Abstract
Background:
Longitudinal studies are needed to clarify whether early adversities are associated with advanced methylation age or if they actually accelerate methylation aging. This study test whether different dimensions of childhood adversity accelerate biological aging from childhood to adulthood, and, if so, via which mechanisms.
Methods:
381 participants provided one blood sample in childhood (average age 15.0; SD=2.3) and another in young adulthood (average age 23.1; SD=2.8). Participants and their parents provided a median of 6 childhood assessments (total=1950 childhood observations), reporting exposures to different types of adversity dimensions (i.e., threat, material deprivation, loss, unpredictability). The blood samples were assayed to estimate DNA methylation age in both childhood and adulthood and also change in methylation age across this period.
Results:
Cross-sectional associations between the childhood adversity dimensions and childhood measures of methylation age were non-significant. In contrast, multiple adversity dimensions were associated with accelerated within-person change in methylation age from adolescence to young adulthood. These associations attenuated in model testing all dimensions at the same time. Accelerated aging increased with increasing number of childhood adversities: Individuals with highest number of adversities experienced 2+ additional years of methylation aging compared to those with no exposure to childhood adversities. The association between total childhood adversity exposure and accelerated aging was partially explained by childhood depressive symptoms, but not anxiety or behavioral symptoms.
Conclusions:
Early adversities accelerate epigenetic aging long after they occur, in proportion to the total number of such experiences, and in a manner consistent with a shared effect that crosses multiple early dimensions of risk.
Keywords: Childhood, adversity, epigenetic, aging, DNA methylation, longitudinal
Introduction
Childhood adversities are common, distressing, and exact a lasting toll on physical and mental health (Copeland, Keeler, Angold, & Costello, 2007; Elizabeth J. Costello, Copeland, Cowell, & Keeler, 2007; Greeson et al., 2011; Widom, 1989). Such experiences have the potential to become physiologically embedded in ways that may persist over time (Copeland, Wolke, et al., 2014; A. Danese et al., 2010; Andrea Danese, Pariante, Caspi, Taylor, & Poulton, 2007; Hertzman & Wiens, 1996). When these changes contribute to a decline in physical integrity that typically occurs with advancing age, they are said to accelerate biological aging. A number of indices of biological aging have been studied. The literature on adversity-associated biological aging has focused on two indices: leukocyte telomere length and DNA methylation (DNAm) age, sometimes referred to as epigenetic age (Colich, Rosen, Williams, & McLaughlin, 2020). Epigenetic age is a person’s predicted age based upon age-related DNA methylation markers (Horvath, 2013). The difference between estimated DNAm age and chronological age may indicate advanced (or delayed) epigenetic age. Advanced DNAm age has been linked to risk factors like poor diet and smoking, diseases like cardiovascular disease and dementia, and even all-cause mortality (Horvath & Raj, 2018). Early adversity has been hypothesized as a risk factor that might advance DNAm aging.
There has been some support for the effects of adversity on advanced DNAm age. Most such studies have used adult methylation data coupled with retrospective measures or childhood adversity. In a meta-analysis of adult populations, retrospectively reported childhood trauma was associated with advanced epigenetic age (Wolf et al., 2018). In that same meta-analysis, lifetime trauma exposure was not associated with DNAm age (but could have been subject to similar recall biases). However, a key weakness of assessing childhood traumatic events retrospectively in adult population is the forgetting and/or recall bias that comes with recalling events decades past (Compton & Lopez, 2014; Moffitt et al., 2010). In contrast, Colich and colleagues (2020) found only two studies of DNAm age that assessed childhood adversity within childhood (Jovanovic et al., 2017; Sumner, Colich, Uddin, Armstrong, & McLaughlin, 2019). Both studies found that early adversity exposures – particularly those involving violence – had cross-sectional associations with advanced methylation age. Another recent study observed such an association in females only (Tang et al., 2020). As such, there is limited literature on studies linking adversity with DNAm age within childhood and studies that have follow up such children into adulthood.
A second significant weakness of studies of the association of childhood adversity with DNAm age is their reliance on single measures of DNAm age. This approach can identify advanced epigenetic age relative to one’s chronological age, but it is unable to reveal whether DNAm aging accelerated following adversity exposure. A number of studies still apply the term accelerated aging when describing advanced age. Importantly, multiple observations allow for tracking change in DNAm over time in response to these early experiences. The current study proposes to study the longitudinal associations of early adversity with within-person change in DNAm age from childhood to adulthood. If such an association is observed, we propose to test whether it is, in part, explained by childhood psychiatric symptoms (i.e., a common outcome of early adversity exposure and also a hypothesized correlate of accelerated aging) (Copeland et al., 2018; McLaughlin et al., 2013; Trotta, Murray, & Fisher, 2015).
A final challenge in understanding associations of childhood adversity with biological aging is adversity heterogeneity. Some studies focus on the effects of individual experiences or categories of experiences (e.g. physical abuse, maltreatment), other look at the effects of all such adverse experiences a child has had (e.g., a cumulative risk), and, more recently, a number of studies have recently focused on particular dimensions of adversity (e.g., threat, unpredictability). All approaches tend to create variables by combining (typically, summing) information about individual adversities or the severity of exposures. The current study will focus on dimensions of adversity proposed under the dimensional model of adversity and psychopathology (DMAP; (McLaughlin, Sheridan, & Lambert, 2014) as well as life history theory (Belsky, Schlomer, & Ellis, 2012). DMAP dimensions include exposure to threatening experiences where there is direct harm or the potential of harm and deprivation where there is a lack of expected inputs parental/child-rearing environment. In their meta-analysis of multiple biological aging indices, Colich and colleagues hypothesized that the childhood adversity dimension of threat would be more likely to be associated with biological aging than a dimension of deprivation (e.g., neglect, food insecurity) (Colich et al., 2020). Deprivation may involve material, cognitive and social aspects but the focus on this analysis will be limited to material deprivation or an inability of the parent to provide financial-based resources. The life history theory emphasizes the in/consistency of early environment over time called Unpredictability. In addition to these dimensions, we have created a dimension involving loss experiences that have been showed to be strongly associated with depressive symptoms (Asselmann, Wittchen, Lieb, Höfler, & Beesdo-Baum, 2015). Thus, this analysis will study the effects of adversity dimensions on biological aging. Finally, we propose to test the impact of a cumulative scale that sums across these adversity dimensions.
Methods
Participants
The Great Smoky Mountains Study is a longitudinal, representative study of children in 11 predominantly rural counties of North Carolina (see (Copeland, Angold, Shanahan, & Costello, 2014)). Three cohorts of children, ages 9, 11, and 13 years, were recruited from a pool of some 12,000 children using a two-stage sampling design, resulting in N = 1,420 participants (49% female). Annual assessments were completed until age 16 and then again at ages 19, 21, 25, and 30. Interviews were completed separately by a parent figure and the participant until age 16, and by the participant only thereafter. Finger-prick blood samples were collected at all assessments and applied to filter paper. Before all interviews, parent and child signed informed consent/assent forms. The study protocol and consent forms for each assessment were approved by the Duke University Medical Center Institutional Review Board and participants received payment for their time.
This study selected a subset of participants based upon availability of having at least one biosample in childhood (ages 9 to 16) and young adulthood (ages 19, 21, 25, or 30), having a range of adversity levels within childhood, and budgetary considerations. In total, 381 participants were included.
Measures
Up to age 16, both the child and parent completed annual structured clinical interviews using the Child and Adolescent Psychiatric Assessment (CAPA; (Angold & Costello, 2000). After age 17 the Young Adult Psychiatric Assessment (YAPA; (Angold et al., 1999) the upward extension of the CAPA, was completed with the participants only.
Childhood adversities were primarily assessed using the Life Events module of the CAPA. The module assesses 16 high magnitude events that meet the DSM PTSD criterion A as involving exposure to death, threatened death or sexual violence and natural disasters, and 14 low magnitude events such as parental divorce or loss of a best friend through a move that are associated with increased MH problems (Copeland et al., 2018). High magnitude events were assessed for lifetime occurrence and more common low magnitude events assessed for 3-month occurrence to maximize recall. Details about the construction and psychometric properties of these sections were described elsewhere (Costello, Messer, Reinherz, Cohen, & Bird, 1998). All childhood adversity dimensions were derived using information collected at all concurrent and prior childhood observation. The 381 participants completed a total of N=1950 childhood observations with a mean of 5.1 observations and a median of 6 observations.
Dimensions were derived assessing threat (e.g., physical abuse, violent death of loved one), material deprivation (e.g., impoverished, no health insurance), loss (e.g., parental divorce, loss of loved one), and unpredictability (e.g., change in parent structure, multiple moves). In addition, we have developed a scale of other adversities not related to these dimensions that are associated with poor short and long-term outcomes for children. Table 1 includes a list of each of the individual childhood adversity dimensions, the individual events that make up each scale, the percent of the sample with one or more such events, the mean number of events, and the range.
Table 1.
Descriptive information about childhood adversity dimensions
| Events included | % with any (N) | M(SD) | Range | |
|---|---|---|---|---|
| Threat | Violent death of loved one, war/terrorism, physical abuse, assault, sexual abuse, rape, domestic violence, chronically unsafe environment, witness to violence | 50.9% (194) | 1.1 (1.4) | 0–5 |
| Material Deprivation | Interviewer rating of basic needs not being met, family poverty, self-reported insufficient finances, no health insurance, limited health coverage, residential instability | 64.8% (247) | 1.5 (1.7) | 0–7 |
| Loss | Death of loved one, loss of best friend breakup with best friend, breakup with boy/girlfriend, divorce/separation | 45.1% (172) | 0.6 (0.8) | 0–4 |
| Unpredictability | Change of school w/o friends, change of parent structure, move 4+ times, reduced standard of living | 42.0% (160) | 0.6 (0.8) | 0–4 |
| Other adversity scale | Serious accident, exposure to noxious agent, diagnosis with serious illness, natural disaster, fire, parental arrest, parental substance use problems, parental mental health problem, parental maternal depression | 69.0% (263) | 1.3 (1.2) | 0–6 |
| Total | Sum of individual adversity variables | 93.7% (357) | 5.1 (3.9) | 0–19 |
N= sample size; M = means; SD = standard deviation.
Information about individual events was aggregated across all available childhood observations. A dimensional/count variable was derived indicating the number of distinct events reported in childhood. The correlations between the individual adversity variables are shown in Table S1 and S2. A cumulative adversity measure was computed by summing scores on the individual adversity dimensions (as well as the other adversity scale). Results are presented for the dimensional/count variables.
Mediators.
Potential mediators for an adversity-DNAm age association include childhood psychiatric symptoms (anxiety, depressive or behavioral symptoms). Participants and a parent were interviewed using the CAPA to assess psychiatric symptoms. The symptom was counted as present if reported by either the parent or child. A three-month “primary period” was selected to minimize forgetting and recall bias (Hardt & Rutter, 2004; Patten, 2003). This analysis used anxiety, depressive, and behavioral symptoms (including conduct, oppositional defiant, attention-deficit/hyperactivity symptoms). The measure of symptoms was taken from the same timepoint as the childhood biosample.
Methylation age.
The current analyses used the last available bloodspot in childhood (<17 years old) and the latest available adult bloodspot (ages 19, 21, 25, and 30). Methylation across the genome was assayed from dried bloodspots, using an optimized protocol for methyl-CG binding domain sequencing (MBD-seq) (Aberg, Chan, & van den Oord, 2020). MBD-seq achieves near complete coverage of all 28 million sites in the blood methylome, but at a fraction of the costs of whole genome bisulfite sequencing (Aberg et al., 2017; Chan et al., 2017). DNAm age was estimated using elastic nets to predict chronological age (in years) from the methylation with parameter alpha set to zero (Han et al., 2018). K-fold cross-validation with k=10 was used to estimate predictive power and to obtain unbiased estimates for each participant. Data from the two timepoints from the same unbiased DNAm age estimates of the k subsets, k − 1 were used as a “training set” to fit the elastic net and obtain regression coefficients. The regression coefficients were then used to estimate chronological age for participants in the “test set.” An annotated table of the top 1000 sites is provide in Table S3. DNAm age was adjusted for the lab technical covariates of the MBD-seq assay (e.g., sample batches, enrichment efficiency (Shabalin et al., 2018)) as well as cell count proportions (i.e., intrinsic DNAm age (Bell et al., 2019)). Despite the restricted age range, the correlation between chronological and DNAm age in this dataset was 0.90. The absolute mean difference was 0.02 (SD=.38). Two methylation age variables were used for analyses: childhood DNAm age and a difference score between the childhood and adulthood DNAm estimates. Additional details about the methylation assay and DNAm estimation is provided in appendix S1.
Analyses
All statistical analyses were completed using linear regression models. These models were implemented using maximum likelihood estimates within SAS PROC GENMOD, a procedure for generalized linear models. To test whether specific childhood adversities affect biological aging, we first tested a series of regression models for each individual adversity dimension and then a model testing all adversity dimensions simultaneously (except the cumulative adversity scale). The models predicting childhood methylation age were adjusted for childhood chronological age, sex, race/ethnicity. The models predicting change in methylation age from childhood to adulthood were adjusted for adult chronological age, the difference between the participant’s age at the time of the childhood and adult timepoint, sex, and race/ethnicity. Coefficients presented are standardized regression coefficients to enable comparison across dimensions.
Follow-up analyses tested whether observed associations were mediated by psychiatric symptoms (anxiety, depressive, or behavioral symptoms). Mediation was tested using the SAS PROCESS macro developed by Hayes to evaluate indirect effects using estimates of the confidence intervals derived from 5,000 bootstrap samples (Hayes, 2012). This approach has been shown to minimize concerns with non-normal sampling distribution and to improve power (Hayes & Scharkow, 2013).
Results
The sample included 381 participants who had methylation assays in both childhood and adulthood. Table 2 provides descriptive information on the demographic makeup of the sample as well as the mean DNAm age at the last childhood observation, the adult observation, and the change in DNAm age from childhood to adulthood. On average, the two observation were 8.2 years apart (SD=3.3).
Table 2.
Descriptive stats on longitudinal methylation sample
| Total | Childhood DNAm age | Adult DNAm age | Change in DNAm age | |
|---|---|---|---|---|
| % (N) | M (SD) | M (SD) | M (SD) | |
| Mean Age | -- | 15.0 (2.3) | 23.1 (2.8) | 8.2 (3.3) |
| Female | 51.4 (196) | 15.1 (2.3) | 23.2 (2.8) | 8.0 (3.2) |
| Male | 48.6 (185) | 14.8 (2.3) | 23.0 (2.8) | 8.3 (3.4) |
| White | 64.3 (245) | 14.9 (2.3) | 23.1 (2.8) | 8.2 (3.2) |
| Black | 4.7 (18) | 15.0 (1.9) | 23.9 (3.7) | 8.9 (4.4) |
| American Indian | 31.0 (118) | 15.1 (2.3) | 23.0 (2.7) | 7.9 (3.2) |
Overall N=381. Average chronological age at childhood obs = 13.9 (SD=1.6). Average chronological age at adult obs = 24.6 (SD=3.6).
Associations between childhood adversity and DNAm age
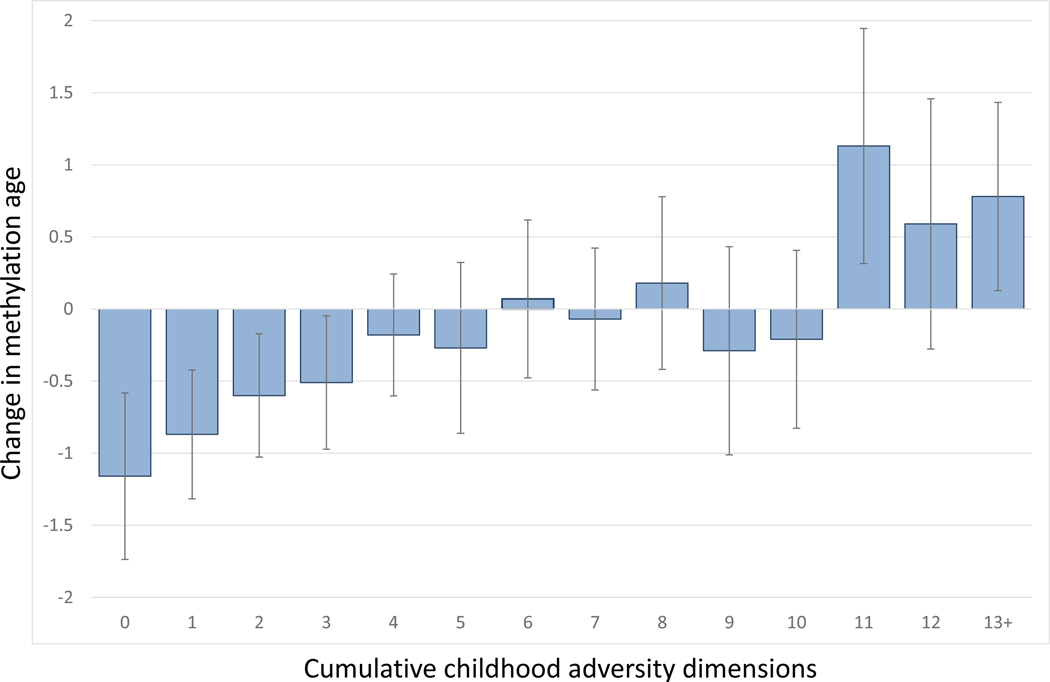
Associations between adversity dimensions and each of the methylation age variables are provided in Table 3 for the individual models and Table 4 for the simultaneous model. In both tables, the first 3 columns display associations (i.e., regression coefficient) with childhood methylation age and the last 3 columns show longitudinal associations with changes in methylation age from childhood to adulthood. None of the childhood adversity dimensions were associated with childhood methylation age in either individual or simultaneous models. In contrast, there was evidence of associations of adversity dimensions with the change in methylation age in univariate models – particularly for the unpredictability dimension- but these associations were attenuated in the model testing all dimensions simultaneously. A cumulative adversity index displayed a strong association with change in DNAm age from childhood to adulthood, B = 0.11, 95%CI = 0.04–0.18, p =0.002. This association met a stringent Bonferroni-type threshold (0.05/12=0.004). Figure 1 shows the association between a scale that sums the adversity dimensions (along with the other adversities) and change in DNAm age: with the endorsement of each additional domain of childhood adversity, DNAm increased such that there was a different of two years between the lowest and highest levels.
Table 3.
Univariate associations between childhood adversity dimensions and methylation aging measures
| Childhood methylation age | Change in methylation age | |||||
|---|---|---|---|---|---|---|
| β | 95%CI | p | β | 95%CI | p | |
| Dimensions | ||||||
| Threat | −0.02 | −0.13–0.07 | 0.58 | 0.08 | −0.01–0.16 | 0.09 |
| Material Deprivation | −0.09 | −0.19–0.01 | 0.06 | 0.07 | −0.01–0.16 | 0.09 |
| Loss | −0.06 | −0.16–0.04 | 0.23 | 0.09 | 0.00–0.18 | 0.04 |
| Unpredictability | −0.07 | −0.17–0.03 | 0.17 | 0.12 | 0.03–0.20 | 0.008 |
| Other adversities | −0.02 | −0.12–0.08 | 0.66 | 0.09 | 0.00–0.17 | 0.04 |
| Cumulative scale | −0.09 | −0.19–0.01 | 0.09 | 0.13 | 0.05–0.22 | 0.002 |
N=381. Tables represents results from 12 regression models. The childhood models were adjusted for childhood age, sex, race/ethnicity. The change in methylation age models were adjusted for adult age, time since the childhood observation, sex, and race/ethnicity. Regression coefficients are standardized.
Table 4.
Associations between all childhood adversity dimensions measures tested simultaneously and biological aging measures
| Childhood DNAm | Change in DNAm | |||||
|---|---|---|---|---|---|---|
| β | 95%CI | p | β | 95%CI | p | |
| Dimensions | ||||||
| Threat | 0.02 | −0.10–0.14 | 0.74 | 0.00 | −0.10–0.10 | 0.98 |
| Material Deprivation | −0.08 | −0.18–0.03 | 0.17 | 0.02 | −0.08–0.11 | 0.70 |
| Loss | −0.05 | −0.16–0.06 | 0.34 | 0.07 | −0.02–0.17 | 0.15 |
| Unpredictability | −0.04 | −0.15–0.07 | 0.52 | 0.09 | −0.01–0.18 | 0.08 |
| Other adversities | −0.01 | −0.11–0.11 | 0.99 | 0.06 | −0.04–0.15 | 0.23 |
Tables represents results from 2 regression models. Each model adjusted for age, sex, race/ethnicity. The change in DNAm models adjusted for time between observations. All childhood adversity are scales of total number of events reported at any point in childhood. Regression coefficients are standardized.
Figure 1.
Associations between cumulative childhood adversity index and changes in DNAm age from childhood to adulthood
Mediation of adversity-DNAm age associations
We tested whether the observed association between total adversity exposure and accelerated methylation aging was mediated by childhood psychiatric symptoms (i.e., depressive, anxiety, or behavioral; see Table 5). Total childhood adversity was strongly associated with all psychiatric symptom scores, but only depressive symptoms were significantly associated with change in DNAm age. Bootstrap confidence intervals for the indirect effect of total adversity on change in DNAm age via depressive symptoms did not include zero, suggesting possible mediation.
Table 5.
Tests of mediation of cumulative adversity scale on change in DNAm age from childhood to adulthood
| Association with cumulative adversity | Association with change in DNAm age | Bootstrapped Indirect effect | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Coeff. | 95% CI | P | Coeff. | 95% CI | P | Coeff. | 95% CI | P | |
| Psychiatric symptoms | |||||||||
| Depression sx. | 0.25 | 0.17–0.33 | <0.001 | 0.29 | 0.07–0.51 | 0.009 | 0.07 | 0.02–0.13 | 0.02 |
| Anxiety sx. | 0.28 | 0.14–0.43 | <0.001 | 0.05 | −0.08–0.18 | 0.46 | 0.01 | −0.02–0.05 | 0.48 |
| Behavioral sx. | 0.54 | 0.33–0.76 | <0.001 | −0.05 | −0.13–0.04 | 0.25 | −0.03 | −0.08–0.03 | 0.27 |
N=381. Med = mediator. Mediation was tested by a series of linear regression models.
Discussion
The aim of this study was to understand how different types of prospectively-assessed childhood adversity dimensions affect longitudinal, within-person changes in methylation age from childhood to adulthood. A number of findings were noteworthy. There was no evidence of association between the childhood adversity dimensions – either individual or cumulatively – on a single measure of methylation age at the end of childhood. In contrast, a number of adversity dimensions showed associations with the within-person, longitudinal measure of change in methylation age over time, but these associations were attenuated in a model testing all dimensions at the same time, suggesting a shared, nonspecific effect of early risk. More adversity exposures were associated with additional accelerated aging: Individuals with the highest level of childhood adversity exposure experienced 2+ additional years of methylation aging as compared to those with the lowest level of childhood adversities. A portion of this association was possibly accounted for by the effect of adversity exposure on depressive symptoms.
Our findings provide strong support for studying longitudinal, within-person changes in methylation age rather than merely comparing between-group differences in age at a single point in time. This is consistent with the notion of methylation age being an index of biological aging and with biological aging being a nonspecific process that is affected by a myriad of intraindividual experiences and exposures. The within-person change design has two primary advantages: 1) It allows individuals to serve as their own control for preexisting differences on confounding variables; and 2) It is more powerful as between person differences are essentially regressed out. Indeed, the only way to determine if an individual’s methylation aging has accelerated or decelerated is to have measured baseline methylation age. The question that this raises is whether one’s methylation age itself or the rate of change in methylation aging will best predict later health outcomes that have been associated with methylation age (Horvath & Raj, 2018). This question will only be answered by longitudinal studies that track methylation age at multiple assessments across the lifespan.
There was some evidence that depressive symptoms at the end of childhood mediated part of the association between total adversity exposure and the accelerated methylation aging. This is consistent with a model wherein symptoms provide an internal index of the strain of a psychosocial experience and it is that internal index (i.e., depressive symptoms) that, over time, may change the biological marker, possible via hyperactivation of stress response systems. Depressive symptoms themselves are known to have effects on different neural and stress response systems (Copeland, Shanahan, Worthman, Angold, & Costello, 2012; Lopez-Duran, Kovacs, & George, 2009; Palmer, Crewther, & Carey, 2015). In contrast, neither anxiety nor behavioral symptoms, which are both known to increase in response to adversity exposure, mediated the adversity-methylation age association. Though more and more examples of accelerated aging are identified, almost nothing is known about the processes by which such accelerated aging takes place, why the associations vary across individuals, and the long-term consequences of accelerated aging.
Conclusions about associations of specific adversity variables should not be drawn based on any one study or even a few. This is particularly the case with adversity variables like threat and material deprivation that are often operationalized differently in different studies (Colich et al., 2020). With those acknowledgements, this study provides little evidence to support specificity of adversity dimensions for methylation aging. This is not surprising, as the adversity dimensions themselves had substantial intercorrelations (rs=0.3–0.5). In models in which multiple adversity variables were tested simultaneously, all significant individual associations were attenuated. This pattern of findings differs from the literature on adversity effects on psychiatric symptoms or imaging outcomes, where specificity has been reported in models testing multiple adversity measures at the same time (Sheridan, Peverill, Finn, & McLaughlin, 2017). For methylation age, however, findings thus far suggest that each additional early adversity contributes in a nonspecific way (Colich et al., 2020; Tang et al., 2020).
Results from this study need to be considered in the context of the following limitations. Although our study sample was ethnically diverse, replication in external samples may be necessary to establish generalizability to other populations. Lifetime assessments of childhood adversities were completed annually through childhood and adolescence, but experiences prior to study enrollment may have been subject to recall bias, and some adverse experience events could have been forgotten. Best efforts were made to harmonize adversity dimensions with measures from prior studies but some differences are unavoidable given the differences in adversities assessed. Finally, there may be unmeasured variables that predict both, childhood adversity exposure and methylation age (e.g., shared genetic liability). Such confounding is minimized with a within-subject design as was used here.
Conclusion
Perhaps the primary public health finding of developmental psychopathology over the past 20 years is the myriad, devastating, long-term effects of early adversity. This study further adds to this literature by suggesting that such experiences age us, long after they occur, and in proportion to the total number of such experiences. Importantly, this work is consistent with the notion of early risk as exerting a shared, nonspecific effect upon accelerated aging. The next steps in the study of adversity-related accelerated aging should focus on who is affected (and why some individuals are not), how they are affected (which cognitive, emotional, and additional biological processes are involved), and what are the health consequences of adversity-related accelerated aging.
Supplementary Material
Table S1. Annotated table of top 1000 CpG sites used for methylation age variable.
Table S2. Correlations between childhood adversity count measures.
Table S3. Correlations between childhood adversity dichotomous measures.
Figure S1. DNAm age-age correlations as a function of the number of sites.
Key points:
Early adversities are associated with advanced methylation age but longitudinal studies are needed to test whether they accelerate methylation aging.
This study found that early adversities age us, long after they occur, in proportion to the total number of such experiences, and in a manner consistent with a shared effect that crosses multiple dimensions. Individuals with highest number of adversities experienced 2+ additional years of methylation aging compared to those with no exposure to childhood adversities.
Adversity-related accelerated aging provided a ready mechanism by which early experience may affect health and functioning across the lifespan.
Acknowledgements
The work presented here was supported by the National Institute of Mental Health (MH080230, MH63970, MH63671, MH48085, MH075766, MH094605, MH104576), the National Institute on Drug Abuse (DA016977, DA011301, DA036523, DA023026), the National Institute of Child health and Development (HD093651) Brain and Behavior Research Foundation (Early Career Award to WEC), and the William T Grant Foundation.
None of the funding organizations had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. W.E.C had full access to all the data in the study, performed all statistical analyses, and takes responsibility for the integrity of the data and the accuracy of the data analysis. None of the authors has biomedical financial interests or potential conflicts of interest.
Footnotes
Conflict of interest statement: No conflicts declared.
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article:
References
- Aberg KA, Chan RF, Shabalin AA, Zhao M, Turecki G, Staunstrup NH, . . . van den Oord EJ (2017). A MBD-seq protocol for large-scale methylome-wide studies with (very) low amounts of DNA. Epigenetics, 12(9), 743–750. doi: 10.1080/15592294.2017.1335849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aberg KA, Chan RF, & van den Oord E. (2020). MBD-seq - realities of a misunderstood method for high-quality methylome-wide association studies. Epigenetics, 15(4), 431–438. doi: 10.1080/15592294.2019.1695339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A, & Costello E. (2000). The Child and Adolescent Psychiatric Assessment (CAPA). Journal of the American Academy of Child and Adolescent Psychiatry, 39, 39–48. [DOI] [PubMed] [Google Scholar]
- Angold A, Cox A, Prendergast M, Rutter M, Simonoff E, Costello EJ, & Ascher BH (1999). The Young Adult Psychiatric Assessment (YAPA). Retrieved from Durham, NC: [Google Scholar]
- Appleyard K, Egeland B, Van Dulmen MHM, & Sroufe LA (2005). When more is not better: the role of cumulative risk in child behavior. Journal of Child Psychology and Psychiatry, 46, 235–245. [DOI] [PubMed] [Google Scholar]
- Asselmann E, Wittchen H-U, Lieb R, Höfler M, & Beesdo-Baum K. (2015). Danger and loss events and the incidence of anxiety and depressive disorders: a prospective-longitudinal community study of adolescents and young adults. Psychological Medicine, 45(1), 153–163. [DOI] [PubMed] [Google Scholar]
- Bell CG, Lowe R, Adams PD, Baccarelli AA, Beck S, Bell JT, . . . Horvath S. (2019). DNA methylation aging clocks: challenges and recommendations. Genome biology, 20(1), 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Schlomer GL, & Ellis BJ (2012). Beyond cumulative risk: distinguishing harshness and unpredictability as determinants of parenting and early life history strategy. Developmental Psychology, 48(3), 662. [DOI] [PubMed] [Google Scholar]
- Chan RF, Shabalin AA, Xie LY, Adkins DE, Zhao M, Turecki G, . . . Van den Oord EJCG (2017). Enrichment methods provide a feasible approach to comprehensive and adequately powered investigations of the brain methylome. Nucleic Acids Res, epub 25 February 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colich NL, Rosen ML, Williams ES, & McLaughlin KA (2020). Biological aging in childhood and adolescence following experiences of threat and deprivation: A systematic review and meta-analysis. Psychological Bulletin, 146(9), 721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton WM, & Lopez MF (2014). Accuracy in Reporting Past Psychiatric Symptoms: The Role of Cross-sectional Studies in Psychiatric Research. JAMA Psychiatry, 71(3), 233–234. [DOI] [PubMed] [Google Scholar]
- Copeland WE, Angold A, Shanahan L, & Costello EJ (2014). Longitudinal Patterns of Anxiety From Childhood to Adulthood: The Great Smoky Mountains Study. Journal of the American Academy of Child and Adolescent Psychiatry, 53(1), 21–33. Retrieved from http://linkinghub.elsevier.com/retrieve/pii/S0890856713006989?showall=true [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Keeler G, Angold A, & Costello EJ (2007). Traumatic Events and Posttraumatic Stress in Childhood. Archives of General Psychiatry, 64, 577–584. Retrieved from http://psych.duhs.duke.edu/library/pdf/20887.pdf [DOI] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Hinesley J, Chan RF, Aberg KA, Fairbank JA, . . . Costello EJ (2018). Association of Childhood Trauma Exposure With Adult Psychiatric Disorders and Functional Outcomes. JAMA Network Open, 1(7), e184493-e184493. doi: 10.1001/jamanetworkopen.2018.4493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Shanahan L, Worthman C, Angold A, & Costello EJ (2012). Cumulative depression episodes predict later C-reactive protein levels: a prospective analysis. Biol Psychiatry, 71(1). Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22047718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland WE, Wolke D, Lereya ST, Shanahan L, Worthman C, & Costello EJ (2014). Childhood bullying involvement predicts low-grade systemic inflammation into adulthood. Proceedings of the National Academy of Sciences. doi: 10.1073/pnas.1323641111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello EJ, Copeland WE, Cowell A, & Keeler G. (2007). Service Costs of Caring for Adolescents With Mental Illness in a Rural Community, 1993–2000. American Journal of Psychiatry, 164(1), 36. Retrieved from http://psych.duhs.duke.edu/library/pdf/21124.pdf [DOI] [PubMed] [Google Scholar]
- Costello EJ, Messer SC, Reinherz HZ, Cohen P, & Bird HR (1998). The prevalence of serious emotional disturbance: A re-analysis of community studies. Journal of Child and Family Studies, 7, 411–432. [Google Scholar]
- Danese A, Caspi A, Williams B, Ambler A, Sugden K, Mika J, . . . Moffitt TE (2010). Biological embedding of stress through inflammation processes in childhood. Molecular Psychiatry, 16(3), 244–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, & Poulton R. (2007). Childhood maltreatment predicts adult inflammation in a life-course study. Proceedings of the National Academy of Sciences, 104(4), 1319–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeson JK, Briggs EC, Kisiel CL, Layne CM, Ake GS 3rd, Ko SJ, . . . Fairbank JA (2011). Complex trauma and mental health in children and adolescents placed in foster care: findings from the National Child Traumatic Stress Network. Child Welfare, 90(6), 91–108. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=22533044 [PubMed] [Google Scholar]
- Han LK, Aghajani M, Clark SL, Chan RF, Hattab MW, Shabalin AA, . . . Jansen R. (2018). Epigenetic aging in major depressive disorder. American Journal of Psychiatry, 175(8), 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt J, & Rutter M. (2004). Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. Journal of Child Psychology and Psychiatry, 45(2), 260–273. doi:doi: 10.1111/j.1469-7610.2004.00218.x [DOI] [PubMed] [Google Scholar]
- Hayes AF (2012). PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling: University of Kansas, KS. [Google Scholar]
- Hayes AF, & Scharkow M. (2013). The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: Does method really matter? Psychological Science, 24(10), 1918–1927. [DOI] [PubMed] [Google Scholar]
- Hertzman C, & Wiens M. (1996). Child development and long-term outcomes: a population health perspective and summary of successful interventions. Social Science & Medicine, 43(7), 1083–1095. [DOI] [PubMed] [Google Scholar]
- Horvath S. (2013). DNA methylation age of human tissues and cell types. Genome biology, 14(10), 3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath S, & Raj K. (2018). DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nature Reviews Genetics, 19(6), 371–384. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Vance LA, Cross D, Knight AK, Kilaru V, Michopoulos V, . . . Smith AK (2017). Exposure to violence accelerates epigenetic aging in children. Scientific reports, 7(1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Duran NL, Kovacs M, & George CJ (2009). Hypothalamic–pituitary–adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology, 34(9), 1272–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Koenen KC, Hill ED, Petukhova M, Sampson NA, Zaslavsky AM, & Kessler RC (2013). Trauma Exposure and Posttraumatic Stress Disorder in a National Sample of Adolescents. Journal of the American Academy of Child & Adolescent Psychiatry, 52(8), 815–830.e814. doi: 10.1016/j.jaac.2013.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, & Lambert HK (2014). Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neuroscience & Biobehavioral Reviews, 47, 578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffitt T, Caspi A, Taylor A, Kokaua J, Milne B, Polanczyk G, & Poulton R. (2010). How common are common mental disorders? Evidence that lifetime prevalence rates are doubled by prospective versus retrospective ascertainment. Psychological Medicine, 40(6), 899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SM, Crewther SG, & Carey LM (2015). A meta-analysis of changes in brain activity in clinical depression. Frontiers in human neuroscience, 8, 1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten SB (2003). Recall bias and major depression lifetime prevalence. Social Psychiatry & Psychiatric Epidemiology, 38(6), 290–296. [DOI] [PubMed] [Google Scholar]
- Shabalin AA, Hattab MW, Clark SL, Chan RF, Kumar G, Aberg KA, . . . Birol I. (2018). RaMWAS: Fast Methylome-Wide Association Study Pipeline for Enrichment Platforms. Bioinformatics. doi: 10.1093/bioinformatics/bty069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Peverill M, Finn AS, & McLaughlin KA (2017). Dimensions of childhood adversity have distinct associations with neural systems underlying executive functioning. Development and Psychopathology, 29(5), 1777–1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner JA, Colich NL, Uddin M, Armstrong D, & McLaughlin KA (2019). Early experiences of threat, but not deprivation, are associated with accelerated biological aging in children and adolescents. Biological Psychiatry, 85(3), 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang R, Howe LD, Suderman M, Relton CL, Crawford AA, & Houtepen LC (2020). Adverse childhood experiences, DNA methylation age acceleration, and cortisol in UK children: a prospective population-based cohort study. Clinical epigenetics, 12, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotta A, Murray R, & Fisher H. (2015). The impact of childhood adversity on the persistence of psychotic symptoms: a systematic review and meta-analysis. Psychological Medicine, 45(12), 2481. [DOI] [PubMed] [Google Scholar]
- Widom CS (1989). Child abuse, neglect, and adult behavior: Research on design and findings on criminality, violence, and child abuse. American Journal of Orthopsychiatry, 59, 355–367. [DOI] [PubMed] [Google Scholar]
- Wolf EJ, Maniates H, Nugent N, Maihofer AX, Armstrong D, Ratanatharathorn A, . . . Lori A. (2018). Traumatic stress and accelerated DNA methylation age: a meta-analysis. Psychoneuroendocrinology, 92, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Annotated table of top 1000 CpG sites used for methylation age variable.
Table S2. Correlations between childhood adversity count measures.
Table S3. Correlations between childhood adversity dichotomous measures.
Figure S1. DNAm age-age correlations as a function of the number of sites.