Abstract
Background
Infantile esotropia (IE) is the inward deviation of the eye. Various aspects of the clinical management of IE are unclear; mainly, the most effective type of intervention and the age at intervention.
Objectives
To examine the effectiveness and optimal timing of surgical and non‐surgical treatment options for IE to improve ocular alignment and achieve or allow the development of binocular single vision.
Search methods
We searched CENTRAL, MEDLINE, Embase, one other database, and three trials registers (November 2021). We did not use any date or language restrictions in the electronic searches for trials.
Selection criteria
We included randomized trials and quasi‐randomized trials comparing any surgical or non‐surgical intervention for IE.
Data collection and analysis
We used standard Cochrane methodology and graded the certainty of the body of evidence for six outcomes using the GRADE classification.
Main results
We included two studies with 234 children with IE. The first study enrolled 110 children (mean age 26.9 ± 14.5 months) with an onset of esotropia before six months of age, and large‐angle IE defined as esotropia of ≥ 40 prism diopters. It was conducted between 2015 and 2018 in a tertiary care hospital in South Africa. It compared a maximum of three botulinum toxin injections with surgical intervention of bimedial rectus muscle recession, and children were followed for six months. There were limitations in study design and implementation; the risk of bias was high, or we had some concerns for most domains.
Surgery may increase the incidence of treatment success, defined as orthophoria or residual esotropia of ≤ 10 prism diopters, compared with botulinum toxin injections, but the evidence was very uncertain (risk ratio (RR) of treatment success 1.88, 95% confidence interval (CI) 1.27 to 2.77; 1 study, 101 participants; very low‐certainty evidence). The results should be read with caution because 23 children with > 60 prism diopters at baseline in the surgery arm also received botulinum toxin at the time of surgery to augment the recessions. There was no evidence of an important difference between surgery and botulinum toxin injections for over‐correction (> 10 prism diopters) of deviation (RR 0.29, 95% CI 0.06 to 1.37; 1 study, 101 participants; very low‐certainty evidence), or additional interventions required (RR 0.66, 95% CI 0.36 to 1.19; 1 study, 101 participants; very low‐certainty evidence). No major complications of surgery were observed in the surgery arm, while children experienced various complications in the botulinum toxin arm, including partial transient ptosis in 9 (16.7%) children, transient vertical deviation in 3 (5.6%) children, and consecutive exotropia in 13 (24.1%) children. No other outcome data for our prespecified outcomes were reported.
The second study enrolled 124 children with onset of esotropia before one year of age in 12 university hospitals in Germany and the Netherlands. It compared bilateral recession with unilateral recession surgeries, and followed children for three months postoperatively. Very low‐certainty evidence suggested that there was no evidence of an important difference between bilateral and unilateral surgeries in the presence of binocular vision (numbers with event unclear, P = 0.35), and over‐correction (RR of having exotropia 1.09, 95% CI 0.45 to 2.63; 1 study, 118 participants). Dissociated vertical deviation, latent nystagmus, or both were observed in 8% to 21% of participants.
Authors' conclusions
Medial rectus recessions may increase the incidence of treatment success compared with botulinum toxin injections alone, but the evidence was very uncertain. No evidence of important difference was found between bilateral surgery and unilateral surgery.
Due to insufficient evidence, it was not possible to resolve the controversies regarding type of surgery, non‐surgical intervention, or age of intervention in this review. There is clearly a need to conduct good quality trials in these areas to improve the evidence base for the management of IE.
Plain language summary
Different treatments for an esotropia (eye turns inward) that occurs within the first six months of life
What is the aim of this review?
The purpose of this Cochrane Review was to find out whether any treatment (e.g. surgery or non‐surgery) is better than another to treat esotropia (eye turns inward) that occurs in children within the first six months of life. This condition is called infantile esotropia. We looked for all relevant studies to answer this question, and found two.
What was studied in the review? Infantile esotropia can affect the vision in the eye, the ability to use the two eyes together (binocularity), and can also be a cosmetic issue to the child or parents. Treatment includes surgical and non‐surgical treatments to reduce how much the eye turns in, and to improve binocularity. Binocularity is the ability to focus on an object with both eyes and only see a single image. This review looked at different treatments, and the timing of each treatment.
What are the main results of the review? We found two relevant studies that enrolled a total of 234 children. One study was from South Africa (110 children). The children received either surgery or botulinum toxin injections, and were followed in six months. Botulinum toxin is a toxin that is used in small amounts to relax muscles, including those in the eyes. This study found that surgery may achieve better good eye alignment, with minimal risk, compared with botulinum toxin injections. But we have very little confidence in the evidence, because we only had one study, with a small number of children, and the study was not well‐designed or conducted.
The other study enrolled 124 children in Germany and the Netherlands. It compared unilateral (i.e. one eye only) and bilateral (i.e. both eyes) surgery. They found that there was no evidence of an important difference in how much the eye turned in or how many children were able to use both eyes to focus on an object between these surgeries. But we have very little confidence in the evidence, because we only had one study, with a small number of children, and the study was not well‐designed or conducted.
Key messages This review does not resolve the controversy regarding the best type of surgery, the value of non‐surgical interventions, or the best age of treatment. It highlights a need for further research in this area.
How up‐to‐date is this review? Information specialist searched for studies that had been published up to 30 November 2021.
Summary of findings
Summary of findings 1. Surgery versus botulinum neurotoxin injections.
| Surgery compared with botulinum toxin injections in children with infantile esotropia | ||||||
|
Patient or population: children with infantile esotropia Settings: tertiary care, single center Intervention: surgery Comparison: botulinum toxin injections | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Botulinum toxin injections | Surgery | |||||
| Treatment success: improvement in the angle of strabismus at 6 months | 370 per 1000 | 696 per 1000 (470 to 1000) |
RR 1.88 (95%CI 1.27 to 2.77) |
101 (1) |
⊕⊝⊝⊝ Very lowa,b | 23 children (48.9%) in the surgery arm, who had > 60 prism diopters at baseline, also received botulinum toxin intraoperatively. |
| Presence and quality of binocularity at 6 months | No outcome data available for this outcome | ‐ | ‐ | ‐ | ||
| Adverse effects (severe, minor) in 6 months | See comments | 101 (1) |
⊕⊝⊝⊝ Very lowa,b | Reported in botulinum toxin arm: partial transient ptosis in 9 (16.7%) children, transient vertical deviation in 3 (5.6%) children, and consecutive exotropia in 13 (24.1%) children | ||
| *The assumed risk is based on the estimate (proportion of participants with the case) in the control group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for study limitations due to high risk of bias in the randomization process, deviations from intended interventions, and missing outcome data bDowngraded two levels for imprecision of results
Summary of findings 2. Bilateral recession versus unilateral recession‐resection.
| Bilateral recession compared with unilateral recession‐resection in children with infantile esotropia | ||||||
|
Patient or population: children with infantile esotropia Settings: 12 university hospitals Intervention: bilateral recession Comparison: unilateral recession‐resection | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Unilateral recession‐resection | Bilateral recession | |||||
| Treatment success: improvement in the angle of strabismus at 6 months | See comments | 118 (1) |
⊕⊝⊝⊝ Very lowa,b | Latent angle: at distance (MD −0.60 degree, 95% CI −2.17 to 0.97); at near (MD 0.20, 95% CI −1.41 to 1.81) at 3 months postoperatively |
||
| Presence and quality of binocularity at 6 months | See comments | Unknown (1) |
⊕⊝⊝⊝ Very lowa,b | Presence of binocular vision: 41.1% in the bilateral group and 35.7% in the unilateral group (P = 0.35) | ||
| Adverse effects (severe, minor) at 6 months | See comments | 118 (1) |
⊕⊝⊝⊝ Very lowa,b | Dissociated vertical deviation 10 cases (8.1%), latent nystagmus 26 cases (21.0%), a combination of both 19 cases (15.3%) | ||
| *The assumed risk is based on the estimate (proportion of participants with the case) in the control group. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level for study limitations, due to some concerns of risk of bias in randomization process and the reported result bDowngraded two levels for imprecision of results
Background
Strabismus (squint) is a misalignment of the eyes in which the visual axes deviate from bifoveal fixation (RCO 2012). It can be subdivided into esotropia (inward deviation), exotropia (outward deviation) or, less commonly, hypertropia (upward deviation), hypotropia (downward deviation) and cyclotropia (torsional deviations ‐ inwards, incyclotorsion or out outwards, excyclotorsion). This review specifically looks at infantile esotropia (IE). Other terms that have been used to describe this condition include congenital esotropia and essential infantile esotropia.
Strabismus is present in approximately 4% of children (Vaughan 1998). The reported incidence of infantile esotropia within the first six months of life varies between 0.1% (Nixon 1985) and 1% (Friedman 1980).
Description of the condition
Infantile esotropia (IE) is a large, constant, stable angle esotropia (the angle indicates the degree/size of the deviation), with an onset within the first six months of life (Kommerell 1988).
Features associated with IE include:
alternating esotropia (fixation can switch from one eye to the other);
cross‐fixation: taking advantage of the crossed position of the eyes so that the right eye is used to look towards the left and the left eye to look towards the right. This can be associated with the appearance of limited abduction (outward movement) of the other eye;
manifest‐latent nystagmus: oscillation of the eyes, which increases when either eye is covered;
asymmetry of optokinetic nystagmus: a following movement followed by a rapid fixation movement in the opposite direction. This can be demonstrated by using a rotating, striped drum;
over‐acting inferior obliques: one of six muscles that move each eye. This usually occurs bilaterally (in either eye) but can be asymmetric, leading to the development of a hypertropia in one or more positions of gaze (most prominent in adduction – inward movement of the eye);
dissociated vertical deviation (DVD): where either eye elevates, resulting in a hypertropia when the amount of light entering it is reduced (Calcutt 1993). This can also occur during a period of inattention or fatigue, and can contribute to the hypertropia in adduction, since the nose can act as an occluder to the image being viewed;
refractive error (focusing error) within normal limits;
suppression: resulting in absence of binocular single vision (BSV) – the simultaneous use of the two eyes to give a single 3‐dimensional image.
loss or reduction of stereopsis: a loss of depth perception related to loss of fusion with an ocular deviation.
Significant amblyopia (a reduction in vision) is rare (Ansons 2001). The main reason for presentation of children with infantile esotropia is usually parental awareness of unacceptable ocular misalignment.
Description of the intervention
Surgery to correct the esotropia involves adjusting the horizontally acting extraocular muscles, and can be divided into three types:
1. unilateral surgery: weakening, usually recession, of the medial rectus, which is responsible for pulling the eye in; and resection (strengthening) of the lateral rectus, which is responsible for pulling the eye out; 2. bilateral surgery: the medial rectus is weakened in both eyes; 3. three or more muscle surgery (horizontal): a combination of recessions and resections.
Surgical adjustment of the vertically acting muscles may also be undertaken to correct any significant hypertropia: 1. weakening of the inferior oblique muscle responsible for pulling the eye up in adduction; 2. weakening of the superior rectus, responsible for pulling the eye up in abduction, adduction, and in the primary (straight ahead) position.
The age at which surgery is performed can vary, and authors have used various terms to describe the timing of surgery. For example, 'ultra early' has been used to describe surgical intervention between four and six months (Helveston 1990), 'early' to describe surgery before the age of two years, and 'late' to describe surgery after the age of two.
The main form of non‐surgical management in IE is botulinum toxin. This drug comes from a bacterium called Clostridium botulinum, which produces toxins that can be used to block muscle contractions. The type of toxin most commonly used for injection into muscles is botulinum toxin A (Scott 1980). The toxin is injected into the medial recti to temporarily paralyze the muscles and weaken their action, allowing the antagonist muscles (the lateral recti) to act unopposed. When the paralytic effect wears off after several months, the alignment may be improved. Another non‐surgical treatment that has been proposed is vision therapy, also referred to as vision training or eye exercises. This is a set of individualized, non‐invasive, therapeutic procedures that are intended to improve the effects of various eye conditions, ranging from strabismic to non‐strabismic binocular vision disorders.
How the intervention might work
Treatment of infantile esotropia aims to improve ocular misalignment. However, another important issue to consider is whether such treatment facilitates and enhances the development of binocular vision. Therefore, there are two aims of intervention:
1. to align the visual axes; 2. to optimize the potential for binocularity. Many authorities believe that alignment to within 10 diopters of orthotropia (straight eyes) by two years of age offers the best prospect for the development of binocular vision (Ing 1980).
Management of the strabismus may be surgical, non‐surgical, or a combination of both. As with other forms of strabismus, it is important to treat any amblyopia or significant refractive error when they exist, but as stated, these are unusual findings in this condition.
Why it is important to do this review
Although a variety of treatment options and strategies are available, clinical guidelines regarding the most effective treatment or age for treatment in IE remain unclear. Therefore, the current evidence on these issues should be systematically evaluated to inform evidence‐based practices for ophthalmologists, orthoptists, optometrists, and children with IE (and their parents).
Objectives
To examine the effectiveness and optimal timing of surgical and non‐surgical treatment options for infantile esotropia to improve ocular alignment and achieve or allow the development of binocular single vision.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized and quasi‐randomized trials that met our inclusion criteria. We provided a narrative summary for relevant, non‐randomized studies in the Discussion.
Types of participants
Participants in the trials were children with infantile esotropia (IE). Participants could have received any treatment for refractive error and amblyopia, but we excluded studies in which participants received prior treatment (surgical or non‐surgical) for the IE. We considered studies in which only subsets of participants were relevant to this review if outcome data were separately reported for these participants, but did not identify such studies.
Types of interventions
We looked at both surgical and non‐surgical interventions. We examined the following comparisons:
-
Surgical
any surgical intervention versus observation alone;
any surgical intervention versus botulinum toxin;
unilateral versus bilateral surgery;
two‐muscle versus three‐ or four‐muscle surgery.
-
Non‐surgical
botulinum toxin versus observation alone;
botulinum pre‐surgical treatment versus surgery alone.
-
Mixed
vision therapy versus surgical intervention or nonsurgical intervention, such as botulinum toxin.
Types of outcome measures
We did not exclude studies solely because there were no outcome data available.
Critical outcomes
1. Proportion of participants with treatment success: improvement in the angle of strabismus
Angle at near (and distance if possible), measured by prism cover test, prism reflections, or synoptophore. Measures within 10 diopters (±) of orthotropia at six months follow‐up were considered to be treatment success.
2. Proportion of participants with binocular vision: presence and quality of binocularity
A wide range of tests exist to diagnose the presence and the quality of binocular vision; a measurement of stereoacuity is considered the 'gold standard'. Certain stereopsis exams may not be undertaken in a certain age group. In this review, all binocular vision test results at six months follow‐up were considered, but were graded into (1) stereoacuity tests; (2) motor fusion, and (3) simultaneous perception.
Other important outcomes
1. Proportion of participants with over‐correction (> 10 prism diopters) of deviation
(i.e. resultant exodeviation) at six months after treatment
2. Proportion of participants with under‐correction (< 10 prism diopters) of deviation
(i.e. residual esodeviation) at six months after treatment
3. Number of additional interventions required
during study period of six months
4. Quality of life measures
We documented any measures of participant or parent/guardian satisfaction or quality of life at six months after treatment.
5. Adverse effects (severe, minor)
We summarized the reported adverse effects of the various interventions, such as effects from surgery (e.g. anterior segment ischemia, conjunctival scarring, inflammation, etc.), or from botulinum toxin injections (e.g. spread of toxin to nearby muscles causing ptosis), and development of amblyopia. These were classified as major (requiring further intervention), and minor (requiring no further intervention). We reported adverse effects as described by study investigators at the longest follow‐up time point.
Follow‐up
We reported the outcome data at six months. When a study did not report outcomes at six months, we considered the outcomes closest to six months, or at the end of study period.
Search methods for identification of studies
Electronic searches
We did not use any date or language restrictions in the following electronic searches for trials.
Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 11) in the Cochrane Library (www.thecochranelibrary.com; searched 30 November 2021; Appendix 1);
MEDLINE Ovid, MEDLINE Ovid In‐Process and Other Non‐Indexed Citations, MEDLINE Ovid Daily, OLDMEDLINE Ovid (January 1950 to 30 November 2021; Appendix 2);
Embase Ovid (January 1980 to 30 November 2021; Appendix 3);
LILACS (Latin American and Caribbean Literature on Health Sciences; January 1982 to 30 November 2021; Appendix 4);
metaRegister of Controlled Trials (mRCT; www.controlled-trials.com; 30 November 2021; Appendix 5);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 30 November 2021; Appendix 6);
World Health Organization International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/search/en; searched 30 November 2021; Appendix 7).
Searching other resources
We searched the reference lists of the included studies. We did not contact individuals or organizations in this field for this review update, to inquire about potentially eligible studies.
Data collection and analysis
Selection of studies
Two review authors independently screened the titles and abstracts retrieved from the database searches, using the web‐based software, Covidence. We obtained full text copies of potentially relevant trials. Two review authors independently assessed the full‐text copies for eligibility according to the Criteria for considering studies for this review. We resolved disagreements by discussion at any stage. If a trial was incomplete, methodology was unclear, or the data were not published, we attempted to contact the trial investigators.
Data extraction and management
Details were extracted from studies on the following:
1. Methods: method of randomization, allocation concealment, masking, and losses to follow up 2. Participants: age, previous treatment, presence of co‐existing ocular disease 3. Interventions: technique used, length of follow‐up 4. Outcomes: difference between intended and actual ocular alignment; minimum six months; presence and quality of binocularity 5. Adverse events and quality of life measures
The two review authors independently extracted data for the primary and secondary outcomes onto paper data collections forms developed by Cochrane Eyes and Vision (CEV). Discrepancies were resolved by discussion. We contacted primary investigators for missing data. One author entered all data into RevMan Web (RevMan Web 2022). Another author independently reviewed the data, to check for inaccuracies.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias, using the RoB 2 tool, following the guidance in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022).
We considered the following domains of bias:
bias arising from the randomization process;
bias due to deviations from intended interventions;
bias due to missing outcome data;
bias in measurement of the outcome;
bias in selection of the reported result.
We judged the risk of bias for critical outcomes and adverse effects as three categories: low risk of bias, high risk of bias, or some concerns. We followed the recommended algorithms to reach an overall risk of bias assessment for each trial. We resolved any discrepancies between review authors by discussion. The consensus decisions for the signaling questions are available upon request.
Measures of treatment effect
We used the risk ratio for dichotomous data (e.g. proportion of participants who achieved alignment within 10 prism diopters of orthotropia), and planned to use mean difference, or standardized mean difference for continuous data when different but similar instruments were used.
Unit of analysis issues
We did not find any unit of analysis issue in this review update.
In the future updates, we will apply the following classification. Trials may randomize one or both eyes to the intervention or comparator. If participants are randomly allocated to treatment, but only one eye per participant is included in the trial, then there is no unit of analysis issue. In these cases, we plan to document how the eye was selected. If participants are randomly allocated to treatment, but both eyes are included and reported, we plan to analyze as clustered data, i.e. adjust for within‐person correlation. If the study is a within‐person study, i.e. one eye is randomly allocated to intervention and the other eye receives the comparator, then we plan to analyze as paired data. We will contact the trial investigators for further information if needed.
Dealing with missing data
We used available case analyses provided in the study.
In future updates, we will use imputed data if they are computed by the trial investigators using an appropriate method, but will not impute missing data ourselves. If ITT data are not available, we will undertake an available case analysis. This assumes that data are missing at random. We will assess whether this assumption is reasonable, by collecting data from each included trial on the number of participants excluded or lost to follow‐up, and reasons for loss to follow‐up by treatment group, if reported.
Assessment of heterogeneity
We examined the overall characteristics of the studies, in particular, the type of participants and types of interventions, to assess the extent to which the studies were similar enough to make combining study results sensible.
In the future updates, we will also examine the forest plots of study results to see how consistent the results of the studies are, in particular, we will consider the size and direction of effects.
Because we included only one study in each analysis, we did not calculate the I² statistic, which is the percentage of the variability in effect estimates due to heterogeneity, rather than sampling error (chance). We will interpret the I2 statistic as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
We will consider that the observed value of I2 depends on the (1) magnitude and direction of effects, and (2) strength of evidence for heterogeneity (e.g. P value from the Chi2 test, or a confidence interval for I2); uncertainty in the value of I2 is substantial when the number of studies is small (Higgins 2022).
Assessment of reporting biases
We assessed selective or incomplete outcome reporting by comparing the outcomes reported in the final report with those specified in the protocol or clinical register. See Assessment of risk of bias in included studies.
Since we did not perform any meta‐analyses, we did not construct funnel plots or consider tests for asymmetry for assessment of publication bias, according to Chapter 13 of the Cochrane Handbook (Page 2022).
Data synthesis
We did not conduct any meta‐analyses, but for future updates, we plan to undertake data analysis according to Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2022). We planned to summarize the data from studies collecting comparable outcome measures with similar follow‐up times.
We will pool data using a random‐effects model in RevMan Web 2022. If data are sparse (e.g. fewer than three small trials), we will use a fixed‐effect model, which will provide a more robust estimate of effect in the case of sparse data.
If there is inconsistency between individual study results, such that a pooled result may not be a good summary of the individual trial results (e.g. the effects are in different directions or I² > 75% and P < 0.1), we will not combine the data. Instead, we will present a narrative summary to describe the pattern of the individual study results (Deeks 2022).
If there is statistical heterogeneity, but all the effect estimates are in the same direction, such that a pooled estimate may seem to provide a good summary of the individual trial results, we may pool the data.
Subgroup analysis and investigation of heterogeneity
We conducted a subgroup analysis based on the degree of esotropia at baseline (e.g. ≤ 60 prism diopters versus > 60 prism diopters).
If there are sufficient trials in future updates, we will also compare the effect of treatment on critical outcomes in the following subgroups: early (6 to 12 months) versus late treatment (after 12 months).
Sensitivity analysis
We did not conduct sensitivity analyses on critical outcomes to examine the robustness of excluding the following studies because we only included one study in each analysis:
Studies at overall high risk of bias
Summary of findings and assessment of the certainty of the evidence
We prepared summary of findings tables for critical outcomes and safety outcomes (i.e. adverse effects). Two authors independently graded the overall certainty of the evidence for each outcome as one of four levels (high, moderate, low, or very low), using the GRADE classification (www.gradeworkinggroup.org/). We downgraded the certainty of the body of evidence if we identify any of the following issues.
High risk of bias among included studies
Indirectness of evidence
Unexplained heterogeneity or inconsistency of results
Imprecision of results (i.e. wide CIs)
High likelihood of publication bias
Results
Description of studies
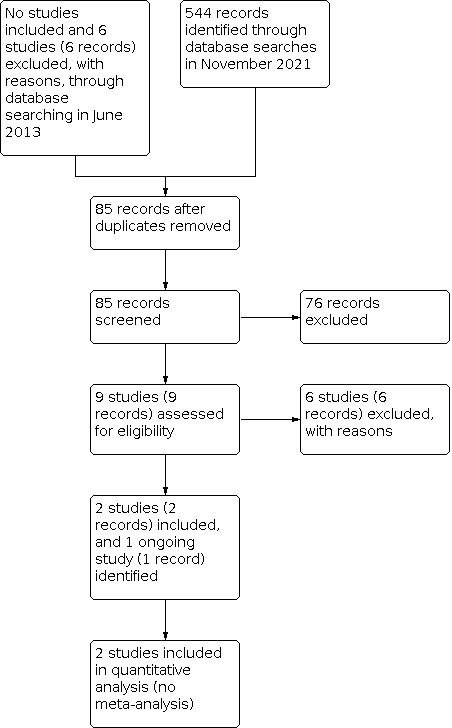
A study selection flow diagram is shown in Figure 1.
1.
PRISMA study flowchart
Results of the search
The detailed results of the original searches were described in the previously published version of this review (Elliott 2013). Briefly, the authors of the previous version of the review did not identify any relevant randomized controlled trials (RCT), and excluded six studies (six reports) after full‐text screening. The June 2013 database searches identified 970 records.
We updated the database searches on 30 November 2021. Of 544 records identified, the CEV Information Specialist removed 459 records because they were duplicates, or irrelevant to the scope of the review, yielding 85 unique records. We screened 85 records of titles and abstracts; nine of which we retrieved full‐text reports for further review. We identified two eligible studies (two records), listed one study (one record) as ongoing, and excluded the remaining six studies (six records). One ongoing study comparing botulinum toxin injection plus strabismus surgery with strabismus surgery alone started in 2018 and plans to enroll 140 participants with infantile esotropia (IE) in France and Switzerland (NCT03459092).
Included studies
We included two studies, in which a total of 234 children with IE were enrolled.
One RCT, conducted in 12 university hospitals in Germany and the Netherlands, compared bilateral recession with unilateral recession–resection in children (mean age 5.8 years) with an onset of esotropia before one year of age (Polling 2009). One hundred and twenty‐four children were enrolled over 48 months; 118 of whom were followed until three months after surgery. This study excluded children with an angle of strabismus larger than 24 degrees or smaller than 10 degrees; any binocular vision; and significant convergence excess, with an angle of strabismus at near fixation more than 1.5 times as large as the angle at distance fixation. The study was funded by the Netherlands Society for Prevention of Blindness, Haags Oogheelkundig Fonds, Stichting Blindenhulp and the Rotterdamse Vereniging Blindenbelangen.
The other included study was conducted between January 2015 and January 2018 in a tertiary care hospital in South Africa (Mayet 2021). One hundred and ten children (mean age 26.9 ± 14.5 months) with an onset of esotropia before six months of age, and large‐angle IE, defined as esotropia of ≥ 40 prism diopters, were assigned to receive either a maximum of three botulinum toxin injections, or surgical intervention of bimedial rectus muscle recession (Mayet 2021). Although study investigators described the study design as a RCT, it was a quasi‐randomized trial, as odd or even numbers were used for allocation. Fifty‐four (98.2%) participants in the botulinum toxin arm and 47 (85.5%) participants in the surgery arm completed the 24‐week follow‐up. Twenty‐one children in the botulinum toxin arm, who did not respond after three injections, were offered surgery after a stable angle measurement on two consecutive visits (one month apart). Twelve children in the surgical arm who did not respond after six weeks received botulinum toxin injections, and four of them were offered additional surgery. The surgery arm was further complicated by the inclusion of two subgroups; children with ≤ 60 prism diopters at baseline received bilateral medial rectus recessions alone, while children with > 60 prism diopters also received botulinum toxin at the time of surgery to augment the recessions. The study was funded by the Anglo‐American Chairman’s Fund.
The detailed information of these studies is shown in the Characteristics of included studies table.
Excluded studies
We excluded twelve studies after screening full‐text reports. We excluded five studies because they were not RCTs, and excluded one study in which participants acquired esotropia after one year of age. We excluded the remaining six studies because their intervention or comparator was irrelevant to this review. We provide the detailed reasons for exclusion in the Characteristics of excluded studies table.
Risk of bias in included studies
We assessed the following domains of risk of bias by using RoB 2 tool for the critical outcomes and safety outcome (i.e. adverse effects) presented in Table 1; Table 2.
Bias arising from the randomization process
Although baseline characteristics were comparable between intervention groups in Mayet 2021, the sequence to allocate the participants was generated by odd or even numbers. Thus, we judged this study at high risk of bias for this domain. Permuted block randomization was used to allocate participants, and participant characteristics were comparable at baseline in Polling 2009. The study did not explain how allocation sequence was concealed until participants were enrolled and assigned to interventions. We judged the study as having some concerns for this domain.
Bias due to deviations from intended interventions
Study personnel and participants could not be masked due to the nature of intervention in both studies. In one study (Polling 2009), there was no other deviation from intended intervention observed (low risk of bias). We judged Mayet 2021 as having some concerns for this domain because data analysis was restricted to children who were followed up for at least 24 weeks after the last procedure.
Bias due to missing outcome data
The outcome data for one randomized participant (1.8%) in the botulinum toxin arm, and eight randomized participants in the surgery arm (14.5%) were not included in the final analysis in Mayet 2021. The reasons for loss to follow‐up or exclusion were not fully described. We judged the study at high risk of bias for this domain. Outcome data were reported for nearly all children (95.2%) in Polling 2009; we judged this study at low risk of bias.
Bias in measurement of the outcome
Complete response (i.e. treatment success) was defined as orthophoria or residual esotropia of ≤ 10 prism diopters in Mayet 2021. Binocular vision was examined at near and distance fixation, using Bagolini striated glasses in Polling 2009. Although both articles failed to describe whether outcome assessors were masked, the outcome data were unlikely to be influenced by knowledge of intervention received. We judged these studies at low risk of bias for this domain.
Bias in selection of the reported result
Protocol, trial register, or prespecified analysis plan was not publicly available for either study. We judged both studies as having some concerns for this domain.
Effects of interventions
Surgery versus botulinum neurotoxin injections
Critical outcomes
Proportion of participants with treatment success: improvement in the angle of strabismus
One study that randomized 110 children (55 children each group) suggested that surgery may increase the incidence of treatment success compared with botulinum toxin injection, but the evidence was very uncertain (Mayet 2021). Thirty‐three (70.2%) children in the surgery arm and 20 (37%) children in the botulinum toxin arm achieved treatment success, defined as orthophoria or residual esotropia of ≤ 10 prism diopters (risk ratio (RR) of treatment success 1.88, 95% CI 1.27 to 2.77; 101 participants; Analysis 1.1). The results should be read with caution because 23 children (48.9%) in the surgery arm, who had > 60 prism diopters at baseline, also received botulinum toxin intraoperatively. We also undertook a subgroup analysis based on the degree of esotropia at baseline. In children with ≤ 60 prism diopters at baseline, the RR of treatment success comparing botulinum toxin with surgery alone was 1.42 (95% CI 0.89 to 2.25, 50 participants), indicating there was no evidence of an important difference between interventions. In children with > 60 prism diopters at baseline, botulinum toxin alone may increase the incidence of treatment success compared with surgery plus botulinum toxin (RR 2.78, 95% CI 1.39 to 5.58; 51 participants).
1.1. Analysis.

Comparison 1: Surgery versus botulinum toxin injections , Outcome 1: Treatment success: improvement in the angle of strabismus
Mayet 2021 also reported that of the 20 children who achieved complete success in the botulinum toxin arm, 11 (55%) children achieved this with one injection, 5 (25%) after the second injection, and 4 (20%) after the third injection.
We judged the certainty of evidence as very low for this outcome, downgrading one level due to high risk of bias, and two levels due to imprecision of results.
Proportion of participants with binocular vision: presence and quality of binocularity
The included studies did not measure this outcome.
Other important outcomes
Proportion of participants with over‐correction (> 10 prism diopters) of deviation
Of the 13 (24.1%) children who initially had an over‐correction with exotropia in the botulinum toxin arm, seven achieved complete success in Mayet 2021. Thus, six children had over‐correction at the end of follow‐up. Two children in the surgery arm had over‐correction. Surgery may have little to no effect on over‐correction compared with botulinum toxin injections (RR 0.29, 95% CI 0.06 to 1.37; 101 participants; Analysis 1.2).
1.2. Analysis.

Comparison 1: Surgery versus botulinum toxin injections , Outcome 2: Proportion of participants with over‐correction (> 10 prism diopters) of deviation
We judged the certainty of evidence as very low for this outcome, downgrading one level due to high risk of bias, and two levels due to imprecision of results.
Proportion of participants with under‐correction (< 10 prism diopters) of deviation
The included studies did not examine this outcome.
Number of additional interventions required
Mayet 2021 did not report the number of additional interventions required, but they reported the proportion of participants who underwent additional interventions.
In the surgery arm, 12 children received 3 U of botulinum toxin as a second procedure after surgery. Eight of the 12 children achieved complete response, while 4 children needed additional surgery. Twenty‐one children in the botulinum toxin arm underwent subsequent surgery. Eleven of the 21 children achieved treatment success, and 2 achieved partial success (i.e. residual esotropia > 10 prism diopters), at 24‐weeks post‐surgery. The risk ratio of having any additional interventions was 0.66 (95% CI 0.36 to 1.19; 101 participants; Analysis 1.3), suggesting there was no evidence of an important difference between two arms.
1.3. Analysis.

Comparison 1: Surgery versus botulinum toxin injections , Outcome 3: Proportion of participants who had additional interventions
We judged the certainty of evidence as very low for this outcome, downgrading one level due to high risk of bias, and two levels due to imprecision of results.
Quality of life measures
The included studies did not examine this outcome.
Adverse effects (severe, minor)
Mayet 2021 did not observe any cases of globe perforation, infections, or chemosis following botulinum toxin injections. Other complications in the botulinum toxin arm included partial transient ptosis in 9 (16.7%) children, transient vertical deviation in 3 (5.6%) children, and consecutive exotropia in 13 (24.1%) children. In the surgical arm, the study authors reported there were no major complications of surgery (e.g slipped or lost muscles, globe perforation, or anesthetic‐related issues).
We judged the certainty of evidence as very low for this outcome, downgrading one level due to high risk of bias, and two levels due to imprecision of results.
Bilateral recession versus unilateral recession‐resection
Critical outcomes
Proportion of participants with treatment success: improvement in the angle of strabismus
Polling 2009 randomized 118 children to either the bilateral group (60 children) or to the unilateral group (58 children). They did not report the proportion of children with treatment success. They reported the mean latent angle at a distance and near. There was no evidence of an important difference in latent angle at a distance (mean difference (MD) ‐0.60 degree, 95% CI ‐2.17 to 0.97, 118 participants), or near (MD 0.20, 95% CI ‐1.41 to 1.81; 118 participants) at three months postoperatively, between those receiving bilateral and unilateral surgeries (Analysis 2.1).
2.1. Analysis.

Comparison 2: Bilateral recession versus unilateral recession–resection, Outcome 1: Mean latent angle (degree)
We judged the certainty of evidence as very low for this outcome, downgrading one level due to high risk of bias, and two levels due to imprecision of results.
Proportion of participants with binocular vision: presence and quality of binocularity
Polling 2009 reported that they found postoperative binocular vision, measured with Bagolini striated glasses, in 41.1% of the bilateral recession group, and 35.7% of the unilateral recession‐resection group (numbers with the event were not explicitly reported; P = 0.35).
We judged the certainty of evidence as very low for this outcome, downgrading one level due to risk of bias, and two levels due to imprecision of results.
Other important outcomes
Proportion of participants with over‐correction (> 10 prism diopters) of deviation
In Polling 2009, nine children treated with bilateral recession, and eight children treated with unilateral recession‐resection experienced an exotropia three months postoperatively (RR 1.09, 95% CI 0.45 to 2.63; 118 participants; Analysis 2.2).
2.2. Analysis.

Comparison 2: Bilateral recession versus unilateral recession–resection, Outcome 2: Proportion of participants with over‐correction (> 10 prism diopters) of deviation
We judged the certainty of evidence as very low for this outcome, downgrading one level due to high risk of bias, and two levels due to imprecision of results.
Proportion of participants with under‐correction (< 10 prism diopters) of deviation
The included studies did not examine this outcome.
Number of additional interventions required
The included studies did not measure this outcome.
Quality of life measures
The included studies did not examine this outcome.
Adverse effects (severe, minor)
Polling 2009 reported that there was no difference in incomitance of the angle of strabismus between the two arms; traction and scarring of the conjunctiva overlying the resected lateral rectus muscle were not reported as complaints.
We judged the certainty of evidence as very low for this outcome, downgrading one level due to high risk of bias, and two levels due to imprecision of results.
Discussion
Summary of main results
In this review update, we identified two eligible studies, which enrolled 234 children with infantile esotropia (IE). One study compared botulinum toxin injections with bimedial rectus muscle recession, with a 24‐week post‐surgery follow‐up. Surgery may increase the incidence of treatment success compared with botulinum toxin injections, but the evidence was very uncertain, due to a small sample size and limitations of the study design and implementation. The evidence was also inconclusive for the risk of over‐correction and the need of additional surgery. No other outcome data for our prespecified outcomes were reported. There were no major complications in either arm, but children who were assigned to the botulinum toxin injection arm appeared to have more complications.
Another study compared bilateral recession with unilateral recession–resection, and followed children for three months postoperatively. There was no evidence of an important difference in latent angle at a distance or near, presence of binocular vision, or over‐correction. Concurrent dissociated vertical deviation, latent nystagmus, or both, were observed in 8% to 21% of the population in this study.
Overall completeness and applicability of evidence
The overall completeness and applicability of the evidence to address all the objectives in this review are low. We identified only two relevant studies for this review, with relatively small sample sizes. Therefore, the evidence found for this review update may not be generalizable regarding interventions for IE.
Quality of the evidence
We graded the certainty of the body of evidence for all reported outcomes as very low, due to high risk of bias and imprecision of results.
There were limitations in study design and implementation in the included studies. In Mayet 2021, we assessed two domains in RoB 2 at high risk of bias. The certainty of the body of evidence was downgraded for imprecision of results because we only included two studies with relatively small sample size in this systematic review.
Potential biases in the review process
We followed the standard processes required by Cochrane to minimize bias, and MECIR reporting standards in conducting this review update (editorial-unit.cochrane.org/mecir). An information specialist performed highly sensitive searches to identify studies. None of the review authors have any financial conflicts of interest.
Agreements and disagreements with other studies or reviews
The aims and outcomes of treatment are to improve ocular alignment and achieve some degree of binocularity. Most authors agree about the ocular alignment and aim for alignment within 10 prism diopters of orthotropia. However, claims of positive demonstrable binocularity after intervention vary widely. This is most likely due to the methods used to assess and confirm the presence of binocularity, and the fact that claims and definition of positive stereopsis and of binocular vision can vary. The early versus late infantile strabismus surgery (ELISS) study used the term 'gross' stereopsis or binocularity, and reported a variety of different tests to measure stereopsis (e.g. Bagolini lenses, Housefly, Titmus, Lang Test, TNO); very few participants in either arm of this trial actually achieved better than this level, i.e. good quality binocularity (Simonsz 2010). Polling's study defined positive binocularity by the presence of a Bagolini cross, i.e. the lowest grade of binocular vision possible. One included study in this review did not examine binocularity as an outcome.
A brief summary of findings from a selection of the current literature on each of the outcome measures is given below.
Surgical interventions
Authors have found that a constant esotropia of > 40 prism diopters in children aged two months to four months either did not spontaneously resolve (Birch 1998), or had a low likelihood of doing so (PEDIG 2002). In 1976, Arnoult and colleagues looked at two groups of children retrospectively; group 1 had all undergone bimedial recessions, group 2 had undergone unilateral surgery (resection of the lateral rectus and recession of the medial rectus [Arnoult 1976]). Although no statistical analysis was documented in this study, the authors concluded that the average postoperative angle of each group was found to be the same, which is in agreement with the findings in Polling 2009.
Although we did not include them in this review update due to different scopes of the review, we identified four randomized controlled trials (RCT) comparing different surgical techniques. Badawi 2014 compared bilateral medial rectus recession with bilateral Y‐split recession of medial recti muscles in 30 children with large‐angle IE. Ten children (67%) who underwent bilateral medial rectus recession, and eleven children (73%) who underwent Y‐splitting technique showed satisfactory results (i.e. orthotropic or residual angles ≤ 15 prism diopters) at the end of six‐month follow‐up. In another RCT, Badawi and colleagues compared Y‐split recession with Decker’s Faden techniques of medial rectus muscles in 50 children with IE (Badawi 2018). All children remained within the satisfactory range at six months. Sixty children with convergence excess esotropia or variable‐angle IE were randomly allocated to either augmented medial rectus muscle recession or medial rectus muscle pulley posterior fixation in Fouad 2016. The authors reported that more children (70%) in the pulley group had successful results than children (40%) in the augmented group (P = 0.037). The fourth RCT compared bimedial rectus muscle recession (7 mm to 8 mm) and bimedial rectus muscle elongation (6.5 mm to 9 mm) in 24 children with large‐angle IE (≥ 70 prism diopters [Ghali 2017]). The study concluded that bimedial rectus muscle elongation is more effective than bimedial rectus muscle recession to treat large‐angle IE, despite a higher level of technical difficulty.
Optometric clinical practice guidelines similarly state that surgical ocular alignment should be considered when the esotropia is large and nonaccommodative (AOA 2010). As such, most authors agree that some form of surgical intervention is necessary to treat IE.
Non‐surgical interventions
In 2010, a single‐center, prospective, non‐randomized study concluded that surgery was more successful than botulinum toxin in the treatment of large‐angle IE; however, in smaller‐angle esotropia (< 30 prism diopters to 35 prism diopters), it was found to be comparable to surgery (de Alba Campomanes 2010). Gursoy 2012 found that there was no difference in binocular alignment with botulinum toxin versus surgery, and proposed that botulinum toxin may be considered a primary treatment for IE.
Botulinum toxin has also been used in addition to surgery, i.e. to augment surgery (bimedial recessions) for large‐angle IE. Lueder 2008 suggested that augmentation with botulinum toxin may be more effective than bimedial recessions alone, especially in large‐angle esotropia (Lueder 2012), which agrees with our findings from Mayet 2021.
Ruiz 2004 retrospectively assessed the role of botulinum toxin prior to surgery. They found it was effective in reducing the amount of further horizontal surgery needed; however, in children under 18 months, injections of 5 U of botulinum toxin induced unbalanced dissociated vertical deviation.
In randomized trials, botulinum toxin has been shown to be a good alternative to surgery for re‐treatment of IE, since it is as successful as surgery in achieving binocularity (sensory and motor fusion, not stereopsis), if carried out within six months of surgery (Tejedor 1999).
Our comprehensive database searches did not identify any RCTs investigating other non‐surgical interventions for IE, including vision therapy. There is consensus surrounding the use of vergence exercises to treat symptomatic convergence insufficiency (Scheiman 2020). However, there is no substantive and comprehensive evidence for interventions for IE.
Age at intervention
The advantages and disadvantages of surgical intervention at an early (< two years of age), or late (> two years of age) stage have been debated in the literature. Some of these are highlighted below.
Early surgery
Advantages: better potential for binocularity, reduced muscle contraction
Disadvantages: may increase the risk of amblyopia, difficulty in obtaining reliable and accurate measurements
Late surgery
Advantages: amblyopia management easier, more reliable measurements
Disadvantages: reduced potential for binocularity, muscle contracture can lead to mechanical component to squint
Prospective cohort studies have found that surgical alignment is associated with better stereopsis (which is considered the gold standard in binocularity) in children who received treatment within the first 24 months of life (early [Birch 1995; Birch 1998]). Wright 1994 proposed even earlier surgery, between the age of 2.5 months and three months, which resulted in good binocularity, and is in agreement with Helveston's proposal of ultra early surgery between four months and six months.
In view of these reports of improved binocularity with surgery in children younger than six months of age, Ing 1995 performed a multi‐center study of the results of children with IE who were operated on at age six months or earlier. He included 16 children with IE, who had been surgically aligned at an average age of 4.2 months, all with a minimum of a four‐year follow‐up. He concluded that surgery by at least six months of age did not lead to a better quality of binocularity than in children who were aligned at age six months. He further concluded that binocularity remains an elusive target, and a rare outcome of treatment for IE.
The early versus late strabismus surgery study (ELISS) was a large, multi‐center, non‐randomized trial, which involved 58 clinics that recruited 231 children with IE to the early surgery group (6 months to 24 months), and 301 to the late group (32 months to 60 months [Simonsz 2005]). This study found that children operated on early had better gross stereopsis at age six compared to the late surgery group. However, this group was operated on more frequently, and there was no significant difference in the angle of the strabismus between the two groups. In 2010, the ELISS study published further results, concluding that the benefit of early surgery for gross binocular vision was balanced by a higher re‐operation rate, and surgery for an occasional child who would have had a spontaneous decrease without surgery (Simonsz 2010). Birch 2006 looked at the long‐term motor and sensory outcomes of children operated on by the age of six months, and concluded that early surgery was associated with a higher prevalence of fusion and stereopsis than surgery after this age. However, Polling 2009 included children who had late surgery (aged three years to eight years), and concluded that 38.4% of them had some degree of gross binocular vision postoperatively.
Gerth 2008 looked at the effects that the timing of surgery for IE had on cortical visual motion processing (by measuring visually evoked potentials), and concluded that early surgery (defined by the authors as at, or before 11 months of age) promoted the development of cortical visual motion processing compared to surgery after this age.
Studies have also looked at the effect of surgery on neuromotor development, as well as vision. Both Drover 2008 and Caputo 2007 conducted studies that looked at the influence of congenital strabismus and surgery on neuromotor development. Both studies concluded that strabismus surgery is beneficial for motor function and development. This is an area for further research.
These highlights show that the current literature on the timing of interventions is still conflicting.
Authors' conclusions
Implications for practice.
Since the previous review conducted in 2013, there have been two randomized controlled trials (RCTs) published on the topic of different surgical interventions, or surgical versus non‐surgical intervention for infantile esotropia (IE).
We found that there was no evidence of an important difference between bilateral surgery and unilateral surgery, but the evidence was very uncertain. While the results of Mayet 2021 support previous suggestions that bimedial rectus muscle recession is the surgical method of choice, lack of consensus remains regarding type of surgery, the role of non‐surgical options in certain populations, and the age of intervention in children with IE.
There seems to be general agreement that any intervention should be undertaken earlier rather than later, however, gross binocular vision still seems possible in those receiving late intervention.
Studies of non‐surgical interventions continue to be undertaken and published, mainly on the use of botulinum toxin. However, the current use of botulinum as a primary intervention for IE remains limited in comparison to surgical intervention, based on the findings in this review. Our searches did not identify any other types of non‐surgical interventions for IE.
Implications for research.
There is clearly a need for good quality trials to be conducted in various areas of IE, to improve the evidence base for the management of this condition. The trials need to be carefully planned, using standardized outcomes; an agreement is needed on what constitutes 'positive binocularity', and what is considered a 'success' in terms of surgical alignment. Ideally, quality of life measures would be incorporated into these trials.
What's new
| Date | Event | Description |
|---|---|---|
| 23 January 2023 | Amended | No new citation; Minor formating errors that do not impact the data or review findings or interpretation. |
History
Protocol first published: Issue 3, 2004 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 7 December 2021 | New citation required and conclusions have changed | One study newly included (Mayet 2021). Relevant sections have been updated. |
| 30 November 2021 | New search has been performed | Electronic searches were updated. |
| 4 July 2011 | New search has been performed | Issue 8 2011: Updated searches yielded no new trials. |
| 28 October 2008 | New search has been performed | Issue 1, 2009: updated searches yielded no new trials. Discussion section updated with new references. |
| 21 August 2008 | Amended | Converted to new review format. |
Risk of bias
Risk of bias for analysis 1.1 Treatment success: improvement in the angle of strabismus.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.1.1 Degree of esotropia ≤ 60 prism diopters at baseline | ||||||||||||
| Mayet 2021 | High risk of bias | Baseline characteristics were comparable between intervention groups, but the sequence to allocate the participants was generated by odd or even numbers. | Some concerns | The study was described as "unblinded study" in which study personnel and participants could not be masked due to the nature of intervention. Data analysis was restricted to children who were followed up for at least 24 weeks after the last procedure. | High risk of bias | The outcome data for one participant (1.8%) in the BNT arm and eight participants in the surgery arm (14.5%) were not included in the final analyses. The reasons of lost to follow‐up or exclusion were not fully described. | Low risk of bias | Complete response (i.e. treatment success) was defined as orthophoria or residual esotropia of ≤10 prism diopters. Although the article failed to describe if outcome assessors were masked, the outcome data should not be influenced by knowledge of intervention received. | Some concerns | Protocol, trial register, or prespecified analysis plan was not publicly available. The study was powered to detect differences in treatment success as planned and reported. Primary outcome of treatment success was consistently reported in Methods and Results. | High risk of bias | See the descriptions shown above. |
| Subgroup 1.1.2 Degree of esotropia > 60 prism diopters at baseline | ||||||||||||
| Mayet 2021 | High risk of bias | Baseline characteristics were comparable between intervention groups, but the sequence to allocate the participants was generated by odd or even numbers. | Some concerns | The study was described as "unblinded study" in which study personnel and participants could not be masked due to the nature of intervention. Data analysis was restricted to children who were followed up for at least 24 weeks after the last procedure. | High risk of bias | The outcome data for one participant (1.8%) in the BNT arm and eight participants in the surgery arm (14.5%) were not included in the final analyses. The reasons of lost to follow‐up or exclusion were not fully described. | Low risk of bias | Complete response (i.e. treatment success) was defined as orthophoria or residual esotropia of ≤10 prism diopters. Although the article failed to describe if outcome assessors were masked, the outcome data should not be influenced by knowledge of intervention received. | Some concerns | Protocol, trial register, or prespecified analysis plan was not publicly available. The study was powered to detect differences in treatment success as planned and reported. Primary outcome of treatment success was consistently reported in Methods and Results. | High risk of bias | See the descriptions shown above. |
Risk of bias for analysis 1.2 Proportion of participants with over‐correction (> 10 prism diopters) of deviation.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Mayet 2021 | High risk of bias | Baseline characteristics were comparable between intervention groups, but the sequence to allocate the participants was generated by odd or even numbers. | Some concerns | The study was described as "unblinded study" in which study personnel and participants could not be masked due to the nature of intervention. Data analysis was restricted to children who were followed up for at least 24 weeks after the last procedure. | High risk of bias | The outcome data for one participant (1.8%) in the BNT arm and eight participants in the surgery arm (14.5%) were not included in the final analyses. The reasons of lost to follow‐up or exclusion were not fully described. | Low risk of bias | Although the article failed to describe if outcome assessors were masked, the outcome data should not be influenced by knowledge of intervention received. | Some concerns | Protocol, trial register, or prespecified analysis plan was not publicly available. | High risk of bias | See the descriptions shown above. |
Risk of bias for analysis 1.3 Proportion of participants who had additional interventions.
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Mayet 2021 | High risk of bias | Baseline characteristics were comparable between intervention groups, but the sequence to allocate the participants was generated by odd or even numbers. | Some concerns | The study was described as "unblinded study" in which study personnel and participants could not be masked due to the nature of intervention. Data analysis was restricted to children who were followed up for at least 24 weeks after the last procedure. | High risk of bias | The outcome data for one participant (1.8%) in the BNT arm and eight participants in the surgery arm (14.5%) were not included in the final analyses. The reasons of lost to follow‐up or exclusion were not fully described. | Low risk of bias | Although the article failed to describe if outcome assessors were masked, the outcome data should not be influenced by knowledge of intervention received. | Some concerns | Protocol, trial register, or prespecified analysis plan was not publicly available. | High risk of bias | See the descriptions shown above. |
Acknowledgements
We thank Iris Gordon, Information Specialist for Cochrane Eyes and Vision (CEV), who created and executed the electronic search strategies. We also thank CEV for support and guidance in preparation of this review. We are grateful to Lorraine Cassidy for her input in to the protocol. We thank Carrie MacEwen, Catey Bunce, Suzanne Brodney‐Folse and Roberta Scherer for their peer review comments on the previous version of this review and protocol. Finally, we thank Sarah Hatt and Anupa Shah for their assistance throughout the review process.
We would also like to thank the following peer reviewers for their comments: for the review manuscript: Susan Cotter (Marshall B. Ketchum University) and Jasleen Jhajj (Nova Southeastern University).
This protocol review update was managed by CEV@US and was signed off for publication by Tianjing Li and Gianni Virgili.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Esotropia] explode all trees #2 esotrop* #3 convergen* near strabism* #4 internal near strabism* #5 #1 or #2 or #3 or #4
Appendix 2. MEDLINE OvidSP search strategy
1 randomized controlled trial.pt. 2 (randomized or randomised).ab,ti. 3 placebo.ab,ti. 4 dt.fs. 5 randomly.ab,ti. 6 trial.ab,ti. 7 groups.ab,ti. 8 or/1‐7 9 exp animals/ 10 exp humans/ 11 9 not (9 and 10) 12 8 not 11 13 exp esotropia/ 14 esotrop$.tw. 15 (strabism$ adj3 convergen$).tw. 16 (strabism$ adj3 internal).tw. 17 or/13‐16 18 12 and 17
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville et al (Glanville 2006).
Appendix 3. Embase OvidSP search strategy
1 exp randomized controlled trial/ 2 exp randomization/ 3 exp double blind procedure/ 4 exp single blind procedure/ 5 random$.tw. 6 or/1‐5 7 (animal or animal experiment).sh. 8 human.sh. 9 7 and 8 10 7 not 9 11 6 not 10 12 exp clinical trial/ 13 (clin$ adj3 trial$).tw. 14 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15 exp placebo/ 16 placebo$.tw. 17 random$.tw. 18 exp experimental design/ 19 exp crossover procedure/ 20 exp control group/ 21 exp latin square design/ 22 or/12‐21 23 22 not 10 24 23 not 11 25 exp comparative study/ 26 exp evaluation/ 27 exp prospective study/ 28 (control$ or propspectiv$ or volunteer$).tw. 29 or/25‐28 30 29 not 10 31 30 not (11 or 23) 32 11 or 24 or 31 33 exp convergent‐strabismus/ 34 esotrop$.tw. 35 (strabism$ adj3 convergen$).tw. 36 (strabism$ adj3 internal).tw. 37 or/33‐36 38 32 and 37
Appendix 4. LILACS search strategy
infantile or congenital and esotrop$ or converge$ or internal and strabism$
Appendix 5. metaRegister of Controlled Trials search strategy
esotropia
Appendix 6. ClinicalTrials.gov search strategy
esotropia
Appendix 7. ICTRP search strategy
esotropia
Data and analyses
Comparison 1. Surgery versus botulinum toxin injections .
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Treatment success: improvement in the angle of strabismus | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.27, 2.77] |
| 1.1.1 Degree of esotropia ≤ 60 prism diopters at baseline | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.89, 2.25] |
| 1.1.2 Degree of esotropia > 60 prism diopters at baseline | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.78 [1.39, 5.58] |
| 1.2 Proportion of participants with over‐correction (> 10 prism diopters) of deviation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.3 Proportion of participants who had additional interventions | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2. Bilateral recession versus unilateral recession–resection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Mean latent angle (degree) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.2 Proportion of participants with over‐correction (> 10 prism diopters) of deviation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Mayet 2021.
| Study characteristics | |
| Methods |
Study design: parallel‐group randomized controlled trial Number randomized (total and per group): 110 participants in total, 55 participants each group Unit of randomization (individual or eye): individual Number analyzed (total and per group): 54 eyes in the botulinum toxin arm and 47 eyes in the surgery arm (101 participants) Unit of analysis (individual or eye): individual Exclusions and losses to follow‐up (total and per group): 9 participants (1 in the botulinum toxin arm and 8 in the surgery arm) were not included in the analysis; only children who were followed for at least 24 weeks after the last procedure were included How were missing data handled?: excluded from analysis Length of follow‐up: at least 24 weeks Reported power calculation (Y/N), if yes, sample size and power: a sample size of 98 (49 per arm) was calculated using a Pearson Chi‐squared test with the proportion of success set at 0.65 for the surgery arm and 0.37 for the BNT arm at a power of 80% and an alpha level of 0.05. |
| Participants |
Country: South Africa Setting: tertiary hospital Baseline characteristics: 1. Botulinum toxin injections N = 54
2. Surgical intervention N = 47
Overall N = 101
Inclusion criteria: children with onset of esotropia before 6 months of age; with large‐angle IE, defined as esotropia of ≥ 40 PD, between the ages of 6 months and 6 years at baseline; informed written parental consent Exclusion criteria: children with significant pattern deviation, neurological impairment and hyperopia of ± 5.00 prism diopters (PD) Baseline equivalence: comparable |
| Interventions |
Intervention‐botulinum toxin injections: "Botulinum toxin (BotoxTM Allergan) was injected in each medial rectus muscle, administered subconjunctively after the muscle was grasped using forceps as described by Benabent 2002. All children received an initial dose of 5 units (U) that was repeated, for a maximum of three injections if the esotropia was > 10 PD at visits at 3, 6, 12, or 24 weeks following the last injection. The dosage at subsequent visits depended on the degree of esotropia, 5 U for deviations ≥ 40 PD and 3 U for deviations < 40 PD." Comparator‐surgery: "In the surgical arm, children underwent standard bilateral medial rectus muscle recession surgery for esotropia ≤ 60 PD. The medial recti were recessed to a maximum of 7 mm with 3 U of botulinum toxin given intraoperatively to each recessed muscle in cases > 60 PD to augment the recessions." |
| Outcomes |
Outcomes reported: complete response defined as orthophoria or residual esotropia of ≤ 10 PD; partial response as residual esotropia of > 10 PD and ≤ 20 PD and deemed acceptable by the parents; failures or non‐response as > 20 PD Adverse outcomes: "Complications in the botulinum toxin arm were partial transient ptosis in 9 children (16.7%), which resolved within 6 to 8 weeks, transient vertical deviation in 3 children (5.6%), and consecutive exotropia in 13 children (24.1%). Seven of the children with exotropia were associated with complete response. There were no cases of globe perforation, infections, or chemosis following botulinum toxin injections." "The two children with exotropia received botulinum toxin to the lateral rectus muscles as initial therapy, followed by bimedial rectus muscle advancement, and had final non‐response. In total, 45 children (95.7%) had a complete response or partial response in the surgery arm. There were no major complications of surgery, such as slipped or lost muscles, globe perforation, or anesthetic‐related issues." Measurement time points: 3, 6, 12, and 24 weeks Other issues with outcome assessment (e.g. quality control for outcomes, if any): none |
| Notes |
Study period: from January 2015 to January 2018 Publication language: English Trial registration: not found Conflicts of interest: "The authors declare that they have no conflict of interest. " Funding source: "the Anglo‐American Chairman’s Fund for grant in facilitating additional surgical lists for the study" |
Polling 2009.
| Study characteristics | |
| Methods |
Study design: parallel‐group randomized controlled trial
Number randomized (total and per group): 124 participants in total (3 participants refused, 1 failed randomization process, and 1 withdrawn by surgeon), resulting in 60 randomized to bilateral group and 59 randomized to unilateral group Unit of randomization (individual or eye): individual Number analyzed (total and per group): 118 participants in total; 60 in bilateral group and 58 randomized to unilateral group Unit of analysis (individual or eye): individual Exclusions and losses to follow‐up (total and per group): 3 participants refused after randomization, 1 failed randomization process, 1 withdrawn by surgeon, 1 participant in unilateral group lost to follow‐up How were missing data handled?: excluded from analysis Length of follow‐up: 3 months Reported power calculation (Y/N), if yes, sample size and power: assuming normal distributions of postoperative angles, a clinically relevant reduction in reoperation of 30% would correspond with a reduction in standard deviation (SD) of 1.8 degrees. The resulting F statistic would be 1.93 (assuming an SD of 5 degree in both groups on average). Inclusion of 120 participants would give the study 80% power at alpha = 0.05 to detect such an F ratio. |
| Participants |
Country: Germany and the Netherlands Setting: university hospital (23 sites) Baseline characteristics: 1. Bilateral recession N = 62
2. Unilateral recession N = 59
Overall N = 121
Inclusion criteria: children aged 3 to 8 years with a normal psychophysical development and onset of esotropia before age 1 year Exclusion criteria: previous strabismus surgery, an angle of strabismus larger than 24 degrees or smaller than 10 degrees, any binocular vision, more than one line logMAR acuity difference between the two eyes, hypermetropia ≥ 6 diopters or myopia ≥ 3 diopters, up‐ or downshoot in (25 degree) adduction > 8 degree, V‐pattern (esotropia measured in 25 degree up‐ and downgaze) > 8 degree, A‐pattern > 5 degree and manifest vertical strabismus > 4 degree; cases with significant convergence excess with an angle of strabismus at near fixation more than 1.5 times as large as the angle at distance fixation Baseline equivalence: comparable |
| Interventions |
Intervention: bilateral recession (BR) Comparator: unilateral recession‐resection (RR) The total relocation of the two muscles in millimeters for both was calculated as follows: the preoperative latent angle of strabismus at distance (in degrees) divided by 1.6. The preoperative latent angle of strabismus measured the day before surgery at distance fixation was the basis for the distance of relocation of the operated muscles. The study committee, consisting of the participating pediatric ophthalmologists, determined this procedure, including the fixed ratio of 1.6 mm per degree of angle of strabismus and the use of the angle measured the day before surgery. The relocation of the muscles by recession or resection was divided equally over the two muscles in either BR or RR. All ophthalmologists had a minimum of 5 years’ experience with both recessions and resections. To evaluate the inter‐operator differences and its accuracy, photos were taken in approximately every tenth participant during two stages of the operation in either recession or resection: (1) after fitting the sutures through the muscle, and (2) before closing the conjunctiva. A millimeter ruler next to the muscle was photographed with the eye. |
| Outcomes |
Outcomes reported: whether the reduction in the latent angle of strabismus at distance fixation was more predictable with either technique, was the variation of the latent angle at distance fixation, 3 months postoperatively; the variation of the mean reduction in the angle of strabismus, divided by the distance of muscle relocation; the presence of binocular vision after surgery Adverse outcomes: "Dissociated vertical deviation (DVD) was reported in 10 cases. Latent nystagmus (LN) was reported in 26 cases. A combination of DVD and LN was reported in 19 cases." Measurement time points: 3 months Other issues with outcome assessment (e.g. quality control for outcomes, if any): none |
| Notes |
Study period: not reported Publication language: English Trial registration: not found Conflicts of interest: "none" declared Funding source: The Netherlands Society for Prevention of Blindness, Haags Oogheelkundig Fonds, Stichting Blindenhulp and the Rotterdamse Vereniging Blindenbelangen supported this study |
BR: bilateral recession DVD: dissociated vertical deviation IE: infantile esotropia LN: Latent nystagmus PD: prism diopters RR: unilateral recession‐resection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arnoult 1976 | Compared 2 groups retrospectively |
| Badawi 2014 | Not intervention and comparator of interest: bilateral medial rectus versus Y‐split recession; surgery is on 2 muscles in both groups |
| Badawi 2018 | Not intervention and comparator of interest: Y‐split recession versus de Decker’s Faden technique; surgery on 2 muscles in both groups |
| Birch 1995 | Prospective cohort, no control group |
| Birch 1998 | 2 cohorts studied ‐ 1 retrospective, 1 prospective; assessed natural history of infantile esotropia |
| Fouad 2016 | Not intervention and comparator of interest: medial rectus muscle recession versus medial rectus muscle pulley posterior fixation; 2 muscle surgery in both groups; included IET and convergence excess ET |
| Ghali 2017 | Not intervention and comparator of interest: bimedial rectus muscle recession versus bimedial rectus muscle elongation |
| Ing 1992 | Not a trial |
| Kushner 1984 | Randomized trial of graded versus standard recessions for infantile esotropia ‐ not one of the interventions looked at in this review |
| NCT03459092 | In this study, participants acquired esotropia after age 1 |
| Simonsz 2005 | Non‐randomized trial |
| TCTR20210210001 | Not intervention and comparator of interest: two different dosages of botulinum toxin |
Characteristics of ongoing studies [ordered by study ID]
NCT03266549.
| Study name | Botulinum toxin augmented surgery vs conventional surgery in the management of large angle horizontal deviations |
| Methods | Parallel‐group, randomized controlled trial |
| Participants | Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Intervention: botulinum toxin augmented surgery
Control: conventional surgery
|
| Outcomes | Primary outcomes :
Secondary outcomes :
|
| Starting date | February 2021 |
| Contact information | Sara Alattar, Msc: alattarsara@yahoo.com |
| Notes |
Differences between protocol and review
We updated the Methods for this review update to follow current Cochrane methodology. We expanded the inclusion criteria for study design to include quasi‐randomized studies. In one identified study, the authors described the study as 'randomized' but the allocation was performed by using odd or even numbers (i.e. quasi‐randomized). After discussion between the authors, we decided to include the study, and judged it at high risk of bias for random allocation. We did not perform any meta‐analysis or sensitivity analysis because only one study was included.
Contributions of authors
Screening search results: LM, SN Appraising quality of papers: LM, SN Extracting data from papers: LM, SN Entering and verifying the data in RevMan Web: LM, SN Analysis of data: LM, SN Interpretation of data: LM, SN Writing the review: LM, SN
Final approval of the document to be published: all review authors Performing previous work that was the foundation of the current study: Sue Elliot (Salisbury Health Care NHS Trust) and Ayad Shafiq (Royal Victoria Infirmary)
Sources of support
Internal sources
-
None, Other
No internal source of support.
External sources
-
National Eye Institute, National Institutes of Health, USA
Methodological support provided by the Cochrane Eyes and Vision US Project, supported by grant 1 U01 EY020522.
-
Queen's University Belfast, UK
Gianni Virgili, Co‐ordinating Editor for Cochrane Eyes and Vision’s work is funded by the Centre for Public Health, Queen’s University of Belfast, Northern Ireland
Declarations of interest
LM: none known SMN: reports a grant from the National Eye Institute, National Institutes of Health, USA; payment to institution JS: no relevant financial interests. Non‐financial interests include employment as a physician with Pepose Vision Institute; affiliation with AAPOS and AAO
Edited (no change to conclusions)
References
References to studies included in this review
Mayet 2021 {published data only}
- Mayet I, Ally N, Alli HD, Tikly M, Williams S. Botulinum neurotoxin injections in essential infantile esotropia – a comparative study with surgery in large-angle deviations. Eye 2021;35(11):3071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Polling 2009 {published data only}
- Polling JR, Eijkemans MJ, Esser J, Gilles U, Kolling GH, Schulz E, et al. A randomised comparison of bilateral recession versus unilateral recession-resection as surgery for infantile esotropia. British Journal of Ophthalmology 2009;93(7):954-7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arnoult 1976 {published data only}
- Arnoult JB, Yeshurun O, Mazow ML. Comparative study of the surgical management of congenital esotropia of 50 prism dioptres or less. Journal of Pediatric Ophthalmology 1976;13(3):129-31. [PubMed] [Google Scholar]
Badawi 2014 {published data only}
- Badawi N, Hegazy K. Comparative study of Y-split recession versus bilateral medial rectus recession for surgical management of infantile esotropia. Clinical Ophthalmology 2014;8:1039-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Badawi 2018 {published data only}
- Badawi N, Ismail AT. Comparative study of y-split recession versus Faden technique for management of infantile esotropia in Egyptians. Journal of Ophthalmology 2018;2018:1‐6. [DOI] [PMC free article] [PubMed]
Birch 1995 {published data only}
- Birch E, Stager D, Everett M. Random dot stereoacuity following surgical correction of infantile esotropia. Journal of Pediatric Ophthalmology and Strabismus 1995;32(4):231-5. [DOI] [PubMed] [Google Scholar]
Birch 1998 {published data only}
- Birch E, Stager D, Wright K, Beck R. The natural history of infantile esotropia during the first six months of life. Journal of American Association for Pediatric Ophthalmology and Strabismus 1998;2(6):325-8. [DOI] [PubMed] [Google Scholar]
Fouad 2016 {published data only}
- Fouad HM, Abdelhakim MA, Awadein A, Elhilali H. Comparison between medial rectus pulley fixation and augmented recession in children with convergence excess and variable-angle infantile esotropia. Journal of American Association for Pediatric Ophthalmology and Strabismus 2016;20(5):405-9.e1. [DOI] [PubMed] [Google Scholar]
Ghali 2017 {published data only}
- Ghali MA. Bimedial rectus muscle elongation versus bimedial rectus muscle recession for the surgical treatment of large-angle infantile esotropia. Clinical Ophthalmology 2017;11:1877-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ing 1992 {published data only}
- Ing MR. Botulinum alignment for congenital esotropia. Transactions of the American Ophthalmological Society 1992;90:361-7. [PMC free article] [PubMed] [Google Scholar]
Kushner 1984 {published data only}
- Kushner B, Morton G. A randomized comparison of surgical procedures for infantile esotropia. American Journal of Ophthalmology 1984;98(1):50-61. [DOI] [PubMed] [Google Scholar]
NCT03459092 {published data only}
- NCT03459092. Botox instead of strabismus surgery (BISS) [A pragmatic, randomized, non-inferiority trial comparing the effectiveness of botulinum toxin-based treatment with conventional strabismus surgery in acquired esotropia]. clinicaltrials.gov/ct2/show/NCT03459092 (first received 8 March 2018).
Simonsz 2005 {published data only}
- Simonsz HJ, Kolling GH, Unnebrink K. Final report of the early vs late infantile strabismus surgery study (ELISSS), a controlled, prospective, multicenter study. Strabismus 2005;13(4):169-99. [DOI] [PubMed] [Google Scholar]
TCTR20210210001 {published data only}
- TCTR20210210001. Effect of two difference doses of botulinum toxin A on infantile esotropia. trialsearch.who.int/Trial2.aspx?TrialID=TCTR20210210001 (first received 10 February 2021).
References to ongoing studies
NCT03266549 {published data only}
- NCT03266549. Botulinum toxin augmented surgery vs conventional surgery in the management of large angle infantile esotropia. clinicaltrials.gov/ct2/show/NCT03266549 (first received 30 August 2017).
Additional references
Ansons 2001
- Ansons AM, Davis H. Diagnosis and Management of Ocular Motility Disorders. 3rd edition. Malden, Mass: Blackwell Science Ltd, 2001. [Google Scholar]
AOA 2010
- American Optometric Association (AOA). Optometric clinical practice guideline. Care of the patient with strabismus: esotropia and exotropia. www.aoa.org/AOA/Documents/Practice%20Management/Clinical%20Guidelines/Consensus-based%20guidelines/Care%20of%20Patient%20with%20Strabismus%20Esotropia%20and%20Exotropia.pdf (accessed 19 August 2022).
Benabent 2002
- Benabent EC, García Hermosa P, Arrazola MT, Alió y Sanz JL. Botulinum toxin injection without electromyographic assistance. Journal of Pediatric Ophthalmology & Strabismus 2002;39(4):231–4. [DOI] [PubMed] [Google Scholar]
Birch 2006
- Birch EE, Stager DR Sr. Long-term motor and sensory outcomes after early surgery for infantile esotropia. Journal of American Association for Pediatric Ophthalmology and Strabismus 2006;10(5):409-13. [DOI] [PubMed] [Google Scholar]
Calcutt 1993
- Calcutt C. Infantile esotropia: current concepts in aetiology and management. British Orthoptic Journal 1993;50:37. [Google Scholar]
Caputo 2007
- Caputo R, Tinelli F, Bancale A, Campa L, Frosini R, Guzzetta A, et al. Motor coordination in children with congenital strabismus: effects of late surgery. European Journal of Paediatric Neurology 2007;11(5):285-91. [DOI] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 05 January 2022. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
de Alba Campomanes 2010
- Alba Campomanes AG, Binenbaum G, Campomanes Eguiarte G. Comparisons of botulinum with surgery as primary treatment for infantile esotropia. Journal of American Association for Pediatric Ophthalmology and Strabismus 2010;14(2):111-6. [DOI] [PubMed] [Google Scholar]
Deeks 2022
- Deeks JJ, Higgins JPT, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Drover 2008
- Drover JR, Stager DR Sr, Morale SE, Leffler JN, Birch EE. Improvement in motor development following surgery for infantile esotropia. Journal of American Association for Pediatric Ophthalmology and Strabismus 2008;12(2):136-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedman 1980
- Friedman Z, Neumann E, Hyams SW, Peleg B. Ophthalmic screening of 38,000 children, age 1 to 2 1/2 years, in child welfare clinics. Journal of Pediatric Ophthalmology & Strabismus 1980;17(4):261-7. [DOI] [PubMed] [Google Scholar]
Gerth 2008
- Gerth C, Mirabella G, Li X, Wright T, Westall T, Colpa L, et al. Timing of surgery for infantile esotropia in humans: effects on cortical motion visual evoked responses. Investigative Ophthalmology and Visual Science 2008;49(8):3432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130-6. [PMC free article] [PubMed] [Google Scholar]
Gursoy 2012
- Gursoy H, Basmak H, Sahin A, Yildirim N, Aydin Y, Colak E. Long-term follow-up of bilateral botulinum toxin injections versus bilateral recessions of the medial rectus muscles for treatment of infantile esotropia. Journal of American Association for Pediatric Ophthalmology and Strabismus 2012;16(3):269-73. [DOI] [PubMed] [Google Scholar]
Helveston 1990
- Helveston EM, Ellis FD, Plager DW, Miller KK. Early surgery for essential infantile esotropia. Journal of Pediatric Ophthalmology & Strabismus 1990;27(3):115-9. [DOI] [PubMed] [Google Scholar]
Higgins 2022
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022)). Cochrane, 2022. www.training.cochrane.org/handbook.
Ing 1980
- Ing MR. Early surgical alignment for congenital esotropia. Ophthalmology 1980;90(2):132-5. [DOI] [PubMed] [Google Scholar]
Ing 1995
- Ing MR. Outcome study of surgical alignment before six months of age for congenital esotropia. Ophthalmology 1995;102(12):2041-5. [DOI] [PubMed] [Google Scholar]
Kommerell 1988
- Kommerell G. Ocular motor phenomena in infantile strabismus. Asymmetry in optokinetic nystagmus and pursuit, latent nystagmus, and dissociated vertical divergence. In: Lennerstrand G, Noorden GK, Campos EC, editors(s). Strabismus and Amblyopia. London, UK: MacMillan Press, 1988:99-109. [Google Scholar]
Lueder 2008
- Lueder GT, Galli ML. Effect of preoperative stability of alignment on outcome of strabismus surgery for infantile esotropia. Journal of American Association for Pediatric Ophthalmology and Strabismus 2008;12(1):66-8. [DOI] [PubMed] [Google Scholar]
Lueder 2012
- Lueder GT, Galli M, Tychsen L, Yildirim C, Pegado V. Long-term results of botulinum toxin-augmented medial rectus recessions for large-angle infantile esotropia. American Journal of Ophthalmology 2012;153(3):560-3. [DOI] [PubMed] [Google Scholar]
Nixon 1985
- Nixon RB, Helveston EM, Miller K, Archer SM, Ellis FD. Incidence of strabismus in neonates. American Journal of Ophthalmology 1985;100(6):798-801. [DOI] [PubMed] [Google Scholar]
Page 2022
- Page MJ, Higgins JPT, Sterne JAC. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
PEDIG 2002
- Pediatric Eye Disease Investigator Group (PEDIG). Spontaneous resolution of early-onset esotropia: experience of the congenital esotropia observational study. American Journal of Ophthalmology 2002;133(1):109-18. [DOI] [PubMed] [Google Scholar]
RCO 2012
- The Royal College of Ophthalmologists (RCO). Guidelines for the management of strabismus in childhood (updated March 2012). www.rcophth.ac.uk/resources-listing/guidelines-for-the-management-of-strabismus-in-childhood/ (accessed 4 November 2022).
RevMan Web 2022 [Computer program]
- Review Manager Web (RevMan Web). Version 4.18.1. The Cochrane Collaboration, 2022. Available at revman.cochrane.org.
Ruiz 2004
- Ruiz MF, Alvarez MT, Sanchez-Garrido CM, Hernaez JM, Rodrigues JM. Surgery and botulinum toxin in congential esotropia. Canadian Journal of Ophthalmology 2004;39(6):639-49. [DOI] [PubMed] [Google Scholar]
Scheiman 2020
- Scheiman M, Kulp MT, Cotter SA, Lawrenson JG, Wang L, Li T. Interventions for convergence insufficiency: a network meta-analysis. Cochrane Database of Systematic Reviews 2020, Issue 12. Art. No: CD006768. [DOI: 10.1002/14651858.CD006768.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Scott 1980
- Scott AB. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology 1980;87(10):1044-9. [DOI] [PubMed] [Google Scholar]
Simonsz 2010
- Simonsz HJ, Eijkemans MJ. Predictive value of age, angle and refraction on rate of reoperation and rate of spontaneous resolution in infantile esotropia. Strabismus 2010;18(3):87-97. [DOI] [PubMed] [Google Scholar]
Tejedor 1999
- Tejedor J, Rodriguez JM. Early re-treatment of infantile esotropia: comparison of botulinum toxin and re-operation. British Journal of Ophthalmology 1999;83(7):783-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vaughan 1998
- Vaughan D, Asbury T, Riordan-Eva P. General Ophthalmology. 15th edition. New York, NY: McGraw-Hill/ Appleton & Lange, 1998. [Google Scholar]
Wright 1994
- Wright KW, Edelman PM, McVey JH, Terry AP, Lin M. High grade stereo-acuity after early surgery for congenital esotropia. Archives of Ophthalmology 1994;112(7):913-9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Elliott 2005
- Elliott S, Shafiq A. Interventions for infantile esotropia. Cochrane Database of Systematic Reviews 2005, Issue 1. Art. No: CD004917. [DOI: 10.1002/14651858.CD004917.pub2] [DOI] [PubMed] [Google Scholar]
Elliott 2013
- Elliott S, Shafiq A. Interventions for infantile esotropia. Cochrane Database of Systematic Reviews 2013, Issue 7. Art. No: CD004917. [DOI: 10.1002/14651858.CD004917.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


