from diabetes mellitus type 2 patients. J Cell Biochem. 2018;119(1): 524–35. [DOI] [PubMed] [Google Scholar]
- 12. Bahlakeh G, Rahbarghazi R, Abedelahi A, Sadigh-Eteghad S, Karimipour M. Neurotrophic factor-secreting cells restored endogenous hippocampal neurogenesis through the Wnt/beta-catenin signaling pathway in AD model mice. Stem Cell Res Ther. 2022;13(1):343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sun S, Zhang Q, Li M, Gao P, Huang K, Beejadhursing R, Jiang W, Lei T, Zhu M, Shu K. GDNF promotes survival and therapeutic efficacy of human adipose-derived mesenchymal stem cells in a mouse model of Parkinson’s disease. Cell Transplant. 2020;29:963689720908512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li M, Sun S, Dangelmajer S, Zhang Q, Wang J, Hu F, Dong F, Kahlert UD, Zhu M, Lei T. Exploiting tumor-intrinsic signals to induce mesenchymal stem cell-mediated suicide gene therapy to fight malignant glioma. Stem Cell Res Ther. 2019;10(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Riaz SK, Iqbal Y, Malik MF. Diagnostic and therapeutic implications of the vascular endothelial growth factor family in cancer. Asian Pac J Cancer Prev. 2015;16(5):1677–82. [DOI] [PubMed] [Google Scholar]
- 16. Liu S, Xu C, Li G, Liu H, Xie J, Tu G, Peng H, Qiu S, Liang S. Vatalanib decrease the positive interaction of VEGF receptor-2 and P2X2/3 receptor in chronic constriction injury rats. Neurochem Int. 2012;60(6):565–72. [DOI] [PubMed] [Google Scholar]
- 17. Selvaraj D, Gangadharan V, Michalski CW, Kurejova M, Stosser S, Srivastava K, Schweizerhof M, Waltenberger J, Ferrara N, Heppenstall P, Shibuya M, et al. A functional role for VEGFR1 expressed in peripheral sensory neurons in cancer pain. Cancer Cell. 2015;27(6):780–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brockington A, Lewis C, Wharton S, Shaw PJ. Vascular endothelial growth factor and the nervous system. Neuropathol Appl Neurobiol. 2004;30(5):427–46. [DOI] [PubMed] [Google Scholar]
- 19. Malykhina AP, Lei Q, Erickson CS, Epstein ML, Saban MR, Davis CA, Saban R. VEGF induces sensory and motor peripheral plasticity, alters bladder function, and promotes visceral sensitivity. BMC Physiol. 2012;12:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hulse RP, Beazley-Long N, Hua J, Kennedy H, Prager J, Bevan H, Qiu Y, Fernandes ES, Gammons MV, Ballmer-Hofer K, Gittenberger de, Groot AC, et al. Regulation of alternative VEGF-A mRNA splicing is a therapeutic target for analgesia. Neurobiol Dis. 2014;71:245–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hamilton JL, Nagao M, Levine BR, Chen D, Olsen BR, Im HJ. Targeting VEGF and its receptors for the treatment of osteoarthritis and associated pain. J Bone Miner Res. 2016;31(5):911–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qiu S, Shi C, Anbazhagan AN, Das V, Arora V, Kc R, Li X, O-Sullivan I, van Wijnen A, Chintharlapalli S, Gott-Velis G, et al. Absence of VEGFR-1/Flt-1 signaling pathway in mice results in insensitivity to discogenic low back pain in an established disc injury mouse model. J Cell Physiol. 2020;235(6): 5305–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee HL, Oh J, Yun Y, Lee HY, You Y, Che L, Lee M, Kim KN, Ha Y. Vascular endothelial growth factor-expressing neural stem cell for the treatment of neuropathic pain. Neuroreport. 2015;26(7):399–404. [DOI] [PubMed] [Google Scholar]
- 24. Lee H-L, Lee HY, Yun Y, Oh J, Che L, Lee M, Ha Y. Hypoxia-specific, VEGF-expressing neural stem cell therapy for safe and effective treatment of neuropathic pain. J Control Release. 2016;226:21–34. [DOI] [PubMed] [Google Scholar]
- 25. Li M, Zeng L, Liu S, Dangelmajer S, Kahlert UD, Huang H, Han Y, Chi X, Zhu M, Lei T. Transforming growth factor-beta promotes homing and therapeutic efficacy of human mesenchymal stem cells to glioblastoma. J Neuropathol Exp Neurol. 2019;78(4):315–25. [DOI] [PubMed] [Google Scholar]
- 26. Guo W, Miyoshi K, Dubner R, Gu M, Li M, Liu J, Yang J, Zou S, Ren K, Noguchi K, Wei F. Spinal 5-HT3 receptors mediate descending facilitation and contribute to behavioral hypersensitivity via a reciprocal neuron-glial signaling cascade. Mol Pain. 2014;10:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diago (CA): Academic Press; 2001. [Google Scholar]
- 28. Bestall SM, Hulse RP, Blackley Z, Swift M, Ved N, Paton K, Beazley-Long N, Bates DO, Donaldson LF. Sensory neuronal sensitisation occurs through HMGB-1-RAGE and TRPV1 in high-glucose conditions. J Cell Sci. 2018;131(14):jcs215939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li M, Zhu M, Xu Q, Ding F, Tian Y, Zhang M. Sensation of TRPV1 via 5-hydroxytryptamine signaling modulates pain hypersensitivity in a 6-hydroxydopamine induced mice model of Parkinson’s disease. Biochem Biophys Res Commun. 2020;521(4):868–73. [DOI] [PubMed] [Google Scholar]
- 30. Ristoiu V, Shibasaki K, Uchida K, Zhou Y, Ton BT, Flonta ML, Tominaga M. Hypoxia-induced sensitization of transient receptor potential vanilloid 1 involves activation of hypoxia-inducible factor-1 alpha and PKC. Pain. 2011;152(4):936–45. [DOI] [PubMed] [Google Scholar]
- 31. Kim YS, Chu Y, Han L, Li M, Li Z, LaVinka PC, Sun S, Tang Z, Park K, Caterina MJ, Ren K, et al. Central terminal sensitization of TRPV1 by descending serotonergic facilitation modulates chronic pain. Neuron. 2014;81(4):873–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee RJ, Springer ML, Blanco-Bose WE, Shaw R, Ursell PC, Blau HM. VEGF gene delivery to myocardium. Circulation. 2000;102(8):898–901. [DOI] [PubMed] [Google Scholar]
- 33. Galland S, Stamenkovic I. Mesenchymal stromal cells in cancer: a review of their immunomodulatory functions and dual effects on tumor progression. J Pathol. 2020;250(5):555–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wessels DJ, Pradhan N, Park YN, Klepitsch MA, Lusche DF, Daniels KJ, Conway KD, Voss ER, Hegde SV, Conway TP, Soll DR. Reciprocal signaling and direct physical interactions between fibroblasts and breast cancer cells in a 3D environment. PLoS ONE. 2019;14(6):e0218854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li M, Luo X, Lv X, Liu V, Zhao G, Zhang X, Cao W, Wang R, Wang W. In vivo human adipose-derived mesenchymal stem cell tracking after intra-articular delivery in a rat osteoarthritis model. Stem Cell Res Ther. 2016;7(1):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bai ZM, Deng XD, Li JD, Li DH, Cao H, Liu ZX, Zhang J. Arterially transplanted mesenchymal stem cells in a mouse reversible unilateral ureteral obstruction model: in vivo bioluminescence imaging and effects on renal fibrosis. Chin Med J (Engl). 2013;126(10):1890–94. [PubMed] [Google Scholar]
- 37. Song SY, Hong J, Go S, Lim S, Sohn HS, Kang M, Jung GJ, Yoon JK, Kang ML, Im GI, Kim BS. Interleukin-4 gene transfection and spheroid formation potentiate therapeutic efficacy of mesenchymal stem cells for osteoarthritis. Adv Healthc Mater. 2020;9(5):e1901612. [DOI] [PubMed] [Google Scholar]
- 38. Zor F, Deveci M, Kilic A, Ozdag MF, Kurt B, Sengezer M, Sönmez TT. Effect of VEGF gene therapy and hyaluronic acid film sheath on peripheral nerve regeneration. Microsurgery. 2014;34(3):209–16. [DOI] [PubMed] [Google Scholar]
- 39. Pereira Lopes FR, Martin PK, Frattini F, Biancalana A, Almeida FM, Tomaz MA, Melo PA, Borojevic R, Han SW, Martinez AM. Double gene therapy with granulocyte colony-stimulating factor and vascular endothelial growth factor acts synergistically to improve nerve regeneration and functional outcome after sciatic nerve injury in mice. Neuroscience. 2013;230:184–97. [DOI] [PubMed] [Google Scholar]
- 40. Pereira Lopes FR, Lisboa BC, Frattini F, Almeida FM, Tomaz MA, Matsumoto PK, Langone F, Lora S, Melo PA, Borojevic R, Han SW, et al. Enhancement of sciatic nerve regeneration after vascular endothelial growth factor (VEGF) gene therapy. Neuropathol Appl Neurobiol. 2011;37(6):600–12. [DOI] [PubMed] [Google Scholar]
- 41. Oh JS, An SS, Gwak S-J, Pennant WA, Kim KN, Yoon DH, Ha Y. Hypoxia-specific VEGF-expressing neural stem cells in spinal cord injury model. Neuroreport. 2012;23(3): 174–78. [DOI] [PubMed] [Google Scholar]
- 42. Matas J, Orrego M, Amenabar D, Infante C, Tapia-Limonchi R, Cadiz MI, Alcayaga-Miranda F, González PL, Muse E, Khoury M, Figueroa FE, et al. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: repeated MSC dosing is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Transl Med. 2019;8(3):215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marrone MC, Morabito A, Giustizieri M, Chiurchiu V, Leuti A, Mattioli M, Marinelli S, Riganti L, Lombardi M, Murana E, Totaro A, et al. TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nat Commun. 2017;8:15292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Su YS, Chiu YY, Lin SY, Chen CC, Sun WH. Serotonin receptor 2B mediates mechanical hyperalgesia by regulating transient receptor potential vanilloid 1. J Mol Neurosci. 2016;59(1):113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tillo M, Erskine L, Cariboni A, Fantin A, Joyce A, Denti L, Ruhrberg C. VEGF189 binds NRP1 and is sufficient for VEGF/NRP1-dependent neuronal patterning in the developing brain. Development. 2015;142(2):314–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pitzer MR, Sortwell CE, Daley BF, McGuire SO, Marchionini D, Fleming M, Collier TJ. Angiogenic and neurotrophic effects of vascular endothelial growth factor (VEGF165): studies of grafted and cultured embryonic ventral mesencephalic cells. Exp Neurol. 2003;182(2):435–45. [DOI] [PubMed] [Google Scholar]
- 47. Silverman WF, Krum JM, Mani N, Rosenstein JM. Vascular, glial and neuronal effects of vascular endothelial growth factor in mesencephalic explant cultures. Neuroscience. 1999;90(4): 1529–41. [DOI] [PubMed] [Google Scholar]
- 48. Yasuhara T, Shingo T, Kobayashi K, Takeuchi A, Yano A, Muraoka K, Matsui T, Miyoshi Y, Hamada H, Date I. Neuroprotective effects of vascular endothelial growth factor (VEGF) upon dopaminergic neurons in a rat model of Parkinson’s disease. Eur J Neurosci. 2004;19(6):1494–504. [DOI] [PubMed] [Google Scholar]