Abstract
Objective
To assess whether an intervention to help patients prioritize goals for their visit would improve patient-provider communication and clinical outcomes.
Design
Randomized controlled pilot study.
Setting
Primary care clinic.
Participants
There were 120 adult hypertensive patients enrolled.
Intervention
Patients were randomized to receive either usual care or a previsit patient activation card developed through a series of focus groups that prompted patients to articulate their needs and set priorities for their clinic visit. Encounters were audiorecorded, transcribed, and assessed using duplicate ratings of patient activation and decision making.
Main outcome measures
The primary outcome was change in medication adherence as measured by pill count at 4 and 12 weeks after the initial visit. Secondary outcomes evaluated patient-provider interaction quality (patient satisfaction, patient activation, shared decision making, patient trust, and physicians’ perceived difficulty of the encounter), functional status, and blood pressure control.
Results
Of the 120 enrolled patients, 106 completed the baseline visit (mean age of 66 years, 53% women, 57% Black, 36% White). Participants had multiple comorbidities (median number of medications = 8). During the visit, there was greater patient activation in the intervention arm than in the control arm (4.4 vs 3.8, P = .047; ratings were based on a scale from 1 to 10). However, after the visit there were no differences in medication adherence (4 weeks: 45.8% vs 49.5%; 12 weeks: 49.4% vs 51.1%), blood pressure control (4 weeks: 133/78 mm Hg vs 131/77 mm Hg; 12 weeks: 129/77 mm Hg vs 129/76 mm Hg), or encounter satisfaction (78.6% vs 73.8% fully satisfied; P = .63). There were also no differences in shared decision making, patients’ trust, or perceived difficulty of the encounter.
Conclusion
A single previsit tool designed to prompt patients to set a prioritized agenda improved patient activation during the visit, but did not affect the quality of the interaction or postvisit patient-centred outcomes.
Résumé
Objectif
Évaluer si une intervention visant à aider les patients à prioriser les objectifs de leur visite améliorerait la communication patient-médecin et les résultats cliniques.
Type d’étude
Une étude expérimentale randomisée contrôlée.
Contexte
Une clinique de soins primaires.
Participants
L’étude a recruté 120 patients adultes souffrant d’hypertension.
Intervention
Les patients ont été choisis aléatoirement pour recevoir soit leurs soins habituels, soit une fiche incitative avant leur visite, élaborée à la suite d’une série de groupes de discussion, les invitant à formuler leurs besoins et à établir leurs priorités pour leur visite clinique. Les conversations durant la visite étaient enregistrées, transcrites et évaluées selon des notes mesurant à la fois la participation et la prise de décisions des patients.
Principaux paramètres à l’étude
Le principal résultat recherché était un changement dans la conformité à la médication en fonction du décompte des médicaments aux semaines 4 et 12 après la visite initiale. Au nombre des résultats secondaires évalués figuraient la qualité de l’interaction entre le patient et le médecin (satisfaction et participation du patient, prise de décisions conjointe, confiance du patient et impression du médecin quant à la difficulté de la rencontre), l’état fonctionnel et le contrôle de la pression artérielle.
Résultats
Parmi les 120 patients inscrits, 106 étaient présents lors de la visite initiale (âge moyen de 66 ans, 53 % de femmes, 57 % de race noire, 36 % de race blanche). Les participants avaient des comorbidités multiples (nombre moyen de médicaments = 8). Durant la visite, la participation était plus grande dans le groupe de l’intervention que dans le groupe témoin (4,4 c. 3,8, p = ,047; les cotes se fondaient sur une échelle de 1 à 10). Toutefois, après la visite, il n’y a eu aucune différence dans la conformité à la médication (semaine 4 : 45,4 c. 49,8 %; semaine 12 : 48,4 c. 52,3 %), le contrôle de la pression artérielle (semaine 4 : 133/78 c. 131/77 mm Hg; semaine 12 : 129/77 c. 129/76 mm Hg) ou la satisfaction concernant la visite (78,6 c. 73,8 % entièrement satisfaits; p = ,63). Il n’y avait pas non plus de différence dans la prise de décisions conjointe, la confiance du patient ou la difficulté perçue de la rencontre.
Conclusion
Un outil à usage unique, conçu pour inciter les patients à établir, avant leur visite, la priorité de leurs objectifs, a amélioré la participation des patients durant la visite, mais n’a pas influencé la qualité de l’interaction ni les résultats centrés sur le patient après la visite.
Patient-provider communication is a complex and challenging interaction, especially if patients have multiple chronic conditions.1 Communication quality affects patient satisfaction, trust, adherence to medications, and potentially other clinical outcomes.2-6 Some interventions have been shown to improve physicians’ communication skills in primary care, although most have not been robust enough to sustain skills in a meaningful way.7
In cancer care, lists of question prompts and patient concern inventories improve the quality of decision-making conversations.8-10 However, it is unproven whether it is possible to enhance primary care management of chronic illness by improving the quality of the patient-provider interaction. One systematic review of 35 trials with the aim of improving patient-provider communication found a range of approaches that could change interactions and that showed some promise for improving patient health.8 However, health outcomes were often subjective, and only 4 trials with health outcomes met predefined quality criteria.
Patient activation and engagement is an emerging area of research. Patients who are more activated and engaged in self-care have better outcomes, including better blood pressure control.10-14 Providers with more positive beliefs about patients’ roles in self-management have more activated patients.15
Studies on how to activate patients in primary care are few. In one study, patients in community mental health centres who were randomized to a 4-hour group education seminar encouraging active participation in their mental health treatment had improved activation and higher satisfaction, but no change in mental health outcomes.16 Senior centre attendees who watched a video encouraging self-management of chronic conditions were more likely to report being more activated for self-care at 6 months and to exhibit healthier behaviour.17 Interventions that involved intensive interaction with an interviewer, such as reflecting on past decisions and doing exercises to help participants develop and prioritize a list of questions for their providers, increased patient activation, but researchers collected no health outcomes.18,19 In another trial, patients were randomized to a computer-based activation intervention to help prepare for their clinic visit. While participants were more likely to disclose stressors during the visit, they were not more activated than controls.20
We conducted a randomized controlled pilot test to assess the efficacy of a patient activation card that prompted patients to reflect on their goals and expectations for the day, and to define a prioritized agenda in writing. Our goal was to develop and test an intervention that was feasible for use in ambulatory care settings. We hypothesized that explicit prompting of patients to reflect on, articulate, and prioritize their agenda would help improve the visit by activating their involvement in the interaction. In addition to assessing outcomes such as trust, satisfaction, and activation, we assessed whether activation led to better medication adherence and hypertension control.21 In particular, we hypothesized that enhanced interaction and activation would improve adherence to antihypertensive medications and would result in improved blood pressure control.22
METHODS
Study design
We conducted a randomized controlled trial that compared usual care with a previsit patient activation card that prompted patients to reflect on, articulate, and prioritize their needs for the visit that day. This protocol was approved by the Walter Reed Army Medical Center Institutional Review Board and is registered on https://clinicaltrials.gov (NCT01606930). Our report adheres to the CONSORT (Consolidated Standards of Reporting Trials) statement.23
Participants
Eligible participants included all adult patients visiting a primary care clinic who had an established patient-provider relationship (defined as more than 2 visits in the preceding year) with 1 of 11 participating clinicians, spoke English, and had at least 3 comorbid medical conditions (one of which was hypertension). Patients were ineligible if they were incapable of completing surveys because of cognitive impairment, as assessed by chart review and by discussion with the patient’s caretaker. Patients were not excluded for lack of English-language proficiency.
Between October 2011 and May 2012, 634 eligible patients were screened and 120 were randomized (Figure 1). Of excluded patients, 246 had scheduling conflicts, 213 did not consent, and 55 were excluded because they were not expected to be in the local area for at least 1 year. Those who did not consent to the trial were similar to enrolled participants with respect to age and sex.
Figure 1.
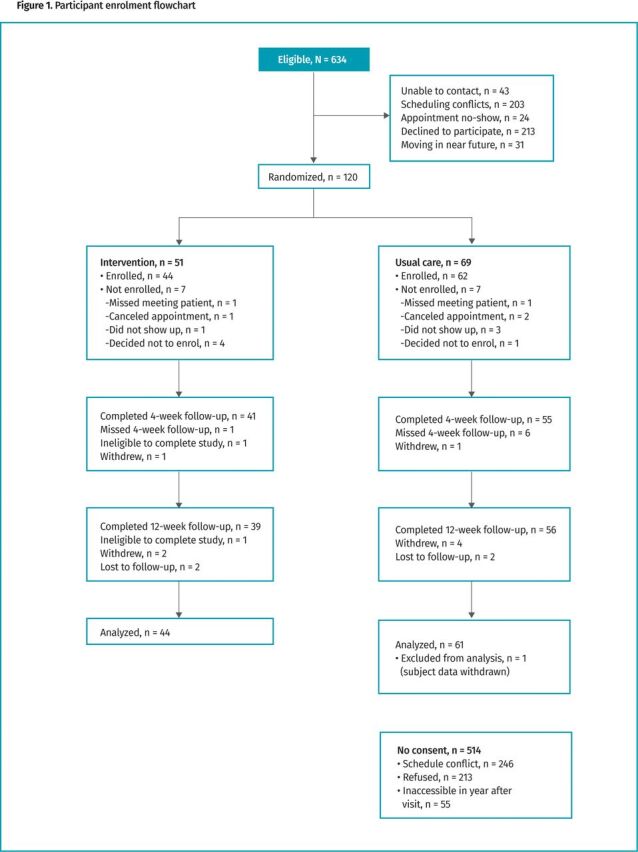
Participant enrolment flowchart
We randomized the 120 participants to 1 of 2 arms using a random numbers table; numbers were placed in numbered, opaque envelopes that were opened by our study coordinator after patients agreed to participate. After participants were enrolled, they completed a series of surveys, and then had 3 serial blood pressure measurements taken.
Intervention development and description
Before this study, we developed a patient activation card using a series of 4 focus groups of physicians (n = 11) and patients (n = 46) from this clinic. Focus groups were audiorecorded and recordings were transcribed. The groups were led by a senior investigator with experience in focus groups (J.H.). The senior investigator used a script approved by the Institutional Review Board to provide consistency across groups. Participants were told that the purpose of the focus groups was to “help us develop some ways of teaching and some materials for teaching that will build better communication.” In addition, focus groups were given a range of possible interventions (eg, coaching, videotapes, letters) and were asked to develop a simple intervention to prime patients for activation during their visit. Focus groups were conducted until data saturation occurred. The final instrument (Figure 2) was designed to prompt patients to reflect on their specific goals for the medical encounter, prioritize those goals, and engage in a discussion with their physician centred on their concerns and expectations. This card was given to patients at least 30 minutes before their encounter, with assistance and coaching available as needed. Patients were encouraged by study personnel to bring this form to their visit and use it to engage their clinician in discussion about their health needs.
Figure 2.
Intervention tool
All participating physicians received 1 hour of training on the importance of addressing patient concerns and expectations. In addition, all participating physicians were briefed on the study’s purpose during the consent process.
Measurements
Blood pressure. We measured blood pressure of participants who had been sitting for 5 minutes before taking the first of the 3 measurements, each taken 5 minutes apart. When then used the average of these measurements. Measurements were taken at baseline and at 4 and 12 weeks thereafter.
Surveys. We surveyed patients before the visit, immediately after the visit, and at 4 and 12 weeks after the visit. Previsit surveys assessed comorbidities, functional status (using the Medical Outcomes Study 6-Item Short Form Health Survey),24 stress, pain severity, medication adherence (using the Morisky Medication Adherence Scale and pill count),25 and health literacy.26 Postvisit surveys included questionnaires that measured trust in the physician27 and visit satisfaction.28 All of these tools have validity evidence, are sensitive to change, and are widely used. Patient surveys at 4 and 12 weeks assessed functional status, stress, pain, trust, and adherence.
Adherence to antihypertensive medications. All participants met with a clinical research pharmacist for adherence assessments of their antihypertensive regimens at baseline and at 4- and 12-weeks’ follow-up. Pill counts were calculated as the number of pills taken (the number of pills dispensed minus the number of pills counted). The number of pills expected to have been taken was calculated by multiplying the daily dose (half, 1, or 2 tablets) by the number of days since the date dispensed.29
Clinician surveys. Clinicians completed the Physician Belief Scale30 and the Freiburg Mindfulness Inventory.31 Immediately after each participant’s visit, physicians rated the difficulty of the encounter using the Difficult Doctor-Patient Relationship Questionnaire.32
Audiorecording coding and measurement of patient activation. Audiorecordings from these encounters were transcribed and then coded by 3 coders who were blinded to the participant study group; the coders assessed the degree of patient activation using the Roter Interaction Analysis System, a validated system that assigns utterances of patients and providers into 26 mutually exclusive domains.33 On the basis of these codes, we defined patient activation as a composite score (1 = low, 10 = high) of ratings in 6 dimensions: relationship symmetry, control of session by the patient, patients’ questions, directedness and confidence in decision making, specific information giving, and patient focus as determined by independent duplicate coding blinded to allocation arm. Each encounter was double coded, with high agreement between coders (Spearman rank correlation = 0.83). Disagreements were discussed, and a final code was based on consensus among the 3 coders.
Outcomes
The primary outcome variable was change in medication adherence as measured by a pill count at 4 and 12 weeks. While the goal of the intervention was to improve the visit interaction, we chose to evaluate the outcome of improved interaction by its effect on a process measure (medication adherence) known to influence patient outcomes. Secondary outcomes included constructs sensitive to the quality of the interaction (patient satisfaction, trust in one’s physician, physician rating of the encounter as “difficult,” and patient activation). Other secondary outcomes included functional status and change in blood pressure at 4 and 12 weeks.
Statistical analysis
The sample size determination (N = 120) for this study was based on a 25% increase in baseline adherence (from 45%), with an α of 0.05 and a β of 0.80, yielding 53 patients per group, with an additional 7 participants per group for expected attrition. This number was based on prior literature on adherence responsiveness to interventions34 coupled with previous findings that a smaller increase in adherence would have a smaller effect on blood pressure control.35 For those lost to follow-up, multiple analyses were performed to assess the sensitivity of our findings in the absence of these data: we tried excluding the data or assuming either a zero change or an average change. A two-tailed χ2 analysis and a t test or the Mann-Whitney U test were used for univariate comparisons of categorical and continuous variables, respectively. Multivariate repeat measure models assessing the effect of our intervention on primary and secondary outcomes (controlling for psychological variables and baseline differences with P < .20) used logistic and linear regression with adjustment for clustering by provider. Analysis was by intention to treat. A P value of .05 or less from a 2-tailed test was considered to indicate statistical significance. All analyses were performed with SPSS, version 22.0.
RESULTS
Characteristics of patients
We identified 634 potentially eligible participants, of whom 120 agreed to participate. Reasons for nonparticipation are in Figure 1. Of the 120 randomized participants, 106 completed the baseline visit (1 then asked to withdraw), 96 completed the 4-week follow-up, and 95 completed the 12-week follow-up. There were 56 women and 49 men who completed the baseline visit. Overall, the cohort was elderly (mean age of 66), mostly Black (57%) or White (36%), and mostly married (73%). Participants had substantial illness burden (Table 1; median of 8 medications), good health literacy (only 8% needed help more than “rarely”), but poor adherence (47% average adherence with hypertensive medications, by pill count). There was general balance in baseline factors between the groups, although the intervention arm trended toward a higher proportion of White people, higher health literacy and adherence, higher functional status, less diabetes, and less pain.
Table 1.
Baseline characteristics of 105 consecutive consenting participants with multiple chronic conditions presenting for periodic visits
| INTERVENTION | |||
|---|---|---|---|
| VARIABLE | PATIENT ACTIVATION CARD (n = 44) | USUAL CARE (n = 61) | P VALUE |
| Age, y | 65.6 | 66.5 | .58 |
| Sex, % female | 52.3 | 54.1 | .85 |
| Ethnicity, % | .64 | ||
| • Black | 50.0 | 62.3 | |
| • White | 43.8 | 29.5 | |
| • Hispanic | 2.1 | 3.3 | |
| • Other | 4.1 | 4.9 | |
| Marital status, % | .70 | ||
| • Married | 70.2 | 76.1 | |
| • Single | 2.1 | 1.5 | |
| • Divorced | 6.4 | 8.9 | |
| • Widowed | 21.3 | 13.5 | |
| Systolic blood pressure, mm Hg* | 139.0 | 136.0 | .35 |
| Diastolic blood pressure, mm Hg* | 81.5 | 80.2 | .49 |
| Hypercholesterolemia, %† | 40.9 | 42.6 | .86 |
| Arthritis, %† | 50.0 | 57.4 | .58 |
| Diabetes, %† | 27.3 | 41.0 | .21 |
| Heart disease (CAD or CHF), %† | 9.1 | 13.1 | .76 |
| Depression or anxiety disorder, %† | 13.6 | 19.7 | .58 |
| COPD, %† | 9.1 | 6.6 | .91 |
| CKD, %† | 11.4 | 14.8 | .83 |
| Current pain level‡ | 2.2 | 3.1 | .13 |
| Health literacy, % never or rarely need help§ | 97.7 | 88.5 | .05 |
| Median no. of medications | 7 | 8 | |
| Adherence, %|| | 51.7 | 42.8 | .10 |
| Recent stress, % | 41.9 | 43.3 | .88 |
| Patient preference for shared decision making, % | 55.8 | 52.5 | .74 |
| Functional status, score¶ | 24.2 | 21.9 | .03 |
CAD—coronary artery disease, CHF—chronic heart failure, CKD—chronic kidney disease, COPD—chronic obstructive pulmonary disease, VAS—visual analog scale.
Blood pressure levels are an average of 3 measurements taken while patient is seated and after 5 minutes of rest.
Data are from self-rated medical history.
Pain levels are based on scores from a 10-point VAS.
This is a self-reported question about how often a patient needs help when reading instructions, pamphlets, or other written material from doctors or pharmacists.
Adherence is mean percentage based on pill count.
Functional status is assessed using the Medical Outcomes Study 6-Item Short Form Health Survey.
Characteristics of physicians
There were 11 participating board-certified staff general internists (mean age 47; 5 White; 6 female) who were experienced (mean time in practice = 18 years) and psychosocially oriented (based on above average scores on mindfulness [37.5] and physicians’ psychosocial beliefs [72.1]).
Primary outcome
There was no difference in medication adherence between intervention and control participants at 4 weeks (45.8% vs 49.5%), or at 12 weeks (49.4% vs 51.1%), or change in adherence over time (Table 2). There was also no difference in blood pressure change or blood pressure control (4 weeks: 133/78 mm Hg vs 131/77 mm Hg; 12 weeks: 129/77 mm Hg vs 129/76 mm Hg).
Table 2.
Primary and secondary outcomes after 4- and 12-weeks’ follow-up for patient activation card arm and usual care arm
| 4 WEEKS (N = 105) | 12 WEEKS (N = 105) | |||||
|---|---|---|---|---|---|---|
| OUTCOMES | PATIENT ACTIVATION CARD (n = 44) |
USUAL CARE (n = 61) |
P VALUE | PATIENT ACTIVATION CARD (n = 44) |
USUAL CARE (n = 61) |
P VALUE |
| Primary outcome, mean (SE) | ||||||
| Adherence, %* | 45.8 (29.0) | 49.5 (26.1) | .51 | 49.4 (27.2) | 51.1 (29.6) | .77 |
| Change in secondary outcomes, mean (SE) | ||||||
| Systolic blood pressure, mm Hg† | -6.04 (2.49) | -4.22 (2.08) | .57‡ | -9.72 (2.85) | -6.18 (2.30) | .33‡ |
| Diastolic blood pressure, mm Hg† | -3.25 (1.63) | -1.88 (1.48) | .54‡ | -4.63 (1.37) | -2.85 (1.35) | .37‡ |
| Trust in physician, score§ | 1.17 (0.58) | 0.38 (0.51) | .31‡ | 0.62 (0.59) | 1.05 (0.50) | .57‡ |
| Functional status, score|| | 0.29 (0.52) | 0.87 (0.40) | .37‡ | 0.79 (0.61) | 0.48 (0.46) | .68‡ |
SE—standard error.
Adherence is mean (SE) percentage based upon pill count.
Blood pressure levels are average (SE) of 3 measurements taken while patient is seated and after 5 minutes of rest.
P values are from an analysis of variance for between-group comparison of change in each variable after 4 and 12 weeks of follow-up.
Changes in trust in physician scores were measured using the Primary Care Assessment Survey.
Changes in functional status scores were measured using the Medical Outcomes Study 6-Item Short Form Health Survey.
Secondary outcomes
Immediately after the visit, there was no difference in patient satisfaction (78.6% vs 73.8% fully satisfied; P = .63). There were also no differences in the degree of shared decision making, patient trust, and physician perceived difficulty of the encounter (Table 3). However, ratings of patient activation from the transcribed audiorecordings found greater activation in the encounters of those receiving the activation card (4.4 vs 3.8, P = .047).
Table 3.
Postvisit patient-centred outcomes for patient activation card arm and usual care arm
| OUTCOMES | PATIENT ACTIVATION CARD (N = 51), MEAN (SD) | USUAL CARE (N = 69), MEAN (SD) | P VALUE* |
|---|---|---|---|
| Patient satisfaction, score† | 23.8 (2.3) | 23.6 (1.9) | .63 |
| Trust in physician, score‡ | 33.7 (3.19) | 32.2 (4.23) | .07 |
| Degree of shared decision making, score§ | 4.7 (2.24) | 4.3 (2.18) | .35 |
| Doctor rating of encounter as difficult, %|| | 18.6 (8) | 24.6 (15) | .47 |
| Patient activation, score¶ | 4.4 (1.57) | 3.8 (1.53) | .047 |
P values are from between-group comparisons after doctor visit.
Patient satisfaction was measured using a 5-point Likert scale with 5 questions ranging from excellent to poor.
The Primary Care Assessment Survey was used to measure trust.
Patient perception of the degree of decision making was measured on the 20-point Reynolds Intellectual Assessment Scales Composite, ranging from 0 (doctor oriented) to 20 (patient oriented).
Doctor rating of encounter as difficult was measured using Difficult Doctor-Patient Relationship Questionnaire-10.
Patient activation is a composite score (1 = low, 10 = high) based on ratings in 6 dimensions: relationship symmetry, control of session by patient, patients’ questions, directedness and confidence in decision making, specific information giving, and patient focus as determined by independent duplicate coding blinded to allocation arm.
DISCUSSION
In this randomized trial of hypertensive primary care patients, we showed that a previsit tool designed to activate patients to reflect on and prioritize their visit agenda improved activation of the patient during the encounter but did not translate into improved medication adherence, blood pressure control, patient satisfaction, or trust. Other dimensions of patient-provider interactions did not improve, such as the degree of shared decision making or the difficulty of the encounter as judged by the physician. This suggests that simple, patient-targeted tools for improving agenda setting and the patient-provider interaction, while able to slightly increase patient activation, are insufficient to make a clinically significant difference on relevant patient outcomes.
These findings are consistent with prior studies, which have shown minimal to no effects of brief interventions to improve patient-provider interactions,8 including 2 focused on improving hypertension control.36,37 While there is limited evidence that better patient-provider relationships are associated with improved adherence,2,3 optimizing those relationships requires more than a simple previsit tool. Given the complexity and bidirectional dynamic of patient-provider interactions, it seems clear that improving this interaction will require more complex interventions that involve both sides of the dyad. This will likely require interventions to develop physician communication skills, patient education on a broader scale, sociocultural enhancement of the system and process of care, as well as previsit tools tailored to the needs of patients at the point of care.
The high health literacy of this population (only 8% had low literacy, compared with 30% to 40% in other studies) could have limited the data’s ability to show an effect. Low literacy has been shown to result in poor patient-provider communication38-41 and poorer health outcomes.42,43 However, studies suggest that health literacy and patient activation are separate constructs that have low correlation with each other,15,44 and that patient activation predicts outcomes better than literacy.15
There are several strengths of this study. First, we created our tool using focus groups of physicians and patients. Second, we randomized participants. Third, we studied multiple dimensions of the interaction with both direct observation and immediate postvisit validated surveys. Finally, we looked at clinical outcomes, measured longitudinally to capture latent effects.
Limitations
This study also has several limitations. First, it was a small sample size from a single academic medical centre, using experienced clinicians who were psychosocially oriented and who had long-term relationships with their patients. It is possible that larger effects could be seen in other settings where potential for improvement in interactions is greater, for instance in less established relationships, such as in acute care, first-visit primary care, or among patients with physicians who are more biomedically oriented. Second, many patients declined to participate. It is likely that those who agreed to participate are different from those who declined (ie, they could be more activated). It is possible that our intervention would have had greater impact on patients who chose not to participate. Third, some imbalances in our baseline variables could indicate ineffective randomization and reflect our small sample size. As a result, it is possible that some of these differences could have resulted in confounding that masked any possible effects, though adjusting for imbalance did not show such effects. Fourth, we gave our control providers a 1-hour training session on addressing patient concerns. This could have reduced the study’s impact, although previous studies on patient-provider communication have found that longer and more intense training is required to change provider behaviour.36,44 Fifth, it is possible that dimensions of activation were missed in our directly observed ratings of patient-provider interactions, and could have been detected if we had used the Patient Activation Measure.14 Finally, this was a very brief intervention focused on patients. This tool could be more effective in the context of a more complex intervention that involved acculturation and repeated use of the tool by both patients and physicians over longer periods.
Conclusion
A single previsit tool designed to prompt patients to develop a prioritized agenda slightly improved patient activation but did not affect other patient-centred outcomes. More robust patient and physician interventions are needed to optimize visit communication in ways that improve outcomes.
Acknowledgment
The findings of this study were presented at the Society of General Internal Medicine conference in Orlando, Fla, in April 2012. The views expressed here are those of the authors only and are not to be construed as those of the US Department of the Army, the US Department of Defense, or the US Department of Veterans Affairs. This protocol was approved by the US Department of Clinical Investigation at the Walter Reed Army Medical Center in Bethesda, MD, and was federally funded (by the Army Medical US Department of the US Department of Defense, and the US Defense Health Research Program) in collaboration with the University of California Susan Samueli Integrative Health Institute in Irvine. The funders did not participate in the study design, analysis of data, or manuscript preparation. The full protocol can be obtained by e-mailing the corresponding author.
Editor’s key points
▸ A single, previsit tool designed to prompt patients to assert a prioritized agenda slightly improved patient activation during the visit, but did not affect other patient-centred outcomes.
▸ Simple, patient-targeted tools for improving agenda setting and patient-provider interactions, while able to slightly increase patient activation, are insufficient to make a clinically significant difference on relevant patient outcomes.
▸ More robust patient and physician interventions could optimize communication in ways that improve outcomes.
Points de repère du rédacteur
▸ Un outil unique, préalable à une visite, conçu pour inciter les patients à établir la priorité de leurs objectifs, a légèrement amélioré la participation des patients durant la visite, mais n’a pas eu d’effets sur les autres résultats axés sur le patient.
▸ Des outils simples et axés sur le patient pour améliorer l’établissement des priorités et les interactions entre le patient et le médecin, bien que susceptibles d’augmenter légèrement la participation du patient, ne suffisent pas pour faire une différence cliniquement significative dans les résultats pertinents pour les patients.
▸ Des interventions plus rigoureuses de la part des patients et des médecins pourraient optimiser la communication de manière à améliorer les résultats.
Footnotes
Contributors
All authors contributed to the concept and design of the study; data gathering, analysis, and interpretation; and preparing the manuscript for submission.
Competing interests
None declared
This article has been peer reviewed.
Cet article a fait l’objet d’une révision par des pairs.
References
- 1.Committee on Diagnostic Error in Health Care; Board on Health Care Services; Institute of Medicine; the National Academies of Sciences, Engineering, and Medicine; Balogh EP, Miller BT, et al., editors. Improving diagnosis in health care. Washington, DC: National Academies Press; 2015. Available from: https://www.ncbi.nlm.nih.gov/books/NBK338596/. Accessed 2021 Dec 8. [PubMed] [Google Scholar]
- 2.Greenfield S, Kaplan SH, Ware JE Jr, Yano EM, Frank HJ.. Patients’ participation in medical care: effects on blood sugar control and quality of life in diabetes. J Gen Intern Med 1988;3(5):448-57. [DOI] [PubMed] [Google Scholar]
- 3.Jackson JL, Kroenke K, Chamberlin J.. Effects of physician awareness of symptom-related expectations and mental disorders. A controlled trial. Arch Fam Med 1999;8(2):135-42. [DOI] [PubMed] [Google Scholar]
- 4.Jackson JL, Chamberlin J, Kroenke K.. Predictors of patient satisfaction. Soc Sci Med 2001;52(4):609-20. [DOI] [PubMed] [Google Scholar]
- 5.Roter DL, Hall JA.. Communication and adherence: moving from prediction to understanding. Med Care 2009;47(8):823-5. [DOI] [PubMed] [Google Scholar]
- 6.Schneider J, Kaplan SH, Greenfield S, Li W, Wilson IB.. Better physician-patient relationships are associated with higher reported adherence to antiretroviral therapy in patients with HIV infection. J Gen Intern Med 2004;19(11):1096-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griffin SJ, Kinmonth AL, Veltman MW, Gillard S, Grant J, Stewart M.. Effect on health-related outcomes of interventions to alter the interaction between patients and practitioners: a systematic review of trials. Ann Fam Med 2004;2(6):595-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dimoska A, Butow PN, Lynch J, Hovey E, Agar M, Beale P, et al. . Implementing patient question-prompt lists into routine cancer care. Patient Educ Couns 2012;86(2):252-8. Epub 2011 Jul 7. [DOI] [PubMed] [Google Scholar]
- 9.Henselmans I, de Haes HC, Smets EM.. Enhancing patient participation in oncology consultations: a best evidence synthesis of patient-targeted interventions. Psychooncology 2013;22(5):961-77. Epub 2012 May 14. [DOI] [PubMed] [Google Scholar]
- 10.Rogers SN, El-Sheikha J, Lowe D.. The development of a Patients Concerns Inventory (PCI) to help reveal patients concerns in the head and neck clinic. Oral Oncol 2009;45(7):555-61. Epub 2008 Nov 22. [DOI] [PubMed] [Google Scholar]
- 11.Greene J, Hibbard JH.. Why does patient activation matter? An examination of the relationships between patient activation and health-related outcomes. J Gen Intern Med 2012;27(5):520-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rask KJ, Ziemer DC, Kohler SA, Hawley JN, Arinde FJ, Barnes CS.. Patient activation is associated with healthy behaviors and ease in managing diabetes in an indigent population. Diabetes Educ 2009;35(4):622-30. Epub 2009 Apr 28. [DOI] [PubMed] [Google Scholar]
- 13.Mosen DM, Schmittdiel J, Hibbard J, Sobel D, Remmers C, Bellows J.. Is patient activation associated with outcomes of care for adults with chronic conditions? J Ambul Care Manage 2007;30(1):21-9. [DOI] [PubMed] [Google Scholar]
- 14.Remmers C, Hibbard J, Mosen DM, Wagenfield M, Hoye RE, Jones C.. Is patient activation associated with future health outcomes and healthcare utilization among patients with diabetes? J Ambul Care Manage 2009;32(4):320-7. [DOI] [PubMed] [Google Scholar]
- 15.Hibbard J. Patient activation and health literacy: what’s the difference? How do each contribute to health outcomes. Stud Health Technol Inform 2017;240:251-62. [PubMed] [Google Scholar]
- 16.Alvarez C, Greene J, Hibbard J, Overton V.. The role of primary care providers in patient activation and engagement in self-management: a cross-sectional analysis. BMC Health Serv Res 2016;16:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lara-Cabrera ML, Salvesen Ø, Nesset MB, De las Cuevas C, Iversen VC, Gråwe RW.. The effect of a brief educational programme added to mental health treatment to improve patient activation: a randomized controlled trial in community mental health centres. Patient Educ Couns 2016;99(5):760-8. Epub 2015 Dec 2. [DOI] [PubMed] [Google Scholar]
- 18.Frosch DL, Rincon D, Ochoa S, Mangione CM.. Activating seniors to improve chronic disease care: results from a pilot intervention study. J Am Geriatr Soc 2010;58(8):1496-503. Epub 2010 Jul 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deen D, Lu WH, Rothstein D, Santana L, Gold MR.. Asking questions: the effect of a brief intervention in community health centers on patient activation. Patient Educ Couns 2011;84(2):257-60. Epub 2010 Aug 25. [DOI] [PubMed] [Google Scholar]
- 20.Deen D, Lu WH, Weintraub MR, Maranda MJ, Elshafey S, Gold MR.. The impact of different modalities for activating patients in a community health center setting. Patient Educ Couns 2012;89(1):178-83. Epub 2012 Jun 9. [DOI] [PubMed] [Google Scholar]
- 21.Wittink MN, Walsh P, Yilmaz S, Mendoza M, Street RL Jr, Chapman BP, et al. . Patient priorities and the doorknob phenomenon in primary care: can technology improve disclosure of patient stressors? Patient Educ Couns 2018;101(2):214-20. Epub 2017 Aug 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shah SJ, Stafford RS.. Patterns of systolic blood pressure control in the United States, 2016. J Gen Intern Med 2018;33(8):1224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boutron I, Altman DG, Moher D, Schulz KF, Ravaud P; CONSORT NPT Group . CONSORT statement for randomized trials of nonpharmacologic treatments: a 2017 update and a CONSORT extension for nonpharmacologic trial abstracts. Ann Intern Med 2017;167(1):40-7. Epub 2017 Jun 20. [DOI] [PubMed] [Google Scholar]
- 24.McHorney CA, Ware JE Jr, Raczek AE.. The MOS 36-Item Short-Form Health Survey (SF-36): II. psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993;31(3):247-63. [DOI] [PubMed] [Google Scholar]
- 25.Morisky DE, Green LW, Levine DM.. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24(1):67-74. [DOI] [PubMed] [Google Scholar]
- 26.Davis TC, Long SW, Jackson RH, Mayeaux EJ, George RB, Murphy PW, et al. . Rapid estimate of adult literacy in medicine: a shortened screening instrument. Fam Med 1993;25(6):391-5. [PubMed] [Google Scholar]
- 27.Anderson LA, Dedrick RF.. Development of the Trust in Physician scale: a measure to assess interpersonal trust in patient-physician relationships. Psychol Rep 1990;67(3 Pt 2):1091-100. [DOI] [PubMed] [Google Scholar]
- 28.Rubin HR, Gandek B, Rogers WH, Kosinski M, McHorney CA, Ware JE Jr.. Patients’ ratings of outpatient visits in different practice settings. Results from the Medical Outcomes Study. JAMA 1993;270(7):835-40. [PubMed] [Google Scholar]
- 29.Lee JK, Grace KA, Taylor AJ.. Effect of a pharmacy care program on medication adherence and persistence, blood pressure, and low-density lipoprotein cholesterol: a randomized controlled trial. JAMA 2006;296(21):2563-71. Epub 2006 Nov 13. [DOI] [PubMed] [Google Scholar]
- 30.Ashworth CD, Williamson P, Montano D.. A scale to measure physician beliefs about psychosocial aspects of patient care. Soc Sci Med 1984;19(11):1235-8. [DOI] [PubMed] [Google Scholar]
- 31.Walach H, Buchheld N, Buttenmüller V, Kleinknecht N, Schmidt S.. Measuring mindfulness—the Freiburg Mindfulness Inventory. Pers Individ Dif 2006;40(8):1543-55. [Google Scholar]
- 32.Hahn SR, Thompson KS, Wills TA, Stern V, Budner NS.. The difficult doctor-patient relationship: somatization, personality and psychopathology. J Clin Epidemiol 1994;47(6):647-57. [DOI] [PubMed] [Google Scholar]
- 33.Roter D. The Roter Interaction Analysis System (RIAS) coding manual. Baltimore, MD: Johns Hopkins University; 1991. [Google Scholar]
- 34.Nieuwlaat R, Wilczynski N, Navarro T, Hobson N, Jeffery R, Keepanasseril A, et al. . Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2014;(11):CD000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gwadry-Sridhar FH, Manias E, Lal L, Salas M, Hughes DA, Ratzki-Leewing A, et al. . Impact of interventions on medication adherence and blood pressure control in patients with essential hypertension: a systematic review by the ISPOR medication adherence and persistence special interest group. Value Health 2013;16(5):863-71. Epub 2013 Jul 4. [DOI] [PubMed] [Google Scholar]
- 36.Cooper LA, Roter DL, Carson KA, Bone LR, Larson SM, Miller ER 3rd, et al. . A randomized trial to improve patient-centered care and hypertension control in underserved primary care patients. J Gen Intern Med 2011;26(11):1297-304. Epub 2011 Jul 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson RA, Huntley A, Hughes RA, Cramer H, Turner KM, Perkins B, et al. . Interventions to support shared decision making for hypertension: a systematic review of controlled studies. Health Expect 2018;21(6):1191-207. Epub 2018 Sep 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallace LS, Rogers ES, Roskos SE, Holiday DB, Weiss BD.. Brief report: screening items to identify patients with limited health literacy skills. J Gen Intern Med 2006;21(8):874-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Powers BJ, Trinh JV, Bosworth HB.. Can this patient read and understand written health information? JAMA 2010;304(1):76-84. [DOI] [PubMed] [Google Scholar]
- 40.Schillinger D, Bindman A, Wang F, Stewart A, Piette J.. Functional health literacy and the quality of physician-patient communication among diabetes patients. Patient Educ Couns 2004;52(3):315-23. [DOI] [PubMed] [Google Scholar]
- 41.Safeer RS, Keenan J.. Health literacy: the gap between physicians and patients. Am Fam Physician 2005;72(3):463-8. [PubMed] [Google Scholar]
- 42.Aboumatar HJ, Carson KA, Beach MC, Roter DL, Cooper LA.. The impact of health literacy on desire for participation in healthcare, medical visit communication, and patient reported outcomes among patients with hypertension. J Gen Intern Med 2013;28(11):1469-76. Epub 2013 May 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mann BK, De Ycaza Singh SA, Dabas R, Davoudi S, Osvath J.. Evaluation of effects of health literacy, numeracy skills, and English proficiency on health outcomes in the population of people with diabetes in East Harlem. Clin Diabetes 2019;37(2):172-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruben BD. Communication theory and health communication practice: the more things change, the more they stay the same. Health Commun 2016;31(1):1-11. Epub 2014 Nov 3. [DOI] [PubMed] [Google Scholar]



