Abstract
Neisseria gonorrhoeae is a gram-negative pathogen that is capable of satisfying its iron requirement with human iron-binding proteins such as transferrin and lactoferrin. Transferrin-iron utilization involves specific binding of human transferrin at the cell surface to what is believed to be a complex of two iron-regulated, transferrin-binding proteins, TbpA and TbpB. The genes encoding these proteins have been cloned and sequenced from a number of pathogenic, gram-negative bacteria. In the current study, we sequenced four additional tbpA genes from other N. gonorrhoeae strains to begin to assess the sequence diversity among gonococci. We compared these sequences to those from other pathogenic bacteria to identify conserved regions that might be important for the structure and function of these receptors. We generated polyclonal mouse sera against synthetic peptides deduced from the TbpA sequence from gonococcal strain FA19. Most of these synthetic peptides were predicted to correspond to surface-exposed regions of TbpA. We found that, while most reacted with denatured TbpA in Western blots, only one antipeptide serum reacted with native TbpA in the context of intact gonococci, consistent with surface exposure of the peptide to which this serum was raised. In addition, we evaluated a panel of gonococcal strains for antigenic diversity using these antipeptide sera.
Virtually all microorganisms require iron as a cofactor for various enzymatic processes (8). Because free iron concentrations are low in oxygenated environments at neutral pH (44) and in plant and animal hosts (55), microbes have evolved intricate and elegant mechanisms by which they scavenge iron from their surroundings. The most common method involves the synthesis of siderophores, which bind iron with high affinity and specificity (44). The process of iron utilization via a siderophore intermediate requires specific binding of the ferric siderophore to a surface-exposed receptor (for reviews, see references 7 and 43). Energy is provided to these TonB-dependent receptors in the form of the proton motive force through the function of an energy-transducing complex of proteins, TonB, ExbB, and ExbD (28, 30, 48).
As an alternative to siderophore synthesis, some bacteria have evolved the capacity to utilize host-derived iron sources by a direct, receptor-mediated mechanism, which is uniquely adapted to the organism's preferred host (for reviews, see references 18 and 24). For example, Actinobacillus pleuropneumoniae, a porcine pathogen, specifically utilizes iron from porcine transferrin (21), whereas Neisseria gonorrhoeae, a strict human pathogen, binds and utilizes the iron specifically from human transferrin (5, 33). N. gonorrhoeae and its close relative Neisseria meningitidis also utilize iron bound to human lactoferrin, hemoglobin, and heme (39). Binding of transferrin, lactoferrin, and hemoglobin to the pathogenic members of the family Neisseriaceae involves two characterized proteins, one of which bears a resemblance to TonB-dependent, siderophore receptors and the other of which is lipidated (1, 3, 4, 6, 15, 34, 35, 47, 54). In the transferrin-iron internalization system, these two proteins have been designated TbpA and TbpB, respectively. A gonococcal mutant lacking both TbpA and TbpB was unable to establish an infection or elicit signs or symptoms of urethritis in a human challenge model of gonococcal infection (16). Although this result strongly implicates the function of the gonococcal transferrin receptor in the initial stages of gonococcal disease, it should be noted that both the tbp mutant and the wild-type strain (FA1090) used in this study were incapable of lactoferrin-iron utilization. Anderson and Sparling have recently created a lactoferrin-positive, transferrin-negative derivative of FA1090 and are currently testing this mutant in the human challenge model to elucidate the role of the lactoferrin receptor in the initiation of urethritis in human males (unpublished data).
Sequence analysis and functional studies of the transferrin receptors (for reviews, see references 14 and 18) have led to the hypothesis that TbpA and TbpB constitute the functional transferrin receptor, which serves as the first point of contact between ferrated transferrin and the bacterium. We have suggested that TbpB increases the efficiency of iron uptake from transferrin by virtue of its specificity for the ferrated ligand (1, 17). Because TbpA is similar to other TonB-dependent receptors, we have proposed that it forms the pore through which transferrin-bound iron enters the periplasm (15, 18). Recent crystal structures of two other TonB-dependent receptors (10, 37) described two functional domains within these outer membrane receptors. The first domain represents the amino-terminal third of the protein and includes the so-called TonB box (53), which has been implicated in a physical association between TonB and outer membrane receptors (2, 27, 32, 52). In addition to the TonB box, this amino-terminal domain contains two other regions that are conserved among TonB-dependent receptors (15). The crystal structures of FepA and FhuA locate this amino-terminal domain not within the plane of the membrane, as previous models suggested (31, 41), but within the lumen of the β-barrel formed by the remaining two-thirds of the protein. This “plug” (37) or internal “hatch” (10) is postulated to limit access into the periplasm and opens only when it simultaneously senses the presence of ligand and cytoplasmic membrane-derived energy.
The neisserial transferrin receptor is subject to intense scrutiny not only to ascertain its mode of operation but also because both components have become viable vaccine candidates for immunoprophylaxis against both meningococcal and gonococcal disease. The transferrin receptors expressed by N. meningitidis strains have been grouped into two distinct classes based on molecular weight and sequence heterogeneity (49). Most strains fall into the high-molecular-weight class, while approximately a quarter of the strains tested expressed a version of TbpB that was significantly smaller. Among a panel of 50 gonococcal strains, all express a TbpB that is closely related to the high-molecular-weight class of meningococcal TbpBs; there is no evidence of a low-molecular-weight species of gonococcal TbpB (13). The purpose of the current study was to begin to assess the antigenic and sequence variability of TbpA species among gonococcal strains, which is a necessary step in the evaluation of TbpA as a potential vaccine component. We sequenced four new gonococcal tbpA genes and compared them to our previously published gonococcal tbpA sequence. We also compared the gonococcal TbpAs with those from N. meningitidis, Haemophilus influenzae, A. pleuropneumoniae, Moraxella catarrhalis, and Pasteurella haemolytica. Sequence variability among gonococcal TbpAs is likely to be highly represented in surface-exposed regions, while sequence conservation among diverse TbpAs might indicate regions that are structurally or functionally constrained. We generated a set of antipeptide sera directed at putatively surface-exposed regions of gonococcal TbpA, which we used to assess the antigenic diversity within a larger group of gonococcal strains. We found that, while most of the antipeptide sera reacted with denatured TbpA, only one serum recognized membrane-bound TbpA, suggesting that the epitope to which this serum was raised was surface exposed in the gonococcus.
MATERIALS AND METHODS
Strains and growth conditions.
The gonococcal strains used in this study are described in Table 1. Gonococci were maintained on Bacto GC medium base (Difco) to which Kellogg's supplement I (29) and 12 μM Fe(NO3)3 were added. Plates were incubated at 35°C in a 5% CO2 atmosphere. To induce iron stress, gonococci were grown in CDM medium (56), to which either no iron or 30% iron-saturated, human transferrin (2.5 μM) (Sigma Aldrich Chemicals, St. Louis, Mo.) was added. In other experiments, iron stress was induced by growth of gonococci on GC medium base plates, to which 10 μM deferoxamine mesylate (Sigma Aldrich Chemicals) was added in place of the Fe(NO3)3 supplement.
TABLE 1.
Strains used in this study
| Organism and strain name | Genotype | Reference or source |
|---|---|---|
| N. gonorrhoeae | ||
| FA19 | Wild type | 40 |
| FA1090 | Wild type | 11 |
| UU1008 | Wild type | 13 |
| FA6747 | tbpA::mTn3Cm | 15 |
| FA6815 | tbpB::Ω | 1 |
| FA6819 | ΔtbpB | 1 |
| Pgh3-2 | Wild type | 13 |
| FA6642 | Wild type | 13 |
| 4102 | Wild type | R. Brunham |
| 4121 | Wild type | R. Brunham |
| 4125 | Wild type | R. Brunham |
| 4141 | Wild type | R. Brunham |
| 4146 | Wild type | R. Brunham |
| 4178 | Wild type | R. Brunham |
| 4196 | Wild type | R. Brunham |
| 4134 | Wild type | R. Brunham |
| N. meningitidis FAM20 | Wild type | 20 |
Molecular biological procedures.
Primers designed to PCR amplify all or portions of tbpA genes from FA1090, UU1008, Pgh3-2, and 4102 chromosomal DNA preparations are as follows. Primers JRW2 (5′-CCAAGGCGAGCGCACCGATG-3′) and TfBP1 (5′-GAGCCCGCCAATGCGCCGCT-3′) were used to amplify the 5′ portion of tbpA, while TfBP27 (5′-CGGTGTATCGGGAAGGATGG-3′) and TfBP34 (5′-GTCGAAATCAGCAAAGGC-3′) were used to amplify the 3′ portion of the gene. In other experiments, we used Hind (5′-CGAAGAGTTGGGCGGATGGTT-3′) and oVCU-7 (5′-CTCGAGGCTCTAGAAACCCCAACGCAG-3′) primers to amplify tbpA in its entirety. PCR products were purified by agarose gel electrophoresis using Magic PCR columns (Promega, Madison, Wis.) and cloned into the pCRII vector (InVitrogen, San Diego, Calif.). Pools of 6 to 10 clones for each PCR product were used as sequencing templates. In some cases, PCR products were not cloned but pooled and sequenced directly. Manual sequencing was performed using Sequenase (United States Biochemical Corporation, Cleveland, Ohio), and automated sequencing was performed at the University of North Carolina at Chapel Hill Automated DNA Sequencing Facility on a Model 373A DNA Sequencer (Applied Biosystems, Foster City, Calif.) using a Taq DyeDeoxy Terminator Cycle sequencing kit (Applied Biosystems). Both strands of the PCR products were sequenced in both directions using specific primers designed from the FA19 tbpA sequence or using vector-specific primers. Sequence analysis was performed using the University of Wisconsin GCG software package (Madison, Wis.) (19). Alignments of the TbpA sequences were constructed from sequences listed in Table 2 using the programs PILEUP and PRETTY. The relatedness tree was constructed using the alignment created by PILEUP and the program GROWTREE.
TABLE 2.
tbpA sequences aligned in this study
| Strain | Organism | Reference or source | Accession no. |
|---|---|---|---|
| FA19 | N. gonorrhoeae | 15 | M96731 |
| FA1090 | N. gonorrhoeae | This study | AF124339 |
| UU1008 | N. gonorrhoeae | This study | AF124338 |
| Pgh3-2 | N. gonorrhoeae | This study | AF241227 |
| 4102 | N. gonorrhoeae | This study | AF240638 |
| M982 | N. meningitidis | 34 | Z15130 |
| B16B6 | N. meningitidis | 34 | Z15129 |
| B:15, P1.16 | N. meningitidis | Unpublished | X75166 |
| MinnA | H. influenzae | 38 | U15052 |
| Eagan | H. influenzae | 38 | U15051 |
| PAK12085 | H. influenzae | 38 | U15053 |
| DL63 | H. influenzae type b | 23 | U10882 |
| h49 | A. pleuropneumoniae | 22 | U16017 |
| h171 | A. pleuropneumoniae | 22 | U16019 |
| Q8 | M. catarrhalis | 42 | AF039312 |
| 4223 | M. catarrhalis | 42 | AF039315 |
| h196 | P. haemolytica | 46 | U73302 |
Antipeptide sera.
Eight peptides of 11 to 14 residues in length were synthesized with the sequences highlighted in green in Fig. 1. These peptides were predicted to be hydrophilic, antigenic, and surface exposed by the program PLOTSTRUCTURE (Genetics Computer Group, Inc., Madison, Wis.). Peptides were conjugated to Pierce's SuperCarrier according to the manufacturer's recommendations (Pierce, Rockford, Ill.). For each peptide conjugate, five mice were immunized subcutaneously in Freund's complete adjuvant and two subsequent times in Freund's incomplete adjuvant. Mice were exsanguinated 12 weeks after the first immunization. Sera from individual mice were maintained separately and screened individually.
FIG. 1.
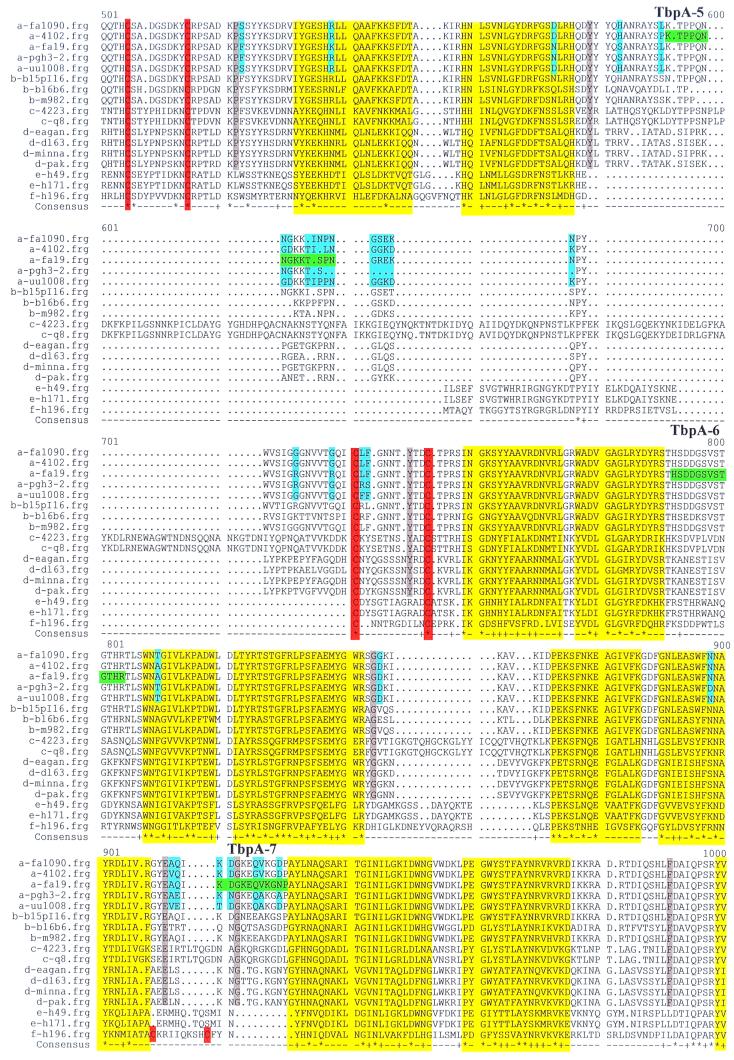
Multiple sequence alignment of TbpA proteins. The letter preceding the sequence name to the left of each line indicates the species from which the TbpA sequence was derived: a, N. gonorrhoeae; b, N. meningitidis; c, M. catarrhalis; d, H. influenzae; e, A. pleuropneumoniae; f, P. haemolytica. Boxed single-letter amino acid designations indicate the mature amino terminus of TbpA, if known. Sequences highlighted in green represent the gonococcal strain FA19 TbpA sequences that were synthesized as peptides to generate antipeptide sera. These peptides are numbered and labeled TbpA-1 through TbpA-8, consecutively, from amino terminus to carboxy terminus. Yellow highlighting represents regions homologous to known transmembrane β-strands in E. coli FepA. Blue shading signifies diversity among TbpAs from five gonococcal strains. Gray shading indicates residues that are unique to TbpAs expressed by the human pathogens. Cysteine residues are highlighted in orange. The black vertical arrow represents the carboxy-terminal end of the so-called plug region, defined by homology with E. coli FepA. Dots indicate positions in which gaps were introduced in the alignment; squiggles indicate positions for which no sequence was available. In the consensus line, the asterisks represent complete identity among all 17 aligned sequences; plus signs indicate conservative replacements at that position in the 17-sequence alignment.
Characterization of antipeptide sera.
Sera obtained following immunizations were screened by Western blotting against whole-cell lysates of gonococcal strains FA19 and FA6815 as previously described (13). Those sera that were negative by Western blotting for reactivity against wild-type TbpA were screened by enzyme-linked immunosorbent assay (ELISA) against homologous peptide. Dilutions of purified peptide were applied to ReactiBind plates (Pierce) according to the manufacturer's recommendations. Dilutions of preimmune sera and hyperimmune sera were screened for reactivity with each peptide by standard procedures. All sera were screened for reactivity against whole-cell dot blots of gonococcal strains FA19 and FA6815 essentially as previously described for solid-phase, transferrin binding assays (17). Bound polyclonal mouse antibodies were detected by addition of a secondary goat anti-mouse immunoglobulin G conjugated to alkaline phosphatase (Sigma Aldrich) followed by development with nitroblue tetrazolium and BCIP (5-bromo-4-chloro-3-indolylphosphate). Immunofluorescence microscopy was performed to verify that antiserum against peptide TbpA-5 bound to surface-exposed epitopes of TbpA on intact gonococci. Iron-stressed, whole cells from gonococcal strains FA19 and FA6747 were mixed with dilutions of preimmune and hyperimmune sera along with immunoglobulin-free bovine serum albumin (Sigma Aldrich) as a nonspecific blocker. Unbound antibody was removed by centrifugation, and then cells with bound antibody were applied to glass microscope slides and immersed in 100% methanol for fixation. Subsequently, slides were probed with a secondary goat anti-mouse antibody conjugated to fluorescein isothiocyanate (Sigma Aldrich) and cells were counterstained with Eriochrome Black (Integrated Diagnostics, Baltimore, Md.). Samples were visualized in an Olympus BHA microscope equipped with a model BH2RFL reflected fluorescence attachment and a model PM-10AD photomicrographic system (Olympus Corp., Melville, N.Y.). Negative controls in these experiments included probing FA6747 (TbpA−) with antiserum against peptide TbpA-5 and probing FA19 with preimmune sera. The positive control was FA19 probed with an antiserum raised against gonococcal PorA.
Nucleotide sequence accession numbers.
The DNA sequences determined in this study were reported to the GenBank database under the accession numbers listed in Table 2.
RESULTS
DNA sequence analysis of tbpA from four gonococcal strains.
The sequences of four gonococcal tbpA genes were determined to begin to assess the extent of interstrain genetic variability of this potential vaccine antigen. The predicted protein sequence of TbpA from gonococcal strain UU1008 was 95.1% identical to the published TbpA sequence (15) from strain FA19, while the TbpA sequence from FA1090 was 96.2% identical to the FA19 TbpA sequence (Table 3). The FA1090 TbpA sequence determined in this study was identical to that identified in the nearly complete, gonococcal genome sequencing project (B. A. Roe, S. P. Lin, L. Song, X. Yuan, S. Clifton, T. Ducey, L. Lewis, and D. W. Dyer, Gonococcal Genome Sequencing Project [http://www.genome.ou.edu/gono.html]). TbpAs from gonococcal strains 4102 and Pgh3-2 were likewise very similar to that of FA19, sharing 96.1 and 97.4% identity, respectively. All of the gonococcal TbpA sequences were closely related to the TbpA sequences from meningococcal strains M982 and B:15, P1.16; pairwise identity scores for these two meningococcal proteins compared with the gonococcal TbpAs ranged from 93.5 to 95.1% (Table 3). In contrast, the TbpA sequence from meningococcal strain B16B6 exhibited only 75.9 to 77% identity compared to the other neisserial TbpAs (Table 3). Thus, all of the gonococcal TbpA sequences determined to date closely resemble the TbpA sequences from meningococcal strains M982 and B:15, P1.16. Figure 1 depicts a multiple sequence alignment comparing the neisserial TbpA proteins determined in this study with those available in GenBank (Table 2). The residues highlighted in blue depict those that are variant among the gonococcal TbpAs. These residues appear in clusters highlighting four hypervariable domains, which correspond to residues 269 to 332, 412 to 447, 583 to 743, and 912 to 929 in the alignment in Fig. 1.
TABLE 3.
Pairwise alignment scores
| Strain | Value for groupa:
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a
|
b
|
c
|
d
|
e
|
f | ||||||||||||
| FA19 | FA1090 | UU1008 | 4102 | Pgh3-2 | M982 | B:15, P1.16 | B16B6 | Q8 | 4223 | DL63 | PAK | MinnA | Eagan | h49 | h171 | h196 | |
| FA19 | 97.5 | 96.7 | 96.5 | 97.9 | 96.2 | 95.7 | 85.1 | 56.2 | 55.9 | 60.6 | 60.2 | 60.0 | 60.0 | 52.9 | 53.3 | 52.5 | |
| FA1090 | 96.2 | 98.8 | 97.6 | 96.0 | 96.6 | 94.8 | 80.0 | 57.1 | 56.3 | 60.6 | 60.8 | 60.8 | 60.8 | 52.8 | 53.4 | 52.5 | |
| UU1008 | 95.1 | 97.3 | 98.5 | 96.0 | 96.9 | 94.4 | 85.8 | 56.6 | 55.3 | 60.6 | 60.7 | 60.1 | 60.1 | 53.3 | 53.8 | 52.3 | |
| 4102 | 96.1 | 97.3 | 98.1 | 96.0 | 94.9 | 94.2 | 80.2 | 56.0 | 55.7 | 61.0 | 60.8 | 60.6 | 60.6 | 52.6 | 53.2 | 52.3 | |
| Pgh3-2 | 97.4 | 95.5 | 95.5 | 95.4 | 95.7 | 95.7 | 80.2 | 56.0 | 55.9 | 60.6 | 60.8 | 60.0 | 60.0 | 53.3 | 53.6 | 51.8 | |
| M982 | 94.5 | 94.3 | 94.2 | 94.2 | 94.2 | 96.9 | 85.8 | ||||||||||
| B:15, P1.16 | 95.1 | 94.3 | 93.5 | 93.6 | 95.1 | 96.7 | 80.5 | ||||||||||
| B16B6 | 76.0 | 76.3 | 75.9 | 76.2 | 76.2 | 77.0 | 76.9 | ||||||||||
| Q8 | 49.2 | 49.9 | 49.2 | 48.9 | 48.9 | ||||||||||||
| 4223 | 48.7 | 49.2 | 48.1 | 48.6 | 48.8 | ||||||||||||
| DL63 | 51.7 | 52.0 | 52.0 | 52.4 | 52.6 | ||||||||||||
| PAK | 51.6 | 52.1 | 52.0 | 52.3 | 52.6 | ||||||||||||
| MinnA | 51.6 | 52.2 | 51.9 | 52.1 | 52.0 | ||||||||||||
| Eagan | 51.6 | 52.2 | 51.9 | 52.1 | 52.0 | ||||||||||||
| h49 | 44.1 | 44.1 | 44.8 | 44.1 | 44.6 | ||||||||||||
| h171 | 44.7 | 44.4 | 45.0 | 44.4 | 45.3 | ||||||||||||
| h196 | 43.7 | 43.2 | 43.4 | 43.2 | 43.0 | ||||||||||||
Strains are grouped by genus and species using the following abbreviations: a, N. gonorrhoeae; b, N. meningitidis; c, M. catarrhalis; d, H. influenzae; e, A. pleuropneumoniae; f, P. haemolytica. Percent similarity scores are shown in boldface; percent identity scores are shown in lightface.
Comparison between gonococcal TbpAs and TbpAs from other genera.
We also compared the gonococcal TbpA sequences determined in this study with those available in GenBank for H. influenzae, A. pleuropneumoniae, M. catarrhalis, and P. haemolytica (Table 2). Identical residues in all 17 aligned sequences (Fig. 1) are indicated by an asterisk in the consensus line, while conserved residues are indicated by a plus sign. Due to their maintenance across genera, these conserved residues are likely to be constrained by the common function, topology, or assembly of these transferrin receptors. Most of the conservation among these proteins was confined to narrow domains that are virtually superimposable with hypothetical transmembrane β-strands (highlighted in yellow in Fig. 1). These putative β-strands were identified by constructing a pairwise alignment between gonococcal TbpA and the Escherichia coli ferric enterobactin receptor, FepA, for which the crystal structure has been determined previously (10). Two other features of TbpA were conserved across genus lines. These included six paired cysteine residues (orange in Fig. 1), which are likely to be located in surface-exposed loops (10, 37, 51), and a 209-residue, amino-terminal domain, which is homologous to the so-called plug or hatch identified in the E. coli TonB-dependent receptors (between the amino terminus and the vertical arrow in Fig. 1). This amino-terminal region not only is well conserved among the 17 sequences aligned in Fig. 1 but also contained three of the seven characterized domains that are shared by all TonB-dependent proteins (15).
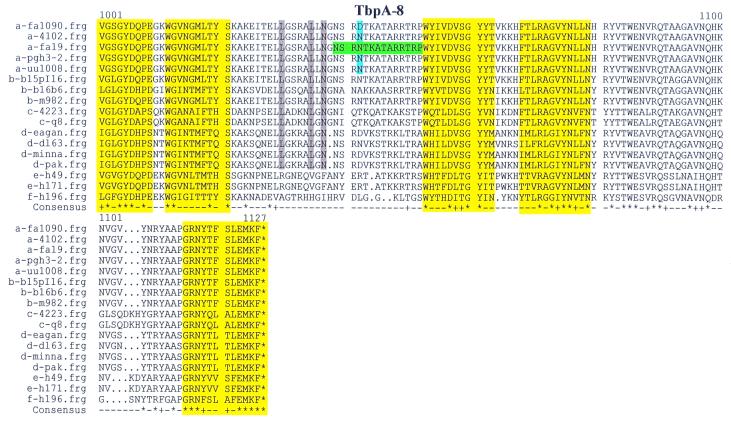
Pairwise identity scores between neisserial TbpAs and TbpAs expressed by other genera ranged from 43% in P. haemolytica to 52.6% in H. influenzae (Table 3). Using these pairwise identity scores, we generated the relatedness tree of TbpA proteins shown in Fig. 2. This tree reflects the relationship between TbpAs expressed by different genera and species; it does not necessarily reflect the overall genetic relatedness between these organisms. Of particular note was the early branching between the TbpAs expressed by the animal pathogens (A. pleuropneumoniae and P. haemolytica) and by the human pathogens. TbpAs expressed by M. catarrhalis strains branched off next, followed by those expressed by H. influenzae strains. The phylogram in Fig. 2 also emphasizes the genetic distance between the TbpA from meningococcal strain B16B6 and the rest of the neisserial TbpAs.
FIG. 2.
Relatedness tree of TbpA proteins. The letter preceding the sequence name indicates the species from which the TbpA sequence was derived: a, N. gonorrhoeae; b, N. meningitidis; c, M. catarrhalis; d, H. influenzae; e, A. pleuropneumoniae; f, P. haemolytica. The scale bar at the bottom represents 10 substitutions per 100 residues.
Reactivities of antipeptide sera.
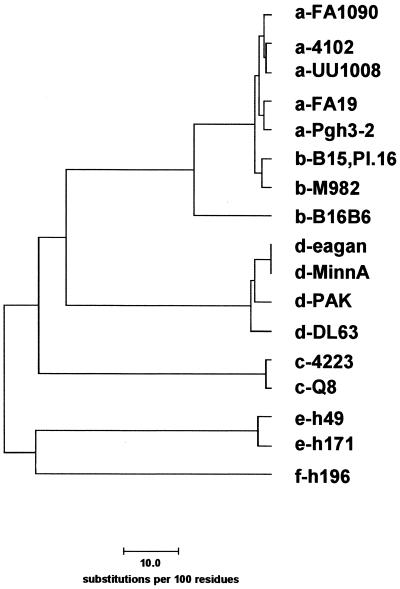
We initially generated antiserum against eight peptides to test our hypothetical topology model of gonococcal TbpA (I. C. Boulton, M. K. Yost, J. E. Anderson, and C. N. Cornelissen, submitted for publication). Peptides were synthesized with the sequences shown in Fig. 1 and were chosen because they were hydrophilic and predicted to be surface exposed and antigenic, with the exception of peptides TbpA-1 and TbpA-2, which were chosen as negative controls in surface exposure experiments. Antipeptide sera were raised in mice and screened by Western blotting against iron-stressed, whole-cell lysates prepared from the wild-type strain and from an isogenic tbpA mutant. Sera elicited against peptides TbpA-2, TbpA-3, TbpA-5, and TbpA-6 recognized TbpA specifically in this assay (Fig. 3). Those sera that were negative by Western blotting were screened by ELISA to determine whether the peptide elicited an immune response. All sera reacted against homologous peptide in an ELISA format, although the anti-TbpA-1 serum did so weakly and thus was not further characterized. These results indicated that antibodies elicited against peptides TbpA-1, TbpA-4, TbpA-7, and TbpA-8, while not reactive against full-length TbpA, were capable of recognizing the immunizing peptide.
FIG. 3.
Antigenic heterogeneity among gonococcal TbpA proteins. The Western blot contains iron-stressed, whole-cell lysates from the following gonococcal strains: FA19, FA1090, UU1008, Pgh3-2, and FA6642 (lanes 1 to 5, respectively). Lanes 6 to 13 contain lysates from the following gonococcal strains obtained from R. Brunham: 4102, 4121, 4125, 4141, 4146, 4178, 4196, and 4134, respectively. Lane 14 contains an iron-stressed, whole-cell lysate from meningococcal strain FAM20. Blots were probed with antisera raised against TbpA-specific peptides as indicated at right. Blots were scanned with an HP ScanJet 4c and annotated with Adobe Photoshop 4.0 software.
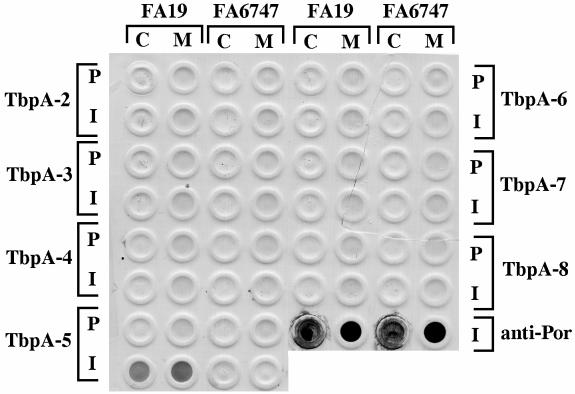
All antipeptide sera, with the exception of that raised against TbpA-1, were screened by whole-cell dot blotting for reactivity against whole, iron-stressed gonococci (Fig. 4). The only serum that specifically reacted in this assay format was raised against peptide TbpA-5 (Fig. 4). Due to the tendency of gonococci to lyse, which could result in apparent surface exposure in dot blots, we confirmed the dot blot result using immunofluorescence microscopy, in which the integrity of the reactive organisms could be assessed by counterstain. As in the dot blot experiment, antiserum raised against TbpA-5 was reactive by this technique against whole, iron-stressed TbpA-expressing gonococci but not against an isogenic tbpA mutant (data not shown). These results indicated that the loop containing peptide TbpA-5 was surface exposed. Counter to our original hypothesis, antiserum raised against the other peptides did not react with whole gonococci (Fig. 4), even though peptides TbpA-3, TbpA-4, TbpA-6, TbpA-7, and TbpA-8 represented epitopes that were predicted to be surface exposed. These sera were likewise not reactive against TbpA expressed by FA6819 in the absence of TbpB (data not shown); thus, the epitopes represented by these peptides were not simply masked by the presence of TbpB. However, it is possible that lipooligosaccharide or some other surface structure could obstruct access by antibody to TbpA even if the peptide-containing epitopes are on the surface.
FIG. 4.
Binding of antipeptide sera to whole, iron-stressed gonococcal cells. All strains tested in this analysis were derivatives of gonococcal strain FA19. Columns of spots contain whole, iron-stressed cells (C) or total membrane preparations (M) of strain FA19 or FA6747, as indicated at top. Rows of spots were probed with preimmune mouse serum (P) or hyperimmune serum (I) from mice immunized with peptides TbpA-2 through TbpA-8, as indicated on either side of the blot. Spots in the lower right corner of the blot (labeled anti-Por) were probed with a PorA monoclonal antibody as a positive control. The blot was scanned with an HP ScanJet 4c and annotated with Adobe Photoshop 4.0 software.
Using antipeptide sera to assess antigenic variability among gonococci.
A panel of 13 gonococcal strains and one meningococcal strain (representative of the low-molecular-weight class) was screened for reactivity with the four Western blot-positive antipeptide sera. As noted previously (13), molecular mass heterogeneity of TbpA among gonococci is limited to between 100 and 103 kDa (Fig. 3). All 13 gonococcal strains expressed a TbpA that reacted with two of the antipeptide sera, those elicited against TbpA-2 and TbpA-6 (Fig. 3). This result suggested that the epitopes represented by these two peptides were well conserved among the gonococcal strains tested. Sera against peptide TbpA-3 reacted with TbpA from 2 out of 13 gonococcal strains, while sera against TbpA-5 reacted with TbpA expressed by 6 out of 13 strains tested. None of the antipeptide sera reacted with the low-molecular-weight TbpA expressed by meningococcal strain FAM20 (Fig. 3, lane 14). These observations are consistent with the sequence heterogeneity depicted in Fig. 1 in that the regions containing peptides TbpA-3 and TbpA-5 were more variable than those containing peptides TbpA-2 and TbpA-6.
DISCUSSION
Since the transferrin-binding proteins have been touted as potential components of a vaccine (36, 50), it is important to assess their interstrain antigenic and sequence variation in addition to defining topology and surface exposure. Gonococcal TbpBs (13) are more diverse than gonococcal TbpAs; however, they do not share the great diversity found among meningococcal TbpBs (49). Meningococcal TbpBs fall into two distinct classes based on molecular mass and sequence characteristics. The low-molecular-weight family, consisting of approximately 26% of tested strains represented by strain B16B6, expresses a TbpB protein of 68 kDa, while the high-molecular-weight family, represented by strain M982, expresses a TbpB of 85 kDa (49). All tested gonococcal strains express a TbpB with M982-like characteristics; there is no evidence of a B16B6-type TbpB (13). In the current study, we assessed the sequence variability of gonococcal TbpAs and found that they are very similar to each other and equally similar to TbpAs of meningococcal strains in the M982 family. In contrast, gonococcal TbpAs share only 75% identity with the TbpA expressed by meningococcal strain B16B6. This observation suggests that both TbpB and TbpA proteins expressed by B16B6-type meningococcal strains are distinct from those expressed by the M982 family. That is, both transferrin-binding proteins can be categorized into either high- or low-molecular-weight classes; meningococcal strain B16B6, as the representative of the low-molecular-weight class for which the sequences of TbpA and TbpB are known, expresses a 68-kDa TbpB protein and a distinct, although not markedly smaller, TbpA protein.
Sequence diversity among gonococcal TbpAs was largely confined to four small regions that could be defined as hypervariable domains, by analogy with other neisserial antigens (12, 25, 26). Two of the four hypervariable domains were in the vicinity of paired cysteine residues in gonococcal TbpA, again reminiscent of another gonococcal antigen, pilin. In gonococcal pilin, a characterized hypervariable region, also known as minicassette 2, is flanked by conserved cysteine residues (25). In addition to sequence variation, regions interspersed between putative transmembrane domains also contain all of the length diversity detected by aligning 17 TbpA sequences from a wide array of bacterial pathogens. The presence of both length and sequence variation in these areas is consistent with their topological assignment as either internal or external putative loops. Two of the antipeptide sera generated in this study detected only a subset of gonococcal TbpAs. Consistent with this observation, the sequences against which these sera were raised lie within the above-defined hypervariable regions. This suggests that TbpA-specific peptide antisera could be used for serotyping, in a manner similar to those utilized currently in porin-specific serotyping schemes.
Stretches of sequence conservation among TbpAs are likely to represent domains of the protein that are constrained by common function or localization. In our alignment of 17 TbpAs, the majority of sequence identity was found within or slightly outside of regions that are homologous to characterized transmembrane β-strands in E. coli FepA (10). Strikingly, none of the putative TbpA transmembrane domains contains any length diversity, suggesting that maintenance of both length and sequence identity is required for the structure and/or function of these receptors. Other pockets of conservation include the putative plug region, which is not only conserved among TbpAs but also well conserved among all members of the TonB-dependent receptor family (10). Six cysteine residues were also conserved among all TbpAs analyzed, although the distance between the first and second cysteine residues varied. Two pairs of cysteine residues were flanked by hypervariable residues; in contrast, the third set of cysteines was flanked by residues that were conserved among the gonococci. Although all of the cysteine residues are likely to reside in extracellular loops (10, 37, 51), it is unclear why some are within apparent hypervariable regions while others are flanked by conserved residues. Of note is the presence of a unique pair of cysteine residues in P. haemolytica TbpA that coincides with one of the four hypervariable regions. The presence of this pair of cysteine residues strengthens our hypothesis that this hypervariable region (containing peptide TbpA-7) is located in an extracellular loop.
By focusing on the putative loop regions interspersed between the transmembrane strands, we can identify several regions that are distinct between the TbpAs expressed by the human pathogens versus those expressed by the animal pathogens. These disparities might highlight epitopes that influence the exquisite species specificity of these receptors. Thus, residues conserved among the TbpAs of human pathogens and divergent in the TbpAs of the animal pathogens might be critical for the specific binding of human transferrin. Residues highlighted in gray in the alignment in Fig. 1 are unique to the TbpAs that recognize human transferrin; therefore, these residues might be critical for this interaction. Alternatively, these residues could be conserved among the human pathogens because these pathogens are more closely related and thus the proteins have not had the evolutionary time to diverge significantly. Another distinction between the TbpAs expressed by the human and animal pathogens is found in both the length and the sequence of the putative loop located between residues 577 and 758 in the alignment shown in Fig. 1. Excluding the pair of cysteine residues, this entire putative loop is unique to TbpAs expressed by the human pathogens. Interestingly, the TbpA sequence of M. catarrhalis features four insertions within this region, adding to the length diversity of this hypothetically surface-exposed loop. The plasticity of this region, which also coincides with the ligand-binding domain of E. coli FepA (45), is consistent with surface exposure. Camouflaging conserved epitopes with sequence and length diversity might be important for evasion of an immune response raised against a receptor that is constrained by the necessity to bind one ligand. The role, if any, that these differences between the TbpAs expressed by human and animal pathogens play in receptor specificity can be tested using current molecular techniques including site-specific mutagenesis and binding-domain swapping.
We initially generated peptide-specific antisera to probe the topology of membrane-associated TbpA. The peptides synthesized were hydrophilic and predicted to be surface exposed and antigenic. While the peptides generated an immune response, seven out of eight antipeptide sera did not recognize native TbpA as presented in the context of the gonococcal outer membrane. The antibodies elicited against peptide TbpA-5 represented the single exception to the poor cross-reactivity between peptide and native protein epitopes. Antibodies generated against peptide TbpA-5 recognized both denatured and native TbpA, supporting the hypothesis that the domain that contains this peptide is surface accessible on intact gonococci. The reason for the relative lack of cross-reactivity between the antipeptide sera and native TbpA remains unclear. The peptides synthesized in this study might have been too short to adequately mimic the conformation of cell-surface-exposed TbpA. Alternatively, cyclizing the peptide immunogens could have increased the frequency with which we obtained sera reactive against conformational epitopes. While this approach was not particularly useful for direct topological mapping, the antipeptide sera generated in this study were helpful in identification of antigenically diverse domains of TbpA. By analogy with other gonococcal antigens, these hypervariable domains are likely to be exposed to the immune system during the course of infection (9). In addition, these peptide-specific antisera could be useful serotyping reagents since they highlight antigenic differences among gonococcal strains.
In this study, we compared the sequence and antigenic characteristics of TbpA from a panel of gonococcal strains. Antigenic and sequence diversity among gonococcal isolates clustered in hypervariable regions, which are postulated to be surface exposed in the gonococcus. By comparing the TbpA proteins expressed by a wide variety of bacterial pathogens, we identified several conserved regions that are likely to be confined by the common function and assembly of these receptors. Many of the conserved domains overlap with areas that are homologous to transmembrane β-strands in FepA. Other conserved features include the amino-terminal 209 residues, a sequence which is analogous to the plug region identified in two crystallized TonB-dependent receptors (10, 37). Buchanan et al. noted that the structure of this amino-terminal region was unique among the TonB-dependent receptors and that the coincidence of conservation and localization suggested that these domains were crucial for the common function of these receptors (10). Another conserved feature identified in the current study of TbpAs was a series of paired cysteine residues, which might be important for the topological stability of several surface-exposed loops (10, 37, 51). Our ongoing studies are aimed at dissection of the surface topology of the transferrin receptor and at delineation of surface-exposed, antigenic domains of TbpA that might elicit a cross-reactive, protective immune response.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grants AI26837 and AI31496 to P.F.S. and AI39523 to C.N.C., both from the National Institute of Allergy and Infectious Diseases.
We thank Deb Palazzi and Jennifer Watson for excellent technical assistance. The TbpA-specific peptides were synthesized and conjugated at ImClone Systems, Inc., New York, N.Y. Immunizations were carried out at Lederle-Praxis Biologicals in Rochester, N.Y. We also appreciate assistance from Kate Nowell, Guy Cabral, Marcia Hobbs, and Ann Jerse in conducting immunofluorescence microscopy experiments.
REFERENCES
- 1.Anderson J E, Sparling P F, Cornelissen C N. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J Bacteriol. 1994;176:3162–3170. doi: 10.1128/jb.176.11.3162-3170.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bell P E, Nau C D, Brown J T, Konisky J, Kadner R J. Genetic suppression demonstrates interaction of TonB protein with outer membrane transport proteins in Escherichia coli. J Bacteriol. 1990;172:3826–3829. doi: 10.1128/jb.172.7.3826-3829.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biswas G D, Anderson J E, Chen C-J, Cornelissen C N, Sparling P F. Identification and functional characterization of the Neisseria gonorrhoeae lbpB gene product. Infect Immun. 1999;67:455–459. doi: 10.1128/iai.67.1.455-459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biswas G D, Sparling P F. Characterization of lbpA, the structural gene for a lactoferrin receptor in Neisseria gonorrhoeae. Infect Immun. 1995;63:2958–2967. doi: 10.1128/iai.63.8.2958-2967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanton K J, Biswas G D, Tsai J, Adams J, Dyer D W, Davis S M, Koch G G, Sen P K, Sparling P F. Genetic evidence that Neisseria gonorrhoeae produces specific receptors for transferrin and lactoferrin. J Bacteriol. 1990;172:5225–5235. doi: 10.1128/jb.172.9.5225-5235.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnah R A, Schryvers A B. Preparation and characterization of Neisseria meningitidis mutants deficient in production of the human lactoferrin-binding proteins LbpA and LbpB. J Bacteriol. 1998;180:3080–3090. doi: 10.1128/jb.180.12.3080-3090.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braun V, Gunter K, Hantke K. Transport of iron across the outer membrane. Biol Met. 1991;4:14–22. doi: 10.1007/BF01135552. [DOI] [PubMed] [Google Scholar]
- 8.Briat J-F. Iron assimilation and storage in prokaryotes. J Gen Microbiol. 1992;138:2475–2483. doi: 10.1099/00221287-138-12-2475. [DOI] [PubMed] [Google Scholar]
- 9.Brunham R C, Plummer F A, Stephens R S. Bacterial antigenic variation, host immune response, and pathogen-host coevolution. Infect Immun. 1993;61:2273–2276. doi: 10.1128/iai.61.6.2273-2276.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchanan S K, Smith B S, Venkatramani L, Xia D, Esser L, Palnitkar M, Chakraborty R, van der Helm D, Deisenhofer J. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat Struct Biol. 1999;6:56–63. doi: 10.1038/4931. [DOI] [PubMed] [Google Scholar]
- 11.Cohen M S, Cannon J G, Jerse A E, Charniga L M, Isbey S F, Whicker L G. Human experimentation with Neisseria gonorrhoeae: rationale, methods, and implications for the biology of infection and vaccine development. J Infect Dis. 1994;169:532–537. doi: 10.1093/infdis/169.3.532. [DOI] [PubMed] [Google Scholar]
- 12.Connell T D, Shaffer D, Cannon J D. Characterization of the repertoire of hypervariable regions in the Protein II (opa) gene family of Neisseria gonorrhoeae. Mol Microbiol. 1990;4:439–449. doi: 10.1111/j.1365-2958.1990.tb00610.x. [DOI] [PubMed] [Google Scholar]
- 13.Cornelissen C N, Anderson J E, Sparling P F. Characterization of the diversity and the transferrin-binding domain of gonococcal transferrin-binding protein 2. Infect Immun. 1997;65:822–828. doi: 10.1128/iai.65.2.822-828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornelissen C N, Anderson J E, Sparling P F. Energy-dependent changes in the gonococcal transferrin receptor. Mol Microbiol. 1997;26:25–35. doi: 10.1046/j.1365-2958.1997.5381914.x. [DOI] [PubMed] [Google Scholar]
- 15.Cornelissen C N, Biswas G D, Tsai J, Paruchuri D K, Thompson S A, Sparling P F. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J Bacteriol. 1992;174:5788–5797. doi: 10.1128/jb.174.18.5788-5797.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornelissen C N, Kelley M, Hobbs M M, Anderson J E, Cannon J G, Cohen M S, Sparling P F. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol Microbiol. 1998;27:611–616. doi: 10.1046/j.1365-2958.1998.00710.x. [DOI] [PubMed] [Google Scholar]
- 17.Cornelissen C N, Sparling P F. Binding and surface exposure characteristics of the gonococcal transferrin receptor are dependent on both transferrin-binding proteins. J Bacteriol. 1996;178:1437–1444. doi: 10.1128/jb.178.5.1437-1444.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornelissen C N, Sparling P F. Iron piracy: acquisition of transferrin-bound iron by bacterial pathogens. Mol Microbiol. 1994;14:843–850. doi: 10.1111/j.1365-2958.1994.tb01320.x. [DOI] [PubMed] [Google Scholar]
- 19.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dyer D W, McKenna W, Woods J P, Sparling P F. Isolation by streptonigrin enrichment and characterization of a transferrin-specific iron uptake mutant of Neisseria meningitidis. Microb Pathog. 1987;3:351–363. doi: 10.1016/0882-4010(87)90005-2. [DOI] [PubMed] [Google Scholar]
- 21.Gonzalez G C, Caamano D L, Schryvers A B. Identification and characterization of a porcine-specific transferrin receptor in Actinobacillus pleuropneumoniae. Mol Microbiol. 1990;4:1173–1179. doi: 10.1111/j.1365-2958.1990.tb00692.x. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez G C, Yu R-H, Rosteck P R, Schryvers A B. Sequence, genetic analysis, and expression of Actinobacillus pleuropneumoniae transferrin receptor genes. Microbiology. 1995;141:2405–2416. doi: 10.1099/13500872-141-10-2405. [DOI] [PubMed] [Google Scholar]
- 23.Gray-Owen S D, Loosmore S, Schryvers A B. Identification and characterization of genes encoding the human transferrin-binding proteins from Haemophilus influenzae. Infect Immun. 1995;63:1201–1210. doi: 10.1128/iai.63.4.1201-1210.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray-Owen S D, Schryvers A B. Bacterial transferrin and lactoferrin receptors. Trends Microbiol. 1996;4:185–191. doi: 10.1016/0966-842x(96)10025-1. [DOI] [PubMed] [Google Scholar]
- 25.Haas R, Meyer T F. The repertoire of silent pilus genes in Neisseria gonorrhoeae: evidence for gene conversion. Cell. 1986;44:107–115. doi: 10.1016/0092-8674(86)90489-7. [DOI] [PubMed] [Google Scholar]
- 26.Hagblom P, Segal E, Billyard E, So M. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. Nature. 1985;315:156–158. doi: 10.1038/315156a0. [DOI] [PubMed] [Google Scholar]
- 27.Heller K J, Kadner R J, Gunther K. Suppression of the btuB451 mutation by mutations in the tonB gene suggests a direct interaction between TonB and TonB-dependent receptor proteins in the outer membrane of Escherichia coli. Gene. 1988;64:147–153. doi: 10.1016/0378-1119(88)90488-x. [DOI] [PubMed] [Google Scholar]
- 28.Kadner R J. Vitamin B12 transport in Escherichia coli: energy coupling between membranes. Mol Microbiol. 1990;4:2027–2033. doi: 10.1111/j.1365-2958.1990.tb00562.x. [DOI] [PubMed] [Google Scholar]
- 29.Kellogg D S, Jr, Peacock W L, Jr, Deacon W E, Brown L, Pirkle C I. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J Bacteriol. 1963;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klebba P E, Rutz J M, Liu J, Murphy C K. Mechanisms of TonB-catalyzed iron transport through the enteric bacterial cell envelope. J Bioenerg Biomembr. 1993;25:603–611. doi: 10.1007/BF00770247. [DOI] [PubMed] [Google Scholar]
- 31.Koebnik R, Braun V. Insertion derivatives containing segments of up to 16 amino acids identify surface- and periplasm-exposed regions of the FhuA outer membrane receptor of Escherichia coli K-12. J Bacteriol. 1993;175:826–839. doi: 10.1128/jb.175.3.826-839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsen R A, Foster-Hartnett D, McIntosh M A, Postle K. Regions of Escherichia coli TonB and FepA proteins essential for in vivo physical interactions. J Bacteriol. 1997;179:3213–3221. doi: 10.1128/jb.179.10.3213-3221.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee B C, Schryvers A B. Specificity of the lactoferrin and transferrin receptors in Neisseria gonorrhoeae. Mol Microbiol. 1988;2:827–829. doi: 10.1111/j.1365-2958.1988.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 34.Legrain M, Mazarin V, Irwin S W, Bouchon B, Quentin-Millet M-J, Jacobs E, Schryvers A B. Cloning and characterization of Neisseria meningitidis genes encoding the transferrin-binding proteins Tbp1 and Tbp2. Gene. 1993;130:73–80. doi: 10.1016/0378-1119(93)90348-7. [DOI] [PubMed] [Google Scholar]
- 35.Lewis L A, Gray E, Wang Y-P, Roe B A, Dyer D W. Molecular characterization of hpuAB, the haemoglobin-haptoglobin-utilization operon of Neisseria meningitidis. Mol Microbiol. 1997;23:737–749. doi: 10.1046/j.1365-2958.1997.2501619.x. [DOI] [PubMed] [Google Scholar]
- 36.Lissolo L, Maitre-Wilmotte G, Dumas P, Mignon M, Danve D, Quentin-Millet M-J. Evaluation of transferrin-binding protein 2 within the transferrin-binding complex as a potential antigen for future meningococcal vaccines. Infect Immun. 1995;63:884–890. doi: 10.1128/iai.63.3.884-890.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Locher K P, Rees B, Koebnik R, Mitschler A, Moulinier L, Rosenbusch J P, Moras D. Transmembrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell. 1998;95:771–778. doi: 10.1016/s0092-8674(00)81700-6. [DOI] [PubMed] [Google Scholar]
- 38.Loosmore S M, Yang Y P, Coleman D C, Shortreed J M, England D M, Harkness R E, Chong P S C, Klein M H. Cloning and expression of the Haemophilus influenzae transferrin receptor genes. Mol Microbiol. 1996;19:575–586. doi: 10.1046/j.1365-2958.1996.406943.x. [DOI] [PubMed] [Google Scholar]
- 39.Mickelsen P A, Blackman E, Sparling P F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from lactoferrin. Infect Immun. 1982;35:915–920. doi: 10.1128/iai.35.3.915-920.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mickelsen P A, Sparling P F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect Immun. 1981;33:555–564. doi: 10.1128/iai.33.2.555-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy C K, Kalve V I, Klebba P E. Surface topology of the Escherichia coli K-12 ferric enterobactin receptor. J Bacteriol. 1990;172:2736–2746. doi: 10.1128/jb.172.5.2736-2746.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Myers L E, Yang Y-P, Du R-P, Wang Q, Harkness R E, Schryvers A B, Klein M H, Loosmoore S M. The transferrin-binding protein B of Moraxella catarrhalis elicits bactericidal antibodies and is a potential vaccine antigen. Infect Immun. 1998;66:4183–4192. doi: 10.1128/iai.66.9.4183-4192.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neilands J B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- 44.Neilands J B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- 45.Newton S M C, Allen J S, Cao Z, Qi Z, Jiang X, Sprencel C, Igo J D, Foster S B, Payne M A, Klebba P E. Double mutagenesis of a positive charge cluster in the ligand-binding site of the ferric enterobactin receptor, FepA. Proc Natl Acad Sci USA. 1997;94:4560–4565. doi: 10.1073/pnas.94.9.4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogunnariwo J A, Woo T K W, Lo R Y C, Gonzalez G C, Schryvers A B. Characterization of the Pasteurella haemolytica transferrin receptor genes and the recombinant receptor proteins. Microb Pathog. 1997;23:273–284. doi: 10.1006/mpat.1997.0156. [DOI] [PubMed] [Google Scholar]
- 47.Pettersson A, Prinz T, Umar A, van der Biezen J, Tommassen J. Molecular characterization of LbpB, the second lactoferrin-binding protein of Neisseria meningitidis. Mol Microbiol. 1998;27:599–610. doi: 10.1046/j.1365-2958.1998.00707.x. [DOI] [PubMed] [Google Scholar]
- 48.Postle K. TonB protein and energy transduction between membranes. J Bioenerg Biomembr. 1993;25:591–601. doi: 10.1007/BF00770246. [DOI] [PubMed] [Google Scholar]
- 49.Rokbi B, Mazarin V, Maitre-Wilmotte G, Quentin-Millet M-J. Identification of two major families of transferrin receptors among Neisseria meningitidis strains based on antigenic and genomic features. FEMS Microbiol Lett. 1993;110:51–58. doi: 10.1111/j.1574-6968.1993.tb06294.x. [DOI] [PubMed] [Google Scholar]
- 50.Rokbi B, Mignon M, Maitre-Wilmotte G, Lissolo L, Danve B, Caugant D A, Quentin-Millet M-J. Evaluation of recombinant transferrin-binding protein B variants from Neisseria meningitidis for their ability to induce cross-reactive and bactericidal antibodies against a genetically diverse collection of serogroup B strains. Infect Immun. 1997;65:55–63. doi: 10.1128/iai.65.1.55-63.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schirmer T, Keller T A, Wang Y-F, Rosenbusch J P. Structural basis for sugar translocation through maltoporin channels at 3.1 Å resolution. Science. 1995;267:512–514. doi: 10.1126/science.7824948. [DOI] [PubMed] [Google Scholar]
- 52.Schoffler H, Braun V. Transport across the outer membrane of Escherichia coli K12 via the FhuA receptor is regulated by the TonB protein of the cytoplasmic membrane. Mol Gen Genet. 1989;217:378–383. doi: 10.1007/BF02464907. [DOI] [PubMed] [Google Scholar]
- 53.Schramm E, Mende J, Braun V, Kamp R M. Nucleotide sequence of the colicin B activity gene cba: consensus peptapeptide among TonB-dependent colicins and receptors. J Bacteriol. 1987;169:3350–3357. doi: 10.1128/jb.169.7.3350-3357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stijilkovic I, Hwa V, de Saint Martin L, O'Gaora P, Nassif X, Heffron F, So M. The Neisseria meningitidis haemoglobin receptor: its role in iron utilization and virulence. Mol Microbiol. 1995;15:531–541. doi: 10.1111/j.1365-2958.1995.tb02266.x. [DOI] [PubMed] [Google Scholar]
- 55.Weinberg E D. Iron and infection. Microbiol Rev. 1978;42:45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.West S E H, Sparling P F. Aerobactin utilization by Neisseria gonorrhoeae and cloning of a genomic DNA fragment that complements Escherichia coli fhuB mutations. J Bacteriol. 1987;169:3414–3421. doi: 10.1128/jb.169.8.3414-3421.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]