Abstract
Objective
To summarize evidence from published systematic reviews evaluating the effect of polypharmacy interventions on clinical and intermediate outcomes. It also summarizes the adverse events that may occur as a result of these interventions.
Data sources
A literature search was conducted using the electronic databases MEDLINE, Embase, CINAHL, Cochrane Central, and Cochrane Database of Systematic Reviews (PROSPERO registration number: CRD42018085767).
Study selection
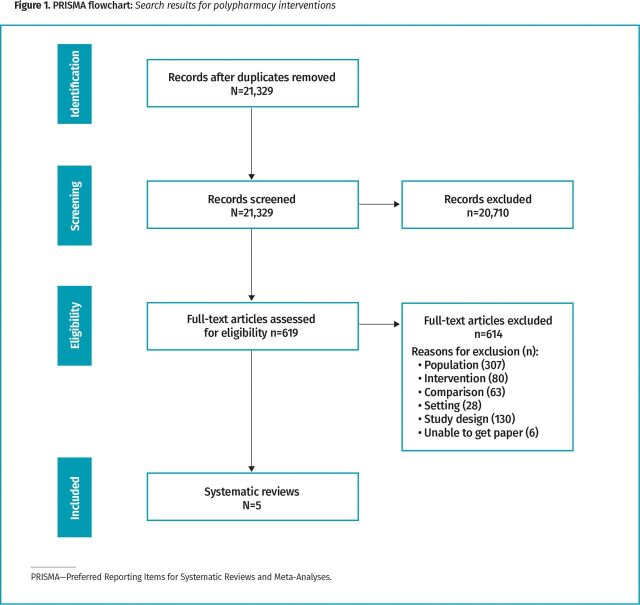
The search yielded a total of 21,329 citations, of which 619 were reviewed as full text and 5 met the selection criteria.
Synthesis
The polypharmacy interventions were found to produce statistically significant reductions in potentially inappropriate prescribing and improved medication adherence; however, the observed effects on clinical and intermediate outcomes were inconsistent. None of the included reviews reported any significant benefit of polypharmacy interventions for quality-of-life outcomes. Specific to health care utilization and cost, polypharmacy interventions reduced health care resource usage and expenditure. The reviews reported no differences in adverse drug events between polypharmacy interventions and usual care groups. The overall certainty of evidence was reported as low to very low across included reviews.
Conclusion
Polypharmacy interventions are associated with reductions in potentially inappropriate prescribing and improvements in medication adherence. However, there is limited evidence of their effectiveness for clinical and intermediate outcomes.
Résumé
Objectif
Résumer les données probantes tirées de revues systématiques publiées qui évaluaient les effets d’interventions en matière de polypharmacie sur les résultats cliniques et intermédiaires. L’article fait aussi la synthèse des événements indésirables qui peuvent se produire à la suite de ces interventions.
Sources d’information
Une recherche documentaire a été effectuée au moyen des bases de données électroniques MEDLINE, Embase, CINAHL, Cochrane Central et de la base de données Cochrane des revues systématiques (numéro d’enregistrement PROSPERO : CRD42018085767).
Sélection des études
La recension a produit 21 329 citations au total. Le texte intégral de 619 d’entre elles a été passé en revue, et 5 articles répondaient aux critères de sélection.
Synthèse
Il a été constaté que les interventions liées à la polypharmacie produisaient des réductions statistiquement significatives dans les prescriptions potentiellement inappropriées et une meilleure adhésion à la médication; par ailleurs, les effets observés sur les résultats cliniques et intermédiaires n’étaient pas uniformes. Aucune des revues incluses ne signalait de bienfaits importants pour les résultats liés à la qualité de vie. En ce qui a trait précisément à l’utilisation des soins de santé et aux coûts, les interventions liées à la polypharmacie réduisaient le recours aux ressources de la santé et les dépenses à cet égard. Les revues ne rapportaient aucune différence dans les événements médicamenteux indésirables entre les interventions en polypharmacie et les groupes de soins habituels. Les revues incluses évaluaient la certitude globale des données probantes comme étant de faible à très faible.
Conclusion
Les interventions liées à la polypharmacie sont corrélées avec des réductions dans les prescriptions potentiellement inadéquates et des améliorations sur le plan de l’adhésion à la médication. Cependant, les données probantes sont limitées quant à leur efficacité pour les résultats cliniques et intermédiaires.
According to the World Health Organization,1 the number of older persons (≥65 years) is expected to reach 1.5 billion by 2050, representing approximately 16% of the population worldwide. Although older adults are healthier today in comparison with those from previous generations, multimorbidity—that is, the presence of 2 or more concurrent chronic medical conditions— continues to rise and is highly prevalent in this population.2
The term polypharmacy is defined as the concurrent use of multiple medications, with the chronic use of 5 or more medications the most commonly considered and clinically relevant minimum.3,4 Twenty-seven percent of older adults living in the community report taking 5 or more medications daily, and a large proportion of them are susceptible to increased risk of adverse health outcomes and drug reactions.3 The number of older adults exposed to polypharmacy continues to rise among this population.5,6 Polypharmacy is associated with negative effects on long-term physical and cognitive functioning,7 drug-drug interactions,8,9 nonadherence,10 adverse health outcomes (eg, falls, cognitive impairment, hospitalization, mortality),5,8,10,11 and medication errors.8,12,13 For example, the risk of adverse health outcomes is estimated to be 13% with the use of 2 medications, 58% with 5 medications, and 82% with 7 or more medications.14
In recent decades, polypharmacy interventions have been developed with the intention to deprescribe, reduce the number or dose of inappropriate medications, and optimize appropriate medication prescription5,6,15 through the use of professional (eg, pharmacist), program-based (eg, medication review clinics), financial (eg, prescribing incentive schemes), and regulatory methods, tools, decision aids, or computer support systems.5,16,17 Such interventions can be explicit (eg, a criteria-based list such as a Beers list of medications that should be avoided in older adults) or implicit (eg, a judgment-based approach in which clinicians use empirical evidence and information from patients to determine the appropriateness of medications).18 These interventions are often tailored, patient-centred, multifaceted, and flexible and have led to fewer hospital admissions, slower decline in quality of life (QOL), resolution of medication-related problems, removal of inappropriately prescribed medications, and improved medication appropriateness and adherence.5,16,19
Although such interventions hold potential for approaches to polypharmacy in primary care, the benefits of such interventions remain unclear in terms of clinical and intermediate outcomes as well as adverse events among older adults living with multimorbidity. As such, the aim of this review of reviews was to summarize the literature related to polypharmacy interventions in older adults living with multimorbidity in the primary care setting. Specifically, this review summarizes the effect of polypharmacy interventions on clinical and intermediate outcomes, and it summarizes the adverse events that occur as a result of these interventions.
Our aim was to understand the effect and, if available, the approaches that seem successful to support the design and implementation of the most effective interventions in managing polypharmacy in older adults for primary care practitioners.5
METHODS
Search strategy
For full details of the review, please see PROSPERO CRD42018085767. A literature search was conducted (Appendix A, available from CFPlus*) using the following electronic databases: MEDLINE, Embase, CINAHL, Cochrane Central, and Cochrane Database of Systematic Reviews. As well, the lists of studies included in the systematic reviews were checked against our search to ensure any relevant citations that had been missed were found and assessed.
Selection criteria
Review studies were included if they met the inclusion criteria outlined in Box 1. Specifically, we selected reviews focused on adults with chronic conditions taking 5 or more medications, where researchers assessed the following: the effect of interventions on clinical outcomes, specifically mortality (all cause) and morbidity (hospitalization, serious adverse events, adverse drug withdrawal events, mobility outcomes including falls and fractures, mood, fatigue, and functional outcomes including instrumental activities of daily living and cognitive functioning); and the effects of the interventions on intermediate outcomes, specifically improvements in blood pressure, glucose control, medication adherence, frailty, reducing polypharmacy, and medication burden. Two team members independently examined the studies based on the titles and abstracts, and a subset was identified for full-text review (Figure 1). Any disagreements between reviewers were resolved through discussions.
Box 1. Inclusion criteria.
Participants or population
Adults with chronic conditions taking 5 or more medications or as indicated by the study
Excluding: pregnant women; children; adults in long-term care or nursing homes
Interventions and exposures
Any polypharmacy intervention in the primary care setting that may include the following: role (ie, pharmacist), a program (medication optimization clinic), tools, decision aids, or computer support systems to deprescribe, taper, or optimize medications. A polypharmacy intervention may be explicit (eg, polypharmacy questionnaire) or implicit (eg, medication review by a pharmacist) in nature
Comparators or controls
Usual care or standard approaches to medication management in primary care
Context
Settings: community-based; primary care; nursing homes or interventions that could easily be conducted in a primary care setting
Outcomes
Clinical outcomes: mortality (all-cause), morbidity (hospitalization, adverse events related to medication), health-related quality of life
Disease-specific risk factors: improvements in cognitive functioning, blood pressure, glucose control, mood, medication adherence, mobility, falls, fatigue, instrumental activities of daily living, frailty, fractures, medication burden
Figure 1.
PRISMA flowchart: Search results for polypharmacy interventions.
Quality assessment
Two authors independently rated the reviews using AMSTAR 2 (A Measurement Tool to Assess Systematic Reviews 2),20 which is a 16-item tool for assessing the methodologic quality of reviews; however, this tool is not intended to provide an overall quality ranking.
Data extraction and synthesis
Data extraction was completed by 1 team member and verified by a second team member. Data were extracted on the aim of the review of reviews, inclusion criteria, polypharmacy intervention characteristics, clinical outcomes, and intermediate outcomes. This included summarizing the overall effectiveness of the polypharmacy interventions. A narrative synthesis was used to summarize the findings across all reviews and a frequency count of coded categories, where relevant, was also performed.
SYNTHESIS
Our search yielded a total of 21,329 citations, of which 619 were reviewed as full text. Five reviews met the selection criteria.5,15,21-23 The included reviews were published between 2014 and 2019. There were 2 narrative summaries and 3 meta-analyses. Particular types of chronic conditions experienced by patients included or targeted in the reviews were not reported in 4 of the 5 reviews. One review outlined the chronic conditions as asthma, diabetes, dyslipidemia, hypertension, cardiovascular disease, and dementia. The mean number of medications taken daily by participants ranged from 5.7 to 9.4. The sample sizes noted in the reviews ranged from 1925 to 61,006 (Table 1).5,15,21-23 Based on AMSTAR 2 quality ratings, 3 studies21-23 were rated low quality, 1 study15 was rated moderate quality, and 1 study5 was rated high quality (Table 2).
Table 1.
Characteristics of included systematic reviews
| STUDY, Y | COUNTRIES | OBJECTIVE | METHODS | PARTICIPANTS | INTERVENTION | FUNDING SOURCE |
|---|---|---|---|---|---|---|
| Johansson et al,15 2016 | NR | To explore the impact of strategies aiming to reduce polypharmacy on mortality, hospitalization, and change in no. of drugs | Analysis type: meta-analysis Setting: GP surgeries, primary care centres, GP outpatient clinic including home-dwelling and community-dwelling participants. Strategies were also carried out in internal medical clinics, hospitals, chronic care geriatric facilities, residential hospitals with continuous care wards, nursing homes, and assisted living facilities Inclusion criteria: age ≥65 y with polypharmacy (≥4 drugs); interventions aimed explicitly at reducing the no. of drugs; patient-relevant outcome measures such as mortality and hospitalization; electronic and nonelectronic interventions as well as mono- and interdisciplinary approaches aimed at the reduction of inappropriate polypharmacy (STOPP interventions); studies explicitly stating the reduction of polypharmacy as an objective or implicitly aimed at the optimization of drug appropriateness by discontinuing inappropriate drugs (eg, tools to detect drug-drug interactions, dosing errors, risk of ADEs, and renal drug dosing). For both study types, the no. of drugs had to be reported at baseline and follow-up Exclusion criteria: studies that did not report no. of drugs; approaches investigating underprescription (eg, START interventions) because a converse effect on drug quantity was expected; interventions focusing on people receiving short-term polypharmacy (eg, terminally ill or receiving cancer chemotherapy); before-and-after studies, interrupted time-series studies, or historically controlled studies, as these study designs have serious limitations Study designs of included studies: 21 RCTs and 4 non-RCTs |
Sample: N=10,980 Total no. of included studies: 25 Mean age range: 69.7 y to 87.7 y Gender range, % male: 20% to 100% Chronic conditions: NR Mean no. of medications reported: 7.4 |
Most studies considered strategies aimed at improving the quality (appropriateness) of the medication regimen by removing inappropriate prescriptions, without explicitly stating the reduction in the no. of drugs as a study objective. Only 5 studies explicitly aimed to reduce the quantity of drugs or the no. of potential or actual drug-related problems, such as nonadherence, expired indication, duplication, inappropriate dosage, off-label use, and contraindications Interventions: pharmacist led (13), physician led (3), multidisciplinary team led (8), physician or pharmacist (1) Description of control or comparator: usual care, other comparable interventions, no intervention Follow-up: 6 wk to 18 mo Outcomes reported:
Quality appraisal: Cochrane ROB GRADE done: yes |
European Union’s Seventh Framework Programme for research technological development and demonstration (grant agreement no. 305 388) |
| Riordan et al,21 2016 | United States, England (United Kingdom), New Zealand | To evaluate studies of pharmacist-led interventions on medication prescribing among community-dwelling older adults receiving primary care to identify the components of successful interventions | Analysis type: narrative synthesis Setting: community Inclusion criteria: all cluster RCTs, quasi-RCTs, controlled before-and-after studies, interrupted timeseries designs. Community-dwelling older adults ≥65 y. Pharmacist-led interventions were included. Comparison group was usual care or other active interventions not focused on medication appropriateness. Primary outcome measure was the change in prescribing appropriateness using a validated explicit or implicit screening tool for the detection of PIP Exclusion criteria: studies that were ongoing, if there was a lack of reply from the author for supplementary information, or if the analysis was purely economic. Studies based on nursing home populations were excluded Study designs of included studies: 4 RCTs, 1 interrupted time series and repeated measures study |
Sample: N=61,006 Total no. of included studies: 5 Mean age range: 64.4 y to 80.4 y Gender range, % male: 28% to 98% Chronic conditions: NR No. of medications reported: overall NR, mean range of 5.7 to 8.2 |
Pharmacist-led interventions were defined as any intervention where the pharmacist had the lead role in an intervention designed to reduce PIP or improve medication appropriateness in primary care Interventions led by: pharmacists Description of control or comparator: usual care or other active interventions not focused on medication appropriateness Follow-up: NR Outcomes reported: the primary outcome measure was change in prescribing appropriateness using a validated explicit or implicit screening tool for the detection of PIP (ie, Beers criteria, STOPP-START, MAI). Secondary outcomes included any clinical or patient self-reported outcomes (eg, QOL, patient satisfaction), change in the no. of medications used, dosage reductions, and medication switches Quality appraisal: Effective Practice and Organisation of Care ROB criteria; Cochrane ROB was used for RCTs GRADE done: no |
Health Research Board SPHeRE/2013/1 |
| Hill-Taylor et al,22 2016 | Ireland, Belgium, Spain, Israel | To update the 2013 systematic review using new evidence from RCTs that assess the effectiveness of STOPP-START criteria on prescribing quality and clinical, humanistic, and economic outcomes in adults ≥65 y | Analysis type: narrative summary Setting: community-dwelling in hospital (patient in transition), n=2 studies; long-term care (patient in stable care), n=2 studies Inclusion criteria: all RCTs involving the prospective application of the STOPP or START criteria on the medication profiles of persons ≥65 y. Studies that measured robust indicators of the clinical, humanistic, and economic impact of the application of the criteria were considered valuable in assessing the effectiveness of the screening tool. Data demonstrating the impact on PIP were also considered primary outcomes Exclusion criteria: non-English, not related to STOPP or START criteria, research not published in peer-reviewed journal, ongoing research, ineligible participant age, ineligible criteria such as modified criteria, truncated criteria, not an RCT, study was retrospective Study design of included studies: RCTs |
Sample: N=1925 Total no. of included studies: 4 Median age range: 74.5 y to 86 y Gender range, % male: 28% to 47% Chronic conditions: NR Mean no. of medications reported: intervention 8.8, control 8.2 |
All studies except 1 (Dalleur et al) used the full 65 STOPP and the full 22 START criteria in their interventions. Dalleur et al also did not use the duplicate therapy STOPP criteria and did not apply the START criteria Interventions led by: pharmacist or physician Description of control or comparator: NR Follow-up: NR Outcomes reported: effects on the appropriateness of prescribing as a result of the intervention were reported by all studies, although reporting and assessment were inconsistent. Clinical outcomes were reported in 3 of the 4 papers, economic indicators in 2, and QOL indicators in 1 Quality appraisal: Cochrane ROB GRADE done: no |
Research grant received from the Drug Evaluation Alliance of Nova Scotia. One author received funding from the Health Research Board (Dublin, Ireland) and Atlantic Philanthropies (Dublin, Ireland) and conducted this research as part of the SPHeRE Programme under grant no. SPHeRE/2013/1 |
| Patterson et al,5 2014 | Australia, Belgium, Canada, Ireland, United States | To determine which interventions, alone or in combination, are effective in improving the appropriate use of polypharmacy and reducing medication-related problems in older people | Analysis type: meta-analysis Setting: hospital setting (7), primary care setting (2), nursing homes (3) Inclusion criteria:
Study designs of included studies: 8 RCTs, 2 cluster RCTs, 2 controlled before-and-after studies |
Sample: N=22,438 Total no. of included studies: 12 Mean age: intervention 76.4 y, control 76.3 y Gender (% male): 35% Chronic conditions: all study participants had more than 1 chronic condition. Conditions included asthma, diabetes, dyslipidemia, hypertension, cardiovascular disease, and dementia No. of medications reported: on average, participants were receiving >4 medications at baseline. In 11 of the 12 studies for which data were available (9878 participants), the mean no. of medications prescribed was 9.4 (intervention participants) and 8.9 (control participants) |
Eleven studies examined multifaceted interventions of pharmaceutical care provided by pharmacists in various settings. One unifaceted study examined computerized decision support provided to GPs in their practices. In hospital settings, pharmacists worked as part of multidisciplinary teams in outpatient clinics, in inpatient services on hospital wards, or as part of the hospital discharge process. In community settings, pharmaceutical care services were provided in community-based family medicine clinics. In nursing homes, pharmacists provided multidisciplinary case conferences, staff education, and a drug therapy management service. Physicians delivered the intervention via a computerized support program in 1 study, whereas in all other studies criteria-based processes were used to develop recommendations for improving the appropriateness of prescribing Participant education was provided as part of the pharmaceutical care intervention in 4 of 6 studies that had face-to-face interventions, and these participants were given directive guidance and specialized medication scheduling tools (eg, monitored dosage systems) to encourage adherence to their prescribed medication regimens Education was provided to prescribers and other team members as part of the intervention in 5 studies Interventions led by: pharmacists (6), physicians (1), team (5) Description of control or comparator: usual care Follow-up: range 1 mo to 12 mo Outcomes reported:
Quality appraisal: Cochrane ROB GRADE done: yes |
No funding |
| Tasai et al,23 2019 | All studies were conducted in high-income Western countries: Denmark, Germany, the Netherlands, Northern Ireland, Portugal, Republic of Ireland, Spain, Sweden, New Zealand, and United States | To assess the impact of medication reviews delivered by community pharmacists to elderly patients on polypharmacy | Analysis type: meta-analysis Setting: community Inclusion criteria: patients ≥65 y who were taking ≥4 prescribed medications; interventions were delivered by community pharmacists; studies measured 1 of these outcomes: hospitalization, ED visits, QOL, adherence. Studies were included regardless of language of publication Exclusion criteria: NR Study design of included studies: RCTs |
Sample: N=4633 Total number of included studies: 4 (3 included in meta-analysis) Mean age range: 74.8 y to 75.9 y Gender, % male: 38.6% Chronic conditions: NR No. of medications reported: ≥7 |
Medication review Interventions led by: community-based pharmacists Description of control or comparator: NR Follow-up: NR Outcomes reported: hospitalization, ED visit, QOL, adherence Quality appraisal: Cochrane Effective Practice and Organisation of Care Group GRADE done: no |
Naresuan University Research Fund |
ADE—adverse drug event; ED—emergency department; GRADE—Grading of Recommendations Assessment, Development and Evaluation; MAI—Medication Appropriateness Index; NR—not reported; PIP—potentially inappropriate prescribing; QOL—quality of life; RCT—randomized controlled trial; ROB—risk of bias; START—Screening Tool to Alert to Right Treatment; STOPP—Screening Tool of Older Persons’ Prescriptions.
Table 2.
AMSTAR 2 ratings
| AMSTAR 2 QUESTIONS | JOHANSSON ET AL,15 2016 | RIORDAN ET AL,21 2016 | HILL-TAYLOR ET AL,22 2016 | PATTERSON ET AL,5 2014 | TASAI ET AL,23 2019 |
|---|---|---|---|---|---|
| Uses elements of PICO | Yes | Yes | Yes | Yes | Yes |
| Methods and protocols defined in advance and deviations reported | Yes | No | Partial yes | Yes | No |
| Authors explained selection of the study designs for inclusion | Yes | Yes | Yes | Yes | Yes |
| Comprehensive literature search strategy used | Yes | Partial yes | Yes | Yes | Partial yes |
| Study selection performed in duplicate | Yes | Yes | Yes | Yes | Yes |
| Duplicate study selection | Yes | Yes | Yes | Yes | Yes |
| Dual data extraction | Yes | No | Yes | Yes | Yes |
| Excluded study list provided | Yes | Yes | No | Yes | No |
| Included studies described adequately | Yes | Yes | Yes | Yes | Yes |
| RCTs: satisfactory ROB technique used | Yes | Yes | Yes | Yes | Partial yes |
| NRSI: satisfactory ROB technique used | Partial yes | No | NA | Yes | NA |
| Funding sources reported | No | No | No | No | No |
| RCT: meta-analysis–appropriate statistical methods | Yes | No meta-analysis conducted | Yes | Yes | Yes |
| NRSI: meta-analysis–appropriate statistical methods | No | NA | No meta-analysis conducted | Yes | NA |
| Impact of ROB on synthesis assessed | Yes | NA | No | Yes | Yes |
| Did the review authors account for ROB in individual studies when interpreting or discussing the results of the review? | No | No | No | Yes | Yes |
| Heterogeneity | Yes | No | Yes | Yes | Yes |
| Conflicts of interest | Yes | Yes | Yes | Yes | Yes |
| Overall rating | Moderate | Low | Low | High | Low |
AMSTAR 2—A Measurement Tool to Assess Systematic Reviews 2; NA—not applicable; NRSI—non-randomized studies of intervention; PICO—patient or population, intervention, comparison, and outcomes; RCT—randomized controlled trial; ROB—risk of bias.
To evaluate different methods for identifying medications suitable for reduction, the reviews included studies that focused on both particular explicit screening tools (criteria-based tools), such as Beers criteria and STOPP-START (Screening Tool of Older Persons’ Prescriptions and Screening Tool to Alert to Right Treatment) criteria, and implicit screening approaches (judgment- or expert opinion–based tools), such as the Medication Appropriateness Index. The primary polypharmacy interventions in most of the studies in included reviews were led by pharmacists (and 2 of the 5 reviews limited their foci to pharmacist-led interventions), while a few also reported physician-led or multidisciplinary team–led interventions (involving GPs, geriatricians, pharmacists, and residential care staff). The operational components of the polypharmacy interventions largely included extended pharmacist consultations, medication reviews, and patient education.
The settings in which the polypharmacy interventions were delivered varied and consisted of the following in combination: acute care hospitals, long-term care facilities (ie, nursing homes, assisted living facilities), primary care (eg, physicians’ offices, community health centres), urgent care centres, outpatient clinics, community and centralized pharmacies, and home health care (ie, care provided in patients’ homes).
The polypharmacy interventions were found to produce statistically significant reductions in potentially inappropriate prescribing (PIP) and improved medication adherence across all 5 reviews; however, the observed effects on downstream clinical and intermediate outcomes were inconsistent. Three reviews assessed allcause mortality as an outcome of interest and reported no differences between intervention and usual care groups across included studies.5,15,22 A trend toward reduced all-cause mortality was reported for longer follow-up periods in 1 review.15 Four reviews evaluated health services utilization, assessing effects on hospitalization, length of stay, readmission, primary care visits, and emergency department visits as outcomes.15,21-23 Only 2 reviews found a benefit for polypharmacy interventions on health services use, in terms of fewer hospitalizations and emergency department visits, when compared with usual care.21,23 None of the 5 reviews reported any significant benefit for polypharmacy interventions in terms of improvements in QOL outcomes (ie, 36-Item Short-Form Health Survey, EuroQol EQ-5D instrument, health-related QOL scale results) when compared with usual care. Specific to health care utilization and cost, 2 reviews noted that polypharmacy interventions reduced the use of health care resources and expenditure.21,22 For adverse drug event outcomes, the reviews reported no significant differences between polypharmacy interventions and usual care groups.5,21,22 The overall quality and certainty of evidence was reported as low to very low across 5 reviews, implying very little confidence in reported effect estimates and that the true effects of polypharmacy interventions might be very different from the estimated effects.
DISCUSSION
To our knowledge, this is the most up-to-date review of reviews that has systematically identified and synthesized a diverse range of evidence about the benefits and relationships of polypharmacy interventions on clinical and intermediate outcomes and the adverse events of such interventions among older adults living with chronic conditions. We systematically identified and synthesized findings across 5 of the highest-quality reviews in the field using the accepted clinically relevant criterion to operationalize polypharmacy (≥5 medications).
Overall, the polypharmacy interventions consistently produced statistically significant reductions in PIP and improved medication adherence; however, these benefits were not shown to produce clinically meaningful changes in either clinical or intermediate outcomes in the studies evaluated.
There are several possible explanations for these findings. First, the evidence across all reviews was of low certainty and did not offer clear findings or conclusions regarding whether the interventions improved clinical and intermediate outcomes. This finding of weak evidence across reviews may be attributed to the heterogeneity and complexity of polypharmacy intervention components, criteria used to assess the appropriateness of medication, study design and settings, sample sizes, short follow-up duration, and diverse range of outcome measures used. Such variability has the potential to limit the overall strength of the conclusions regarding the influence of the interventions on the clinical outcomes and intermediate outcomes. Some reviews were more narrowly focused, with 2 focused on pharmacist-led interventions and 1 focused only on the effectiveness of studies using 1 medication screening list (STOPP-START criteria). Only 2 reviews took a broad view of intervention types. Second, implementation appears key to the success or lack of success of the same intervention type. In studies looking at pharmacist-led interventions, a lack of an effective operationalized pathway for teamwork or communication between health professionals conducting medication reviews and the prescriber may have influenced any effect. Similarly, the review of the STOPP-START tool concluded that success depended heavily on the implementation of the tool. Thus, it may be the implementation of the intervention elements and not the intervention element per se. The reviews identified several interprofessional barriers that likely had an impact on effectiveness of polypharmacy interventions, such as lack of information sharing (ie, access to patients’ clinical information) and lack of collaboration across multidisciplinary teams, particularly for pharmacist-led interventions where the pharmacists’ recommendations were not at times implemented by corresponding health care providers. Third, studies may not have had the sample size or adequate duration to be able to demonstrate downstream effects. Fourth, it is possible that, once established, the negative associations of polypharmacy have limited reversibility with reduction in medications. Fifth, it is possible that negative associations of polypharmacy are highly confounded by the effects of multimorbidity. Finally, reviews reported inconsistency across the primary focus of polypharmacy interventions (ie, some studies focused on reducing the number of medications while others focused on improving the overall appropriateness of prescribing); however, none of the polypharmacy interventions used a combined approach as their primary focus.24
Implications for current practice and future research
The findings from this review of reviews have several implications for practice and research, particularly as polypharmacy continues to rise with the prevalence of multiple chronic comorbid conditions among the older adult population.
Specific to practice, as shown by this review of reviews, polypharmacy interventions have the potential to reduce PIP and improve medication adherence. These outcomes alone are important in clinical practice, as the reverse of these (inappropriate prescribing and medication nonadherence) can have negative effects. This highlights the imperative for health care providers to incorporate polypharmacy interventions in their day-to-day clinical practices. Further, the sheer number of studies that have examined the effects of polypharmacy interventions demonstrates that these types of interventions are possible to implement in a range of settings (primary care, long-term care, hospitals, etc); however, success or lack of success was closely tied to actual implementation, so assessing the feasibility and practicality of implementation in primary care settings and effective models for interprofessional teamwork is essential initial groundwork.
The challenge for researchers is to identify how a reduction in PIP and improvement in medication adherence as a result of polypharmacy interventions might translate into changes in both clinical and intermediate outcomes. The degree of reversibility of negative clinical outcomes associated with polypharmacy and the time to see an effect on clinically important outcomes need closer examination. It may not be surprising that if largely medication-focused interventions are chosen, the effect will be seen in medication-focused outcome measures.
Despite the rhetoric around patient priorities in care, few if any interventions incorporated this. Given that the aim of these complex interventions is to improve patient-relevant outcomes, but they currently have a weak effect on these, it would be interesting to test interventions to reduce medication numbers that are led not just by medication-guided appropriateness, but also by patient-guided appropriateness (priorities and preferences) to see whether this might improve the effect on patient-relevant outcomes, including QOL.
It will be necessary for future research to examine potential mechanisms by which polypharmacy interventions may lead to potential improvements in clinical and intermediate outcomes, and not just PIP and medication adherence. Mechanisms may include examining theoretical underpinnings of the intervention, duration, focus, type and number of components, dose, mode of delivery and teaching method, and level of patient involvement, among other characteristics.
The implementation of polypharmacy interventions can be quite challenging. This review of reviews highlights some barriers related to implementation that will need examination by future researchers. Addressing implementation challenges and testing solutions will promote fidelity and the likelihood of success, and it will increase the internal validity, construct validity, external validity, and certainty of statistical conclusions in polypharmacy intervention research. Finally, the certainty of evidence across reviews was found to be of low quality, highlighting the importance for future research to attend to the rigour of methodologic aspects of studies.
Strengths and limitations
The review provided a comprehensive overview of the literature regarding polypharmacy interventions and the influence on clinical and intermediate outcomes for older adults living with multiple chronic conditions. The review identified a limited number of reviews synthesizing data from an array of studies and research designs, settings, chronic conditions, polypharmacy interventions, and outcomes. Synthesizing these findings has generated a comprehensive, evidence-based review of polypharmacy interventions. However, limitations are noteworthy in this review of reviews. First, we used a more stringent criterion to operationalize polypharmacy definition across reviews (ie, ≥5 medications). However, the use of this criterion is considered more relevant for clinical outcomes and supported in literature. There may, however, be strategies that focus on deprescribing single medications and classes that, if they have a significant effect on important outcomes, could be successfully used in a broader polypharmacy-based approach. Second, given the summarized nature of evidence in a review of reviews where individual studies are not reviewed, we are unable to comment on the most effective intervention strategies or elements of efficacious interventions. Third, across reviews, limited information was provided regarding the types of chronic conditions and the effect of polypharmacy interventions on clinical and intermediate outcomes, resulting in some challenges in the synthesis of the evidence. Fourth, there is a possibility of overlap or duplication of polypharmacy intervention studies across reviews. Finally, in this review of reviews, we were unable to synthesize or meta-analyze data, which may have provided valuable empirical evidence and further strengthened the findings.
Conclusion
Polypharmacy interventions are associated with reductions in PIP and improved medication adherence. However, there is limited evidence of their effectiveness for clinical outcomes of importance to patients. Findings from this review highlight the importance of further high-quality research on polypharmacy intervention characteristics, as these are complex interventions. Understanding the influence of the intervention characteristics on clinical and intermediate outcomes will help guide and refine clinical practice. Further, understanding the implementation of these intervention characteristics may be just as, if not more important than, studying the characteristics themselves.
Supplementary Material
Acknowledgments
This work was supported by funding from the Labarge Centre for Mobility in Aging–McMaster Institute for Research on Aging and the Canadian Institutes of Health Research (#PJT 148971).
Editor’s key points
▸ Polypharmacy interventions are associated with reductions in potentially inappropriate prescribing and improved medication adherence. However, evidence of their effectiveness for patient-relevant clinical and intermediate outcomes is limited.
▸ Implementation of interventions has an important effect on success, with lack of communication between health providers and lack of an effective operationalized pathway for teamwork identified as barriers.
▸ High-quality research is needed on effective polypharmacy intervention and implementation characteristics and on whether reduction of polypharmacy can reverse negative clinical outcomes.
Points de repère du rédacteur
▸ Les interventions liées à la polypharmacie sont corrélées avec des reductions dans les prescriptions potentiellement inappropriées et une meilleure adhésion à la médication. Toutefois, les données probantes sur leur efficacité quant aux résultats cliniques et intermédiaires relatifs aux patients sont limitées.
▸ La mise en œuvre des interventions influe considérablement sur leur réussite; le manque de communication entre les professionnels de la santé et l’absence d’un cheminement opérationnel efficace pour le travail en équipe ont été identifiés comme des obstacles.
▸ Des recherches de grande qualité sont nécessaires sur les interventions efficaces liées à la polypharmacie et sur les caractéristiques de leur implantation, et pour savoir si la reduction de la polypharmacie peut inverser les issues cliniques défavorables.
Footnotes
Appendix A is available from https://www.cfp.ca. Go to the full text of the article online and click on the CFPlus tab.
Contributors
All authors contributed to the concept and design of the study; data gathering, analysis, and interpretation; and preparing the manuscript for submission.
Competing interests
None declared
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
References
- 1.Global health and aging. Bethesda, MD: National Institute on Aging, National Institutes of Health, World Health Organization; 2011. Available from: https://www.nia.nih.gov/sites/default/files/2017-06/global_health_aging.pdf. Accessed 2022 Jun 9. [Google Scholar]
- 2.Tinetti ME, Fried TR, Boyd CM.. Designing health care for the most common chronic condition—multimorbidity. JAMA 2012;307(23):2493-4. Erratum in: JAMA 2012;308(3):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reason B, Terner M, Moses McKeag A, Tipper B, Webster G.. The impact of polypharmacy on the health of Canadian seniors. Fam Pract 2012;29(4):427-32. Epub 2012 Jan 5. [DOI] [PubMed] [Google Scholar]
- 4.Masnoon N, Shakib S, Kalisch-Ellett L, Caughey GE.. What is polypharmacy? A systematic review of definitions. BMC Geriatr 2017;17(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patterson SM, Cadogan CA, Kerse N, Cardwell CR, Bradley MC, Ryan C, et al. . Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev 2014;(10):CD008165. Update in: Cochrane Database Syst Rev 2018;9(9):CD008165. [DOI] [PubMed] [Google Scholar]
- 6.Urfer M, Elzi L, Dell-Kuster S, Bassetti S.. Intervention to improve appropriate prescribing and reduce polypharmacy in elderly patients admitted to an internal medicine unit. PLoS One 2016;11(11):e0166359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koyama A, Steinman M, Ensrud K, Hillier TA, Yaffe K.. Long-term cognitive and functional effects of potentially inappropriate medications in older women. J Gerontol A Biol Sci Med Sci 2014;69(4):423-9. Epub 2013 Nov 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charlesworth CJ, Smit E, Lee DSH, Alramadhan F, Odden MC.. Polypharmacy among adults aged 65 years and older in the United States: 1988-2010. J Gerontol A Biol Sci Med Sci 2015;70(8):989-95. Epub 2015 Mar 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajjar ER, Cafiero AC, Hanlon JT.. Polypharmacy in elderly patients. Am J Geriatr Pharmacother 2007;5(4):345-51. [DOI] [PubMed] [Google Scholar]
- 10.Maher RL, Hanlon J, Hajjar ER.. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf 2014;13(1):57-65. Epub 2013 Sep 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah BM, Hajjar ER.. Polypharmacy, adverse drug reactions, and geriatric syndromes. Clin Geriatr Med 2012;28(2):173-86. [DOI] [PubMed] [Google Scholar]
- 12.Fried TR, O’Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK.. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc 2014;62(12):2261-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stawicki S, Gerlach AT.. Polypharmacy and medication errors: stop, listen, look, and analyze. OPUS 12 Sci 2009;3(1):6-10. [Google Scholar]
- 14.Fulton MM, Allen ER.. Polypharmacy in the elderly: a literature review. J Am Acad Nurse Pract 2005;17(4):123-32. [DOI] [PubMed] [Google Scholar]
- 15.Johansson T, Abuzahra ME, Keller S, Mann E, Faller B, Sommerauer C, et al. . Impact of strategies to reduce polypharmacy on clinically relevant endpoints: a systematic review and meta-analysis. Brit J Clin Pharmacol 2016;82(2):532-48. Epub 2016 May 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cooper JA, Cadogan CA, Patterson SM, Kerse N, Bradley MC, Ryan C, et al. . Interventions to improve the appropriate use of polypharmacy in older people: a Cochrane systematic review. BMJ Open 2015;5(12):e009235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fick DM, Maclean JR, Rodriguez NA, Short L, Vanden Heuvel R, Waller JL, et al. . A randomized study to decrease the use of potentially inappropriate medications among community-dwelling older adults in a southeastern managed care organization. Am J Manag Care 2004;10(11 Pt 1):761-8. [PubMed] [Google Scholar]
- 18.Spinewine A, Schmader KE, Barber N, Hughes C, Lapane KL, Swine C, et al. . Appropriate prescribing in elderly people: how well can it be measured and optimised? Lancet 2007;370(9582):173-84. [DOI] [PubMed] [Google Scholar]
- 19.American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015;63(11):2227-46. Epub 2015 Oct 8. [DOI] [PubMed] [Google Scholar]
- 20.Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, et al. . AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riordan DO, Walsh KA, Galvin R, Sinnott C, Kearney PM, Byrne S.. The effect of pharmacist-led interventions in optimising prescribing in older adults in primary care: a systematic review. SAGE Open Med 2016;4:2050312116652568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill-Taylor B, Walsh KA, Stewart S, Hayden J, Byrne S, Sketris IS.. Effectiveness of the STOPP/START (Screening Tool of Older Persons’ potentially inappropriate Prescriptions/Screening Tool to Alert doctors to the Right Treatment) criteria: systematic review and meta-analysis of randomized controlled studies. J Clin Pharm Ther 2016;41(2):158-69. Epub 2016 Mar 17. [DOI] [PubMed] [Google Scholar]
- 23.Tasai S, Kumpat N, Dilokthornsakul P, Chaiyakunapruk N, Saini B, Dhippayom T.. Impact of medication reviews delivered by community pharmacist to elderly patients on polypharmacy: a meta-analysis of randomized controlled trials. J Patient Saf 2021;17(4):290-8. Epub 2019 Mar 27. [DOI] [PubMed] [Google Scholar]
- 24.Rankin A, Cadogan CA, In Ryan C, Clyne B, Smith SM, Hughes CM.. Core outcome set for trials aimed at improving the appropriateness of polypharmacy in older people in primary care. J Am Geriatr Soc 2018;66(6):1206-12. Epub 2018 Feb 20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.