Abstract
Context
Neuromuscular training (NMT) facilitates the acquisition of new movement patterns that reduce the anterior cruciate ligament injury risk. However, the neural mechanisms underlying these changes are unknown.
Objective
To determine the relationship between brain activation and biomechanical changes after NMT with biofeedback.
Design
Cohort study.
Setting
Research laboratory.
Patients or Other Participants
Twenty female high school soccer athletes, with 10 in an augmented NMT group and 10 in a control (no training) group.
Main Outcome Measure(s)
Ten participants completed 6 weeks of NMT augmented with real-time biofeedback to reduce knee injury-risk movements, and 10 participants pursued no training. Augmented neuromuscular training (aNMT) was implemented with visual biofeedback that responded in real time to injury-risk biomechanical variables. A drop vertical jump with 3-dimensional motion capture was used to assess injury-risk neuromuscular changes before and after the 6-week intervention. Brain-activation changes were measured using functional magnetic resonance imaging during unilateral knee and multijoint motor tasks.
Results
After aNMT, sensory (precuneus), visual-spatial (lingual gyrus), and motor-planning (premotor) brain activity increased for knee-specific movement; sensorimotor cortex activity for multijoint movement decreased. The knee-abduction moment during landing also decreased (4.66 ± 5.45 newton meters; P = .02; Hedges g = 0.82) in the aNMT group but did not change in the control group (P > .05). The training-induced increased brain activity with isolated knee movement was associated with decreases in knee-abduction moment (r = 0.67; P = .036) and sensorimotor cortex activity for multijoint movement (r = 0.87; P = .001). No change in brain activity was observed in the control group (P > .05).
Conclusions
The relationship between neural changes observed across tasks and reduced knee abduction suggests that aNMT facilitated recruitment of sensory integration centers to support reduced injury-risk mechanics and improve sensorimotor neural efficiency for multijoint control. Further research is warranted to determine if this training-related multimodal neuroplasticity enhances neuromuscular control during more complex sport-specific activities.
Keywords: functional magnetic resonance imaging, motion capture, injury prevention, neuroimaging, sensorimotor control, landing neuromuscular control
The majority of sport-related anterior cruciate ligament (ACL) injuries are noncontact, commonly resulting from motor-coordination errors that lead to excessive dynamic knee valgus (medial collapse resulting in elevated knee-abduction moments or loading).1 The motor-coordination cause of ACL injury is exemplified by video analyses of injury events occurring in the athletic field of play, which often demonstrate that factors such as a ball or another player in close proximity or distracted attention (or a combination of these) are involved in most noncontact ACL injuries.1 These external distractors may affect the ability of the central nervous system (CNS) to anticipate and prepare for high-risk situations (eg, rapid changes in direction) while avoiding compromising knee positions (eg, excessive knee valgus).2 As compromised motor coordination, distracted attention, and decreased neurocognitive ability may all contribute to the mechanism of noncontact ACL injuries, considering the CNS in injury-prevention efforts may enhance injury-risk reduction.2 However, despite previous studies3,4 demonstrating that altered CNS function may predispose athletes to ACL injury, prevention strategies have not been updated to reflect these risk factors.
The current standard of care for preventing ACL injuries is neuromuscular training (NMT), and although it is moderately effective when implemented, injury-resistant movement is not always achieved or sustained,5 especially in high-risk female athletes.6 Further, the ACL injury incidence has remained unchanged and is actually increasing in female athletes.7 This is due in part to a lack of program adherence, education, and instruction,8 compounded by the unchanged effectiveness of NMT for ACL injury prevention in nearly a decade,9 remaining at approximately 100 athletes required to be treated to prevent 1 ACL injury. Although NMT incorporates progressive exercises (including balance, strength, and plyometric activities) that have positive effects on physical and mental preparation for sport,8 the injury-prevention focus of traditional NMT is limited to improving the body mechanics associated with injury risk. These movements are typically evaluated using standardized biomechanics laboratory testing,10 which neglects critical aspects of neural function that may contribute to the injury risk and the neuroplasticity associated with the acquisition of injury-resistant movement patterns.3,4 Knowledge of ACL injury-prevention neurotherapeutic targets may inform the design and implementation of more effective strategies to target the neural activity that results in motor coordination leading to injury.
Initial studies on the effects of NMT, augmented with real-time, interactive visual biofeedback, showed improved injury-risk biomechanics during squatting relative to sham biofeedback and transfer of those improved mechanics to landing.11 Further, resting-state brain sensorimotor connectivity enhancements after this augmented neuromuscular training (aNMT) were associated with improvements in injury-risk landing mechanics.12 However, no researchers have yet evaluated the effects of interventions on neural activity during a knee sensorimotor task. Thus, the purpose of our preliminary study was to identify (1) neural activity changes in isolated knee joint and multijoint motor tasks after aNMT and (2) the association between those neural changes and injury-risk movement strategies13 in high school female soccer players.
METHODS
Participants
Of the initial 38 participants, 25 were allocated to the intervention arm; 7 of these were ineligible for neuroimaging due to orthodontic braces. Of the remaining 18 in the intervention arm, 2 athletes did not complete the tasks correctly or had testing challenges involving technology (scanner failure, unable to hear the metronome for task regulation and timing) or comprehension (fell asleep, did not understand the instructions); 1 athlete experienced a combination of challenges. The remaining 15 girls underwent first-level neuroimaging analyses and quality assurance to evaluate the usability of the functional magnetic resonance imaging (fMRI) data for subsequent higher-level analyses. Specifically, excessive head motion and data quality assurance was determined via conservative criteria: >2 mm of absolute head motion or >0.3 mm of relative head motion for either task at either time point; adequate blood-oxygenation-level–dependent signal-model fit, with stable baseline and task-activation profiles; or excessive task-correlated head motion. This resulted in 5 additional athletes being excluded for left-sided movements and 8 for right-sided movements. Therefore, all higher-level neuroimaging analyses used left-side motor-task data only as it yielded the most acceptable data (n = 10) for this preliminary report. The control group contained 13 participants, with 3 excluded for excessive head motion based on the same quality control criteria. In the final analysis, 20 athletes were chosen for the study, with 10 in each group (Table 1). Specifically, 10 participants completed 6 weeks of aNMT with real-time biofeedback designed to reduce knee injury-risk movements (Figure 1),11,12 and 10 served as control individuals who received no intervention. All athletes underwent fMRI and biomechanical testing preintervention and postintervention (6–7 weeks apart for both cohorts). Participants (and a parent or legal guardian if under 18 years) completed an MRI screening form and provided informed assent or consent, respectively. The protocol was approved by Cincinnati Children's Hospital Medical Center Institutional Review Board.
Table 1.
Participant Demographics and Knee-Abduction Moment Data by Group, Mean ± SD
| Characteristic |
Intervention (n = 10) |
Control (n = 10) |
| Age, y | 15.7 ± 1.06 | 16.2 ± 0.63 |
| Height, cm | 164.2 ± 7.24 | 165.2 ± 4.24 |
| Weight, kg | 55.82 ± 9.02 | 60.07 ± 9.54 |
| Body mass index, kg/m2 | 20.34 ± 2.25 | 22.0 ± 3.22 |
| Knee-abduction moment, nm | ||
| Preintervention | 16.91 ± 11.63 | 12.13 ± 8.81 |
| Postintervention | 12.24 ± 10.27 | 9.64 ± 5.99 |
| Difference | 4.66 ± 5.45a | 2.49 ± 6.76 |
Indicates significant difference (P < .05).
Figure 1.
Three-dimensional rendering of visual biofeedback stimulus for augmented neuromuscular training intervention (aNMT). The aNMT stimulus is a real-time, interactive biofeedback stimulus responsive to and driven by select biomechanical variables identified in previous research11 as contributing to injury risk. The specific variables driving aNMT biofeedback for this study were a function of the following variables: (1) trunk lean, (2) knee-to-hip joint extensor moment force ratio, (3) knee-abduction moment of force, and (4) vertical ground reaction force ratio. These variables were calculated in real time and used to render a visual geometric shape (rectangle displayed on a projector screen). The feedback shape changed in real time according to the biomechanical variables as the athlete performed an exercise. The desired outcome for athletes was to move and produce a perfectly symmetric stimulus shape that corresponded to low injury-risk biomechanics. Deviations of the biomechanical variables from the desired injury-resistant movement-pattern goal values yielded specific, systematic distortions of the feedback shape.
Augmented Neuromuscular Training
During the 6-week training period, participants in the aNMT group completed 3 training sessions per week on nonconsecutive days, for a total of 18 possible training sessions. Each session lasted 90 minutes, starting with an agility-ladder 5-minute warm-up and then a standard NMT program involving stations of weightlifting, plyometrics, core development, and speed training.14 Athletes were organized into small groups of 6 to 8 girls to complete 2 of the 4 stations (45 minutes each) during each session and were led through the program by 1 or 2 instructors per group. For 2 sessions each week, the athletes performed biofeedback training individually by pursuing 1 of 6 exercises while maintaining the goal shape of a dynamic visual stimulus—a rectangular shape that was mapped and transformed in real time (lag < 20 milliseconds) as a function of key biomechanical specifications (Figure 1).11 After completing 2 sessions of an exercise, the participant progressed to the next exercise in the following order: body squats, followed by single-legged Romanian dead lifts, pistol squats, overhead squats, squat jumps, and, finally, tuck jumps. Each session consisted of 3 sets of 10 repetitions with biofeedback. The shape was presented to the girls on a projector screen, and their only instruction was to maintain this shape. Unknown to the athlete, the goal shape corresponded to movement patterns associated with a low injury risk. If she moved using biomechanics associated with a higher ACL injury risk (eg, knee valgus, asymmetric loading, insufficient knee or hip flexion), then the rectangular stimulus became distorted in a manner that reflected the severity of the deficit (Figure 1).11 The average number of training sessions attended by participants was 15.0 ± 1.9, with 83.3% NMT session compliance, and all athletes completed all 12 sessions of biofeedback training (100% aNMT compliance). Girls in the control group did not participate in the training and performed their regular off-season activities.
Preintervention and Postintervention Landing Biomechanical Analysis
All participants performed 3 drop vertical jumps before and after the 6-week training period at the Human Performance Laboratory of the Cincinnati Children's Sports Medicine Biodynamics Center. Thirty-one retroreflective markers (B&L Engineering) were secured to specific locations throughout the body for 3-dimensional (3D) motion capture.10 For the drop vertical-jump trials, athletes stood on top of a 31-cm box with their feet 35 cm apart. They stepped off the box with both feet and onto 2 force plates (model BMS600900; Advanced Mechanical Technology, Inc) sampled at 1200 Hz. Immediately after landing on the force plates, the girls were instructed to perform a maximum vertical jump while raising both hands to attempt to grab a vertically suspended basketball that was aligned with their previously recorded maximum jump height. Participants were allowed familiarization trials, and any incorrectly performed trials were repeated until 3 were correctly performed. The marker trajectories were recorded using a high-speed motion-analysis system with 39 digital cameras (Raptor-E; Motion Analysis Corp) at 240 Hz with a data-collection computer (model Z840; Hewlett Packard Development Co). Data were postprocessed using Cortex software (version 6.2; Motion Analysis Corp). For each athlete, the external peak knee-abduction moment (pKAM)13 was computed using an inverse-dynamic analysis in Visual3D (C-Motion, Inc) and averaged bilaterally over the 3 trials.
Key Points
Neuromuscular training augmented with biofeedback reduced the peak knee-abduction moment during landing.
Neuromuscular training–associated neuroplasticity increased sensory, visual-spatial, and motor-planning activity for knee movement and reduced sensorimotor cortex activity for loaded multijoint movement.
Neural activity changes from augmented neuromuscular training may enhance novel intervention pathways to facilitate injury-risk reduction.
Magnetic Resonance Imaging Set-Up
On the same day as the fMRI, participants first completed a mock scanner session during which they were trained on knee motor tasks with a standardized video and practiced with experimenter guidance. All girls wore standardized shorts and socks without shoes to control for skin tactile feedback. Athletes were positioned supine on the MRI table with their legs in a custom apparatus using padding and straps to reduce head motion.15 To further reduce head motion and increase comfort, they were placed in the head coil with earplugs, headphones, and foam pads between the head and coil. Inside the head coil, a mirror was placed so that the girls could see the projector screen for task instructions. In addition, fluidized positioners (models 14010004, 1401007, 1401011; Mölnlycke) were placed under each girl's back, and hook-and-loop straps secured the torso. To standardize arm placement and reduce accessory movement, participants held handlebars that were secured to the sides of the MRI table.
Isolated Knee Extension-Flexion Motor Task
The isolated knee task consisted of unilateral knee extension-flexion paced by a metronome at 1.2 Hz over a preset range of motion (0°–45°) with foam wedges under the knees to ensure a starting position of 45° of flexion. Participants wore ankle braces to prevent ankle movement. Each girl contracted her quadriceps muscle to raise her leg to extension and then lowered the leg without touching the heel to the table, so that the heel was approximately 1 in (2.54 cm) above the table (to decrease abrupt table contact and limit accessory head motion). During the task, athletes were given cues on the projector and sounds via the metronome to guide their leg into flexion and extension for 18 repetitions (36 clicks, with each click indicating the move into extension or flexion) over each 30-second movement block. Each individual completed 4 movement blocks on each leg as separate runs, with an interspersed rest; the starting leg was randomly selected.
Multijoint Leg Press Against Resistance (Ankle, Knee, and Hip Extension-Flexion)
The multijoint leg-press task consisted of a combined unilateral ankle, knee, and hip extension-flexion movement under load.16 For this task, athletes lay supine on the MRI table in a leg-press testing apparatus made up of 2 pedals on a track (allowing both sides to be set up for movement, but the left and right sides were tested independently). Participant restraints were similar to those of the knee motor task but with the ankle braces removed. Each girl flexed a single knee to approximately 45° of flexion (with concurrent hip and ankle flexion) with the feet moving along the track and then extended that knee to approximately 0° of flexion with the resistance of about 20% of body weight via resistance bands.15 Similar to the knee motor task, metronome sounds (1.2 Hz) and visually displayed numeric cues helped guide the participants during the tasks to ensure smooth transitions between each 30-second movement block and 30-second rest.
Magnetic Resonance Imaging Data Acquisition and Analyses
Neuroimaging was acquired on an Ingenia scanner (model 3.0 T; Philips Medical Systems) using a 32-channel phased-array head coil. Data for fMRI were acquired with a gradient-echo planar imaging sequence using a periodic block design in which 30 seconds of motor task was interspersed with 30 seconds of rest. Twenty frames were acquired per cycle for a total of 4 movement cycles for each leg captured in separate runs, with a 3-second repetition time, a 3.75- × 3.75-mm in-plane resolution, and a 5-mm slice thickness for 38 axial slices (field of view = 240 × 240 mm, matrix = 64 × 64). A 3D high-resolution T1-weighted image (repetition time = 8.3 milliseconds, echo time = 3.7 milliseconds, field of view = 256 × 256 mm, matrix = 256 × 256, slice thickness = 1 mm, 176 slices) was collected for registration.
Neuroimaging analyses were performed using the Functional MRI of the Brain (FMRIB) software library (FSL; version 5.0.10; Oxford Centre).17,18 Data were spatially registered and smoothed to correct for head-motion artifact and improve sensitivity in quantifying functional activation.19 This included standard preprocessing applied to individual data, including nonbrain removal, spatial smoothing using a Gaussian kernel of 5-mm full width at half maximum, and standard motion correction.20 Realignment parameters21 (3 rotations and 3 translations) from the motion-correction procedure were included as covariates to account for confounding effects of head movement. High-pass temporal filtering at 120 seconds and time-series statistical analysis were carried out using a linear model with local autocorrelation correction.22 Functional images were coregistered with the respective high-resolution T1-weighted image and normalized to a standard Montreal Neuroimaging Institute 152 template using the FMRIB nonlinear image registration tool.20,21 First-level analysis of lower extremity movement relative to rest was performed using a general linear model analysis cluster-significance threshold of P < .05 (random field cluster–multiple comparisons corrected) and z > 2.3.
Statistical Analysis
Baseline demographics and pKAM were compared between groups (aNMT, control) using independent t tests to determine any differences before the intervention (α set a priori at P < .05). Due to the preliminary nature of this report with a small sample size, we conducted paired t tests (preintervention and postintervention) separately for each group to assess longitudinal changes in pKAM (α set a priori at P < .05 and the Hedges g effect size reported). For brain activity, baseline between-groups comparisons (aNMT > control and control > aNMT) were completed with voxel-wise independent t tests; longitudinal within-group changes with voxel-wise paired t tests (postintervention > preintervention and preintervention > postintervention) were completed with α set a priori at P < .05 (random field cluster–multiple comparisons corrected) and cluster z > 2.3. Two Pearson correlation analyses (1 for each fMRI task) were used to determine the relationship between preintervention and postintervention neural activity changes (% signal change of the average activity for all clusters different from preintervention to postintervention) and pKAM changes (preintervention-postintervention absolute difference).
RESULTS
No demographic differences were present between groups (P values > .05; Table 1). Additionally, the groups did not differ at baseline for pKAM (P > .05) or brain activity (z < 2.3, P > .05). After training, the intervention group reduced pKAM (P = .02, Hedges g = 0.82). During the knee task, the intervention group increased brain activity in 3 clusters associated with sensory, visual-spatial, and motor planning after aNMT relative to baseline (Figure 2; Table 2). During the multijoint leg press, the intervention group decreased activity in 1 cluster localized to the primary motor and somatosensory cortex activity after aNMT relative to baseline (Figure 3; Table 2). No longitudinal changes in neural activity were observed for the control group (z < 2.3, P > .05) or pKAM (P > .05).
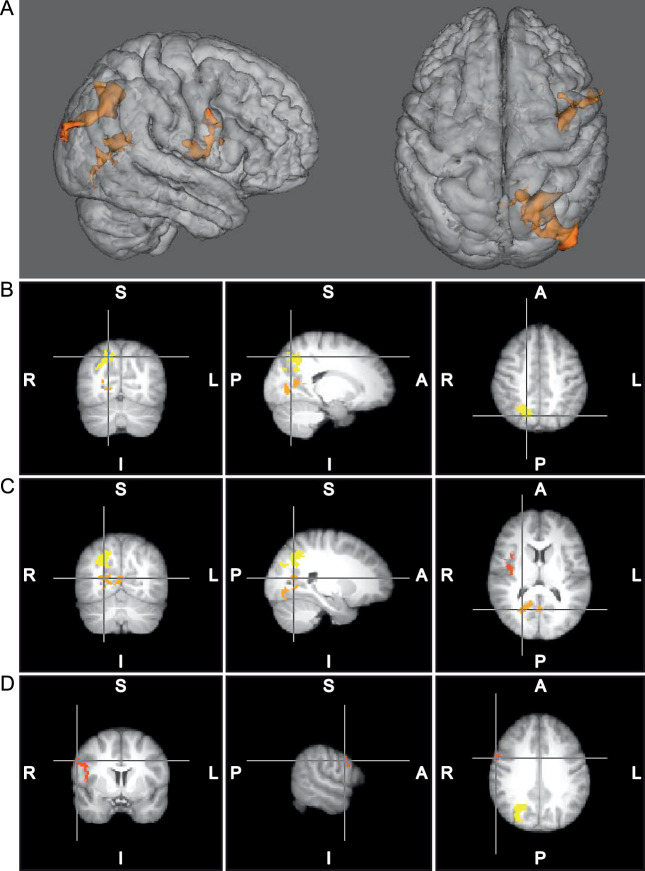
Figure 2.
A, Three-dimensional representation of clusters of increased activation for the left isolated knee task after augmented neuromuscular training (n = 10); the control group (n = 10) had no change with time. B, Yellow = cluster 1, primarily precuneus and parietal activity. C, Orange = cluster 2, primarily lingual gyrus and intracalcarine cortex activity. D, Red = cluster 3, secondary somatosensory and premotor activity.
Table 2.
Neuroplasticity of Augmented Neuromuscular Training for Left Knee and Multijoint Motor Tasks
| Cluster |
Brain Regionsa |
Side |
Voxels (No.) |
P Value |
Montreal Neuroimaging Institute Coordinate of Peak Voxelb |
z Statistic Maximum |
||
|
x
|
y
|
z
|
||||||
| Isolated knee movement | ||||||||
| 1 | Precuneus, superior parietal cortex, and superior lateral occipital cortex | Right | 964 | <.001 | 18 | −68 | 46 | 3.38 |
| 2 | Lingual gyrus, precuneus intracalcarine cortex, and supracalcarine cortex | Right | 461 | .023 | 24 | −64 | 12 | 3.33 |
| 3 | Secondary somatosensory cortex and premotor cortex | Right | 416 | .040 | 60 | 4 | 34 | 3.4 |
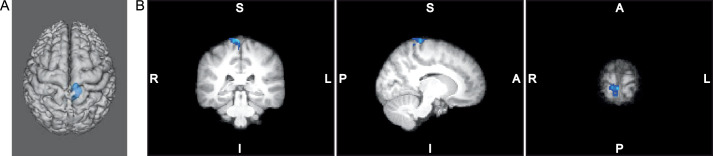
| Multijoint movement | ||||||||
| 1 | Precentral gyrus (primary motor cortex) and postcentral gyrus (primary somatosensory cortex) | Right | 380 | <.001 | 6 | −34 | 80 | 6.88 |
Abbreviations: FSLeyes, FSL image viewer for 3-dimensional and 4-dimensional data; FSL, Functional Magnetic Resonance Imaging of the Brain software library.
Brain regions with significant activation set a priori at P <.05 random-effects analysis, random field cluster–corrected for multiple comparisons, and z threshold set at z > 2.3. Anatomical regions identified with FSLeyes and FSL function atlasquery based on peak voxel location and >5% probability of cluster of activity in anatomical region based on the Harvard-Oxford Cortical Structural Atlas17,23–25 (cluster 1 [both movements] and 2) and Juelich Histological Atlas26–28 (cluster 3).
x, y, and z are based on the Montreal Neuroimaging Institute standard brain template coordinates.
Figure 3.
A, Three-dimensional representation. B, Two-dimensional triplanar view of the primary somatosensory and motor cortex activation decrease postintervention (blue) for the left multijoint leg press in the augmented neuromuscular training group (n = 10).
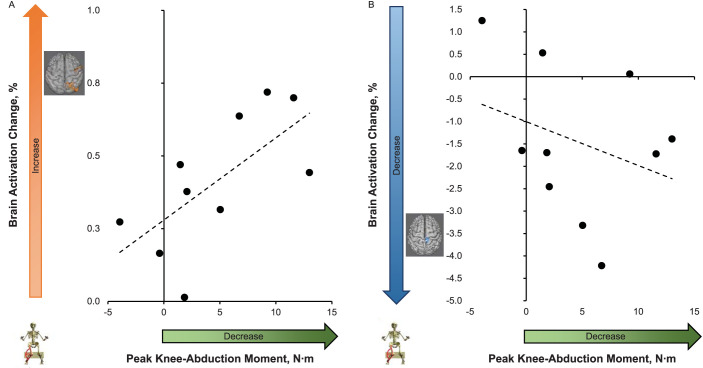
The preintervention-to-postintervention increase in knee sensory integration brain activity, averaged across all 3 clusters (sensory, visual-spatial, and motor planning), was correlated with the training-induced reduction of pKAM (ie, dynamic knee valgus) during landing (r = 0.67, P = .036; Figure 4). The preintervention-postintervention increased activity during the knee task was also correlated with the decreased primary motor and somatosensory cortex brain activity during the multijoint leg-press task (r = 0.87, P = .001). However, the preintervention-to- postintervention changes in pKAM during landing were not associated with changes in brain activity for the multijoint leg press (r = −0.32, P = .38; Figure 4). Head motion during the isolated knee task was 0.23 ± 0.07 mm of absolute motion and 0.06 ± 0.02 mm of relative motion averaged across both time points. Head motion during the multijoint leg-press task was 0.36 ± 0.17 mm of absolute motion and 0.08 ± 0.04 mm of relative motion averaged across both time points.
Figure 4.
Relationship between brain activation changes (% signal change of only voxels identified in Table 2 as different from preintervention to postintervention with respect to each motor task, y axis) and landing injury risk (peak knee-abduction moment [Nm]) difference from pre- to postneuromuscular training (x axis). Larger x-axis values indicate a greater decrease in knee-abduction moment from pretest to posttest. Larger y-axis values indicate an increase in brain activation and smaller values indicate a decrease in brain activation from pretest to posttest. A, Knee-task brain activity (averaged across all 3 identified clusters) increased. B, Multijoint leg-press task brain activity decreased across the 1 identified cluster.
DISCUSSION
Female athletes who completed 6 weeks of aNMT reduced pKAM during landing, with associated increased neural-activity changes. Specifically, reduced pKAM was associated with increased neural activity during the isolated knee task in the contralateral precuneus and secondary somatosensory and superior parietal cortices (areas integrating sensorimotor coordination and limb spatial awareness)29–31; lingual gyrus, lateral occipital cortex, and supracalcarine and intracalcarine cortices (areas processing congruent sensory and visual feedback and cross-modal integration for motor control)32,33; and premotor cortex (area engaging in complex motor planning).34,35 Further, aNMT reduced neural activity during the multijoint task in the contralateral primary motor and somatosensory cortices (referred to as the sensorimotor cortex, with a collective role in motor execution, organization, and direct sensory perception).
Isolated Knee Extension-Flexion Neural-Activity Changes With aNMT
Augmented NMT resulted in increased brain activity in multiple sensory integration regions during the isolated knee task, taxing the quadriceps to maintain knee positional and timing control. That increase in brain activity (all 3 clusters averaged) was associated with decreased pKAM during the drop-jump landing. This correlation may have indicated an adaptive relationship between neural-activity changes and injury-risk reduction after aNMT. Of note, aNMT used biofeedback that linked proprioceptive processing, visual feedback, and motor processing, and therefore, the observed neural-activity changes may have been related to a mechanistic underpinning of aNMT. Recently, an fMRI experiment using the same isolated knee-movement paradigm showed that individuals with biomechanics classified as high injury risk exhibited less precuneus and parietal cortex activity.36 Less neural activity in these regions was attributed, in part, to reductions in proprioceptive and spatial awareness, which may have impaired the ability to sense out-of-plane movements (excessive valgus) and make rapid motor corrections. The ability of aNMT to engage these sensory integration regions for knee motor control may support such biofeedback-augmented approaches to reducing injury-risk coordination that are secondary to deficits in sensory processing. Alternatively, as we did not test a sham control group, it was possible that it was not the biofeedback but the NMT or the combination that induced these effects or the combination of both.37,38
Previous researchers38,39 using a similar neuroimaging paradigm found greater lingual gyrus activity in individuals with a history of ACL reconstruction (ACLR) relative to matched control individuals. Greater neural activity in the extrastriate visual regions was suggested to be evidence of a sensory reweighting effect from knee injury, by which disruptions in joint mechanoreception promoted shifts in neural cross-modal sensory processing biasing toward visual-spatial brain activity for movement control.33,41 In comparing the postinjury results with the effects of aNMT, it is important to consider differences in experimental design and the relative nature of the blood-oxygenation-level–dependent signal-contrast interpretation (the data cannot be compared in absolute terms across study designs but depends on the contrasts such as group [ACLR versus control] or within-participant change [the current report]). In an earlier case-control investigation,39 greater lingual gyrus activity in ACLR participants was assessed relative to control participants; in this study, we found a within-participant increase in lingual gyrus activity after aNMT training using a biofeedback tool that specifically engaged cross-modal proprioceptive visual processing. Thus, the greater overall anatomical regional activity did not mean the lingual gyrus was activating to the same relative degree in both studies, nor was it engaged in the exact same neural process or changed in the same way. However, these findings do support the potential of both ACLR and aNMT to affect cross-modal sensory processing through different pathways.
The ACLR-specific lingual gyrus activity39,40 occurred more inferiorly than the increase after aNMT, and as such, was more associated with extrastriate body properties for limb representation,42 with connectivity to the temporal and frontal cortex. The more superior aNMT activation included the intracalcarine and supracalcarine cortices, with connectivity to the parietal lobe and precuneus, and was more specific for matching proprioceptive cues with visual information (cross modal) as opposed to visual limb representation.43,44 Therefore, despite similar overall anatomical region activity between aNMT effects and ACLR relative to control group differences, unique subregions were likely being activated with nuanced but related functionality and different connectivity and concurrent activity across the brain.45 The ability to modulate cross-modal neural activity via aNMT could be a viable target for rehabilitation to modify sensory reweighting by linking visual-spatial with knee proprioceptive neural processing. This strategy may improve neuromuscular coordination without the explicit reliance on matched visual cues with knee-joint position typically performed in rehabilitation. This biofeedback could potentially shift the cross-modal processing pathway from relying on explicit attention and visual limb representation to relying on the integration of proprioceptive afferent feedback.
The increased premotor activity concurrent with sensory activity after aNMT may have indicated engagement of a motor learning circuit specific to imitation and modeling due to the biofeedback training.46 Although this imitation typically requires a similar model (human), previous authors47,48 demonstrated that with practice, objects can be neurologically integrated, similar to body segments; thus, the biofeedback, despite being an ambiguous rectangle, may have induced a similar effect for movement modeling. In our study, elevated premotor activity may have also been a downstream consequence of changes in movement planning secondary to alterations in activity among cross-modal (lingual gyrus and supracalcarine cortex) and sensory (precuneus) regions for knee motor coordination. In addition, the lateral nature of neural activation pointed to increased motor planning and secondary sensory processing activity in response to external stimuli (similar to the rectangular goal target in aNMT), whereas more medial activity indicated programming motion from internal cues.49,50
Loaded Multijoint Leg-Press Neural-Activity Changes With aNMT
In some respects, the multijoint leg-press task provided a higher-fidelity assay than previous fMRI paradigms by capturing neural activity for loaded, combined ankle, knee, and hip motion.15 Along with the added multijoint requirements and resistance likely increasing motor output (motor cortex) demand, the restricted range of motion due to the foot-pedal tracks may have reduced the probability of detecting sensory processing changes relative to the isolated knee task (during which several sensory integration regions increased in activity after aNMT).16 The sensorimotor cortex efficiency (ie, decreased neural activity) response from aNMT to control the ankle, knee, and hip under load may have supported an improved neuromuscular capability not directly linked to pKAM during landing (as it was not correlated with pKAM changes). Therefore, the efficiency response could have been secondary to increasing muscular strength from NMT as opposed to directly affecting injury-risk coordination. A similar neural efficiency response has been reported in highly trained athletes executing motor tasks.51 In these studies, high-level karate athletes required less brain activation to stand on 1 leg than their nonathletic counterparts, indicating that the extensive training needed to remain stable on 1 leg over time made the task less neurologically demanding. Standard NMT could have had a similar effect, as the load and coordination demand of the NMT exercises increased over the 6 weeks; the multijoint leg press became relatively less neurologically complex, requiring less sensorimotor cortex activity to sustain performance. Additionally, the biofeedback targeting injury-risk movement may not have been the driver of efficiency, as the reduction in activity was not significantly related to pKAM adaptations; it could have simply been secondary to increased lower extremity muscle strength and capacity (or the sensorimotor cortex efficiency was a downstream effect of sensory processing changes for isolated knee movement).
In a previous investigation,36 those classified as high injury risk displayed increased cognitive-motor neural regions (frontal cortex and posterior cingulate gyrus) during the same multijoint leg-press task. Because aNMT was not successful in directly modulating activity in these specific brain regions, further intervention refinement may be needed to incorporate the increased cognitive challenge during motor execution along with focused coordination training. Although sensorimotor cortex efficiency was not significantly related to pKAM changes, it was possible the sensory visual-motor–planning increase for knee-joint positioning was supporting the sensorimotor cortex efficiency response with the vital sensory and preparatory motor information to fine tune motor action, possibly facilitating reduced injury-risk motions. This idea is speculative, but we hypothesize that sensorimotor cortex efficiency could increase the capability for elevated task complexity and play a role in the transfer of injury-resistant movement patterns to more demanding sport scenarios with more extensive cognitive and perceptual demands.52
Limitations
Limitations of our research included an inability to deconstruct whether the observed effects were due to the unique biofeedback, the NMT, or some combination thereof or to generalize the results beyond female adolescent athletes. We analyzed data from the left side only due to the novel nature of the neuroimaging paradigm and prioritizing the side that yielded the most data that passed quality assurance. However, future authors should examine the effects of limb dominance, lateralization, and side-to-side asymmetries on neural activity and biofeedback training effects. The primary biomechanical variable prospectively associated with ACL injury and used as a marker for injury-risk neuromuscular control in the current study was pKAM.13 Knee-abduction mechanics may not predict injury risk in all populations.53 However, associated knee-valgus motion was commonly identified during noncontact ACL injury1 and was a frequent intervention target for reducing the ACL injury risk.8 Hence, despite the need for future research with complementary biomechanical assessments (eg, global movement profiles), these preliminary data provided initial support for the ability of aNMT to improve NMT, potentially by promoting adaptive neuroplasticity.
CONCLUSIONS
This preliminary report from the Train the Brain Project for ACL injury prevention indicated that 6 weeks of aNMT resulted in greater sensory-associated processing and motor-planning neural activity for isolated knee movement and decreased sensorimotor cortex activation for loaded multijoint movement, which may facilitate improved movement strategies (reduced pKAM) in high school female soccer players. Future confirmatory research with larger sample sizes can validate or refute these neuromuscular adaptations to optimize training techniques for ACL injury prevention.
ACKNOWLEDGMENTS
For their support of and assistance with this study, we recognize the following people. From Seton High School (Cincinnati, OH), we thank Ron Quinn, Lisa Larosa, Holly Laiveling, and the entire soccer coaching staff as well as the Seton administration and athletic director Wendy Smith. From Madeira High School (Madeira, OH), we thank soccer head coach Dan Brady, athletic director Joe Kimling, and principal David Kennedy. We appreciate the soccer parents and players for participating in and enthusiastically supporting the project as well as their patience with the testing, scheduling, and follow-up testing. Special acknowledgment goes to athletic trainers Cindy Busse, ATC (Seton High School), and Glenna Knapp, ATC (Madeira High School). Without their time and commitment and their passion for the health and well-being of their student-athletes, this study would not have been possible.
We also thank Christopher D. Riehm, PhD, for designing and rendering Figure 1.
Funding Statement
FINANCIAL DISCLOSURES Dustin R. Grooms, PhD, ATC, CSCS, has current and ongoing funding support from the National Institutes of Health (NIH)/National Center for Complementary and Integrative Health (Award R21AT009339-02), NIH/National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH/NIAMS; Awards NIAMS; R01AR076153 and R01AR077248), and the US Department of Defense Congressionally Directed Medical Research Programs Peer Reviewed Orthopaedic Research Program (Award OR170266), research award (81XWH-18-1-0707). Michael A. Riley, PhD, has current and ongoing funding support from NIH/NIAMS (Awards U01AR067997, R01AR076153) and NIH/National Institute on Deafness and Other Communication Disorders (Award R01DC017301). Gregory D. Myer, PhD, CSCS, consults with commercial entities to support commercialization strategies and applications to the US Food and Drug Administration but has no financial interest in the commercialization of the products. Dr Myer's institution receives current and ongoing grant funding from National Institutes of Health/NIAMS grants U01AR067997, R01 AR070474, R01AR055563, R01AR076153, R01 AR077248, and industry-sponsored research funding related to injury prevention and sport performance to his institution. Drs Myer, Riley, and Kiefer are inventors of a biofeedback technology similar to that used in this study (US patent 11,350,854, "Augmented neuromuscular training system and method," application approved June 7, 2022, software copyrighted). The technology was designed to enhance rehabilitation and prevent injuries and generates licensing royalties. This work was directly supported by the NIH/NIAMS (Awards U01AR067997, R01AR076153, and R01AR077248). The funders played no role in study design, data collection, analysis, decision to publish, or manuscript preparation.
REFERENCES
- 1.Krosshaug T, Nakamae A, Boden BP, et al. Mechanisms of anterior cruciate ligament injury in basketball: video analysis of 39 cases. Am J Sports Med . 2007;35(3):359–367. doi: 10.1177/0363546506293899. [DOI] [PubMed] [Google Scholar]
- 2.Swanik CB. Brains and sprains: the brain's role in noncontact anterior cruciate ligament injuries. J Athl Train . 2015;50(10):1100–1102. doi: 10.4085/1062-6050-50.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swanik CB, Covassin T, Stearne DJ, Schatz P. The relationship between neurocognitive function and noncontact anterior cruciate ligament injuries. Am J Sports Med . 2007;35(6):943–948. doi: 10.1177/0363546507299532. [DOI] [PubMed] [Google Scholar]
- 4.Diekfuss JA, Grooms DR, Yuan W, et al. Does brain functional connectivity contribute to musculoskeletal injury? A preliminary prospective analysis of a neural biomarker of ACL injury risk. J Sci Med Sport . 2019;22(2):169–174. doi: 10.1016/j.jsams.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Padua DA, DiStefano LJ, Marshall SW, Beutler AI, de la Motte SJ, DiStefano MJ. Retention of movement pattern changes after a lower extremity injury prevention program is affected by program duration. Am J Sports Med . 2012;40(2):300–306. doi: 10.1177/0363546511425474. [DOI] [PubMed] [Google Scholar]
- 6.Åkerlund I, Waldén M, Sonesson S, Lindblom H, Hägglund M. High compliance with the injury prevention exercise programme Knee Control is associated with a greater injury preventive effect in male, but not in female, youth floorball players. Knee Surg Sports Traumatol Arthrosc . 2022;30(4):1480–1490. doi: 10.1007/s00167-021-06644-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck NA, Lawrence JTR, Nordin JD, DeFor TA, Tompkins M. ACL tears in school-aged children and adolescents over 20 years. Pediatrics . 2017;139(3):e20161877. doi: 10.1542/peds.2016-1877. [DOI] [PubMed] [Google Scholar]
- 8.Arundale AJH, Silvers-Granelli HJ, Myklebust G. ACL injury prevention: where have we come from and where are we going? J Orthop Res . 2022;40(1):43–54. doi: 10.1002/jor.25058. [DOI] [PubMed] [Google Scholar]
- 9.Sugimoto D, Myer GD, McKeon JM, Hewett TE. Evaluation of the effectiveness of neuromuscular training to reduce anterior cruciate ligament injury in female athletes: a critical review of relative risk reduction and numbers-needed-to-treat analyses. Br J Sports Med . 2012;46(14):979–988. doi: 10.1136/bjsports-2011-090895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford KR, Myer GD, Hewett TE. Reliability of landing 3D motion analysis: implications for longitudinal analyses. Med Sci Sports Exerc . 2007;39(11):2021–2028. doi: 10.1249/mss.0b013e318149332d. [DOI] [PubMed] [Google Scholar]
- 11.Bonnette S, DiCesare CA, Kiefer AW, et al. A technical report on the development of a real-time visual biofeedback system to optimize motor learning and movement deficit correction. J Sports Sci Med . 2020;19(1):84–94. [PMC free article] [PubMed] [Google Scholar]
- 12.Diekfuss JA, Grooms DR, Bonnette S, et al. Real-time biofeedback integrated into neuromuscular training reduces high-risk knee biomechanics and increases functional brain connectivity: a preliminary longitudinal investigation. Psychophysiology . 2020;57(5):e13545. doi: 10.1111/psyp.13545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med . 2005;33(4):492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 14.Myer GD, Ford KR, Palumbo JP, Hewett TE. Neuromuscular training improves performance and lower-extremity biomechanics in female athletes. J Strength Cond Res . 2005;19(1):51–60. doi: 10.1519/13643.1. [DOI] [PubMed] [Google Scholar]
- 15.Grooms DR, Diekfuss JA, Ellis JD, et al. A novel approach to evaluate brain activation for lower extremity motor control. J Neuroimaging . 2019;29(5):580–588. doi: 10.1111/jon.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anand M, Diekfuss JA, Slutsky-Ganesh AB, et al. Integrated 3D motion analysis with functional magnetic resonance neuroimaging to identify neural correlates of lower extremity movement. J Neurosci Methods . 2021;355(5):109108. doi: 10.1016/j.jneumeth.2021.109108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage . 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage . 2004;23(supple 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 19.Smith AJ, Blumenfeld H, Behar KL, Rothman DL, Shulman RG, Hyder F. Cerebral energetics and spiking frequency: the neurophysiological basis of fMRI. Proc Natl Acad Sci U S A . 2002;99(16):10765–10770. doi: 10.1073/pnas.132272199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage . 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- 21.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal . 2001;5(2):143–156. doi: 10.1016/S1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 22.Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage . 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- 23.Makris N, Goldstein JM, Kennedy D, et al. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res . 2006;83(2–3):155–171. doi: 10.1016/j.schres.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Frazier JA, Chiu S, Breeze JL, et al. Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry . 2005;162(7):1256–1265. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein JM, Seidman LJ, Makris N, et al. Hypothalamic abnormalities in schizophrenia: sex effects and genetic vulnerability. Biol Psychiatry . 2007;61(8):935–945. doi: 10.1016/j.biopsych.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 26.Eickhoff SB, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage . 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 27.Eickhoff SB, Heim S, Zilles K, Amunts K. Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. NeuroImage . 2006;32(2):570–582. doi: 10.1016/j.neuroimage.2006.04.204. [DOI] [PubMed] [Google Scholar]
- 28.Eickhoff SB, Paus T, Caspers S, et al. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. NeuroImage . 2007;36(3):511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- 29.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain . 2006;129(3):564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 30.Wenderoth N, Debaere F, Sunaert S, Swinnen SP. The role of anterior cingulate cortex and precuneus in the coordination of motor behaviour. Eur J Neurosci . 2005;22(1):235–246. doi: 10.1111/j.1460-9568.2005.04176.x. [DOI] [PubMed] [Google Scholar]
- 31.Oshio R, Tanaka S, Sadato N, Sokabe M, Hanakawa T, Honda M. Differential effect of double-pulse TMS applied to dorsal premotor cortex and precuneus during internal operation of visuospatial information. Neuroimage . 2010;49(1):1108–1115. doi: 10.1016/j.neuroimage.2009.07.034. [DOI] [PubMed] [Google Scholar]
- 32.Peuskens H, Vanrie J, Verfaillie K, Orban GA. Specificity of regions processing biological motion. Eur J Neurosci . 2005;21(10):2864–2875. doi: 10.1111/j.1460-9568.2005.04106.x. [DOI] [PubMed] [Google Scholar]
- 33.Macaluso E, Frith CD, Driver J. Modulation of human visual cortex by crossmodal spatial attention. Science . 2000;289(5482):1206–1208. doi: 10.1126/science.289.5482.1206. [DOI] [PubMed] [Google Scholar]
- 34.Ball T, Schreiber A, Feige B, Wagner M, Lücking CH, Kristeva-Feige R. The role of higher-order motor areas in voluntary movement as revealed by high-resolution EEG and fMRI. Neuroimage . 1999;10(6):682–694. doi: 10.1006/nimg.1999.0507. [DOI] [PubMed] [Google Scholar]
- 35.Nachev P, Wydell H, O'Neill K, Husain M, Kennard C. The role of the pre-supplementary motor area in the control of action. Neuroimage . 2007;36(3–3):T155–T163. doi: 10.1016/j.neuroimage.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grooms DR, Diekfuss JA, Slutsky-Ganesh AB, et al. Preliminary report on the Train the Brain project sensorimotor neural correlates of anterior cruciate ligament injury risk biomechanics Part I [published online ahead of print March 10. J Athl Train . 2022]. [DOI] [PMC free article] [PubMed]
- 37.Realtime Sensorimotor Feedback for Injury Prevention Assessed in Virtual Reality ClinicalTrialsgov identifier NCT02933008. Updated October 8, 2021. Accessed August 8, 2022. https://clinicaltrials.gov/ct2/show/NCT02933008?id=NCT02933008&draw=2&rank=1.
- 38.Neuroplastic Mechanisms Underlying Augmented Neuromuscular Training ClinicalTrialsgov Identifier NCT04069520. Updated October 21, 2021. Accessed August 8, 2022. https://clinicaltrials.gov/ct2/show/NCT04069520?id=NCT04069520&draw=2&rank=1.
- 39.Grooms DR, Page SJ, Nichols-Larsen DS, Chaudhari AMW, White SE, Onate JA. Neuroplasticity associated with anterior cruciate ligament reconstruction. J Orthop Sports Phys Ther . 2017;47(3):180–189. doi: 10.2519/jospt.2017.7003. [DOI] [PubMed] [Google Scholar]
- 40.Criss CR, Onate JA, Grooms DR. Neural activity for hip-knee control in those with anterior cruciate ligament reconstruction: a task-based functional connectivity analysis. Neurosci Lett . 2020;730:134985. doi: 10.1016/j.neulet.2020.134985. [DOI] [PubMed] [Google Scholar]
- 41.Macaluso E, Driver J. Spatial attention and crossmodal interactions between vision and touch. Neuropsychologia . 2001;39(12):1304–1316. doi: 10.1016/S0028-3932(01)00119-1. [DOI] [PubMed] [Google Scholar]
- 42.Astafiev SV, Stanley CM, Shulman GL, Corbetta M. Extrastriate body area in human occipital cortex responds to the performance of motor actions. Nat Neurosci . 2004;7(5):542–548. doi: 10.1038/nn1241. [DOI] [PubMed] [Google Scholar]
- 43.Calvert GA. Crossmodal processing in the human brain: insights from functional neuroimaging studies. Cereb Cortex . 2001;11(12):1110–1123. doi: 10.1093/cercor/11.12.1110. [DOI] [PubMed] [Google Scholar]
- 44.Limanowski J, Blankenburg F. Integration of visual and proprioceptive limb position information in human posterior parietal, premotor, and extrastriate cortex. J Neurosci . 2016;36(9):2582–2589. doi: 10.1523/JNEUROSCI.3987-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zimmermann M, Mars RB, de Lange FP, Toni I, Verhagen L. Is the extrastriate body area part of the dorsal visuomotor stream? Brain Struct Funct . 2018;223(1):31–46. doi: 10.1007/s00429-017-1469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buccino G, Vogt S, Ritzl A, et al. Neural circuits underlying imitation learning of hand actions: an event-related fMRI study. Neuron . 2004;42(2):323–334. doi: 10.1016/s0896-6273(04)00181-3. [DOI] [PubMed] [Google Scholar]
- 47.Gallivan JP, McLean DA, Valyear KF, Culham JC. Decoding the neural mechanisms of human tool use. eLife . https://doi.org/10.7554/eLife00425 . 2013;2:e00425. doi: 10.7554/eLife.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ehrsson HH, Rosén B, Stockselius A, Ragnö C, Köhler P, Lundborg G. Upper limb amputees can be induced to experience a rubber hand as their own. Brain . 2008;131(12):3443–3452. doi: 10.1093/brain/awn297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halsband U, Matsuzaka Y, Tanji J. Neuronal activity in the primate supplementary, pre-supplementary and premotor cortex during externally and internally instructed sequential movements. Neurosci Res . 1994;20(2):149–155. doi: 10.1016/0168-0102(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 50.Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. Neuroimage . 2002;15(2):373–385. doi: 10.1006/nimg.2001.0976. [DOI] [PubMed] [Google Scholar]
- 51.Del Percio C, Babiloni C, Marzano N, et al. “Neural efficiency” of athletes' brain for upright standing: a high-resolution EEG study. Brain Res Bull . 2009;79(3–4):193–200. doi: 10.1016/j.brainresbull.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Grooms DR, Kiefer AW, Riley MA, et al. Brain-behavior mechanisms for the transfer of neuromuscular training adaptions to simulated sport: initial findings from the Train the Brain project. J Sport Rehabil . 27(5)1–5. 2018. [DOI] [PMC free article] [PubMed]
- 53.Krosshaug T, Steffen K, Kristianslund E, et al. The vertical drop jump is a poor screening test for ACL injuries in female elite soccer and handball players: a prospective cohort study of 710 athletes. Am J Sports Med . 2016;44(4):874–883. doi: 10.1177/0363546515625048. [DOI] [PubMed] [Google Scholar]