Abstract
Background
The intensity of inflammation during COVID-19 is related to adverse outcomes. Proprotein convertase subtilisin/kexin type 9 (PCSK9) is involved in low-density lipoprotein receptor homeostasis, with potential influence on vascular inflammation and on COVID-19 inflammatory response.
Objectives
The goal of this study was to investigate the impact of PCSK9 inhibition vs placebo on clinical and laboratory outcomes in patients with severe COVID-19.
Methods
In this double-blind, placebo-controlled, multicenter pilot trial, 60 patients hospitalized for severe COVID-19, with ground-glass opacity pneumonia and arterial partial oxygen pressure to fraction of inspired oxygen ratio ≤300 mm Hg, were randomized 1:1 to receive a single 140-mg subcutaneous injection of evolocumab or placebo. The primary endpoint was death or need for intubation at 30 days. The main secondary endpoint was change in circulating interleukin (IL)-6 at 7 and 30 days from baseline.
Results
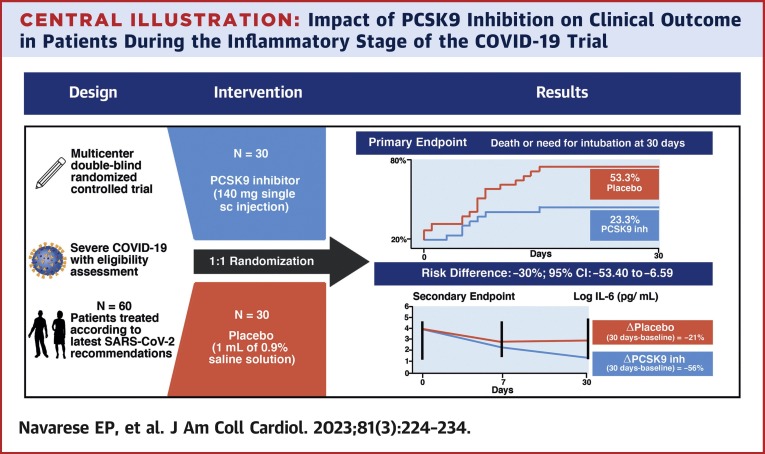
Patients randomized to receive the PCSK9 inhibitor had lower rates of death or need for intubation within 30 days vs placebo (23.3% vs 53.3%, risk difference: –30%; 95% CI: –53.40% to –6.59%). Serum IL-6 across time was lower with the PCSK9 inhibitor than with placebo (30-day decline: –56% vs –21%). Patients with baseline IL-6 above the median had lower mortality with PCSK9 inhibition vs placebo (risk difference: –37.50%; 95% CI: –68.20% to –6.70%).
Conclusions
PCSK9 inhibition compared with placebo reduced the primary endpoint of death or need for intubation and IL-6 levels in severe COVID-19. Patients with more intense inflammation at randomization had better survival with PCSK9 inhibition vs placebo, indicating that inflammatory intensity may drive therapeutic benefits. (Impact of PCSK9 Inhibition on Clinical Outcome in Patients During the Inflammatory Stage of the COVID-19 [IMPACT-SIRIO 5]; NCT04941105)
Key Words: COVID-19, death, interleukin-6, intubation, PCSK9 inhibition, randomized controlled trial
Abbreviations and Acronyms: ICU, intensive care unit; IL, interleukin; LDL-C, low-density lipoprotein cholesterol; LPS, lipopolysaccharide; PCSK9, proprotein convertase subtilisin/kexin type 9; RD, risk difference
Central Illustration
Severe COVID-19 can cause acute respiratory distress syndrome and systemic inflammation that may develop into a cytokine storm.1 The degree of immune dysregulation portends worse prognoses and increased mortality.2, 3, 4, 5, 6 Targeting crucial aspects of the inflammatory response during COVID-19 may represent an effective treatment strategy, particularly in critically ill patients.7 Interleukin (IL)-6 is a major driver of the inflammatory response in patients with COVID-19, and increased IL-6 levels predict a more severe course.8 , 9
Proprotein convertase subtilisin/kexin type 9 (PCSK9) is an enzyme involved in the homeostasis of low-density lipoprotein (LDL) receptors with potential influence on vascular inflammation.10 During acute inflammation, accumulation of oxidized LDL and apolipoprotein B promotes cholesterol crystal generation in macrophages that in turn activate the inflammasome complex and the release of inflammatory cytokines.8 PCSK9 can directly activate proinflammatory signaling pathways inducing cytokine production.11 In patients with septic shock, lower PCSK9 function implied blunted inflammatory responses and overall improved outcomes.12 Subjects with PCSK9 loss-of-function genotype have been characterized by lower PCSK9 levels and enhanced resolution of infections.13 PCSK9 inhibitors are powerful lipid-lowering agents that may curtail the inflammatory response in patients with COVID-19 through reduction of LDL-cholesterol (LDL-C) and/or inhibition of PCSK9. Experimental and clinical data suggest that PCSK9 inhibitors may exert anti-inflammatory effects related to interference with the IL-6–mediated inflammatory pathway triggered by PCSK9.10 , 12, 13, 14, 15, 16, 17, 18
We sought to determine the impact of PCSK9 inhibition vs placebo on clinical and laboratory outcomes in patients with severe COVID-19 characterized by pneumonia associated with respiratory failure and heightened inflammatory response.
Methods
Trial Design and Patient Population
We designed a pilot study aimed at testing for the first time the effect of PCSK9 inhibition vs placebo in COVID-19. IMPACT-SIRIO 5 (Impact of PCSK9 Inhibition on Clinical Outcome in Patients During the Inflammatory Stage of the COVID-19; NCT04941105) is a pilot, double-blind, placebo-controlled, multicenter, randomized, investigator-initiated clinical trial evaluating the impact of a PCSK9 inhibitor on clinical and laboratory outcomes in patients with severe COVID-19. Eligible patients were randomly assigned in a 1:1 ratio to receive either a PCSK9 inhibitor or placebo, and followed up for 30 days. The study was approved by an independent Ethics Committee of the Nicolaus Copernicus University of Poland. Written informed consent was obtained from all patients. The full study rationale is reported in the Supplemental Appendix.19
Briefly, patients hospitalized for COVID-19 in 4 clinical sites underwent screening for eligibility. Eligible patients were required to have SARS-CoV-2 infection confirmed by real-time reverse transcription polymerase chain reaction. Further inclusion criteria were age ≥18 years and COVID-19–associated pneumonia with typical radiologic changes, including ground-glass opacities and consolidations with bilateral and peripheral distribution.20 In addition, patients had to present with an arterial partial oxygen pressure to fraction of inspired oxygen ratio ≤300 mm Hg and serum levels of IL-6 above the upper reference limit. Key exclusion criteria were other known active infections (including systemic antibiotic therapy for presumed superimposed bacterial pneumonia without confirmed culture), clinical conditions contraindicating PCSK9 inhibitors, survival expectancy <48 hours, estimated glomerular filtration rate <30 mL/min/1.73 m2, absolute neutrophil count <2,000/mm3, platelet count <50,000/mm3, and pregnancy.
Full details regarding the inclusion and exclusion criteria are listed in the trial protocol (Supplemental Appendix).19 The participating sites were the Departments of Cardiology, Internal Medicine, and Infectious Diseases of the Nicolaus Copernicus University, Bydgoszcz, Poland, and the Department of Lung/Infectious Diseases, Oncology and Tuberculosis of the Nicolaus Copernicus University, Toruń, Poland.
Laboratory parameters were measured in fresh serum samples with the Atellica ci8200 analyzer (Siemens Healthineers) using commercially available tests. Interleukin levels were measured by using a fully automated single-step direct sandwich immunoassay with acridinium-ester chemiluminescent technology. Patients were treated according to the latest management recommendations for SARS-CoV-2 infection.21 Trial conduction followed the Declaration of Helsinki, the guideline for Good Clinical Practice by the International Council for Harmonisation Committee for Medicinal Products for Human Use (GCP CHMP/ICH/135/95), and local regulations.
Given the lack of previous experience with PCSK9 inhibitor therapy in COVID-19, a sample size of 60 patients was elected based on several ongoing trials on lipid-lowering therapies in COVID-1922 and on guidelines regarding sample sizes for pilot trials, which recommend 20 to 50 patients.23, 24, 25 The sample size was considered adequate to test the effect of PCSK9 inhibition vs placebo in a severe COVID-19 setting in which events such as death or need for intubation are frequently observed (range 18% to 67%).26
Endpoints
Clinical and laboratory outcomes
The primary clinical study endpoint was death or need for intubation within 30 days. The main secondary endpoint was the change in serum IL-6 concentrations from baseline to 7 and 30 days measured in survivors to those time points.
Other secondary endpoints
Other secondary endpoints included individual components of the primary endpoint and durations of oxygen therapy, hospital stay, intubation, noninvasive ventilation, or high-flow nasal cannula.
Randomized interventions
After patient enrollment, allocation to PCSK9 inhibitor or placebo was performed by connection to a prespecified Web link. The random allocation sequence was generated by computer software. Dedicated research nurses not involved as trial investigators administered study treatments (PCSK9 inhibitor or placebo) blindly according to randomization. Treatment injections with autoinjectors were masked to patients and investigators with the use of black plastic envelopes. The patient lists and code numbers were kept locked until the end of the trial. The subsequent trial phases, involving data collection, patient monitoring, and clinical procedures, were performed by clinicians or investigators unaware of assigned treatment. Thus, nurses, patients, physicians, and investigators were unaware of allocated treatment until after study conclusion.
Patients randomized to the PCSK9 inhibitor arm received a single 1 mL subcutaneous injection containing 140 mg of evolocumab. Patients randomized to the placebo arm received a single 1 mL subcutaneous injection of 0.9% saline solution.
Statistical Analyses
Primary efficacy analysis was on an intention-to-treat basis. Data distribution was checked by using the Kolmogorov-Smirnov test with data presented as median with IQR or mean ± SD as appropriate. Logarithmic transformation was applied to non-normally distributed IL-6 concentrations before analysis.
The primary endpoint was a composite of death or intubation within 30 days from randomization. Outcome differences in the 2 arms were analyzed according to risk difference (RD) expressed in percentage points with 95% CIs. Cumulative incidence curves were used to display differences between study groups. A prespecified analysis was performed stratifying the rates of death after PCSK9 inhibitor or placebo by baseline IL-6 concentrations below or above the median value. The correlation between baseline IL-6 and LDL-C was tested by using a Spearman coefficient. A waterfall plot was constructed displaying the greatest percent reductions across time of IL-6 levels in the individual patients compared with baseline. To determine whether the PCSK9 inhibitor effect on clinical outcomes (30-day death or need for intubation) was closely tied to the inflammatory marker (IL-6), we conducted a logistic regression model with treatment (PCSK9 inhibitor vs placebo) and with IL-6 as interaction term. Marginal probabilities of death or intubation were reported for each treatment group.
Analyses were conducted by using R Project version 4.0.3 (R Foundation for Statistical Computing) for statistical computing and IBM SPSS Statistics for Windows version 26.0 (IBM SPSS Statistics, IBM Corporation).
Results
Patient Enrollment and Characteristics
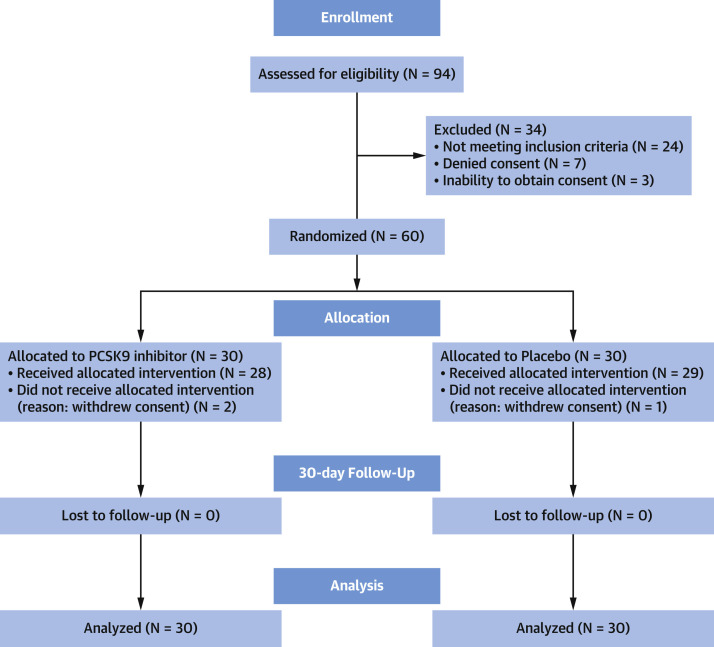
Enrollment was conducted from June 2021 to May 2022. During this period, the Pomeranian Voivodeship in northern Poland, where the trial was conducted, experienced low rates of severe COVID-19. The Consolidated Standards of Reporting Trials flowchart of patient disposition is presented in Figure 1 : 30 patients were assigned to receive the PCSK9 inhibitor (93% [n = 28] actually received the drug) and 30 to receive placebo (96% [n = 29] actually received placebo). All patients were admitted to an intensive care unit at the time of enrollment.
Figure 1.
Trial Flowchart
The processes of screening for eligibility, randomization to assigned treatment, and intention-to-treat analysis are displayed. PCSK9 = proprotein convertase subtilisin/kexin type 9.
Baseline characteristics (Table 1 ) were balanced between the PCSK9 inhibitor and placebo groups in terms of age (66.07 ± 12.09 years vs 66.23 ± 11.85 years, respectively), sex (37% vs 40% female), and body mass index (29.94 ± 5.40 kg/m2 and 29.80 ± 5.21 kg/m2). Underlying cardiovascular disease was present in 20% vs 27%, prior chronic obstructive lung disease in 10% vs 7%, and diabetes mellitus in 27% vs 30%. Mean days from symptom onset to randomization were 7.80 ± 2.55 vs 8.80 ± 4.17. Other demographic, clinical, and laboratory characteristics were likewise balanced between the 2 groups. No selective IL-6 inhibitors were administered in the 2 groups. During the trial, therapeutic measures against COVID-19 and its sequelae (including steroids, remdesivir, antibiotics, heparin, and aspirin) were administered in a balanced way to the 2 treatment groups.
Table 1.
Baseline Characteristics of the Enrolled Study Population
| PCSK9 Inhibitor Group (n = 30) | Placebo Group (n = 30) | |
|---|---|---|
| Age, y | 66.07 ± 12.09 | 66.23 ± 11.85 |
| Female | 11 (37) | 12 (40) |
| BMI, kg/m2 | 29.94 ± 5.40 | 29.80 ± 5.21 |
| Cardiovascular disease | 6 (20) | 8 (27) |
| Diabetes | 8 (27) | 9 (30) |
| COPD | 3 (10) | 2 (7) |
| Chronic kidney disease | 1 (3) | 2 (7) |
| LDL-C, mg/dL | 78.43 ± 26.10 | 77.23 ± 30.9 |
| Acetylsalicylic acid | 8 (27) | 8 (27) |
| P2Y12 inhibitor | 1 (3) | 0 (0) |
| Angiotensin-converting enzyme inhibitor | 10 (33) | 12 (40) |
| Beta-blockers | 15 (50) | 13 (43) |
| Statins | 11 (36.7) | 10 (33.3) |
| Days from illness onset to randomization | 7.80 ± 2.55 | 8.80 ± 4.17 |
| Vaccinated | 30 (100) | 30 (100) |
| Oxygenation index | 101.22 (83.52-157.82) | 107.75 (75.52-160.31) |
| Unfractionated heparin | 30 (100) | 28 (93) |
| Systolic blood pressure, mm Hg | 128.88 ± 16.90 | 123.55 ± 17.95 |
| Diastolic blood pressure, mm Hg | 71.07 ± 9.85 | 72.13 ± 13.73 |
| Remdesivir | 8 (27) | 10 (33) |
| Steroids | 25 (83) | 26 (87) |
| Antibiotics | 27 (90) | 28 (93.3) |
| Leukocytes (×103/μL) | 6.6 (5.29-9.41) | 7.85 (5.86-10.7) |
| Neutrophils (×103/μL) | 5.01 (4.21-7.73) | 6.85 (4.7-8.46) |
| Platelets (×103/μL) | 248.07 ± 104.23 | 258.93 ± 89.27 |
| Hemoglobin, g/dL | 12.59 ± 1.43 | 12.96 ± 1.94 |
| CRP, mg/dL | 129.21 (93.5-180.8) | 132.29 (59.38-182.0) |
| D-dimer, ng/mL | 1093.47 (643.01-1,675.25) | 1233 (910-2,429.25) |
| Fibrinogen, mg/dL | 541 (484-709) | 606 (508-761) |
Values are mean ± SD, n (%), or median (IQR).
BMI = body mass index; COPD = chronic obstructive pulmonary disease; CRP = C-reactive protein; LDL = low-density lipoprotein cholesterol; PCSK9 = proprotein convertase subtilisin/kexin type 9.
Clinical and Laboratory Endpoints
Primary clinical outcome
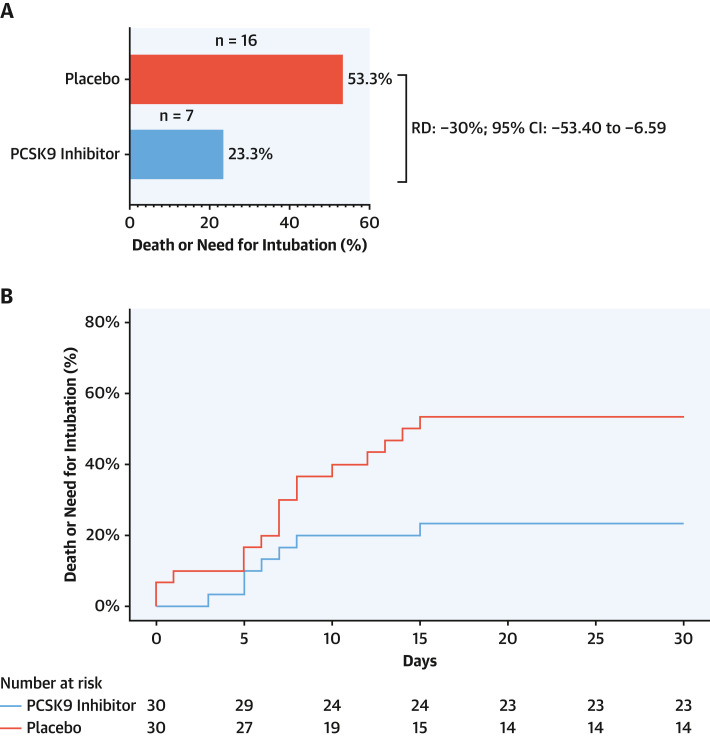
The rate of the primary clinical endpoint (30-day death or need for intubation) was lower in patients allocated to receive the PCSK9 inhibitor than in those allocated to receive placebo (23.3% vs 53.3%; RD: −30%; 95% CI: −53.40% to −6.59%) (Figure 2A ). The cumulative incidence curves of the effect of PCSK9 inhibitor vs placebo at 30 days are presented in Figure 2B.
Figure 2.
Death or Need for Intubation
Death or need for intubation (primary endpoint) at 30 days among patients administered a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor or placebo. (A) Rates of primary endpoint in the PCSK9 inhibitor and placebo arms. (B) Cumulative incidence curves of 30-day death or need for intubation in the intention-to-treat population. n = number of events; RD = risk difference.
The prespecified per-protocol analysis of the primary endpoint yielded results consistent with the intention-to-treat analysis: 22.3% vs 53.3%; RD: −31.11%; 95% CI: −54.87% to −7.34% (Supplemental Figure 1).
Secondary clinical endpoints
Secondary clinical outcomes are presented in Table 2 . The median duration of oxygen therapy was significantly shorter in the PCSK9 inhibitor group compared with the placebo group: 13 days (9-21 days) vs 20 days (11-23 days). Similarly, patients allocated to the PCSK9 inhibitor group had a shorter hospital stay: 16 days (13-23 days) vs 22 days (15-27 days). A numerically lower mortality was observed in the PCSK9 inhibitor vs placebo group: 16.7% vs 33.3%; RD: −16.6%; 95% CI: −38.17% to 4.83% (Supplemental Figure 2).
Table 2.
Secondary Clinical Endpoints
| PCSK9 Inhibitor (n = 30) | Placebo (n = 30) | |
|---|---|---|
| Death | 5 (16.7) | 10 (33.3) |
| Need for intubation | 6 (20) | 13 (43.3) |
| Duration of oxygen therapy, d | 13 (9-21) | 20 (11-23) |
| Duration of hospital stay, d | 16 (13-23) | 22 (15-27) |
| Intubation duration, d | 10 (3-24) | 19 (10-25) |
| Time with noninvasive mechanical ventilation or high-flow nasal cannula, d | 0 (0-1) | 0 (0-1) |
Values are n (%) or mean (range).
PCSK9 = proprotein convertase subtilisin/kexin type 9.
Serum IL-6 and LDL-C concentrations
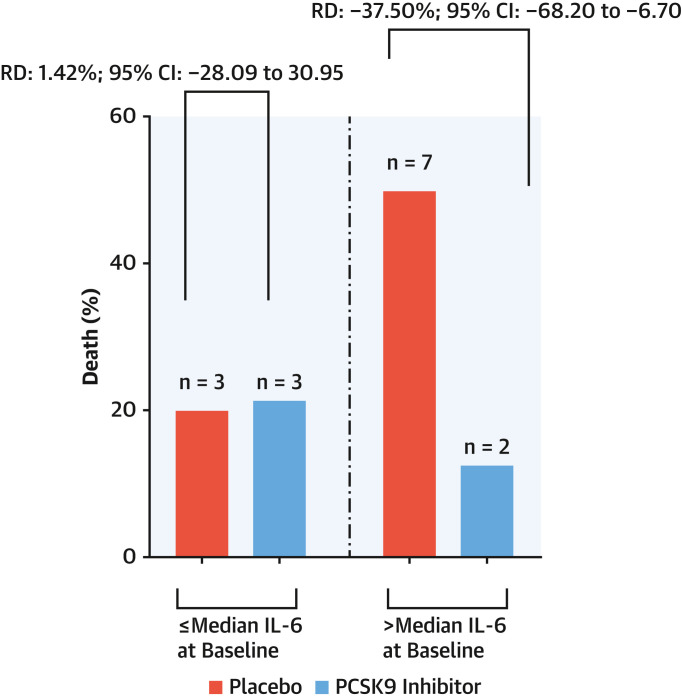
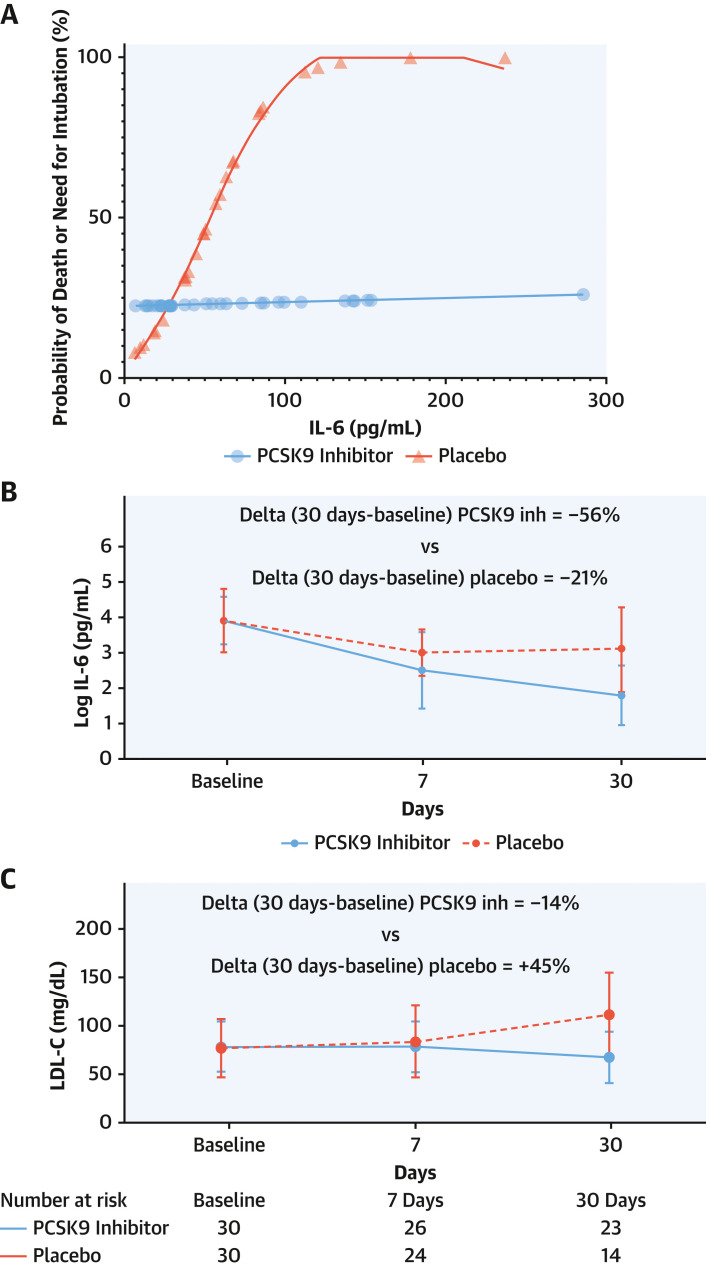
Median IL-6 concentration at baseline was 51.29 pg/mL (24.70-86.90 pg/mL). Patients presenting IL-6 concentrations above the baseline median attained a mortality reduction with the PCSK9 inhibitor vs placebo (12.5% vs 50%; RD: −37.50%; 95% CI: −68.20% to −6.70%) (Figure 3 ). Marginal probabilities of death or need for intubation in the 2 arms showed that, with progressively higher IL-6 levels at baseline, the probability of a clinical event increased in the placebo group but not in the PCSK9 inhibitor arm (Figure 4A ). PCSK9 inhibition reduced the levels of serum IL-6 more than placebo across the explored time points, such that the magnitude of IL-6 reduction at 30 days was more pronounced with the PCSK9 inhibitor (delta = −56%) than with placebo (delta = −21%) (Figure 4B).
Figure 3.
Death Rates by Baseline IL-6
Thirty-day rates of death in patients randomized to receive PCSK9 inhibitor or placebo, stratified according to median serum IL-6 levels ≤51.29 pg/mL and >51.29 pg/mL at baseline. IL = interleukin; other abbreviations as in Figure 2.
Figure 4.
Primary Endpoint Marginal Probabilities and Biomarker Changes According to Treatment Arm
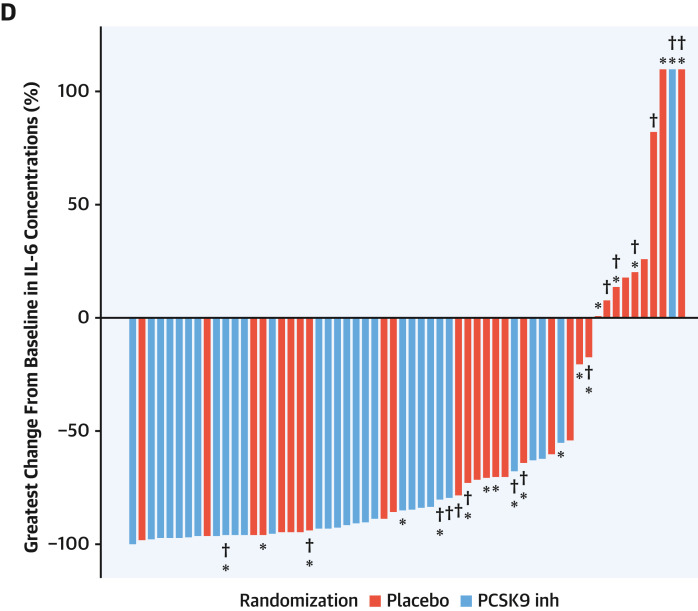
(A) Individual marginal probabilities of death or need for intubation in the PCSK9 inhibitor or placebo arm across levels of baseline serum IL-6. Serum IL-6 concentrations (B) and low-density lipoprotein cholesterol (LDL-C) levels (C) at baseline and at 7 and 30 days after randomization in the PCSK9 inhibitor and placebo arms. (D) Waterfall plot for greatest percent change in serum IL-6 levels with PCSK9 inhibitor or placebo up to 30 days compared with baseline. ∗Denotes need for intubation. †Denotes a fatal event. Inh = inhibitor; other abbreviations as in Figure 2.
Temporal analysis of LDL-C levels showed a progressive decrease in the PCSK9 inhibitor arm, whereas the opposite pattern emerged in the placebo arm (Figure 4C). The greatest reduction of IL-6 across 7 and 30 days vs baseline in each individual patient is displayed in Figure 4D. Sixty percent of patients allocated to the PCSK9 inhibitor group had a >90% decrease of IL-6 levels vs baseline, whereas this reduction was noted in 27% of patients allocated to placebo. Part of the decrease in IL-6 levels from baseline in both treatment groups was likely due to perturbation by infection, with recovery of unperturbed baseline levels over 1 month.
A moderate direct correlation was found between IL-6 and LDL-C concentrations at baseline (rho = 0.50) (Supplemental Figure 3). Other explored cytokines and inflammatory markers, including IL-10 and IL-18, also showed correlations with LDL-C at baseline (Supplemental Table 1).
Adverse Events
At day 30, no side effects were reported in relation to PCSK9 inhibitor or placebo administration. No documented thrombotic, serious adverse arrhythmic, or myocarditis event occurred in the investigated population.
Discussion
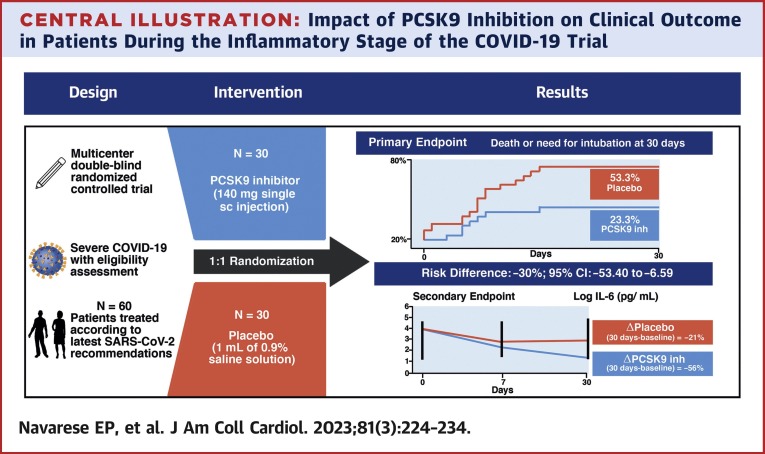
In this investigator-initiated, multicenter, double-blind, placebo-controlled, randomized pilot trial, the PCSK9 inhibitor was beneficial with respect to the primary endpoint of death or need for intubation at 30 days in hospitalized patients with severe COVID-19. The pattern of IL-6 changes was affected by PCSK9 inhibitor administration, as the magnitude of IL-6 reduction at 30 days was more pronounced with the PCSK9 inhibitor vs placebo (Central Illustration ). To our knowledge, this study is the first to address the role of PCSK9 monoclonal antibodies in severe COVID-19 through a prospective, randomized controlled trial.
Central Illustration.
Impact of PCSK9 Inhibition on Clinical Outcome in Patients During the Inflammatory Stage of the COVID-19 Trial
In this double-blind, placebo-controlled randomized pilot trial conducted in patients with severe COVID-19, a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor reduced the primary endpoint of death or need for intubation and serum interleukin (IL)-6 levels at 30 days compared with placebo. inh = inhibitor; N = number of patients; sc = subcutaneous.
The inflammatory response to SARS-CoV-2, with cytokine generation, is an immune dysregulation that, if extreme, can lead to multiorgan failure and death. Increased IL-6 levels have been shown to predict disease severity, risk of intubation, and mortality in patients with COVID-19.9 , 27 , 28 PCSK9 increases the expression of Toll-like receptor 4 through the activation of nuclear factor kappa-light-chain-enhancer of activated B-cell transcription factor, leading to the expression of proinflammatory cytokines such as IL-6.11 , 29 Blockade of PCSK9-mediated nuclear factor kappa-light-chain-enhancer of activated B-cell activation may thus interfere upstream in the sequence of inflammatory events in severe COVID-19,30 , 31 promoting broad inhibition of the inflammatory cascade. PCSK9 has been associated with increased inflammatory response, particularly in the lungs, and decreased survival in animal models with sepsis.16
In the present study, PCSK9 inhibitor administration produced relatively modest reductions in LDL-C concentrations at 30 days, whereas an increase in LDL-C levels was noted in the placebo arm. It is known that acute-phase inflammatory reactions, including those in COVID-19, depress LDL-C levels.32 Thus, it can be speculated that the rise in LDL-C in the placebo group at 30 days and the blunted decline in LDL-C in the evolocumab group reflect a time-dependent waning of the acute-phase effects on LDL-C, superimposed on treatment effects.
Although we found a moderate association between baseline levels of LDL-C and IL-6 in the present study, it is possible that the greatest benefit of PCSK9 inhibition in severe COVID-19 resides in mechanisms distinct from increased LDL receptor expression, including direct inhibition of PCSK9-triggered inflammation.11 Such interpretation would explain why the positive findings of the present study have not been consistently observed in trials involving other lipid-lowering agents such as statins.33
In addition to reduced mortality or need for intubation at 30 days with the PCSK9 inhibitor treatment compared with placebo, a prespecified analysis on 30-day mortality alone in the subset of patients with greatest degree of inflammation at randomization (ie, with baseline IL-6 values above the median of 51.29 pg/mL) showed survival benefit with the PCSK9 inhibitor. Although our study was not powered to address mortality alone, its reduction with PCSK9 inhibitor in patients presenting higher baseline IL-6 levels suggests that inflammatory intensity may drive therapeutic benefits. This hypothesis is supported by our analysis of probabilities of death or intubation across increasing levels of IL-6. There was a distinct pattern of greater probability of adverse events at 30 days in the placebo group vs. a relatively flat event rate in patients treated with the PCSK9 inhibitor with increasing IL-6 concentrations at baseline (Figure 4A).
The correlation between reduced inflammation, improved prognosis, and PCSK9 inhibition found in this study seems worthy of further investigation not only in COVID-19 but also other diseases (eg, viral, bacterial, rheumatic) characterized by heightened inflammation. Our study data suggest a role for personalization of PCSK9 therapy and/or stratification of outcomes by measurement of an inflammation marker. In addition to their role as lipid-lowering therapy, PCSK9 monoclonal antibodies might have a role as direct inhibitors of PCSK9, a powerful trigger of the inflammatory cascade. A potential clinical benefit of PCSK9 inhibitors might thus be hypothesized in inflammatory states, with benefits proportional to the intensity of inflammation.
Study Limitations
The present study was not designed to address a detailed profiling of the changes of biomarkers other than IL-6 across time. The prespecified analysis of mortality alone stratified according to baseline IL-6 concentrations should be viewed as exploratory. This pilot trial was designed to assess trial procedures and treatments. The study was not designed to statistically test any hypothesis of superior efficacy or safety of PCSK9 inhibition in patients with COVID-19. Therefore, no formal sample size calculation was provided, and no P values are presented in this paper. All findings should be interpreted as hypothesis generating. Further large-scale data are needed to confirm the findings, which, however, are consistent with COVID-19 cytokine storm reduction leading to improved survival.34
Conclusions
The findings in this double-blind, randomized, pilot trial conducted in patients with severe COVID-19 indicate that administration of a single 140-mg dose of PCSK9 inhibitor may reduce the incidence of the composite of death or need for intubation at 30 days, without raising safety concerns, compared with placebo. The decrease in IL-6 levels at 30 days was greater with the PCSK9 inhibitor than with placebo. Mortality was reduced with the PCSK9 inhibitor in patients with high baseline inflammation (as assessed by IL-6 levels), indicating that inflammatory intensity may drive therapeutic benefits. PCSK9 inhibition may represent a novel therapeutic pathway in addition to currently recommended therapeutic approaches for severe COVID-19.35 The relevant findings of this pilot trial justify a larger study in patients with COVID-19 and other inflammatory conditions, in view of the potential implications.
Perspectives.
COMPETENCY IN PATIENT CARE AND PROCEDURAL SKILLS: In patients with severe COVID-19 infection, PCSK9 inhibition reduced the composite outcome of 30-day mortality or intubation as well as serum IL-6 levels compared with placebo, and in those with the highest levels of inflammation, PCSK9 inhibition reduced mortality.
TRANSLATIONAL OUTLOOK: Future large-scale trials should confirm the clinical benefit of PCSK9 inhibition in patients with COVID-19 and clarify the pathophysiological mechanisms contributing to improved outcomes.
Funding Support and Author Disclosures
This research was supported by the Collegium Medicum of Nicolaus Copernicus University (grant ID ZES.WL.2.2021). Dr Navarese has received speaker and consultancy fees from Amgen, Sanofi-Regeneron, Bayer; and has received grants from Abbott, outside the submitted work. Dr Gurbel has received grants and personal fees from Bayer HealthCare LLC, Otitopic Inc, Amgen, Janssen, U.S. WorldMeds LLC, Instrumentation Laboratory, Haemonetics, Medicure Inc, Idorsia Pharmaceuticals, Hikari Dx, and Novartis; has received personal fees from UpToDate, outside the submitted work; has a patent issued (Detection of Restenosis Risk in Patients and Assessment of Cardiac Health and Thrombotic Risk in a Patient); and is an expert witness in litigation involving clopidogrel. Dr Gorog has received institutional research grants from Bayer and Bristol Myers Squibb; and has received speaker fees from AstraZeneca, Bayer, and Boehringer Ingelheim. Dr Andreotti has received lecture/consultancy fees from Amgen, Bayer, BMS/Pfizer, and Daiichi-Sankyo. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Listen to this manuscript's audio summary by Editor-in-Chief Dr Valentin Fuster onwww.jacc.org/journal/jacc.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For supplemental Methods, figures, and a table, please see the online version of this paper.
Appendix
References
- 1.Xie Y., Wang Z., Liao H., Marley G., Wu D., Tang W. Epidemiologic, clinical, and laboratory findings of the COVID-19 in the current pandemic: systematic review and meta-analysis. BMC Infect Dis. 2020;20:1–12. doi: 10.1186/s12879-020-05371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behrens E.M., Koretzky G.A. Cytokine storm syndrome: looking toward the precision medicine era. Arthritis Rheumatol. 2017;69:1135–1143. doi: 10.1002/art.40071. [DOI] [PubMed] [Google Scholar]
- 3.Corradini E., Ventura P., Ageno W., et al. Clinical factors associated with death in 3044 COVID-19 patients managed in internal medicine wards in Italy: results from the SIMI-COVID-19 study of the Italian Society of Internal Medicine (SIMI) Intern Emerg Med. 2021;16:1005–1015. doi: 10.1007/s11739-021-02742-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G., Greco M., Zanella A., et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180:1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zangrillo A., Beretta L., Scandroglio A.M., et al. Characteristics, treatment, outcomes and cause of death of invasively ventilated patients with COVID-19 ARDS in Milan, Italy. Crit Care Resusc. 2020;22:200–211. doi: 10.1016/S1441-2772(23)00387-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X., Yu Y., Xu J., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montazersaheb S., Hosseiniyan Khatibi S.M., Hejazi M.S., et al. COVID-19 infection: an overview on cytokine storm and related interventions. Virol J. 2022;19:92. doi: 10.1186/s12985-022-01814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabaka P., Koščálová A., Straka I., et al. Role of interleukin 6 as a predictive factor for a severe course of Covid-19: retrospective data analysis of patients from a long-term care facility during Covid-19 outbreak. BMC Infect Dis. 2021;21:308. doi: 10.1186/s12879-021-05945-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorog D.A., Storey R.F., Gurbel P.A., et al. Current and novel biomarkers of thrombotic risk in COVID-19: a consensus statement from the International COVID-19 Thrombosis Biomarkers Colloquium. Nat Rev Cardiol. 2022;19:475–495. doi: 10.1038/s41569-021-00665-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navarese E.P., Kołodziejczak M., Dimitroulis D., et al. From proprotein convertase subtilisin/kexin type 9 to its inhibition: state-of-the-art and clinical implications. Eur Heart J Cardiovasc Pharmacother. 2016;2:44–53. doi: 10.1093/ehjcvp/pvv045. [DOI] [PubMed] [Google Scholar]
- 11.Momtazi-Borojeni A.A., Sabouri-Rad S., Gotto A.M., et al. PCSK9 and inflammation: a review of experimental and clinical evidence. Eur Heart J Cardiovasc Pharmacother. 2019;5:237–245. doi: 10.1093/ehjcvp/pvz022. [DOI] [PubMed] [Google Scholar]
- 12.Walley K.R., Thain K.R., Russell J.A., et al. PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci Transl Med. 2014;6 doi: 10.1126/scitranslmed.3008782. 258ra143-258ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genga K.R., Lo C., Cirstea M.S., et al. Impact of PCSK9 loss-of-function genotype on 1-year mortality and recurrent infection in sepsis survivors. EBioMedicine. 2018;38:257–264. doi: 10.1016/j.ebiom.2018.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricci C., Ruscica M., Camera M., et al. PCSK9 induces a pro-inflammatory response in macrophages. Sci Rep. 2018;8:2267. doi: 10.1038/s41598-018-20425-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang C.L., Zeng Y.D., Hu Z.X., Liang H. PCSK9 promotes the secretion of pro-inflammatory cytokines by macrophages to aggravate H/R-induced cardiomyocyte injury via activating NF-κB signalling. Gen Physiol Biophys. 2020;39:123–134. doi: 10.4149/gpb-2019057. [DOI] [PubMed] [Google Scholar]
- 16.Dwivedi D.J., Grin P.M., Khan M., et al. Differential expression of PCSK9 modulates infection, inflammation, and coagulation in a murine model of sepsis. Shock. 2016;46:672–680. doi: 10.1097/SHK.0000000000000682. [DOI] [PubMed] [Google Scholar]
- 17.Schuster S., Rubil S., Endres M., et al. Anti-PCSK9 antibodies inhibit pro-atherogenic mechanisms in APOE∗3Leiden.CETP mice. Sci Rep. 2019;9 doi: 10.1038/s41598-019-47242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mach F., Baigent C., Catapano A.L., et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk—the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Eur Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 19.Kubica J., Podhajski P., Magielski P., et al. IMPACT of PCSK9 inhibition on clinical outcome in patients during the inflammatory stage of the SARS-COV-2 infection: rationale and protocol of the IMPACT-SIRIO 5 study. Cardiol J. 2022;29:140. doi: 10.5603/CJ.a2021.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Churruca M., Martínez-Besteiro E., Couñago F., Landete P. COVID-19 pneumonia: a review of typical radiological characteristics. World J Radiol. 2021;13:327–343. doi: 10.4329/wjr.v13.i10.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.COVID-19 Treatment Guidelines Panel Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/ [PubMed]
- 22.Talasaz A.H., Sadeghipour P., Aghakouchakzadeh M., et al. Investigating lipid-modulating agents for prevention or treatment of COVID-19: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;78:1635–1654. doi: 10.1016/j.jacc.2021.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sim J., Lewis M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J Clin Epidemiol. 2012;65:301–308. doi: 10.1016/j.jclinepi.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Browne R.H. On the use of a pilot sample for sample size determination. Stat Med. 1995;14:1933–1940. doi: 10.1002/sim.4780141709. [DOI] [PubMed] [Google Scholar]
- 25.Julious S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharmaceut Stat. 2005;4:287–291. [Google Scholar]
- 26.Vaschetto R., Barone-Adesi F., Racca F., et al. Outcomes of COVID-19 patients treated with continuous positive airway pressure outside the intensive care unit. ERJ Open Research. 2021;7 doi: 10.1183/23120541.00541-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aziz M., Fatima R., Assaly R. Elevated interleukin-6 and severe COVID-19: a meta-analysis. J Med Virol. 2020;92:2283–2285. doi: 10.1002/jmv.25948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laguna-Goya R., Utrero-Rico A., Talayero P., et al. IL-6-based mortality risk model for hospitalized patients with COVID-19. J Allergy Clin Immunol. 2020;146:799–807. doi: 10.1016/j.jaci.2020.07.009. e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen F., Castranova V., Shi X., Demers L.M. New insights into the role of nuclear factor-kappaB, a ubiquitous transcription factor in the initiation of diseases. Clin Chem. 1999;45:7–17. [PubMed] [Google Scholar]
- 30.Rosas I.O., Bräu N., Waters M., et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384:1503–1516. doi: 10.1056/NEJMoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang L, Li Y, Cheng Z, Lv Z, Luo S, Xia Y. PCSK9 promotes endothelial dysfunction during sepsis via the TLR4/MyD88/NF-κB and NLRP3 pathways. Inflammation. Published online August 5, 2022. https://doi.org/10.1007/s10753-022-01715-z [DOI] [PubMed]
- 32.Wei X., Zeng W., Su J., et al. Hypolipidemia is associated with the severity of COVID-19. J Clin Lipidol. 2020;14:297–304. doi: 10.1016/j.jacl.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghati N., Bhatnagar S., Mahendran M., et al. Statin and aspirin as adjuvant therapy in hospitalised patients with SARS-CoV-2 infection: a randomised clinical trial (RESIST trial) BMC Infect Dis. 2022;22:606. doi: 10.1186/s12879-022-07570-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cron R.Q., Caricchio R., Chatham W.W. Calming the cytokine storm in COVID-19. Nat Med. 2021;27:1674–1675. doi: 10.1038/s41591-021-01500-9. [DOI] [PubMed] [Google Scholar]
- 35.Horby P., Lim W.S., Emberson J.R., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.