Abstract
Background
Hepatitis countermeasures are being promoted by governments in Japan. We aimed to develop performance indicators (PIs) to assess the process and outcome of such countermeasures implemented for the prevention of viral hepatitis-related liver cancer at the national and prefectural government levels.
Methods
We developed 19 PIs for hepatitis countermeasures implemented by local governments, covering the morbidity and mortality of liver cancer, hepatitis testing, subsidy programs for examinations and antiviral treatment, and education on hepatitis patient care to healthcare workers. We analyzed the PIs for each prefecture from Fiscal Year (FY) 2018–2020.
Results
The morbidity and mortality of liver cancer significantly decreased in the study period. The percentage of municipalities conducting hepatitis screening was already high at 95% in FY2017. The usage rate of government-subsidized screenings did not change. The subsidy usage rate for periodic viral hepatitis examination significantly increased. Meanwhile, the subsidy usage rate for antiviral treatment of hepatitis B increased, whereas that for hepatitis C decreased. The number of certified healthcare workers providing care for hepatitis patients increased significantly, and these workers were efficiently placed at regional core centers, institutions specialized in liver diseases, health care centers, and municipal governments. Liver cancer mortality was positively correlated with hepatitis screening, subsidies for periodic examinations, and the number of hepatitis medical care coordinators but was negatively correlated with subsidies for anti-HCV therapy, suggesting that rigorous countermeasures were implemented in prefectures with high liver cancer mortality.
Conclusions
The developed PIs could be a useful tool for monitoring government efforts and achievements, thereby providing basic data for setting practical goals in liver cancer prevention.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00535-023-01956-1.
Keywords: Hepatitis B virus, Hepatitis C virus, Viral hepatitis, Liver cancer, Performance indicators
Introduction
Approximately, 257 million people are estimated to be infected with hepatitis B virus (HBV) and 71 million infected with hepatitis C virus (HCV) all over the world [1]. Both viruses cause liver cirrhosis and liver cancer. Liver cancer is the second most common cause of cancer deaths in the Asia-Pacific Region, and approximately 78% of liver cancer are due to chronic hepatitis B or C [1]. In Japan, HCV infection (60.3%) and HBV infection (12.9%) were the leading and third-most common causes of liver cancer in the analysis from 2008 to 2016 [2]. Therefore, effective countermeasures for HBV- or HCV-infected people are necessary to reduce mortality from liver cancer. Japan has begun implementing various hepatitis countermeasures since early 2000 s, as a 5-year project on publicly funded national screening of HBV and HCV infection among residents aged 40 years and over was implemented during 2002–2006. As a result of these countermeasures, the estimated number of people with viral hepatitis in Japan has been trending downward for both HBV and HCV, decreasing from 1.31 to 1.47 million for HBV and 1.69–2.20 million for HCV in 2000 to 1.12–1.27 million for HBV and 0.98–1.58 million for HCV in 2011 [3, 4]. In addition to the decrease between 2000 and 2011, the release of many new antiviral drugs after 2011 that are potent inhibitors of viral replication has made control of HBV and HCV infection much easier than before. Therefore, it is critical to increase the rate of hepatitis virus screening, detect infected individuals, have those individuals promptly undergo antiviral therapy at institutions specialized in liver disease care, and provide education on hepatitis patient care to healthcare workers in order to reduce mortality from liver diseases such as cirrhosis and cancer.
In Japan, the Basic Act on Hepatitis Control (Act No. 97 of 2009) was enacted in January 2010 [5], specific guidelines for hepatitis control were established in May 2011, and the Basic Guidelines for Promotion of Control Measures for Hepatitis (Ministry of Health, Labour and Welfare [MHLW] Notification No. 160 of 2011) were established. The guidelines were also revised in June 2016 (MHLW Notification No. 278 of 2016) and in March 2022 (MHLW Notification No. 62 of 2022) [6]. The revised 2022 guidelines recommended that stakeholders related to hepatitis care promote hepatitis countermeasures more efficiently to reduce the regional disparities in hepatitis countermeasures. In other words, efficient collaboration between local governments (prefectural and municipal governments), regional core centers for the management of liver disease, institutions specialized in liver diseases, and primary care physicians is necessary to deliver high-quality hepatitis care. Local governments, especially prefectural governments, have a particularly important role among these groups in creating hepatitis countermeasures, raising awareness of hepatitis, promoting hepatitis screening, setting hepatitis-related performance targets, and distributing subsidies for testing and treatment as well as the recruitment, training, and placement of healthcare workers. Direct comparisons of hepatitis countermeasures among Japan’s 47 prefectures are difficult because of differences in morbidity and mortality from liver cancer, in the prevalence of hepatitis virus, in the etiology of liver cancer, and in infrastructure for hepatitis care [2, 3, 7], and each prefectural government has to perform different ways to control viral hepatitis-related disease based on the circumstances. To assess each prefecture’s efforts on controlling viral hepatitis and subsequently reducing liver cancer mortality, we aimed to develop performance indicators (PIs) reflecting achievements related to various aspects of hepatitis countermeasures.
We developed 19 PIs and analyzed their changes at the prefectural level over the 3-year period from Fiscal Year (FY) 2017 to FY2019 (FY2016 to FY2018 for one indicator). We also determined which PIs correlated with liver cancer mortality at the prefectural level. Active monitoring and assessment of these PIs might provide essential information about the success of countermeasures aimed at preventing liver cancer implemented at the prefectural and national levels.
Methods
Development of PIs for local government programs
In FY2017, we developed draft indicators for local hepatitis control programs, divided into three areas: (1) hepatitis screening (S), (2) follow-up with individuals who screen positive for hepatitis virus (FU), and (3) policies (P). A research group supported by a Health and Labor Science Research Grant developed a draft of the PIs, which was reviewed by external committee members, including hepatologists, occupational health physicians, and hepatitis patient advocacy groups as well as MHLW officials and local (prefectural and municipal) government officials in charge of hepatitis countermeasures. In FY2018, we finalized a total of 19 indicators (7 related to screening, 3 to follow-up, and 9 to policies; Table 1) and began using these indicators to evaluate program performance between FY2016 and FY2019 (hereinafter, “FY” is omitted).
Table 1.
Summary of the 19 performance indicators
| Indicator | Item | Numerator | Denominator |
|---|---|---|---|
| S1 | Rate of municipalities conducting hepatitis screening (through the Health Promotion Service) | Number of municipalities conducting hepatitis screening 1) | Number of municipalities 8) |
| S2 | Hepatitis screening rate per 100,000 adults aged 40 years or older (through the Health Promotion Service, calculated separately for HBV and HCV) | Number of people screened for hepatitis (through the Health Promotion Service) 2) | 100,000 individuals in the total population aged 40 years or older 9) |
| S3 | Hepatitis screening rate per 100,000 adults (through the Specific Infectious Disease Testing Service, calculated separately for HBV and HCV) | Number of people screened for hepatitis (through the Specific Infectious Disease Testing Service) 3) | 100,000 individuals in the total adult population 9) |
| S4 | Liver cancer incidence (morbidity) per 100,000 population (crude and age-adjusted) | Number of people with liver cancer 4) | 100,000 population |
| S5 | Liver cancer mortality per 100,000 population (crude and age-adjusted) | Number of deaths from liver cancer 5) | 100,000 population |
| S6 | Usage rate of subsidies for nucleos(t)ide analog therapy for hepatitis B | Number of patients receiving subsidy for nucleos(t)ide analog therapy 6) | Number of patients treated for hepatitis B (hepatitis and compensated or uncompensated cirrhosis) in FY2018 according to a FY2020 study based on a national database 10) |
| S7 | Usage rate of subsidies for DAA therapy for hepatitis C | Number of patients receiving subsidies for DAA therapy 6) | Number of patients treated for hepatitis C (hepatitis and compensated or uncompensated cirrhosis) in FY2018 according to a FY2020 study based on a national database 10) |
| FU1 | Usage rate of subsidies for initial detailed examinations per 100,000 adults | Number of individuals who received subsidies for initial detailed examinations 7) | 100,000 individuals in the total adult population 9) |
| FU2 | Rate of municipalities conducting follow-up | Number of municipalities conducting follow-up 1) | Number of municipalities 8) |
| FU3 | Usage rate of subsidies for periodic examinations per 100,000 adults | Number of individuals in each prefecture receiving subsidies for periodic examinations | 100,000 individuals in the total adult population 9) |
| P1 | Whether or not the prefecture had created a plan and numerical targets for countermeasures against hepatitis 1) | N/A | N/A |
| P2 | Whether or not the prefecture’s council responsible for hepatitis countermeasures held meetings 1) | N/A | N/A |
| P3 | Total number of certified hepatitis medical care coordinators (HMCCs) per 100,000 adults | Total number of certified HMCCs1) | 100,000 individuals in the total adult population 9) |
| P4 | Number of newly certified HMCCs per 100,000 adults in each year | Number of newly certified HMCCs a year 1) | 100,000 individuals in the total adult population 9) |
| P5 | Whether or not the local government had a system for renewing HMCC certification 1) | N/A | N/A |
| P6 | HMCC staffing rate at regional core centers for the management of liver disease | Number of regional core centers with at least one HMCC 1) | Number of regional core centers 1) |
| P7 | HMCC staffing rate at institutions specialized in liver diseases | Number of institutions specialized in liver diseases with at least one HMCC 1) | Number of institutions specialized in liver diseases |
| P8 | HMCC staffing rate at public health centers | Number of public health centers with at least one HMCC 1) | Number of public health centers 1) |
| P9 | HMCC staffing rate at municipal governments | Number of municipal governments with at least one HMCC 1) | Number of municipalities 8) |
The data sources used to calculate indicators 1) through 10) are listed in the Supplementary Information
HBV hepatitis B virus, HCV hepatitis C virus, DAA direct-acting antivirals, HMCC hepatitis medical care coordinators, N/A not applicable
Screening (S)
Hepatitis screening programs implemented by local governments fall into two main categories: those implemented by municipalities through the Health Promotion Service, and those implemented by public health centers, prefectures, and ordinance-designated cities through the Specific Infectious Disease Testing Service [8, 9]. S1 is the rate of municipalities conducting hepatitis screening through the Health Promotion Service, S2 is the hepatitis screening rate per 100,000 adults aged 40 years or older through the Health Promotion Service, and S3 is the hepatitis screening rate per 100,000 adults through the Specific Infectious Disease Testing Service. S2 and S3 were determined individually for HBV and HCV infection.
S4 and S5 are related to morbidity and mortality from liver cancer. S4 is the incidence (morbidity) of liver cancer per 100,000 population (crude and age-adjusted), and S5 is the liver cancer mortality rate per 100,000 population (crude and age-adjusted).
S5 and S6 are related to usage rate of subsidies for antiviral treatment. In Japan, patients undergoing antiviral therapy (interferon or nucleos(t)ide analogs for hepatitis B and DAA for hepatitis C) are eligible to receive subsidies to reduce their out-of-pocket medical expenses [9, 10], and most of such patients use this subsidy program. S6 is the usage rate of subsidies for nucleos(t)ide analog therapy for hepatitis B, and S7 is the usage rate of subsidies for direct-acting antiviral (DAA) therapy for hepatitis C.
Follow-up (FU)
To prevent the progression to cirrhosis and liver cancer as well as to detect and treat these diseases at earlier stages, it is important to direct people who screen positive for hepatitis to promptly seek an initial detailed examination and to undergo periodic examinations. To this end, the subsidies are provided for initial detailed examinations and periodic examinations. The initial-examination subsidy covers the cost of all necessary examinations when people who screen positive for hepatitis visit a specialist physician. The subsidy for periodic examinations is available up to twice a year for patients with chronic hepatitis, cirrhosis, or liver cancer caused by viral hepatitis (including those undergoing follow-up for eradicated HCV) [9, 10]. Importantly, individuals who apply for these subsidies must consent to participate in a follow-up project in which staff from local governments or core centers periodically confirm whether they have visited and received treatment at a medical institution. Those who have not sought medical attention are encouraged to do so by phone or an in-person visit, as necessary. Various follow-up efforts have been implemented in different regions [11, 12]. Based on this context, we developed three follow-up PIs: FU1 is the subsidy usage rate for initial detailed examinations per 100,000 adults, FU2 is the rate of municipalities conducting follow-ups, and FU3 is the subsidy usage rate for periodic examinations per 100,000 adults.
Policies (P)
P1 is whether or not the prefecture had created a plan and specific numerical targets for hepatitis measures, and P2 is whether or not the prefecture’s council responsible for hepatitis countermeasures held meetings. P3 through P9 are related to hepatitis medical care coordinators (HMCCs) [13]. HMCCs are professionals who are expected to ensure the efficient implementation of hepatitis countermeasures. The health care workers such as physicians, nurses, local government officials, and pharmacists can be qualified as HMCCs based on the regulation on each prefecture. They provide basic information about hepatitis to residents and patients from their respective professional perspectives and encourage people to be screened and seek appropriate medical care depend on their positions. In response to a strong request from MHLW in 2017 [14], prefectures across Japan began putting greater effort into training HMCCs. P3 is the total number of certified HMCCs per adult population, and P4 is the number of new HMCCs certified in a given fiscal year per adult population. It is also important for HMCCs to stay up to date with the latest information on hepatitis; thus, we thought that one way to achieve this might be to set criteria for renewing HMCC certification. Hence, P5 is whether or not the local government has a system for renewing HMCC certification. P6, P7, P8, and P9 are the HMCC staffing rate at regional core centers for the management of liver diseases, institutions specialized in liver diseases, public health centers, and municipal governments, respectively.
Data collection
The necessary data (numerator and denominator) for calculating each PI are shown in Table 1, and the respective data sources are shown in the supplementary information. There were no missing data for the PIs of all 47 prefectures.
Statistical analysis
Comparisons of PIs between 2 years were analyzed by repeated measures analysis of variance and the trend analysis through 3 years was performed by Jonckheere–Terpstra test. Subjects were grouped according to crude liver cancer mortality in 2019, and comparisons of PI between groups were performed in the same way. Correlation coefficients were calculated between each PI and the other 18 PIs. To analyze the relationships of PIs in terms of crude liver cancer mortality, multivariate regression analysis with forward-stepwise selections of variables was performed. P < 0.05 was considered significant. All statistical analyses were performed using GraphPad Prism 9.3.1 (GraphPad Software; La Jolla, CA) or IBM SPSS Statistics version 27 (IBM, Armonk, NY).
Results
Hepatitis screening
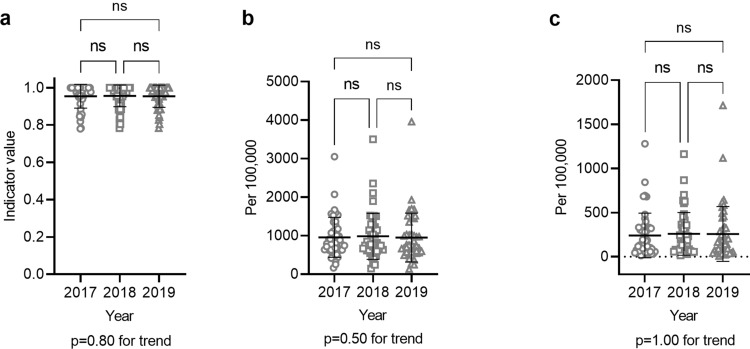
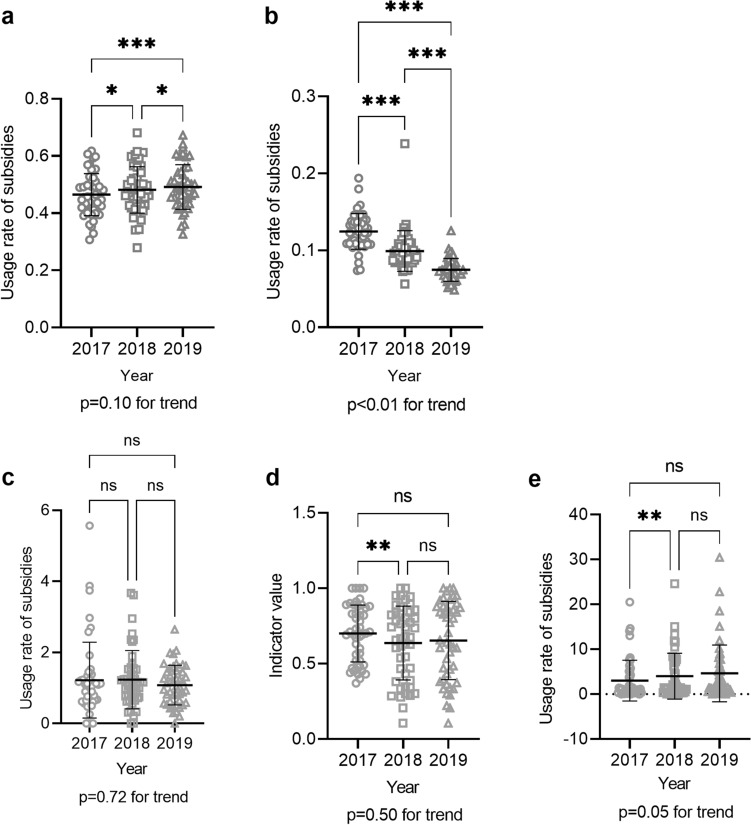
S1, the percentage of municipalities conducting hepatitis screening through the Health Promotion Service, was already high at 95% in 2017, the first year of the 3-year study period, and did not change significantly over the study period (Fig. 1a). This shows that municipalities were already conducting hepatitis screening in many prefectures. S2, the hepatitis screening rate through the Health Promotion Service, and S3, the hepatitis screening rate through the Specific Infectious Disease Testing Service, did not change significantly for either HBV and HCV tests over the 3-year study period (Fig. 1b and c, Supplementary Fig. 1), suggesting a need for further publicity campaigns to encourage people to take advantage of these hepatitis screening opportunities. S4 included both the crude and age-adjusted incidences of liver cancer, both of which significantly decreased every year between any 2 years of the study period (Fig. 2a). S5 included the crude and age-adjusted mortalities from liver cancer, both of which significantly decreased every year between any 2 years of the study period. (Fig. 2b). S6, the usage rate of subsidies for nucleos(t)ide analog therapy for hepatitis B, increased significantly between any years of the study period (Fig. 3a). S7, the usage rate of subsidies for DAA therapy for hepatitis C, decreased significantly between any years of the study period (Fig. 3b).
Fig. 1.
Indicators related to hepatitis screening (S1–S3). a S1 The rate of municipalities conducting hepatitis screening through the Health Promotion Service was calculated for each prefecture, and the value for each prefecture was plotted for each fiscal year. b S2 The hepatitis B virus (HBV) screening rate per 100,000 adults aged 40 years or older through the Health Promotion Service was plotted for each prefecture for each fiscal year. c S3 The HBV screening rate per 100,000 adults through the Specific Infectious Disease Testing Service was plotted for each prefecture for each fiscal year. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ns not significant; error bars show standard deviation
Fig. 2.
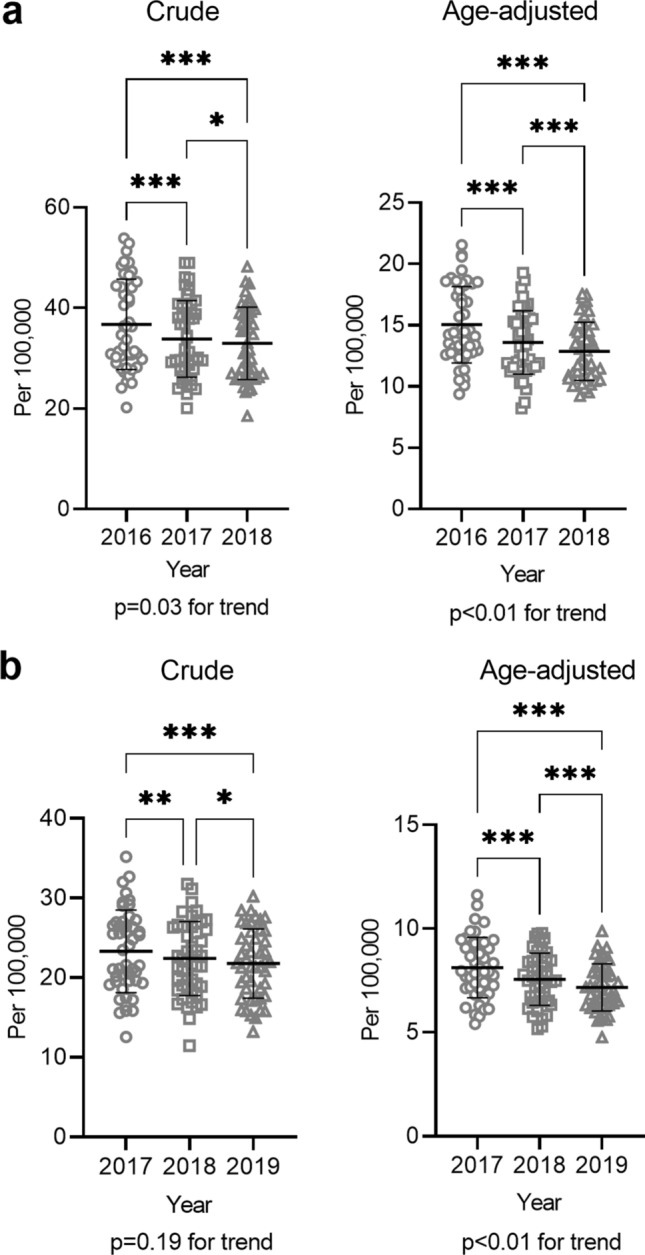
Indicators related to morbidity and mortality from liver cancer (S4, S5). a S4 The crude (left) and age-adjusted (right) incidences of liver cancer per 100,000 population were plotted for each prefecture for each fiscal year. b S5 The crude (left) and age-adjusted (right) mortality rates from liver cancer per 100,000 population was plotted for each prefecture for each fiscal year. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ns not significant; error bars show standard deviation
Fig. 3.
Indicators related to antiviral therapy (S6, S7) and follow-up (FU1–FU3). a S6: the usage rate of subsidies for nucleos(t)ide analog therapy for hepatitis B was plotted for each prefecture for each fiscal year. b S7: the usage rate of subsidies for DAA therapy for hepatitis C was plotted for each prefecture for each fiscal year. c FU1: the usage rate of subsidies for initial detailed examinations per 100,000 adults was plotted for each prefecture for each fiscal year. d FU2: the percentage of municipalities conducting follow-ups was calculated for each prefecture, and the value for each prefecture was plotted for each fiscal year. e FU3: the usage rate of subsidies for periodic examinations per 100,000 adults was plotted for each prefecture for each fiscal year. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ns not significant; error bars show standard deviation
Follow-up of individuals who screened positive for hepatitis
FU1, the subsidy usage rate for initial detailed examinations, did not change significantly over the 3 year study period (Fig. 3c). FU2, the rate of municipalities conducting follow-ups, decreased significantly from 0.7 in 2017 to 0.64 in 2018 (Fig. 3d), demonstrating a need for improvement in this area. FU3, the subsidy usage rate for periodic examinations, increased significantly between 2017 and 2018, suggesting that the program had become more widespread (Fig. 3e).
Policies
Regarding P1, all prefectures created a plan for implementing hepatitis countermeasures during the 3-year study period. It is also important to set specific numerical targets rather than abstract goals in order to assess the effectiveness and success of countermeasures. However, there were five prefectures in 2017 and 2018 as well as four in 2019 that did not set specific numerical targets (Table 2, top section). Regarding P2, all prefectures established a council to handle hepatitis countermeasures, and all such councils met in 2017 and 2018. However, in 2019, the councils in 11 prefectures did not meet (Table 2, middle), probably due to the impact of COVID-19 pandemic.
Table 2.
Indicators related to hepatitis control policies (P1, P2, P5)
| Year | Plan | Numerical targets | ||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| 2017 | 47 | 0 | 42 | 5 |
| 2018 | 47 | 0 | 42 | 5 |
| 2019 | 47 | 0 | 43 | 4 |
| Year | Yes | No |
|---|---|---|
| 2017 | 47 | 0 |
| 2018 | 47 | 0 |
| 2019 | 36 | 11 |
| Year | Yes | No |
|---|---|---|
| 2017 | 16 | 31 |
| 2018 | 21 | 26 |
| 2019 | 23 | 24 |
P1 Whether or not the prefecture had created a plan and numerical targets for hepatitis countermeasures
P2 Whether or not the prefecture’s council for hepatitis countermeasures met
P5 Whether or not the local government had a system for renewing HMCC certification
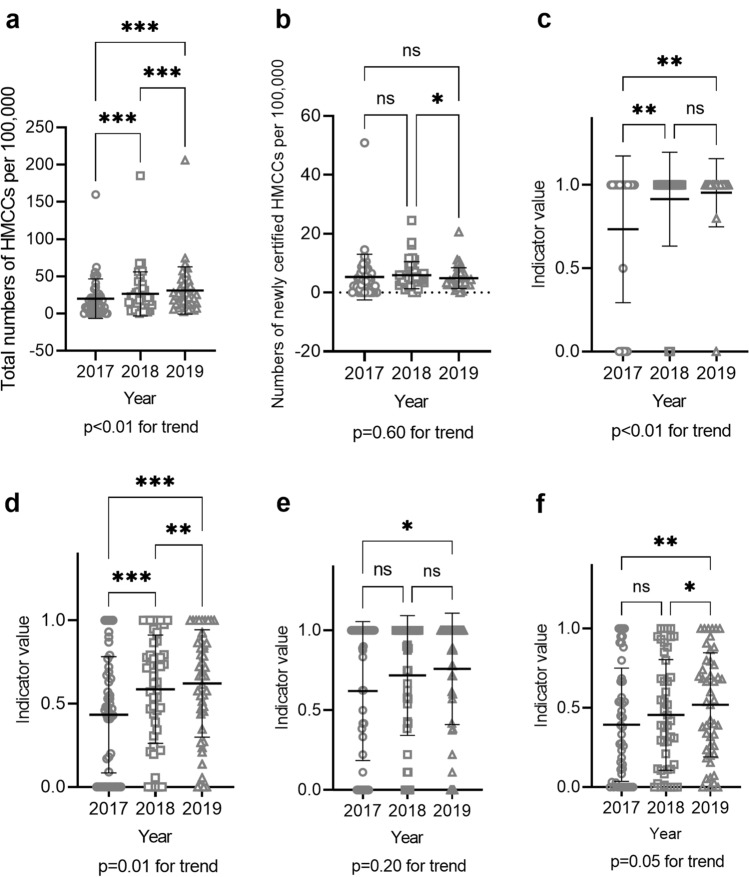
P3, the total number of certified HMCCs per 100,000 adults, increased significantly between any 2 years of the study period (Fig. 4a). P4, the number of newly certified HMCCs per 100,000 adults a year, decreased significantly between 2018 and 2019 (Fig. 4b). P5, whether or not the local government had a system for renewing HMCC certification, showed an increasing trend, with 16 having such systems in 2017, 21 in 2018, and 23 in 2019 (Table 2, bottom). P6, the HMCC staffing rate at regional core centers, dramatically increased from 0.73 in 2017 to 0.95 in 2018 (Fig. 4c). P7, the staffing rate at institutions specialized in liver diseases, was 0.43 in 2017, 0.59 in 2018, and 0.62 in 2019, showing a significant increase every year (Fig. 4d). P8, the staffing rate at public health centers, was 0.62 in 2017, 0.72 in 2018, and 0.76 in 2019, showing a significant increase between 2017 and 2019 (Fig. 4e). P8, the staffing rate at municipal governments, was 0.39 in 2017, 0.46 in 2018, and 0.52 in 2019, showing a significant increase between 2018 and 2019 (Fig. 4f). The trend analysis showed that the staffing rate at regional core centers and institutions specialized in liver diseases significantly increased over this period. The HMCC staffing rate at regional core centers improved up to a sufficiently high level but that at institutions specialized in liver diseases, public health centers, and municipal governments, especially the last two, needs further improvement.
Fig. 4.
Indicators related to training of hepatitis medical care coordinators (HMCCs) (P3, P4, P6–P9). a P3: the total number of certified HMCCs per 100,000 adults was plotted for each prefecture for each fiscal year. b P4: the number of newly certified HMCCs per 100,000 adults was plotted for each prefecture for each fiscal year. c P6: the rate of regional core centers for the management of liver disease staffed with at least one HMCC was plotted for each prefecture for each fiscal year. d P7: the rate of institutions specialized in liver diseases care staffed with at least one HMCC was plotted for each prefecture for each fiscal year. e P8: the rate of public health centers staffed with at least one HMCC was plotted for each prefecture for each fiscal year. f P9: the rate of municipal governments staffed with at least one HMCC was plotted for each prefecture for each fiscal year. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ns not significant; error bars show standard deviation
Correlation analysis
Next, we analyzed the correlation between each PI and the other 18 using the 2019 data and observed several statistically significant correlations, as shown in Supplementary Table 1. Among them, we focused on S5 (crude liver cancer mortality) because it is one of the most accurately calculated PIs and because death due to liver cancer is the most critical consequence of hepatitis virus infection. Crude liver cancer mortality statistically correlated with the following PIs: S3, S4 (both crude and age-adjusted), S5 (age-adjusted), S7, FU3, P3, and P4. It positively correlated with S3, S4, FU3, P3, and P4 and negatively correlated with S7 (Supplementary Fig. 2 and Supplementary Table 2). Furthermore, S4 (crude liver cancer morbidity) showed a similar correlation pattern with crude liver cancer mortality because it positively correlated with S5 (both crude and age-adjusted), FU3, P3, and P4 and negatively correlated with S7 (Supplementary Table 2). Multivariate regression analysis revealed that S7 and P3 significantly correlated with crude liver cancer mortality (Supplementary Table 2).
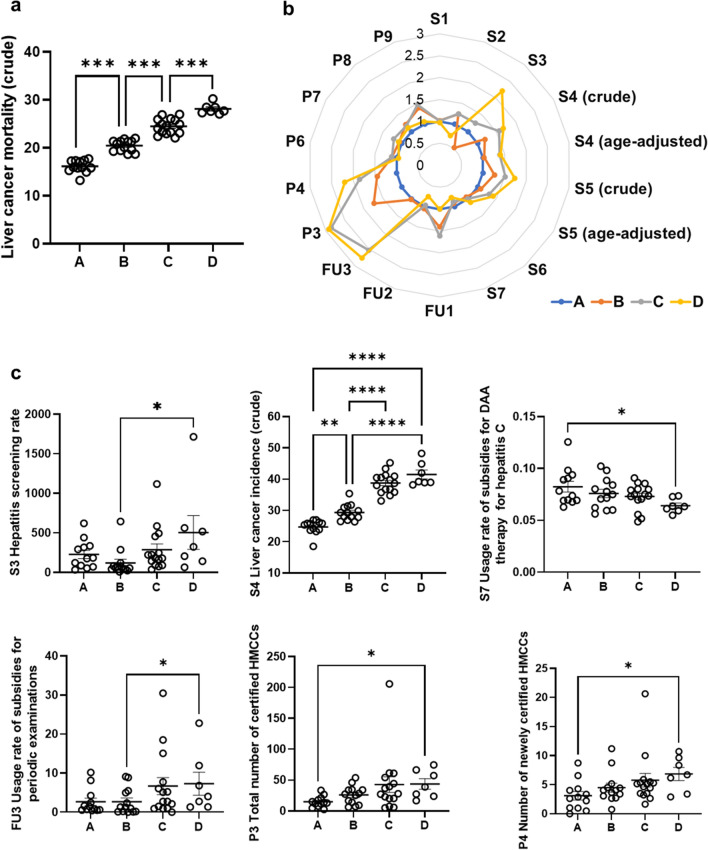
To further analyze the differences in PIs among prefectures according to liver cancer mortality, we categorized all the prefectures into four groups, A–D (Fig. 5a). In this grouping, the difference between the maximum and minimum crude liver cancer mortality was evenly divided into four groups, with group A as the lowest and D as the highest. The PIs of groups B, C, and D were normalized to those of group 1, with group A PIs set to 1, thereby showing the differences in all PIs among the four groups (Fig. 5b). We also compared the differences in PIs for which we observed a significant correlation with crude liver cancer mortality in Supplementary Table 2. Crude liver cancer incidence (S4) among groups showed a similar trend with crude liver cancer mortality. S3 and FU3 of group D were significantly higher than those of group B. S7 of group D was significantly lower than that of group A. P3 and P4 of group D were significantly higher than those of group A (Fig. 5c).
Fig. 5.
Performance indicators related to crude liver cancer mortality in 2019. S5 Crude liver cancer mortality was divided into four groups based on the mortality rate in 2019. A = less than 18, 12 prefectures; B = 18–22, 13 prefectures; C = 22–27, 15 prefectures; D = more than 27, 7 prefectures. The differences between groups were analyzed by one-way analysis of variance. a histogram was generated to compare the difference of several PIs among the four groups. PIs of groups B, C, and D were normalized to those of group 1, with group A PIs set to 1. S4 was the data from 2018, and the other PIs were from 2019. b Differences in several PIs, including S3 (HBV), S4, S7, FU3, P3, and P4, between groups were analyzed by one-way analysis of variance. S4 was the data from 2018, and the other PIs were from 2019. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. ns not significant; error bars show standard error
Discussion
Hepatitis B or C virus infection is one of the main causes of liver cancer in Japan and around the world. To prevent the development of virus-induced liver cancer, eradication of HCV or suppressing HBV replication in patients is necessary in the clinic. To this end, many antiviral medications, including generic versions, have been approved and registered in countries regardless of income level; therefore, it is fair to say that getting access to powerful antivirals is no longer a barrier to achieving the WHO goal of eliminating hepatitis virus by 2030 [15]. At the same time, it is critical to increase the rate of hepatitis virus testing in order to detect undiagnosed individuals and connect them with hepatologists who will provide appropriate antiviral therapy, thereby helping to reduce morbidity and mortality in patients with HBV/HCV-associated liver diseases. In Japan, prefectural governments play an extremely important role in hepatitis countermeasures because individual prefectures have a responsibility to implement countermeasures in accordance with the national policy. However, an unbiased comparison of prefectural countermeasures is difficult because each prefecture has different infrastructure for hepatitis care. To evaluate the progress and achievements of hepatitis countermeasures at the prefectural and national levels, we developed hepatitis PIs and analyzed their changes over 3 years.
Similar clinical indicators, which have appeared as quality indicators or key performance indicators, have been developed for application to various medical areas, including general health care capacity [16], emergency departments [17], intensive care units [18], medication management services [19], and radiation oncology [20]. Such indicators allow medical providers to monitor and improve their own performance internally. Another important role for indicators is to provide comparative information across all medical providers. The hepatitis PIs developed herein are quite unique because they enable us to assess various aspects of countermeasure processes and outcomes within and among prefectures and to provide a comparative view nationwide for policymakers.
Indicators for the morbidity (S4) and mortality (S5) of liver cancer revealed that both significantly decreased in crude and age-adjusted numbers over the 3-year study period (Fig. 2). These results are supported by a nationwide survey, which showed that liver cancer mortality peaked in Japan in the early 2000s and has gradually decreased ever since [3]. It is thus plausible to consider that the various hepatitis countermeasures implemented in Japan to date have worked effectively. One of the limitations of the PIs is the lack of indicators reflecting morbidity and mortality of HBV/HCV-related liver cirrhosis and liver failure. Further nationwide surveys are needed to collect reliable data on the morbidity and mortality of advanced liver diseases in Japan.
The percentage of hepatitis patients receiving antiviral therapy is a crucial indicator for hepatitis control. Therefore, it is important to estimate the number of people with hepatitis and the number of people receiving antiviral therapy as accurately as possible. We used the National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB), an archive of anonymized electronic insurance claims, to obtain data on hepatitis patients for this study and to calculate the number of people with hepatitis B and hepatitis C as of 2018 [21]. It is important to note that the number of patients calculated from the NDB is only an approximation because insurance claims may not always reflect the correct diseases. To calculate the number of patients who received antiviral therapy, we used the number of people enrolled in a medical expense subsidy program for hepatitis care [9, 10] because most such patients use this subsidy program to reduce their out-of-pocket medical expenses. The usage rate of subsidies for nucleos(t)ide analog therapy for hepatitis B increased significantly during the study period (Fig. 3a), suggesting that the number of HBV-positive patients receiving treatment increased over time. In contrast, the usage rate of subsidies for DAA therapy for hepatitis C decreased significantly (Fig. 3b), one reason for which is the reduction of untreated HCV-positive patients who are eligible for DAA therapy. Highly efficient DAA therapy was introduced as standard clinical practice in Japan in 2014 and many patients were treated afterwards.
To determine the PIs impacting liver cancer mortality, a correlation analysis of the 2019 data was performed. Crude liver cancer mortality significantly correlated positively with S3 (hepatitis screening rate), FU3 (usage rate of subsidies for periodic examinations), P3 (total number of certified HMCCs), and P4 (number of newly certified HMCCs in each year) and negatively correlated with S7 (usage rate of subsidies for DAA therapy for hepatitis C) (Supplementary Fig. 2 and Supplementary Table 2). Comparisons among the four prefecture groups categorized by liver cancer mortality showed similar trends in the correlation analysis (Fig. 5). It seems to be the opposite correlation of liver cancer morbidity and mortality (S4 and S5) with testing (S3), follow-ups (FU3), education for HMCCs (P3 and P4), and treatment (S7). Given that morbidity and mortality are outcomes of countermeasures and medical care thus far, reduction of liver cancer deaths has been a crucial target of action in prefectures with a high mortality. Thus, it is likely that more rigorous action has been taken in such prefectures compared with others, thereby improving testing, follow-up, education, and treatment. A negative correlation was found between liver cancer mortality and treatment subsidies (S7), suggesting that most patients with HCV had already been treated by DAA between 2014 and 2018 and that the number of remaining untreated patients is small in prefectures with higher liver cancer mortality. It must be noted that individuals can be diagnosed as having liver cancer first, then screened for hepatitis virus for searching etiology, and undergo antiviral therapy or surveillance for recurrence after diagnosis, in other words, the present analysis does not take care of the order by which these PIs were performed. These results indicate that the intensive efforts/countermeasures of the prefectures with higher liver cancer mortality to reduce it have been well reflected in several PIs, however, the present study does not show the direct evidence that these intensive efforts/countermeasures will surely result in the reduction on liver cancer mortality. Since it would take some time for these intensive efforts/countermeasures to contribute to reductions in liver cancer mortality, these PIs should be continuously monitored over time.
The hepatitis virus infection is still the leading cause of liver cancer in Japan, however, the rate of viral hepatitis-related liver cancer is decreasing, as it decreased from 85.3 to 64.4% between 2008 and 2016. Instead, the rate of nonalcoholic steatohepatitis (NASH)/nonalcoholic fatty liver disease (NAFLD) has dramatically increased as it increased from 8.5 to 18.6% at the same period [2]. Thus, to further reduce the morality from liver cancer in near future, the effective countermeasures and the relevant PIs to identify the patients with NASH/NAFLD and follow up those are urgently needed.
In summary, we developed and evaluated PIs for hepatitis countermeasures implemented over a 3-year period. Continuous assessment of indicators should help to clarify the progress of hepatitis countermeasures in each prefecture as well as issues that need to be addressed. Periodically reviewing and updating PIs will be necessary to further improve their feasibility and reliability because the circumstances of viral hepatitis patients are likely to change over time. Active usage of PIs by local and national governments might promote hepatitis countermeasures aimed at preventing liver cancer across Japan, thereby reducing regional disparities and increasing access to high-quality hepatitis care.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This study was funded by a Health, Labour and Welfare Policy Research Grant (Policy Research for Eliminating Hepatitis) for “Development of Indicators for Evaluating the Pathology of Hepatitis and Application of those Indicators to Measures against Hepatitis” (Principal researcher: Tatsuya Kanto, FY2017–2019) and “New Approaches to Enhancing Comprehensive Measures against Hepatitis” (Principal researcher: Tatsuya Kanto, grant #20HC2002, FY2020–2021).
Author contributions
All the authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Tetsuro Shimakami, Junko Tanaka, and Tatsuya Kanto. The first draft of the manuscript was written by Tetsuro Shimakami, and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. WHO Global hepatitis report, 2017. https://www.who.int/publications/i/item/global-hepatitis-report-2017 (accessed May Nov 22, 2022).
- 2.Enomoto H, Ueno Y, Hiasa Y, et al. The transition in the etiologies of hepatocellular carcinoma-complicated liver cirrhosis in a nationwide survey of Japan. J Gastroenterol. 2021;56:158–167. doi: 10.1007/s00535-020-01748-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka J, Akita T, Ko K, et al. Countermeasures against viral hepatitis B and C in Japan: an epidemiological point of view. Hepatol Res. 2019;49:990–1002. doi: 10.1111/hepr.13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanaka J, Akita T, Ohisa M, et al. Trends in the total numbers of HBV and HCV carriers in Japan from 2000 to 2011. J Viral Hepat. 2018;25:363–372. doi: 10.1111/jvh.12828. [DOI] [PubMed] [Google Scholar]
- 5.Ministry of Health, Labour and Welfare. Basic Act on Hepatitis Control https://elaws.e-gov.go.jp/document?lawid=421AC1000000097Accessed September 7th, 2022. (in Japanese)
- 6.Ministry of Health, Labour and Welfare. Basic Guidelines for Promotion of Control Measures for Hepatitis. https://www.mhlw.go.jp/hourei/doc/hourei/H220308H0021.pdf Accessed September 7th, 2022. (in Japanese)
- 7.Setoyama H, Korenaga M, Kitayama Y, et al. Nationwide survey on activities of regional core centers for the management of liver disease in Japan: cumulative analyses by the Hepatitis Information Center 2009–2017. Hepatol Res. 2020;50:165–173. doi: 10.1111/hepr.13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korenaga M, Kanto T. Testing, diagnosis of viral hepatitis, and the follow-up policy in Japan. Glob Health Med. 2021;3:308–313. doi: 10.35772/ghm.2021.01072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oza N, Isoda H, Ono T, et al. Current activities and future directions of comprehensive hepatitis control measures in Japan: the supportive role of the hepatitis information center in building a solid foundation. Hepatol Res. 2017;47:487–496. doi: 10.1111/hepr.12897. [DOI] [PubMed] [Google Scholar]
- 10.Setoyama H, Tanaka Y, Kanto T. Seamless support from screening to anti-HCV treatment and HCC/ decompensated cirrhosis: subsidy programs for HCV elimination. Glob Health Med. 2021;3:335–342. doi: 10.35772/ghm.2021.01079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kikuchi M, Sawabe M, Aoyagi H, et al. Development of an intervention system for linkage-to-care and follow-up for hepatitis B and C virus carriers. Hepatol Int. 2022;16:68–80. doi: 10.1007/s12072-021-10269-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimakami T, Kaneko S. Use of information and communication technology in the support of viral hepatitis patients in Japan. Glob Health Med. 2021;3:314–320. doi: 10.35772/ghm.2021.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isoda H, Eguchi Y, Takahashi H. Hepatitis medical care coordinators: comprehensive and seamless support for patients with hepatitis. Glob Health Med. 2021;3:343–350. doi: 10.35772/ghm.2021.01073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The director of the HSB of Ministry of Health, Labour and Welfare. HSB Notification No. 0425–4. Notification regarding training and utilization of hepatitis medical care coordinators. Ministry of Health, Labour and Welfare. 2017. https://www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou09/pdf/hourei-170425-1.pdf Accessed September 7th, 2022. (in Japanese)
- 15.World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. Towards ending viral hepatitis. World Health Organization. 2016. https://apps.who.int/iris/handle/10665/246177 Accessed September 7th, 2022.
- 16.Gibberd R, Hancock S, Howley P, et al. Using indicators to quantify the potential to improve the quality of health care. Int J Qual Health Care. 2004;16(Suppl 1):i37–43. doi: 10.1093/intqhc/mzh019. [DOI] [PubMed] [Google Scholar]
- 17.Burkett E, Martin-Khan MG, Gray LC. Quality indicators in the care of older persons in the emergency department: a systematic review of the literature. Australas J Ageing. 2017;36:286–298. doi: 10.1111/ajag.12451. [DOI] [PubMed] [Google Scholar]
- 18.Rewa OG, Stelfox HT, Ingolfsson A, et al. Indicators of intensive care unit capacity strain: a systematic review. Crit Care. 2018;22:86. doi: 10.1186/s13054-018-1975-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lima TM, Aguiar PM, Storpirtis S. Development and validation of key performance indicators for medication management services provided for outpatients. Res Social Adm Pharm. 2019;15:1080–1087. doi: 10.1016/j.sapharm.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Lopez Torrecilla J, Marin IBS, Ruiz-Alonso A, et al. Quality indicators in radiation oncology: proposal of the Spanish society of radiation oncology (SEOR) for a continuous improvement of the quality of care in oncology. Clin Transl Oncol. 2019;21:519–533. doi: 10.1007/s12094-018-1943-z. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka J, Kurusu A. Analysis of patients who have received (or are receiving) drugs or medical treatment for hepatitis B or C virus-related liver disease using the NDB. Health and Labour Sciences Research Grant (Policy Research for Eliminating Hepatitis) Epidemiological study on the incidence of hepatitis infection and measures for eliminating hepatitis FY2020 Report. 281–317. 2021. https://mhlw-grants.niph.go.jp/system/files/report_pdf/202021004A-buntan23.pdf Accessed September 7th, 2022. (in Japanese)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.