Abstract
Mast cell (MC) degranulation has been implicated in the side effect profile of a variety of clinically useful agents. Thus, after intrathecal delivery, formation of space-occupying, meningeally-derived masses may be related to local MC degranulation. We systematically characterized degranulating effects of opioid and nonopioid analgesics on cutaneous flares in the dog and in primary human MC (hMC) cultures.
Methods:
Dogs were anesthetized with IV propofol and received intradermal (ID) injections (50 μL). Flare diameters were measured at 30 min. Drugs showing flare responses were tested after intramuscular (IM) cromolyn (10 mg/kg), a MC stabilizer. Human primary MCs (human cord blood CD34+/CD45+ cells) were employed and β-hexosaminidase in cell-free supernatants were measured to assess degranulation.
Results:
A significant skin flare for several classes of agents was observed including opioids, ziconotide, ketamine, ST-91, neostigmine, adenosine, bupivacaine, lidocaine, MK-801 and 48/80. Tizanidine, fentanyl, alfentanil, gabapentin and baclofen produced no flare. Flare produced by all ID agents, except adenosine, bupivacaine and lidocaine, was reduced by cromolyn. Naloxone had no effect upon opiate or 48/80 evoked flares. In hMC studies, 48/80 resulted in a concentration-dependent release of β-hexosaminidase. The rank order of drug-induced hMC β-hexosaminidase release was similar to that for flares.
Conclusions:
A variety of therapeutically useful drugs degranulate MCs. This action may account for side effects such as the intrathecal granuloma resulting from spinally-delivered opioids. This degranulating effect may be useful in predicting potential intrathecal toxicity in the development of novel agents.
Keywords: MCs, Degranulation, Analgesics, Opiates, Calcium Channel, NMDA Antagonist
1. Introduction
Spinal delivery of opioids can produce a potent analgesia (Deer et al., 2017a; Deer et al., 2017b). An important adverse effect that may arise with the chronic infusion of several opioids (notably morphine and hydromorphone) is the development of space-occupying masses (granulomas) in the intrathecal space of guinea pigs (Eddinger et al., 2016), dogs (Yaksh et al., 2003), sheep (Gradert et al., 2003) and, importantly, humans (Yaksh et al., 2002; Deer et al., 2017c). Our work has shown that these masses arise from proliferating fibroblasts and inflammatory cells that migrate from the adjacent meninges (Yaksh et al., 2003). We have shown that the intrathecal granuloma has several characteristic properties: i) the granuloma is produced by morphine, hydromorphone and methadone, but not fentanyl and alfentanil (Allen et al., 2006a; Yaksh et al., 2013b) in a concentration-dependent fashion (Allen et al., 2006b), ii) the morphine-associated intrathecal mass is not prevented by cotreatment with the opiate antagonist naltrexone (Yaksh et al., 2013a), which, consistent with the absence of a granuloma inducing effect of several potent opiates (fentanyl and alfentanil) suggests an effect independent of an opiate receptor (Allen et al., 2006b; Yaksh et al., 2013b); and, iii) is reduced by co-treatment with a MC stabilizer (cromolyn), suggesting a role for local (meningeal) MCs (Yaksh et al., 2013a).
MCs are key effector cells in a variety of inflammatory processes (Theoharides and Cochrane, 2004) and their activation has been shown to play an important role in a range of pathologies, including skin (Asero et al., 2017), pulmonary components in allergic response (Kubo, 2017), atherosclerosis (Conti et al., 2017) and pain (Kaur et al., 2017). MC degranulation leads to the secretion of a large number of biologically active products, including amines, cytokines and peptidases (Vukman et al., 2017). While MCs can be activated by allergens that bind to IgE attached to the FcεRI receptor (Amin, 2012), it is also clear that such activation can be achieved by nonallergic stimuli, including a variety of drugs (Bischoff, 2007). It is known that certain opiates, such as morphine, but not others, such as fentanil, degranulate MCs and produce local vasodilation in a manner not reversed by naloxone (Levy et al., 1989; Barke and Hough, 1993; Blunk et al., 2004). This association is provocative as it suggests that, to the degree an agent yields a flare after intradermal delivery or evokes MC degranulation, it may be more likely to result in an intrathecal mass after intrathecal infusion.
In the present study we systematically examined the effects of agents delivered intradermally on canine cutaneous flare and on the degranulation of human primary skin MCs as measured by the release of ß-hexosaminidase (Fukuishi et al., 2014). Three classes of agents were examined: i) commonly used intrathecal opioids, ii) sodium and calcium channel blockers and iii) a variety of agents that have been employed for spinal delivery in preclinical and clinical subjects.
2. Materials and methods
All studies involving animals were accomplished under protocols approved by the Institutional Animal Care and Use Committee of the University of California, San Diego (UCSD). Human MCs were obtained under protocols approved by the UCSD human studies Institutional Review Board.
2.1. Dog cutaneous flare
2.1.1. Animals
Male beagle dogs (Ridglan Farms Inc., Mt. Horeb, WI, or equivalent), 12–16 months of age and weighing approximately 9–16 kg, were housed individually in runs with wood shavings and given ad libitum access to food and water. For the study, dogs received atropine (0.04 mg/kg, IM) prior to anesthesia induced with propofol (5 μg kg−1 min−1). Animals were intubated and anesthesia maintained under spontaneous ventilation with 1.0–2.0% isoflurane and 60% N20/40% O2 (approximate values). Animals were continuously monitored during anesthesia for oxygen saturation, inspired and end tidal values of isoflurane, CO2, N2O and oxygen, and heart and respiratory rates. Animals were placed in dorsal recumbency and body temperature was maintained with an underbody heating pad. The chest and upper abdomen were clipped and surgically prepped.
2.1.2. Drug delivery protocol
Injection sites on the chest and abdomen were lightly marked with ink. Intradermal injections on the chest and upper abdomen were performed with single use sterile 30 ga/0.5 mL insulin syringes. Two rows of 9 or 10 injections each in a volume of 50 μL were made (cranial to caudal), lateral to the midline at time 0. Resulting skin flares were measured at time 0, 10 and 30 min post-injection without knowledge of injectate. After completion of the study sequence, subcutaneous lactated Ringers solution (100–300 mL) was given and animals were recovered.
2.1.3. Drug delivery sequences
Each study drug was examined at three concentrations in each of 4–5 animals. In each study with each animal, up to 5 different agents were randomly assigned to be delivered along with a vehicle injection (saline) and a positive control (compound 48/80). Drugs and concentrations delivered are shown in Table 1. Sodium chloride (0.9%) was the vehicle for all drugs. In separate experiments, the effects of the MC stabilizer cromolyn (10 mg/kg, IM) were examined to assess the role of MC degranulation in the observed flare. Selection of the 10 mg/kg dose was based on a preliminary dose-ranging study in which a dose-dependent block was observed at 7.5, 10 and 15 mg/kg IM. The administration of 7.5 mg/kg produced a partial block of compound 48/80 (1 mg/mL) induced flare (Yaksh et al., 2013a), while 10 mg/kg resulted in a near complete block of flare; the 15 mg/kg dose resulted in emesis. To determine the role of the opioid receptor in the observed flare effects, animals were pretreated with the opioid antagonist naltrexone (1 mg/kg, IM) given 30 min prior to intradermal injection of the highest concentrations of test articles employed. This dose of naltrexone was chosen given previous work showing that this systemic dose would prevent the robust analgesic effects of intrathecal morphine in the dog (Yaksh et al., 2013a).
Table 1.
Drugs employed in the flare and degranulation studies.
| Agent | Source | Action | MW | Dog Flare: mg/mL mg/mL (mM) |
Human MC: (μM) |
|---|---|---|---|---|---|
| Adenosine | 3 | Adenosine ag | 267 | 0.1, 1, 5 (0.04, 4.0, 40 mM) |
10 |
| Alfentanil citrate | 2 | μ-Opioid ag | 529 | 0.2, 2, 20 (0.4, 3.8, 37.8) |
10, 1 |
| Baclofen | 3 | GABA B ag | 213 | 0.1, 1, 4 (0.47, 4.7, 47 mM) |
5, 10 |
| Bupivacaine HCl | 3 | Sodium chan blkr | 288 | 1, 10, 50 (3.5, 35, 175 mM) |
5, 10 |
| Clonidine HCl | 3 | Alpha 2 ag | 230 | 0.1, 1, 10 (0.04, 0.4, 4 mM) |
5, 10 |
| Fentanyl Citrate | 2 | μ-Opioid ag | 529 | 0.02, 0.2, 2 (0.04, 0.4, 3.8) |
1, 10 |
| Gabapentin | 5 | a2∂ ag | 171 | 0.1, 1, 10 (0.58, 5.8, 58 mM) |
5, 10 |
| Hydromorphone HCl | 3 | μ-Opioid ag | 322 | 0.1, 1, 10 (0.3, 3, 30 mM) |
1, 10 |
| Ketamine HCl | 7 | NMDA antag | 238 | 0.1, 1, 10 (0.04, 0.4, 4 mM) |
5, 10 |
| Lidocaine HCl | 3 | Sodium ch blkr | 234 | 1.0, 10.0, 50.0 (0.4, 4, 20 mM) |
10 |
| Methadone HCl | 2 | μ-Opioid ag | 0.1, 1.10 | ||
| MK-801 Hydrogen Maleate | 3 | NMDA antag | 221 | 0.1, 1, 6 (0.5, 4.5, 27 mM) |
5, 10 |
| Morphine sulfate | 1 | μ-Opioid ag | 758 (2) | 0.1, 0.1, 10 (0.27, 2, 7, 27 mM) |
0.1, 1, 10 |
| Morphine-3-glucuronide | 2 | Opioid metabolite (inactive) | 461 | 0.1, 1, 4 (0.25, 2.5, 25 mM) |
10 |
| Morphine-6-glucuronide | 2 | μ-Opioid ag (Opioid metabolite) | 461 | 0.1, 1, 10 (0.25, 2.5, 25 mM) |
10 |
| Neostigmine bromide | 6 | Chase inhib | 223 | 0.1, 1, 10 (0.5, 4.5, 5 mM) |
5, 10 |
| ST-91, 2(2,6-diethylphenylamino)-2-imidazoline, | 4 | Alpha 1/2 ag | 254 | 0.01, 0.1, 1, 10 (0.04, 0.4, 4, 40 mM) |
10 |
| Tizanidine HCl | 3 | Alpha 2 ag | 254 | 0.1, 1, 10 (0.4, 4, 40 mM) |
5, 10 |
| Ziconotide | Elan Pharm. | N type Ca chan blkr | 2639 | 0.2, 2.0, 20 (μg/mL) (0.5, 5.3, 53 μM) |
0.01, 0.1, 1, 10 |
| H-Cys-Lys-Gly-Lys-Gly-Ala-Lys-Cys-Ser-Arg-Leu-Met-Tyr-Asp-Cys-Cys-Thr-Gly-Ser-Cys-Arg-Ser-Gly-Lys-Cys-NH2 | |||||
| Antagonists/vehicles | |||||
| Cromolyn sodium | 3 | MC stabilizer | 468 | 10 mg/kg (IM) pre-tx | |
| Naltrexone HCl | 3 | Pan opiate antag | 327 | 1 mg/kg | |
| Compound 48/80 | 3 | Positive Control | 165 ? | 1 mg/mL | |
| Saline | 1 | Vehicle (Flare) | 0.9% | ||
| PBS | Vehicle (MC) |
Source: 1: Hospira, Lake Forest, IL; 2: NIDA; 3: Sigma Chemical, St. Louis, MO; 4: Santa Cruz Biotechnology, Dallas, TX; 5: Toronto Research Biochemical, Toronto, CA; 6: Aldrich; 7: RBI, Natick, MA; IM: Intramuscular
2.1.4. Assessment of flare response
Flare areas (A) were calculated in square millimeters as an oval (A = 3.14 a * b, where a = half-length of long axis and b = half-length of short axis). This procedure was repeated at sites not recognized as being previously injected up to 6 times for each animal with a minimum of 5 days for recovery between injections. This protocol was similar to those previously described to assess flare in dogs (Becker et al., 1985; Rubinstein et al., 1990; Kirshenbaum and Metcalfe, 2006; Yaksh et al., 2013a). Data for analysis are presented as scattergrams with the mean and standard deviation (SD).
2.2. Human mast cell culture
2.2.1. Culture system
To assess drug effects upon human mast cell (hMC) degranulation, primary human mast cells were derived from human umbilical cord blood positive for CD34+ and CD45+ antigens (Astarte Biologics) (Kirshenbaum and Metcalfe, 2006). Briefly, CD34+ CD45+ cells were cultured in serum-free culture media (Stemline II, Sigma) containing recombinant human stem cell factor (100 ng/mL, R&D Systems), recombinant human IL-6 (100 ng/mL, R&D Systems), and recombinant human IL-3 (20 ng/mL, R&D Systems, first week only). After 10 weeks, hMCs were consistently generated as confirmed by the expression of CD117 and FcεRI. Cell maturation was confirmed by metachromatic staining with toluidine blue. The purity of hMCs was > 98%.
2.2.2. Degranulation studies
MC degranulation was assessed by measuring the activity of ß-hexosaminidase in the supernatants (Schwartz et al., 1979; Schick and Austen, 1989; Suzuki and Verma, 2008) of 4 × 104 human MCs in 100 μl saline phosphate buffer (0.9% NaCl, 10 mM NaH2PO4, 45 mM glucose) incubated for 2 h with different drugs. For each sample assayed, supernatant aliquots (20 μl) were mixed with substrate solution (100 μl), which consisted of 1 mM 4-methylumbelliferyl-2-acetamide-2-deoxy-β-D-glucopyranoside (Calbiochem) in 0.1 M sodium citrate buffer (pH 4.5), and were incubated for 2 h at 37 °C. The reaction was then stopped by the addition of 12 μl of 0.2 M glycine (pH 10.7). The reaction mixtures were excited at 365 nm and measured at 460 nm in a fluorescence plate reader (Gemini EM microplate spectrofluorometer, Molecular Devices). To determine the total cellular content of this enzyme, an equivalent number of cells were lysed with 1% triton-X-100 (Sigma). Release of ß-hexosaminidase was calculated as the percentage of the total enzyme content. Compound 48/80 was used to promote MC degranulation in an IgE-independent way. Exposure of hMCs to compound 48/80 (15 μM) resulted in an increase in MC degranulation as measured by increases in the fraction of releasable ß-hexoseaminidase in the media, which was linearly related to the number of hMCs in the wells. Based on these initial studies, subsequent release studies were typically carried out with 4 × 104 cells/well. In any given run, the individual data are presented as the % of the maximum possible effect where the concentrations of ß–hexosaminidase are normalized by the following formula: (Release with drug – PBS release)/48/80 release – PBS release) × 100.
2.3. Drugs
The agents employed, their source and the concentrations examined are given in Table 1.
2.4. Statistical analysis
For the cutaneous flare and hMC degranulation, comparisons were made across treatments using one-way ANOVA with post hoc comparison to the saline (vehicle control) group using the Dunnett multiple comparison test. For specific post hoc comparisons between the flare-inducing agent alone and with a co-treatment, multiple two-tailed t-tests were performed, with a Bonferroni correction for alpha buildup performed for each set of tests. The GraphPad Prism software package (v.4.0c for Mac OS X; GraphPad Software Inc., La Jolla, CA) was used for all analyses.
3. Results
3.1. Intradermal flare studies
All dogs thrived throughout the study series. Anesthetic sessions and recoveries were uneventful. Some test sessions resulted in a slow anesthetic recovery, as when high concentrations of opioid test articles were given. Animals receiving neostigmine displayed muscle fasciculations while under anesthesia and during anesthetic recovery, again, likely reflecting a systemic effect generated by the highest concentration. Injection sites were normal by the following day. ST-91 at 10 mg/mL produced a local superficial necrosis at the injection site. That concentration was considered intolerable and lower concentrations (1 mg/mL or less) were employed thereafter.
3.1.1. Compound 48/80
Intradermal injections of kcompound 48/80 (1 mg/mL) but not saline vehicle resulted in a significant flare. This effect was prevented by pretreatment with cromolyn 10 mg/kg IM (Fig. 1A, Supplementary Fig. 1B).
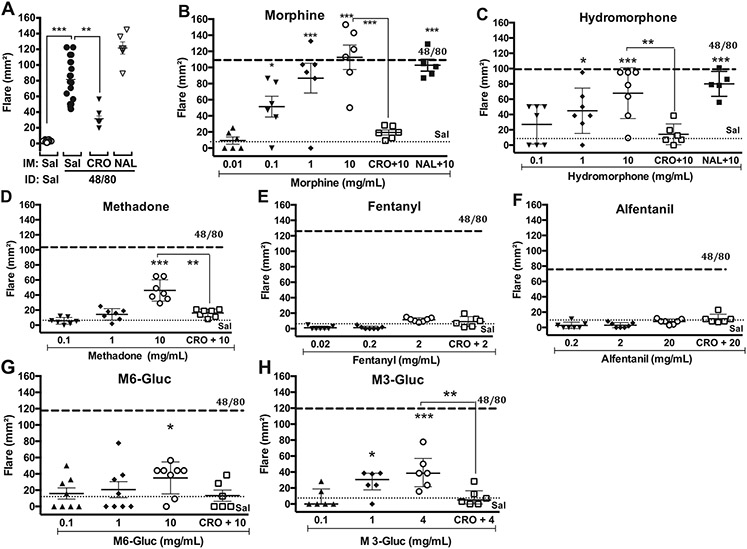
Fig. 1.
Scattergrams showing mean ± SD of effects of intradermal (ID) agents on the flare response (mm2) in dog at 30 min after ID injection. A: Flare produced by intradermal (ID) vehicle or 48/80 either with Saline (Sal) control, cromolyn (CRO: 10 mg/kg) or naloxone (NAL: 1 mg/kg). Flare produced by ID drug given alone or after intramuscular (IM) CRO (10 mg/kg) or IM NAL (1 mg/kg) for B: morphine; C: hydromorphone; D: methadone given alone or after IM CRO (10 mg/kg); E: fentanyl; F: Alfentanil; G: morphine-6-glucuronide (M6-Gluc); H: morphine-3-glucuronide (M3-Gluc). Dashed line and dotted line indicate the mean response to ID compound 48/80 (1 mg/mL) or saline (Sal), respectively. One-way ANOVA, followed by Dunnett's Multiple Comparison vs. saline. *: p < 0.05: **: p < 0.01: ***: p < 0.001: ****: p < 0.0001.
3.1.2. Opioids
Intradermal injection of morphine (Fig. 1B), hydromorphone (Fig. 1C), methadone (Fig. 1D) or morphine metabolites morphine-6-glucuronide (Fig. 1G) and morphine-3-glucuronide (Fig. 1H) resulted in a significant concentration-dependent skin flare 30 min after injection. Neither fentanil (Fig. 1E) nor alfentanil (Fig. 1F) were different from their respective saline controls. The rank order of flare size observed at the highest concentrations employed was: morphine (10 mg/mL), hydromorphone (10 mg/mL), methadone (10 mg/mL), morphine-3-glucronide (4 mg/mL), morphine-6-glucuronide (10 mg/mL) > fentanyl (2 mg/mL), alfentanil (20 mg/mL).
3.1.3. Non-opioid analgesics
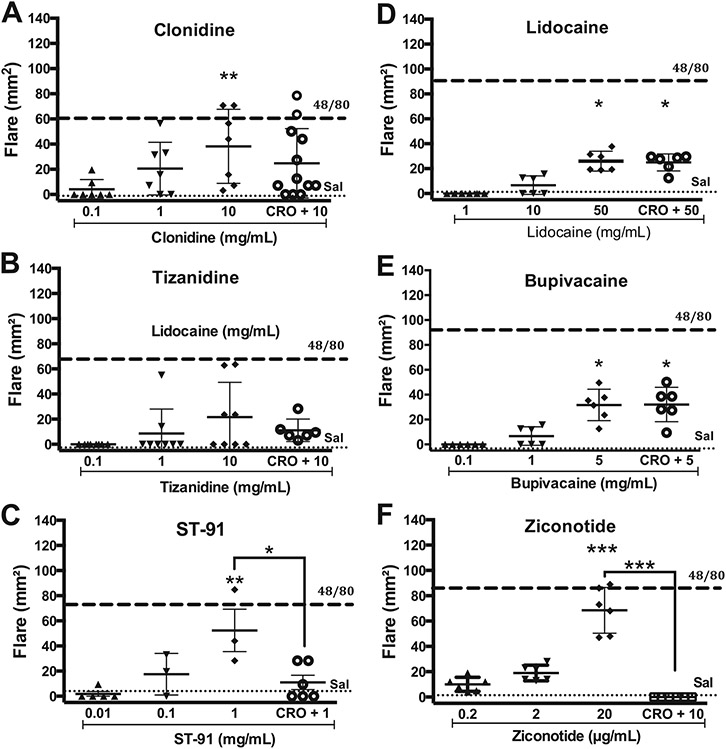
Intradermal injection of non-opioid analgesics, including the alpha 2 adrenergic agonists clonidine (Fig. 2A), tizanidine (Fig. 2B), ST-91 (Fig. 2C) and ST-91 at 10 mg/mL produced only a modest flare. The ST-91 effect resulted in an acute whitening of the skin at the injection site suggestive of a local vasoconstriction. At the highest doses these skin sites displayed a local ulceration over the next 24 h that healed completely during the following 4–7 days.
Fig. 2.
Scattergrams showing the mean ± SD of effects of intradermal (ID) agents on the flare response (mm2) in the dog at 30 min after ID injection of drug given alone or after intramuscular (IM) CRO (10 mg/kg). A: Clonidine; B: Tizanidine; C: ST-91; D: Lidocaine; E: Bupivacaine or F: Ziconotide. Dashed line and dotted line indicate the mean response to ID compound 48/80 (1 mg/mL) or saline (Sal), respectively. One-way ANOVA, followed by Dunnett's Multiple Comparison vs. saline. *: p < 0.05; **: p < 0.01; ***: p < 0.001; ****: p < 0.0001.
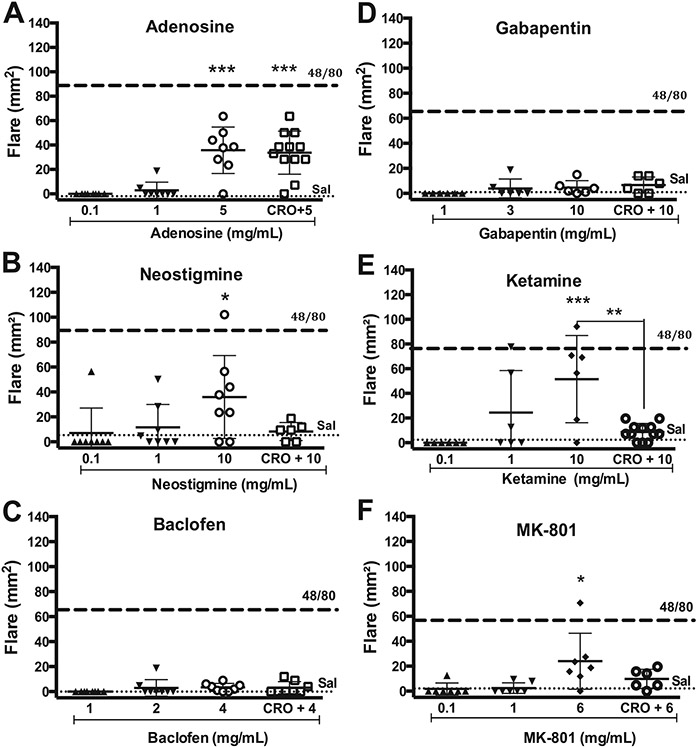
Other agents used intrathecally such as adenosine (Fig. 3A) and neostigmine (Fig. 3B) resulted in a significant flare as compared to saline control at 30 min post-injection. In contrast, neither baclofen (Fig. 3C) nor gabapentin (Fig. 3D), at the highest concentrations employed, had any effect as compared to the vehicle control (Fig. 4).
Fig. 3.
Scattergrams showing the mean ± SD of effects of intradermal (ID) agents on the flare response (mm2) in the dog at 30 min after ID injection of drug given alone or after intramuscular (IM) CRO (10 mg/kg). A: Adenosine; B: Neostigmine; C: Baclofen; D: Gabapentin; E: Ketamine or F: MK-801. Dashed line and dotted line indicate the mean response to ID compound 48/80 (1 mg/mL) or saline (Sal), respectively. One-way ANOVA, followed by Dunnett's Multiple Comparison vs. saline. *: p < 0.05; **: p < 0.01; ***: p < 0.001; ****: p < 0.0001.
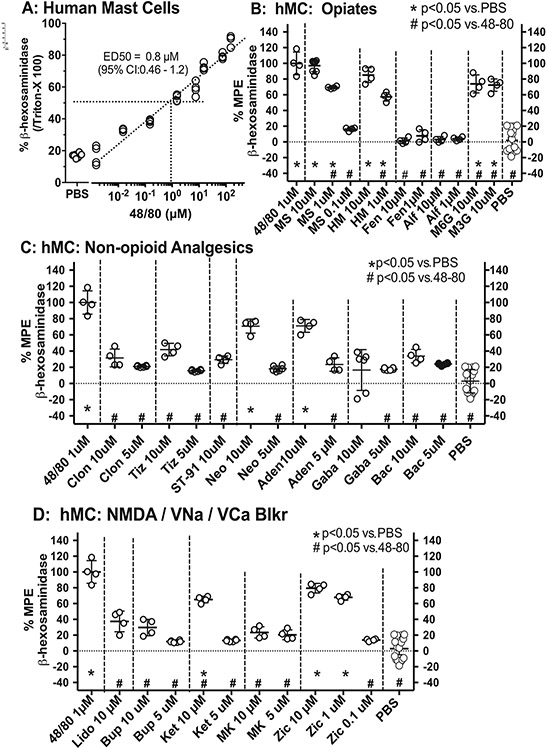
Fig. 4.
A: Scattergrams showing concentration-effect line for 48/80 plotting the ß-hexoseamindiase release in human mast cell (hMC) cultures evoked by 48/80, where release is expressed as a percent of the total release produced by Triton X vs. log 48/80 concentrations. The release of ß-hexoseamindiase in hMC produced by B: Opiates; C: non-opioid analgesics; D: NMDA and voltage gated sodium and calcium channel antagonists. Release is expressed as the percent of the maximum possible effect (%MPE), where MPE is the drug-evoked release by PBS divided by the difference between the release-evoked 48/80 and PBS. One-way ANOVA with post hoc comparisons performed with Dunnett's. *: p < 0.05 compared with release evoked by PBS and #: p < 0.05 for release as compared to 48/80.
3.1.4. Channel blockers
Intradermal injection of the channel blockers ziconotide, ketamine, MK-801, bupivacaine and lidocaine (Fig. 5) resulted in a significant flare as compared to saline control at 30 min post-injection.
Fig. 5.
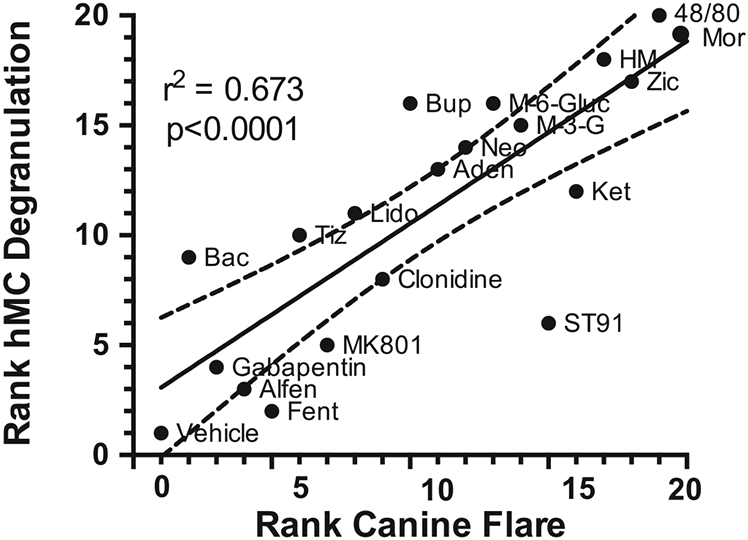
The in vivo and ex vivo effects of these drugs was plotted in rank order of flare (1 lowest to 20 highest) produced at the highest dose examined versus the rank order of hMC ß-hexoseaminidase releasing effects (1 lowest to 20 highest) of the highest high concentration (10 μM) examined. Regression analysis revealed a high and statistically significant correlation coefficient (r2 = 0.673).
3.2. Antagonists
3.2.1. Cromolyn
Following acquisition of the concentration response curves of the analgesic agents, the MC stabilizer cromolyn (10 mg/kg, IM) was given 30 min prior to intradermal injection at the highest concentrations of test article employed. As shown in Figs. 1-3, cromolyn pretreatment produced a significant decrease in flare size as compared to the agent alone for compound 48/80, morphine, hydromorphone, morphine-3-glucuronide, ST-91, ziconotide, and ketamine, or the agent in the presence of cromolyn failed to produce a significant increase in flare relative to vehicle control (morphine-6-glucuronide, clonidine, tizanidine, neostigmine, MK-801). In contrast, the flares produced by adenosine, bupivacaine and lidocaine were not altered in the presence of cromolyn.
3.3. Naloxone
The opioid antagonist naloxone (1 mg/kg, IM) was given 30 min prior to intradermal injection of compound 48/80 (Fig. 1A), morphine (Fig. 1B) or hydromorphone (Fig. 1C). As indicated, naloxone at this dose failed to alter the flare following any of these compounds.
3.4. Human MC degramilation
3.4.1. Compound 48/80
Exposure of hMCs to compound 48/80 (10−2 to 102 μM) resulted in a concentration-dependent increase in the fraction of releasable β-hexoseaminidase in the culture media (Fig. 4A). Based on these initial concentration-response studies, subsequent studies employed 1 μM compound 48/80, which released approximately 50% of the total pool of β-hexoseaminidase released by detergent treatment.
3.4.2. Opioids
Exposure of hMCs to mu opioids (morphine, hydromorphone) resulted in a robust concentration-dependent increase in MC degranulation as compared to vehicle (PBS). Morphine-3-glucuronide and morphine-6-glucuronide at the single concentration (10 μM) tested also resulted in a significant release. In contrast, neither fentanyl nor alfentanil had any effect at the highest concentration employed (Fig. 4B). Methadone was not studied in the hMC preparation.
3.4.3. Non-opioid analgesics
Exposure of hMCs to alpha 2 agonists clonidine, tizanidine, and ST-91, the GABAB agonist baclofen, the AChase inhibitor neostigmine, adenosine, and the a2∂ ligand gabapentin at the highest concentrations examined resulted in MC degranulation as compared to vehicle (PBS) (Fig. 4C).
3.4.4. Channel blockers
Exposure of hMCs to the voltage-gated sodium channel blockers lidocaine and bupivacaine, the NMDA antagonists MK-801 and ketamine and a voltage-gated N-type Ca channel blocker ziconotide at the highest concentrations examined resulted in MC degranulation as compared to vehicle (PBS) (Fig. 4D).
3.5. Relative activity
The rank order of flare produced at the highest dose of each drug was plotted against the rank order of hMC β-hexoseaminidase releasing effects at the highest concentration (10 μM) examined (Fig. 5). Regression analysis revealed a high and statistically significant correlation coefficient (r2 = 0.673).
4. Discussion
In the present studies, molecules that have been shown to have a spinal action altering pain or spasticity were examined for 1) their cromolyn-sensitive flare response after intradermal delivery and 2) the degranulation of hMC cultures. The outcomes of the in vivo in combination with in vitro experiments suggest a clear effect mediated by MCs. Importantly, the relative efficacy in producing cutaneous flare showed a significant covariance with the potency of these agents in degranulating human MCs (CD34+ CD45+) derived from umbilical cord blood. Among these agents were morphine, hydromorphone, methadone and the morphine 3/6 glucuronides. This profile of opioid-evoked degranulation has been reported by others (Rosow et al., 1982; Tharp et al., 1987; Marone et al., 1993). Other agents producing flare and hMC degranulation were ketamine, neostigmine and ST-91. Ketamine and MK-801 (NMDA antagonists) both evoked small cromolyn-reversed mild flares that were prevented with cromolyn pretreatment; ketamine, but not MK-801, showed a significant effect upon hMC degranulation. Given the lack of an effect of MK-801, these data do not support a role for NMDA antagonism in mediating the flare and degranulating effects of ketamine. Previous work has shown that ketamine will evoke histamine release (Marone et al., 1993) and will produce local dilation in the mesenteric circulation ((Brookes et al., 2002) but see (Brookes et al., 2004)). Lidocaine and bupivacaine both produced small flares that were cromolyn-insensitive and did not result in hMC degranulation. Local anesthetics have typically been shown to block evoked release from MCs (Yanagi et al., 1996), while others have shown a reduction of MCs at wound sites (Rodrigues et al., 2011) and some to apparently evoke release (Yanagi et al., 1997; Tufek et al., 2013). Ziconotide displayed a potent cromolyn-reversed flare and hMC degranulation. Previous work has shown that systemic ziconotide yields a MC-mediated hypotension (Bowersox et al., 1992) and blocked nerve stimulation-evoked vasoconstriction (Wright et al., 2000). ST-91 is a polar analogue of the alpha 2 agonist clonidine that resulted in a cromolyn-sensitive flare. This agent also resulted in a local whitening around the injection site suggestive of local vasoconstriction. The alpha2-adrenergic agonists clonidine and tizanidine displayed mild flare effects, but little or no effect upon release from hMCs. Previous work showed that clonidine, by an alpha2 adrenoceptor, dose-dependently suppressed the wheal and flare reaction initiated by albumin (Lindgren et al., 1987). Adenosine resulted in a potent flare response that was cromolyn-insensitive and resulted in a potent release from hMC. It has been shown that adenosine, through an A3 receptor, can activate MCs (Ramkumar et al., 1993), while acting through other adenosine receptors, notably the A2a receptor, will produce potent dilation, suggesting that in the present case, the direct vasodilatory effect likely obscured the dilation secondary to MC degranulation (Layland et al., 2014). Importantly, a number of agents had no effect on flare or hMC degranulation. These included the opioids alfentanil and fentanyl, the alpha 2 delta-targeted agent gabapentin and the GABAB agonist and antispasticity agent baclofen.
4.1. Mechanisms of MC activation
The mechanisms whereby MC degranulation may be initiated include i) activation of high-affinity receptors for the Fc region of IgE, through specific receptors (Borriello et al., 2014); ii) a receptor-dependent interaction with binding sites mediated by cationic charges of some molecules which act as receptor mimetic agents to trigger MCs through Gi/o-coupled receptors activating phospholipase C and intracellular calcium mobilization (Veien et al., 2000; Solinski et al., 2014; Yu et al., 2016). Such signaling pathways have been shown for morphine in MCs (Klinker and Seifert, 1997), The so-called MC stabilizers such as cromolyn may act by preventing this G-protein activation (Klinker and Seifert, 1997). A number of receptors may be activated by charged molecules and initiate downstream signaling through a Gi/o coupling mediated by phospholipase C. Two such cationic-sensitive families are the Mas-related, gen-like receptors (MrgX) and the formyl receptors on MCs (Gupta et al., 2015; McNeil et al., 2015; Subramanian et al., 2016). In spite of interest in these G-protein-coupled MC-degranulating receptors, Additional work is required to determine what role these specific receptors play in the degranulating process initiated by various intrathecal agents.
While the specific mechanisms leading to MC degranulation remain to be determined, an important issue was the assessment of the role played by opioid receptors on degranulation and flare. Several lines of evidence specifically argue against a role for a classical opioid receptor activation. i) Flare was produced by morphine, hydromorphone, methadone and morphine-6-glucuronide, but not by fentanyl or alfentanil, which are potent mu opioid agonists; ii) the effects of several opioids on MC degranulation produced by morphine and hydromorphone were not reversed or prevented by naloxone pretreatment. These observations argue that these opioid effects were not mediated by a classical opioid receptor. This profile of opioid-evoked degranulation has been reported by others (Rosow et al., 1982; Tharp et al., 1987; Marone et al., 1993).
4.2. Consequences of opioid-induced MC degranulation
MCs play a pervasive role in inflammation. Thus, their degranulation: i) releases vasodilators (histamine and serotonin) leading to local flare and hypotension (Muldoon et al., 1987; Levy et al., 1989; Barke and Hough, 1993; Treuren et al., 1993; Blunk et al., 2004), ii) increases vascular permeability (tumor necrosis factor: TNF, tryptase and chymase) (King and Miller, 1984; Scudamore et al., 1995); and iii) enhances fibroblast proliferation, chemotaxis and collagen synthesis (Gruber et al., 1997; Garbuzenko et al., 2002; Kohyama et al., 2010). As opiate molecules such as morphine can robustly degranulate MCs, such effects may play a role in a variety of opiate-initiated pathologies in different organ systems. Thus, in lung, MC tryptase induces fibroblast proliferation via activation of PAR-2. In kidney, morphine increased proliferation of fibroblasts (Singhal et al., 1998), which may account for its proposed role in renal interstitial fibrosis. In skin, MC deficient mice or the inhibition of MC degranulation reduces fibrocyte response and fibrosis (Thevenot et al., 2011; Avula et al., 2014), thus leading to a decrease in scar formation in skin wound healing (Chen et al., 2014).
Another important area has been the potential role of meningeal MC degranulation on the formation of intrathecal granulomas in human and animal models receiving chronic intrathecal delivery of several opioids (Allen et al., 2006a; Allen et al., 2006b; Yaksh et al., 2013a). It has been hypothesized that, as cited above, in skin, lung and kidney, opioid-induced degranulation of MCs may lead to fibroblast proliferation and collagen secretion forming a space-occupying mass (Yaksh et al., 2013a; Eddinger et al., 2016). The present studies suggest that agents known to result in granulomas, such as morphine, hydromorphone and methadone, have a greater likelihood of producing granulomas than agents such as fentanyl and alfentanil, which do not produce MC degranulation (Eddinger et al., 2016; Deer et al., 2017a; Deer et al., 2017b). Thus, as noted in the data summary in Table 2 that reflects a summary of data from a variety published studies, the morphine concentration delivered by chronic infusion employed in dog to produce maximum increases in thermal escape latency is around 1.0 mg/mL (Allen et al., 2006a). In the canine model, granulomas were observed at infusion concentrations as low as 1–2 mg/mL, while 12 mg/mL in the canine model is considered to result in virtually a 100% incidence (Yaksh et al., 2003; Yaksh et al., 2013a). These infusion concentrations in the 10–15 kg canine model result in lumbar cerebrospinal fluid (CSF) morphine concentrations corresponding to 6 and 42 μg/mL, respectively (Allen et al., 2006b). In humans, the analgesic dose of intrathecal morphine varies widely with infusion concentrations of 1 to 2 mg/mL being employed; Infumorph®, a commonly used FDA-approved injectable morphine for intrathecal infusion, is available at concentrations of 10 and 25 mg/mL. The relationship of dose to incidence of granuloma varies widely (Coffey and Burchiel, 2002; Yaksh et al., 2002; Deer et al., 2012; Deer et al., 2017a), but it is generally conceded that the incidence rises with infusion concentrations and time. Consensus statements have indicated that the maximum recommended morphine concentration should be 20 mg/mL (Deer et al., 2017a). In lumbar CSF sampling studies of patients receiving continuous intrathecal morphine, this infusion concentration has been shown to produce lumbar CSF morphine concentrations of 20–30 μg/mL (61–91 μM). We note that morphine produced a significant flare at concentrations of 0.1 mg/mL (300 μM) in the cutaneous canine model. In vitro studies on canine meninges displayed significant histamine release at morphine concentrations of 100 μM. These numbers, while reflecting a variety of assumptions, converge on the conclusion that morphine at analgesic concentrations in the dog and human may approach concentrations that yield cutaneous cromolyn-sensitive flares produced by MC degranulation. We would contrast this with a similar analysis of ziconotide. This highly charged peptide is a potent MC degranulating agent. However, ziconotide has not been shown to initiate pathology in canine models at doses as high as 0.06 mg/h (23 nmol) (Skov et al., 2007). The analgesic dose as measured by thermal escape in dog is < 1 μg/h (0.38 nmol/h) (Yaksh et al., 2012), producing lumbar CSF concentrations of approximately 0.15 nmol/mL while the human analgesic dose is reported to be approximately 0.125–0.21 μg/h (0.05–0.08 nmol/h) (Wermeling et al., 2003; Deer et al., 2017a). Sampling of CSF after infusion of 5 μg/h of ziconotide in humans revealed peak CSF concentrations of approximately 400 ng/mL (0.15 nmol/mL) (Wermeling et al., 2003). As noted in the present study, the canine flare-inducing concentration for ziconotide is 20 μg/mL (7.5 nM). These approximations support the useful hypothesis that analgesic concentrations less than those required to produce MC degranulation will reduce the likelihood of an intrathecal mass formation.
Table 2.
Summary of in vivo work showing concentrations producing analgesia and/or toxicity after intrathecal infusion, flare after intradermal delivery in dogs.
| MW | Human and dog IT Analgesia Antispasticity a,b |
Reference | IT canine toxicologyb |
Reference | In vivo: canine Intra- dermal Flare c |
j | |
|---|---|---|---|---|---|---|---|
| mg * mL−1/mM | mg * mL−1/mM | mg/mL−1/mM | |||||
| Adenosine | 267 | 0.25/ H,B | > 3/11 | (Chiari et al., 1999) | 1/3.7 | j | |
| Alfentanil Citrate | 529 | 4/7.6 C,I | (Yaksh et al., 2012) | > 20/ > 38 | (Yaksh et al., 2012) | > 20/ > 38 | (Yaksh et al., 2012)j |
| Baclofen HCl | 213 | 1/4.7, C,I 0.3/9.4 H,I c |
(Sabbe et al., 1993) (Boster et al., 2016) |
> 2/ > 9.4 | (Sabbe et al., 1993) | > 4/19 | j |
| Bupivacaine HCl | 325 | 1.5/ 4.6 C,I 7.5/23 H,I |
(Kroin et al., 1987) | > 3.7/ > 11.4 | (Kroin et al., 1987) | 50/154 | j |
| Clonidine HCl | 267 | 2.5/9.4 C,I 0.12/0.4 H,I |
(Kroin et al., 1987; Kroin et al., 2003) (Uhle et al., 2000) |
> 2/ > 7.5 | (Kroin et al., 2003) | 10/38 | j |
| DPDPE | 645 | 3/4.6 C,I | (Horais et al., 2003) | 6/9.3 | (Horais et al., 2003) | 10/16 | j |
| Fentanyl Citrate | 529 | 0.3/0.6 H,I 0.1/ H,Id |
(Allen et al., 2006b) (Willis and Doleys, 1999) |
> 2/ > 3.8 | (Allen et al., 2006b) | > 2/ > 3.8 | j |
| Gabapentin | 171 | ND | 80 mg/mLf | Hildebrand, unpubg | > 10/ > 59 | j | |
| Hydromor phone HCl | 322 | 0.1/0.3 | (Allen et al., 2006b) | 3/9.3 | (Allen et al., 2006b) | 1/3.1 | j |
| Ketamine HCl | 274 | ND | 4.2/15 | (Yaksh et al., 2008) | 10/36 | j | |
| Lidocaine HCl | 271 | ND | ND | 50/185 | |||
| Methadone HCl | 346 | 3/8.6 C,I | (Allen et al., 2006b) | 1/2.9 | (Allen et al., 2006b) | 10/29 | (Yaksh et al., 2013a),j |
| MK-801 | 337 | ND | 0.4/1.2 | (Yaksh et al., 2008) | 6/18 | j | |
| Morphine Sulfate | 329 | 0.1/0.3 C,I | (Allen et al., 2006b) | 3/9.1 | (Yaksh et al., 2003) | 0.1/0.3 | (Yaksh et al., 2013a), j |
| Morphine 3 gluc | 461 | ND | ND | 1/2.2 | |||
| Morphine 6 gluc | 461 | ND | ND | 10/22 | |||
| Neostigmine Methylsulfate | 334 | 0.075/0.22, H,B | (Lauretti et al., 1998) | > 1/ > 3.0 | (Yaksh et al., 1995) | 10/30 | |
| ST-91 HCl | 253 | 4/15.8 C,I | Yaksh, unpubh | < 18/ < 71 | Yaksh, unpubh | 1/4.0 | |
| Tizanidine HCl | 290 | 2.5/9.4 C,I | (Kroin et al., 2003) | 6/20.6 | (Kroin et al., 2003) | 10/34 | |
| Ziconotide | 2639 | < 0.001/0.0004 C,Ii 0.003e/0.001 H,I |
(Wermeling et al., 2003; Yaksh et al., 2012; Deer et al., 2017a) | > 0.006i | (Skov et al., 2007) | 0.020/0.008 |
Doses represent best estimates based on reported doses or the mean of a distribution.
H: human; C: Canine: B: bolus; I: Infusion.
Delivered in 2 mg/mL.
per day.
Dose is 0.005 mg/24 h.
Carried out in sheep.
Keith Hildebrand, Medtronic, personal communication.
T.L. Yaksh, unpublished observations.
mg/h.
unpublished data in this paper.
5. Conclusions
These results support the hypothesis that a variety of analgesic agents will yield degranulation of MCs and cromolyn-sensitive dermal flares. For the opioids these effects are independent of an opioid receptor. Importantly, these data suggest that after intrathecal delivery, analgesic concentrations less than those required to produce MC degranulation have a diminished risk of intrathecal granuloma formation in dogs and humans.
Supplementary Material
Abbreviations
- A
Area
- hMC
human mast cells
- ID
intradermal
- IL
interleukin
- IM
intramuscular
- IT
intrathecal
- IV
intravenous
- MrgX
Mas-related, gen-like receptors
- NMDA
n-methyl d-aspartate
- PAR
proteinase activated receptors
- PBS
phosphate buffered saline
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.taap.2017.10.017.
Transparency document
The http://dx.doi.org/10.1016/j.taap.2017.10.017 associated with this article can be found in online version.
Conflict of interest statement
A formal Laboratory Service Agreement was entered into by and between The Regents of the University of California, San Diego and Medtronic PLC. and a study was conducted in the laboratory of Tony Yaksh, UCSD faculty member, with funding from Medtronic. A manuscript resulted from this work and is being submitted to the journal Toxicology and Applied Pharmacology for consideration for publication. The authors include the PI Dr. Yaksh, and 2 Medtronic scientific research staff, Drs. Keith Hildebrand and Linda Page.
Portions of the data shown in this paper were presented in a poster at the Society for Neuroscience annual meeting, Washington DC, November 2017.
References
- Allen JW, Horais KA, Tozier NA, Wegner K, Corbeil JA, Mattrey RF, Rossi SS, Yaksh TL, 2006a. Time course and role of morphine dose and concentration in intrathecal granuloma formation in dogs - a combined magnetic resonance imaging and histopathology investigation. Anesthesiology 105, 581–589. [DOI] [PubMed] [Google Scholar]
- Allen JW, Horais KA, Tozier NA, Yaksh TL, 2006b. Opiate pharmacology of intrathecal granulomas. Anesthesiology 105, 590–598. [DOI] [PubMed] [Google Scholar]
- Amin K, 2012. The role of mast cells in allergic inflammation. Respir. Med 106, 9–14. [DOI] [PubMed] [Google Scholar]
- Asero R, Tedeschi A, Marzano AV, Cugno M, 2017. Chronic urticaria: a focus on pathogenesis. F1000Res 6, 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avula MN, Rao AN, McGill LD, Grainger DW, Solzbacher F, 2014. Foreign body response to subcutaneous biomaterial implants in a mast cell-deficient Kit(w-Sh) murine model. Acta Biomater. 10, 1856–1863. [DOI] [PubMed] [Google Scholar]
- Barke KE, Hough LB, 1993. Opiates, mast cells and histamine release. Life Sci. 53, 1391–1399. [DOI] [PubMed] [Google Scholar]
- Becker AB, Chung KF, McDonald DM, Lazarus SC, Frick OL, Gold WM, 1985. Mast cell heterogeneity in dog skin. Anat. Rec 213 (477–480), 530–571. [DOI] [PubMed] [Google Scholar]
- Bischoff SC, 2007. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nat. Rev. Immunol 7, 93–104. [DOI] [PubMed] [Google Scholar]
- Blunk JA, Schmelz M, Zeck S, Skov P, Likar R, Koppert W, 2004. Opioid-induced mast cell activation and vascular responses is not mediated by mu-opioid receptors: an in vivo microdialysis study in human skin. Anesth. Analg 98, 364–370 (table of contents). [DOI] [PubMed] [Google Scholar]
- Borriello F, Granata F, Varricchi G, Genovese A, Triggiani M, Marone G, 2014. Immunopharmacological modulation of mast cells. Curr. Opin. Pharmacol 17, 45–57. [DOI] [PubMed] [Google Scholar]
- Boster AL, Adair RL, Gooch JL, Nelson ME, Toomer A, Urquidez J, Saulino M, 2016. Best practices for intrathecal baclofen therapy: dosing and long-term management. Neuromodulation 19, 623–631. [DOI] [PubMed] [Google Scholar]
- Bowersox SS, Singh T, Nadasdi L, Zukowska-Grojec Z, Valentino K, Hoffman BB, 1992. Cardiovascular effects of omega-conopeptides in conscious rats: mechanisms of action. J. Cardiovasc. Pharmacol 20, 756–764. [PubMed] [Google Scholar]
- Brookes ZL, Brown NJ, Reilly CS, 2002. Differential effects of intravenous anaesthetic agents on the response of rat mesenteric microcirculation in vivo after haemorrhage. Br. J. Anaesth 88, 255–263. [DOI] [PubMed] [Google Scholar]
- Brookes ZL, Reilly CS, Brown NJ, 2004. Differential effects of propofol, ketamine, and thiopental anaesthesia on the skeletal muscle microcirculation of normotensive and hypertensive rats in vivo. Br. J. Anaesth 93, 249–256. [DOI] [PubMed] [Google Scholar]
- Chen L, Schrementi ME, Ranzer MJ, Wilgus TA, DiPietro LA, 2014. Blockade of mast cell activation reduces cutaneous scar formation. PLoS One 9, e85226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiari A, Yaksh TL, Myers RR, Provencher J, Moore L, Lee CS, Eisenach JC, 1999. Preclinical toxicity screening of intrathecal adenosine in rats and dogs. Anesthesiology 91, 824–832. [DOI] [PubMed] [Google Scholar]
- Coffey RJ, Burchiel K, 2002. Inflammatory mass lesions associated with intrathecal drug infusion catheters: report and observations on 41 patients. Neurosurgery 50, 78–86 (discussion 86-77). [DOI] [PubMed] [Google Scholar]
- Conti P, Lessiani G, Kritas SK, Ronconi G, Caraffa A, Theoharides TC, 2017. Mast cells emerge as mediators of atherosclerosis: Special emphasis on IL-37 inhibition. Tissue Cell 49, 393–400. [DOI] [PubMed] [Google Scholar]
- Deer T, Rauck RL, Kim P, Saulino MF, Wallace M, Grigsby EJ, Huang IZ, Mori F, Vanhove GF, McDowell G, 2017a. Effectiveness and Safety of Intrathecal Ziconotide: Interim Analysis of the Patient Registry of Intrathecal Ziconotide Management (PRIZM). Pain Pract. [DOI] [PubMed] [Google Scholar]
- Deer TR, Levy R, Prager J, Buchser E, Burton A, Caraway D, Cousins M, De Andres J, Diwan S, Erdek M, Grigsby E, Huntoon M, Jacobs MS, Kim P, Kumar K, Leong M, Liem L, McDowell GC 2nd, Panchal S, Rauck R, Saulino M, Sitzman BT, Staats P, Stanton-Hicks M, Stearns L, Wallace M, Willis KD, Witt W, Yaksh T, Mekhail N, 2012. Polyanalgesic Consensus Conference–2012: recommendations to reduce morbidity and mortality in intrathecal drug delivery in the treatment of chronic pain. Neuromodulation 15, 467–482 (discussion 482). [DOI] [PubMed] [Google Scholar]
- Deer TR, Pope JE, Hayek SM, Bux A, Buchser E, Eldabe S, De Andres JA, Erdek M, Patin D, Grider JS, Doleys DM, Jacobs MS, Yaksh TL, Poree L, Wallace MS, Prager J, Rauck R, DeLeon O, Diwan S, Falowski SM, Gazelka HM, Kim P, Leong M, Levy RM, McDowell II G, McRoberts P, Naidu R, Narouze S, Perruchoud C, Rosen SM, Rosenberg WS, Saulino M, Staats P, Stearns LJ, Willis D, Krames E, Huntoon M, Mekhail N, 2017b. The Polyanalgesic Consensus Conference (PACC): recommendations on intrathecal drug infusion systems best practices and guidelines. Neuromodulation 20, 96–132. [DOI] [PubMed] [Google Scholar]
- Deer TR, Pope JE, Hayek SM, Lamer TJ, Veizi IE, Erdek M, Wallace MS, Grider JS, Levy RM, Prager J, Rosen SM, Saulino M, Yaksh TL, De Andres JA, Abejon Gonzalez D, Vesper J, Schu S, Simpson B, Mekhail N, 2017c. The Polyanalgesic Consensus Conference (PACC): recommendations for intrathecal drug delivery: guidance for improving safety and mitigating risks. Neuromodulation 20, 155–176. [DOI] [PubMed] [Google Scholar]
- Eddinger KA, Rondon ES, Shubayev VI, Grafe MR, Scadeng M, Hildebrand KR, Page LM, Malkmus SA, Steinauer JJ, Yaksh TL, 2016. Intrathecal catheterization and drug delivery in guinea pigs: a small-animal model for morphine-evoked granuloma formation. Anesthesiology 125, 378–394. [DOI] [PubMed] [Google Scholar]
- Fukuishi N, Murakami S, Ohno A, Yamanaka N, Matsui N, Fukutsuji K, Yamada S, Itoh K, Akagi M, 2014. Does beta-hexosaminidase function only as a degranulation indicator in mast cells? The primary role of beta-hexosaminidase in mast cell granules. J. Immunol 193, 1886–1894. [DOI] [PubMed] [Google Scholar]
- Garbuzenko E, Nagler A, Pickholtz D, Gillery P, Reich R, Maquart FX, Levi-Schaffer F, 2002. Human mast cells stimulate fibroblast proliferation, collagen synthesis and lattice contraction: a direct role for mast cells in skin fibrosis. Clin. Exp. Allergy 32, 237–246. [DOI] [PubMed] [Google Scholar]
- Gradert TL, Baze WB, Satterfield WC, Hildebrand KR, Johansen MJ, Hassenbusch SJ, 2003. Safety of chronic intrathecal morphine infusion in a sheep model. Anesthesiology 99, 188–198. [DOI] [PubMed] [Google Scholar]
- Gruber BL, Kew RR, Jelaska A, Marchese MJ, Garlick J, Ren SL, Schwartz LB, Korn JH, 1997. Human mast cells activate fibroblasts – tryptase is a fibrogenic factor stimulating collagen messenger ribonucleic acid synthesis and fibroblast chemotaxis. J. Immunol 158, 2310–2317. [PubMed] [Google Scholar]
- Gupta K, Kotian A, Subramanian H, Daniell H, Ali H, 2015. Activation of human mast cells by retrocyclin and protegrin highlight their immunomodulatory and antimicrobial properties. Oncotarget 6, 28573–28587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horais K, Hruby V, Rossi S, Cizkova D, Meschter C, Dorr R, Yaksh TL, 2003. Effects of chronic intrathecal infusion of a partial differential opioid agonist in dogs. Toxicol. Sci 71, 263–275. [DOI] [PubMed] [Google Scholar]
- Kaur G, Singh N, Jaggi AS, 2017. Mast cells in neuropathic pain: an increasing spectrum of their involvement in pathophysiology. Rev. Neurosci [DOI] [PubMed] [Google Scholar]
- King SJ, Miller HR, 1984. Anaphylactic release of mucosal mast cell protease and its relationship to gut permeability in Nippostrongylus-primed rats. Immunology 51, 653–660. [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum AS, Metcalfe DD, 2006. Growth of human mast cells from bone marrow and peripheral blood-derived CD34+ pluripotent progenitor cells. Methods Mol. Biol 315, 105–112. [DOI] [PubMed] [Google Scholar]
- Klinker JF, Seifert R, 1997. Morphine and muscle relaxants are receptor-independent G-protein activators and cromolyn is an inhibitor of stimulated G-protein activity. Inflamm. Res 46, 46–50. [DOI] [PubMed] [Google Scholar]
- Kohyama T, Yamauchi Y, Takizawa H, Kamitani S, Kawasaki S, Nagase T, 2010. Histamine stimulates human lung fibroblast migration. Mol. Cell. Biochem 337, 77–81. [DOI] [PubMed] [Google Scholar]
- Kroin JS, McCarthy RJ, Penn RD, Kerns JM, Ivankovich AD, 1987. The effect of chronic subarachnoid bupivacaine infusion in dogs. Anesthesiology 66, 737–742. [DOI] [PubMed] [Google Scholar]
- Kroin JS, McCarthy RJ, Penn RD, Lubenow TJ, Ivankovich AD, 2003. Continuous intrathecal clonidine and tizanidine in conscious dogs: analgesic and hemodynamic effects. Anesth. Analg 96, 776–782 (table of contents). [DOI] [PubMed] [Google Scholar]
- Kubo M, 2017. Innate and adaptive type 2 immunity in lung allergic inflammation. Immunol. Rev 278, 162–172. [DOI] [PubMed] [Google Scholar]
- Lauretti GR, Hood DD, Eisenach JC, Pfeifer BL, 1998. A multi-center study of intrathecal neostigmine for analgesia following vaginal hysterectomy. Anesthesiology 89, 913–918. [DOI] [PubMed] [Google Scholar]
- Layland J, Carrick D, Lee M, Oldroyd K, Berry C, 2014. Adenosine: physiology, pharmacology, and clinical applications. JACC Cardiovasc. Interv 7, 581–591. [DOI] [PubMed] [Google Scholar]
- Levy JH, Brister NW, Shearin A, Ziegler J, Hug CC Jr., Adelson DM, Walker BF, 1989. Wheal and flare responses to opioids in humans. Anesthesiology 70, 756–760. [DOI] [PubMed] [Google Scholar]
- Lindgren BR, Anderson CD, Frodin T, Andersson RG, 1987. Inhibitory effects of clonidine on allergic reactions in guinea-pig skin. Eur. J. Pharmacol 134, 339–343. [DOI] [PubMed] [Google Scholar]
- Marone G, Stellato C, Mastronardi P, Mazzarella B, 1993. Mechanisms of activation of human mast cells and basophils by general anesthetic drugs. Ann. Fr. Anesth. Reanim 12, 116–125. [DOI] [PubMed] [Google Scholar]
- McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, Dong X, 2015. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 519, 237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon SM, Freas W, Mahla ME, Donlon MA, 1987. Plasma histamine and catecholamine levels during hypotension induced by morphine and compound 48/80. J. Cardiovasc. Pharmacol 9, 578–583. [DOI] [PubMed] [Google Scholar]
- Ramkumar V, Stiles GL, Beaven MA, Ali H, 1993. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J. Biol. Chem 268, 16887–16890. [PubMed] [Google Scholar]
- Rodrigues FV, Hochman B, Wood VT, Simoes MJ, Juliano Y, Ferreira LM, 2011. Effects of lidocaine with epinephrine or with buffer on wound healing in rat skin. Wound Repair Regen. 19, 223–228. [DOI] [PubMed] [Google Scholar]
- Rosow CE, Moss J, Philbin DM, Savarese JJ, 1982. Histamine release during morphine and fentanyl anesthesia. Anesthesiology 56, 93–96. [DOI] [PubMed] [Google Scholar]
- Rubinstein I, Nadel JA, Graf PD, Caughey GH, 1990. Mast cell chymase potentiates histamine-induced wheal formation in the skin of ragweed-allergic dogs. J. Clin. Invest 86, 555–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbe MB, Grafe MR, Pfeifer BL, Mirzai THM, Yaksh TL, 1993. Toxicology of baclofen continuously infused into the spinal intrathecal space of the dog. Neurotoxicology 14, 397–410. [PubMed] [Google Scholar]
- Schick B, Austen KF, 1989. Modulation of chymase-mediated rat serosal mast cell degranulation by trypsin or diisopropyl fluorophosphate. Immunology 66, 434–438. [PMC free article] [PubMed] [Google Scholar]
- Schwartz LB, Austen KF, Wasserman SI, 1979. Immunologic release of beta-hexosaminidase and beta-glucuronidase from purified rat serosal mast cells. J. Immunol 123, 1445–1450. [PubMed] [Google Scholar]
- Scudamore CL, Thornton EM, Mcmillan L, Newlands GFJ, Miller HRP, 1995. Release of the mucosal mast-cell granule chymase, rat mast-cell protease-ii, during anaphylaxis is associated with the rapid development of paracellular permeability to macromolecules in rat jejunum. J. Exp. Med 182, 1871–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal PC, Sharma P, Sanwal V, Prasad A, Kapasi A, Ranjan R, Franki N, Reddy K, Gibbons N, 1998. Morphine modulates proliferation of kidney fibroblasts. Kidney Int. 53, 350–357. [DOI] [PubMed] [Google Scholar]
- Skov MJ, Beck JC, de Kater AW, Shopp GM, 2007. Nonclinical safety of ziconotide: an intrathecal analgesic of a new pharmaceutical class. Int. J. Toxicol 26, 411–421. [DOI] [PubMed] [Google Scholar]
- Solinski HJ, Gudermann T, Breit A, 2014. Pharmacology and signaling of MAS-related G protein-coupled receptors. Pharmacol. Rev 66, 570–597. [DOI] [PubMed] [Google Scholar]
- Subramanian H, Gupta K, Ali H, 2016. Roles of mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J. Allergy Clin. Immunol 138, 700–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Verma IM, 2008. Phosphorylation of SNAP-23 by IkappaB kinase 2 regulates mast cell degranulation. Cell 134, 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharp MD, Kageysobotka A, Fox CC, Marone G, Lichtenstein LM, Sullivan TJ, 1987. Functional-heterogeneity of human mast-cells from different anatomic sites – invitro responses to morphine-sulfate. J. Allergy Clin. Immunol 79, 646–653. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Cochrane DE, 2004. Critical role of mast cells in inflammatory diseases and the effect of acute stress. J. Neuroimmunol 146, 1–12. [DOI] [PubMed] [Google Scholar]
- Thevenot PT, Baker DW, Weng H, Sun MW, Tang L, 2011. The pivotal role of fibrocytes and mast cells in mediating fibrotic reactions to biomaterials. Biomaterials 32, 8394–8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treuren BC, Galletly DC, Robinson BJ, Short TG, Ure RW, 1993. The influence of the H1 and H2 receptor antagonists, terfenadine and ranitidine on the hypotensive and gastric pH effects of the histamine releasing drugs, morphine and tubocurarine. Anaesthesia 48, 758–762. [DOI] [PubMed] [Google Scholar]
- Tufek A, Kaya S, Tokgoz O, Firat U, Evliyaoglu O, Celik F, Karaman H, 2013. The protective effect of dexmedetomidine on bupivacaine-induced sciatic nerve inflammation is mediated by mast cells. Clin. Invest. Med 36, E95–E102. [DOI] [PubMed] [Google Scholar]
- Uhle EI, Becker R, Gatscher S, Bertalanffy H, 2000. Continuous intrathecal clonidine administration for the treatment of neuropathic pain. Stereotact. Funct. Neurosurg 75, 167–175. [DOI] [PubMed] [Google Scholar]
- Veien M, Szlam F, Holden JT, Yamaguchi K, Denson DD, Levy JH, 2000. Mechanisms of nonimmunological histamine and tryptase release from human cutaneous mast cells. Anesthesiology 92, 1074–1081. [DOI] [PubMed] [Google Scholar]
- Vukman KV, Forsonits A, Oszvald A, Toth EA, Buzas EI, 2017. Mast cell secretome: soluble and vesicular components. Semin. Cell Dev. Biol 67, 65–73. [DOI] [PubMed] [Google Scholar]
- Wermeling D, Drass M, Ellis D, Mayo M, McGuire D, O'Connell D, Hale V, Chao S, 2003. Pharmacokinetics and pharmacodynamics of intrathecal ziconotide in chronic pain patients. J. Clin. Pharmacol 43, 624–636. [PubMed] [Google Scholar]
- Willis KD, Doleys DM, 1999. The effects of long-term intraspinal infusion therapy with noncancer pain patients: evaluation of patient, significant-other, and clinic staff appraisals. Neuromodulation 2, 241–253. [DOI] [PubMed] [Google Scholar]
- Wright CE, Robertson AD, Whorlow SL, Angus JA, 2000. Cardiovascular and autonomic effects of omega-conotoxins MVIIA and CVID in conscious rabbits and isolated tissue assays. Br. J. Pharmacol 131, 1325–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Allen JW, Veesart SL, Horais KA, Malkmus SA, Scadeng M, Steinauer JJ, Rossi SS, 2013a. Role of meningeal mast cells in intrathecal morphine-evoked granuloma formation. Anesthesiology 118, 664–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, de Kater A, Dean R, Best BM, Miljanich GP, 2012. Pharmacokinetic analysis of ziconotide (SNX-111), an intrathecal N-type calcium channel blocking analgesic, delivered by bolus and infusion in the dog. Neuromodulation 15, 508–519 (discussion 519). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Grafe MR, Malkmus S, Rathbun ML, Eisenach JC, 1995. Studies on the safety of chronically administered intrathecal neostigmine methylsulfate in rats and dogs. Anesthesiology 82, 412–427. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Hassenbusch S, Burchiel K, Hildebrand KR, Page LM, Coffey RJ, 2002. Inflammatory masses associated with intrathecal drug infusion: a review of preclinical evidence and human data. Pain Med. 3, 300–312. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Horais KA, Tozier NA, Allen JW, Rathbun M, Rossi SS, Sommer C, Meschter C, Richter PJ, Hildebrand KR, 2003. Chronically infused intrathecal morphine in dogs. Anesthesiology 99, 174–187. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Steinauer JJ, Veesart SL, Malkmus SA, 2013b. Alfentanil: correlations between absence of effect upon subcutaneous mast cells and absence of granuloma formation after intrathecal infusion in the dog. Neuromodulation 16, 459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksh TL, Tozier N, Horais KA, Malkmus S, Rathbun M, Lafranco L, Eisenach J, 2008. Toxicology profile of N-methyl-d-aspartate antagonists delivered by intrathecal infusion in the canine model. Anesthesiology 108, 938–949. [DOI] [PubMed] [Google Scholar]
- Yanagi H, Ozawa R, Kobayashi M, Sankawa H, Saito H, 1997. Effect of dibucaine and lidocaine on histamine release from mouse bone marrow-derived cultured mast cells. Masui 46, 16–22. [PubMed] [Google Scholar]
- Yanagi H, Sankawa H, Saito H, Iikura Y, 1996. Effect of lidocaine on histamine release and Ca2+ mobilization from mast cells and basophils. Acta Anaesthesiol. Scand 40, 1138–1144. [DOI] [PubMed] [Google Scholar]
- Yu Y, Blokhuis BR, Garssen J, Redegeld FA, 2016. Non-IgE mediated mast cell activation. Eur. J. Pharmacol 778, 33–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.