Abstract
Membrane proteins (MPs) play key roles in cellular signaling pathways and are responsible for intercellular and intracellular interactions. Dysfunctional MPs are directly related to the pathogenesis of various diseases, and they have been exploited as one of the most sought‐after targets in the pharmaceutical industry. However, working with MPs is difficult given that their amphiphilic nature requires protection from biological membrane or membrane mimetics. Polymersomes are bilayered nano‐vesicles made of self‐assembled block copolymers that have been widely used as cell membrane mimetics for MP reconstitution and in engineering of artificial cells. This review highlights the prevailing trend in the application of polymersomes in MP study and drug discovery. We begin with a review on the techniques for synthesis and characterization of polymersomes as well as methods of MP insertion to form proteopolymersomes. Next, we review the structural and functional analysis of the different types of MPs reconstituted in polymersomes, including membrane transport proteins, MP complexes, and membrane receptors. We then summarize the factors affecting reconstitution efficiency and the quality of reconstituted MPs for structural and functional studies. Additionally, we discuss the potential in using proteopolymersomes as platforms for high‐throughput screening (HTS) in drug discovery to identify modulators of MPs. We conclude by providing future perspectives and recommendations on advancing the study of MPs and drug development using proteopolymersomes.
Keywords: biophysical characterization, drug discovery, high‐throughput screening, incorporation, liposome, nano‐vesicle, polymersome, proteoliposome, proteopolymersome, reconstitution
1. INTRODUCTION
Membrane proteins (MPs) constitute 20%–30% of all proteins encoded by the genome of various organisms 1 , 2 , 3 and represent the targets of most pharmacological agents. 4 , 5 , 6 MPs include signal transducers, channel proteins, metabolite transporters, cell surface receptors, enzymes, and anchors. Dysfunctional MPs are associated with various diseases including cancers, autoimmune diseases, and neurological disorders. 7 Therefore, understanding both the structural and functional effects of MPs is of great importance. Currently, 6.5% of the over 181,969 entries of protein structures in the Protein Data Bank are MPs with structures deposited in different databases. 3 , 8 , 9 Of these, only less than 2% have high‐resolution structures consistently found in all databases. 6 , 10 The dearth of studies that focus on MPs can be contributed by various factors. First, MPs are usually unstable and require a bilayer membrane for them to be folded correctly during protein translation. Second, it is difficult to obtain stable and functional MPs of interest in high yields, as MPs are usually low in numbers and tend to aggregate in the cytoplasm, despite attempts at protein overexpression. 6 , 11 Importantly, MPs are generally insoluble in aqueous solution due to the incompatibility between the hydrophobic nature of MP surfaces associated with lipid membranes and the hydrophilicity of solvent molecules. The use of amphiphilic agents is thus necessary to extract MPs from the native membranes and maintain them in a stable soluble form. Hence, there is a need to develop synthetic membrane platforms that mimic native biological membrane to provide amphiphilic environments for the MPs and maintain their structural and functional integrity for in vitro protein studies. 12 , 13 , 14 , 15
Conventional methods of MP study include the usage of protein tethered lipid bilayer and supported planar lipid bilayer membranes. 16 , 17 However, these systems have limitations such as incompatibility between tethered molecules and extra‐membranous domains, inaccessibility of region occupied by tethered molecules as well as uncontrollable orientation of inserted MPs and constraints on their biological functions. 16 , 17 Therefore, cell membrane mimetics with vesicular morphologies known as nano‐vesicles have been increasingly used to overcome these limitations. 18 While liposomes are composed of natural nontoxic phospholipids, polymersomes are formed by amphiphilic block copolymers. 19 , 20 Both types of nano‐vesicles are analogous to biological membrane and suitable for MP residence. 17 , 18 , 20 , 21 , 22 These nano‐vesicles, or small unilamellar vesicles (SUV), have a size of 20–100 nm and have the lowest interfacial area and highest configurational entropy as compared to other morphologies. This makes them more energetically favorable for MP reconstitution. 20 They also have an increased stability over large unilamellar vesicles (LUVs, >100 nm in size) and giant unilamellar vesicles (GUVs, >1 μm in size). 20 Additionally, they contain a concentration gradient, which can play a key role in determining the functions of pore‐forming channel MPs. 23 While liposomes have been widely used and reviewed for their use in MP reconstitution and the related structural and functional studies, 17 they are limited by low stability. 22 To overcome this limitation, polymersomes have been increasingly adopted for MP studies because of their superior stability. 22 , 23 , 24 Liposomes and polymersomes with reconstituted MPs are termed as proteoliposomes and proteopolymersomes, respectively.
Apart from finding a suitable membrane support, it is crucial to ensure that the inserted MPs are folded in the correct orientation and maintain their biological functions, in order to facilitate further characterizations of these MPs. 14 , 15 Hence, it is imperative to optimize chemical constituents used in the formation of polymersomes or hybrid polymer‐lipid systems, 25 , 26 , 27 , 28 , 29 , 30 MP production methods, and parameters used in the reconstitution process. 17 , 31 , 32 The reconstitution process plays a key role in determining the efficiency of reconstitution, the quality of the inserted MPs, as well as the resolution and capacity of the methods used to study these MPs. 17 , 31 In this article, we will review the use of polymersomes in MP structural and functional studies, as well as their translational application in high‐throughput screening (HTS) for drug discovery (Figure 1). We start by introducing the synthesis and characterization of polymersomes and methods of MP reconstitution to form proteopolymersomes. We then summarize the use of proteopolymersomes in studying both the structures and functions of channel proteins, MP complexes, and membrane receptors. Additionally, we provide a comprehensive list of factors affecting the efficiency of MP insertion and the quality of the inserted MPs. Finally, we discuss the feasibility and current applications of proteo‐nano‐vesicles in HTS. We conclude by providing future prospects in using polymersomes to engineer artificial cells as well as laying out a roadmap with recommendations for using proteopolymersomes in drug discovery pipeline.
FIGURE 1.

Polymersomes as platforms for MP study and drug discovery. Polymersomes, which are made up of block copolymers, can mimic biological membranes for reconstitution or incorporation of MPs, including channels, receptors, and protein complexes to form proteopolymersomes (center). Proteopolymersomes can be used to study the structure–function relationship of MPs including the characterization of (a) receptor‐ligand binding through the use of surface plasmon resonance (SPR), 33 (b) channel transport function through conducting fluorescent dye leakage assay, and (c) MP structure by nuclear magnetic resonance (NMR).Source: Figure 1c is reproduced with permission from reference 34, Copyright 2018, Springer Nature. (d) Proteopolymersomes can also be used in high‐throughput screening (HTS) for drug discovery to identify modulators of MPs. Schematics were created with BioRender.com.
2. SYNTHESIS AND CHARACTERIZATION OF POLYMERSOMES
Polymersomes are spherical nanovesicular systems with polymer shells of 5–50 nm in thickness and are formed by the self‐assembly of amphiphilic block copolymers. 35 , 36 , 37 , 38 The polymersome membrane provides a physical barrier that isolates the encapsulated materials from external biological environment, while allowing controlled release or exchange of biological molecules due to the presence of a concentration gradient. A major difference between polymersomes and liposomes lies in the chemical versatility to control the thickness of the membranes where liposomes are limited to a membrane thickness of up to 5 nm, while polymersomes can have membrane thickness of up to 50 nm, depending on the type of block copolymers used. 24 This suggests that polymersomes could potentially accommodate larger and higher amounts of MPs than liposomes, although it is important to consider the hydrophobic mismatch that might be present during MP insertion. 24 Due to the higher molecular weight of constituent block polymers and the potential of forming cross‐linking structures through UV irradiation, 39 , 40 polymersomes usually have enhanced mechanical properties, 41 , 42 higher stability, 43 , 44 lower dissociation rates, lower permeability, 44 and limited leakage 45 compared to liposomes (Figure 2a). 20 , 22 Furthermore, their dense hydrophilic polymer brush‐like coronas increases their resistance to degradation and have longer circulation half‐lives in vivo. 48
FIGURE 2.
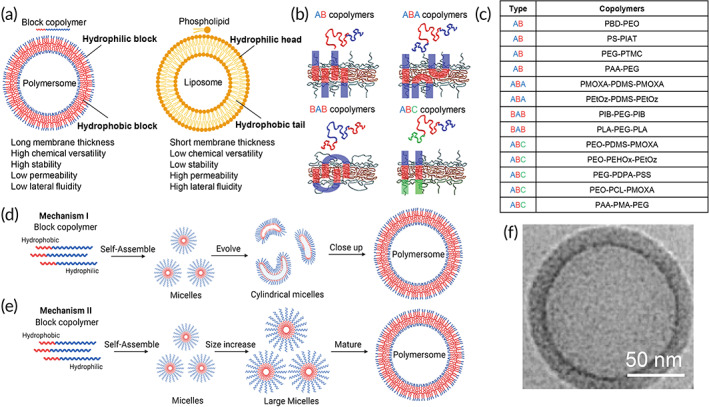
Properties of polymersomes and their formation mechanisms and characterization. (a) Comparison of vesicle properties between polymersomes and liposomes. 20 (b) Polymersomes are formed by self‐assembly of block copolymers into a vesicular structure. Various compositions of diblock copolymers (AB) and triblock copolymers (ABA, BAB, and ABC) are used in the formation of polymersomes. Source: Reproduced with permission from reference 46, Copyright 2012, Elsevier. (c) A list of chemical constituents of diblock and triblock copolymers used in polymersome synthesis. (d,e) Schematics of two different proposed mechanisms for polymersome formation where (d) spherical micelles are first formed from the self‐assembly of block copolymers, which are then further self‐assembled into micelles with cylindrical or disk morphologies that can wrap around to form a vesicular shape; and (e) small spherical micelles are formed from rapid self‐assembly of block copolymers which then grow into larger micelles. Source: Figure 2d,e is modified and reproduced with permission from reference 44, Copyright 2011, Springer Nature. (f) Cryo‐TEM images of polymeromes formed by PEO‐PBD copolymer. The hydrophobic cores of PBD are the darker areas. Scale bar represents 50 nm. Source: Modified and reproduced with permission from reference 47, Copyright 2002, ACS Publications. Schematics were created with BioRender.com.
2.1. Types of copolymers used in polymersome synthesis
Diblock (AB) and triblock (ABA, BAB, and ABC) copolymers 35 , 36 , 37 , 38 are usually used in polymersome synthesis, with A and C being the hydrophilic blocks and B being the hydrophobic block (Figure 2b,c). 46 , 47 Control over the polymer block length and the hydrophilic to hydrophobic block ratio allow for tuning of membrane thickness, morphology, rigidity, and permeability of the polymersome. 23 , 37 , 49 , 50
2.1.1. Diblock copolymers
The most commonly used diblock polymers is poly(butadiene)‐b‐poly(ethylene oxide) (PBD‐PEO)‐based. 47 , 49 , 51 Their ability to provide more fluidity over other diblock copolymers make them suitable for studying membrane receptors. 52 , 53 Polystyrene‐b‐poly(isocyanoalanine[2‐thiophen‐3‐yl‐ethyl]amide) (PS‐PIAT) diblock copolymers self‐assemble into an intrinsically porous bilayer, 54 and have been used to overcome the issue of lower permeability in polymersomes, allowing the function of larger channel or pore‐forming proteins to be tested. Other forms of diblock polymers that have been used in MP studies include poly(ethylene glycol)‐b‐poly(trimethylene carbonate) (PEG‐PTMC) 55 and poly (methyl acrylate)‐b‐poly(ethylene glycol) (PAA‐PEG). 56
2.1.2. Triblock copolymers
Poly(2‐methyloxazoline)‐poly(dimethylsiloxane)‐poly(2‐methyloxazoline) (PMOXA‐PDMS‐PMOXA) 57 , 58 is the most commonly used triblock (ABA) polymer in polymersome synthesis for MP studies. ABA polymers can change their conformation to adapt to the MP length to overcome hydrophobic mismatch, as demonstrated in reconstitution of outer membrane porin F (OmpF) protein in PMOXA‐PDMS‐PMOXA 59 and ATP synthase, or bacteriorhodopsin (BR) reconstitution in poly(2‐ethyl‐2‐oxazoline)‐b‐poly(dimethylsiloxane)‐b‐poly(2‐ethyl‐2‐oxazoline) (PEtOz‐PDMS‐PEtOz). 60 , 61 , 62 To create a polymeric nanocompartment with low permeability, polyisobutylene‐polyethylene glycol‐polyisobutylene (PIB‐PEG‐PIB) (BAB) with the PIB unit being impermeable to many molecules, 63 has been used in the formation of polymersomes with the insertion of an Escherichia coli (E. coli) outer MP. 64 Poly(lactic acid)‐poly(ethylene glycol)‐poly(lactic acid) (PLA–PEG–PLA) is another type of BAB polymer, which has been used to synthesize polymersomes as nanocarriers for delivery of hydrophilic and hydrophobic drugs. 65
To account for the membrane asymmetry in lipid composition, poly(ethylene oxide)‐b‐poly(dimethylsiloxane)‐b‐poly(2‐methyloxazoline) (PEO‐PDMS‐PMOXA) (ABC) is used. 66 , 67 ABC polymers can adopt a mixture of hairpin or transmembrane orientations due to steric hindrance and are useful for MP study as they can change their chemical composition to influence the orientation of the inserted integral proteins upon the application of external fields such as electric fields to its membrane leaflets. 68 Recently, an one‐pot synthesis method of a new ABC triblock terpolymer, poly(ethylene oxide)‐block‐poly(2‐(3‐ethylheptyl)‐2‐oxazoline)‐block‐poly(2‐ethyl‐2‐oxazoline) (PEO‐PEHOx‐PEtOz), using sequential microwave‐assisted polymerization has been reported. 69 The asymmetry of the formed polymersomes can be adjusted by varying the ratio of PEO to PEtOz and potentially be used for directed insertion of MPs. In another study, poly(ethylene glycol)‐poly(diisopropylaminoethyl methacrylate)‐b‐poly(styrenesulfonate) (PEG‐PDPA‐PSS) has been used for directed insertion of proteorhodopsin (PR). 70 Other types of ABC polymers, including poly(ethylene oxide)‐b‐polycaprolactone‐b‐poly(2‐methyl‐2‐oxazoline) (PEO‐PCL‐PMOXA) 71 and PAA‐PMA‐PEG 56 have also demonstrated success in forming polymersomes and may offer new avenues for MPs study in novel applications.
2.2. Synthesis of polymersomes
There are two different proposed mechanisms for the formation of polymersomes where (i) spherical micelles are first formed from the self‐assembly of block copolymers, which are then further self‐assembled into micelles with cylindrical or disk morphologies that can wrap around to form a vesicular shape (Figure 2d); and (ii) small spherical micelles are formed from rapid self‐assembly of block copolymers, which then grow into larger micelles and polymersomes (Figure 2e). 72 Specifically, polymersomes can be synthesized from different copolymers via solvent‐displacement, polymer film rehydration, solid rehydration, or electroformation techniques. 43 , 67 , 73 In solvent displacement method, the polymer is dissolved in a suitable organic solvent and added dropwise to an aqueous buffer and stirred vigorously to form an emulsion. While being a simple and fast method, the polydispersity of polymersome sizes is high, 74 and residual organic solvents may denature most amphiphilic MPs and result in low reconstitution efficiency. 75 To overcome the use of organic solvents, polymer rehydration technique has been developed, where the polymer solution is first dried to remove traces of organic solvents before rehydration in aqueous buffers. Polyethyleneoxide‐polyethylethylene (PEO‐PEE)‐based polymersomes generated using the polymer film rehydration method yields small polymersomes with a size of about 100 nm but with a broad size distribution. 45 In solid rehydration, the polymer is made into bulk powder form before rehydration in aqueous buffers. However, it requires stronger and longer agitation time for complete rehydration. 45 Electroformation is another method commonly used to synthesize PMOXA‐PDMS‐PMOXA and PB‐PEO polymersomes, 76 , 77 but this method results in polymersomes in a larger size range of 10–40 μm. 78 Other techniques include 3D soft‐confined solvent annealing, 79 droplet microfluidic that have been used to produce PEG–PLA‐based polymersomes, 80 and gel‐assisted rehydration where polymer solutions are spread across dehydrated agarose films and subsequently rehydrated in aqueous buffers. 81
2.3. Characterization of polymersomes
The hydrodynamic radius, size distribution, and morphology of the formed polymersomes can be characterized by dynamic light scattering (DLS), static light scattering (SLS), optical microscopy, and transmission electron microscopy (TEM). 82 High‐throughput scattering methods such as combinatorial small‐angle X‐ray scattering (SAXS) or wide‐angle x‐ray scattering (WAXS) can provide information about structural features of colloidal size, including membrane bilayer thickness and internal structure. 83 The small‐angle neutron scattering (SANS) technique can study the morphology and thermodynamics of polymer blends and copolymers in polymersomes, as well as the polymersome structure. 84 Optical microscopy can only resolve polymersomes larger than 1 μm in diameter, 85 while higher resolution imaging tools such as TEM, cryo‐TEM, and freeze fracture cryo‐scanning electron microscopy (FF‐Cryo‐SEM) are able to give about a 1000‐fold increase in resolution and a 100‐fold increase in depth of field. 85 In particular, cryo‐TEM can avoid the drying process associated artifacts in electron microscopy sample preparation and can provide the information regarding the size, morphology, and bilayer thickness of polymersomes (Figure 2f). 83 Atomic force microscopy (AFM) can also be used to characterize the mechanical properties of polymersomes. 83
3. STRATEGIES FOR MP INSERTION TO FORM PROTEOPOLYMERSOMES
The reconstitution or insertion of MPs in polymersomes has emerged as a powerful tool in studying the structure and functionality of MPs. 86 To retain the structural integrity of MPs and confer their biological functionalities, MPs have to be preserved in amphiphilic environment similar to their native environment such as the use of detergents to prevent denaturation. The protein–detergent–membrane interaction play a key role in MP insertion, which is affected by the different methods of protein production and purification, the type and amount of detergents used, and the different physicochemical properties of polymersomes, including their fluidity and flexibility. MPs can be reconstituted via three major methods: (1) cell‐based protein production and detergent mediated reconstitution, 87 (2) cell‐free co‐translational protein production and direct incorporation, 53 and (3) reconstitution by destabilization of vesicles or supported planar bilayer membranes. 88 , 89 , 90 , 91 , 92 , 93 Following reconstitution, purification steps such as dialysis, gel filtration or size exclusion chromatography (SEC), centrifugation, and bio‐beads aided procedures should be carried out to remove excess detergents and other reagents to enhance the formation of stable proteopolymersomes.
3.1. Cell‐based protein production and detergent mediated reconstitution
The recombinant MPs are first purified from plasmid transformed bacteria cultures, and the purified MPs are solubilized with detergents and emulsified with excess polymers via self‐assembly, followed by detergent removal (Figure 3a). 32 , 94 The addition of detergents allows for ease of MPs solubilization and keeps them in a native environment to facilitate MPs folding and stabilization. Upon protein reconstitution, the detergent molecules need to be removed to aid in the formation of stable vesicles, and residual detergent may also inhibit protein activity. 94 Multiple MPs have been reconstituted into polymersomes through this approach with common detergent removal methods including dialysis, 95 gel filtration or SEC, 86 , 87 centrifugation, 52 or bio‐beads aided procedures (Table 1). 86 , 87 In the dialysis method, the MPs and polymersome emulsion are dialyzed against a larger volume of buffer to remove the excess detergents. 95 For gel filtration or SEC‐mediated detergent removal, the MP‐polymersome solution is passed through a gel‐exclusion column which separate and elute the proteopolymersomes before the detergent. Different sized columns can be used ranging from Sephadex G25 to G200. 94 This technique has the advantage of being rapid but can lead to a broader size distribution in proteopolymersomes. Using the centrifugation approach, the excess detergents as well as free MPs are filtered through centrifugal filtration cartridges of a certain molecular weight cut‐off. 90 For bio‐beads mediated detergent removal, the beads are used to physically adsorb and sequester excess detergents, where the detergent‐bound beads can subsequently be removed by centrifugation or filtration. 94 The choice of detergent removal method and its efficiency are dependent on the type of detergent used during the MP reconstitution process. 94 , 114 Detergents with a high critical micelle concentration (CMC), such as cholate and octyl glucoside, tend to form smaller micelles and make them easier to remove via the process of dialysis or by SEC. 94 , 114 Detergents with a lower CMC, such as Triton‐X 100 which forms larger micelles, are less likely to be removed by dialysis or SEC and hence are more often removed via bio‐beads aided process. 94 , 114 Some limitations associated with cell‐based protein production or MP overexpression are low yield, cell cytotoxicity, protein aggregation, and misfolding, which can in turn result in polymer membrane overcrowding. 115
FIGURE 3.
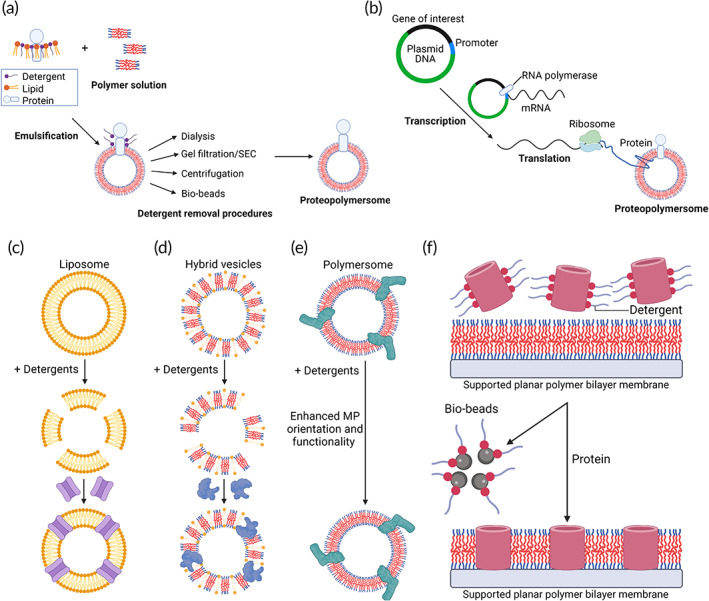
MP insertion strategies to form proteopolymersomes. (a) Detergent‐mediated reconstitution of MPs into polymersomes. MPs from native membranes are purified, solubilized, and stabilized by detergents. The MP solution is then mixed with polymers dissolved in organic solvent to form an emulsification with a mixture of polymer–protein–detergent micelles. When detergent is removed from the micellar solutions via procedures such as dialysis, gel filtration/SEC, centrifugation or with the use of bio‐beads, MPs are reconstituted into vesicles forming proteopolymersomes. Source: Modified and reproduced with permission from reference 87, Copyright 2002, SciELO. (b) Spontaneous incorporation of MPs into polymersome to form proteopolymersomes through cell‐free protein synthesis by adding complementary DNA encoding the protein of interest and polymersomes directly to an in vitro expression mixture, including RNA polymerase and ribosome. Source: Modified and reproduced with permission from reference 53, Copyright 2012, John Wiley and Sons. (c–e) Vesicle destabilization by detergents in (c) liposome with reconstitution of NorA multidrug efflux transporter as an example, 88 (d) hybrid vesicle made of lipids and polymers with reconstitution of Cyt‐bo3 ubiquinol oxidase as an example, 89 and (e) polymersome with enhanced orientation and improved functionality of NADH:ubiquinone oxidoreductase (Complex I) as an example. 90 (f) Destabilization of supported planar polymer bilayer membrane by bio‐beads for MP reconstitution with functional insertion of M1oK1 as an example. Source: Modified and reproduced with permission from reference 91, Copyright 2014, Elsevier. Schematics were created with BioRender.com.
TABLE 1.
List of proteopolymersomes based membrane protein studies
| Membrane transport proteins | Block copolymers | Protein production | Insertion method; purification method | (A) Proteopolymersome characterization | References |
|---|---|---|---|---|---|
| (B) MP structural studies | |||||
| (C) MP functional studies | |||||
| Outer membrane protein F (OmpF) | PMOXA–PDMS–PMOXA | Cell based | Detergent mediated; gel filtration/SEC | (A) Cryo‐TEM, DLS, SLS, TEM, AFM | 26, 96, 97 |
| (B) N/A | |||||
| (C) Iodometry to monitor ampicillin hydrolysis by β‐lactamase; LSM | |||||
| OmpF 6His | PMOXA–PDMS–PMOXA | Cell based | Detergent mediated; gel filtration/SEC | (A) DLS | 98 |
| (B) CD | |||||
| (C) Leakage assay of fluorescent dye | |||||
| OmpF‐S‐S‐CF | PMOXA6–PDMS44–PMOXA6 | Cell based | Detergent mediated; dialysis | (A) SLS, DLS, Cryo‐TEM | 99 |
| (B) N/A | |||||
| (C) Fluorescence generated when using AmR as a substrate for HRP, FCS, EPR | |||||
| Aquaporins (AQPs) | PMOXA11–PDMS34 | Cell based | Detergent mediated; dialysis | (A) DLS | 95 |
| (B) N/A | |||||
| (C) SFLS, DLS | |||||
| AQPZ | PMOXA15–PDMS110–PMOXA15 | Cell based | Detergent mediated; bio‐beads | (A) DLS, FETEM | 100 |
| (B) N/A | |||||
| (C) SFLS | |||||
| AQP0 | PEO14–PB22, PEO14–PB22, PMOXA20–PDMS42–PMOXA20 PMOXA12–PDMS55–PMOXA12 | Cell based | Detergent mediated; gel filtration/SEC or Dialysis | (A) EM, RS | 101, 102 |
| (B) N/A | |||||
| (C) SFLS | |||||
|
Ferric hydroxamate uptake protein component A (FhuA) FhuA Δ1–129 FhuA Δ1–160 |
PMOXA–PDMS–PMOXA | Cell based | Detergent mediated; bio‐beads or dialysis | (A) ITC, DLS | 27, 103 |
| (B) CD | |||||
| (C) FCS | |||||
| FhuA Δ1–159 | PIB1000–PEG6000–PIB1000 | Cell based | Detergent mediated; gel filtration/SEC | (A) DLS, Cryo‐TEM | 64 |
| (B) CD | |||||
| (C) Absorbance detection at 370 nm of TMB oxidation product when using TMB as a substrate for encapsulated HRP | |||||
| Gramicidin A (gA) | PMOXA–PDMS–PMOXA | Cell based | Detergent mediated; gel filtration/SEC | (A) TEM, SLS | 104 |
| (B) N/A | |||||
| (C) SFLS; Fluorescence spectroscopy on changes of the ANG‐2 dye specific for Na+ transport and APG‐2 dye specific for K+ transport | |||||
| Ionomycin | PMOXA6–PDMS44–PMOXA6 | Cell based | Detergent mediated; gel filtration/SEC | (A) TEM | 105 |
| (B) N/A | |||||
| (C) SFLS; Fluorescence spectroscopy on changes in the calcium sensitive ACG dye due to influx of Ca2+ ions | |||||
| KcsA | PMOXA–PDMS–PMOXA | Cell based | Detergent mediated; dialysis | (A) FCS | 59 |
| (B) Z‐scan fluorescence correlation spectroscopy | |||||
| (C) N/A | |||||
| Maltoporin (LamB) | PMOXA–PDMS–PMOXA | Cell based | Detergent mediated; gel filtration/SEC | (A) Langmuir trough | 28 |
| (B) N/A | |||||
| (C) Fluorescence spectroscopy monitoring the change in fluorescently labeled DNA released into the vesicle | |||||
| Nucleoside‐specific porin (TsX) | PMOXA20–PDMS54 PMOXA20 | Cell based | Detergent mediated; gel filtration/SEC | (A) DLS, Gel electrophoresis | 106 |
| (B) N/A | |||||
| (C) Fluorescence due to hydrolysis of prodrug 2‐fluoroadenosine to 2‐fluoroadenine | |||||
| Large conductance mechano‐sensitive ion channel (MscL) |
Hybrid vesicles: (a) 1,2‐dioleoyl‐sn‐glycero‐3‐phosphocholine (DOPC) (b) PEO9‐b‐PBD12; PEO14‐b‐PBD22; PEO24‐b‐PBD36 |
Cell free | Co‐translational incorporation; gel filtration/SEC | (A) Western blotting | 51 |
| (B) mEGFP fluorescence due to proper folding | |||||
| (C) Leakage assay of fluorescent dye | |||||
| α‐Hemolysin | PBD‐PEO | Cell free | Co‐translational incorporation; centrifugation | (A) SEM | 52 |
| (B) N/A | |||||
| (C) Leakage assay of fluorescent dye | |||||
| NADH: ubiquinone oxidoreductase (Complex I) | PMOXA(9–64)–PDMS(23–165)–PMOXA(9–64) | Cell based | Detergent mediated with vesicle destabilization; bio‐beads | (A) EPR, BCA | 90 |
| (B) N/A | |||||
| (C) NADH/Ferricyanide or NADH/Decylubiquinone or NADH:Ubiquinone 2/AQ oxido‐reductase activity assay; Complex I inhibition assay | |||||
| FoF1‐ATPase and BR | PEtOz−PDMS−PEtOz | Cell based | Detergent mediated; dialysis | (A) TEM | 61, 62, 107 |
| (B) N/A | |||||
| (C) Production of photoinduced electrochemical proton gradient; ATP synthesis activity | |||||
| Proteorhodopsin (PR) | PEG–PDPA–PSS | Cell based | Detergent mediated; centrifugation | (A) TEM | 70 |
| (B) PR would orientate with negatively charged PSS | |||||
| (C) Light‐activated pH changes | |||||
| Proteorhodopsin (PR) | PS‐b‐P4MVP2 | Cell based | Detergent mediated; centrifugation | (A) TEM | 108 |
| (B) SAXS, RS, ssNMR | |||||
| (C) Time‐resolved visible spectroscopy (flash photolysis) | |||||
| Cytochrome bo3 (Cyt‐bo3) | Hybrid vesicles: (a) PBd22‐b‐PEO14, (b) POPC | Cell based | Detergent mediated or vesicle destabilization; bio‐beads | (A) DLS and TEM | 89 |
| (B) N/A | |||||
| (C) Decylubiquinone oxidation reaction initial rate | |||||
| NaAtm1 P‐glycoprotein (PgP) | Hybrid vesicles: (a) Palmitoyl‐oleoyl‐phosphatidylcholine, (b) PBD‐PEO | Cell based | Detergent mediated; bio‐beads | (A) Flotation in a sucrose density gradient | 30 |
| (B) N/A | |||||
| (C) Passive permeability to a fluorescent probe |
| Membrane receptors | Block copolymers | Protein production | Insertion method; purification method | (A) Proteopolymersome characterization | References |
|---|---|---|---|---|---|
| (B) MP structural studies | |||||
| (C) MP functional studies | |||||
| Dopamine receptor D2 (DRD2) |
PBD–PEO PMOXA–PDMS–PMOXA |
Cell free | Co‐translational incorporation; centrifugation | (A) Western blotting | 53 |
| (B) Conformational antibody binding (SPR) | |||||
| (C) Native ligand binding and replacement assay (compete dye‐labeled ligand with unlabeled ligand) | |||||
| C‐X‐C chemokine receptor type 4 (CXCR4) | PB–PEO | Cell free | Co‐translational incorporation; centrifugation | (A) Western blotting | 109 |
| (B) Conformational antibody binding (SPR) | |||||
| (C) Native ligand binding | |||||
| Glucagon‐like peptide‐1 receptor (GLP‐1R) |
PBD–PEO PMOXA–PDMS–PMOXA |
Cell free | Co‐translational incorporation; dialysis | (A) Western blotting, TEM, DLS, SEC | 110 |
| (B) Conformational antibody binding (SPR) | |||||
| (C) Native ligand binding, radioligand saturation binding assay | |||||
| Claudin‐2 (Cldn‐2) | PBD–PEO | Cell free | Co‐translational incorporation; centrifugation | (A) SEM, Western blotting | 52 |
| (B) Conformation antibody binding (SPR) | |||||
| (C) N/A | |||||
| Major histocompatibility complex I (MHC‐I) | PMOXA–PDMS–PMOXA | Cell free | Co‐translational incorporation; bio‐beads | (A) Fluorescent microscopy images of antibody binding | 111 |
| (B) Conformational antibody binding (SPR) | |||||
| (C) T‐cell activation | |||||
| Peptide anchors | PMOXA–PDMS–PMOXA | Cell based | Detergent mediated; gel filtration/SEC | (A) SEC, confocal microscopy, tryptophan fluorescence measurements | 112, 113 |
| (B) Intact with membrane (nonpore forming), CD | |||||
| (C) N/A |
Abbreviations: ACG, Asante Calcium Green; AFM, atomic force microscopy; AmR, Amplex UltraRed; ANG‐2,Asante NaTRIUM Green‐2; APG‐2, Asante Potassium Green‐2; BCA, bicinchoninic acid protein assay; CD, circular dichroism; DLS, dynamic light scattering; EM, electron microscopy; EPR, electron paramagnetic resonance; FCS, fluorescence correlation spectroscopy; FETEM, field emission transmission electron microscopy; HRP, horse radish peroxidase; ITC, isothermal calorimetry; LSM, laser scanning microscopy; RS, Raman spectroscopy; SAXS, small angle x‐ray scattering; SEC, size exclusion chromatography; SFLS, stopped flow light scattering kinetics; SLS, static light scattering; SPR, surface plasmon resonance; ssNMR, solid‐state NMR spectroscopy; TEM, transmission electron microscopy; TMB, 3,3′,5,5′‐tetramethylbenzidine.
3.2. Cell‐free co‐translational protein production and direct incorporation
The MP of interest is expressed from a plasmid and directly incorporated into the polymersome (Figure 3b). 116 In this method, the cDNA coding for the MP of interest and reaction mixtures containing necessary components for protein translation are added to polymersomes in solution and incubated at elevated temperatures for a few hours. A typical reaction mixture is composed of a cell extract from E. coli, wheat germ, or rabbit reticulocytes, containing components such as ribosomes, translation factors, aminoacyl‐tRNA synthetases, and tRNAs, which are required for production of protein. 117 , 118 , 119 A more recent development is cell‐free protein synthesis using recombinant elements (PURE) system, which comprises individually purified components of the E. coli translation apparatus. 120 The PURE system does not contain cell extract and results in less degradation of cDNA template as well as protein products, thereby allowing for more efficient incorporation of MPs. 120 The cell‐free method also allows direct access to reaction conditions, where additional agents which aid the reconstitution process such as detergents or protein folding catalysts can be included. 115 The cell‐free method overcomes the issues associated with conventional overexpression and reconstitution of MPs into membrane models, such as low protein yields, cytotoxicity, misfolding, and aggregation. 121 , 122 Upon reconstitution, the proteopolymersome size and morphology can be further fine‐tuned through freeze–thaw, extrusion, and sonication methods. 94 , 114 Polymersomes without MPs, as well as excess cell‐free expression reaction reagents, can be removed from proteopolymersomes by methods similar to detergent removal including dialysis, 110 gel filtration or SEC, 86 , 87 centrifugation, 53 and bio‐beads mediated process. 111 A limitation of the direct incorporate approach is that the necessary posttranslational modifications, which are required for the formation of fully functional proteins may not occur, unless known enzymes responsible for these processes are added to the reaction mixture. 123
3.3. Reconstitution by destabilization of vesicles or supported planar bilayer membranes
Membrane destabilization by detergents has been used to reconstitute MPs in liposomes (Figure 3c) 88 and hybrid vesicles (Figure 3d), 89 as wells as a way to enhance the orientation and functionality of reconstituted MPs in polymersomes (Figure 3e). 90 While the use of detergents and their removal are also necessary in this approach, the key difference lies in the vesicles or proteo‐vesicles being formed first, followed by the addition of detergents to perturb the integrity of the vesicles to allow for solubilized MPs insertion 88 or reorientation of the inserted MPs. 90 Multidrug resistance (MDR) transporter NorA was incorporated in liposomes made from E. coli polar lipid crude extract by destabilization using detergents. 88 Liposome destabilization was achieved by the stepwise addition of Triton X‐100 and mixed with NorA protein solution, and bio‐beads were added for detergent removal. 88 Cytochrome bo3 (Cyt‐bo3) has been incorporated in hybrid vesicles made of PBD‐PEO and POPC using detergent‐mediated reconstitution. Hybrid vesicles are first formed by extrusion and destabilized by gradual addition of small concentrations of Triton X‐100 detergent. At the brink where the detergent started to break up the integrity of the hybrid vesicles, Cyt‐bo3 solutions were added and incorporated into the vesicles, where the excess detergents are then removed by bio‐beads. 89 NADH:ubiquinone oxidoreductase (Complex I) was incorporated in PMOXA‐PDMS‐PMOXA polymers using detergent‐mediated reconstitution. Partial destabilization of the polymer membrane by adding Triton X‐100 detergent allows for rearrangement of the inserted Complex I to enhance its structural orientation with a considerable fraction of vesicles remained intact. 90
Other types of membrane destabilization methods, such as voltage and bio‐beads mediated destabilization, have been used to reconstitute MPs on supported planar lipid or polymer bilayer membranes. Bio‐beads mediated MP insertion has been used for the insertion of MloK1, a cyclic nucleotide‐modulated potassium channel from Mesorhizobium loti, into supported PDMS‐PMOXA‐based polymeric membranes (Figure 3f). 91 To achieve functional insertion of M1oK1, both the protein and the polymer membrane were destabilized by bio‐beads. The bio‐beads provided the driving force for the insertion of the MP into the polymer membrane. The conductance across M1oK1 increased only when protein reconstitution was carried out in the presence of bio‐beads. 91 Voltage destabilization is another approach that has been suggested with the insertion of α‐hemolysin into supported planar polymer membranes made of PB‐PEO diblock copolymers as an example. 92 , 93
Cell‐based protein production followed by detergent‐mediated reconstitution has been the predominantly used method in MP insertion. The adoption of the cell‐free co‐translational incorporation approach, which overcomes limitations in cell‐based protein production, has been on a rise. The membrane destabilization method is still largely limited to MP reconstitution in liposomes, hybrid vesicles or planar membrane bilayers. Regardless of the methods used, reproducibility and predictability are two important requirements to fulfill in the engineering of proteopolymersomes to allow for accurate acquisition of biological information related to the MPs of interest and their applications such as in engineering of artificial cells and drug discovery. In general, the proteopolymersomes formed should have bilayer thickness that match MP hydrophobic domain, high mechanical strength, good stability, and conformation flexibility to adapt to MP insertion and functionality.
4. MEMBRANE TRANSPORT PROTEINS
Membrane transport proteins are MPs that play important roles in maintaining the physiological function of cells. There are two different types of transport (passive and active) across cell membranes. Passive transport requires no energy input as transport follows a concentration gradient and examples include channel proteins. 124 In contrast, active transport requires energy, most commonly from ATP hydrolysis by primary active transporters, which include proton pumps. Active transport is used to carry substances into a cell against the concentration gradient. 125 Liposomes have been used to study membrane transport proteins, in particular channel proteins; however, their highly fluid and leaky nature hinders the retention of molecules, often resulting in inaccurate measurement of these protein functions. 20 , 125 Polymersomes can overcome these issues with their low passive permeability to low‐molecular‐weight solute, 44 and have been used widely by researchers to reconstitute and incorporate channel proteins or porins. 99 Apart from studying the functional activity of channel proteins, the activity of protein complexes can also be modeled and studied with proteopolymersomes. These complexes include primary active transporters and MP coupling systems such as NADH:ubiquinone oxidoreductase (Complex I), F0F1‐ATPase, and proton pumps. We have summarized the various types of channel proteins for passive transport and protein complexes for active transport studied in polymersomes (Table 1).
4.1. Channel proteins for passive transport
4.1.1. OmpF
The outer membrane protein F (OmpF) is a MP that functions as a passive diffusion channel in E. coli and assembles to form a highly stable trimer in membranes. OmpF functions as the main route of outer membrane penetration for many antibiotics, hence studying its structure and function can be of clinical importance in determining bacterial resistance mechanisms and therapeutic advancements. 126 OmpF is the first MP successfully reconstituted with full functionality into PMOXA–PDMS–PMOXA membranes. 25 , 26 , 96 , 97 , 106 The OmpF reconstitution efficiency is increased with homogenous distribution of MPs and polymers coupled with slow controlled removal of surfactants. 26 The successful passage of antibiotics, such as ampicillin, demonstrates the functional reconstitution of OmpF in polymersomes. 25 , 26 OmpF function has also been determined through monitoring the conversion of passaged substrates with no antibacterial activity into substrates with bacterial activity or antibiotics by the encapsulated penicillin acylase enzyme. 97 OmpF containing double mutants (K89C and R270C) with SAMSA fluorescein conjugation through disulfide bonding termed as OmpF‐S‐S‐CF is reconstituted into PMOXA‐PDMS‐PMOXA polymersomes via the rehydration method (Figure 4a). 99 The successful insertion of OmpF into polymersomes is evaluated using fluorescence correlation spectroscopy (FCS) to determine whether there is an increase in diffusion time (Figure 4b), 99 or by electron paramagnetic resonance (EPR) which has a broad spectrum, indicative of low mobility due to successful MP reconstitution. The protein functions are determined via encapsulating horse radish peroxidase (HRP) in polymersomes and monitoring for changes in fluorescence due to the formation of resorufin‐like product upon successful diffusion of Amplex UltraRed (AmR), a substrate for HRP. The reconstituted proteopolymersomes show good biocompatibility in a zebrafish embryo model, demonstrating potential use of these polymersomes as cellular implants in living organisms. 99 Other modified OmpF such as OmpF 6His has also been successfully reconstituted in PMOXA–PDMS–PMOXA polymersomes. 98 The structure of the OmpF 6His is determined with circular dichroism (CD) in solution, which indicates that OmpF 6His adopts a β‐barrel stable structure in proteopolymersome. Functional reconstitution of OmpF 6His is determined through measuring a significant release of encapsulated acridine orange outside of the proteopolymersomes when the pH was increased from 5 to 7 across the OmpF, which allows for protons to pass through and result in changes in acridine orange. 98
FIGURE 4.
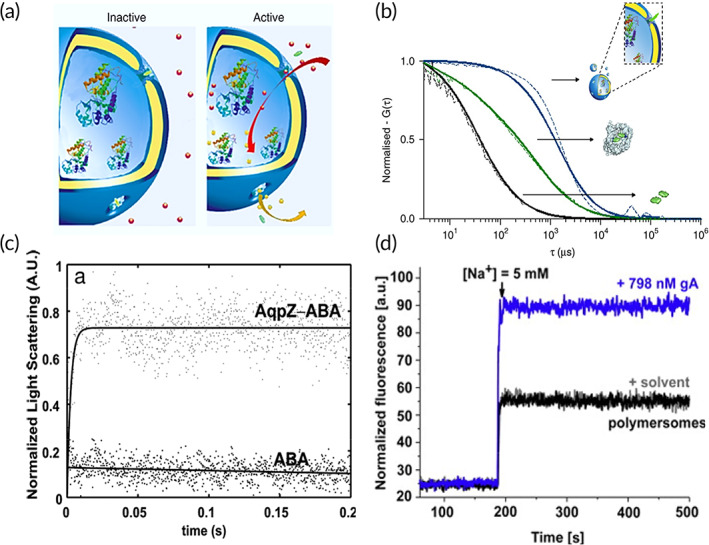
Structural and functional studies of channel proteins with passive diffusion using polymersomes. (a) Schematic of polymersomes with channel proteins reconstituted to allow passage of solutes and selective permeability of ions when the channel proteins are active. Example shown is an OmpF proteopolymersome. (b) Insertion of SAMSA fluorescein (SAMSA‐CF) conjugated OmpF with K89C and R270C double mutations through disulfide bonding (OmpF‐S‐S‐CF) was evaluated with fluorescence correlation spectroscopy. Reconstitution of OmpF‐S‐S‐CF into polymersome (blue) increases protein diffusion time, compared to OmpF‐S‐S‐CF in surfactant (1% octyl‐glucopyranoside/1% OG) (green), and SAMSA‐CF only control (black). Dotted line refers to experimental autocorrelation curves and solid line refers to fitted curve. Source: Figure 3a,b is reproduced with permission from reference 99, Copyright 2018, Springer Nature. (c) Stopped‐flow light‐scattering experiment to characterize vesicle permeability in aquaporin Z (AqpZ) proteopolymersomes. The initial rise rates are used to calculate the permeability and there is an increase in relative light scattering when AqpZ is reconstituted in the polymersomes. Source: Reproduced with permission from reference 101, Copyright 2007, National Academy of Sciences, USA. (d) Measurements of Na+ influx in ANG‐2 (Na+ specific dye) loaded polymersomes before and after reconstitution of gramicidin A (gA). The presence of gA allows for higher influx of Na+ ions into the polymersomes, resulting in an increase in fluorescence intensity of the ANG‐2. Source: Reproduced with permission from reference 104, Copyright 2015, Elsevier
4.1.2. Aquaporins
Another widely studied class of channel proteins is the aquaporins (AQPs), which are water channels that can mediate bidirectional, transmembrane water flow in the presence of an osmotic gradient. Its dysfunction is associated with multiple human diseases, such as glaucoma, cancer, epilepsy, and obesity. 127 Several AQPs have been reconstituted into PMOXA‐PDMS copolymer‐based polymersomes via detergent‐mediated reconstitution including AQP1‐5, which are highly specific for water and AQP3, 7, 9, and 10, which mediate glycerol flux. 95 The functionality of reconstituted AQPs as solute transporters of water or glycerol is studied with stopped flow light scattering kinetics, where a hyperosmotic gradient is first imposed across the membrane of the AQP proteopolymersomes, and then a hypertonic gradient is applied. Outflow from the polymersome results in faster shrinking and increase in light scattering, indicating higher water permeability as a result of functional AQP reconstitution. 95 Aquaporin Z (AQPZ), which can maintain water permeability while retaining uncharged solutes (i.e., glucose, glycerol, salt, and urea), is reconstituted in PMOXA‐PDMS‐PMOXA polymersomes, where it shows 90 times higher water permeability than polymersomes without AQPZ insertion, as well as high rejection rates of salt, glycerol, and urea (Figure 4c). 101 However, AQPZ incorporation has a limiting concentration at a protein‐to‐polymer ratio of 1:50, where a further increase in protein concentration decreases water permeability instead of enhancing it. 101 This limit in ratio could be due to the method of reconstitution used, where a higher detergent concentration used in the AQPZ stock solution can lead to reduced AQPZ reconstitution efficiency. This can be overcome by using slow detergent removal methods or other reconstitution methods. 101
In a similar study, AQPZ is reconstituted into disulfide‐functionalized PMOXA‐PDMS‐PMOXA copolymer via film rehydration technique, and the vesicle shrinkage or permeability is determined to be 4680 μm/s. 100 Further studies show that AQPZ water permeability can be improved when reconstituted in PMOXA‐PDMS‐PMOXA membranes with a larger hydrophobic thickness, due to a decrease in Arrhenius activation energies for water transport across the AQPZ. 128 For structural studies of AQPZ, SAXS has been used to determine AQPZ structure that has been reconstituted in PBD‐PEO polymersomes with different molecular weights. SAXS indicates that AQPZ reconstitution in PB45‐PEO14 leads to a minor difference in average hydrophobic vesicle wall thickness, which could indicate a dimpling or puckering of polymers close to the incorporated AQPZs. On the other hand, in PB33‐PEO18, micelle‐formation tendency is reduced when AQPZ is incorporated. 129 The lens specific water channel aquaporin 0 (AQP0) was reconstituted in PEO‐PB and PMOXA‐PDMS‐PMOXA polymersomes with varying copolymer block lengths, where the proteopolymersome size and morphology are optimized through increasing polymer dissolution and reducing detergent removal rate. 102 The successful incorporation of AQP0 in PEO‐PB and PMOXA‐PDMS‐PMOXA is determined with electron microscopy (EM), and the water permeability of AQP0 determined using stopped flow light scattering measurements showed permeability of 189.7 ± 61.3 μm/s, 102 which is high compared to the measured permeability of other reported polymersomes, such as 2.5 μm/s for poly(ethyl ethylene)‐poly(ethylene oxide) (PEE‐PEO). 130 This could be due to smaller hydrophobic repeat units in the PEO‐PB polymer compared to other polymer‐based polymersomes. 102
Apart from MP studies, AQP incorporated polymersomes also can be applied in industrial water purification processes. For instance, AQPZ reconstituted PMOXA‐PDMS‐PMOXA proteopolymersomes are covalently immobilized onto the surface of a porous ultrafiltration cellulose acetate membrane, followed by in situ surface imprinting polymerization to generate a thin imprinted polymer layer. 101 Forward osmosis and nanofiltration functionality were also tested and determined that AQPZ imprinted membrane had salt rejections above 50% and has a membrane selectivity of water to salt, demonstrating AQPZ facilitated water transport and salt rejection. 131
4.1.3. FhuA
Polymersomes have also been used to study transmembrane protein ferric hydroxamate uptake protein component A (FhuA), which is one of the largest β‐barrel channel proteins. In E.coli, FhuA mediates the active transport of ferrichrome‐bound iron and it also acts as the receptor for bacteriophages. Truncated variants of FhuA (FhuA Δ1–129 and FhuA Δ1–160) has been reconstituted in PMOXA‐PDMS‐PMOXA polymersomes using cell‐based reconstitution, and the activity of FhuA has been determined through monitoring the passage of sulforhodamine dye into polymersomes, 27 or release of calcein dye out of the polymersomes via fluorescence spectroscopy. 103 The direction of FhuA Δ1–160 insertion has been determined through measuring endodermic changes using isothermal titration calorimetry (ITC) in PMOXA‐PDMS‐PMOXA polymersomes. 132 In a separate study, FhuA Δ1‐159 has been reconstituted into thick PIB‐PEG‐PIB polymersome membranes. 64 To overcome the problem of hydrophobic mismatch that is seen during insertion of FhuA Δ1‐159, the length of MP can be matched to the thickness of the polymersome by doubling the last five amino acids of each of the 22 β‐sheets before the more regular periplasmatic β‐turns, which can lead to an 1 nm increase to become extended FhuA Δ1‐159 (FhuA Δ1‐159 Ext). 64 The secondary protein structure of reconstituted FhuA Δ1‐159 Ext is determined through CD spectroscopy, which shows β‐barrel folding, indicative of correct folding. The functional activity of FhuA Δ1‐159 Ext is proven via kinetic analysis of 3,3′,5,5′‐tetramethylbenzidine (TMB) uptake by encapsulated HRP. 64
4.1.4. Ion channels
Ion channels, such as gramicidin A (gA), 104 ionomycin, 105 and KcsA 59 have also been studied in polymersomes. Ion channel gA, which allows for the transport of protons and monovalent ions, is reconstituted in a series of PMOXA–PDMS–PMOXA polymersomes with membrane thickness ranging from 9.2 to 16.2 nm, where membranes thicker than 12.1 nm did not result in successful reconstitution of gA protein, potentially due to hydrophobic mismatch of the protein to polymersome membrane. 104 The functionality of gA is investigated through encapsulation of pyranine, a pH‐sensitive dye in polymersomes, where quenching of fluorescence intensity indicates gA activity due to transport of protons into the polymersomes. Other methods such as monitoring for fluorescence changes of the Asante NaTRIUM Green‐2 (ANG‐2) dye that is specific for Na+ transport and Asante Potassium Green‐2 (APG‐2) dye that is specific for K+ transport have also been used to determine gA functionality (Figure 4d). 104 Ionomycin, which allows for transport of Ca2+ ions, has been incorporated in PMOXA–PDMS–PMOXA‐based polymersomes or polymeric GUVs via film rehydration, and its transport functionality is studied through analyzing fluorescent increases in the calcium sensitive Asante Calcium Green (ACG) dye due to influx of Ca2+ ions into the polymersome. 105 In addition, the permeability of ionomycin can be determined with stopped flow apparatus. 105 KcsA, which allows for transport of K+ ions, has also been studied in PMOXA‐PDMS‐PMOXA polymersomes. However, the incorporation efficiency of the KcsA is only 5%, potentially due to the long drying process during electroformation, which results in aggregation and eventual degradation of the KcsA channel. KcsA insertion is confirmed with measuring the free lateral diffusion inside the polymer membrane with z‐scan FCS, where an increase in diffusion rate indicates incorrect incorporation due to protein aggregation. 59
4.1.5. Maltoporin/LamB
Maltoporin or LamB is a trimeric channel in the outer cell wall of Gram‐negative bacteria that specifically transport maltose and maltodextrins and also serves as a receptor for phage λ. LamB was reconstituted into PMOXA–PDMS–PMOXA polymersomes through mixing the LamB and vesicles solution together to mimic and analyze the mechanisms of phage genome transfer into bacteria through phage binding to trigger release of DNA into the polymersome. 28 LamB functionality is determined through monitoring the change in Oxazole Yellow (YO‐PRO‐1) fluorescently labeled DNA released into the vesicle before and after phage addition. The addition of phage results in a steep increase in the fluorescence intensity, indicating that the protein is functional in inducing the injection of viral DNA. 28 This successful reconstitution of LamB in polymersomes can serve as polymeric nanocontainer that is able to translocate DNA across a synthetic membrane, which can potentially be applied in gene delivery and therapeutic applications.
4.1.6. TsX
TsX is nucleoside‐specific channel‐forming outer membrane porin that allows the specific transport of nucleosides and nucleotides. TsX has been reconstituted into PMOXA‐PDMS‐PMOXA polymersomes. To determine its nucleoside specific activity, the transport of prodrug 2‐fluoroadenosine into the polymersomes via TsX was monitored via its hydrolysis to 2‐fluoroadenine 106 with a reducing sugar assay. TsX reconstituted proteopolymersomes are also used to deliver thymidine phosphorylase (TP) as an enzyme therapy strategy for mitochondrial neurogastrointestinal encephalomyopathy, where TsX functions as a channel to allow for the transport of enzyme substrate thymidine and product thymine through the polymersome. The TP enzyme activity can be determined through monitoring for thymine formation through determining the difference in absorption between thymidine substrate and thymine product at 290 nm. 133
4.1.7. MscL (hybrid vesicles)
MscL is a large‐conductance mechanosensitive ion channel found in prokaryotic and eukaryotic cell membranes and play an important role in rapidly regulating turgor pressure around the cell in response to increased membrane tension. Hybrid vesicles consisting of DOPC with varying concentrations of PEO‐PBD diblock copolymer are used to reconstitute and study the folding of α‐helical MscL. 51 MscL protein is incorporated into the hybrid membrane via cell‐free expression using a construct of MscL tagged with monomeric enhanced green fluorescent protein (mEGFP) at the C‐terminus as well as a translation system. Proper folding of MscL results in an increase of GFP fluorescence intensity. The functional activity of MscL incorporation is investigated through a calcein dye release through measuring the amount of calcein release from the vesicle via fluorescence spectroscopy. 51
The ability to add pores or synthetic channels to polymersomes could lead to novel membrane composites with unique selectivity and permeability. For instance, α‐hemolysin, involved in pore formation, has been inserted into PBD‐PEO polymersomes using cell‐free co‐translational incorporation approach, which increased permeability to encapsulated calcein dye. 52 In addition to porins, synthetic pores self‐assembled from either a dendritic dipeptide or a dendritic ester have also been successfully synthesized into stable helical pores in PEO‐PBD polymersomes to enhance polymersomes permeability. 134 Similarly, synthetic porins made from carbon nanotubes have also been incorporated in PBD‐PEO copolymer‐based polymersomes. 135 Other functional modifications to polymeric membrane include incorporation of multiple channel proteins such as AlkL, OmpW, OprG and TodX, PhoE and FocA in PMOXA‐PDMS‐PMOXA polymersomes, where the combination of TodX and PhoE led to the most significant improvement in mass transfer compared to polymersomes without MPs. 136 This study primary focuses on improving mass transfer of polymersomes and not biophysical characterization of the reconstituted MPs. Other applications of channel proteins reconstituted polymersomes include being nanoreactors, where the channel proteins allow for selective permeation of water, nucleotides, and molecules into polymersomes to facilitate enzymatic reactions. 96 , 105 , 136
4.2. Protein complexes for active transport
4.2.1. NADH:ubiquinone oxidoreductase (Complex I)
Amphiphilic block copolymer PMOXA–PDMS–PMOXA was used to study the electron‐transfer activity of bacterial respiratory enzyme complex NADH:ubiquinone oxidoreductase (Complex I). 90 Complex I couples the transfer of electrons from NADH to ubiquinone performed by a series of redox centers with a translocation of protons across the membrane. EPR, a well‐known technique to detect free radicals, was used to detect the presence of radical anions of the electron acceptors, which accounts for the in situ activity of Complex I in proteopolymersomes (Figure 5a). 90 , 137 NADH/ferricyanide oxidoreductase activity assay proved that a high fraction of Complex I was inserted with desired orientation, favoring electron transfer from the vesicles into their membranes (Figure 5b). 90 Furthermore, ubiquinone 2 (CoQ2), known to be involved in the natural mechanism of energy conversion as an electron acceptor, was used to indicate the amount of electron transfer from the vesicles into their membranes. The addition of NADH to the proteopolymersome solution generated an EPR spectrum of CoQ2 with a significantly higher intensity, indicating the incorporation of more reduced forms of CoQ2 in the proteopolymersomes, which proves that Complex I mediate the electron transfers when reconstituted in the polymer membrane. 90
FIGURE 5.
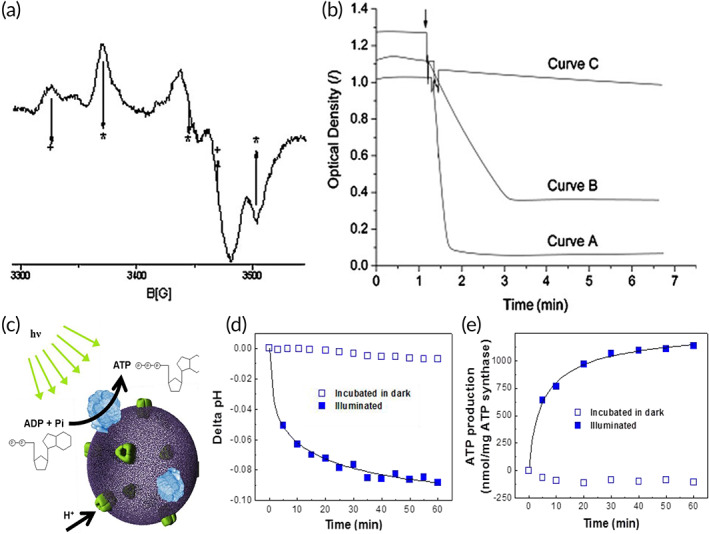
Structural and functional studies of membrane protein complexes with active transport in polymersomes. (a) Electron paramagnetic resonance (EPR) spectrum of NADH:ubiquinone oxidoreductase (Complex I) reconstituted in polymersomes. The EPR spectrum of Complex I in polymersome is similar to that of native Complex I solubilized in surfactants, indicating that chain of electron transfer was not affected by the reconstitution process. The five arrows with + and * indicate the signature of EPR spectrum specific to native Complex I. 137 (b) Measurement of NADH/ferricyanide oxidoreductase activity to determine the preferential orientation of Complex I in polymersomes. The activity of native Complex I solubilized in surfactants (Curve A) is preserved after incorporation in the polymer membrane (Curve B), and Curve C indicates no activity from empty polymersomes. The reduction of activity in proteopolymersomes is due to reduced fraction of incorporated protein, as well as partially unoriented Complex I. Source: Figure 4a,b is reproduced with permission from reference 90, Copyright 2010, John Wiley and Sons. (c) Schematic representation of an ATP‐producing polymersome based on bacteriorhodopsin (BR)‐ATP synthase coupling system. (d) Intravesicular pH change with respect to light illumination as a measure of proton pumping activity of BR reconstituted polymersomes. (e) Photosynthetic ATP production in the BR‐ATP synthase reconstituted polymersomes under illuminated condition, which is coupled with proton pumping activity of BR. Source: Figure 4c–e is reproduced with permission from reference 61, Copyright 2005, American Chemical Society, as well as from reference 138, Copyright 2013, MDPI
4.2.2. ATP synthase and bacteriorhodopsin (BR)
ATP synthase is composed of two domains, the membrane integrated F0 and the soluble F1. Coupling activity between the F0 and F1 complexes drives proton movement toward the F1 side of the membrane, resulting in ATP synthesis (Figure 5c). 61 , 138 The rotating activity of FoF1‐ATPase in the amphiphilic triblock copolymer PEtOz−PDMS−PEtOz can be maintained to synthesize ATP, using the photoinduced proton gradient generated from BR activity. 61 , 62 , 107 This is the first successful biosynthesis through coupled reactions between reconstituted transmembrane proteins in a single proteopolymersome, and the first to demonstrate motor functionality in a polymer membrane. The production of photoinduced electrochemical proton gradients from both BR and BR‐ATP synthase reconstituted proteopolymersomes can be measured by the addition of pyranine inside the proteopolymersomes. The relative fluorescence intensity ratio at 456 nm and 402 nm indicates the H+ ion concentration, and hence the internal pH in proteopolymersomes can be quantified (Figure 5d). 61 , 138 A bioluminescence assay using luciferin and luciferase is used to quantify ATP production, since luciferase catalyzes the oxidation of luciferin by consuming ATP and shows that ATP production increases significantly with increasing light incubation, indicating functional reconstitution of ATP synthase (Figure 5e). 61 , 62 , 107 , 138 However, proteopolymersome‐based studies of FoF1‐ATPase are limited by difficulties involved in the reconstitution process, such as low membrane permeability due to its synthetic nature and material inhomogeneity, thereby preventing continuous substrate and products transport across the channel protein and reduction in enzymatic reactions. Furthermore, some reconstitution conditions can be harsh to the FoF1‐ATPase, which is made up of multiple subunits that can be easily disrupted. Therefore, there is a need for better optimized membranes such as hybrid vesicles formed by the blends of lipids and block copolymers that can result in better reconstitution of such MP complexes. 139
4.2.3. Proton pump—proteorhodopsin
Purified light‐activated photo pump proteorhodopsin (PR) can be reconstituted in polymersomes formed from PEG‐PDPA‐PSS. 70 PR has a distinct polarity where the intracellular side has a slight positive charge, which is further increased through engineering a decahistidine‐tag at this side. On the other hand, the extracellular side bears a slightly negative charge. As a result, incorporation of PR into the polymersome allowed for its directed insertion where the PR would orientate with the negatively charged PSS group. This functionality of PR is confirmed by a light‐dependent pH change of the proteopolymersome solution, indicating the intended orientation. 70 In another study, PR is reconstituted in polystyrene‐b‐poly(4‐vinyl‐N‐methylpyridine iodide)2 (PS‐P4MVP2) polymersomes via spontaneous reconstitution at pH 7.4. 108 The membrane bilayer thickness is around 3.4–4.4 nm depending on increasing PS chain length, while the length of PR is less than 3.5 nm, indicating that hydrophobic mismatch may occur during reconstitution. 108 However, the results show successful PR reconstitution, suggesting that the polymer membrane is conformationally active to match the hydrophobic domain of PR. The reconstitution and packing of PR in these proteopolymersomes are investigated with SAXS, revealing a two‐dimensional hexagonally packed PR lattice in individual proteopolymersome membrane bilayers, consistent with previously conducted orientation studies. The secondary structure and structural stability of PR was further confirmed using Raman and solid‐state NMR (ssNMR) spectroscopy through labeling with13C and15N radioisotopes. 108 Time‐resolved visible spectroscopy through flash‐photolysis was used to determine PR functionality through monitoring whether it maintained key photocycle steps and turnover kinetics, where they showed that the PR reconstituted in proteopolymersomes retained the presence of M intermediate at 420 nm, absence of strong signals from the 13‐cis‐dark state at 600 nm, and relatively fast photocycle turnover kinetics. 108
4.2.4. Proton pump—cytochrome bo3 (hybrid vesicles)
The MP cytochrome bo3 (Cyt‐bo3), a redox‐reaction driven proton pump that couples oxygen reduction to proton transport, has been studied in hybrid lipid vesicles made from diblock copolymer PBD‐PEO and 1‐palmitoyl‐2‐oleoyl‐sn‐glycero‐3‐phosphocholine (POPC) phospholipid, with varying percentages. 89 Hybrid vesicles are used because they can combine both the higher stability of polymer components and the more annular and biocompatible lipid bilayer. The hybrid vesicle is formed via optimization of the reconstitution techniques, where extrusion of the hybrid vesicles, followed by gradual destabilization of the vesicles by small amounts of detergents, and eventual incorporation of the MP yielded spherical vesicles with size between 75 and 116 nm, confirmed with DLS and TEM. 89 To determine the optimal ratio between POPC and PBd22‐PEO14 that enables the highest Cyt‐bo3 activity in the hybrid vesicles, the initial rates of decylubiquinone oxidation are measured via absorbance reading at 275 nm, where an equimolar ratio between POPC and PBD‐PEO yields the best hybrid vesicle with Cyt‐bo3 having high initial activity and slow loss in activity. 89 Comparatively, Cyt‐bo3 is not functionally reconstituted in PBD‐PEO only based polymersomes, due to the poor biocompatibility of its membrane, indicating the need for hybrid vesicles that combines POPC liposomes biocompatibility to high stability of the PBD‐PEO polymersomes. 89 In a similar study with Cyt‐bo3 reconstitution in hybrid vesicles, the authors further investigated the hybrid membrane characteristics and showed that these membranes have less permeability than lipid bilayers, and 50 mol% PBD‐PEO hybrid vesicles have high initial reconstituted activity and retain around 20% of initial activity after 500 days. 140 Cyt‐bo3 has also been reconstituted in PDMS‐g‐PEO with and without phosphatidylcholine (PC) and showed that it had the highest activity in hybrid vesicles, as measured by the level of oxygen reduction, while the activity in either polymersomes or liposomes was about the same. 141
4.2.5. NaAtm1 and human P‐glycoprotein (hybrid vesicles)
ATP binding cassette (ABC) proteins including Novosphingobium aromaticivorans Atm1 protein, which mediates the active efflux of toxic metals complexed to glutathione, and human P‐glycoprotein (Pgp), which transports hydrophobic drugs, have been reconstituted and studied separately in hybrid vesicles consisting of both phospholipids and PBD‐PEO. 30 Reconstitution of either human Pgp or Atm1 protein is confirmed by density gradient centrifugation, as well as low passive permeability to a fluorescent probe (calcein acetomethoxyl‐ester) (C‐AM). Functional reconstitution of Atm1 or Pgp proteins is determined by ATPase functional assay which measures the liberation of inorganic phosphate. 30
Besides the examples on Cyt‐bo3 proton‐pumping oxygen reductase and ABC transporters, transmembrane protein complexes have a primary application of ATP production, which is coupled to active transport of protons under light stimulation. 142 Research has focused on optimizing artificial photosynthetic systems for ATP production to advance toward engineering of artificial cells. A limitation of the current approach lies in ATP being produced outside proteopolymersomes or proteoliposomes, which does not allow for more quantitative mechanistic studies such as mimicking in‐cell biochemical reactions. An improvement to this has been reported in a study using liposome GUVs to produce ATP where multilayer vesicles were formed like the structure of plant cells and ATP was harvested in the inner membranes to drive actin polymerization and carbon fixation continuously. 143 More MPs capable of energy harvesting could be reconstituted in polymersomes 94 to study their energy production capability as well as expand the research on artificial cells that can perform generation and consumption of energy all within themselves. 144 , 145
5. MEMBRANE RECEPTORS
Membrane receptors are specialized protein molecules attached to or integrated into the cell membrane. Membrane receptors play important roles such as facilitating communication between the cell and the extracellular environment through interaction with specific ligands including hormones and neurotransmitters. 146 Membrane receptors have been studied in liposomes; however, the incorporated proteins are unstable and hinder the measurements of receptor functions. 147 Hence, receptor‐based proteopolymersome systems have been engineered with reconstitution of receptors that are responsible for signal transduction (G‐protein‐coupled receptors, GPCRs), cell–cell communication (Cldn2), immune response (major histocompatibility complex I, MHC‐I) and cell adhesion (peptide anchors) (Table 1).
5.1. GPCRs (DRD2, CXCR4, and GLP‐1R)
GPCRs represent the largest class of MPs in the human genome and play a key role in mediating most of our physiological responses to neurotransmitters, hormones, and external stimuli. Hence, they are potential therapeutic targets for a broad spectrum of diseases and the study of their structure–function relationship is important. 148 Several proteopolymersome systems with GPCRs incorporation have been generated through cell‐free synthesis, including the reconstitution of dopamine receptor D2 (DRD2), 53 chemokine C‐X‐C receptor 4 (CXCR4) 109 and glucagon‐like peptide‐1 receptor (GLP‐1R) into polymersomes formed by PMOXA‐PDMS‐PMOXA or PBD‐PEO block copolymers. 110 In these proteopolymersomes, successful GPCR insertion is characterized by flow cytometry, SEC, and Western blots. The physiologically correct folding and orientation of reconstituted GPCR is confirmed by binding of respective conformational specific antibodies and native or synthetic ligands (Figure 6a), 53 as characterized by surface plasmon resonance (SPR), flow cytometry, I‐125 radioactive ligand binding or fluorescence‐based assays, with non‐GPCR proteopolymersomes or polymersomes without MP incorporation used as controls which showed no binding. 53 , 109 , 110
FIGURE 6.
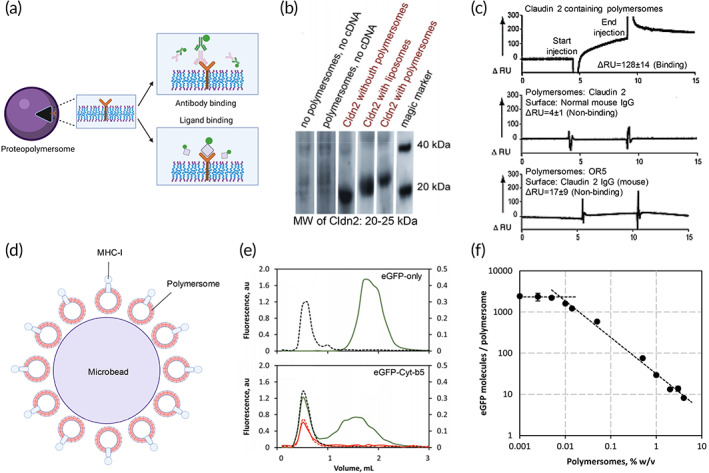
Characterization of membrane receptor‐based proteopolymersomes and their applications. (a) The structure–function relationship of membrane receptors is characterized by binding of conformational antibodies and native ligands to illustrate the proper folding and functions of the receptors, respectively. Schematic was created with BioRender.com. Source: Reproduced with permission from reference 53, Copyright 2012, John Wiley and Sons.(b) Western blot of in vitro expressed claudin‐2 (Cldn2) in the absence or presence of polymersomes or liposomes. (c) SPR measurements showing the binding of Cldn2 reconstituted proteopolymersomes to anti‐Cldn2 functionalized surface but not normal mouse IgG surface. There is also no significant binding between empty polymersomes without Cldn‐2 expression and the anti‐Cldn2 surface.Source: Figure 5b,c is reproduced with permission from reference 52, Copyright 2011, Springer Nature.(d) Engineering of an artificial antigen‐presenting cell by cell‐free in vitro synthesis and incorporation of MHC‐I into polymersomes (red vesicles) and the attachment of MHC‐I proteopolymersomes onto the 3D surface of microbead as a support (purple), forming MHC‐I proteopolymersome‐beads. 111 (e) Size‐exclusion chromatography (SEC) characterization of eGFP (top) and eGFP‐fused transmembrane domain of the rabbit cytochrome b5 (Cyt‐b5) (bottom) proteopolymersomes. Black dashes represent quantification of polymersomes by measuring light absorbance at 350 nm. Green and red solid lines show the presence of eGFP characterized by fluorescence signal (green, made fresh; red, after 6 weeks of storage). (f) There is an inversely proportional dependency of the immobilized eGFP‐Cyt‐b5 molecules per polymersome with increasing polymersome concentration. The polymersome surface area becomes limiting below 0.05% w/v. Source: Figure 5e,f is reproduced with permission from reference 113, Copyright 2016, Springer Nature. Schematics were created with BioRender.com.
In the individual system, the binding of dansyl‐labeled dopamine to DRD2 proteopolymersome illustrates a half‐maximal effective concentration (EC50) of 30 μM, which is much higher than the known EC50 of its native ligand dopamine in the nanomolar range. 149 While it is not discussed, this discrepancy can be due to the low amount of protein incorporation at only 25%, the presence of dansyl label leading to steric hindrance, and potential protein misfolding due to cell‐free synthesis that resulted in reduced ligand binding capacity. In the CXCR4 system, comparable dissociation constants of native ligand SDF‐1α in CXCR4 proteopolymersome (8.4 nM) and native membrane (1.4 nM) are identified. It was suggested that the lower affinity of the ligand for proteopolymersome could be due to the absence of G proteins in the synthetic system, which may affect CXCR4 conformation and alter ligand binding affinity. 150 In the GLP‐1R study, the binding affinity (Kd) of N‐terminal extracellular domain specific antibody is 18.6 nM, which indicates that some GLP‐1R assumed a correct orientation due to the accessibility to the N‐terminal domain. However, there is also some binding of the 1D4 antibody to the C‐terminal C9 tag, suggesting the presence of reversely incorporated GLP‐1R in the proteopolymersomes. 110 In addition, the low SPR response units during antibody binding show the presence of a low percentage of GLP‐1R incorporation into polymersomes. To promote folding of GLP‐1R for enhanced functionality, Fos‐choline 14 (Fos14) detergent is introduced, which functions as a chemical chaperone. Fos14 assists the folding of GLP‐1R and mediates a more stable incorporation of GLP‐1R into the polymersomes. 110 Radioligand competition binding assay between125I‐labeled GLP‐1 as tracer and native peptide ligand exendin‐4 confirms the functionality of these Fos14‐assisted GLP‐1R proteopolymersomes. The Kd of GLP‐1R proteopolymersomes (54.3 nM) determined is similar to that of GLP‐1R in native membrane (37.8 nM). 110
5.2. Claudin‐2
Claudin‐2 (Cldn2) is a transmembrane receptor that promotes cell–cell adhesion by forming homodimer with another molecule in neighboring cell. 151 Cldn2 is a component of the tight junction and forms cation‐selective and water permeable paracellular channel. 151 It also acts as a signal modulator and integrator that affects cell proliferation and migration, which may be relevant in both cancer biology and tissue regeneration. 151 Cldn2 is inserted into PBD‐PEO polymersomes using a cell‐free in vitro synthesis method and characterized for reconstitution using SEM and Western blots (Figure 6b). 52 Staphylococcal α‐hemolysin, which is a pore‐forming MP, is used as a positive control through dye leakage assay to demonstrate spontaneous MP insertion into PBD‐PEO polymersome. Cldn2 proteopolymersome is also characterized by monitoring the binding of specific antibodies against Cldn2 using SPR. SPR measurements indicate that there is binding between Cldn2 proteopolymersomes and the immobilized anti‐Cldn2 IgG (ΔRU of 128 ± 14) but not with normal mouse IgG (ΔRU of 4 ± 1) functionalized surface (Figure 6c). 52 There is no significant binding between polymersomes without Cldn‐2 expression and the anti‐Cldn2 IgG functionalized surface (ΔRU of 17 ± 9). Cldn2 has also been reconstituted into liposomes for direct comparison between the functionality of incorporated protein in both types of nano‐vesicles. The increased binding to anti‐Cldn2 by Cldn2 proteopolymersomes as compared to Cldn2 proteoliposomes not only indicates the correct folding and orientation of reconstituted Cldn2 but also the enhanced stability of protein insertion into polymersomes than liposomes for MP studies. 52
5.3. MHC‐I
To induce immune‐modulatory response, it is essential for MHC‐I proteins to be expressed on the extracellular‐side of antigen‐presenting cells (APCs) for molecular recognition of pathogens by T cells. Artificial APCs, which can behave as polymer‐based synthetic immunological synapses, are often used to enhance MHC‐I antigen presentation. 152 A new type of artificial APC is developed using cell free in vitro synthesis method of incorporation of MHC‐I molecule H‐2Kb preloaded with chicken ovalbumin (OVA) into the bilayer membranes of ABA‐RBOE‐PS‐SA nano‐vesicle beads that are made from self‐assembly of block copolymers (Figure 6d). 111 After confirming the structure and function of the incorporated MHC‐I, the MHC‐I H‐2Kb‐OVA proteopolymersomes serve as artificial APCs to promote antigen recognition and immunological synapse formation in CD8+ T cells isolated from OT‐I transgenic mice and induced T‐cell activation. 111 The engineered MHC‐I proteopolymersome represents a promising platform for studying and quantifying the spatio‐functional interactions between artificial APC and T‐cell and hence can have further applications such as HTS of T‐cell regulating compounds. In another study, pH‐responsive nanoparticles composed of triblock copolymers ([BMA‐co‐DEAEMA]‐b‐[DMA‐co‐PDSMA] polymers) doped with pyridyl disulfide functionalized monomer (PDSMA) for antigen conjugation are incorporated with MHC‐I, for use as artificial APCs. 153 Although different from MHC‐I proteopolymersomes, the MHC‐I conjugated nanoparticles are able to enhance MHC‐I antigen uptake in dendritic cells, consistent with that observed in MHC‐I proteopolymersomes. 153
5.4. Peptide anchors (CecA, Cyt‐b5, Vam3p, lysis protein L)
Amphiphilic peptides have been used as anchors to decorate polymersome for additional surface functionality including anti‐microbial activity as well as for membrane surface anchoring of water soluble proteins. 154 , 155 An example is the reconstitution of a fusion protein (CecA‐eGFP) based on the antibacterial peptide Cecropin A (CecA) and the enhanced green fluorescent protein (eGFP) into polymersomes formed by triblock copolymer polyisobutylene‐polyethylene glycol‐polyisobutylene (PIB–PEG–PIB). 112 Successful reconstitution of CecA into polymersomes is characterized by the folding of a random coil into α‐helix in presence of polymersomes detected by CD and the co‐localization of CecA and polymersomes as shown through SEC and tryptophan fluorescence measurements. 112 A follow‐up study has shown a similar reconstitution of natural peptide anchors including eGFP fused transmembrane domains of cytochrome b5 (Cyt‐b5), viral lysis protein L of the bacteriophage MS2, and yeast syntaxin VAM3 (Vam3p) with CecA‐eGFP as a positive control. 113 The presence of natural peptide anchors allows the tethering of water‐soluble protein or enzyme to membranes. These natural peptides are reconstituted into PMOXA–PDMS–PMOXA polymersomes. The display of eGFP on the surface of polymersomes illustrates the proper insertion of the peptide anchors into the polymeric membranes and co‐localization of these peptides and polymersomes is shown through SEC (Figure 6e). 113 The study also shows an inversely proportional dependency of the immobilized eGFP‐Cyt‐b5 molecules per polymersome with increasing polymersome concentration where the polymersome surface area becomes limiting below 0.05% w/v (Figure 6f). 113 Importantly, these peptide anchors do not form pores or disintegrate the membranes, illustrating their potential to anchor water soluble proteins on membrane surface. 113 , 154
While the above membrane receptor studies are conducted in proteopolymersomes, there are other receptor‐based studies performed in proteoliposomes as well as in lipid and polymer bilayers. 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 Some of these important MP complexes, such as β‐site amyloid precursor protein (APP) cleaving enzyme 1 (BACE1) and γ‐secretase, 156 , 157 , 158 , 159 , 160 may be further studied in polymersome for structural and functional comparison in different nano‐vesicles. The techniques used in these liposome‐related MP studies, including FCS, fluorescence recovery after photobleaching (FRAP), single molecule tracking (SMT), total internal reflection fluorescence spectroscopy (TIRFS), fluorescence resonance energy transfer (FRET) and continuous‐wave EPR (CW‐EPR), could also be applied to the characterization of receptor–ligand interactions and changes in MP conformations and oligomeric states in proteopolymersomes. 161 , 162 , 163
6. FACTORS AFFECTING MP STUDIES IN PROTEOPOLYMERSOMES
A good cell membrane mimetic should be morphologically similar to the biological bilayer membrane, equivalent thickness in a liquid crystalline phase, without any change in the membrane fluidity, which may affect the equilibrium distribution of the different MPs. 164 There are several factors that affect the folding, function, and dynamic equilibrium of MPs in proteopolymersomes. We will discuss three key groups of factors below, including membrane composition, MP expression and reconstitution system, and protein states (Table 2).
TABLE 2.
Factors affecting membrane protein (MP) reconstitution and quality of structural and functional characterization
| Optimization parameters | Factors affecting MP reconstitution | Effects on MP structural and functional characterization | MPs | References |
|---|---|---|---|---|
| Membrane composition | Polymer composition/asymmetricity | Determine the physiologic orientation and function of the inserted MP; molecular weights for different polymer blocks can facilitate efficient protein encapsulation and stabilization |
AQP0 PR |
29, 70, 165 |
| Polymer flexibility/curvature | Determine the physiologic orientation and function of the inserted MP |
AQP0 OmpF |
29, 166, 167 | |
| Polymer membrane thickness | Increased thickness increases MP conformational stability | Complex I | 28, 90, 166, 168 | |
| Membrane protein expression and reconstitution system | Cell‐based protein purification and reconstitution |
Advantages: Addition of detergents allows for ease of MP solubilization and can facilitate MP folding Disadvantages: Cytotoxicity, misfolding, and aggregation |
OmpF Cldn‐2 |
169, 170, 171 |
| Cell‐free co‐translational incorporation |
Advantages: No cytotoxicity, pure and homogenous protein formation, can be solubilized with milder detergents, less chance of protein misfolding Disadvantages: Lower final protein yields |
OmpF Cldn‐2 |
169, 170, 171 | |
| Hydrophobic mismatch | Decrease the equilibrium concentration or activity of MP | OmpF | 29, 64, 167, 172, 173 | |
| Detergent concentration | High concentrations may decrease MP activity |
NaAtm1 Pgp FhuA OmpF |
30, 101, 132 | |
| Rate of detergent removal | Slow rate of removal results in smaller polymersomes; fast rate of removal results in larger polymersomes | AQP0 | 102 | |
| Size of vesicles | Smaller vesicles are more compatible with biological membranes and increases MP stability; large vesicle interior volume lowers MP membrane concentration, and increases NMR structural study difficulty | Cldn‐2 | 52, 174, 175 | |
| Preformed vesicles | Limit the number of MP that can be incorporated due to high energetic expenditure | AQP0 | 102 | |
| Polymer/lipid‐to‐protein ratio | A low polymer/lipid to MP ratio increases the quantity of MPs available in polymersomes for study; a ratio of 1:1 has been shown to have high NMR sensitivity | MsCL Influenza M2 proton channel | 176, 177 | |
| Protein amount/concentration | High protein concentration increases MP reconstitution; saturated protein concentration decreases MP reconstitution |
OmpF TsX |
25, 26, 27, 28, 60, 96, 97, 102, 106 | |
| Protein states | Monomers/purple membrane | Determine the physiologic orientation and function of the inserted MP | BR/F0F1‐ATP synthase | 107 |
| Environmental condition | Light illumination increases MP activity | PR | 60, 61, 62, 107 | |
| Immobilization of polymersomes on surfaces/free flowing polymersomes | MP activity decreases when polymersomes are immobilized | OmpF | 96 | |
| Correlation time of the MP–surfactant complex (PSC)/Tumbling rate | Determine the ability of protein structure to be resolved by NMR. Fast‐tumbling small PSCs (<100 kDa) can be studied by solution NMR; slow reorienting aggregates are more suitable to be characterized by solid‐state NMR | DAGK | 178, 179 |
6.1. Membrane composition
The asymmetricity of copolymers used in polymersome synthesis could result in different thickness and fluidity of the formed polymersomes, thereby affecting the orientation and functionality of the inserted MPs. A study with aquaporins with His‐tag shows that the percentages of nonphysiological orientation as characterized by the exposure of His‐tag to the external solution for lipids and ABA triblock copolymer were near 50%, which is in reasonable agreement with random insertion into the membranes. However, for ABC and CBA triblock polymers, there was a preferred physiologic orientation of 72% in the ABC system, and a nonphysiologic orientation of 81% in the CBA system (Figure 7a). 29 In addition, designing triblock copolymers with different molecular weights for the A and C blocks can facilitate efficient protein encapsulation and stabilization. This can be done through having the longer end with a higher molecular weight segregating on the outside of the polymersome due to a larger radius of curvature with a differing volume fraction, and the smaller end segregating on the inside of the polymersome. 165 The conformational freedom and flexibility of the polymers are key factors to promote MP incorporation without involving a loss of free energy. Hydrophobic mismatch between the polymer and MP affects protein structure and functionality (Figure 7b). 166 For instance, in an OmpF reconstituted proteopolymersome, a thin 3 nm polymer bilayer matches with the protein length and results in functional protein without deformation of the polymer membrane. In contrast, a 6 nm polymer bilayer shows a strong negative mismatch, resulting in symmetric deformations in the upper and the lower leaflets, and could potentially lead to an expulsion of the MP. 167 A membrane formed from amphiphilic block copolymers can withstand larger hydrophobic mismatches of more than 22% in the membrane thickness than lipid‐based membranes, which can typically only withstand mismatches of 2%–3%. 167 Thus, increased flexibility of polymeric membranes can lead to a more successful biomolecule insertion. On the other hand, PR has been functionally reconstituted into highly stiff and stable glassy block copolymer membranes with the polystyrene hydrophobic block, 167 indicating that the conformation and flexibility also depends on the type of MPs inserted.
FIGURE 7.
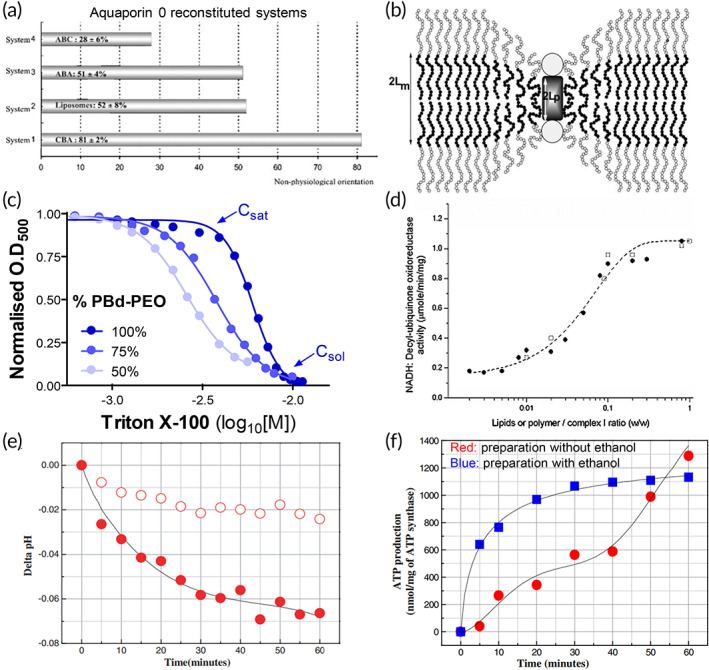
Factors affecting reconstitution efficiency and quality of membrane protein study. (a) Polymer asymmetricity affects orientation of aquaporin 0 insertion into polymersomes. Source: Reproduced with permission from reference 29, Copyright 2004, John Wiley and Sons. (b) Hydrophobic mismatch between the polymer and membrane protein near an inclusion in a polymeric bilayer. The chains in the unperturbed bilayer are highly stretched in order for much shorter proteins to match the thickness without undergoing significant compression when compared to the free chain radius of gyration. 2Lm is the thickness of a flat bilayer and 2Lp is the inclusion thickness. Source: Reproduced with permission from reference 166, Copyright 2003, Elsevier. (c) Higher detergent concentrations are required by vesicles made up by a higher proportion of block copolymers (PBd‐PEO) to reach saturation (Csat) and solubilization (Csol) points, indicating that there is an increase in stability with inclusion of block copolymers in vesicles. Source: Reproduced with permission from reference 30, Copyright 2020, MDPI. (d) Effect of increasing lipid/polymer to NADH:ubiquinone oxidoreductase (Complex I) concentration on decyl‐ubiquinone oxidoreductase activity. Increasing lipid/polymer to membrane protein ratio results in more functional activity. Source: Reproduced with permission from reference 90, Copyright 2010, John Wiley and Sons. (e) Preparation method controls proton vectoriality in bacteriorhodopsin (BP) proteopolymersomes. Incorporation with BR monomer in the absence of ethanol results in the light‐induced change in pH exhibiting negative values with increase in illumination time. Solid circles indicate illuminated condition, while hollow circles indicate dark‐incubated. (f) Protein states affect the functional activity of the reconstituted membrane proteins in polymersomes. BR‐ATP synthase exhibits an acceleration of ATP synthesis when polymersomes are prepared without ethanol (red). Preparation of polymersomes with ethanol (blue) shows an initial increase in ATP synthesis which decreases with time. Source: Figure 7e,f is reproduced with permission from reference 107, Copyright 2006, IOP Publishing
Nano‐vesicles, in particular the SUVs, are more stable, have a smaller curvature than GUVs, and provide a local environment that is more similar to that of biological membranes for MPs. This property makes them more suitable for MP studies, particular for structural studies. 20 , 43 , 180 In addition, a key advantage of these nano‐vesicles lies in the clearly defined compartments segregated by the copolymer layers, which creates a concentration gradient that allows the transport of solutes and hence the measurement of the activity of pore‐forming proteins across the membrane. 104 , 123 , 181 Hence, controlling the size of the polymersomes is important in determining their applications and methods used to study MPs in proteopolymersomes. 182 Polymersomes, although stable and come with many benefits, are not always favorable environments for MP reconstitution, and in some cases, modifications to the membrane environment are required to achieve the desired functions. 30 , 183 These issues motivate the modification of polymersome properties to enhance their bio‐functionality, such as blending block copolymers and phospholipids to create hybrid vesicles, with the goal of combining the best features of these two materials such as having the chemical versatility and robustness of polymersomes with the biocompatibility and biofunctionality of liposomes (Figure 7c). 30 , 183 , 184
6.2. MP expression and reconstitution system
Cell‐free protein expression systems have been widely adopted to produce structurally intact mammalian MPs, 185 and to overcome the limitations of the conventional protein production with use of E. coli or yeast. 116 , 176 Cell‐free protein production generates a large amount of properly folded and biologically active proteins to be mixed with sufficient copolymers for optimal reconstitution for extensive MP studies. While cell‐free approaches come with many advantages, the required enzymes supplemented in vitro which may be inferior to the quality control systems found in cells, and may contribute to making some misfolded and inactive MPs in the mixture that confounds quantifications of MP properties. 186 Therefore, it is important to optimize the protein expression and reconstitution system to use for MP studies depending on the quantity and stability of the MPs required. Choice of detergents and organic solvents in reconstitution may affect the stability of MPs, and hence the efficiency of reconstitution for MP studies. The use of detergents is important in the extraction of large quantities of MPs which is required for techniques such as NMR to achieve a significant detection signal. However, high concentration of detergents used can also lead to formation of detergent micelles which can destabilize MPs. Consequently, careful control of detergent concentration needs to be done to increase MPs stability. 166 , 176 , 187 Organic solvents are necessary for polymersomes solubilization and are used to mix with the MPs solution to facilitate protein reconstitution. However, the presence of organic solvents can denature MPs, hence decreasing their functional activity. Therefore, new methods such as polymer rehydration and droplet microfluidics, which eliminates the need for organic solvents, have been discovered for reconstitution to improve MPs quality and activity in multiple studies. 27 , 43 , 61 , 62 , 90 , 107 The lipid/polymer‐to‐protein concentration and ratio also affects MP activity. 188 For instance, a ratio of 1:1 results in the highest NADH‐decylubiquinone oxireductase activity (Figure 7d). 90
6.3. Protein states
The protein states of MPs used also affect its orientation of insertion in the polymersomes. This can be seen through the comparison between insertion of BR in monomeric state and BR in the form of purple membrane (PM). 61 , 62 , 107 Using the BR monomer, results showed that light‐induced pH exhibited negative values with increasing illumination time (Figure 7e). This indicates that protons were being pumped inwards into the core of the polymersomes, which is opposite to the outward pumping with BR in purple membrane. This also shows that BR molecules are preferentially positioned with the C‐terminus facing outward and inward in the proteopolymersomes when reconstituted with BR monomer and BR in purple membrane respectively. 61 , 62 , 107 Changes in protein states also affect the functional activity of MPs. In the case of proteopolymersomes of BR in PM state, BR‐ATP synthase exhibited an acceleration of ATP synthesis (Figure 7f). 107 When the reconstituted BR is in the monomeric state, BR‐ATP synthase activity had an initial slow activity and increased progressively over the course of 30 min when the rate decreased. 107
In a reconstituted proteopolymersome, changes in protein concentration used or amphiphile‐to‐MP ratio can affect proteopolymersomes morphologies, quality of protein crystals needed for MP structural study, 102 , 176 as well as MP orientation and activities, 52 , 61 , 62 , 107 and quantities available in proteopolymersomes for study. The use of excellent quality of protein crystals has also been shown to allow the elucidation of small molecule interactions with influenza M2 proton channels in lipid bilayers as well as the determination of the high‐resolution structures of their complexes. 177 The correlation time of the protein‐surfactant complex (PSC) also affects the ability of MPs to be resolved by NMR, where the fast‐tumbling small PSCs below 100 kDa (e.g., diacylglycerol kinase [DAGK] in detergent micelles) can be studied by solution NMR, 178 while slow reorienting aggregates are more open to ssNMR. 179 Finally, there is a difference in the functionality of MPs inserted into free vesicles as compared to immobilized vesicles. It is observed that MPs in immobilized vesicles have 6.5 times lower activity than the free vesicles in solution. 189 Two possible reasons can explain this phenomenon. First, it can be due to the presence of an unstirred aqueous layer at the polymer‐membrane solution interface leading to the formation of a diffusional barrier for otherwise rapidly permeating substrate. 189 Second, the positioning of the nanoreactors toward the surface and the immobilization on the solid support may result in a reduced accessibility of the MPs. 96
7. CELL MEMBRANE MIMETICS AS PLATFORMS FOR HTS IN DRUG DISCOVERY
New in vitro tools and models that can directly monitor the structural and functional properties of MPs are increasingly needed to enable the identification of novel lead compounds that can guide preclinical drug developments. Currently, majority of the HTS campaigns in drug discovery make use of cell‐based biosensors and related secondary assays to identify small molecule modulators that target MPs. Although cell‐based platforms have multiple advantages including being more physiologically relevant, nonspecific targeting of drug compounds remains as a major limitation as drug compounds can interact with multiple proteins or targets in the cells. They also have the disadvantage of random insertion of gene of interest into the cell genome that can disrupt the expression of some endogenous proteins. 190 , 191 , 192 , 193 Hence, the characterization of the interactions between drug candidates and MPs using a cell‐free system to directly observe their functional modulation and structural perturbation in a high‐throughput setting can greatly facilitate the speed, specificity, and quality of drug discovery. 192 MP inserted nano‐vesicles such as proteopolymersomes and proteoliposomes, either freely residing in microplates or immobilized onto a membrane bilayer, serve as excellent cell‐free HTS platforms (Figure 8a). 196 , 197
FIGURE 8.
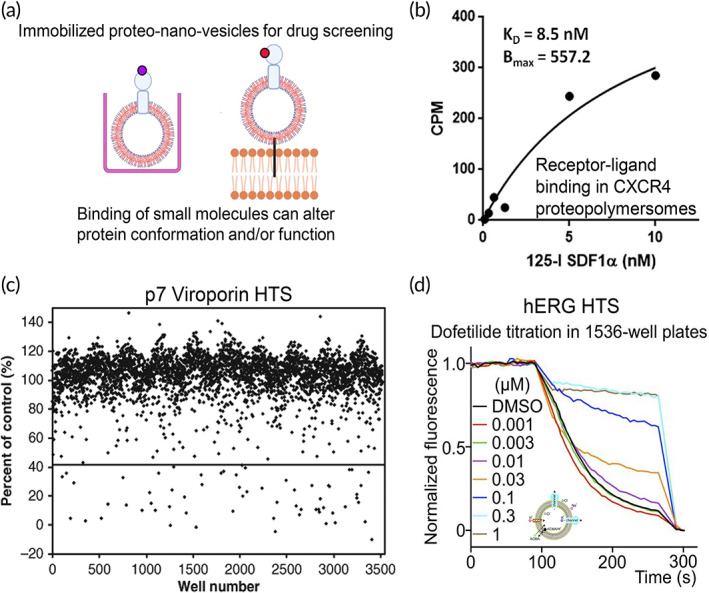
Proteopolymersomes and proteoliposomes as high‐throughput screening (HTS) platforms for drug discovery. (a) Immobilized proteopolymeromes or proteoliposomes, either through free‐flowing proteo‐nanovesicles residing in multiwell plates (left) or tethering of proteo‐nanovesicles to a bilayer support (right), can be used for HTS drug discovery. Binding of small molecules can alter membrane protein conformation and/or function. (b) Saturation binding of iodine‐125 (125I) radiolabeled SDF1α ligand to CXCR4 receptor incorporated proteopolymersomes as a proof‐of‐concept study that the proteopolymersomes can be utilized to screen for small molecule binders that modulate membrane receptor structures and functions. Source: Reproduced with permission from reference 109, Copyright 2014, PLOS. (c) HTS drug discovery of 3520 compounds using Hepatitis C Virus p7 Viroporin proteoliposomes and a fluorescence dye permeability assay. A 1.8% hit rate is observed. Source: Reproduced with permission from reference 194, Copyright 2011, SAGE. (d) A HTS platform in 1536‐well plates developed using hERG channel proteoliposomes and a fluorescence dye permeability assay. A positive control compound, dofetilide, is illustrated with an increase in dye permeability, indicating the ability of the HTS platform to be used to screen for compounds that modulate the hERG channel. Source: Reproduced with permission from reference 195, Copyright 2016, National Academy of Sciences, USA. Schematics were created with BioRender.com.
7.1. Structural and conformational screening
HTS of small molecules can be performed based on either modulating the protein–protein interactions (PPIs) 198 , 199 or perturbation of protein conformational dynamics, which directly corresponds to protein functions. 200 , 201 For example, multiple receptors form preligand dimers or undergo conformational changes upon ligand binding to exert their functions. The disruption of these interactions or alterations in their conformational states can lead to either inhibition or activation of the receptor. 202 Biophysical techniques can be adopted to measure PPIs and protein conformational change for MP studies, such as using FRET 203 or SPR 204 , 205 , 206 in binding screens. While not yet established in proteopolymersome, the feasibility of this approach has been demonstrated through FRET measurement of the dimerization of transmembrane fibroblast growth factor receptor 3 (FGFR3) helix incorporated in proteoliposomes, where the determined thermodynamic parameters are directly relevant to the biological processes in cell membranes. 159 This suggests that the compound induced modulation of MPs incorporated in proteoliposomes can be monitored through FRET changes, which directly reflect structural changes of MPs that correlate to their functions. In addition, high‐throughput binding screens can be conducted using SPR 204 , 205 , 206 to identify small molecule inhibitors/activators that modulate PPIs. For example, SPR has been used to measure the binding of native ligands on Cldn2, DRD2, CXCR4, and GLP‐1R proteopolymersomes (Figure 8b). 52 , 53 , 109 These platforms can also be optimized and adopted for HTS of active drug compounds that bind to these proteopolymersomes and alter the ligand binding capability to the reconstituted MPs, although no such study has been reported up to date. Nevertheless, studies have shown that it is feasible to detect compounds that alter structures and functions of the reconstituted MPs in proteoliposomes immobilized on SPR chips. 87 , 197 SPR measurements and screening have been done in immobilized lipid bilayers with reconstituted full‐length BACE1 and the interaction between the BACE1 and several inhibitors is confirmed by this SPR biosensor‐based assay. 207
7.2. Functional screening
In MP functional screening, a direct method is to screen for compounds that modulate channel proteins through leakage assay of fluorescent dyes. While the use of proteopolymersome in functional screening of compounds targeting channel proteins has not yet been developed, this functional HTS strategy has been widely adopted in proteoliposomes; hence, a similar strategy can be potentially adopted. 20 Examples include the development of novel cell‐free HTS methods for hepatitis C virus p7 viroporin 194 and K+ channel proteins 195 (Figure 8c,d). Specifically, a low‐throughput proteoliposome‐based fluorescent dye permeability assay was modified, optimized, and converted to a robust HTS assay to screen for compounds capable of interfering with p7 channel function (Figure 8c). 194 To eliminate nonspecific hits, melittin channel‐forming peptide is used in a counter screen. 194 Similarly, a proteoliposome flux assay using a fluorescent dye was applied in a HTS and the study identified new activators and inhibitors of four different K+ channels. GIRK2, TRAAK, Slo1 and hERG, all of which are important MPs to control ion homeostasis and cell signaling (Figure 8d). 195 In another study, hERG channel is expressed through a cell‐free expression system and integrated into a biomimetic lipid bilayer platform. The properly folded and functional hERG channel is used for probing the channel and drug interactions through a fluorescence polarization assay and can be adopted for HTS to discover novel channel blockers. 208 All these studies illustrate the potential of using proteopolymersome for similar functional screening studies.
Once lead compounds are identified, high‐resolution studies are required to understand the binding sites of small molecule modulators as well as how they perturb the MP of interest. An example of a high‐resolution study is using solid‐state MAS NMR to investigate the binding and structural change of a small molecule water channel inhibitor (NSC13691) to AQPZ (Figure 9a–c) in proteoliposomes, together with their functional inhibition in a stopped‐flow water permeability assay (Figure 9d). 34 Future directions in HTS for drug discovery can include mass production of nano‐vesicles 209 and construction of cell‐like structures such as membrane‐based nanoreactors, artificial cells, 210 or synthetic cell chassis, and the exploitation of microfluidic devices to increase the throughput in MP studies and drug discovery. 211
FIGURE 9.
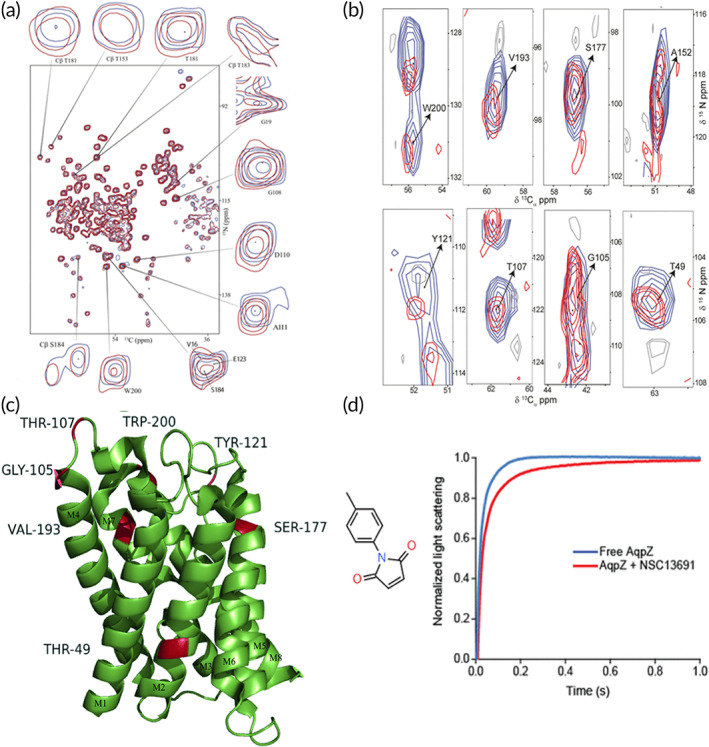
Structure‐based elucidation of inhibitor binding site in AqpZ proteoliposome by solid‐state MAS NMR. (a) Two dimensional (2D)15N–13Cα spectra of AqpZ with inhibitor (NSC13691) (red) and without inhibitor (blue) recorded by solid‐state MAS NMR illustrating that AqpZ–drug interaction leads to perturbation in chemical shift of AqpZ structure with significant perturbations being highlighted. (b) Contour plots of 2D planes (15N, 13Cα) corresponding to AqpZ with inhibitor (red) and without inhibitor (blue) extracted from 3D NCACX spectra with the assigned peaks indicating significant chemical shift perturbations of more than 0.2 ppm in both 13Cα and 15 N planes. (c) Mapping of the ApZ–drug interaction site onto the crystal structure (1RC2). The residues that have undergone significant chemical shift perturbations have been highlighted in red. (d) The inhibitor NSC13691 blocks AqpZ function as characterized by a stopped‐flow water permeability assay in AqpZ proteoliposomes. A reduced water permeability of AqpZ is observed as characterized by a significant decrease in the rate constant of the AqpZ proteoliposomes shrinkage. Source: Figure 9a–d is reproduced with permission from reference 34, Copyright 2018, Springer Nature
8. SUMMARY AND FUTURE PERSPECTIVES
In this review, we provided extensive insights into MP studies using proteopolymersomes, specifically in the types of proteins studied, techniques used to study MP structures and functions and complications involved in these studies. Multiple factors affecting MP studies in proteopolymersomes suggest that the silver‐lining in engineering proteopolymersomes that provide the most relevant structural and functional information lies in detailed optimization and controllability of the processes for amphiphilic polymers self‐assembly, protein synthesis, and reconstitution. The advancement to incorporate enzymes, chaperone proteins, and DNA to these synthetic membranes to generate artificial cells 212 , 213 or nanoreactors 214 , 215 , 216 will further propel promising platforms not only to study MPs but also investigate a wide range of cell functions and processes that initiate from the formation of proteopolymersomes.
We further detailed the feasibility and current implementation of reconstituted MPs in synthetic membranes, including proteopolymersomes and MP bound polymeric bilayer, to be used in HTS for therapeutic discovery. Although multiple examples in using proteo‐nanovesicles to study the modulation of MP structures and functions by pharmaceutical compounds have been reported, there are limited studies using proteo‐nanovesicles, in particular proteopolymersomes, as HTS platforms in drug discovery. The main obstacles in using proteopolymersomes in the HTS process lie in the lack of robust and scalable production methods to produce large batches of proteopolymersomes with perfect monodispersity and reproducibility. 217 , 218 Once these obstacles are overcome, we propose that proteopolymersomes are suitable to be used as HTS platforms to discover novel therapeutics targeting the reconstituted protein of interest and provide corresponding recommendations as well as a roadmap for using proteopolymersomes in drug discovery pipeline (Figure 10).
Development of proteopolymersome‐based screening platform: Exploiting the use of polymersomes in drug discovery can potentially be adapted to all membrane‐bound protein targets through careful selection of suitable reconstitution methods and polymer mixtures to engineer the desired proteopolymersome as a screening platform (Figure 10a). This platform, in principle, allows the incorporation of any MP for which the complementary DNA is available. An initial characterization of the engineered proteopolymersomes should be performed to ensure good quality control. To achieve inserted MPs of better quality, chaperones or enzymes may be added to assist the MP folding. 110 Furthermore, other cellular components such as organized metabolic reactions and gene expression mechanisms may be included in distinct spatial compartments in the proteopolymersomes 18 , 218 to achieve the engineering of synthetic cells as a screening platform, which would be compatible in their biological functionalities to drug screening using living cells. This is followed by the characterization of the structure and function of the reconstituted MP to validate its physiological folding and functions. The proteopolymersome constructed will need to be coupled to a detection method such as fluorescence or luminescence measurements to establish the screen platform with sensing capability (Figure 10b). The adaptation into high‐throughput formats, including accurate dispensing of proteopolymersomes into multiwells, calibrations, and determination of limit of detection should be optimized.
Validation of the screening platform and benchmarking against known biological data: The proteopolymersomes based screening platform can be tested with positive controls known to modulate the MP structure (e.g., conformational dynamics) and function (e.g., ligand binding) to ensure that the MPs are responding to all protein‐specific stimuli and to determine the compound efficacy or binding affinities to the MPs (Figure 10c). Testing with positive controls will also determine the assay quality Z‐factor and coefficient of variation of the screening platform. 219 Negative controls that are known to target other MPs should also be tested to ensure that they do not perturb the target MPs of interest and there is high specificity of compounds to the reconstituted proteopolymersomes. Mutations in MPs that might change their signals as well as other proteins such as native ligands can be used to validate the MP functions in proteopolymersomes. These parameters can be benchmarked with known biological data or results obtained from other cell‐based assays that have been previously conducted to assess the performance of the screening platform (Figure 10d). If the experimental values fall outside the acceptable range of the known data, the engineering of proteopolymersomes must be further optimized to obtain data that corroborate with other studies to ensure accuracy and consistency.
Automation‐assisted HTS: The established screening platform with high mechanical stability can be used in combination with automations such as multiwell plate‐readers, microfluidics or SPR to assist drug screening using proteopolymersomes, which will facilitate high‐throughput and rapid turnover in identifying MP modulators (Figure 10e). New technologies enabling HTS and the scaling up of the production of proteopolymersomes for drug screening can be developed to facilitate the hit identification process. Hit compounds can undergo further structural optimization by medicinal chemistry to improve on the pharmacological properties of the potential drug candidates (Figure 10f). These compound analogs should be tested with proteopolymersomes to ensure that they are still targeting the protein of interest. Furthermore, the binding sites and the chemical groups of the analogs that interact with the MPs can be elucidated by structural studies using proteopolymersomes. This will allow chemists to make better informed decisions in designing the structures of the analogs for improved binding and potency.
Assessment of the efficacy of hit compounds in cell‐based assays and animal models: The efficacy of the hit compounds should be determined using secondary or orthogonal assays typically through cell‐based studies (Figure 10g) as well as in appropriate animal models (Figure 10h). Both the functionality and specificity of the hit compounds should be determined at this stage. Typically, the hit compounds are specific if it does not have an effect in cells and animal models containing the knockout of the MPs of interest. The use of proteopolymersomes in drug discovery has an added advantage of having higher specificity than conventional cell‐based biosensors containing other biological components. From these studies, the pharmacokinetics, pharmacodynamics, and drug biodistribution should also be determined.
Elucidation of the mechanism of action of the lead drug candidate using proteopolymersomes: The protein–drug interaction sites and whether the drug acts through a competitive or allosteric mechanism can be elucidated using proteopolymersomes (Figure 10i). Proteopolymersomes containing mutations in the protein of interest can be utilized to confirm the binding sites where a lack of key binding residues will reduce drug binding. The binding sites of the lead drug candidates can be determined by obtaining high‐resolution crystal structure of drug bound proteins by cryo‐EM or x‐ray crystallography. Structural perturbation or conformational change of protein of interest induced by the drug candidates can be resolved by NMR spectroscopy. Furthermore, protein complexes or coupling systems in proteopolymersomes would enable the investigation of the effect of drugs not only on the target MPs but also on other downstream proteins in the pathways.
FIGURE 10.
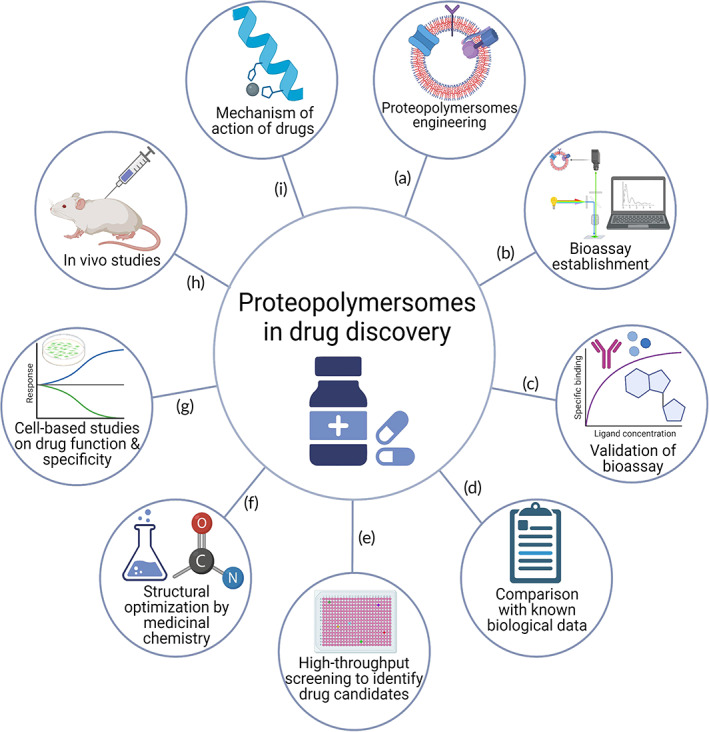
Prospective research directions and a roadmap for using proteopolymersomes in drug discovery. (a) Proteopolymersomes engineering using suitable block copolymers and gene/protein or interest. (b) Bioassay establishment with proteopolymersomes using appropriate detection methods such as FRET measurement for structural change or fluorescent leakage assay for channel functional assay. (c) Validation of bioassay using positive controls that are known to alter protein structure or modulate protein function in proteopolymersomes. (d) Compare the structural and functional data obtained from proteopolymersomes with known biological data from literature to benchmark the performance of the bioassay established with proteopolymersomes to decide whether to proceed with high‐throughput screening (HTS) or more optimizations may be required. (e) HTS of suitable chemical libraries using proteopolymersomes to identify hit compounds or potential drug candidates for further testing. (f) Potential drug candidates may undergo structural optimization by medicinal chemistry to obtain analogs with improved pharmacological properties, which will be tested in proteopolymersomes for their functions. (g) Cell‐based studies will be conducted to determine the efficacy of the potential drug candidates in modulating cellular functions as well as elucidate their specificity to the protein target. (h) Use of animal models such as mice to validate the efficacy of potential drug candidates in vivo. (i) To elucidate the mechanism of action of the potential drug candidates using proteopolymersomes, including their binding sites, how they perturb the protein of interest as well as whether they act through competitive or allosteric mechanisms. Schematics were created with BioRender.com.
AUTHOR CONTRIBUTIONS
Chih Hung Lo: Conceptualization (lead); funding acquisition (equal); writing – original draft (equal); writing – review and editing (equal). Jialiu Zeng: Funding acquisition (equal); writing – original draft (equal); writing – review and editing (equal).
CONFLICT OF INTERESTS
There are no conflicts of interest to declare.
ACKNOWLEDGMENTS
The authors thank Lance M. O'Connor from the University of Minnesota for proofreading the manuscript. This study is supported by a Lee Kong Chian School of Medicine Dean's Postdoctoral Fellowship to Chih Hung Lo (Grant Award Number 021207‐00001) from Nanyang Technological University (NTU) Singapore. This study is also supported by a Presidential Postdoctoral Fellowship to Jialiu Zeng (Grant Award Number 021229‐00001) from NTU Singapore.
Lo CH, Zeng J. Application of polymersomes in membrane protein study and drug discovery: Progress, strategies, and perspectives. Bioeng Transl Med. 2023;8(1):e10350. doi: 10.1002/btm2.10350
Chih Hung Lo and Jialiu Zeng contributed equally to this study.
Funding information Lee Kong Chian School of Medicine Dean's Postdoctoral Fellowship, Nanyang Technological University (NTU), Singapore, Grant/Award Number: 021207‐00001; Presidential Postdoctoral Fellowship, Nanyang Technological University (NTU), Singapore, Grant/Award Number: 021229‐00001
Contributor Information
Chih Hung Lo, Email: chihhung.lo@ntu.edu.sg.
Jialiu Zeng, Email: jialiu.zeng@ntu.edu.sg.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Fagerberg L, Jonasson K, von Heijne G, Uhlén M, Berglund L. Prediction of the human membrane proteome. Proteomics. 2010;10(6):1141‐1149. doi: 10.1002/pmic.200900258 [DOI] [PubMed] [Google Scholar]
- 2. Pedro AQ, Queiroz JA, Passarinha LA. Smoothing membrane protein structure determination by initial upstream stage improvements. Appl Microbiol Biotechnol. 2019;103(14):5483‐5500. doi: 10.1007/s00253-019-09873-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roumia AF, Tsirigos KD, Theodoropoulou MC, Tamposis IA, Hamodrakas SJ, Bagos PG. OMPdb: a global hub of Beta‐barrel outer membrane proteins. Front Bioinform. 2021;1:9. doi: 10.3389/fbinf.2021.646581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gong J, Chen Y, Pu F, et al. Understanding membrane protein drug targets in computational perspective. Curr Drug Targets. 2019;20(5):551‐564. [DOI] [PubMed] [Google Scholar]
- 5. Gulezian E, Crivello C, Bednenko J, et al. Membrane protein production and formulation for drug discovery. Trends Pharmacol Sci. 2021;42(8):657‐674. doi: 10.1016/j.tips.2021.05.006 [DOI] [PubMed] [Google Scholar]
- 6. Pandey A, Shin K, Patterson RE, Liu X‐Q, Rainey JK. Current strategies for protein production and purification enabling membrane protein structural biology. Biochem Cell Biol. 2016;94(6):507‐527. doi: 10.1139/bcb-2015-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marinko JT, Huang H, Penn WD, Capra JA, Schlebach JP, Sanders CR. Folding and misfolding of human membrane proteins in health and disease: from single molecules to cellular proteostasis. Chem Rev. 2019;119(9):5537‐5606. doi: 10.1021/acs.chemrev.8b00532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Newport TD, Sansom MSP, Stansfeld PJ. The MemProtMD database: a resource for membrane‐embedded protein structures and their lipid interactions. Nucleic Acids Res. 2019;47(D1):D390‐D397. doi: 10.1093/nar/gky1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nastou KC, Tsaousis GN, Iconomidou VA. PerMemDB: a database for eukaryotic peripheral membrane proteins. Biochim Biophys Acta ‐ Biomembr. 2020;1862(2):183076. doi: 10.1016/j.bbamem.2019.183076 [DOI] [PubMed] [Google Scholar]
- 10. Shimizu K, Cao W, Saad G, Shoji M, Terada T. Comparative analysis of membrane protein structure databases. Biochim Biophys Acta ‐ Biomembr. 2018;1860(5):1077‐1091. doi: 10.1016/j.bbamem.2018.01.005 [DOI] [PubMed] [Google Scholar]
- 11. Wiseman DN, Otchere A, Patel JH, et al. Expression and purification of recombinant G protein‐coupled receptors: a review. Protein Expr Purif. 2020;167:105524. doi: 10.1016/j.pep.2019.105524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rawlings AE. Membrane proteins: always an insoluble problem? Biochem Soc Trans. 2016;44(3):790‐795. doi: 10.1042/BST20160025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen A, Majdinasab EJ, Fiori MC, Liang H, Altenberg GA. Polymer‐encased nanodiscs and polymer nanodiscs: new platforms for membrane protein research and applications. Front Bioeng Biotechnol. 2020;8:1329. doi: 10.3389/fbioe.2020.598450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thoma J, Burmann BM. Fake it ’Till You make it‐the pursuit of suitable membrane Mimetics for membrane protein biophysics. Int J Mol Sci. 2020;22(1):50. doi: 10.3390/ijms22010050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chorev DS, Robinson CV. The importance of the membrane for biophysical measurements. Nat Chem Biol. 2020;16(12):1285‐1292. doi: 10.1038/s41589-020-0574-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Girard‐Egrot AP, Maniti O. Why do tethered‐bilayer lipid membranes suit for functional membrane protein reincorporation? Appl Sci. 2021;11(11):4876‐4911. doi: 10.3390/app11114876 [DOI] [Google Scholar]
- 17. Shen H‐H, Lithgow T, Martin L. Reconstitution of membrane proteins into model membranes: seeking better ways to retain protein activities. Int J Mol Sci. 2013;14(1):1589‐1607. doi: 10.3390/ijms14011589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamat NP, Katz JS, Hammer DA. Engineering polymersome protocells. J Phys Chem Lett. 2011;2(13):1612‐1623. doi: 10.1021/jz200640x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Akbarzadeh A, Rezaei‐Sadabady R, Davaran S, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102. doi: 10.1186/1556-276X-8-102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rideau E, Dimova R, Schwille P, Wurm FR, Landfester K. Liposomes and polymersomes: a comparative review towards cell mimicking. Chem Soc Rev. 2018;47(23):8572‐8610. doi: 10.1039/C8CS00162F [DOI] [PubMed] [Google Scholar]
- 21. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2020;20:101‐124. doi: 10.1038/s41573-020-0090-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aibani N, Khan TN, Callan B. Liposome mimicking polymersomes; a comparative study of the merits of polymersomes in terms of formulation and stability. Int J Pharm X. 2020;2:100040. doi: 10.1016/j.ijpx.2019.100040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller AJ, Pearce AK, Foster JC, O'Reilly RK. Probing and tuning the permeability of polymersomes. ACS Cent Sci. 2021;7(1):30‐38. doi: 10.1021/acscentsci.0c01196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matoori S, Leroux J‐C. Twenty‐five years of polymersomes: lost in translation? Mater Horiz. 2020;7(5):1297‐1309. doi: 10.1039/C9MH01669D [DOI] [Google Scholar]
- 25. Nardin C, Thoeni S, Widmer J, Winterhalter M, Meier W. Nanoreactors based on (polymerized) ABA‐triblock copolymer vesicles. Chem Commun. 2000;15:1433‐1434. doi: 10.1039/B004280N [DOI] [Google Scholar]
- 26. Nardin C, Widmer J, Winterhalter M, Meier W. Amphiphilic block copolymer nanocontainers as bioreactors. Eur Phys J E. 2001;4(4):403‐410. doi: 10.1007/s101890170095 [DOI] [Google Scholar]
- 27. Nallani M, Benito S, Onaca O, et al. A nanocompartment system (synthosome) designed for biotechnological applications. J Biotechnol. 2006;123(1):50‐59. doi: 10.1016/j.jbiotec.2005.10.025 [DOI] [PubMed] [Google Scholar]
- 28. Graff A, Sauer M, Van Gelder P, Meier W. Virus‐assisted loading of polymer nanocontainer. Proc Natl Acad Sci. 2002;99(8):5064 LP‐5068. doi: 10.1073/pnas.062654499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stoenescu R, Graff A, Meier W. Asymmetric ABC‐triblock copolymer membranes induce a directed insertion of membrane proteins. Macromol Biosci. 2004;4(10):930‐935. doi: 10.1002/mabi.200400065 [DOI] [PubMed] [Google Scholar]
- 30. Rottet S, Iqbal S, Beales PA, et al. Characterisation of hybrid polymersome vesicles containing the efflux pumps NaAtm1 or P‐glycoprotein. Polymers (Basel). 2020;12(5):1049. doi: 10.3390/polym12051049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zemella A, Thoring L, Hoffmeister C, Kubick S. Cell‐free protein synthesis: pros and cons of prokaryotic and eukaryotic systems. Chembiochem. 2015;16(17):2420‐2431. doi: 10.1002/cbic.201500340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Skrzypek R, Iqbal S, Callaghan R. Methods of reconstitution to investigate membrane protein function. Methods. 2018;147:126‐141. doi: 10.1016/j.ymeth.2018.02.012 [DOI] [PubMed] [Google Scholar]
- 33. RBM S. Chapter 1 introduction to surface plasmon resonance. Handbook of Surface Plasmon Resonance. The Royal Society of Chemistry; 2017:1‐26. doi: 10.1039/9781788010283-00001 [DOI] [Google Scholar]
- 34. Phillips M, To J, Yamazaki T, Nagashima T, Torres J, Pervushin K. Binding of a small molecule water channel inhibitor to aquaporin Z examined by solid‐state MAS NMR. J Biomol NMR. 2018;71(2):91‐100. doi: 10.1007/s10858-018-0195-0 [DOI] [PubMed] [Google Scholar]
- 35. Discher DE, Ortiz V, Srinivas G, et al. Emerging applications of polymersomes in delivery: from molecular dynamics to shrinkage of tumors. Prog Polym Sci. 2007;32(8):838‐857. doi: 10.1016/j.progpolymsci.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Najer A, Wu D, Vasquez D, Palivan CG, Meier W. Polymer nanocompartments in broad‐spectrum medical applications. Nanomedicine (Lond). 2013;8(3):425‐447. doi: 10.2217/nnm.13.11 [DOI] [PubMed] [Google Scholar]
- 37. Discher DE, Ahmed F. Polymersomes. Annu Rev Biomed Eng. 2006;8(1):323‐341. doi: 10.1146/annurev.bioeng.8.061505.095838 [DOI] [PubMed] [Google Scholar]
- 38. Takahashi R, Miwa S, Rössel C, et al. Polymersome formation induced by encapsulation of water‐insoluble molecules within ABC triblock terpolymers. Polym Chem. 2020;11(20):3446‐3452. doi: 10.1039/D0PY00426J [DOI] [Google Scholar]
- 39. Wang X, Liu G, Hu J, Zhang G, Liu S. Concurrent block copolymer polymersome stabilization and bilayer permeabilization by stimuli‐regulated “traceless” crosslinking. Angew Chem Int Ed Engl. 2014;53(12):3138‐3142. doi: 10.1002/anie.201310589 [DOI] [PubMed] [Google Scholar]
- 40. Discher BM, Bermudez H, Hammer DA, Discher DE, Won Y‐Y, Bates FS. Cross‐linked Polymersome membranes: vesicles with broadly adjustable properties. J Phys Chem B. 2002;106(11):2848‐2854. doi: 10.1021/jp011958z [DOI] [Google Scholar]
- 41. Duncanson WJ, Lin T, Abate AR, Seiffert S, Shah RK, Weitz DA. Microfluidic synthesis of advanced microparticles for encapsulation and controlled release. Lab Chip. 2012;12(12):2135‐2145. doi: 10.1039/C2LC21164E [DOI] [PubMed] [Google Scholar]
- 42. Chakraborty K, Shinoda W, Loverde SM. Molecular simulation of the shape deformation of a polymersome. Soft Matter. 2020;16(13):3234‐3244. doi: 10.1039/C9SM02165E [DOI] [PubMed] [Google Scholar]
- 43. Kita‐Tokarczyk K, Grumelard J, Haefele T, Meier W. Block copolymer vesicles—using concepts from polymer chemistry to mimic biomembranes. Polymer (Guildf). 2005;46(11):3540‐3563. doi: 10.1016/j.polymer.2005.02.083 [DOI] [Google Scholar]
- 44. Le Meins J‐F, Sandre O, Lecommandoux S. Recent trends in the tuning of polymersomes' membrane properties. Eur Phys J E Soft Matter. 2011;34(2):14. doi: 10.1140/epje/i2011-11014-y [DOI] [PubMed] [Google Scholar]
- 45. Lee JC, Bermudez H, Discher BM, et al. Preparation, stability, and in vitro performance of vesicles made with diblock copolymers. Biotechnol Bioeng. 2001;73(2):135‐145. doi: 10.1002/bit.1045 [DOI] [PubMed] [Google Scholar]
- 46. Lee JS, Feijen J. Polymersomes for drug delivery: design, formation and characterization. J Control Release. 2012;161(2):473‐483. doi: 10.1016/j.jconrel.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 47. Bermudez H, Brannan AK, Hammer DA, Bates FS, Discher DE. Molecular weight dependence of polymersome membrane structure, elasticity, and stability. Macromolecules. 2002;35(21):8203‐8208. doi: 10.1021/ma020669l [DOI] [Google Scholar]
- 48. Leong J, Teo JY, Aakalu VK, Yang YY, Kong H. Engineering polymersomes for diagnostics and therapy. Adv Healthc Mater. 2018;7(8):e1701276. doi: 10.1002/adhm.201701276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ahmed F, Discher DE. Self‐porating polymersomes of PEG–PLA and PEG–PCL: hydrolysis‐triggered controlled release vesicles. J Control Release. 2004;96(1):37‐53. doi: 10.1016/j.jconrel.2003.12.021 [DOI] [PubMed] [Google Scholar]
- 50. Zhang X, Contini C, Constantinou AP, Doutch JJ, Georgiou TK. How does the hydrophobic content of methacrylate ABA triblock copolymers affect polymersome formation? J Polym Sci. 2021;59(15):1724‐1731. doi: 10.1002/pol.20210371 [DOI] [Google Scholar]
- 51. Jacobs ML, Boyd MA, Kamat NP. Diblock copolymers enhance folding of a mechanosensitive membrane protein during cell‐free expression. Proc Natl Acad Sci. 2019;116(10):4031‐4036. doi: 10.1073/pnas.1814775116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nallani M, Andreasson‐Ochsner M, Tan C‐WD, et al. Proteopolymersomes: in vitro production of a membrane protein in polymersome membranes. Biointerphases. 2011;6(4):153‐157. doi: 10.1116/1.3644384 [DOI] [PubMed] [Google Scholar]
- 53. May S, Andreasson‐Ochsner M, Fu Z, et al. In vitro expressed GPCR inserted in polymersome membranes for ligand‐binding studies. Angew Chem Int Ed Engl. 2013;52(2):749‐753. doi: 10.1002/anie.201204645 [DOI] [PubMed] [Google Scholar]
- 54. Kuiper SM, Nallani M, Vriezema DM, et al. Enzymes containing porous polymersomes as nano reaction vessels for cascade reactions. Org Biomol Chem. 2008;6(23):4315‐4318. doi: 10.1039/B811196K [DOI] [PubMed] [Google Scholar]
- 55. Lebleu C, Rodrigues L, Guigner J‐M, Brûlet A, Garanger E, Lecommandoux S. Self‐assembly of PEG‐b‐PTMC copolymers: micelles and polymersomes size control. Langmuir. 2019;35(41):13364‐13374. doi: 10.1021/acs.langmuir.9b02264 [DOI] [PubMed] [Google Scholar]
- 56. Mason AF, Thordarson P. Polymersomes with asymmetric membranes based on readily accessible Di‐ and triblock copolymers synthesized via SET‐LRP. ACS Macro Lett. 2016;5(10):1172‐1175. doi: 10.1021/acsmacrolett.6b00747 [DOI] [PubMed] [Google Scholar]
- 57. Nardin C, Hirt T, Leukel J, Meier W. Polymerized ABA triblock copolymer vesicles. Langmuir. 2000;16(3):1035‐1041. doi: 10.1021/la990951u [DOI] [Google Scholar]
- 58. Nardin C, Winterhalter M, Meier W. Giant free‐standing ABA triblock copolymer membranes. Langmuir. 2000;16(20):7708‐7712. doi: 10.1021/la000204t [DOI] [Google Scholar]
- 59. Itel F, Najer A, Palivan CG, Meier W. Dynamics of membrane proteins within synthetic polymer membranes with large hydrophobic mismatch. Nano Lett. 2015;15(6):3871‐3878. doi: 10.1021/acs.nanolett.5b00699 [DOI] [PubMed] [Google Scholar]
- 60. Choi H‐J, Lee H, Montemagno CD. Toward hybrid proteo‐polymeric vesicles generating a photoinduced proton gradient for biofuel cells. Nanotechnology. 2005;16(9):1589‐1597. doi: 10.1088/0957-4484/16/9/031 [DOI] [Google Scholar]
- 61. Choi H‐J, Montemagno CD. Artificial organelle: ATP synthesis from cellular mimetic polymersomes. Nano Lett. 2005;5(12):2538‐2542. doi: 10.1021/nl051896e [DOI] [PubMed] [Google Scholar]
- 62. Choi H‐J, Montemagno CD. Reconstruction of cellular processes in nanoscale artificial organelles solvent‐free incorporation of proteins into block copolymers. 2006 Sixth IEEE Conference on Nanotechnology. Vol 1. IEEE; 2006:150‐153. doi: 10.1109/NANO.2006.247593 [DOI] [Google Scholar]
- 63. Puskas JE, Chen Y, Dahman Y, Padavan D. Polyisobutylene‐based biomaterials. J Polym Sci Part A Polym Chem. 2004;42(13):3091‐3109. doi: 10.1002/pola.20114 [DOI] [Google Scholar]
- 64. Muhammad N, Dworeck T, Fioroni M, Schwaneberg U. Engineering of the E. coli outer membrane protein FhuA to overcome the hydrophobic mismatch in thick polymeric membranes. J Nanobiotechnol. 2011;9:8. doi: 10.1186/1477-3155-9-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Danafar H, Rostamizadeh K, Davaran S, Hamidi M. PLA‐PEG‐PLA copolymer‐based polymersomes as nanocarriers for delivery of hydrophilic and hydrophobic drugs: preparation and evaluation with atorvastatin and lisinopril. Drug Dev Ind Pharm. 2014;40(10):1411‐1420. doi: 10.3109/03639045.2013.828223 [DOI] [PubMed] [Google Scholar]
- 66. Zhao Y, Li X, Zhao X, et al. Asymmetrical polymer vesicles for drug delivery and other applications. Front Pharmacol. 2017;8:374. doi: 10.3389/fphar.2017.00374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hu Y, Qiu L. Polymersomes: preparation and characterization. Methods Mol Biol. 2019;2000:247‐265. doi: 10.1007/978-1-4939-9516-5_17 [DOI] [PubMed] [Google Scholar]
- 68. Wang C, Zhao T, Li Y, Huang G, White MA, Gao J. Investigation of endosome and lysosome biology by ultra pH‐sensitive nanoprobes. Adv Drug Deliv Rev. 2017;113:87‐96. doi: 10.1016/j.addr.2016.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Daubian D, Fillion A, Gaitzsch J, Meier W. One‐pot synthesis of an amphiphilic ABC triblock copolymer PEO‐b‐PEHOx‐b‐PEtOz and its self‐assembly into nanoscopic asymmetric polymersomes. Macromolecules. 2020;53(24):11040‐11050. doi: 10.1021/acs.macromol.0c02301 [DOI] [Google Scholar]
- 70. Gaitzsch J, Hirschi S, Freimann S, Fotiadis D, Meier W. Directed insertion of light‐activated proteorhodopsin into asymmetric polymersomes from an ABC block copolymer. Nano Lett. 2019;19(4):2503‐2508. doi: 10.1021/acs.nanolett.9b00161 [DOI] [PubMed] [Google Scholar]
- 71. Konishcheva EV, Zhumaev UE, Meier WP. PEO‐b‐PCL‐b‐PMOXA triblock copolymers: from synthesis to microscale polymersomes with asymmetric membrane. Macromolecules. 2017;50(4):1512‐1520. doi: 10.1021/acs.macromol.6b02743 [DOI] [Google Scholar]
- 72. Uneyama T. Density functional simulation of spontaneous formation of vesicle in block copolymer solutions. J Chem Phys. 2007;126(11):114902. doi: 10.1063/1.2463426 [DOI] [PubMed] [Google Scholar]
- 73. Liao J, Wang C, Wang Y, Luo F, Qian Z. Recent advances in formation, properties, and applications of polymersomes. Curr Pharm des. 2012;18(23):3432‐3441. doi: 10.2174/138161212801227050 [DOI] [PubMed] [Google Scholar]
- 74. Bartenstein JE, Robertson J, Battaglia G, Briscoe WH. Stability of polymersomes prepared by size exclusion chromatography and extrusion. Colloids Surf A Physicochem Eng Asp. 2016;506:739‐746. doi: 10.1016/j.colsurfa.2016.07.032 [DOI] [Google Scholar]
- 75. Girard P, Pécréaux J, Lenoir G, Falson P, Rigaud J‐L, Bassereau P. A new method for the reconstitution of membrane proteins into giant unilamellar vesicles. Biophys J. 2004;87(1):419‐429. doi: 10.1529/biophysj.104.040360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ruysschaert T, Sonnen AFP, Haefele T, Meier W, Winterhalter M, Fournier D. Hybrid nanocapsules: interactions of ABA block copolymers with liposomes. J Am Chem Soc. 2005;127(17):6242‐6247. doi: 10.1021/ja043600x [DOI] [PubMed] [Google Scholar]
- 77. Lim S, de Hoog H‐P, Parikh A, Nallani M, Liedberg B. Hybrid, nanoscale phospholipid/block copolymer vesicles. Polymers (Basel). 2013;5(3):1102‐1114. doi: 10.3390/polym5031102 [DOI] [Google Scholar]
- 78. Pereno V, Carugo D, Bau L, et al. Electroformation of giant unilamellar vesicles on stainless steel electrodes. ACS Omega. 2017;2(3):994‐1002. doi: 10.1021/acsomega.6b00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mao X, Li H, Kim J, et al. Polymersome formation by solvent annealing‐induced structural reengineering under 3D soft confinement. Nano Res. 2021;14(12):4644‐4649. doi: 10.1007/s12274-021-3396-x [DOI] [Google Scholar]
- 80. Martino C, Kim S‐H, Horsfall L, et al. Protein expression, aggregation, and triggered release from polymersomes as artificial cell‐like structures. Angew Chem Int Ed Engl. 2012;51(26):6416‐6420. doi: 10.1002/anie.201201443 [DOI] [PubMed] [Google Scholar]
- 81. Greene AC, Sasaki DY, Bachand GD. Forming giant‐sized polymersomes using gel‐assisted rehydration. J Vis Exp. 2016;111:54051. doi: 10.3791/54051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Marsden HR, Gabrielli L, Kros A. Rapid preparation of polymersomes by a water addition/solvent evaporation method. Polym Chem. 2010;1(9):1512‐1518. doi: 10.1039/C0PY00172D [DOI] [Google Scholar]
- 83. Habel J, Ogbonna A, Larsen N, et al. Selecting analytical tools for characterization of polymersomes in aqueous solution. RSC Adv. 2015;5(97):79924‐79946. doi: 10.1039/C5RA16403F [DOI] [Google Scholar]
- 84. Matter Y, Enea R, Casse O, Lee CC, Baryza J, Meier W. Amphiphilic PEG‐b‐PMCL‐b‐PDMAEMA triblock copolymers: from synthesis to Physico‐chemistry of self‐assembled structures. Macromol Chem Phys. 2011;212(9):937‐949. doi: 10.1002/macp.201000661 [DOI] [Google Scholar]
- 85. Kim KT, Meeuwissen SA, Nolte RJM, van Hest JCM. Smart nanocontainers and nanoreactors. Nanoscale. 2010;2(6):844‐858. doi: 10.1039/B9NR00409B [DOI] [PubMed] [Google Scholar]
- 86. Wang L, Tonggu L. Membrane protein reconstitution for functional and structural studies. Sci China Life Sci. 2015;58(1):66‐74. doi: 10.1007/s11427-014-4769-0 [DOI] [PubMed] [Google Scholar]
- 87. Rigaud J‐L. Membrane proteins: functional and structural studies using reconstituted proteoliposomes and 2‐D crystals. Braz J Med Biol Res. 2002;35(7):753‐766. doi: 10.1590/s0100-879x2002000700001 [DOI] [PubMed] [Google Scholar]
- 88. Simeonov P, Werner S, Haupt C, Tanabe M, Bacia K. Membrane protein reconstitution into liposomes guided by dual‐color fluorescence cross‐correlation spectroscopy. Biophys Chem. 2013;184:37‐43. doi: 10.1016/j.bpc.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 89. Khan S, Li M, Muench SP, Jeuken LJC, Beales PA. Durable proteo‐hybrid vesicles for the extended functional lifetime of membrane proteins in bionanotechnology. Chem Commun. 2016;52(73):11020‐11023. doi: 10.1039/C6CC04207D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Graff A, Fraysse‐Ailhas C, Palivan CG, et al. Amphiphilic copolymer membranes promote NADH:ubiquinone oxidoreductase activity: towards an electron‐transfer nanodevice. Macromol Chem Phys. 2010;211(2):229‐238. doi: 10.1002/macp.200900517 [DOI] [Google Scholar]
- 91. Kowal JŁ, Kowal JK, Wu D, Stahlberg H, Palivan CG, Meier WP. Functional surface engineering by nucleotide‐modulated potassium channel insertion into polymer membranes attached to solid supports. Biomaterials. 2014;35(26):7286‐7294. doi: 10.1016/j.biomaterials.2014.05.043 [DOI] [PubMed] [Google Scholar]
- 92. Zhang X, Fu W, Palivan CG, Meier W. Natural channel protein inserts and functions in a completely artificial, solid‐supported bilayer membrane. Sci Rep. 2013;3(1):2196. doi: 10.1038/srep02196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Yorulmaz Avsar S, Kyropoulou M, Di Leone S, Schoenenberger C‐A, Meier WP, Palivan CG. Biomolecules turn self‐assembling amphiphilic block co‐polymer platforms into biomimetic interfaces. Front Chem. 2019;6:645. doi: 10.3389/fchem.2018.00645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Rigaud J‐L, Pitard B, Levy D. Reconstitution of membrane proteins into liposomes: application to energy‐transducing membrane proteins. Biochim Biophys Acta. 1995;1231(3):223‐246. doi: 10.1016/0005-2728(95)00091-V [DOI] [PubMed] [Google Scholar]
- 95. Bjørkskov FB, Krabbe SL, Nurup CN, et al. Purification and functional comparison of nine human aquaporins produced in Saccharomyces cerevisiae for the purpose of biophysical characterization. Sci Rep. 2017;7(1):16899. doi: 10.1038/s41598-017-17095-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Grzelakowski M, Onaca O, Rigler P, Kumar M, Meier W. Immobilized protein–polymer Nanoreactors. Small. 2009;5(22):2545‐2548. doi: 10.1002/smll.200900603 [DOI] [PubMed] [Google Scholar]
- 97. Langowska K, Palivan CG, Meier W. Polymer nanoreactors shown to produce and release antibiotics locally. Chem Commun. 2013;49(2):128‐130. doi: 10.1039/C2CC36345C [DOI] [PubMed] [Google Scholar]
- 98. Ihle S, Onaca O, Rigler P, et al. Nanocompartments with a pH release system based on an engineered OmpF channel protein. Soft Matter. 2011;7(2):532‐539. doi: 10.1039/C0SM00679C [DOI] [Google Scholar]
- 99. Einfalt T, Witzigmann D, Edlinger C, et al. Biomimetic artificial organelles with in vitro and in vivo activity triggered by reduction in microenvironment. Nat Commun. 2018;9(1):1127. doi: 10.1038/s41467-018-03560-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Duong PHH, Chung T‐S, Jeyaseelan K, et al. Planar biomimetic aquaporin‐incorporated triblock copolymer membranes on porous alumina supports for nanofiltration. J Membr Sci. 2012;409‐410:34‐43. doi: 10.1016/j.memsci.2012.03.004 [DOI] [Google Scholar]
- 101. Kumar M, Grzelakowski M, Zilles J, Clark M, Meier W. Highly permeable polymeric membranes based on the incorporation of the functional water channel protein aquaporin Z. Proc Natl Acad Sci. 2007;104(52):20719‐20724. doi: 10.1073/pnas.0708762104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kumar M, Habel JEO, Shen Y, Meier WP, Walz T. High‐density reconstitution of functional water channels into vesicular and planar block copolymer membranes. J Am Chem Soc. 2012;134(45):18631‐18637. doi: 10.1021/ja304721r [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Mirzaei Garakani T, Liu Z, Glebe U, et al. In situ monitoring of membrane protein insertion into block copolymer vesicle membranes and their spreading via potential‐assisted approach. ACS Appl Mater Interfaces. 2019;11(32):29276‐29289. doi: 10.1021/acsami.9b09302 [DOI] [PubMed] [Google Scholar]
- 104. Lomora M, Garni M, Itel F, Tanner P, Spulber M, Palivan CG. Polymersomes with engineered ion selective permeability as stimuli‐responsive nanocompartments with preserved architecture. Biomaterials. 2015;53:406‐414. doi: 10.1016/j.biomaterials.2015.02.080 [DOI] [PubMed] [Google Scholar]
- 105. Lomora M, Itel F, Dinu IA, Palivan CG. Selective ion‐permeable membranes by insertion of biopores into polymersomes. Phys Chem Chem Phys. 2015;17(24):15538‐15546. doi: 10.1039/C4CP05879H [DOI] [PubMed] [Google Scholar]
- 106. Ranquin A, Versées W, Meier W, Steyaert J, Van Gelder P. Therapeutic nanoreactors: combining chemistry and biology in a novel triblock copolymer drug delivery system. Nano Lett. 2005;5(11):2220‐2224. doi: 10.1021/nl051523d [DOI] [PubMed] [Google Scholar]
- 107. Choi H‐J, Germain J, Montemagno CD. Effects of different reconstitution procedures on membrane protein activities in proteopolymersomes. Nanotechnology. 2006;17(8):1825‐1830. doi: 10.1088/0957-4484/17/8/003 [DOI] [Google Scholar]
- 108. Kuang L, Fernandes DA, O'Halloran M, et al. “Frozen” block copolymer nanomembranes with light‐driven proton pumping performance. ACS Nano. 2014;8(1):537‐545. doi: 10.1021/nn4059852 [DOI] [PubMed] [Google Scholar]
- 109. de Hoog H‐PM, Lin JieRong EM, Banerjee S, Décaillot FM, Nallani M. Conformational antibody binding to a native, cell‐free expressed GPCR in block copolymer membranes. PLoS One. 2014;9(10):e110847. doi: 10.1371/journal.pone.0110847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Hu Z. Construction of polymersome‐based artificial cell membrane; 2017. https://dr.ntu.edu.sg/bitstream/10356/69959/1/Thesis_HUZHAOLONG_Amended.pdf Published Online
- 111. Cheng J, Nallani M. Solid supported artificial cell membrane system; 2016. https://www.freepatentsonline.com/20160109434.pdf Published Online
- 112. Noor M, Dworeck T, Schenk A, Shinde P, Fioroni M, Schwaneberg U. Polymersome surface decoration by an EGFP fusion protein employing Cecropin A as peptide “anchor.”. J Biotechnol. 2012;157(1):31‐37. doi: 10.1016/j.jbiotec.2011.10.005 [DOI] [PubMed] [Google Scholar]
- 113. Klermund L, Poschenrieder ST, Castiglione K. Simple surface functionalization of polymersomes using non‐antibacterial peptide anchors. J Nanobiotechnol. 2016;14(1):48. doi: 10.1186/s12951-016-0205-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Rigaud J‐L, Lévy D. Reconstitution of membrane proteins into liposomes. Methods Enzymol. 2003;372:65‐86. doi: 10.1016/S0076-6879(03)72004-7 [DOI] [PubMed] [Google Scholar]
- 115. Katzen F, Peterson TC, Kudlicki W. Membrane protein expression: no cells required. Trends Biotechnol. 2009;27(8):455‐460. doi: 10.1016/j.tibtech.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 116. Garenne D, Haines MC, Romantseva EF, Freemont P, Strychalski EA, Noireaux V. Cell‐free gene expression. Nat Rev Methods Prim. 2021;1(1):49. doi: 10.1038/s43586-021-00046-x [DOI] [Google Scholar]
- 117. Khambhati K, Bhattacharjee G, Gohil N, Braddick D, Kulkarni V, Singh V. Exploring the potential of cell‐free protein synthesis for extending the abilities of biological systems. Front Bioeng Biotechnol. 2019;7:248. doi: 10.3389/fbioe.2019.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Worst EG, Exner MP, De Simone A, et al. Cell‐free expression with the toxic amino acid canavanine. Bioorg Med Chem Lett. 2015;25(17):3658‐3660. doi: 10.1016/j.bmcl.2015.06.045 [DOI] [PubMed] [Google Scholar]
- 119. Lee K‐H, Kim D‐M. Recent advances in development of cell‐free protein synthesis systems for fast and efficient production of recombinant proteins. FEMS Microbiol Lett. 2018;365(17):fny174. doi: 10.1093/femsle/fny174 [DOI] [PubMed] [Google Scholar]
- 120. Kuruma Y, Nishiyama K‐I, Shimizu Y, Müller M, Ueda T. Development of a minimal cell‐free translation system for the synthesis of presecretory and integral membrane proteins. Biotechnol Prog. 2005;21(4):1243‐1251. doi: 10.1021/bp049553u [DOI] [PubMed] [Google Scholar]
- 121. Kaneda M, Nomura SM, Ichinose S, et al. Direct formation of proteo‐liposomes by in vitro synthesis and cellular cytosolic delivery with connexin‐expressing liposomes. Biomaterials. 2009;30(23–24):3971‐3977. doi: 10.1016/j.biomaterials.2009.04.006 [DOI] [PubMed] [Google Scholar]
- 122. Focke PJ, Hein C, Hoffmann B, et al. Combining in vitro folding with cell free protein synthesis for membrane protein expression. Biochemistry. 2016;55(30):4212‐4219. doi: 10.1021/acs.biochem.6b00488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Schmitt C, Lippert AH, Bonakdar N, Sandoghdar V, Voll LM. Compartmentalization and transport in synthetic vesicles. Front Bioeng Biotechnol. 2016;4:19. doi: 10.3389/fbioe.2016.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Subramanyam P, Colecraft HM. Ion channel engineering: perspectives and strategies. J Mol Biol. 2015;427(1):190‐204. doi: 10.1016/j.jmb.2014.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Principles of membrane transport. Molecular Biology of the Cell. 4th ed. Garland Science; 2002. [Google Scholar]
- 126. Choi U, Lee C‐R. Distinct roles of outer membrane porins in antibiotic resistance and membrane integrity in Escherichia coli . Front Microbiol. 2019;10:953. doi: 10.3389/fmicb.2019.00953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Verkman AS. Aquaporins in clinical medicine. Annu Rev Med. 2012;63:303‐316. doi: 10.1146/annurev-med-043010-193843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Xie W, Wei Jun Low J, Armugam A, Jeyaseelan K, Tong YW. Regulation of aquaporin Z osmotic permeability in ABA tri‐block copolymer. AIMS Biophys. 2015;2(3):381‐397. [Google Scholar]
- 129. Habel J, Hansen M, Kynde S, et al. Aquaporin‐based biomimetic polymeric membranes: approaches and challenges. Membranes. 2015;5(3):307‐351. doi: 10.3390/membranes5030307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Discher BM, Won Y‐Y, Ege DS, et al. Polymersomes: tough vesicles made from Diblock copolymers. Science (80‐). 1999;284(5417):1143‐1146. doi: 10.1126/science.284.5417.1143 [DOI] [PubMed] [Google Scholar]
- 131. Xie W, He F, Wang B, et al. An aquaporin‐based vesicle‐embedded polymeric membrane for low energy water filtration. J Mater Chem A. 2013;1(26):7592‐7600. doi: 10.1039/C3TA10731K [DOI] [Google Scholar]
- 132. Onaca O, Sarkar P, Roccatano D, et al. Functionalized nanocompartments (synthosomes) with a reduction‐triggered release system. Angew Chem Int Ed Engl. 2008;47(37):7029‐7031. doi: 10.1002/anie.200801076 [DOI] [PubMed] [Google Scholar]
- 133. De Vocht C, Ranquin A, Willaert R, et al. Assessment of stability, toxicity and immunogenicity of new polymeric nanoreactors for use in enzyme replacement therapy of MNGIE. J Control Release. 2009;137(3):246‐254. doi: 10.1016/j.jconrel.2009.03.020 [DOI] [PubMed] [Google Scholar]
- 134. Kim AJ, Kaucher MS, Davis KP, et al. Proton transport from dendritic helical‐pore‐incorporated Polymersomes. Adv Funct Mater. 2009;19(18):2930‐2936. doi: 10.1002/adfm.200900076 [DOI] [Google Scholar]
- 135. Sanborn JR, Chen X, Yao Y‐C, et al. Carbon nanotube porins in amphiphilic block copolymers as fully synthetic mimics of biological membranes. Adv Mater. 2018;30(51):1803355. doi: 10.1002/adma.201803355 [DOI] [PubMed] [Google Scholar]
- 136. Schwarzer TS, Klermund L, Wang G, Castiglione K. Membrane functionalization of polymersomes: alleviating mass transport limitations by integrating multiple selective membrane transporters for the diffusion of chemically diverse molecules. Nanotechnology. 2018;29(44):44LT01. doi: 10.1088/1361-6528/aadb7e [DOI] [PubMed] [Google Scholar]
- 137. Leif H, Sled VD, Ohnishi T, Weiss H, Friedrich T. Isolation and characterization of the proton‐translocating NADH: ubiquinone oxidoreductase from Escherichia coli . Eur J Biochem. 1995;230(2):538‐548. doi: 10.1111/j.1432-1033.1995.0538h.x [DOI] [PubMed] [Google Scholar]
- 138. Choi HJ, Montemagno CD. Recent progress in advanced nanobiological materials for energy and environmental applications. Materials (Basel). 2013;6(12):5821‐5856. doi: 10.3390/ma6125821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Jia Y, Li J. Reconstitution of FoF1‐ATPase‐based biomimetic systems. Nat Rev Chem. 2019;3(6):361‐374. doi: 10.1038/s41570-019-0100-8 [DOI] [Google Scholar]
- 140. Seneviratne R, Khan S, Moscrop E, et al. A reconstitution method for integral membrane proteins in hybrid lipid‐polymer vesicles for enhanced functional durability. Methods. 2018;147:142‐149. doi: 10.1016/j.ymeth.2018.01.021 [DOI] [PubMed] [Google Scholar]
- 141. Marušič N, Otrin L, Zhao Z, et al. Constructing artificial respiratory chain in polymer compartments: insights into the interplay between Bo (3) oxidase and the membrane. Proc Natl Acad Sci U S A. 2020;117(26):15006‐15017. doi: 10.1073/pnas.1919306117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Xie G, Wen L, Jiang L. Biomimetic smart nanochannels for power harvesting. Nano Res. 2016;9(1):59‐71. doi: 10.1007/s12274-016-0993-1 [DOI] [Google Scholar]
- 143. Lee KY, Park S‐J, Lee KA, et al. Photosynthetic artificial organelles sustain and control ATP‐dependent reactions in a protocellular system. Nat Biotechnol. 2018;36(6):530‐535. doi: 10.1038/nbt.4140 [DOI] [PubMed] [Google Scholar]
- 144. Berhanu S, Ueda T, Kuruma Y. Artificial photosynthetic cell producing energy for protein synthesis. Nat Commun. 2019;10(1):1325. doi: 10.1038/s41467-019-09147-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Wang C, Yang J, Lu Y. Modularize and unite: toward creating a functional artificial cell. Front Mol Biosci. 2021;8:198976. doi: 10.3389/fmolb.2021.781986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Heldin C‐H, Lu B, Evans R, Gutkind JS. Signals and receptors. Cold Spring Harb Perspect Biol. 2016;8(4):a005900. doi: 10.1101/cshperspect.a005900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Serebryany E, Zhu GA, Yan ECY. Artificial membrane‐like environments for in vitro studies of purified G‐protein coupled receptors. Biochim Biophys Acta. 2012;1818(2):225‐233. doi: 10.1016/j.bbamem.2011.07.047 [DOI] [PubMed] [Google Scholar]
- 148. Rosenbaum DM, Rasmussen SGF, Kobilka BK. The structure and function of G‐protein‐coupled receptors. Nature. 2009;459(7245):356‐363. doi: 10.1038/nature08144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Cravchik A, Sibley DR, Gejman PV. Functional analysis of the human D2 dopamine receptor missense variants. J Biol Chem. 1996;271(42):26013‐26017. doi: 10.1074/jbc.271.42.26013 [DOI] [PubMed] [Google Scholar]
- 150. Baribaud F, Edwards TG, Sharron M, et al. Antigenically distinct conformations of CXCR4. J Virol. 2001;75(19):8957‐8967. doi: 10.1128/JVI.75.19.8957-8967.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Venugopal S, Anwer S, Szászi K. Claudin‐2: roles beyond permeability functions. Int J Mol Sci. 2019;20(22):5655. doi: 10.3390/ijms20225655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Yang W, Deng H, Zhu S, et al. Size‐transformable antigen‐presenting cell–mimicking nanovesicles potentiate effective cancer immunotherapy. Sci Adv. 2020;6(50):eabd1631. doi: 10.1126/sciadv.abd1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Wilson JT, Postma A, Keller S, et al. Enhancement of MHC‐I antigen presentation via architectural control of pH‐responsive, endosomolytic polymer nanoparticles. AAPS J. 2015;17(2):358‐369. doi: 10.1208/s12248-014-9697-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Lee SY, Choi JH, Xu Z. Microbial cell‐surface display. Trends Biotechnol. 2003;21(1):45‐52. doi: 10.1016/s0167-7799(02)00006-9 [DOI] [PubMed] [Google Scholar]
- 155. van Bloois E, Winter RT, Kolmar H, Fraaije MW. Decorating microbes: surface display of proteins on Escherichia coli . Trends Biotechnol. 2011;29(2):79‐86. doi: 10.1016/j.tibtech.2010.11.003 [DOI] [PubMed] [Google Scholar]
- 156. Ahn K, Shelton CC, Tian Y, et al. Activation and intrinsic gamma‐secretase activity of presenilin 1. Proc Natl Acad Sci U S A. 2010;107(50):21435‐21440. doi: 10.1073/pnas.1013246107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Roder F, Waichman S, Paterok D, et al. Reconstitution of membrane proteins into polymer‐supported membranes for probing diffusion and interactions by single molecule techniques. Anal Chem. 2011;83(17):6792‐6799. doi: 10.1021/ac201294v [DOI] [PubMed] [Google Scholar]
- 158. Shah S, Lee S‐F, Tabuchi K, et al. Nicastrin functions as a γ‐secretase‐substrate receptor. Cell. 2005;122(3):435‐447. doi: 10.1016/j.cell.2005.05.022 [DOI] [PubMed] [Google Scholar]
- 159. You M, Li E, Wimley WC, Hristova K. Forster resonance energy transfer in liposomes: measurements of transmembrane helix dimerization in the native bilayer environment. Anal Biochem. 2005;340(1):154‐164. doi: 10.1016/j.ab.2005.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Yu L, Wang W, Ling S, et al. CW‐EPR studies revealed different motional properties and oligomeric states of the integrin β1a transmembrane domain in detergent micelles or liposomes. Sci Rep. 2015;5(1):7848. doi: 10.1038/srep07848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Kiene K, Schenk SH, Porta F, et al. PDMS‐b‐PMOXA polymersomes for hepatocyte targeting and assessment of toxicity. Eur J Pharm Biopharm. 2017;119:322‐332. doi: 10.1016/j.ejpb.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 162. Pang Z, Lu W, Gao H, et al. Preparation and brain delivery property of biodegradable polymersomes conjugated with OX26. J Control Release. 2008;128(2):120‐127. doi: 10.1016/j.jconrel.2008.03.007 [DOI] [PubMed] [Google Scholar]
- 163. Broz P, Benito SM, Saw C, et al. Cell targeting by a generic receptor‐targeted polymer nanocontainer platform. J Control Release. 2005;102(2):475‐488. doi: 10.1016/j.jconrel.2004.10.014 [DOI] [PubMed] [Google Scholar]
- 164. Cross TA, Sharma M, Yi M, Zhou H‐X. Influence of solubilizing environments on membrane protein structures. Trends Biochem Sci. 2011;36(2):117‐125. doi: 10.1016/j.tibs.2010.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Peyret A, Zhao H, Lecommandoux S. Preparation and properties of asymmetric synthetic membranes based on lipid and polymer self‐assembly. Langmuir. 2018;34(11):3376‐3385. doi: 10.1021/acs.langmuir.7b04233 [DOI] [PubMed] [Google Scholar]
- 166. Pata V, Dan N. The effect of chain length on protein solubilization in polymer‐based vesicles (polymersomes). Biophys J. 2003;85(4):2111‐2118. doi: 10.1016/S0006-3495(03)74639-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Srinivas G, Discher DE, Klein ML. Key roles for chain flexibility in block copolymer membranes that contain pores or make tubes. Nano Lett. 2005;5(12):2343‐2349. doi: 10.1021/nl051515x [DOI] [PubMed] [Google Scholar]
- 168. Warren NJ, Armes SP. Polymerization‐induced self‐assembly of block copolymer nano‐objects via RAFT aqueous dispersion polymerization. J Am Chem Soc. 2014;136(29):10174‐10185. doi: 10.1021/ja502843f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Shinoda T, Shinya N, Ito K, et al. Cell‐free methods to produce structurally intact mammalian membrane proteins. Sci Rep. 2016;6(1):30442. doi: 10.1038/srep30442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Klammt C, Schwarz D, Löhr F, Schneider B, Dötsch V, Bernhard F. Cell‐free expression as an emerging technique for the large scale production of integral membrane protein. FEBS J. 2006;273(18):4141‐4153. doi: 10.1111/j.1742-4658.2006.05432.x [DOI] [PubMed] [Google Scholar]
- 171. Kuruma Y, Ueda T. The PURE system for the cell‐free synthesis of membrane proteins. Nat Protoc. 2015;10(9):1328‐1344. doi: 10.1038/nprot.2015.082 [DOI] [PubMed] [Google Scholar]
- 172. González‐Pérez A, Stibius KB, Vissing T, Nielsen CH, Mouritsen OG. Biomimetic triblock copolymer membrane arrays: A stable template for functional membrane proteins. Langmuir. 2009;25(18):10447‐10450. doi: 10.1021/la902417m [DOI] [PubMed] [Google Scholar]
- 173. Mobashery N, Nielsen C, Andersen OS. The conformational preference of gramicidin channels is a function of lipid bilayer thickness. FEBS Lett. 1997;412(1):15‐20. doi: 10.1016/s0014-5793(97)00709-6 [DOI] [PubMed] [Google Scholar]
- 174. Bloom M, Burnell EE, MacKay AL, Nichol CP, Valic MI, Weeks G. Fatty acyl chain order in lecithin model membranes determined from proton magnetic resonance. Biochemistry. 1978;17(26):5750‐5762. doi: 10.1021/bi00619a024 [DOI] [PubMed] [Google Scholar]
- 175. Lengyel JS, Milne JLS, Subramaniam S. Electron tomography in nanoparticle imaging and analysis. Nanomedicine (London). 2008;3(1):125‐131. doi: 10.2217/17435889.3.1.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Abdine A, Verhoeven MA, Park K‐H, et al. Structural study of the membrane protein MscL using cell‐free expression and solid‐state NMR. J Magn Reson. 2010;204(1):155‐159. doi: 10.1016/j.jmr.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 177. Cady SD, Schmidt‐Rohr K, Wang J, Soto CS, Degrado WF, Hong M. Structure of the amantadine binding site of influenza M2 proton channels in lipid bilayers. Nature. 2010;463(7281):689‐692. doi: 10.1038/nature08722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Vinogradova O, Sönnichsen F, Sanders CR. On choosing a detergent for solution NMR studies of membrane proteins. J Biomol NMR. 1998;11(4):381‐386. doi: 10.1023/a:1008289624496 [DOI] [PubMed] [Google Scholar]
- 179. McDermott A. Structure and dynamics of membrane proteins by magic angle spinning solid‐state NMR. Annu Rev Biophys. 2009;38:385‐403. doi: 10.1146/annurev.biophys.050708.133719 [DOI] [PubMed] [Google Scholar]
- 180. Mineev KS, Nadezhdin KD. Membrane mimetics for solution NMR studies of membrane proteins. Nanotechnol Rev. 2017;6(1):15‐32. doi: 10.1515/ntrev-2016-0074 [DOI] [Google Scholar]
- 181. de Hoog H‐PM, Nallani M, Tomczak N. Self‐assembled architectures with multiple aqueous compartments. Soft Matter. 2012;8(17):4552‐4561. doi: 10.1039/C2SM06934B [DOI] [Google Scholar]
- 182. Bleul R, Thiermann R, Maskos M. Techniques To control polymersome size. Macromolecules. 2015;48(20):7396‐7409. doi: 10.1021/acs.macromol.5b01500 [DOI] [Google Scholar]
- 183. Beales PA, Khan S, Muench SP, Jeuken LJC. Durable vesicles for reconstitution of membrane proteins in biotechnology. Biochem Soc Trans. 2017;45(1):15‐26. doi: 10.1042/BST20160019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Men Y, Peng F, Tu Y, van Hest JCM, Wilson DA. Methods for production of uniform small‐sized polymersome with rigid membrane. Polym Chem. 2016;7(24):3977‐3982. doi: 10.1039/C6PY00668J [DOI] [Google Scholar]
- 185. Arachea BT, Sun Z, Potente N, Malik R, Isailovic D, Viola RE. Detergent selection for enhanced extraction of membrane proteins. Protein Expr Purif. 2012;86(1):12‐20. doi: 10.1016/j.pep.2012.08.016 [DOI] [PubMed] [Google Scholar]
- 186. Drew D, Fröderberg L, Baars L, de Gier J‐WL. Assembly and overexpression of membrane proteins in Escherichia coli . Biochim Biophys Acta. 2003;1610(1):3‐10. doi: 10.1016/s0005-2736(02)00707-1 [DOI] [PubMed] [Google Scholar]
- 187. Górecki R, Antenucci F, Norinkevicius K, et al. Effect of detergents on morphology, size distribution, and concentration of copolymer‐based polymersomes. Langmuir. 2021;37(6):2079‐2090. doi: 10.1021/acs.langmuir.0c03044 [DOI] [PubMed] [Google Scholar]
- 188. Poschenrieder ST, Hanzlik M, Castiglione K. Polymersome formation mechanism and formation rate in stirred‐tank reactors. J Appl Polym Sci. 2018;135(14):46077. doi: 10.1002/app.46077 [DOI] [Google Scholar]
- 189. Sundaram PV. An analysis and interpretation of the Michaelis‐menten kinetics of immobilized enzymes perturbed by film diffusion. J Solid Phase Biochem. 1978;3(3):241‐246. doi: 10.1007/BF02991850 [DOI] [Google Scholar]
- 190. Maddox CB, Rasmussen L, White EL. Adapting cell‐based assays to the high throughput screening platform: problems encountered and lessons learned. JALA Charlottesv Va. 2008;13(3):168‐173. doi: 10.1016/j.jala.2008.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Zhang Z, Guan N, Li T, Mais DE, Wang M. Quality control of cell‐based high‐throughput drug screening. Acta Pharm Sin B. 2012;2(5):429‐438. doi: 10.1016/j.apsb.2012.03.006 [DOI] [Google Scholar]
- 192. Contreras‐Llano LE, Tan C. High‐throughput screening of biomolecules using cell‐free gene expression systems. Synth Biol. 2018;3(1):ysy012. doi: 10.1093/synbio/ysy012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Saeui CT, Mathew MP, Liu L, Urias E, Yarema KJ. Cell surface and membrane engineering: emerging technologies and applications. J Funct Biomater. 2015;6(2):454‐485. doi: 10.3390/jfb6020454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Gervais C, Dô F, Cantin A, et al. Development and validation of a high‐throughput screening assay for the hepatitis C virus p7 viroporin. J Biomol Screen. 2011;16(3):363‐369. doi: 10.1177/1087057110396215 [DOI] [PubMed] [Google Scholar]
- 195. Su Z, Brown EC, Wang W, MacKinnon R. Novel cell‐free high‐throughput screening method for pharmacological tools targeting K+ channels. Proc Natl Acad Sci U S A. 2016;113(20):5748‐5753. doi: 10.1073/pnas.1602815113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Przybyło M, Borowik T, Langner M. Application of liposome based sensors in high‐throughput screening systems. Comb Chem High Throughput Screen. 2007;10(6):441‐450. doi: 10.2174/138620707781996439 [DOI] [PubMed] [Google Scholar]
- 197. Bally M, Bailey K, Sugihara K, Grieshaber D, Vörös J, Städler B. Liposome and lipid bilayer arrays towards biosensing applications. Small. 2010;6(22):2481‐2497. doi: 10.1002/smll.201000644 [DOI] [PubMed] [Google Scholar]
- 198. Lo CH, Vunnam N, Lewis AK, et al. An innovative high‐throughput screening approach for discovery of small molecules that inhibit TNF receptors. SLAS Discov Adv Life Sci R&D. 2017;22(8):950‐961. doi: 10.1177/2472555217706478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Lo CH, Schaaf TM, Thomas DD, Sachs JN. Fluorescence‐based TNFR1 biosensor for monitoring receptor structural and conformational dynamics and discovery of small molecule modulators. Methods Mol Biol. 2021;2248:121‐137. doi: 10.1007/978-1-0716-1130-2_9 [DOI] [PubMed] [Google Scholar]
- 200. Lo CH, Schaaf TM, Grant BD, et al. Noncompetitive inhibitors of TNFR1 probe conformational activation states. Sci Signal. 2019;12(592):eaav5637. doi: 10.1126/scisignal.aav5637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Lo CH, Huber EC, Sachs JN. Conformational states of TNFR1 as a molecular switch for receptor function. Protein Sci. 2020;29(6):1401‐1415. doi: 10.1002/pro.3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Latorraca NR, Venkatakrishnan AJ, Dror RO. GPCR dynamics: structures in motion. Chem Rev. 2017;117(1):139‐155. doi: 10.1021/acs.chemrev.6b00177 [DOI] [PubMed] [Google Scholar]
- 203. Cooper MA. Advances in membrane receptor screening and analysis. J Mol Recognit. 2004;17(4):286‐315. doi: 10.1002/jmr.675 [DOI] [PubMed] [Google Scholar]
- 204. Patching SG. Surface plasmon resonance spectroscopy for characterisation of membrane protein‐ligand interactions and its potential for drug discovery. Biochim Biophys Acta. 2014;1838(1 Pt A):43‐55. doi: 10.1016/j.bbamem.2013.04.028 [DOI] [PubMed] [Google Scholar]
- 205. Ryu H, Fuwad A, Yoon S, et al. Biomimetic membranes with transmembrane proteins: state‐of‐the‐art in transmembrane protein applications. Int J Mol Sci. 2019;20(6):1437. doi: 10.3390/ijms20061437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Maynard JA, Lindquist NC, Sutherland JN, et al. Surface plasmon resonance for high‐throughput ligand screening of membrane‐bound proteins. Biotechnol J. 2009;4(11):1542‐1558. doi: 10.1002/biot.200900195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Christopeit T, Stenberg G, Gossas T, et al. A surface plasmon resonance‐based biosensor with full‐length BACE1 in a reconstituted membrane. Anal Biochem. 2011;414(1):14‐22. doi: 10.1016/j.ab.2011.02.041 [DOI] [PubMed] [Google Scholar]
- 208. Arslan Yildiz A, Kang C, Sinner E‐K. Biomimetic membrane platform containing hERG potassium channel and its application to drug screening. Analyst. 2013;138(7):2007‐2012. doi: 10.1039/C3AN36159D [DOI] [PubMed] [Google Scholar]
- 209. Poschenrieder ST, Schiebel SK, Castiglione K. Polymersomes for biotechnological applications: large‐scale production of nano‐scale vesicles. Eng Life Sci. 2017;17(1):58‐70. doi: 10.1002/elsc.201600100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Zhu Y, Guo X, Liu J, Li F, Yang D. Emerging advances of cell‐free systems toward artificial cells. Small Methods. 2020;4(10):2000406. doi: 10.1002/smtd.202000406 [DOI] [Google Scholar]
- 211. Damiati S. New opportunities for creating man‐made bioarchitectures utilizing microfluidics. Biomed Microdevices. 2019;21(3):62. doi: 10.1007/s10544-019-0415-8 [DOI] [PubMed] [Google Scholar]
- 212. Gumz H, Boye S, Iyisan B, et al. Toward functional synthetic cells: in‐depth study of nanoparticle and enzyme diffusion through a cross‐linked Polymersome membrane. Adv Sci. 2019;6(7):1801299. doi: 10.1002/advs.201801299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Luo R, Göpfrich K, Platzman I, Spatz JP. DNA‐based assembly of multi‐compartment polymersome networks. Adv Funct Mater. 2020;30(46):2003480. doi: 10.1002/adfm.202003480 [DOI] [Google Scholar]
- 214. Blackman LD, Varlas S, Arno MC, Fayter A, Gibson MI, O'Reilly RK. Permeable protein‐loaded polymersome cascade nanoreactors by polymerization‐induced self‐assembly. ACS Macro Lett. 2017;6(11):1263‐1267. doi: 10.1021/acsmacrolett.7b00725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Moreno S, Voit B, Gaitzsch J. The chemistry of cross‐linked polymeric vesicles and their functionalization towards biocatalytic nanoreactors. Colloid Polym Sci. 2020;299:309‐324. doi: 10.1007/s00396-020-04681-w [DOI] [Google Scholar]
- 216. Kim J, Kim KT. Polymersome‐based modular nanoreactors with size‐selective transmembrane permeability. ACS Appl Mater Interfaces. 2020;12(20):23502‐23513. doi: 10.1021/acsami.0c05637 [DOI] [PubMed] [Google Scholar]
- 217. Garanger E, Lecommandoux S. Towards bioactive nanovehicles based on protein polymers. Angew Chem Int Ed. 2012;51(13):3060‐3062. doi: 10.1002/anie.201107734 [DOI] [PubMed] [Google Scholar]
- 218. De Oliveira H, Thevenot J, Lecommandoux S. Smart polymersomes for therapy and diagnosis: fast progress toward multifunctional biomimetic nanomedicines. WIREs Nanomed Nanobiotechnol. 2012;4(5):525‐546. doi: 10.1002/wnan.1183 [DOI] [PubMed] [Google Scholar]
- 219. Zhang JH, Chung TD, Oldenburg KR. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4(2):67‐73. doi: 10.1177/108705719900400206 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


