Abstract
Background:
The World Health Organization recommends universal birth dose vaccination for hepatitis B virus (HBV), yet only 3 provinces and territories in Canada provide birth dose vaccination, and Canadian-born children in Ontario are acquiring HBV before adolescent vaccination. We sought to determine whether birth and/or infant HBV vaccination is cost-effective.
Methods:
We used a dynamic HBV model that incorporates population by year, disease stage, sex and the influence of immigration to quantify the disease and economic burden of chronic HBV infection in Ontario from 2020 to 2050. We compared 4 vaccination scenarios, which included a birth dose vaccine and variations of the 2 subsequent doses (either alone or as a part of the hexavalent vaccine) and a hexavalent-only strategy in infancy with the current adolescent vaccination strategy. Our costing estimates were based on values from 2020.
Results:
All 4 infant vaccination approaches prevented an additional 550–560 acute and 160 chronic pediatric HBV infections from 2020 to 2050 compared with adolescent vaccination. Whereas birth dose could be cost-effective, incorporating vaccination into a hexavalent vaccine was cost saving. By 2050, the hexavalent approach led to $428 000 in cost savings per disability-adjusted life years averted.
Interpretation:
At the current prevalence in Ontario, a switch to birth dose or infant dose will be cost-effective or even cost saving. Introducing any form of infant HBV immunization in Ontario will prevent acute and chronic pediatric HBV infections.
In Ontario, hepatitis B virus (HBV) ranks fourth on the list of infectious diseases with the greatest burden of illness by years of life lost,1 and can lead to cirrhosis and liver failure or hepatocellular carcinoma. Infants who acquire HBV have a more than 90% risk of progression to chronic infection.2 The World Health Organization (WHO) has prioritized birth dose vaccination for HBV as a key tenet of the strategy for HBV elimination. Vaccination within 24 hours of birth and 2 additional infant doses is more than 90% effective at preventing transmission and has decreased global prevalence from 5% to 1% in children under 5 years of age.2 However, even after 30 years and adoption among more than 100 countries (including the United States, United Kingdom and Australia),3 only 3 provinces and territories in Canada provide birth dose vaccination: 5 vaccinate in infancy and 5 in adolescence, including in Ontario.4 It is hypothesized that the rationale for adolescent vaccination is based on 4 assumptions: all pregnant women are screened, all infants born to mothers who are positive for HBV receive postexposure immunization, sexual contact is the only other risk factor, and immunity from birth and infant vaccination wanes.5
The National Advisory Committee on Immunization has proposed that each province must monitor for inadequate prenatal screening and preventable pediatric infections.5 At present, Ontario does not have a centralized database to show that all children born to a mother who is positive for HBV receive birth doses and 2 subsequent doses, in addition to hepatitis B immune globulin (HBIg). Our previous research showed that children born in Canada who live in Ontario are acquiring HBV before age 12 (6 cases per 1000); and these infections would likely be prevented with universal birth dose vaccination. This number reflects only diagnosed infections among children, but most children are never tested, making this an underestimate of pediatric infections acquired in Ontario.6 Children may have been infected through vertical transmission when prenatal screening was missed, or horizontal transmission from contacts who may not have been aware of their infection. Prevalence of HBV is particularly high among newcomers to Canada from HBV-endemic regions.7–9 Although children should be vaccinated at birth if a household contact or caregiver is known to carry HBV, a high proportion of those living with HBV have not been diagnosed because HBV is largely asymptomatic and not part of the routine Canadian immigration medical examination.10,11 Several long-term follow-up studies in Asia have reported that people who received birth dose HBV vaccination had similar infection rates as those who were vaccinated in adolescence.12–14
We used the PRoGReSs model,1 a dynamic HBV model that incorporates population by year, disease stage, sex and the influence of immigration. We compared vaccination timing based on direct and health outcomes to determine which approaches would be cost-effective compared with current adolescent vaccination from 2020 to 2050.
Methods
Study design and setting
To consider the cost and public health implications of a policy change in Ontario, the PRoGReSs model was used. The model has been shown to predict the impact of immunization on both reduced disease transmission and overall disease burden reliably,15 with results from this model previously validated using longitudinal age-specific prevalence of hepatitis B surface antigen (HBsAg) in 3 countries (US, Iran and China).16 This model has received feedback from experts in 94 countries, has aided in national elimination planning around the world and has not only been peer reviewed in multiple publications but also underwent annual reviews by other modelling groups while part of the Vaccine Impact Modelling Consortium.15–22 We based our model inputs on literature review, administrative data, institutional internal data and expert consensus (Appendix 1, Supplementary Table 1S, available at www.cmajopen.ca/content/11/1/E24/suppl/DC1). We entered Ontario population,23 mortality and historical data in the Ontario HBV disease burden and transmission model (PRoGReSs model), including prevalence of HBsAg by age and sex, HBeAg prevalence and rate of high viral load among women of child-bearing potential, HBIg and birth dose for infants born to mothers who were positive for HBV, the annual number of HBV-related liver transplants, and treatment and diagnosis rates (Appendix 1, Supplementary Methods).16 We used the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist for reporting economic evaluations of health interventions.24
The HBV PRoGReSs model is a dynamic Markov disease burden and transmission model that tracks the distribution of the prevalence of HBsAg by sex, age (1-yr age cohorts), year (1950–2050), disease stage (acute, chronic, cirrhosis, decompensated cirrhosis, hepatocellular carcinoma and death) and viral load (categorical) and accounts for background mortality, disease progression rates, spontaneous viral clearance and fulminant hepatitis16 (Appendix 1, Figure 1S, Figure 2S). Disease transmission was calculated both horizontally and vertically (by vertical transmission rates). We used data from Statistics Canada23 and the United Nation’s Department of Economic and Social Affairs, Population Division25 for annual estimates of background population and mortality by sex and 1-year age cohort from 1950 to 2050. We applied epidemiologic estimates for HBV (Table 1) to the background Ontario population.
Table 1:
Model parameters: disease and economic inputs for Ontario
| Parameter | Year | Value | Source | Low | High |
|---|---|---|---|---|---|
| Disease burden input | |||||
| HBsAg+ prevalence | 2017 | 0.80 (determined) | 0.29% | 1.6% | |
| HBsAg+ prevalence (male:female) | 2017 | 1.38 | Kwong et al. 20171 | – | – |
| HBeAg+ among HBsAg+ women of child-bearing age | 2012–2016 | 18.9 | WHO 20222 | 9.1% | 24.0% |
| Viral load ≥ 20 000 UI/mL among people who are HBeAg+ | 2002–2008 | 90 | WHO 20213 | – | – |
| Viral load ≥ 20 000 UI/mL among people who are HBeAg– | 2002–2008 | 13 | WHO 20213 | – | – |
| Total diagnosed | 2003–2013 | 39 623* | – | – | |
| Newly diagnosed | 2017 | 1878 | Kwong et al. 20171 | – | – |
| Total treated | 2018 | 6520† | Kwong et al. 2017,1 PHAC 20224 | – | – |
| Annual liver transplants | 2016 | 264‡ | – | – | |
| Liver transplants due to HBV | 2016 | 9.3‡ | – | – | |
| HBV vaccination timely birth and 3-dose coverage rates for infants born to HBsAg+ mothers | 2016 | 93.7* | NACI 20215 | – | – |
| HBIg coverage rate for infants born to HBsAg+ mothers who also received timely birth dose | 2016 | 93.7* | – | – | |
| Economic input | |||||
| Disability weight | |||||
| Decompensated cirrhosis | 2020 | 0.178 | Biondi et al. 20206 | – | – |
| HCC | 2020 | 0.466 | Biondi et al. 20206 | – | – |
| Liver transplant | 2020 | 0.024 | Biondi et al. 20206 | – | – |
| Value of a statistical life-year, 2020 Can$ | |||||
| GDP per capita | 2020 | 62 138 | Coffin et al. 20197 | – | – |
| Screening and laboratory cost per test, 2020 Can$ | |||||
| HBsAg | 2020 | 10.25§ | |||
| HBeAg | 2020 | 10.25§ | |||
| Viral load testing | 2020 | 100.00* | |||
| ALT testing | 2020 | 10.00§ | |||
| CBC/creatinine/bilirubin | 2020 | 29.00§ | |||
| Abdominal ultrasonography | 2020 | 135.9 | Statistics Canada 20178 | 68.39 | 271.8 |
| HBV treatment and prophylaxis cost, 2020 Can$ | |||||
| Treatment (annual) | 2020 | 5770 | Wong et al. 20139 | 3509 | 8031 |
| HBV vaccination (children) | 2020 | 11.16* | 10.92 | 11.40 | |
| HBV vaccination (adult) | 2020 | 7.44* | 7.28 | 7.60 | |
| HBIg | 2020 | 287 | Lapointe-Shaw 202110 | 230 | 344 |
| Annual health state cost, 2020 Can$ | |||||
| Chronic HBV | 2020 | 1150 | Health Management Branch 200911 | 1048 | 1341 |
| Compensated cirrhosis | 2020 | 2517 | Health Management Branch 200911 | 2013 | 3760 |
| Decompensated cirrhosis | 2020 | 15 113 | Health Management Branch 200911 | 11 184 | 22 059 |
| HCC | 2020 | 17 970 | Health Management Branch 200911 | 14 279 | 23 134 |
| Liver transplantation | 2020 | 133 346 | Health Management Branch 200911 | 126 969 | 143 801 |
| After liver transplantation | 2020 | 51 475 | Health Management Branch 200911 | 45 015 | 62 035 |
Note: ALT = alanine transaminase, CBC = complete blood cell count, GDP = gross domestic product, HBeAg = hepatitis B e antigen, HBIg = hepatitis B immune globulin, HBsAg = hepatitis B surface antigen, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, NACI = National Advisory Committee on Immunization, WHO = World Health Organization.
Expert consensus achieved through Delphi process (Appendix 1, Supplementary Table 1S, available at www.cmajopen.ca/content/11/1/E24/suppl/DC1).
Treatment rate for British Columbia based on internal review from the BC Centre for Disease Control applied to data from Ontario, with adjustment for population and prevalence rate ratio.
Unpublished data: internal analysis conducted at London Health Sciences Centre and Toronto General Hospital within hepatology divisions from 2001 to 2017.
Provincial reimbursement.
Cost adjusted based on 67% of the infected population receiving abdominal ultrasonography based on age guidelines (≥ 40 yr for men and ≥ 50 yr for women) and model-estimated proportion of patients in this age range.
We assumed a male-to-female ratio of 1.38,6,8,26 considered the impact of immigration and adjusted for reported prevalence of HBsAg and hepatitis B e antigen (HBeAg) among women who were pregnant,6 including the proportion with high viral load (≥ 20 000 IU/mL),27 we estimated the HBsAg prevalence in Ontario at 0.80% in 2017 (Table 2). In that year, there were an estimated 39 623 previously diagnosed cases and 1878 newly diagnosed cases.26,28 In 2016, the population treated with antivirals was estimated at 11 600 patients and an estimated 25 HBV-related liver transplants were performed in Ontario (Table 2).
Table 2:
General population hepatitis B virus immunization intervention scenarios for Ontario
| Scenario | Administration | Predicted immunization coverage | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| 2021 | 2022–2023 | 2024–2050 | 2021 | 2022–2023 | 2024–2050 | ||
|
|
|
||||||
| Birth dose coverage, % | Three-dose coverage, % | ||||||
| 1. Base (current adolescent schedule) | Two pediatric HBV vaccine doses in grade 7 | 0 | 0 | 0 | 0 | 0 | 0 |
|
| |||||||
| 2. Three individual doses (0, 1 and 6 mo) | Three pediatric HBV vaccine doses in the first year of life | 75 | 90 | 95 | 75 | 90 | 95 |
|
| |||||||
| 3. Two individual, 1 combined (0, 1 and 6 mo) | Two pediatric HBV vaccine doses, final dose hexavalent vaccine on the same schedule as current pentavalent vaccine | 75 | 90 | 95 | 75 | 90 | 95 |
|
| |||||||
| 4. One individual, 2 combined (0, 2 and 6 mo) | One dose of pediatric HBV vaccine, 2 doses of hexavalent vaccine on the same schedule as current pentavalent vaccine | 75 | 90 | 95 | 75 | 90 | 95 |
|
| |||||||
| 5. Three combined (2, 4 and 6 mo) | Three doses of hexavalent vaccine on the same schedule as current pentavalent vaccine | 0 | 0 | 0 | 75 | 90 | 95 |
Note: HBV = hepatitis B virus.
We included the impact of diagnostics, treatment and immunization on disease and economic burden in our modelling. Ontario introduced universal adolescent vaccination in 1997,29 and immunization coverage data for Ontario were either directly available from Public Health Ontario or were linearly extrapolated (Appendix 1, Supplementary Table 2S). For infants born to mothers who were positive for HBsAg, 93.7%6 received birth dose coverage within the first 24 hours of life, 3-dose coverage and HBIg coverage based on current comprehensive immunization programs.29 Thirty-eight percent of eligible women received antiviral treatment during their third trimester.6
We assessed the HBV disease burden and economic impacts under 5 scenarios. We modelled a base scenario and 4 general population vaccination strategies from 2020 to 2050 under the following conditions (Table 2): current 2-dose adolescent vaccination used in Ontario (base case; scenario 1); birth dose vaccination and individual vaccinations at 1 and 6 months (3 separate vaccines; scenario 2); birth dose vaccination, vaccination at 1 month and a hexavalent 6-month vaccination (DTaP-HB-IPV-Hib; scenario 3) (the recommended WHO schedule with the third dose as a part of the hexavalent); birth dose vaccination, with hexavalent doses at 2 and 6 months (scenario 4); and hexavalent vaccination at 2, 4 and 6 months (currently used in other provinces; scenario 5). Scenario details and a description of the calculation of uncertainty are described in Appendix 1, Supplementary Methods IV and V.
Statistical analysis
We applied cost data to disease burden outcomes to determine the economic impact of each scenario. Direct costs included those for health care, screening, immunization, diagnosis and treatment. Health care costs by disease stage were reported previously;30 however, we adjusted these to remove the reported cost of medications for cases classified as chronic HBV (Fibrosis score of F0–F3) and compensated cirrhosis, as the treated population was tracked separately. Costs for HBV medication were reported previously31 and were adjusted based on the distribution of patients by treatment regimen at the Toronto Centre for Liver Disease from 2010 to 2019. We based indirect costs on the value of a statistical life year (gross domestic product [GDP] per capita = Can$62 138, http://api.worldbank.org/v2/en/country/CAN?downloadformat=csv; Table 2) and disability-adjusted life years [DALYs]32 incurred owing to years of life lost because of premature mortality and years lived with disability (Table 2). All costs were inflated to 2020 Canadian dollars based on the consumer price index for health care,33 and indirect costs were discounted at a standard 3% rate.32 We analyzed economic outcomes to 2050 to account for lag time between the implementation of infant immunization on disease burden effects and economic changes.
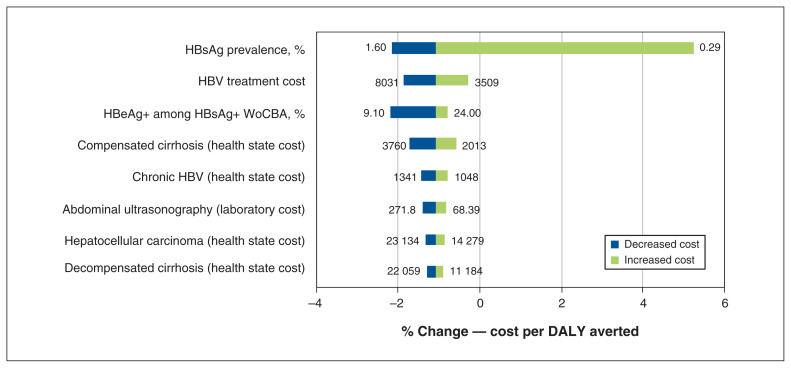
We used Crystal Ball release 11.1.2.3.500 in our sensitivity analysis to calculate high-level disease burden and economic impact outcomes. We used β-PERT distributions for all uncertainty intervals. We used a Monte Carlo simulation to estimate key drivers of uncertainty for the cost per DALY averted for scenario 5 compared with the base scenario, with input ranges based on published estimates and expert input (Table 1).16 The key drivers of uncertainty (Figure 1) account for more than 99% of the variation around cost per DALY averted when comparing the hexavalent approach to the adolescent vaccination.
Figure 1:
Comparison of key drivers of uncertainty (model inputs) for % change in cost per disability-adjusted life year (DALY) averted for scenario 1 (base) to scenario 5 (vaccines at 2, 4 and 6 mo as a part of the hexavalent approach to adolescent vaccination). Note: HBeAg = hepatitis B e antigen, HBsAg = hepatitis B surface antigen, HBV = hepatitis B virus, WoCBA = women of child-bearing age.
Ethics approval
This study did not include people and did not require ethics approval.
Results
With the current adolescent vaccination strategy, we found that acute HBV infections were projected to decrease from 110 in 2020 to 16 in 2050 (85% decline); this was largely due to an overall decrease in unvaccinated adults because of immunization rates of over 70% in school-based programs in Ontario since 1997. Despite this, 1500 acute and 520 chronic infections (Table 3) would still occur over the 30-year time frame owing to infections in the pediatric population under 12 years of age, new infections in those who either did not receive or were not eligible for adolescent vaccination and imported cases as a result of immigration. In comparison, all infant scenarios prevented 560–570 acute and 160 and chronic cases by 2050 (Table 3). As a result of those already infected in 2020, imported cases and new adult chronic infections, liver-related deaths increased until 2042 when they peaked at 780 deaths, declining thereafter.
Table 3:
Number and impact of acute and chronic cases of hepatitis B virus, by scenario
| Scenario | No. of cases of acute HBV | Cumulative burden 2020–2050 | ||||
|---|---|---|---|---|---|---|
| No. of cases of chronic HBV | Cumulative direct costs, millions of Can$ | Cumulative costs averted, millions of Can$ | DALYs averted | Cost per DALY averted, Can$ | ||
| 1. Base(current adolescent schedule) | 1500 | 520 | 3333 | – | – | – |
| 2. Three individual doses (0, 1 and 6 mo) | 940 | 360 | 3424 | 91 | 54 | 1 675 000 |
| 3. Two individual, 1 combined (0, 1 and 6 mo) | 940 | 360 | 3370 | 62 | 54 | 671 000 |
| 4. One individual, 2 combined (0, 2 and 6 mo) | 940 | 360 | 3339 | 6 | 54 | 103 000 |
| 5. Three combined (2, 4 and 6 mo) | 950 | 370 | 3310 | −23 | 54 | −428 000 |
Note: DALY = disability-adjusted life year, HBV = hepatitis B virus.
We compared both annual costs and cumulative direct costs (2020–2050) between scenarios. For all proposed scenarios, we found that annual direct medical costs were estimated at $142 million (in 2020 Canadian dollars) and were projected to decrease by about 50%. This is partially related to an annual 3% discount rate but would occur owing to a decrease in prevalence with any vaccination strategy. Direct medical costs for adolescent vaccination over 30 years was $3.333 million (Table 3). These costs decreased depending on the type of alternative vaccination strategy used and in a stepwise fashion depending on whether the HBV vaccine was given as a separate dose or as part of the hexavalent vaccine. For example, for the scenario involving the recommended birth dose schedule and delivery of all 3 as separate vaccines, direct costs increased to $3.424 million, whereas if the hexavalent strategy was included, direct costs were less than adolescent immunization at $3.310 million (Table 3). Annual cost savings peaked in 2022, with savings ranging from $1.3 to $4.4 million.
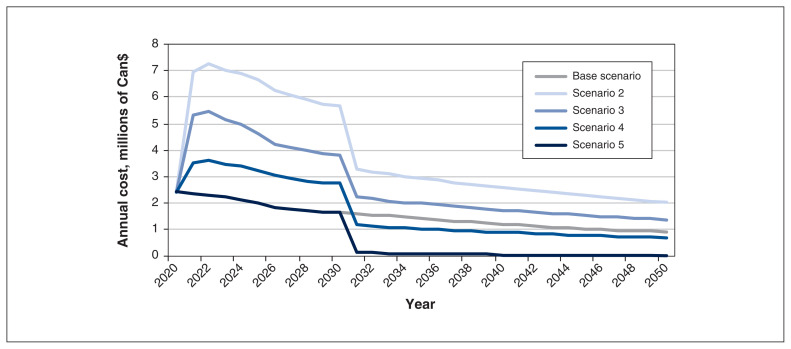
We found that annual immunization costs were estimated at $2.4 million in 2020 under all scenarios and declined by 65% for adolescent vaccination to $0.91 million in 2050 because of the application of a standard 3% discount rate. Costs were incurred during the 12-year catch-up period (infant and birth plus adolescent) for all scenarios (Figure 2). Annual costs for the 2 strategies that included birth dose and 1-month vaccine dosing had greater costs every year from 2020 to 2050, leading to cumulative immunization costs increasing by up to 160% (from $46 to $119 million). However, the 2 strategies that had at least 2 of 3 vaccine doses as part of the hexavalent immunization had costs below the current adolescent costs by 2030 (Figure 2). The hexavalent strategy also decreased cumulative costs by 50%, from $46 to $23 million. Cumulative immunization costs represented 1.4% of direct medical costs for adolescent vaccination and between 0.70% (3 hexavalent immunizations) and 3.5% (3 individual doses including birth dose) of costs under the vaccination scenarios.
Figure 2:
Costs for hepatitis B virus (HBV)–related immunization in Ontario from 2020 to 2050, by scenario.
Indirect costs were based on the value of a statistical life year ($62 138) applied to DALYs incurred by each scenario. Indirect costs accounted for 75% of total (direct and indirect) costs in 2020 ($600 million), peaking at 85% of annual total costs in 2045 under all scenarios. Total indirect and direct costs were $18.431 million for adolescent vaccination, increasing to $18.522 million for 3 individual doses and $18.408 million when using the 3-dose hexavalent approach (Table 4). Total cumulative costs were $62 million higher with 3 individual doses than for adolescent vaccination, whereas 3-dose hexavalent strategy led to cost savings of $23 million by 2050 (Table 3).
Table 4:
Cumulative costs related to hepatitis B virus in Ontario, by category and scenario (2020–2050)
| Scenario | Direct cost, millions of Can$ | Total indirect cost, millions of Can$ | Total costs, millions of Can$ | ||||
|---|---|---|---|---|---|---|---|
| Health care | Treatment and laboratory | Screening | Immunization | Total direct cost | |||
| 1 | 2246 | 932 | 109 | 46 | 3333 | 15 098 | 18 431 |
| 2 | 2245 | 950 | 111 | 119 | 3424 | 15 098 | 18 522 |
| 3 | 2245 | 932 | 109 | 83 | 3370 | 15 098 | 18 467 |
| 4 | 2245 | 932 | 109 | 52 | 3339 | 15 098 | 18 436 |
| 5 | 2245 | 932 | 109 | 23 | 3310 | 15 098 | 18 408 |
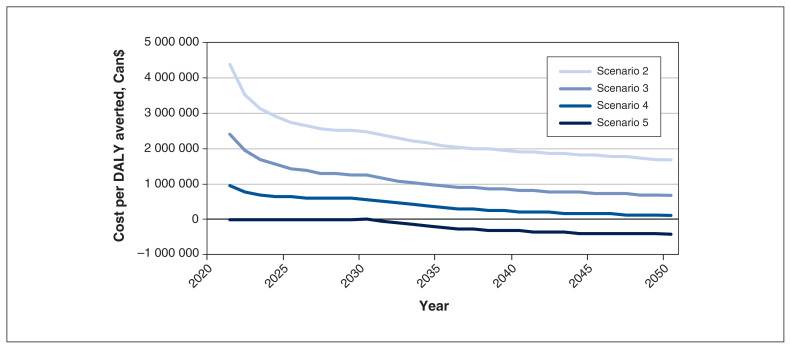
We found that about 3% of all DALYs were incurred from years lived with disability, and 97% were incurred owing to years of life lost (liver-related death). In 2020, there were an estimated 9650 annual DALYs incurred owing to HBV, increasing to 11 100 annual DALYs by 2050 with the current adolescent strategy, an increase of 15%. This increase is largely the result of the impacts of the liver-related disability and death for the current chronically infected population, as well as chronic infections among newcomers to Canada between 2020 and 2050. Total cumulative DALYs using adolescent vaccination were estimated at 360 000 during 2020–2050 (Figure 3) and, by 2050, all 4 infant scenarios had averted the same number of DALYs (≥ 54 DALYs) (Table 3). As the model did not use a lifetime horizon, the major contributor to DALYs averted was immunization costs from 2020 to 2050. Cost per DALY incurred ranged from $104 000 to $1.68 million (birth dose plus separate individual vaccines at 1 mo and 6 mo), whereas hexavalent dosing at 2, 4 and 6 months was cost saving at $428 000 per DALY averted (Table 3). We conducted a sensitivity analysis for the cost per DALY averted in the hexavalent approach compared with adolescent vaccination (Appendix 1, Supplementary Methods V). Prevalence of HBsAg, the cost of HBV treatment and the prevalence of HBeAg among women of child-bearing age who were positive for HBsAg were the key drivers of uncertainty, accounting for 95% of variation (Figure 1). Prevalence alone accounted for more than 80% of observed uncertainty (Appendix 1, Supplementary Material Section III).
Figure 3:
Cost per disability-adjusted life year (DALY) averted in Ontario from 2020 to 2050, by scenario.
Interpretation
In 2020, we reported that there is epidemiologic evidence to reconsider the current adolescent HBV vaccination strategy in Ontario.6 Herein, we have shown that incorporating infant immunization into our current vaccination schedule would prevent acute and chronic infections. Incorporating immunization into a hexavalent vaccine given at 2, 4 and 6 months could also be cost saving, based on the assumptions made in our modelling.
We evaluated 5 scenarios for HBV vaccination strategies. The impact of immigration, as it relates to immigration to Canada from HBV-endemic countries, was included. Although birth or infant vaccination does not eliminate the impact of imported cases, it could prevent early childhood transmission from caregivers who may have unknown HBV. All vaccination strategies led to an overall decline in chronic cases by 2050. However, switching to any form of birth or infant vaccination would prevent 37% to 38% of acute and 30% to 31% of chronic cases in Ontario by 2050. All models include continuation of HBV screening of pregnant women (about 94%), and universal birth dose and HBIg for children born to mothers known to be HBsAg positive.
To switch from 2-dose adolescent to 3-dose birth or infant vaccination, there would be a 12-year period where vaccination was occurring in both groups to ensure all children were vaccinated. A single birth dose strategy was cost-effective, as the cost per DALY was less than the standard willingness-to-pay threshold of 2 times GDP per capita ($124 000).34 In this approach, the hexavalent vaccine at 2 and 6 months would replace our current pentavalent strategy that does not include HBV, costing less per year than the adolescent strategy after the 12-year catch-up period (after 2032). However, switching to a hexavalent approach at 2, 4 and 6 months now would not lead to additional immunization costs and thus no additional costs during the catch-up period (Figure 2).
Visits for pediatric immunizations are typically incorporated into well-baby visits in primary care and pediatrics or at local health units, whereas school-based adolescent vaccination requires additional infrastructure. Even in the context of school vaccination programs, adolescent immunization coverage is suboptimal and much lower than infant. The 2017 Childhood National Immunization Coverage Survey (published by the Public Health Agency of Canada in 2019) reported an uptake of other 3-dose infant programs of 90%,35 whereas HBV adolescent coverage in Ontario in recent years has been as low as 67% (Appendix 1, Table 2S). Furthermore, during the COVID-19 pandemic, school-based vaccination rates were severely disrupted, with low rates of 16.8% and 25% for 2019–2020 and 2020–2021, respectively).36 Not only would incorporating HBV vaccination into well-baby visits reduce the number of injections children would receive and be cost saving, but this approach would follow the provincial strategy for primary care providers to administer most immunizations in Ontario and increase overall HBV immunization coverage.37
There is existing evidence that birth dose or infant immunization reduces both acute and chronic HBV in children; yet adoption varies by province. British Columbia has been immunizing children in infancy for 20 years,38 and early analysis of the impact of infant vaccination showed benefit.39 Nunavut, a region with historically high prevalence, has also been providing birth dose and infant vaccination for over 20 years. The first serosurvey results in the postvaccination era were published in 2017 and documented a reduction in the prevalence of hepatitis B core antibody (a marker of exposure) from 19.8% to 1.8%, and a decrease in HBsAg prevalence from 2.5% to 0.3%.40
Limitations
We assumed that estimates of HBV prevalence for foreign-born Ontario residents to be similar to the prevalence in their country of origin;41 however, this assumption may not be accurate in some populations.42 Our model likely underestimates the cost of disease burden because it does not account for HIV/HBV, HBV/hepatitis C virus and HBV/hepatitis D virus co-infections, all of which can lead to faster disease progression. Although hepatocellular carcinoma can still develop after clearance of HBsAg,43,44 the current model does not account for the small number of people with chronic infection who clear HBsAg.45–48 Using a 30-year time horizon, most HBV-related deaths and costs of end-of-life care are not included. A lifetime time horizon would increase benefits without increasing costs, making all strategies more cost-effective. Although we did not evaluate using this model, additional cost savings are likely, including eliminating the logistics, supplies and personnel for the school-based HBV vaccination program; the likely higher cost of adult versus pediatric vaccine doses; and the buying power of Ontario, which could potentially benefit other Canadian jurisdictions that use the hexavalent vaccine.
Conclusion
We have shown previously that children born in Canada and living in Ontario are acquiring HBV before adolescent immunization; a lifelong disease that is completely preventable. Here we show that birth dose vaccination is cost-effective and infant immunization is cost saving. Considering the minimal increase in cost, a shift to birth dose vaccination is necessary to achieve the fewest number of preventable new infections in children. Based on these data, a policy change to include birth dose HBV immunization is needed and should become the standard of care as well as publicly funded.
Supplementary Material
Acknowledgement
The authors thank their clinical and scientific collaborators for supplying expert opinion and previously acquired data to assist with the inputs for the model.
Footnotes
Competing interests: Harry Janssen has received consultant fees and honoraria from Aligos, Antios, Arbutus, Eiger, Gilead Sciences, GSK, Janssen, Merck, Roche, VBI Vaccines, Vir Biotechnology and Viroclinics. Jordan Feld has received consultant fees from AbbVie, Antios, Arbutus, Eiger, Enanta, BlueJay, GSK, Janssen and Roche. Mia Biondi has received honoraria from Gilead and AbbVie. Hemant Shah has received consultant fees and honoraria from AbbVie, Gilead, Intercept and Lupin SCOPE. He has also received travel support and is a member of the advisory boards of AbbVie, Gilead, Intercept and Lupin. Homie Razavi was a member of the advisory boards of Gilead, AbbVie, Abbott, Merck, Janssen, Roche and VBI Vaccines. He is also a member of the board of directors of the Center for Disease Analysis Foundation. No other competing interests were declared.
This article has been peer reviewed.
Contributors: Mia Biondi, Chris Estes and Devin Razavi-Shearer contributed equally to this work. Mia Biondi, Chris Estes, Devin Razavi-Shearer, Homie Razavi and Jordan Feld conceived and designed the study. Mia Biondi, Chris Estes, Devin Razavi-Shearer, Kanwar Sahdra, Nechama Lipton and Jordan Feld retrieved the data. Chris Estes and Devin Razavi-Shearer ran the model, supervised by Mia Biondi, Jordan Feld and Homie Razavi. All of the authors reviewed the manuscript critically for important intellectual content, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Funding: This study was supported by the Viral Hepatitis Care Network. Mia Biondi has received grants from Gilead and AbbVie. The Center for Disease Analysis has received grants from Gilead Sciences, AbbVie, Hep-Quant, World Health Organization–Zeshan Foundation, EndHep 2030, Pan American Health Organization, John C. Martin Foundation and Merck. Hemant Shah has received grants from Boehringer Ingelheim, Canadian Liver Foundation, and Ontario Ministry of Health and Ministry of Long-Term Care. Harry Janssen has received grants from AbbVie, Gilead Sciences, GSK, Janssen, Roche and Vir Biotechnology. Jordan Feld has received grants from AbbVie, Gilead, GSK, Janssen, Roche, Eiger and Enanta.
Data sharing: All data will be available to other researchers upon review and approval of the “reason for the request” by Mia Biondi and Jordan Feld. Requests for data should be sent to mbiondi@yorku.ca.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/11/1/E24/suppl/DC1.
References
- 1.Kwong JC, Ratnasingham S, Campitelli MA, et al. The impact of infection on population health: results of the Ontario burden of infectious diseases study. PLoS One. 2012;7:e44103. doi: 10.1371/journal.pone.0044103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hepatitis B [news] Geneva: World Health Organization; [accessed 2020 Feb. 5]. modified 2022 June 24. Available https://www.who.int/news-room/fact-sheets/detail/hepatitis-b. [Google Scholar]
- 3.Immunization coverage [news] Geneva: World Health Organization; [accessed 2020 Dec. 31]. modified 2021 July 15. Available https://www.who.int/news-room/fact-sheets/detail/immunization-coverage. [Google Scholar]
- 4.Provincial and territorial routine and catch-up vaccination schedule for infants and children in Canada. Ottawa: Public Health Agency of Canada; [accessed 2019 Nov 11]. modified 2022 July 7. Available: https://www.canada.ca/en/public-health/services/provincial-territorial-immunization-information/provincial-territorial-routine-vaccination-programs-infants-children.html. [Google Scholar]
- 5.Advisory Committee. An Statement (ACS) National Advisory Committee on Immunization (NACI): update on the recommended use of hepatitis B vaccine. Ottawa: Public Health Agency of Canada; 2017. [accessed 2019 Nov 11]. modified 2021 July 5. Available: https://www.canada.ca/en/public-health/services/publications/healthy-living/update-recommended-use-hepatitis-b-vaccine.html#a5. [Google Scholar]
- 6.Biondi MJ, Marchand-Austin A, Cronin K, et al. Prenatal hepatitis B screening, and hepatitis B burden among children, in Ontario: a descriptive study. CMAJ. 2020;192:E1299–305. doi: 10.1503/cmaj.200290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffin CS, Ramji A, Cooper CL, et al. Canadian HBV Network. Epidemiologic and clinical features of chronic hepatitis B virus infection in 8 Canadian provinces: a descriptive study by the Canadian HBV Network. CMAJ Open. 2019;7:E610–7. doi: 10.9778/cmajo.20190103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Census Profile, 2016 Census. Ottawa: Statistics Canada; 2017. [accessed 2019 Apr 1]. modified 2021 Aug 12. Available https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/index.cfm?Lang=E. [Google Scholar]
- 9.Wong WW, Woo G, Heathcote EJ, et al. Disease burden of chronic hepatitis B among immigrants in Canada. Can J Gastroenterol. 2013;27:137–47. doi: 10.1155/2013/924640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lapointe-Shaw L, Chung H, Holder L, et al. Diagnosis of chronic hepatitis B pericomplication: risk factors and trends over time. Hepatology. 2021;73:2141–54. doi: 10.1002/hep.31557. [DOI] [PubMed] [Google Scholar]
- 11.Health Management Branch. Handbook for designated medical practitioners. Government of Canada; 2009. [accessed 2021 May 13]. Available: https://publications.gc.ca/collections/collection_2011/cic/Ci4-11-2011-eng.pdf. [Google Scholar]
- 12.Ni Y-H. Natural history of hepatitis B virus infection: pediatric perspective. J Gastroenterol. 2011;46:1–8. doi: 10.1007/s00535-010-0304-7. [DOI] [PubMed] [Google Scholar]
- 13.Yim HJ, Lok AS-F. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology. 2006;43(Suppl 1):S173–81. doi: 10.1002/hep.20956. [DOI] [PubMed] [Google Scholar]
- 14.Haber BA, Block JM, Jonas MM, et al. Hepatitis B Foundation. Recommendations for screening, monitoring, and referral of pediatric chronic hepatitis B. Pediatrics. 2009;124:e1007–13. doi: 10.1542/peds.2009-0567. [DOI] [PubMed] [Google Scholar]
- 15.de Villiers MJ, Gamkrelidze I, Hallett TB, et al. Modelling hepatitis B virus infection and impact of timely birth dose vaccine: a comparison of two simulation models. PLoS One. 2020;15:e0237525. doi: 10.1371/journal.pone.0237525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383–403. doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 17.Li X, Mukandavire C, Cucunubá ZM, et al. Vaccine Impact Modelling Consortium. Estimating the health impact of vaccination against ten pathogens in 98 low-income and middle-income countries from 2000 to 2030: a modelling study. Lancet. 2021;397:398–408. doi: 10.1016/S0140-6736(20)32657-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hepatitis B and C in the spotlight: a public health response in the Americas. Washington (DC): Pan American Health Organization; 2016. [Google Scholar]
- 19.Lim Y-S, Ahn S-H, Shim J-J, et al. Impact of expanding hepatitis B treatment guidelines: a modelling and economic impact analysis. Aliment Pharmacol Ther. 2022;56:519–28. doi: 10.1111/apt.17052. [DOI] [PubMed] [Google Scholar]
- 20.Sanai FM, Alghamdi M, Dugan E, et al. A tool to measure the economic impact of hepatitis B elimination: a case study in Saudi Arabia. J Infect Public Health. 2020;13:1715–23. doi: 10.1016/j.jiph.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Global hepatitis report, 2017. Geneva: World Health Organization; 2017. [Google Scholar]
- 22.Le L-V, Blach S, Rewari B, et al. Liver Int. 2021. Dec 11, Progress towards achieving viral hepatitis B and C elimination in the Asia and Pacific region: results from modelling and global reporting. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23.Table 17-10-0005-01: Population estimates on July 1st, by age and sex. Ottawa: Statistics Canada; [accessed 2021 Mar. 18]. Available https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1710000501. [Google Scholar]
- 24.Husereau D, Drummond M, Petrou S, et al. ISPOR Health Economic Evaluation Publication Guidelines-CHEERS Good Reporting Practices Task Force. Consolidated Health Economic Evaluation Reporting Standards (CHEERS): explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16:231–50. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 25.United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019. New York: United Nations; 2019. [accessed 2021 Sept. 20]. Available https://population.un.org/wpp/publications/Files/WPP2019_Highlights.pdf? [Google Scholar]
- 26.Report on hepatitis B and C in Canada: 2017. Ottawa: Public Health Agency of Canada; [accessed 2020 Jan. 5]. modified 2019 Sept 18. Available: https://www.canada.ca/en/services/health/publications/diseases-conditions/report-hepatitis-b-c-canada-2017.html? [Google Scholar]
- 27.Wiseman E, Fraser MA, Holden S, et al. Perinatal transmission of hepatitis B virus: an Australian experience. Med J Aust. 2009;190:489–92. doi: 10.5694/j.1326-5377.2009.tb02524.x. [DOI] [PubMed] [Google Scholar]
- 28.Nanwa N, Kwong JC, Feld JJ, et al. A population-based matched cohort study evaluating the health care costs of hepatitis B virus (HBV) in Ontario, Canada. Canadian Liver Journal. 2022 May 2; doi: 10.3138/canlivj-2021-0029.. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hepatitis B immunization. Toronto: Public Health Ontario; 2017. [accessed 2020 Jan. 5]. Available: https://www.publichealthontario.ca/-/media/documents/h/2017/hepb-technical-report.pdf? [Google Scholar]
- 30.Gagnon YM, Levy AR, Iloeje UH, et al. Treatment costs in Canada of health conditions resulting from chronic hepatitis B infection. J Clin Gastroenterol. 2004;38(Suppl 3):S179–86. doi: 10.1097/00004836-200411003-00011. [DOI] [PubMed] [Google Scholar]
- 31.He J, Bowen JM, Xie F, et al. Cost-effectiveness analysis of antiviral treatments for HBeAg-positive chronic hepatitis B in Canada. Value Health. 2012;15:894–906. doi: 10.1016/j.jval.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 32.Global Health Estimates Technical Paper WHO/DDI/DNA/GHE/2020.3. Geneva: World Health Organization; 2020. [accessed 2021 Sept. 21]. WHO methods and data sources for global burden of disease estimates 2000–2019. Available https://cdn.who.int/media/docs/default-source/gho-documents/global-health-estimates/ghe2019_daly-methods.pdf?sfvrsn=31b25009_7. [Google Scholar]
- 33.Table 18-10-0004-01: Consumer Price Index, monthly, not seasonally adjusted. Ottawa: Statistics Canada; 2022. [accessed 2021 Apr. 5]. Available https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1810000401. [Google Scholar]
- 34.Tan-Torres Edejer T, Baltussen R, Adam T, editors. Making choices in health: WHO guide to cost-effectiveness analysis. Geneva: World Health Organization; 2003. [accessed 2020 Dec. 23]. Available https://apps.who.int/iris/bitstream/handle/10665/42699/9241546018.pdf;jsessionid=4A70C65C925E0E4B6CADCDCA1F58E7D2?sequence=1. [Google Scholar]
- 35.Vaccine coverage in Canadian children: results from the 2017 Childhood National Immunization Coverage Survey (cNICS) Ottawa: Public Health Agency of Canada; 2019. [accessed 2020 Dec. 23]. Available: https://www.canada.ca/content/dam/phac-aspc/documents/services/publications/healthy-living/2017-vaccine-uptake-canadian-children-survey/2017-vaccine-uptake-canadian-children-survey-eng.pdf. [Google Scholar]
- 36.Immunization coverage report for school-based programs in Ontario: 2019–20 and 2020–21 school years. Toronto: Public Health Ontario; 2022. [accessed 2022 Nov. 30]. Available: https://www.publichealthontario.ca/-/media/documents/i/2021/immunization-coverage-2019-2021.pdf?sc_lang=en. [Google Scholar]
- 37.About immunization 2020: modernizing Ontario’s publicly funded immunization program. Toronto: Ministry of Health and Long-Term Care; 2020. [accessed 2020 Dec. 23]. Available: https://www.health.gov.on.ca/en/common/ministry/publications/reports/immunization_2020/immunization_2020_report.pdf. [Google Scholar]
- 38.History of immunization in BC. Vancouver: BC Centre for Disease Control; 2020. [accessed 2020 Dec. 23]. Available: http://www.bccdc.ca/resource-gallery/Documents/Guidelines%20and%20Forms/Guidelines%20and%20Manuals/Epid/CD%20Manual/Chapter%202%20-%20Imms/HistoryImmunization.pdf. [Google Scholar]
- 39.Mackie CO, Buxton JA, Tadwalkar S, et al. Hepatitis B immunization strategies: timing is everything. CMAJ. 2009;180:196–202. doi: 10.1503/cmaj.081112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huynh C, Minuk GY, Uhanova J, et al. Serological and molecular epidemiological outcomes after two decades of universal infant hepatitis B virus (HBV) vaccination in Nunavut, Canada. Vaccine. 2017;35:4515–22. doi: 10.1016/j.vaccine.2017.07.040. [DOI] [PubMed] [Google Scholar]
- 41.Sharma S, Carballo M, Feld JJ, et al. Immigration and viral hepatitis. J Hepatol. 2015;63:515–22. doi: 10.1016/j.jhep.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 42.Ahmad AA, Falla AM, Duffell E, et al. Estimating the scale of chronic hepatitis B virus infection among migrants in EU/EEA countries. BMC Infect Dis. 2018;18:34. doi: 10.1186/s12879-017-2921-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adachi H, Kaneko S, Matsushita E, et al. Clearance of HBsAg in seven patients with chronic hepatitis B. Hepatology. 1992;16:1334–7. doi: 10.1002/hep.1840160605. [DOI] [PubMed] [Google Scholar]
- 44.Ahn SH, Park YN, Park JY, et al. Long-term clinical and histological outcomes in patients with spontaneous hepatitis B surface antigen seroclearance. J Hepatol. 2005;42:188–94. doi: 10.1016/j.jhep.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 45.Chen C-J, Yang H-I. Natural history of chronic hepatitis B REVEALed. J Gastroenterol Hepatol. 2011;26:628–38. doi: 10.1111/j.1440-1746.2011.06695.x. [DOI] [PubMed] [Google Scholar]
- 46.Chu C-M, Liaw Y-F. HBsAg seroclearance in asymptomatic carriers of high endemic areas: appreciably high rates during a long-term follow-up. Hepatology. 2007;45:1187–92. doi: 10.1002/hep.21612. [DOI] [PubMed] [Google Scholar]
- 47.Gigi E, Lalla T, Orphanou E, et al. Long term follow-up of a large cohort of inactive HBsAg (+)/HBeAg (−)/anti-HBe (+) carriers in Greece. J Gastrointestin Liver Dis. 2007;16:19–22. [PubMed] [Google Scholar]
- 48.Zacharakis GH, Koskinas J, Kotsiou S, et al. Natural history of chronic HBV infection: a cohort study with up to 12 years follow-up in North Greece (part of the Interreg I–II/EC-project) J Med Virol. 2005;77:173–9. doi: 10.1002/jmv.20434. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.